Synthesis of Keratin Nanoparticles Extracted from Human Hair through Hydrolysis with Concentrated Sulfuric Acid: Characterization and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
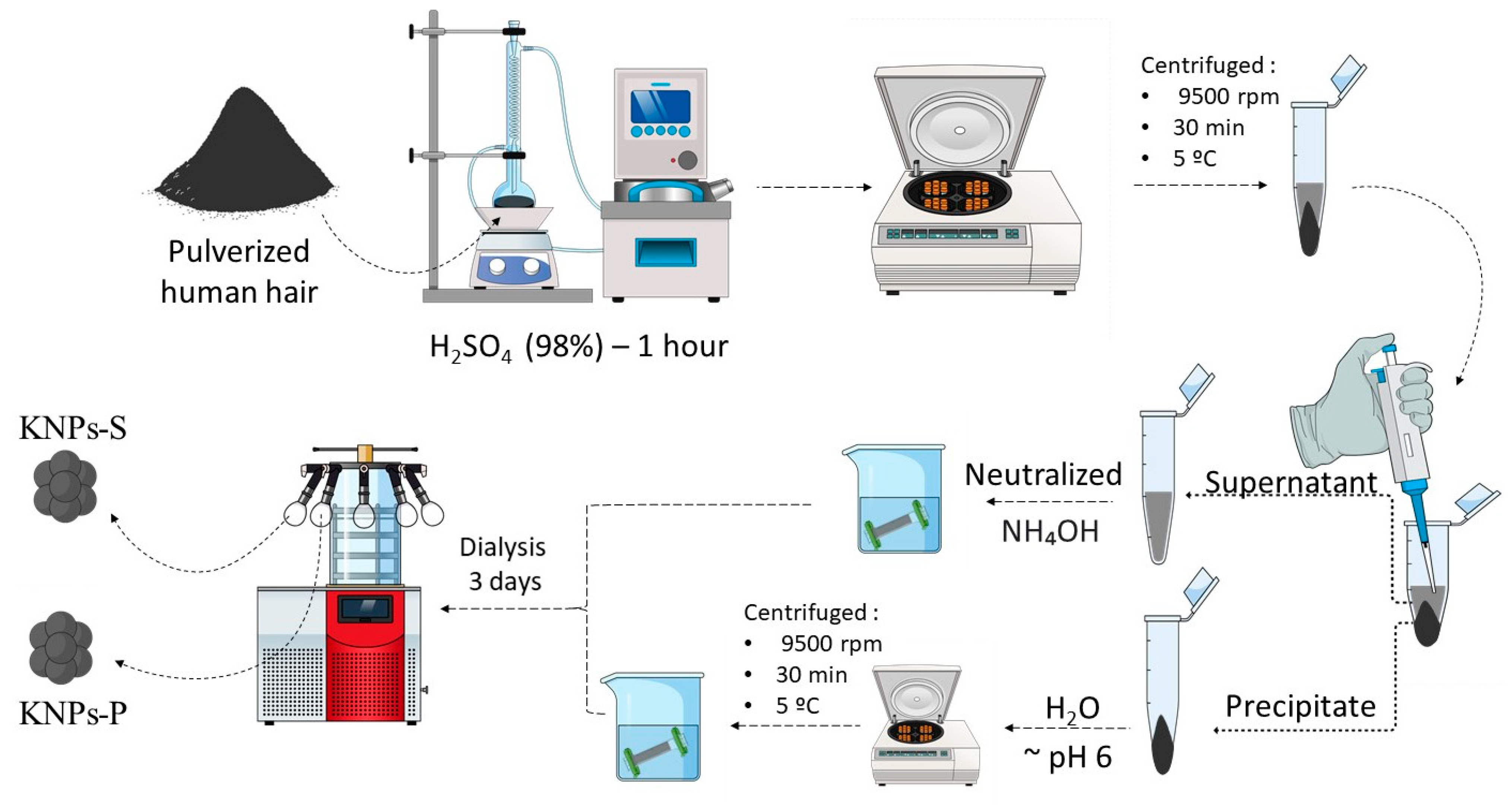
2.2.1. Pre-Treatment of Human Hair
2.2.2. Extraction and Synthesis of Keratin Nanoparticles (KNPs)
2.3. Characterization
2.4. Cytotoxicity
2.5. Hemolysis Assay
3. Results and Discussion
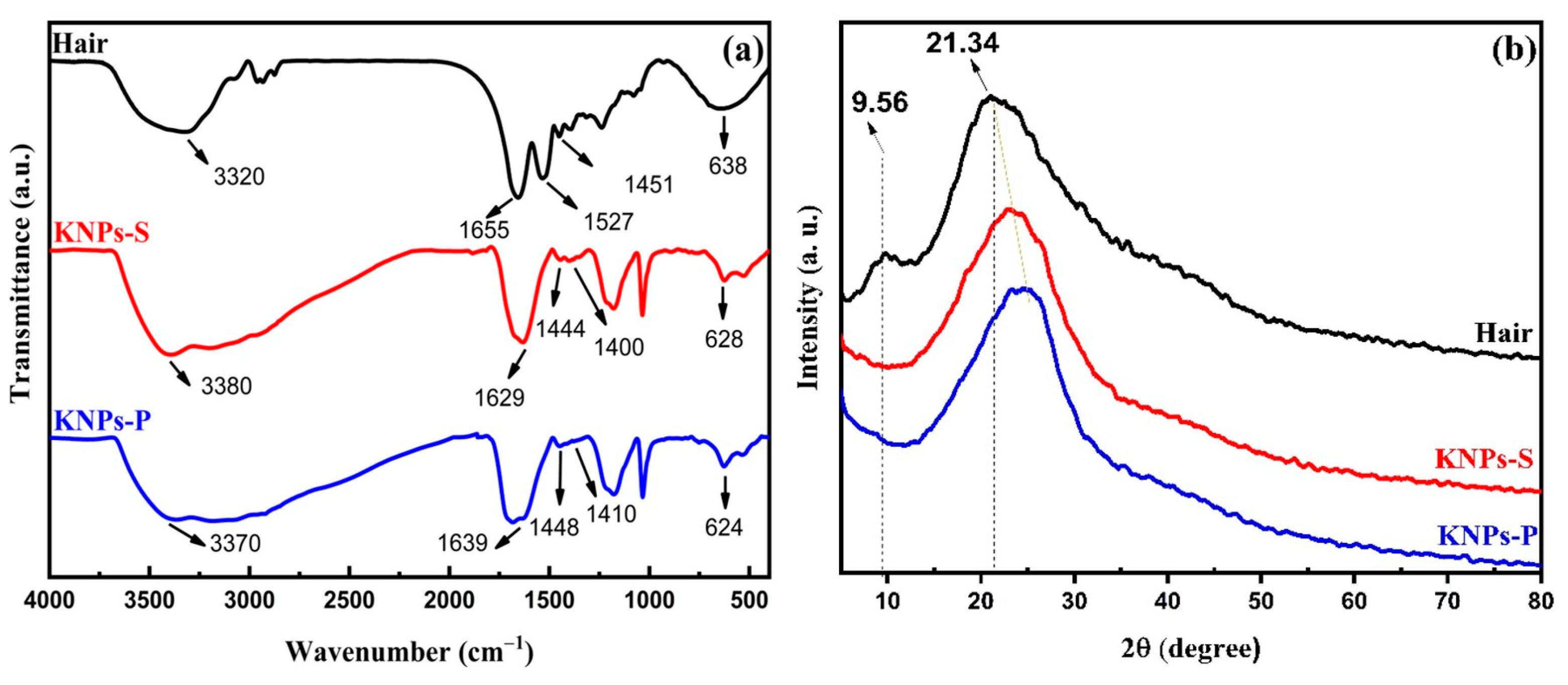
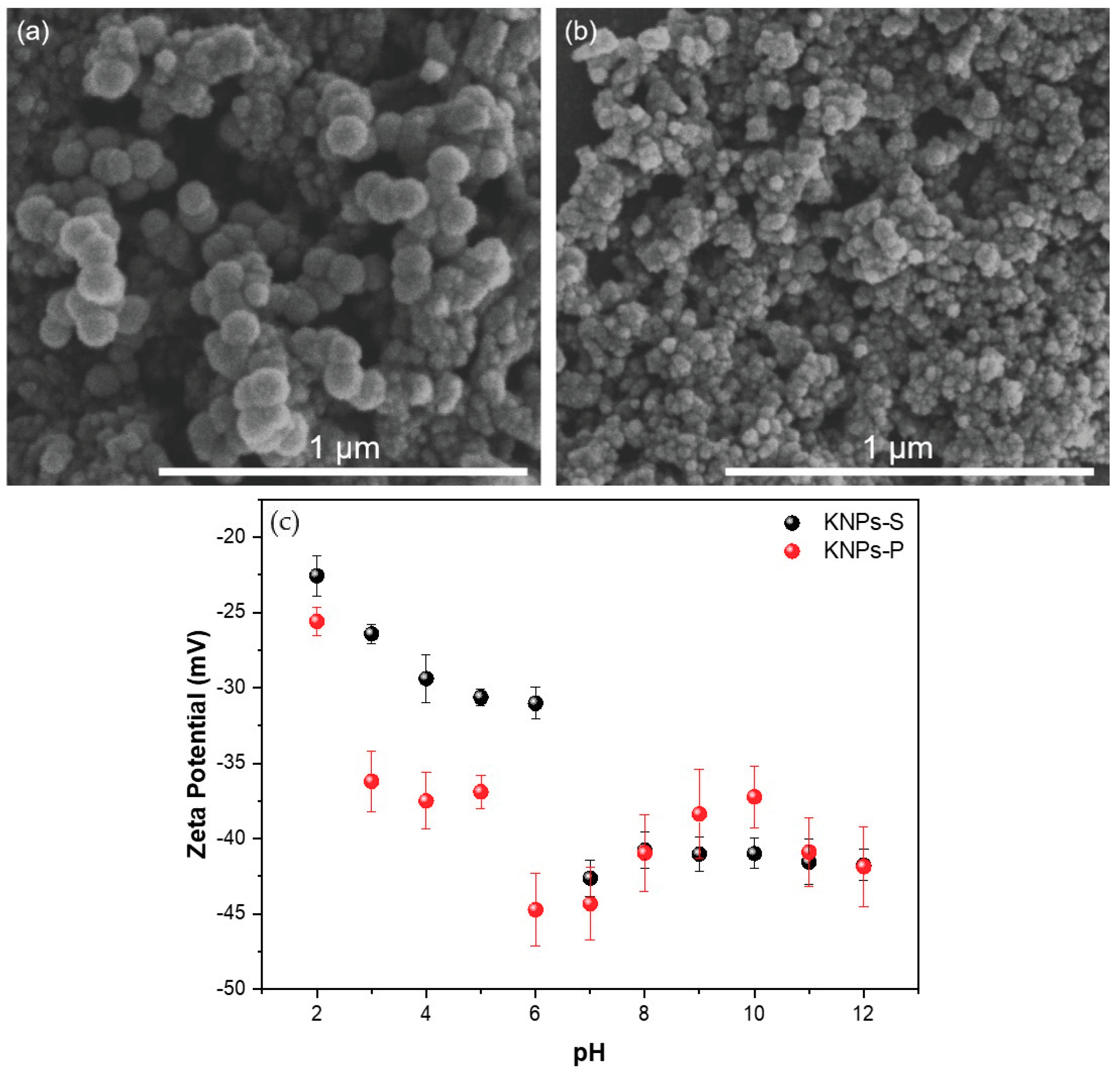
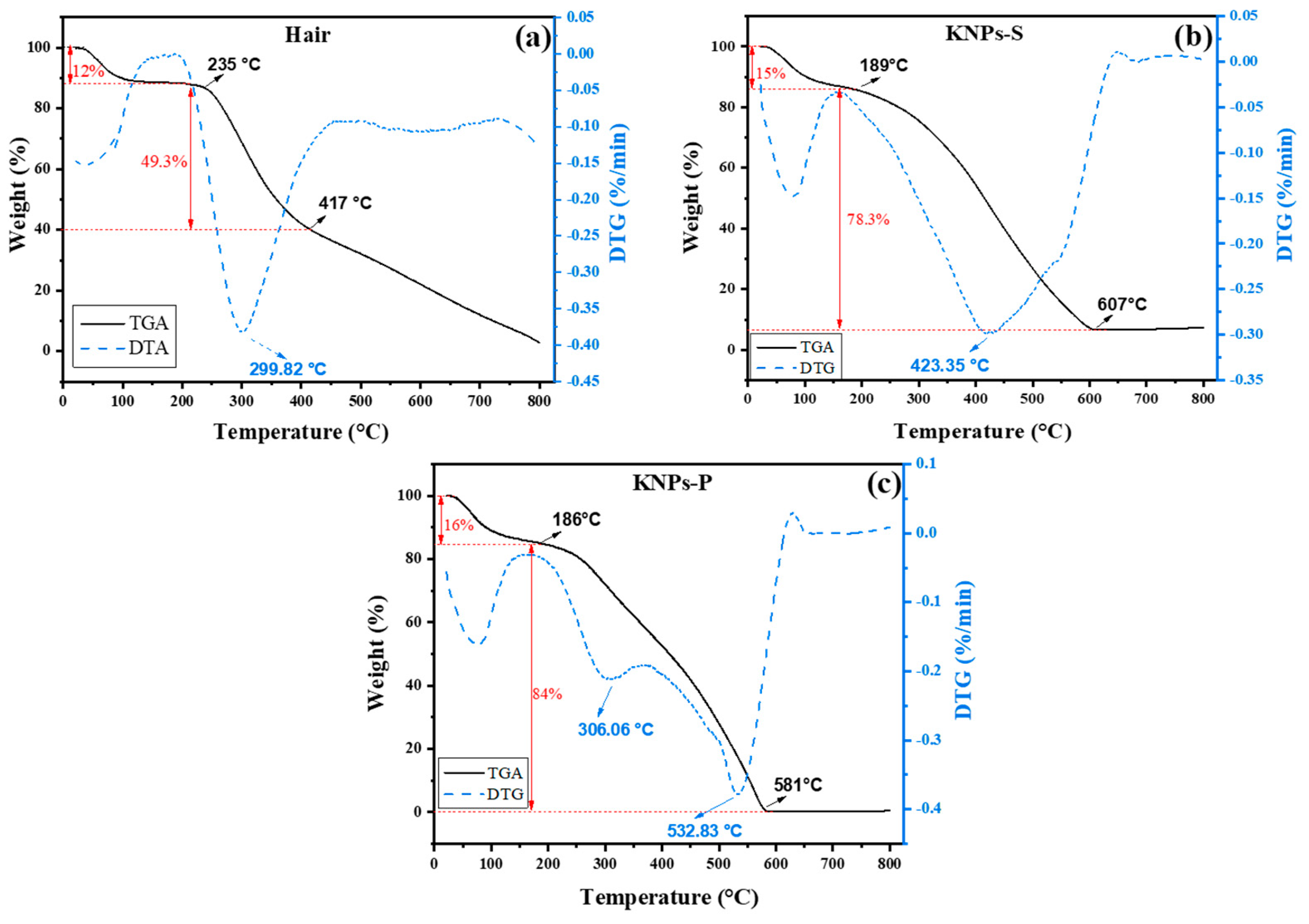
3.1. Characterization
3.2. Cytotoxicity
3.3. Hemolytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaitanya Reddy, C.; Khilji, I.A.; Gupta, A.; Bhuyar, P.; Mahmood, S.; Saeed AL-Japairai, K.A.; Chua, G.K. Valorization of Keratin Waste Biomass and Its Potential Applications. J. Water Process Eng. 2021, 40, 101707. [Google Scholar] [CrossRef]
- Ferroni, C.; Varchi, G. Keratin-Based Nanoparticles as Drug Delivery Carriers. Appl. Sci. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Kokot, S. Keratin. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 4363–4368. ISBN 978-0-08-043152-9. [Google Scholar]
- Xu, S.; Sang, L.; Zhang, Y.; Wang, X.; Li, X. Biological Evaluation of Human Hair Keratin Scaffolds for Skin Wound Repair and Regeneration. Mater. Sci. Eng. C 2013, 33, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin—Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of Keratin Biomaterial Containing Silver Nanoparticles as a Potential Wound Dressing in Full-thickness Skin Wound Model in Diabetic Mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.K.; Mija, A. Keratin Associations with Synthetic, Biosynthetic and Natural Polymers: An Extensive Review. Polymers 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and Application of Keratin from Natural Resources: A Review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef]
- Diwan, H.; Sah, M.K. Exploring the Potential of Keratin-Based Biomaterials in Orthopedic Tissue Engineering: A Comprehensive Review. Emergent Mater. 2023, 6, 1441–1460. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Li, Y.; Xu, H.-J.; Li, W.; Wang, J.; Zhang, Y. Valorization of Livestock Keratin Waste: Application in Agricultural Fields. Int. J. Environ. Res. Public Health 2022, 19, 6681. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhang, S. A Multifunctional Eco-Friendly Fertilizer Used Keratin-Based Superabsorbent as Coatings for Slow-Release Urea and Remediation of Contaminated Soil. Prog. Org. Coat. 2021, 154, 106158. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Seghir, B.B.; Hemmami, H.; Zeghoud, S.; Laouini, S.E.; Rebiai, A.; Amor, I.B.; Souici, I.; Beki, A. Preparation Methods Keratin and Nanoparticles Keratin from Wool: A Review. Alger. J. Chem. Eng. AJCE 2020, 1, 5–11. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, M.; Liu, P. Keratin-Based Drug-Protein Conjugate with Acid-Labile and Reduction-Cleavable Linkages in Series for Tumor Intracellular DOX Delivery. J. Ind. Eng. Chem. 2019, 80, 739–748. [Google Scholar] [CrossRef]
- Onifade, A.A.; Al-Sane, N.A.; Al-Musallam, A.A.; Al-Zarban, S. A Review: Potentials for Biotechnological Applications of Keratin-Degrading Microorganisms and Their Enzymes for Nutritional Improvement of Feathers and Other Keratins as Livestock Feed Resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Qin, C.; Zhao, Z.; Yuan, H.; Wei, J.; Nie, Y. Experimental and Theoretical Study on the Extraction of Keratin from Human Hair Using Protic Ionic Liquids. J. Mol. Liq. 2022, 368, 120626. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Valorization of Keratin Based Waste. Process Saf. Environ. Prot. 2018, 115, 85–98. [Google Scholar] [CrossRef]
- Ye, J.-P.; Gong, J.-S.; Su, C.; Liu, Y.-G.; Jiang, M.; Pan, H.; Li, R.-Y.; Geng, Y.; Xu, Z.-H.; Shi, J.-S. Fabrication and Characterization of High Molecular Keratin Based Nanofibrous Membranes for Wound Healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial Enzymes Catalyzing Keratin Degradation: Classification, Structure, Function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef]
- Sarma, A. Biological Importance and Pharmaceutical Significance of Keratin: A Review. Int. J. Biol. Macromol. 2022, 219, 395–413. [Google Scholar] [CrossRef]
- Anil, S.; Sweety, V.; Vikas, B.; Joseph, B. Cytotoxicity and Cell Viability Assessment of Biomaterials. In Cytotoxicity—Understanding Cellular Damage and Response; IntechOpen: London, UK, 2023; ISBN 978-1-80356-245-2. [Google Scholar]
- Silva, O.A.; Pellá, M.G.; Popat, K.C.; Kipper, M.J.; Rubira, A.F.; Martins, A.F.; Follmann, H.D.M.; Silva, R. Rod-Shaped Keratin Nanoparticles Extracted from Human Hair by Acid Hydrolysis as Photothermally Triggered Berberine Delivery System. Adv. Powder Technol. 2022, 33, 103353. [Google Scholar] [CrossRef]
- Truzzi, F.; Mandrioli, D.; Gnudi, F.; Scheepers, P.T.J.; Silbergeld, E.K.; Belpoggi, F.; Dinelli, G. Comparative Evaluation of the Cytotoxicity of Glyphosate-Based Herbicides and Glycine in L929 and Caco2 Cells. Front. Public Health 2021, 9, 643898. [Google Scholar] [CrossRef] [PubMed]
- Tentor, F.R.; de Oliveira, J.H.; Scariot, D.B.; Lazarin-Bidóia, D.; Bonafé, E.G.; Nakamura, C.V.; Venter, S.A.S.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Scaffolds Based on Chitosan/Pectin Thermosensitive Hydrogels Containing Gold Nanoparticles. Int. J. Biol. Macromol. 2017, 102, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of pH-Responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs. J. Vis. Exp. 2013, 73, e50166. [Google Scholar] [CrossRef]
- Widmer, F.; Wright, L.C.; Obando, D.; Handke, R.; Ganendren, R.; Ellis, D.H.; Sorrell, T.C. Hexadecylphosphocholine (Miltefosine) Has Broad-Spectrum Fungicidal Activity and Is Efficacious in a Mouse Model of Cryptococcosis. Antimicrob. Agents Chemother. 2006, 50, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Nathani, A.; Rajendran, A.; Sharma, C.S.; Lenka, N.; Gupta, P. Investigation of Human Hair Keratin-Based Nanofibrous Scaffold for Skin Tissue Engineering Application. Drug Deliv. Transl. Res. 2024, 14, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Hao, S.; Hu, W.; Wang, M.; Zang, Z.; Zhu, L.; Du, J.; Tang, X. Human Hair Keratin for Physically Transient Resistive Switching Memory Devices. J. Mater. Chem. C 2019, 7, 3315–3321. [Google Scholar] [CrossRef]
- Shui-qing, J.; Lin, Z.; Haixia, W.; Gang, C. Study on Effective Extraction of Keratin from Human Hair Wastes. Integr. Ferroelectr. 2017, 180, 102–107. [Google Scholar] [CrossRef]
- Agarwal, V.; Panicker, A.G.; Indrakumar, S.; Chatterjee, K. Comparative Study of Keratin Extraction from Human Hair. Int. J. Biol. Macromol. 2019, 133, 382–390. [Google Scholar] [CrossRef]
- Gniadecka, M.; Nielsen, O.F.; Christensen, D.H.; Wulf, H.C. Structure of Water, Proteins, and Lipids in Intact Human Skin, Hair, and Nail. J. Investig. Dermatol. 1998, 110, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Wang, Y.; Li, P.; Yuan, J.; Shen, J. Preparation of Keratin/Chlorhexidine Complex Nanoparticles for Long-Term and Dual Stimuli-Responsive Release. RSC Adv. 2015, 5, 82334–82341. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Staroszczyk, H.; Śliwińska, A. Solubilization of Keratins and Functional Properties of Their Isolates and Hydrolysates. J. Food Biochem. 2018, 42, e12494. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and Properties of Granular Cold-Water-Soluble Porous Starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure Keratin Membrane and Fibers from Chicken Feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Vijayaraghavan, R.; Patti, A.F.; MacFarlane, D.R. Distillable Protic Ionic Liquids for Keratin Dissolution and Recovery. ACS Sustain. Chem. Eng. 2014, 2, 1888–1894. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and Chemical Stability of Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Tavakkoli Yaraki, M.; Farrokhnia, A.; Bamdad, M. Keratin Nanoparticles Obtained from Human Hair for Removal of Crystal Violet from Aqueous Solution: Optimized by Taguchi Method. Int. J. Biol. Macromol. 2020, 143, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and Kinetics Study of Spherical Cellulose Nanocrystal Extracted from Cotton Cloth Waste by Acid Hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, Y.; Qin, M.; Wu, K.; Fu, Y.; Zhao, J. Nanocrystalline Cellulose from Aspen Kraft Pulp and Its Application in Deinked Pulp. Int. J. Biol. Macromol. 2013, 60, 241–247. [Google Scholar] [CrossRef]
- Shao, X.; Wei, X.; Song, X.; Hao, L.; Cai, X.; Zhang, Z.; Peng, Q.; Lin, Y. Independent Effect of Polymeric Nanoparticle Zeta Potential/Surface Charge, on Their Cytotoxicity and Affinity to Cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Prevention of Nanoparticle Aggregation during Freeze-Drying. J. Control. Release 2017, 248, 153. [Google Scholar] [CrossRef] [PubMed]
- Degobert, G.; Aydin, D. Lyophilization of Nanocapsules: Instability Sources, Formulation and Process Parameters. Pharmaceutics 2021, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Preparation and Characterization of Nanocomposites of Polyvinyl Alcohol/Cellulose Nanowhiskers/Chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Pakdel, M.; Moosavi-Nejad, Z.; Kermanshahi, R.K.; Hosano, H. Self-Assembled Uniform Keratin Nanoparticles as Building Blocks for Nanofibrils and Nanolayers Derived from Industrial Feather Waste. J. Clean. Prod. 2022, 335, 130331. [Google Scholar] [CrossRef]
- Liu, L.-R.; Huang, M.-C.; Lee, Z.-J.; Wei, Y. Rational Design of Keratin Nanoparticles Utilizing Diverse Hair Protein Fractions for Controlled Drug Release. J. Taiwan Inst. Chem. Eng. 2023, 160, 105240. [Google Scholar] [CrossRef]
- Mothé, M.G.; Viana, L.M.; Mothé, C.G. Thermal Property Study of Keratin from Industrial Residue by Extraction, Processing and Application. J. Therm. Anal. Calorim. 2018, 131, 417–426. [Google Scholar] [CrossRef]
- Ge, N.; Zhang, Y.; Zhang, H.; Zhu, R.; Shi, X. Preparation and Characterization of Rabbit Hair Keratin Hydrogel. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1040, 012003. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Methods of Keratin Extraction from Poultry Feathers and Their Effects on Antioxidant Activity of Extracted Keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef]
- Li, Y.; Zhi, X.; Lin, J.; You, X.; Yuan, J. Preparation and Characterization of DOX Loaded Keratin Nanoparticles for pH/GSH Dual Responsive Release. Mater. Sci. Eng. C 2017, 73, 189–197. [Google Scholar] [CrossRef]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental Considerations on The Cytotoxicity of Nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.K.; Loizidou, M.; Seifalian, A.M. Toxicology and Clinical Potential of Nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-Dependent Toxicity and Cell Interaction Mechanisms of Gold Nanoparticles on Mouse Fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef]
- Jonavičė, U.; Romenskaja, D.; Kriaučiūnaitė, K.; Jarmalavičiūtė, A.; Pajarskienė, J.; Kašėta, V.; Tunaitis, V.; Malm, T.; Giniatullin, R.; Pivoriūnas, A. Extracellular Vesicles from Human Teeth Stem Cells Trigger ATP Release and Promote Migration of Human Microglia through P2X4 Receptor/MFG-E8-Dependent Mechanisms. Int. J. Mol. Sci. 2021, 22, 10970. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.; Alexa, O. Biocompatibility Assessment of Biomaterials Used in Orthopedic Devices: An Overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Fröhlich, E. The Role of Nanoparticle Size in Hemocompatibility. Toxicology 2009, 258, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-F.; Lu, T.-Y.; Liu, Y.-C.; Liu, T.-H.; Feng, C.-C.; Lin, C.-W.; Yang, K.-C.; Wei, Y.; Yu, J. Keratin-Associated Protein Nanoparticles as Hemostatic Agents. ACS Appl. Nano Mater. 2021, 4, 12798–12806. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Zhi, X.; Li, P.; Jiang, X.; Yuan, J. Triple Stimuli-Responsive Keratin Nanoparticles as Carriers for Drug and Potential Nitric Oxide Release. Mater. Sci. Eng. C 2018, 91, 606–614. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, P.; Li, Y.; Li, P.; Yuan, J.; Lin, J. One-Step Fabricated Keratin Nanoparticles as pH and Redox-Responsive Drug Nanocarriers. J. Biomater. Sci. Polym. Ed. 2018, 29, 1920–1934. [Google Scholar] [CrossRef]
- Mukherjee, A.; Pal, S.; Parhi, S.; Karki, S.; Ingole, P.G.; Ghosh, P. One-Pot Extraction of Bioresources from Human Hair via a Zero-Waste Green Route. ACS Omega 2023, 8, 15759–15768. [Google Scholar] [CrossRef]
| Samples | CC50 (µg/mL) | HC50 (µg/mL) |
|---|---|---|
| KNPs-P | 246.21 ± 44.8 | >1000 |
| KNPs-S | 176.67 ± 68.2 | >1000 |
| Samples | Concentration (µg/mL) | Hemolytic Activity (%) |
|---|---|---|
| KNPs-P | 1000 | 3.55 ± 6.14 |
| 500 | 0.64 ± 0.90 | |
| 250 | 0.57 ± 0.98 | |
| 125 | 2.34 ± 3.30 | |
| 62.5 | 2.34 ± 3.30 | |
| KNPs-S | 1000 | 13.6 ± 0.42 |
| 500 | 6.60 ± 3.07 | |
| 250 | 3.19 ± 0.73 | |
| 125 | 0.43 ± 9.32 | |
| 62.5 | 1.49 ± 2.10 |
| Extraction Agent | Temp. (°C)/Time | Zeta (mv) | Size (nm) | Cytotoxicity CC50 (µg/mL) | Hemolysis (%) | Ref. |
|---|---|---|---|---|---|---|
| H2SO4 | 100 °C/1 h | −25.60 ± 0.92 to −41.87 ± 3.63 | KNPs-P: 72 ± 5 | 246.21 ± 44.8 | 3.55 ± 6.14 | This study |
| H2SO4 | 100 °C/1 h | −22.56 ± 1.33 to −41.76 ± 3.03 | KNPs-S: 27 ± 5 | 176.67 ± 68.2 | 13.6 ± 0.42 | this study |
| Na2S | K1 = 40 °C/4.5 h | −19.0 ± 3.5 | - | - | - | [32] |
| C2H4O3 | K2 = Room temp./overnight | −24.5 ± 10.9 | - | - | - | [32] |
| C2H4O2S | K3 = 37 °C/15 h | −20 ± 6 | - | - | - | [32] |
| CH4N2O | K4 = 50 °C/3 days | −17.7 ± 4.7 | - | - | - | [32] |
| HCl | 100 °C/1 h | 20.2 to −34.4 | 190 ± 20 | >2000 | - | [23] |
| NH2C(CH2OH)3, DTT, C2H6O and CH4N2O | 50 °C/72 h | −34.4 ± 8.1 | 190–600 | - | 0.30 ± 0.15, 1.11 ± 1.10 and 1.80 ± 0.65% | [60] |
| [BMIM]Cl and HCl | 175 °C/3 h | - | - | - | 1.8 ± 0.5% | [63] |
| CH4N2O, CH4N2S, NH2C(CH2OH)3 and 2-mercaptoetanol | 50 °C/3 days | 63.7 | - | - | - | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, O.A.; Rossin, A.R.S.; Lima, A.M.d.O.; Valente, A.D.; Garcia, F.P.; Nakamura, C.V.; Follmann, H.D.M.; Silva, R.; Martins, A.F. Synthesis of Keratin Nanoparticles Extracted from Human Hair through Hydrolysis with Concentrated Sulfuric Acid: Characterization and Cytotoxicity. Materials 2024, 17, 3759. https://doi.org/10.3390/ma17153759
Silva OA, Rossin ARS, Lima AMdO, Valente AD, Garcia FP, Nakamura CV, Follmann HDM, Silva R, Martins AF. Synthesis of Keratin Nanoparticles Extracted from Human Hair through Hydrolysis with Concentrated Sulfuric Acid: Characterization and Cytotoxicity. Materials. 2024; 17(15):3759. https://doi.org/10.3390/ma17153759
Chicago/Turabian StyleSilva, Otavio A., Ariane R. S. Rossin, Antônia M. de Oliveira Lima, Andressa D. Valente, Francielle P. Garcia, Celso V. Nakamura, Heveline D. M. Follmann, Rafael Silva, and Alessandro F. Martins. 2024. "Synthesis of Keratin Nanoparticles Extracted from Human Hair through Hydrolysis with Concentrated Sulfuric Acid: Characterization and Cytotoxicity" Materials 17, no. 15: 3759. https://doi.org/10.3390/ma17153759







