Effect of Activated Siliceous Wastes Incorporated as Mineral Admixtures on the Rheological Properties of Cement Paste: Insights into Their Physicochemical Interactions in Suspension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silicate Minerals
2.2. Cement and PCE Superplasticizers
2.3. Chemical Reagents
2.4. Preparation
2.5. Experimental Methods
3. Results and Discussion
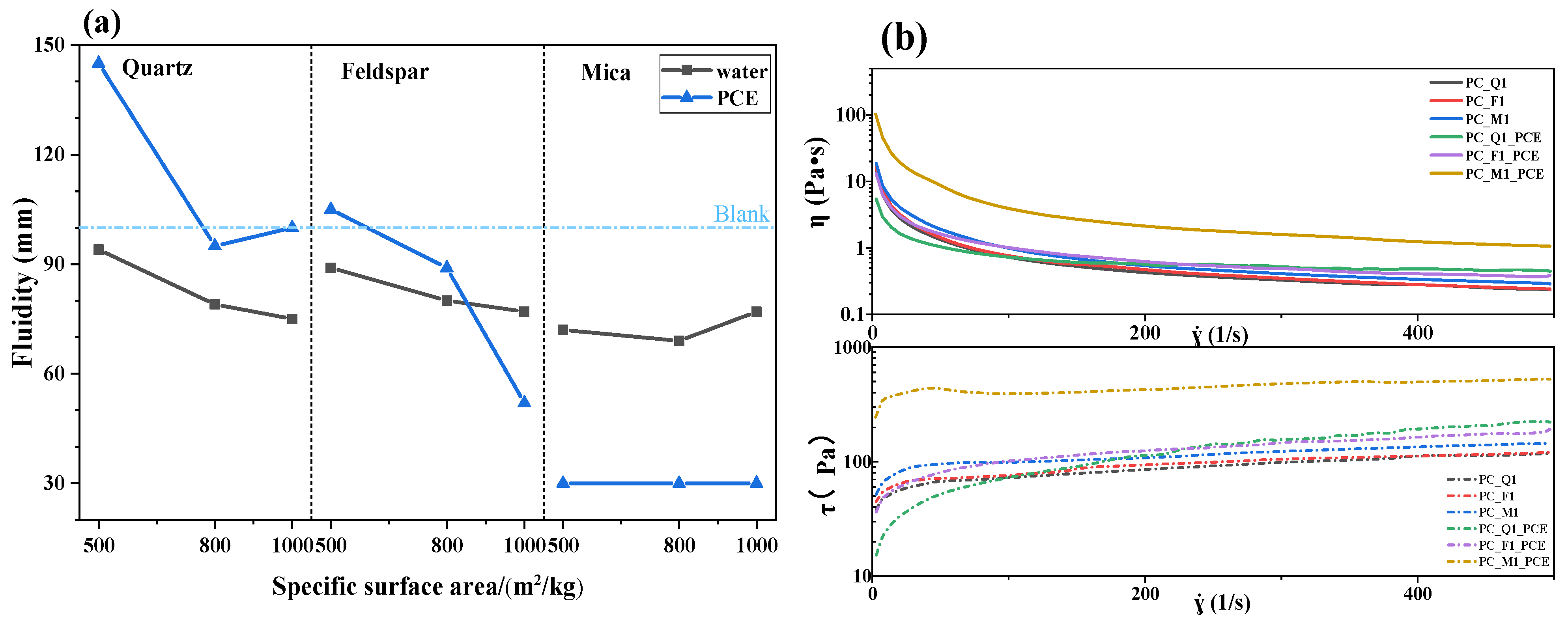
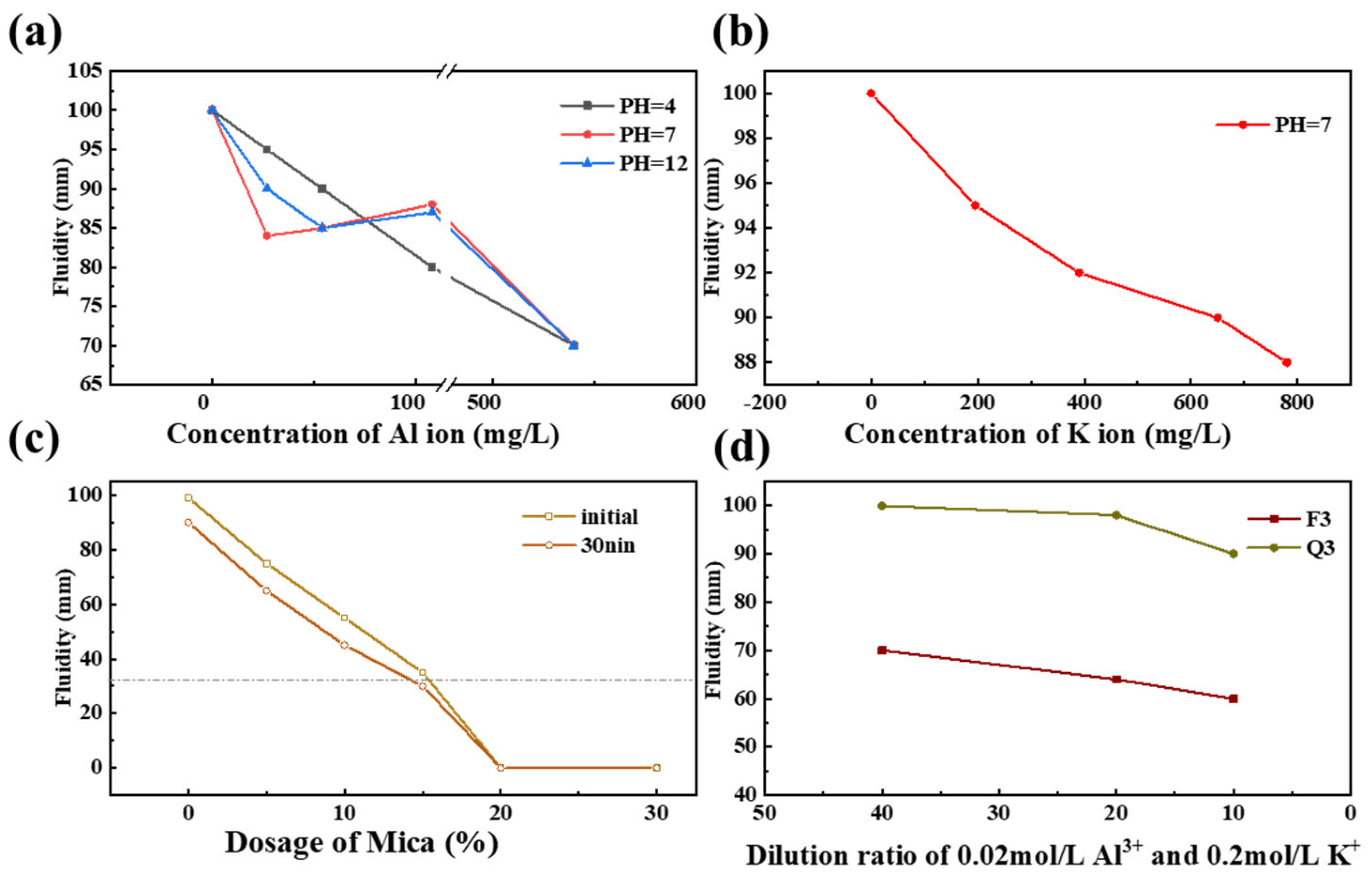
3.1. Fluidity of the Cement Paste Blended with Silicate Minerals
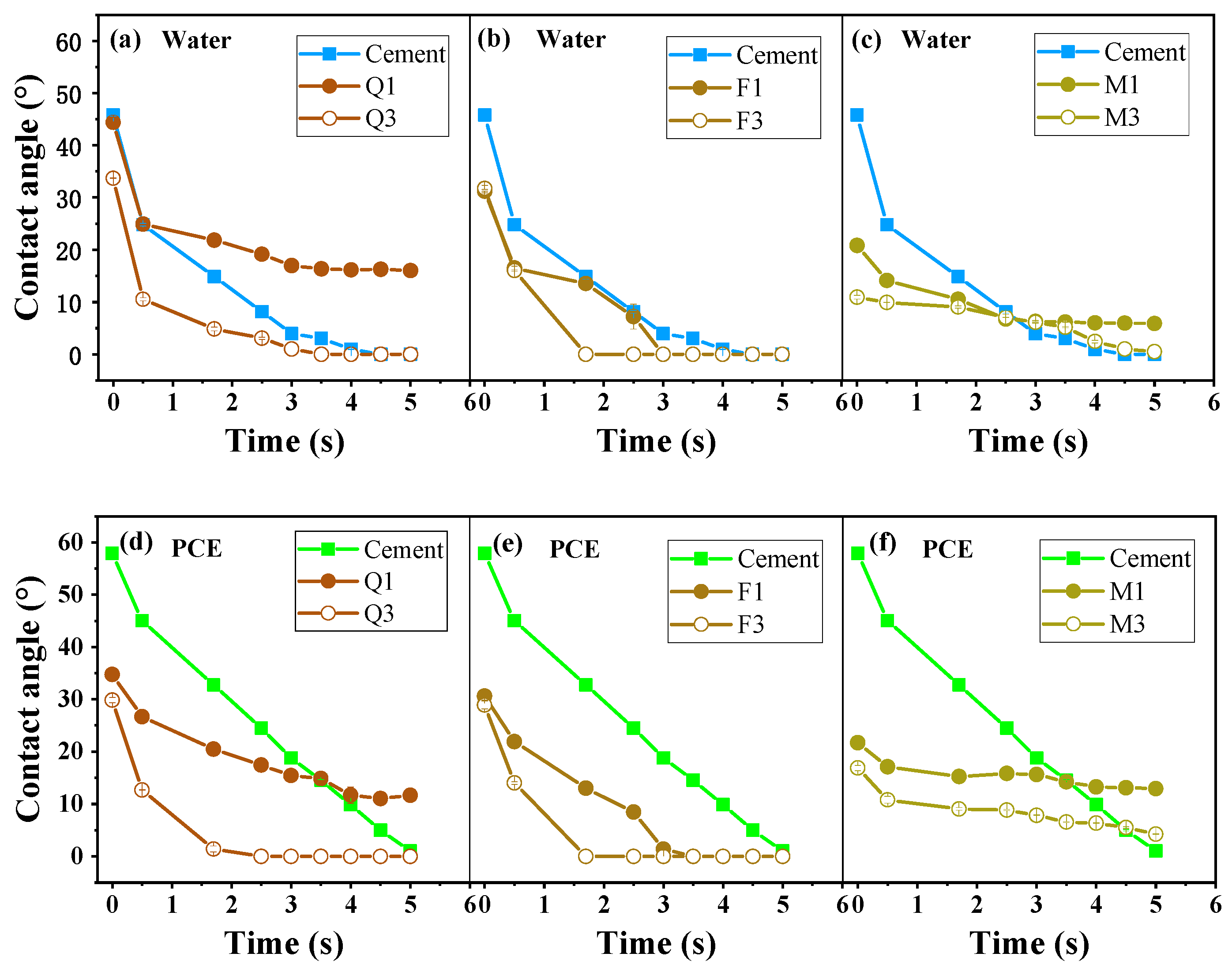
3.2. Wettability of Silicate Minerals
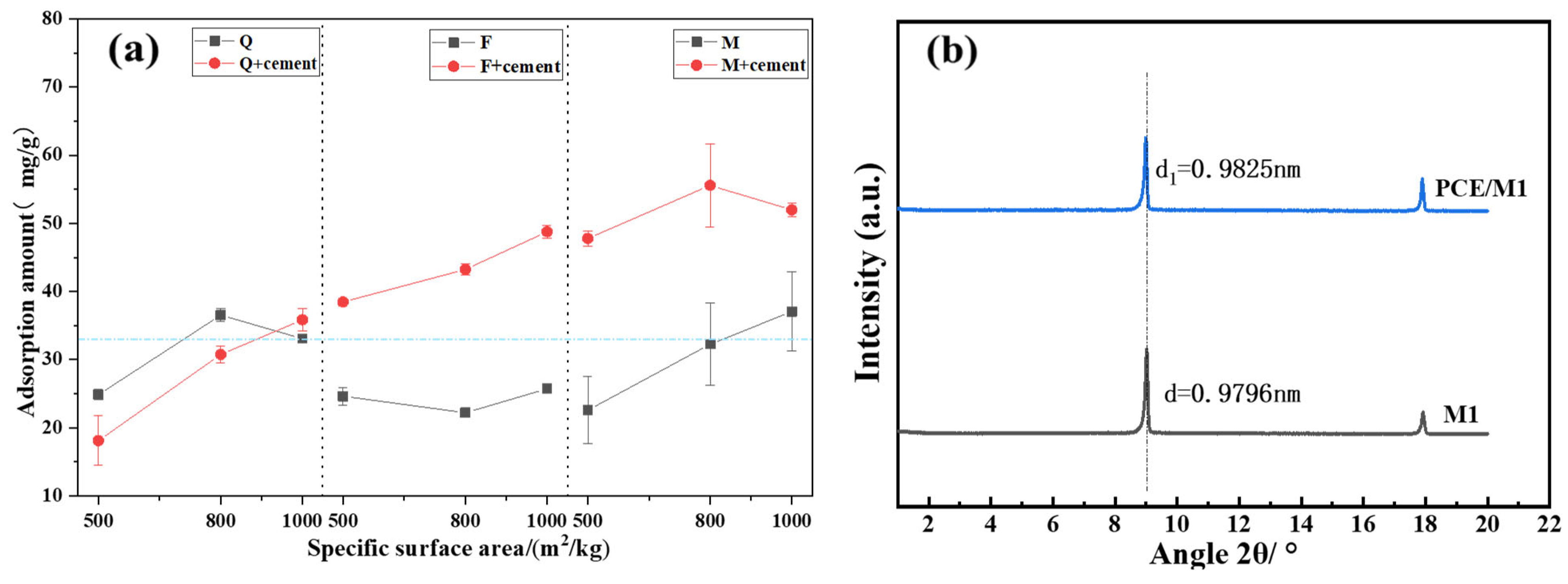
3.3. Adsorption of PCE on Silicate Minerals
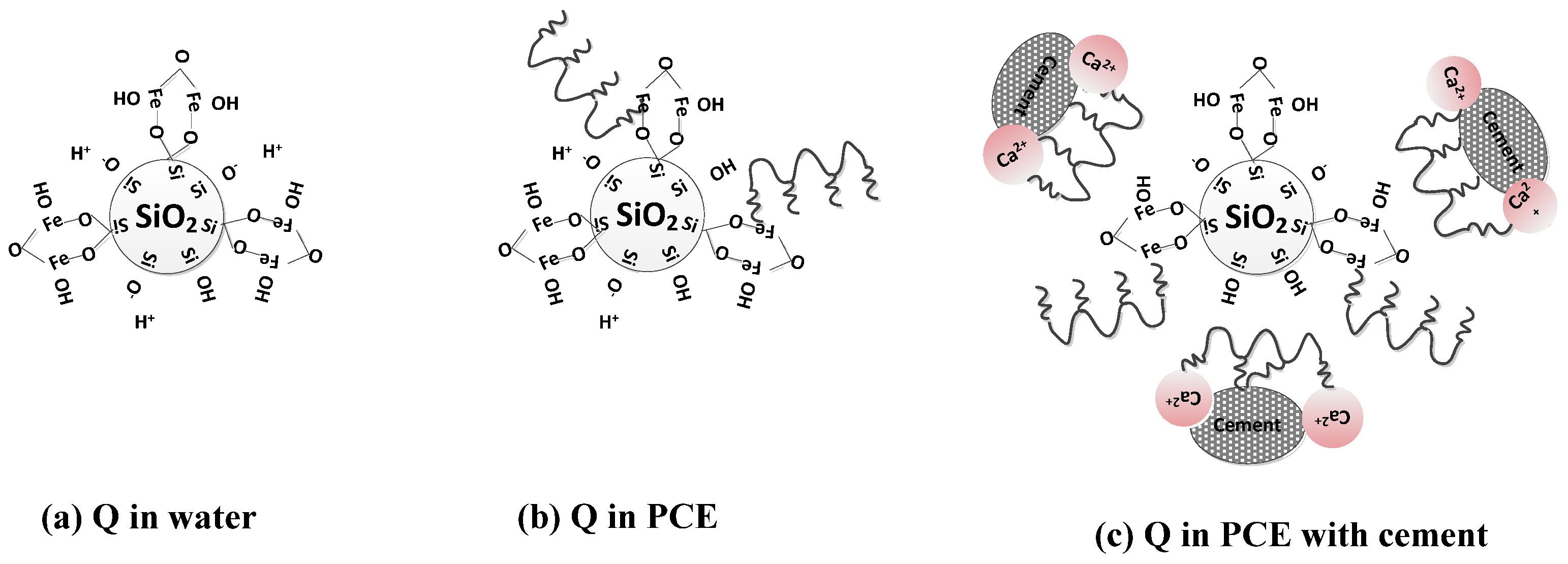
3.4. Mechanistic Study
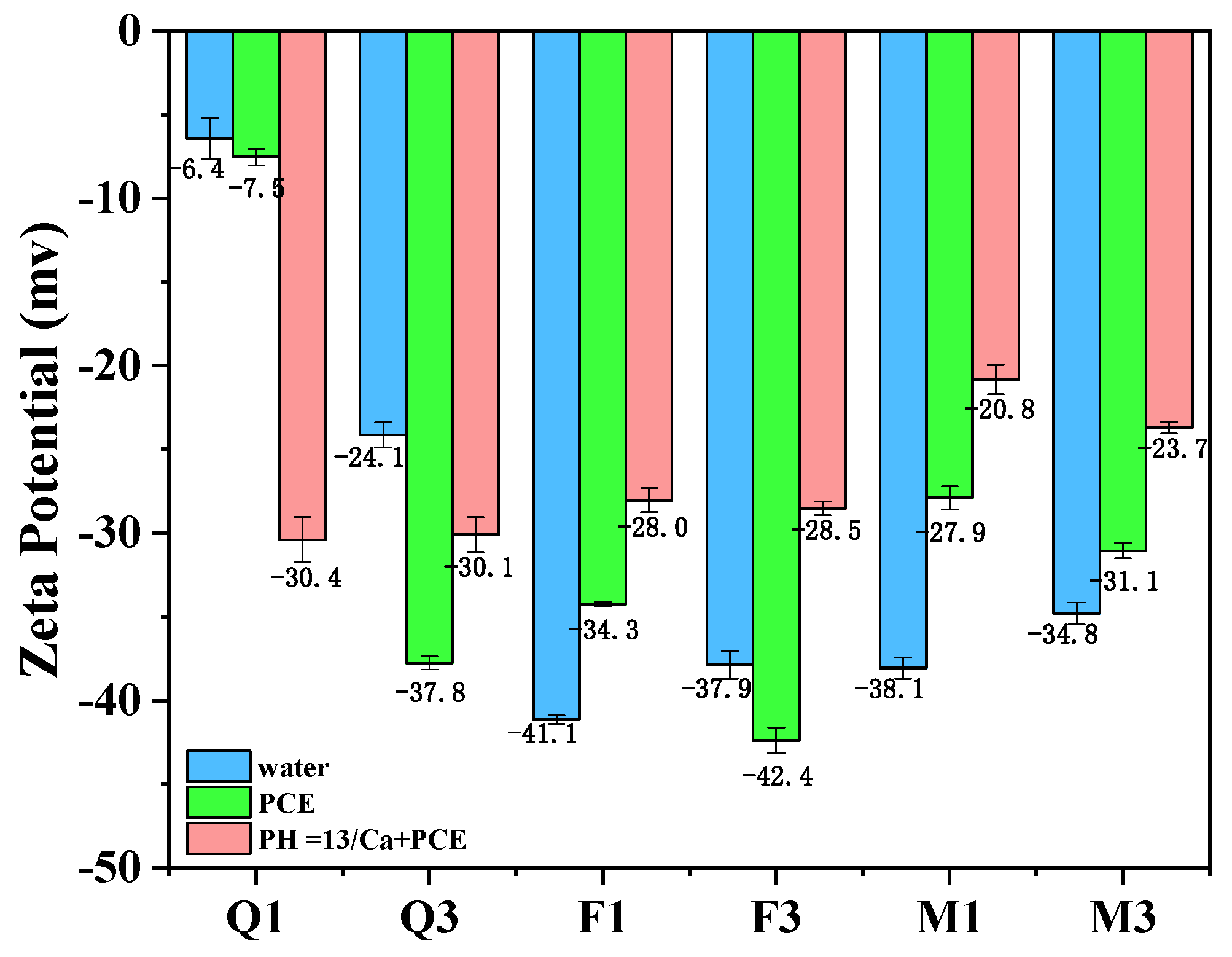
3.4.1. Zeta Potential of Silicate Minerals
3.4.2. Ionic Coordination of Silicate Mineral Admixtures
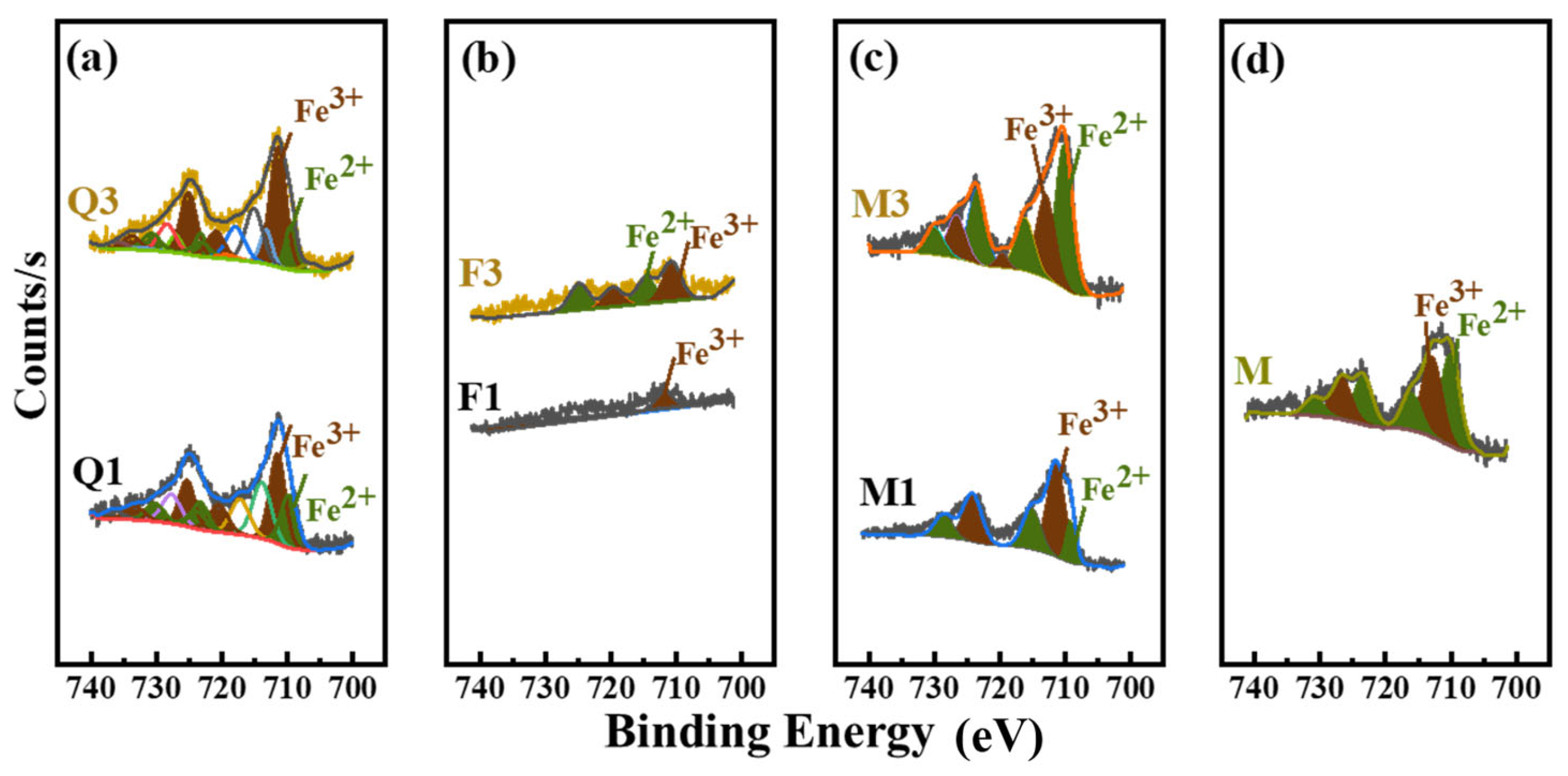
3.4.3. Dissolved Metal Cation of Silicate Minerals
3.4.4. The Hydrolysis and Potential Equilibrium of Silicate Minerals
4. Conclusions
- (1)
- Activated quartz hydrophilicity is similar to cement when it comes into contact with water. The water demand and PCE absorption are the lowest. Optimal flowability can be achieved at a specific surface area of 535 m2/kg. Increasing the specific surface area leads to coordination between minerals and Fe ions in grinding media, which leads to enhanced hydrophilicity and PCE adsorption.
- (2)
- The capillary adsorption of activated feldspar results in a reduction in free water within the blended cement. Activated mica demonstrated strong hydrophilicity, a high metal cation solution, and a lamellar structure, which increased the shear stress and reduced flowability. Consequently, 15 wt% mica causes a complete loss of flowability.
- (3)
- With cement hydration, the solubility of metal cations such as Al3+, K+, and Si4+ in activated mineral admixtures is facilitated, and the Ca2+ produced by hydration will be adsorbed on the mineral’s surface. This results in a significant reduction in cement slurry fluidity due to the increased levels of PCE.
- (4)
- PCE can adsorb to the surface of cement particles to improve slurry properties but cannot adsorb directly to mineral admixtures. A specialized polymer additive for siliceous waste admixture, possibly with cationic groups, needs to be prepared to improve fluidity. The utilization of siliceous wastes will result in a meaningful contribution to environmental protection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.B.; Zhou, Z.Y.; Wang, Y.L.; Zhou, Q.S.; Qi, T.G.; Liu, G.H.; Peng, Z.H. Enrichment and separation of iron minerals in gibbsitic bauxite residue based onreductive Bayer digestion. Trans. Nonferr. Met. Soc. China 2020, 30, 1980–1990. [Google Scholar] [CrossRef]
- Simonsen AM, T.; Solismaa, S.; Hansen, H.K.; Jensen, P.E. Evaluation of mine tailings’ potential as supplementary cementitious materials based on chemical, mineralogical and physical characteristics. Waste Manag. 2020, 102, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Black, L.; Purnell, P. Is carbon dioxide pricing a driver in concrete mix design. Mag. Concr. Res. 2016, 68, 561–567. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, H.J.; Peng, T.J.; Zheng, W.M. Preparation of porous glass-ceramics from coal fly ash and asbestos tailings by high-temperature pore-forming. Waste Manag. 2020, 106, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Saedi, A.; Jamshidi-Zanjani, A.; Darban, A.K. A review on different methods of activating tailings to improve their cementitious property as cemented paste and reusability. J. Environ. Manag. 2020, 270, 110881. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Wang, Z.; Yao, J.; Cong, X.; Anning, C.; Lyu, X. Pozzolanic activity and hydration properties of feldspar after mechanical activation. Powder Technol. 2021, 383, 167–174. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Jia, Z.; Sun, S.; Anning, C.; Qiu, J.; Lyu, X. Effect of anhydrite on hydration properties of mechanically activated muscovite in the presence of calcium oxide. Appl. Clay Sci. 2020, 196, 105742. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Zhang, J.; Wang, J.; Lyu, X. Effects of mechanical grinding on pozzolanic activity and hydration properties of quartz. Adv. Powder Technol. 2020, 31, 4500–4509. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, H.; Wang, J.; Feng, E.; Zhang, Y.; Lyu, X. Mechanical activation of siliceous tailings and its application as cement admixtures. Miner. Eng. 2022, 177, 107366. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, Q.; Wang, W.; Huang, Y.; Deng, J.; Xu, Z. Study on Flotation Tailings of Kaolinite-type Pyrite when Used as Cement Admixture and Concrete Admixture. Procedia Environ. Sci. 2016, 31, 644–652. [Google Scholar] [CrossRef]
- Wong, R.C.K.; Gillott, J.E.; Law, S.; Thomas, M.J.; Poon, C.S. Calcined oil sands fine tailings as a supplementary cementing material for concrete. Cem. Concr. Res. 2004, 34, 1235–1242. [Google Scholar] [CrossRef]
- Ince, C. Reusing gold-mine tailings in cement mortars: Mechanical properties and socio-economic developments for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2019, 238, 117871. [Google Scholar] [CrossRef]
- de Carvalho, J.M.F.; Schmidt, W.; Kuehne, H.C.; Peixoto, R.A.F. Influence of high—Charge and low—Charge PCE—Based superplasticizers on Portland cement pastes containing particle—Size designed recycled mineral admixtures. J. Build. Eng. 2020, 32, 101515. [Google Scholar] [CrossRef]
- Shi, L.; Kuang, J.; Liu, S.; Lu, Y.; Yan, J. Research status of building materials application of copper tailings and rule of influence of mineral composition. Silic. Rep. 2022, 41, 3511–3524. [Google Scholar]
- Ma, B.; Yang, H.; Tan, H.; Dai, Z. Adsorption characteristics of cement and clay minerals on different water reducing agents. J. Silic. 2013, 41, 328–333. [Google Scholar]
- Norvell, J.K.; Stewart, J.G.; Juenger, M.C.; Fowler, D.W. Influence of clays and clay-sized particles on concrete performance. J. Mater. Civ. Eng. 2007, 19, 1053–1059. [Google Scholar] [CrossRef]
- Luo, K.; Guo, L.; Chen, C.; Lin, C.; Lei, J. Adsorption capacity of clay layered silicate minerals on polycarboxylate superplasticizer. Non-Met. Miner. 2017, 40, 99–101. [Google Scholar]
- Wang, L. Study on the Effect of Clay Minerals on the Performance of Polycarboxylic Acid Water Reducing Agent and the Mechanism. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2013. [Google Scholar]
- Wang, Z.; Kao, Y.; Wang, L.; Hu, Q.; Jia, X. Effect and mechanism of monomineral clay on the dispersibility of polycarboxylic acid water reducing agent. J. Constr. Mater. 2015, 18, 879–887. [Google Scholar]
- Li, R.; Lei, L.; Plank, J. Influence of PCE superplasticizers on the fresh properties of low carbon cements containing calcined clays: A comparative study of calcined clays from three different sources. Cem. Concr. Compos. 2023, 139, 105072. [Google Scholar] [CrossRef]
- Vargas, F.; Lopez, M. Development of a new supplementary cementitious materialfrom the activation of copper tailings: Mechanical performance and analysis of factors. J. Clean. Prod. 2018, 182, 427–436. [Google Scholar] [CrossRef]
- GB/T 8074-2008; Testing Method for Specific Surface of Cement-Blaine Method. Standards Press of China: Beijing, China, 2008.
- GB 8076-2008; Appendix A, Concrete Admixtures. Standards Press of China: Beijing, China, 2008.
- GB/T 8077-2012; Method for Testing Uniformity of Concrete Admixture. Standards Press of China: Beijing, China, 2012.
- Feng, H. Study on Interaction Mechanism of Polycarboxylic Acid Superplasticizer with Stone Powder in Ledong Haikou Area, Hainan Province. Master’s Thesis, Hainan University, Haikou, China, 2018. (In Chinese). [Google Scholar]
- Zhang, G.; Liu, J.; Sui, B.; Chen, C. Mechanism Research and application of siliceous sand Modifiers. Mater. Rev. 2017, 31, 56–62. (In Chinese) [Google Scholar]
- Cocco, O.; Garroni, S.; Enzo, S.; Pia, G.; Meloni, P.; Delogu, F. Ball Milling of Silica-Based Pyroclastic Scoriae: Measurement of Mechanochemical Reactivity by Radical Scavenging. J. Phys. Chem. C 2018, 122, 2773–2782. [Google Scholar] [CrossRef]
- Hochstrasser, G.; Antonini, J.F. Surface States of Pristine Silica Surfaces: I. ESR Studies of Es′ Dangling Bonds and of CO2– Adsorbed Radicals. Surf. Sci. 1972, 32, 644–664. [Google Scholar] [CrossRef]
- Narayanasamy, J.; Kubicki, J.D. Mechanism of Hydroxyl Radical Generation from a Silica Surface: Molecular Orbital Calculation. J. Phys. Chem. B 2005, 109, 21796–21807. [Google Scholar] [CrossRef] [PubMed]
- Saruwatari, K.; Kameda, J.; Tanaka, H. Generation of HydrogenIons and Hydrogen Gas in Quartz–water Crushing Experiments: An Example of Chemical Processes in Active Faults. Phys. Chem. Miner. 2004, 31, 176–182. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Z.; Li, J.; Li, Q.; Wang, Z.; Luo, X. Infrared spectroscopy and XPS analysis compared the activation mechanism of Ca2+ and Fe3+ on quartz. Spectrosc. Spectrosc. 2020, 40, 1876–1882. (In Chinese) [Google Scholar]
- Xiong, W.; Yang, X.; Duan, W.; Wang, D.; Zuo, Y. Effect of soluble chlorine salts on the rheology of cement slurry mixed with different structural polycarboxylic acid water reducing agents. New Build. Mater. 2011, 38, 90–93. [Google Scholar]
- Li, C.; Feng, N. Study on the compatibility of comb-shaped polycarboxylic acid-based water reducing agent with cement. J. Constr. Mater. 2004, 3, 252–260. [Google Scholar]
- Zhang, J.; Ye, H.; Gao, X.; Wu, W. Adsorption and desorption of polycarboxylate ether superplasticizer in fresh cementitious materials blended with mineral admixtures. J. Mater. Res. Technol. 2022, 17, 1740–1751. [Google Scholar] [CrossRef]
- Gülgönül, I.; Karagüzel, C.; Çelik, M.S. Surface vs. bulk analyses of various feldspars and their significance to flotation. Int. J. Miner. Process. 2007, 86, 68–74. [Google Scholar] [CrossRef]
- Maslova, M.V.; Gerasimova, L.G.; Forsling, W. Surface Properties of Cleaved Mica. Colloid J. 2004, 66, 322–328. [Google Scholar] [CrossRef]
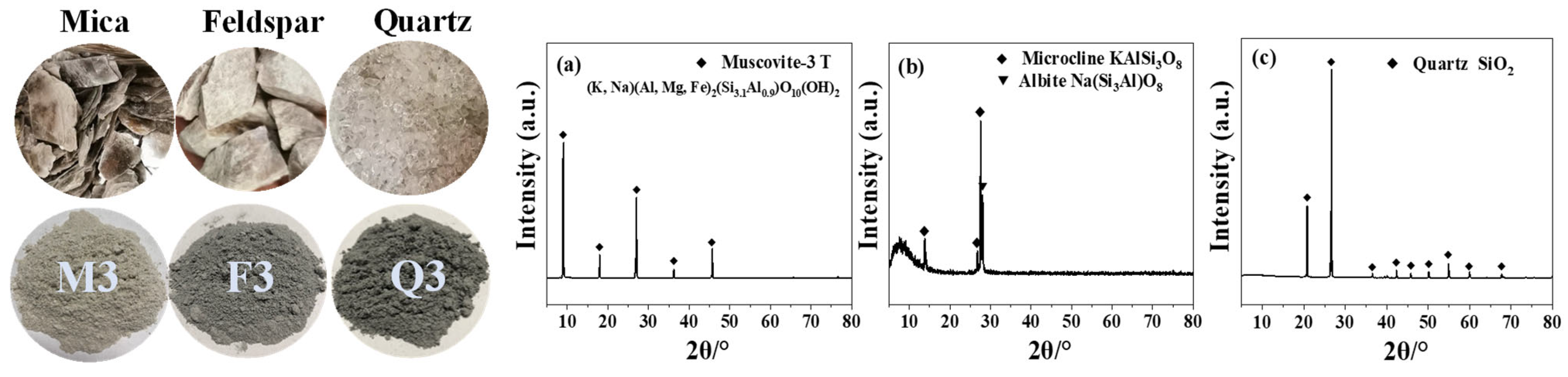
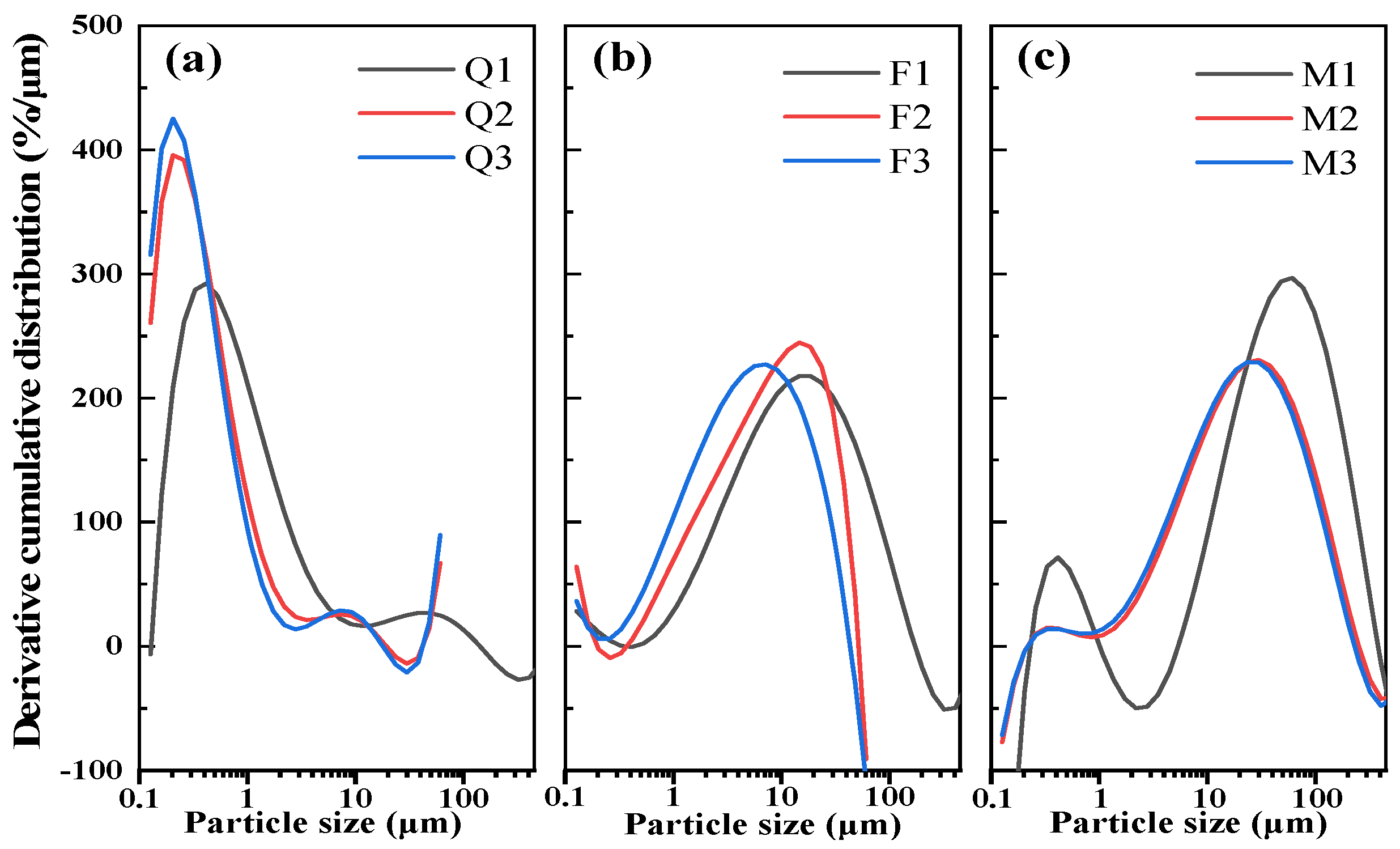


| Sample | Mineral | Grinding Time (min) | Blaine Specific Surface Area (m2/kg) |
|---|---|---|---|
| Q1 | Quartz | 10 | 535 |
| Q2 | 15 | 880 | |
| Q3 | 20 | 1016 | |
| F1 | Feldspar | 15 | 518 |
| F2 | 20 | 785 | |
| F3 | 25 | 1014 | |
| M1 | Mica | 30 | 463 |
| M2 | 45 | 833 | |
| M3 | 60 | 960 |
| Sample | PC | Quartz | Feldspar | Mica | PCE | Water |
|---|---|---|---|---|---|---|
| PC (Blank) | 500 | 0 | 0 | 0 | 0 | 250 |
| PC_30Q | 350 | 150 | 0 | 0 | 0 | 250 |
| PC_30F | 350 | 0 | 150 | 0 | 0 | 250 |
| PC_30M | 350 | 0 | 0 | 150 | 0 | 250 |
| PC_PCE (Blank) | 500 | 0 | 0 | 0 | 1.13 | 170 |
| PC_30Q_PCE | 350 | 150 | 0 | 0 | 1.13 | 170 |
| PC_30F_PCE | 350 | 0 | 150 | 0 | 1.13 | 170 |
| PC_30M_PCE | 350 | 0 | 0 | 150 | 1.13 | 170 |
| Sample | PC_Q1 | PC_F1 | PC_M1 | PC_Q1_PCE | PC_F1_PCE | PC_M1_PCE |
|---|---|---|---|---|---|---|
| τ0 (Pa) | 12.15 | 24.46 | 30.36 | 3.12 | 11.84 | 138.5 |
| Al3+ | Fe3+ | K+ | Li+ | Ca2+ | Si4+ | |
|---|---|---|---|---|---|---|
| Q3 | 0.16 | 0.5 | 8.66 | - | 0.21 | 13.17 |
| F3 | 10.83 | 0.26 | 285.93 | - | 0.34 | 30.98 |
| M3 | 23.92 | 0.1 | 437.78 | 2.86 | 0.07 | 24.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Kuang, J.; Qiu, T. Effect of Activated Siliceous Wastes Incorporated as Mineral Admixtures on the Rheological Properties of Cement Paste: Insights into Their Physicochemical Interactions in Suspension. Materials 2024, 17, 3781. https://doi.org/10.3390/ma17153781
Shi L, Kuang J, Qiu T. Effect of Activated Siliceous Wastes Incorporated as Mineral Admixtures on the Rheological Properties of Cement Paste: Insights into Their Physicochemical Interactions in Suspension. Materials. 2024; 17(15):3781. https://doi.org/10.3390/ma17153781
Chicago/Turabian StyleShi, Linyun, Jingzhong Kuang, and Tingsheng Qiu. 2024. "Effect of Activated Siliceous Wastes Incorporated as Mineral Admixtures on the Rheological Properties of Cement Paste: Insights into Their Physicochemical Interactions in Suspension" Materials 17, no. 15: 3781. https://doi.org/10.3390/ma17153781




