Fabrication of Magnetic Poly(L-lactide) (PLLA)/Fe3O4 Composite Electrospun Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Methods
3. Results and Discussion
3.1. Effect of Molecular Weight (PLLA) on Electrospun Fibers
3.2. Effect of Polymer Concentration on Diameters of Electrospun Fibers
3.3. Effect of Electrospinning Mode on Diameter of Fibers
3.4. Thermogravimetric Analysis (TGA)
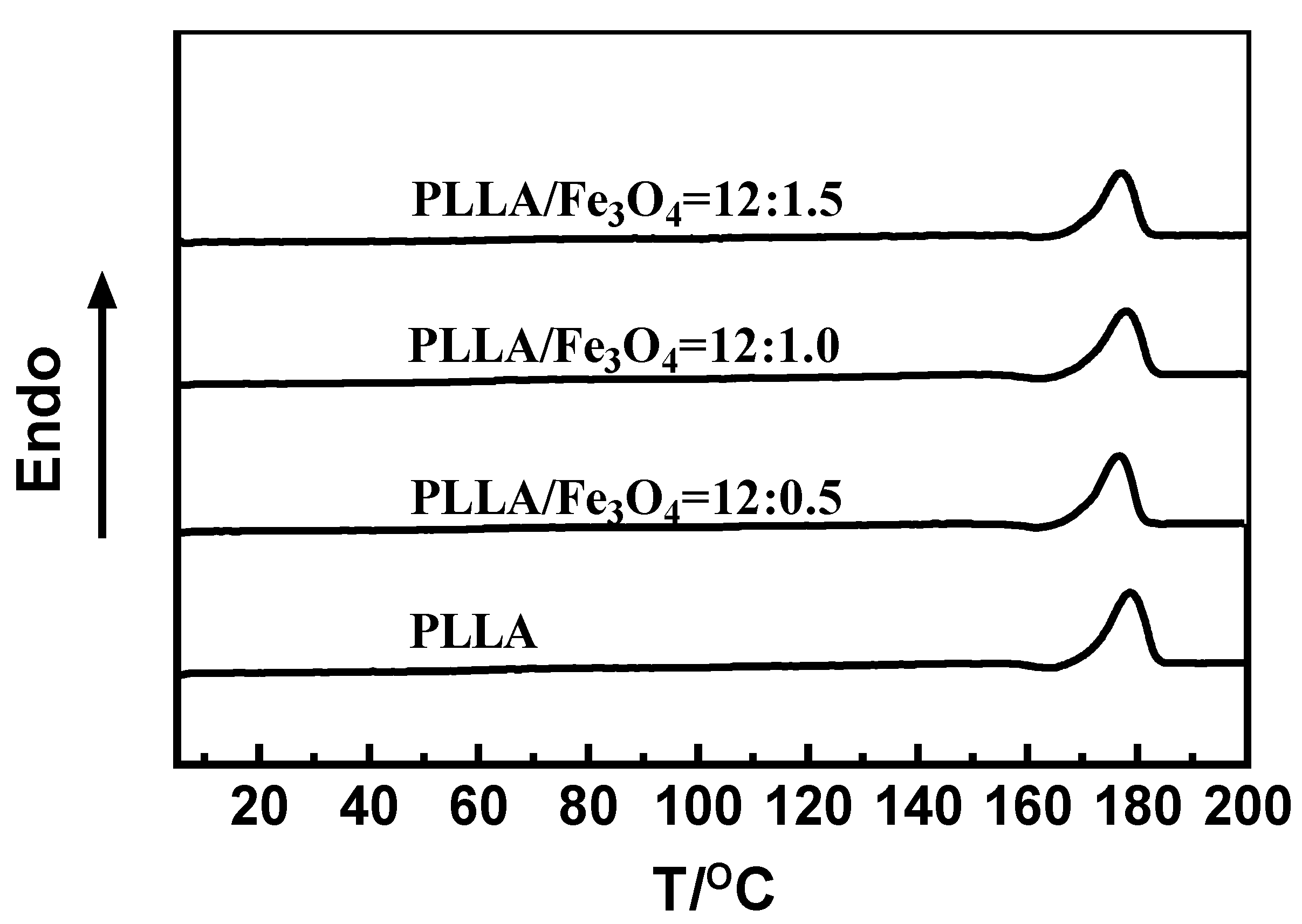
3.5. Differential Scanning Calorimetry (DSC)
3.6. Infrared Spectroscopic Analysis (FTIR)
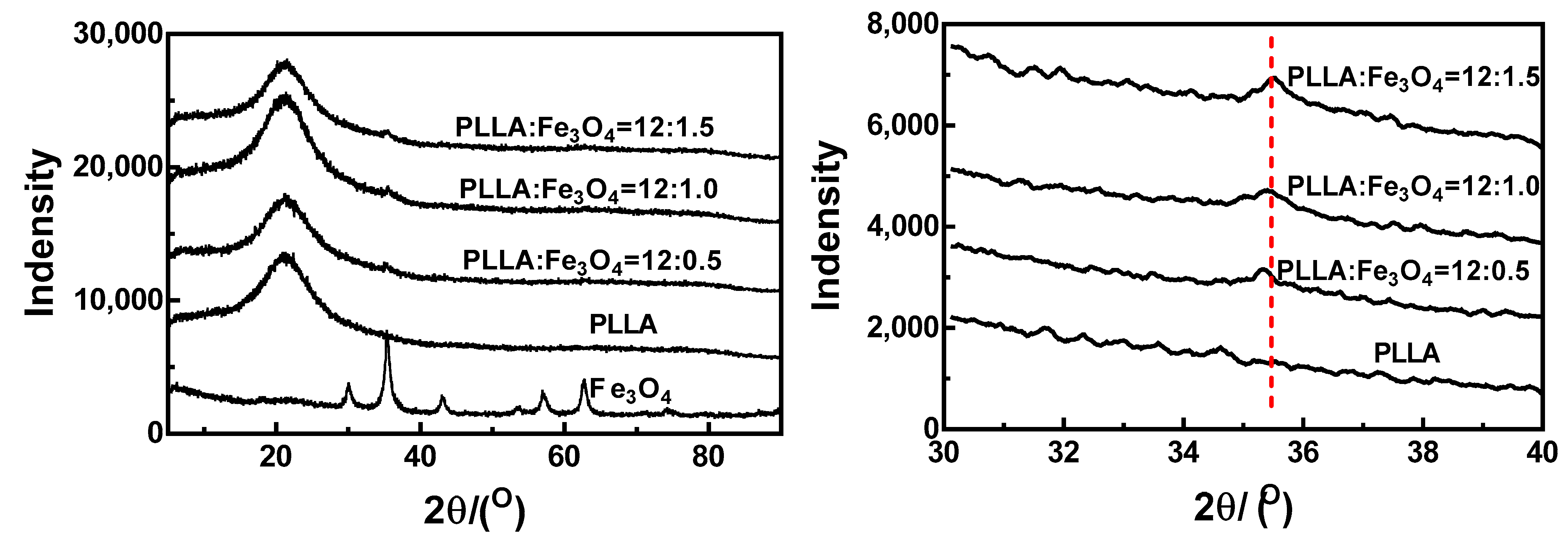
3.7. X-ray Analysis (XRD)
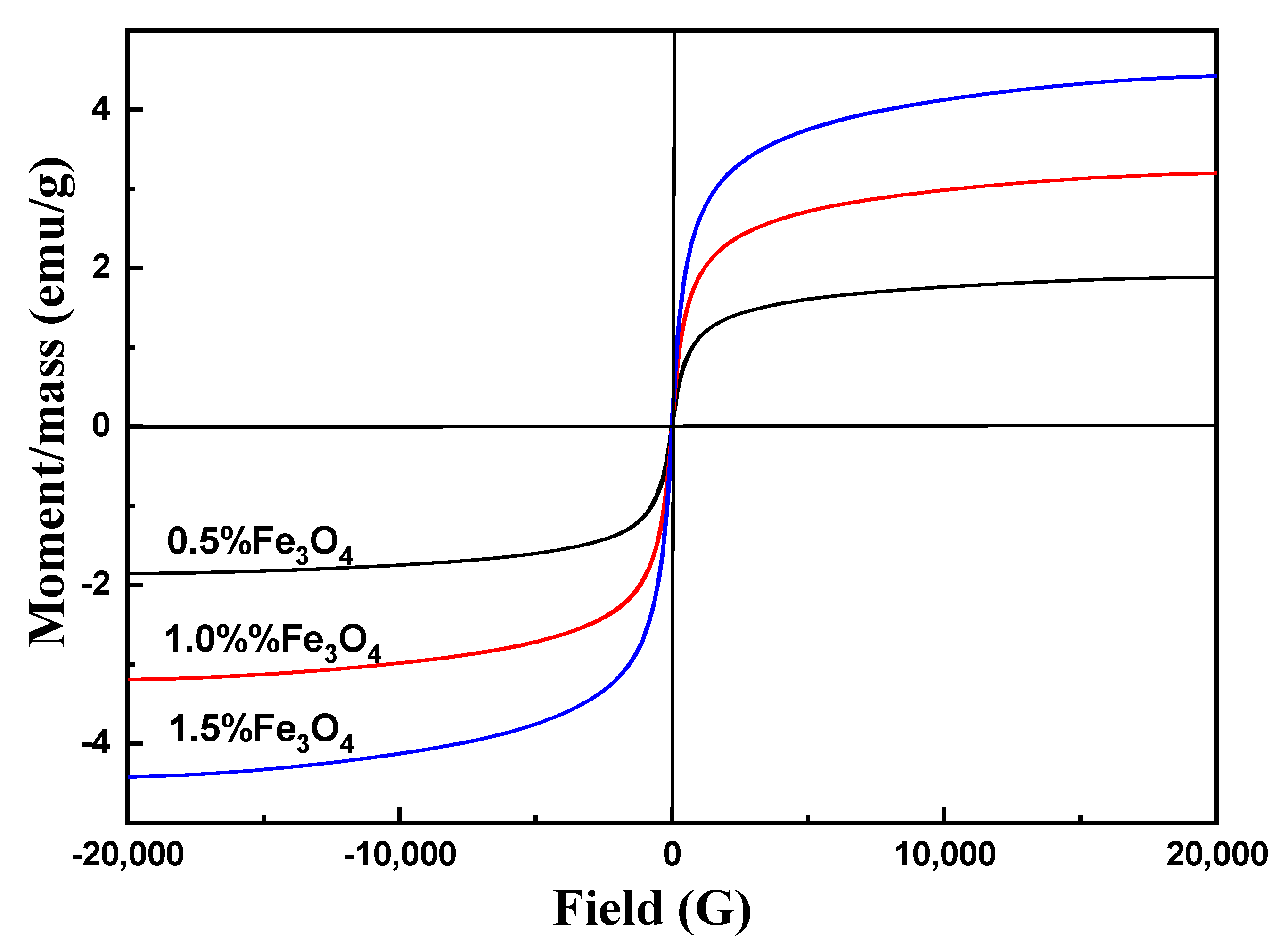
3.8. Magnetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous porous PLLA/PCL fibrous scaffold for bone tissue regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Sadraei, S.M.; Mansoori, R.; Singh Chauhan, N.P.; Sargazi, G. Nanomaterials supported by polymers for tissue engineering applications: A review. Heliyon 2022, 8, e12193. [Google Scholar] [CrossRef]
- Kotlarz, M.; Melo, P.; Ferreira, A.M.; Gentile, P.; Dalgarno, K. Cell seeding via bioprinted hydrogels supports cell migration into porous apatite-wollastonite bioceramic scaffolds for bone tissue engineering. Biomater. Adv. 2023, 153, 213532. [Google Scholar] [CrossRef]
- González, S.G.; Vlad, M.D.; López, J.L.; Aguado, E.F. Novel bio-inspired 3D porous scaffold intended for bone-tissue engineering: Design and in silico characterisation of histomorphometric, mechanical and mass-transport properties. Mater. Desig. 2023, 225, 111467. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Cano-Vicent, A.; Sabater, I.S.R.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio 2022, 16, 100412. [Google Scholar] [CrossRef]
- Entz, L.; Falgayrac, G.; Chauveau, C.; Pasquier, G.; Lucas, S. The extracellular matrix of human bone marrow adipocytes and glucose concentration differentially alter mineralization quality without impairing osteoblastogenesis. Bone Rep. 2022, 17, 101622. [Google Scholar] [CrossRef]
- Seo Lee, J.; Nah, H.; Lee, D.; An, S.-H.; Ko, W.-K.; Jin Lee, S.; Nyoung Heo, D. Immediately implantable extracellular matrix-enriched osteoinductive hydrogel-laden 3D-printed scaffold for promoting vascularized bone regeneration in vivo. Mater. Des. 2022, 219, 110801. [Google Scholar] [CrossRef]
- da Silva, D.M.; Barroca, N.; Pinto, S.C.; Semitela, Â.; de Sousa, B.M.; Martins, P.A.D.; Marques, P.A.A.P. Decellularized extracellular matrix-based 3D nanofibrous scaffolds functionalized with polydopamine-reduced graphene oxide for neural tissue engineering. Chem. Eng. J. 2023, 472. [Google Scholar] [CrossRef]
- Feng, B.; Ji, T.; Wang, X.; Fu, W.; Ye, L.; Zhang, H.; Li, F. Engineering cartilage tissue based on cartilage-derived extracellular matrix cECM/PCL hybrid nanofibrous scaffold. Mater. Des. 2020, 193, 108773. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Nowack, B. Probabilistic modelling of nanobiomaterial release from medical applications into the environment. Environ. Int. 2021, 146, 106184. [Google Scholar] [CrossRef]
- Lian, S.; Lamprou, D.; Zhao, M. Electrospinning technologies for the delivery of Biopharmaceuticals: Current status and future trends. Int. J. Pharm. 2024, 651, 123641. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Madden, P.W.; Patriarca, E.; Ahearne, M. Optimization and evaluation of oxygen-plasma-modified, aligned, poly (capital JE, Ukrainian- caprolactone) and silk fibroin nanofibrous scaffold for corneal stromal regeneration. Biomater. Biosyst. 2023, 12, 100083. [Google Scholar] [CrossRef]
- Dorati, R.; Conti, B.; Colzani, B.; Dondi, D.; Lazzaroni, S.; Modena, T.; Genta, I. Ivermectin controlled release implants based on poly-D, l -lactide and poly-ε-caprolactone. J. Drug Deliv. Sci. Technol. 2018, 46, 101–110. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Hasirci, V. Cell behavior on the alginate-coated PLLA/PLGA scaffolds. Int. J. Biol. Macromol. 2019, 124, 444–450. [Google Scholar] [CrossRef]
- Qiao, T.; Jiang, S.; Song, P.; Song, X.; Liu, Q.; Wang, L.; Chen, X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloids Surf. B Biointerfaces 2016, 146, 221–227. [Google Scholar] [CrossRef]
- Zahir, L.; Kida, T.; Tanaka, R.; Nakayama, Y.; Shiono, T.; Kawasaki, N.; Nakayama, A. Synthesis and properties of biodegradable thermoplastic elastomers using 2-Methyl-1,3-propanediol, succinic acid and lactide. Polym. Degrad. Stab. 2020, 181. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.H.; Awad, G.A.S. Moxifloxacin-loaded in situ synthesized Bioceramic/Poly(L-lactide-co-epsilon-caprolactone) composite scaffolds for treatment of osteomyelitis and orthopedic regeneration. Int. J. Pharm. 2021, 602, 120662. [Google Scholar] [CrossRef]
- Qiang, N.; Lin, W.; Zhou, X.; Liu, Z.; Lu, M.; Qiu, S.; Tang, S.; Zhu, J. Electrospun fibers derived from peptide coupled amphiphilic copolymers for dorsal root ganglion (DRG) outgrowth. Gels 2021, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Momeni, P.; Nourisefat, M.; Farzaneh, A.; Shahrousvand, M.; Abdi, M.H. The engineering, drug release, and in vitro evaluations of the PLLA/HPC/Calendula Officinalis electrospun nanofibers optimized by Response Surface Methodology. Heliyon 2024, 10, e23218. [Google Scholar] [CrossRef]
- Frydlova, B.; Fajstavr, D.; Slepickova Kasalkova, N.; Rimpelova, S.; Svobodova Pavlickova, V.; Svorcik, V.; Slepicka, P. Replicated biopolymer pattern on PLLA-Ag basis with an excellent antibacterial response. Heliyon 2023, 9, e21566. [Google Scholar] [CrossRef]
- Estrada, R.G.; Multigner, M.; Fagali, N.; Lozano, R.M.; Munoz, M.; Cifuentes, S.C.; Lieblich, M. Metastable FeMg particles for controlling degradation rate, mechanical properties, and biocompatibility of Poly(l-lactic) acid (PLLA) for orthopedic applications. Heliyon 2023, 9, e22552. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Li, W.; Zeng, G.; Yan, J.; Liu, X.; Jiang, X.; Yang, J.; Sun, D. One-pot green synthesis of I@CNDs-Fe3O4 hybrid nanoparticles from kelp for multi-modal imaging in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112037. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Pazik, R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef]
- Niu, Z.; Murakonda, G.K.; Jarubula, R.; Dai, M. Fabrication of Graphene oxide-Fe3O4 nanocomposites for application in bone regeneration and treatment of leukemia. J. Drug Deliv. Sci. Technol. 2021, 63, 102412. [Google Scholar] [CrossRef]
- Ren, R.; Guo, J.; Song, H.; Wei, Y.; Luo, C.; Zhang, Y.; Xiong, W. A novel implant surface modification mode of Fe3O4-containing TiO2 nanorods with sinusoidal electromagnetic field for osteoblastogenesis and angiogenesis. Mater. Today Bio 2023, 19, 100590. [Google Scholar] [CrossRef]
- Komlev, A.S.; Gimaev, R.R.; Zverev, V.I. Smart magnetocaloric coatings for implants: Controlled drug release for targeted delivery. Phys. Open 2021, 7, 100063. [Google Scholar] [CrossRef]
- Kubota, M.; Yokoi, T.; Ogawa, T.; Saito, S.; Furuya, M.; Yokota, K.; Kawashita, M. In-vitro heat-generating and apatite-forming abilities of PMMA bone cement containing TiO2 and Fe3O4. Ceram. Int. 2021, 47, 12292–12299. [Google Scholar] [CrossRef]
- Huan, W.; Dong, M.; Li, J.; Xie, M.; Huang, Y.; Carlini, R.; Yang, Y. Magnetic properties of Al3+ doped Fe3O4 by solid-solid reaction and biological applications of GQDs grafted MNPs. J. Magn. Magn. Mater. 2023, 587, 171360. [Google Scholar] [CrossRef]
- Baladi, M.; Amiri, M.; Salavati-Niasari, M. Green sol–gel auto-combustion synthesis, characterization and study of cytotoxicity and anticancer activity of ErFeO3/Fe3O4/rGO nanocomposite. Arab. J. Chem. 2023, 16, 104575. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, J.; Hu, Y.; Xia, Z.; Cai, K. Osteogenic differentiation of the MSCs on silk fibroin hydrogel loaded Fe3O4@PAA NPs in static magnetic field environment. Colloids Surf. B Biointerfaces 2022, 220, 112947. [Google Scholar] [CrossRef]
- Ura, D.P.; Stachewicz, U. Direct electrospinning of short polymer fibers: Factors affecting size and quality. Compos. Part A Appl. Sci. Manuf. 2024, 181, 108138. [Google Scholar] [CrossRef]
- Fathona, I.W.; Yabuki, A. A simple one-step fabrication of short polymer nanofibers via electrospinning. J. Mater. Sci. 2014, 49, 3519–3528. [Google Scholar] [CrossRef]
- Navarro Oliva, F.S.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedoui, F. Nanoparticle size and surface chemistry effects on mechanical and physical properties of nano-reinforced polymers: The case of PVDF-Fe3O4 nano-composites. Polym. Test. 2023, 117, 107851. [Google Scholar] [CrossRef]
- Hingrajiya, R.D.; Patel, M.P. Fe3O4 modified chitosan based co-polymeric magnetic composite hydrogel: Synthesis, characterization and evaluation for the removal of methylene blue from aqueous solutions. Int. J. Biol. Macromol. 2023, 244, 125251. [Google Scholar] [CrossRef]
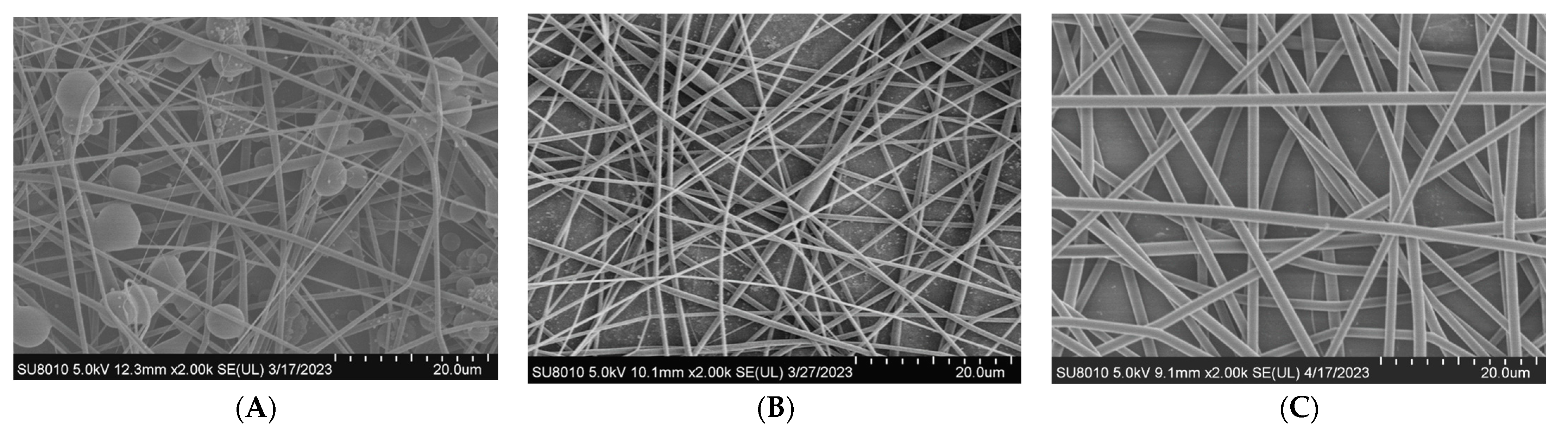

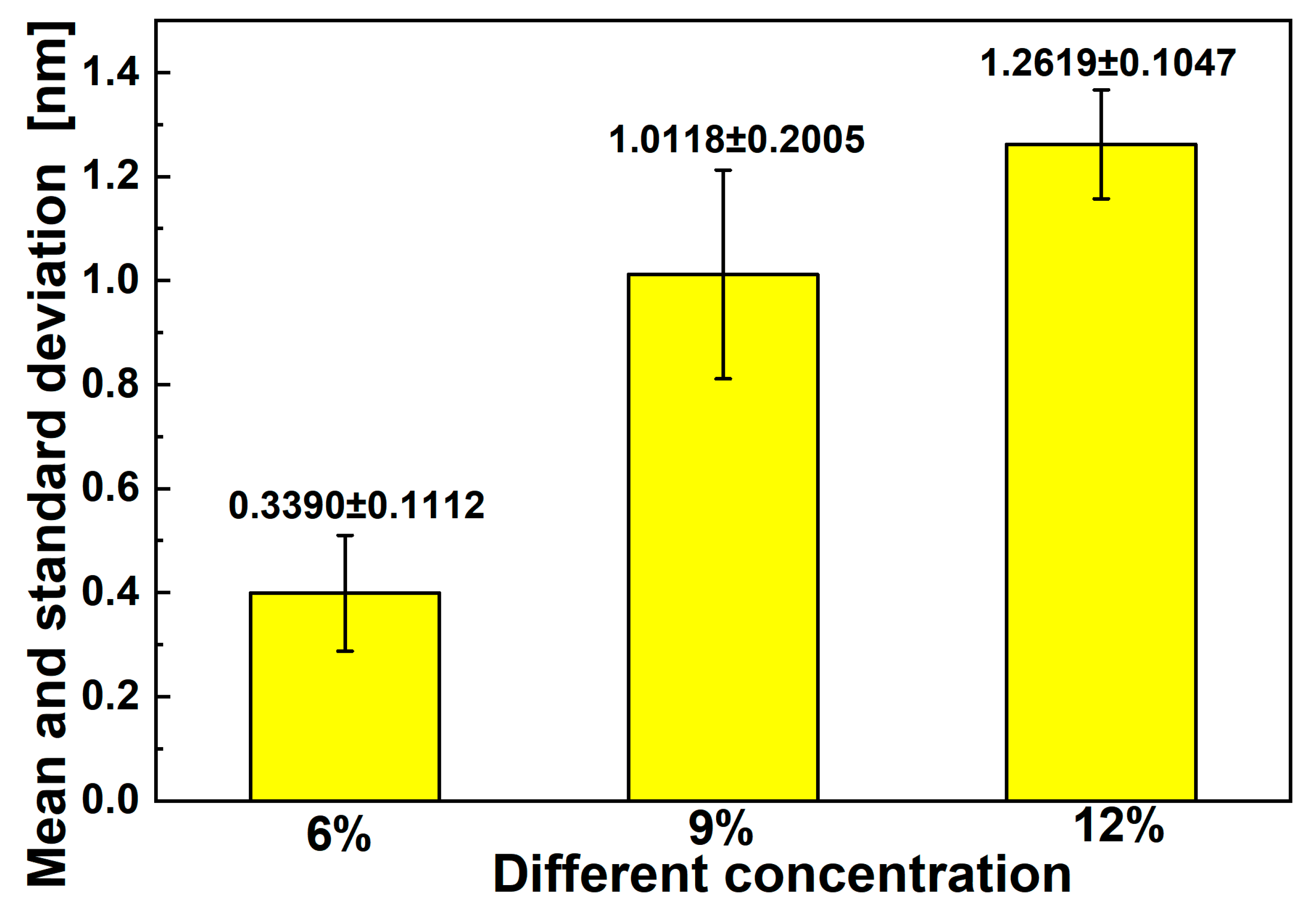




| Number | Composition (Mass Ratio) | Mass/Volume (w/v%) |
|---|---|---|
| 1 | PLLA/Fe3O4 = 12:0 | 6 |
| 2 | PLLA/Fe3O4 = 12:0 | 9 |
| 3 | PLLA/Fe3O4 = 12:0 | 12 |
| 4 | PLLA/Fe3O4 = 12:0.5 | 12 |
| 5 | PLLA/Fe3O4 = 12:1 | 12 |
| 6 | PLLA/Fe3O4 = 12:1.5 | 12 |
| Number | Composition | Mass/Volume (w/v%) | Voltage (kV) | Mn |
|---|---|---|---|---|
| 1 | PLLA | 12 | 10 | 20,000 |
| 2 | PLLA | 12 | 10 | 83,000 |
| 3 | PLLA | 12 | 10 | 170,000 |
| Number | Composition | Mass/Volume (w/v%) | Voltage (kV) | Collector Type |
|---|---|---|---|---|
| 1 | PLLA | 8 | 10 | random electrospun fibers |
| 2 | PLLA | 10 | 10 | random electrospun fibers |
| 3 | PLLA | 12 | 10 | random electrospun fibers |
| Number | Composition | Mass/Volume (w/v%) | Voltage (kV) | Collector Type |
|---|---|---|---|---|
| 1 | PLLA | 12 | 12 | random |
| 2 | PLLA | 12 | 12 | orientation |
| Samples | Thermal Decomposition Temperature (°C) | Maximum Decomposition Temperature (°C) |
|---|---|---|
| PLLA | 266.2 | 346.2 |
| PLLA/Fe3O4 = 12:0.5 | 252.1 | 307.1 |
| PLLA/Fe3O4 = 12:1 | 246.9 | 294.9 |
| PLLA/Fe3O4 = 12:1.5 | 232.8 | 286.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zheng, Y.; Lin, L.; Liu, X.; Qiang, N. Fabrication of Magnetic Poly(L-lactide) (PLLA)/Fe3O4 Composite Electrospun Fibers. Materials 2024, 17, 3773. https://doi.org/10.3390/ma17153773
Liu Z, Zheng Y, Lin L, Liu X, Qiang N. Fabrication of Magnetic Poly(L-lactide) (PLLA)/Fe3O4 Composite Electrospun Fibers. Materials. 2024; 17(15):3773. https://doi.org/10.3390/ma17153773
Chicago/Turabian StyleLiu, Zhu, Yufu Zheng, Lizhong Lin, Xiaofei Liu, and Na Qiang. 2024. "Fabrication of Magnetic Poly(L-lactide) (PLLA)/Fe3O4 Composite Electrospun Fibers" Materials 17, no. 15: 3773. https://doi.org/10.3390/ma17153773
APA StyleLiu, Z., Zheng, Y., Lin, L., Liu, X., & Qiang, N. (2024). Fabrication of Magnetic Poly(L-lactide) (PLLA)/Fe3O4 Composite Electrospun Fibers. Materials, 17(15), 3773. https://doi.org/10.3390/ma17153773











