Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash
Abstract
1. Introduction
2. Research Methods
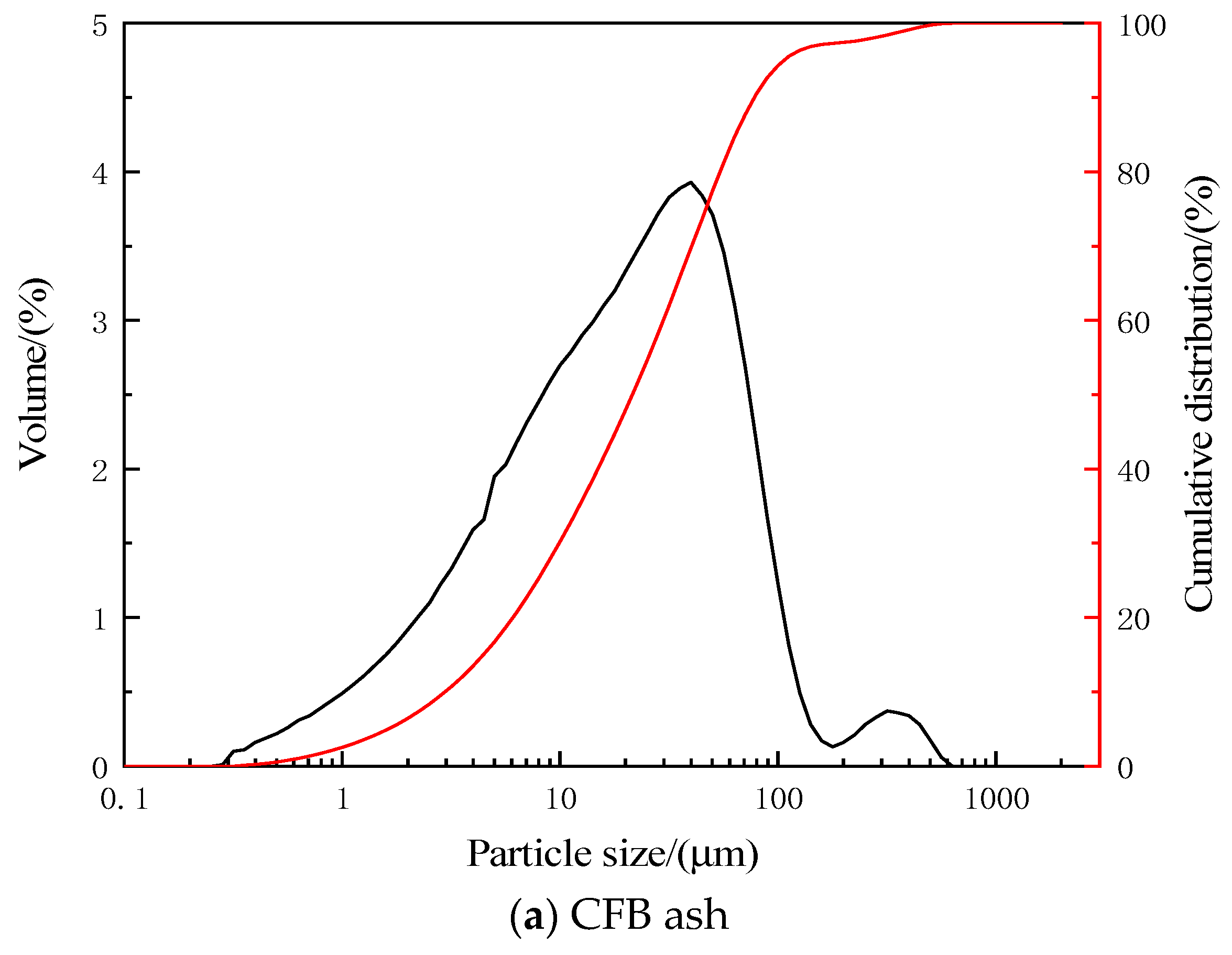
2.1. Raw Materials
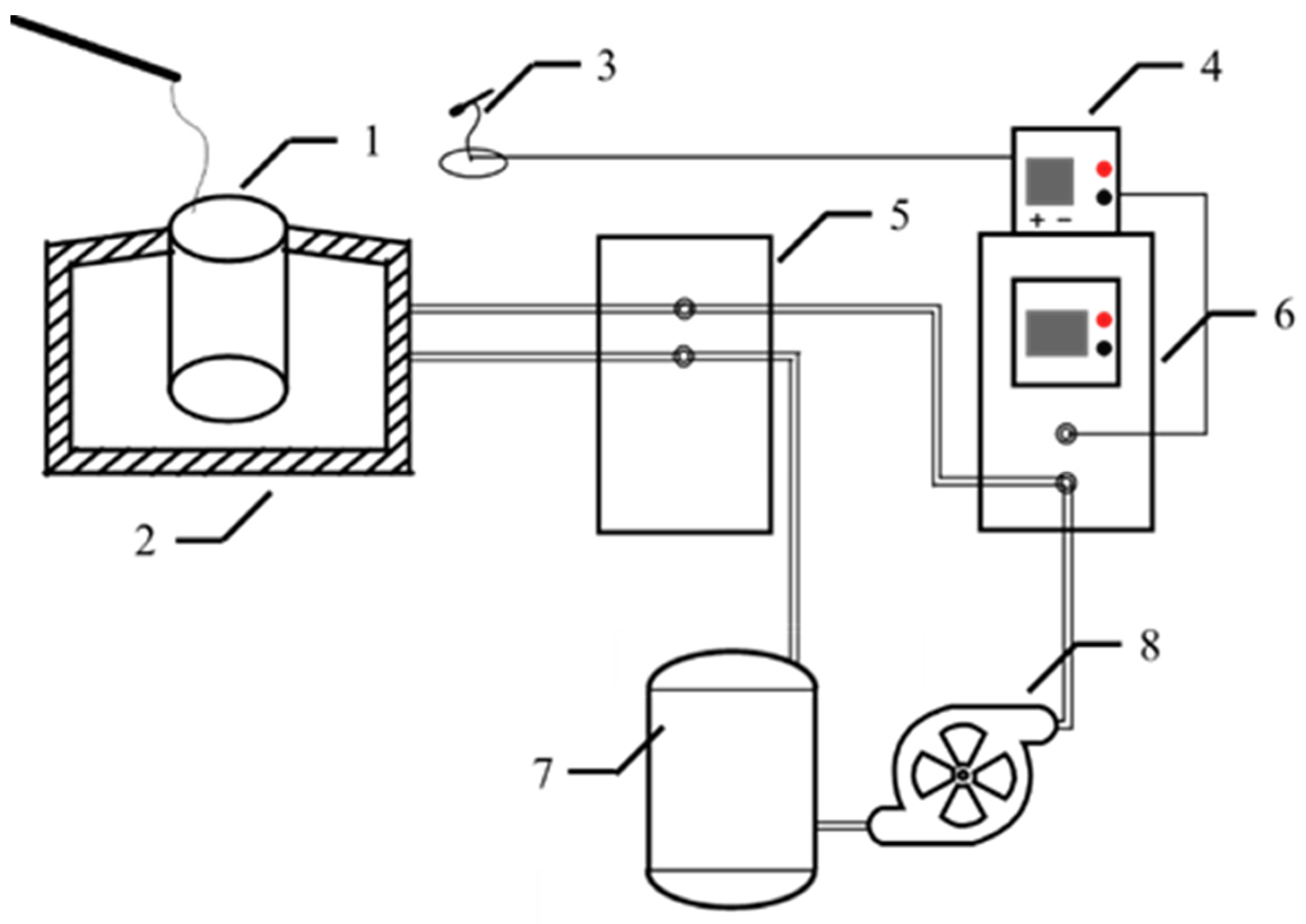
2.2. Experimental Process
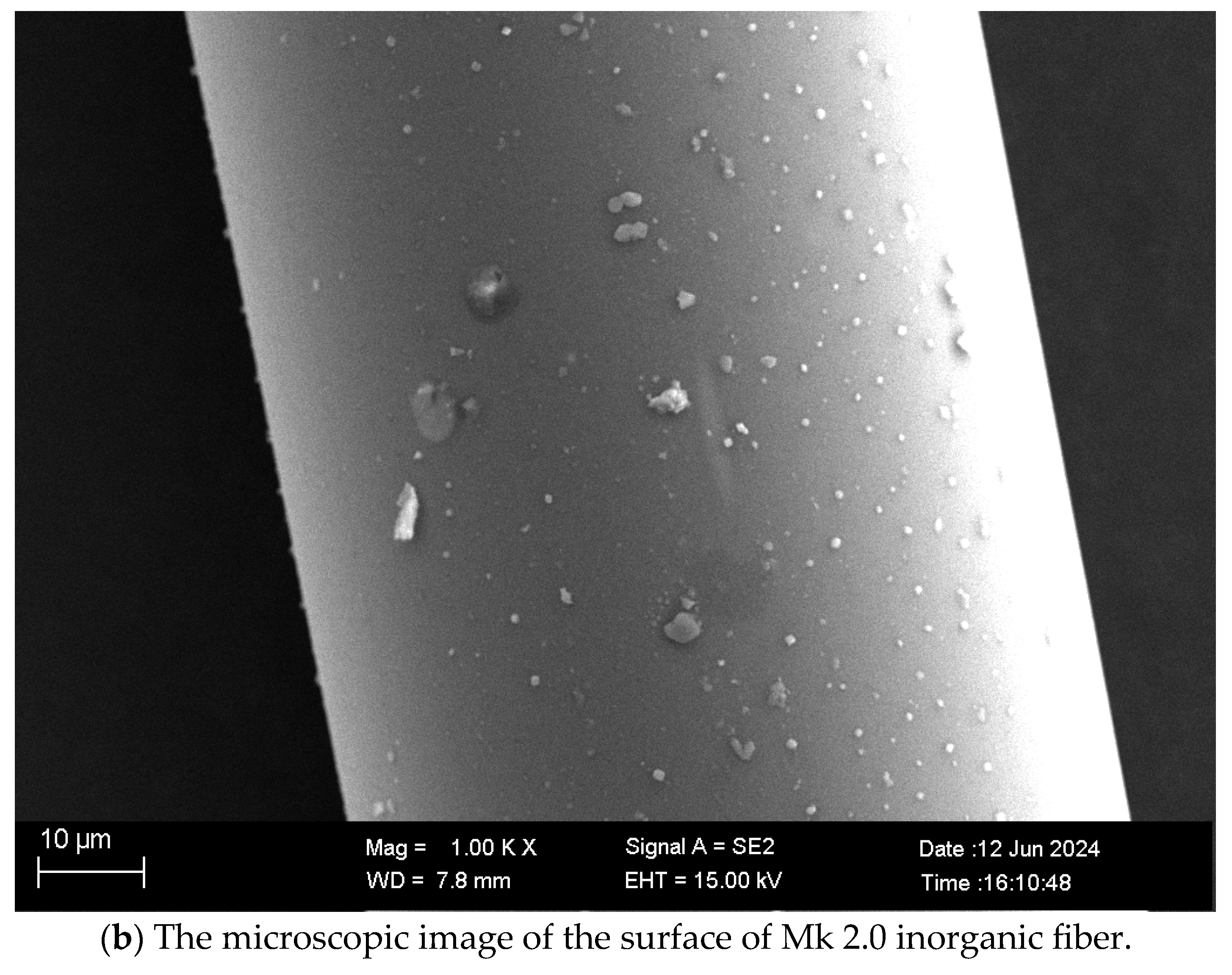
2.3. Characterization of Microstructure
2.4. FactSage Simulation Analysis
3. Results and Discussion
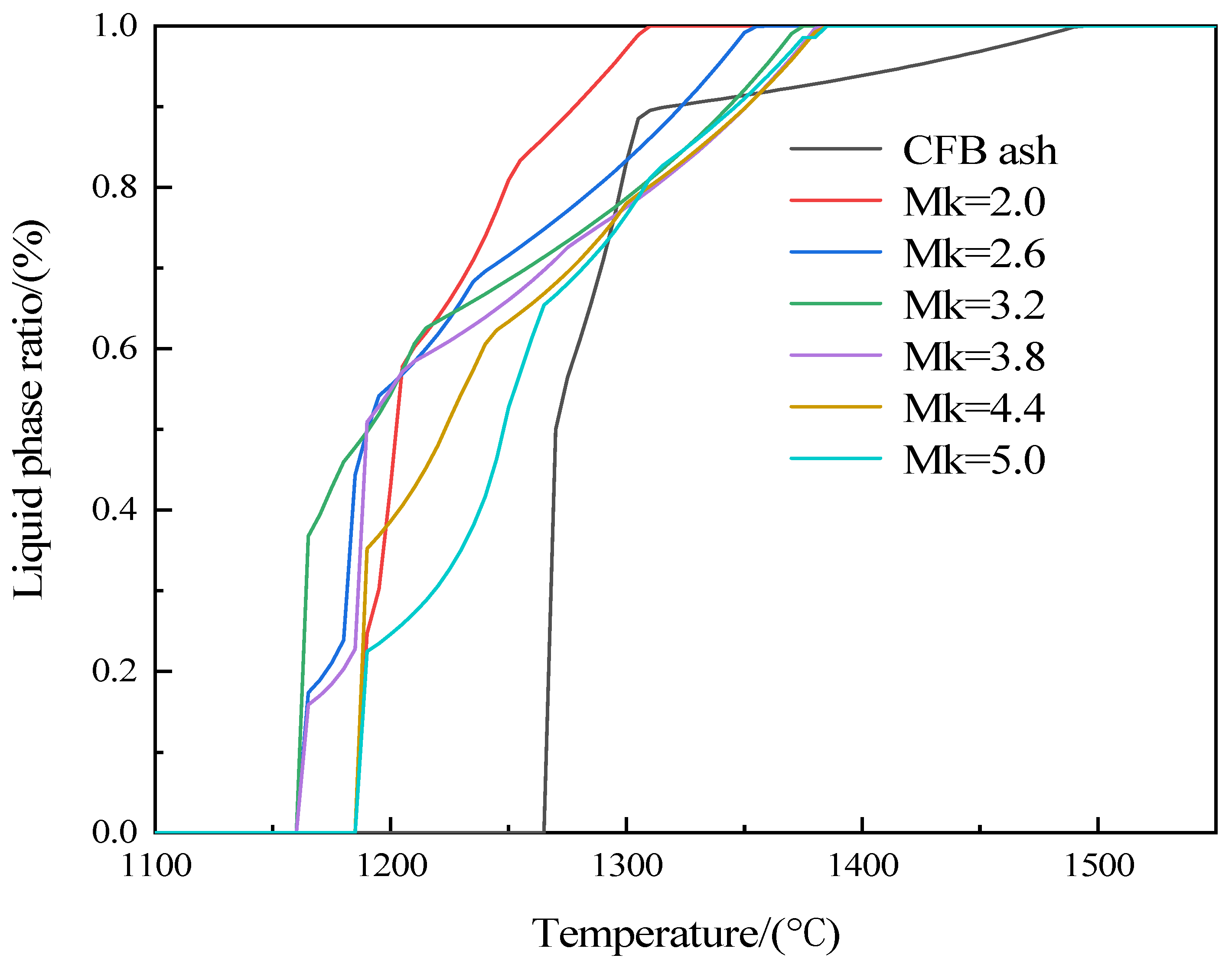
3.1. Melting Characteristic Analysis
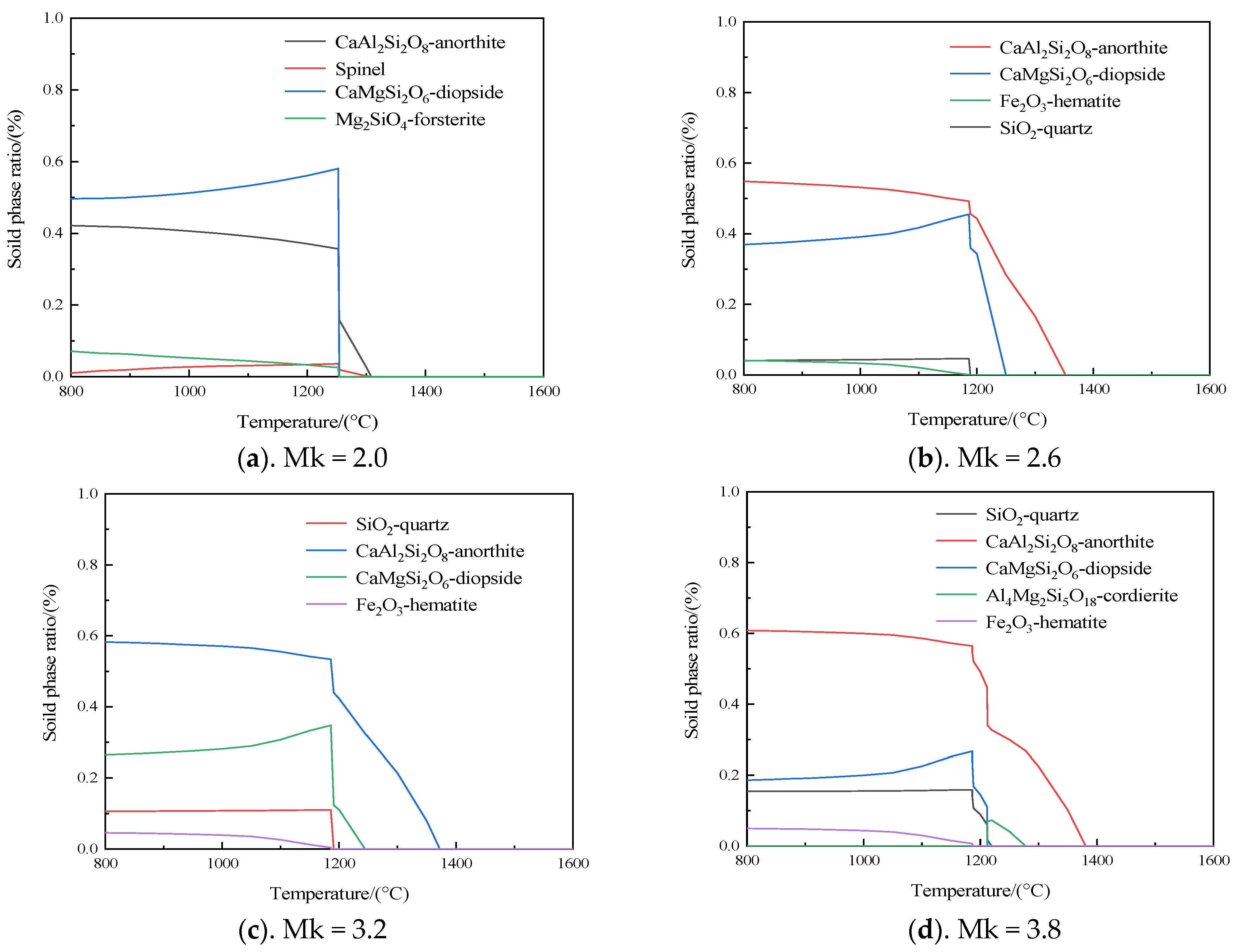
3.2. The Mineral Evolution Patterns of Samples with Different Mk with Temperature Changes
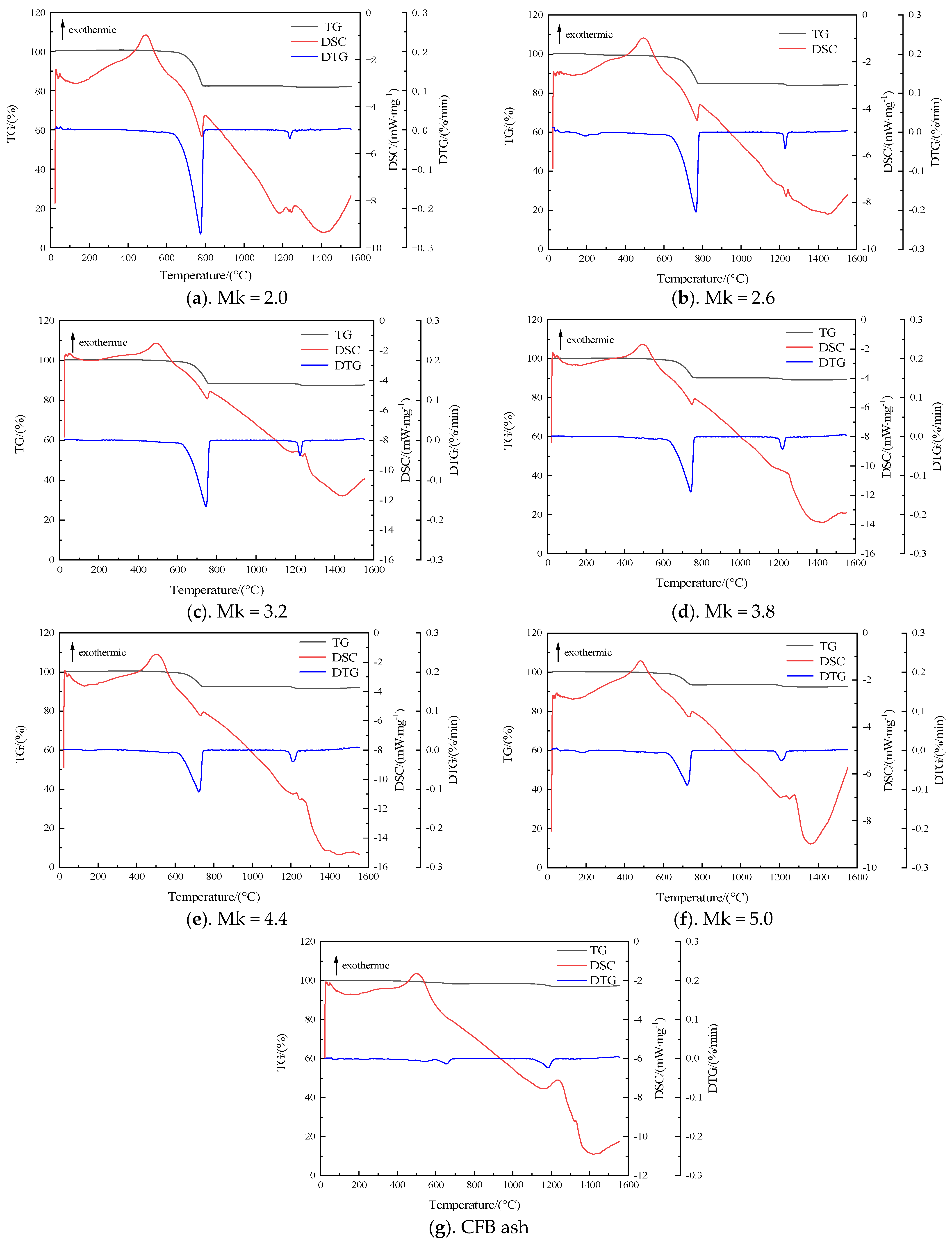
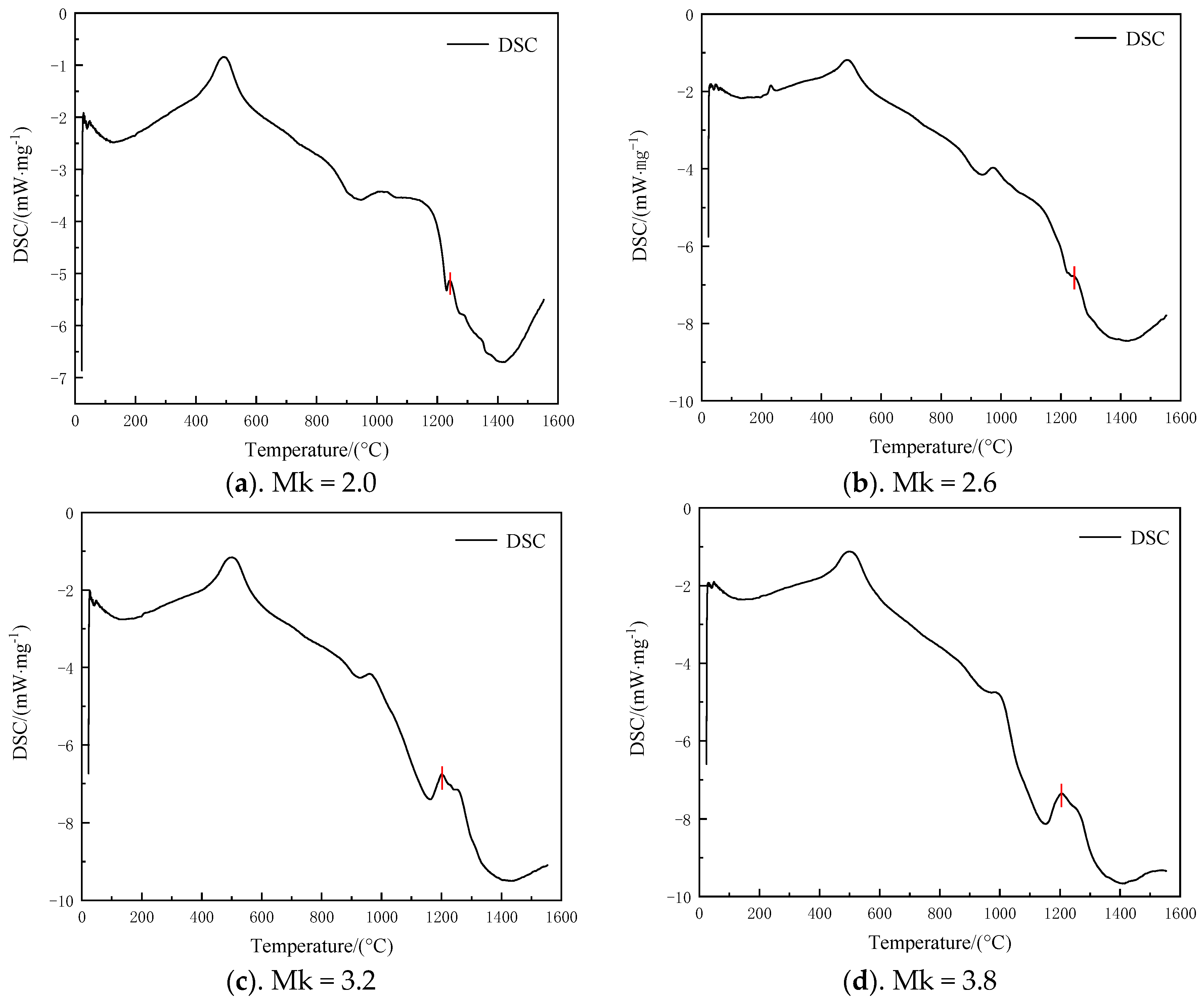
3.3. High-Temperature Melting Characterization Analysis by TG-DSC
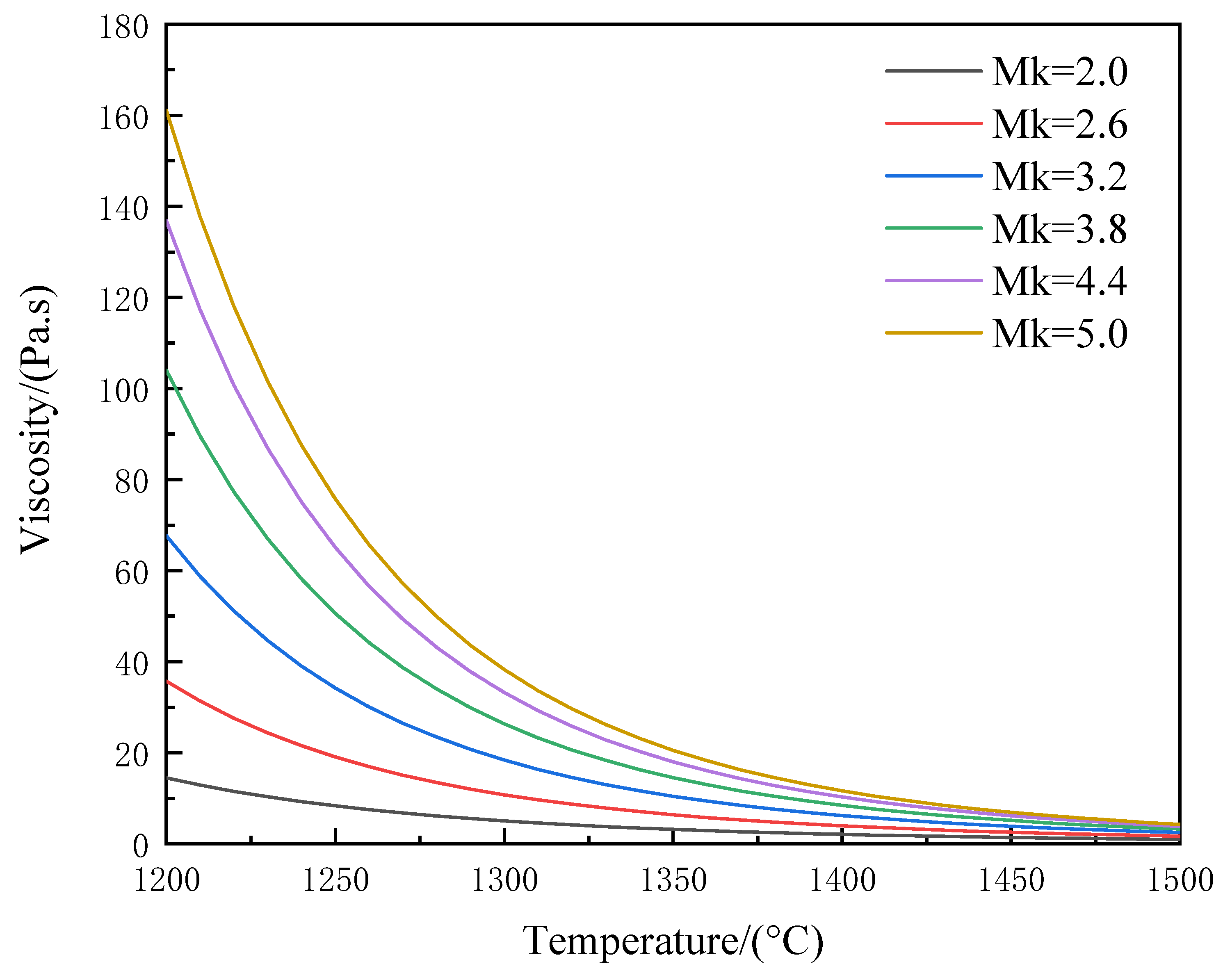
3.4. Viscosity–Temperature Characterization
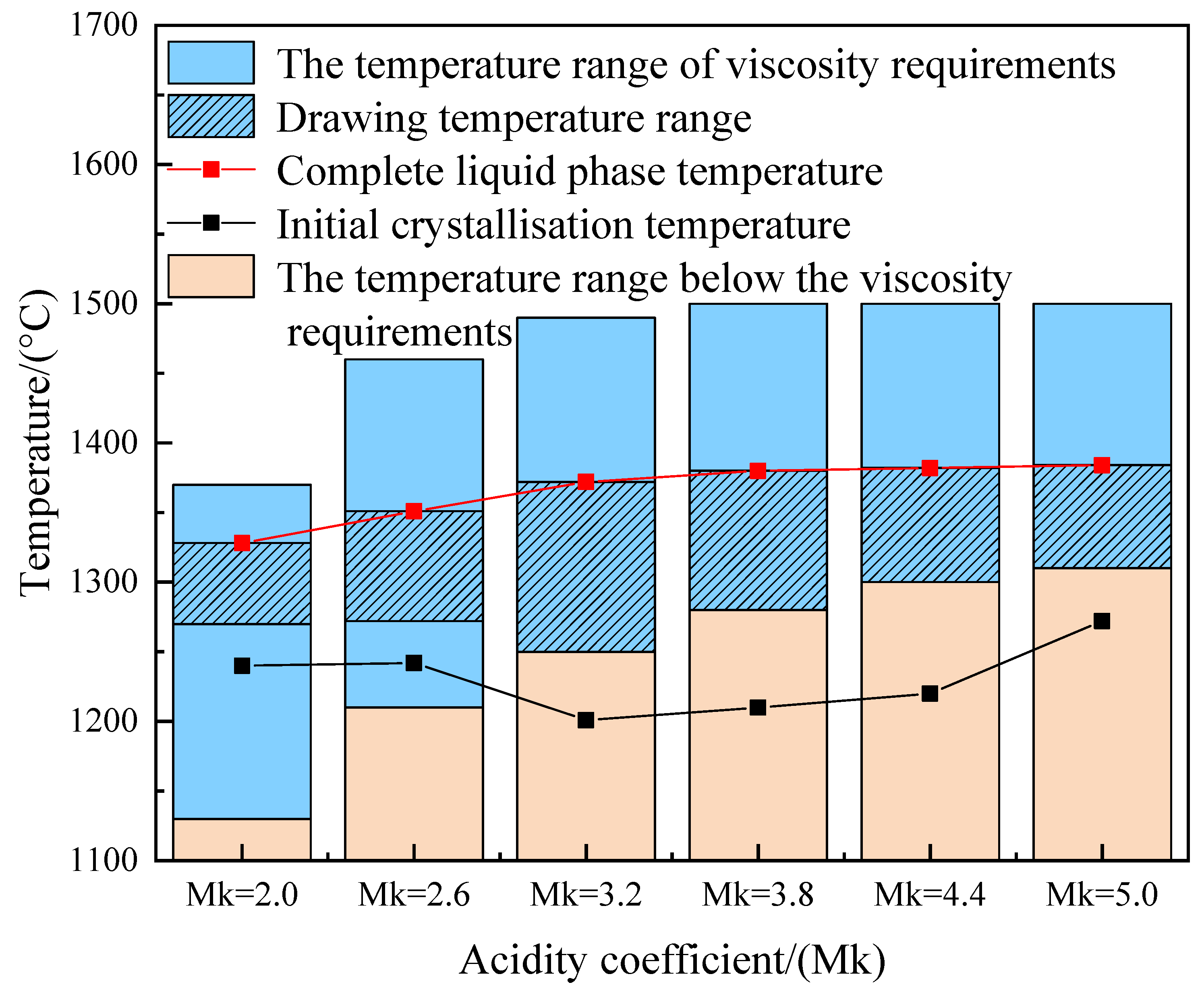
3.5. Determination of Fiber Drawing Temperature Analysis
3.6. Mechanical Property Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiele, J.J.; Traber, M.G.; Re, R.; Yan, L.; Delfosse, L. Co-combustion of coal and municipal solid waste in a circulating fluidized bed. Fuel 1998, 77, 1311–1315. [Google Scholar]
- Yue, G.; Lv, J.; Xu, P.; Hu, X.; Lin, X.; Chen, Y.; Li, J. Development Status and Prospect analysis of Circulating Fluidized Bed combustion. Electr. Power China 2016, 49, 1–13. [Google Scholar]
- Xu, D.J.; Shan, J.H.; Zhao, C.Q.; Zhou, N.; Liu, J.Y. Comparative study on performance of fly ash and fly ash in circulating fluidized bed (CFB). Compr. Util. Fly Ash 2022, 36, 105–108. [Google Scholar]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 28, 122443. [Google Scholar] [CrossRef]
- Zhou, M.; Ye, Q.; Chen, X.; Zhang, J.; Chen, L.; Wang, H. Composition design and properties of CFB ash mortar. Bull. Silic. 2022, 41, 425–432. [Google Scholar]
- Xiao, X.B.; Yang, H.R.; Zhang, H.; Lu, J.F.; Yue, G.X. Research on carbon content in fly ash from circulating fluidized bed boilers. Energy Fuels 2005, 19, 1520–1525. [Google Scholar] [CrossRef]
- Konieczynski, J.; Stec, K. The occurrence of trace elements in flue gases from circulating fluidized bed boilers. Arch. Environ. Prot. 2010, 36, 3–19. [Google Scholar]
- Lee, S.H.; Kim, G.S. Self-Cementitious Hydration of Circulating Fluidized Bed Combustion Fly Ash. J. Korean Ceram. Soc. 2017, 54, 128–136. [Google Scholar] [CrossRef]
- Du, X.; Huang, Z.; Ding, Y.; Xu, W.; Zhang, M.; Wei, L.; Yang, H. Feasibility Study of Grinding Circulating Fluidized Bed Ash as Cement Admixture. Materials 2022, 15, 5610. [Google Scholar] [CrossRef]
- Cui, Y.F.; Gao, K.K.; Zhang, P. Experimental and Statistical Study on Mechanical Characteristics of Geopolymer Concrete. Materials 2020, 13, 1651. [Google Scholar] [CrossRef]
- Lin, W.T.; Lin, K.L.; Chen, K.; Korniejenko, K.; Hebda, M.; Lach, M. Circulation Fluidized Bed Combustion Fly Ash as Partial Replacement of Fine Aggregates in Roller Compacted Concrete. Materials 2020, 12, 4204. [Google Scholar] [CrossRef] [PubMed]
- Xia, J. Study on the design and key properties of CFB ash grouting filling materials. Highroad 2021, 66, 99–104. [Google Scholar]
- Ma, Z.; Zhang, X.; Guo, Y.; Cheng, F. Research progress on characteristics and element dissolution behaviors of circulating gluidized bed-derived fly ash. Chem. Ind. Eng. Prog. 2021, 40, 3058–3071. [Google Scholar]
- Shoppert, A.; Loginova, I.; Valeev, D. Kinetics Study of Al Extraction from Desilicated Coal Fly Ash by NaOH at Atmospheric Pressure. Materials 2022, 14, 7700. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Lu, Z.; Li, J.; Song, K. Comparative study on the performance of curing sulfur ash in circulating fluidized bed. J. Wuhan Univ. Technol. 2014, 36, 6–13. [Google Scholar]
- Shao, Z.; Yuan, S.; Guo, X.; Wang, F. Research on development and application of circulating fluidized bed boiler technology. Power Stn. Syst. Eng. 2023, 39, 31–32. [Google Scholar]
- Li, N.; Liu, Y.; Cui, Z.; Hu, L.; Jiang, L. Properties and applications of basalt fiber. Synth. Fiber 2022, 51, 16–23. [Google Scholar]
- Chen, J.; Pan, Q.; Wei, Y.; Luo, Y. Experimental Study on Flexural Behavior of RC Piles with Basalt Fiber-Reinforced Polymer Bars and Load Carrying Capacity Calculation. Buildings 2024, 14, 1328. [Google Scholar] [CrossRef]
- Song, P.; Gao, H.; Wang, L.; Zheng, Z.; Cheng, Z.; Wang, Z. Analysis of basic characteristics and application prospect of basalt fiber. Miner. Prot. Util. 2022, 42, 173–178. [Google Scholar]
- Qi, F.; Li, J.; Li, C.; Wei, H.; Gao, Y. Research review of continuous basalt fiber. Hi-Tech Fiber Appl. 2006, 30, 42–46. [Google Scholar]
- Novitskii, A.G.; Efremov, M.V. Technological Aspects of the Suitability of Rocks from Different Deposits for the Production of Continuous Basalt Fiber. Glass Ceram. 2013, 69, 409–412. [Google Scholar] [CrossRef]
- Pu, M.; Peng, X.; Feng, X.; Chen, C. Research status of continuous basalt fiber ore raw materials in Sichuan Province. China Nonmet. Miner. Ind. Guide 2024, 44, 16–19. [Google Scholar]
- Zhang, J.; Xu, X.; Liu, Z. Characteristics of basalt ore for continuous fiber in Emeishan Basalt Formation, Sichuan Province. Hi-Tech Fiber Appl. 2019, 44, 52–59. [Google Scholar]
- Ma, Q.; Ding, L.; Wang, Q.; Yu, Y.; Lida, L.; Li, H. Preparation and characterization of continuous fly ash derived glass fibers with improved tensile strength. Mater. Lett. 2018, 231, 119–121. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Sohn, J.; Park, H. Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J. Clean. Prod. 2018, 203, 957–965. [Google Scholar] [CrossRef]
- Wen, X. Study on Preparation and Melting Characteristics of Fly Ash Based Inorganic Fiber. Master’s Thesis, Shanxi University, Taiyuan, China, 2020. [Google Scholar]
- National Public Service Platform for Standards Information. Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=DD3D95E5C16A71EBE05397BE0A0AF33F (accessed on 30 July 2024).
- Jamshaid, H.; Mishra, R. A green material from rock: Basalt fiber—A review. J. Text. Inst. 2016, 7, 107. [Google Scholar] [CrossRef]
- Li, J.; Dang, X. Preparation of basalt continuous fiber. Synth. Fiber Ind. 2007, 17, 35–37. [Google Scholar]
- Zhang, J.; Wang, Z.; Cheng, F. Research progress of solid waste based inorganic fibers. Mater. Rev. 2021, 35, 7019–7026. [Google Scholar]
- Knox, L.J.; Whitwell, J.C. Studies of breaking-stress distributions, Part I: The weak-link theory and alternate models. Text. Res. J. 1971, 41, 510–517. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, P. Study on strength and dispersion of basalt fiber. J. Nantong Univ. Nat. Sci. Ed. 2013, 12, 55–59. [Google Scholar]
- Deák, T.; Czigány, T. Chemical Composition and Mechanical Properties of Basalt and Glass Fibers: A Comparison. Text. Res. J. 2009, 79, 645–651. [Google Scholar] [CrossRef]






| (wt%) | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | SO3 | TiO2 | MgO | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| CFB Ash | 54.19 | 23.64 | 7.64 | 5.85 | 2.50 | 1.77 | 1.61 | 1.08 | 0.93 |
| Mk = 2.0 | 41.68 | 18.07 | 19.59 | 4.52 | 1.91 | 1.35 | 1.24 | 10.29 | 0.72 |
| Mk = 2.6 | 44.75 | 19.44 | 16.66 | 4.85 | 2.05 | 1.46 | 1.33 | 8.03 | 0.77 |
| Mk = 3.2 | 46.91 | 20.40 | 14.60 | 5.07 | 2.15 | 1.53 | 1.39 | 6.44 | 0.81 |
| Mk = 3.8 | 48.51 | 21.11 | 13.07 | 5.25 | 2.23 | 1.58 | 1.44 | 5.26 | 0.83 |
| Mk = 4.4 | 49.75 | 21.66 | 11.88 | 5.38 | 2.29 | 1.62 | 1.48 | 4.35 | 0.85 |
| Mk = 5.0 | 50.73 | 22.10 | 10.95 | 5.49 | 2.33 | 1.66 | 1.51 | 3.62 | 0.87 |
| (°C) | Initial Liquid Phase Formation Temperature | Complete Liquid Phase Temperature |
|---|---|---|
| CFB Ash | 1273.00 | 1499.00 |
| Mk = 2.0 | 1253.00 | 1318.00 |
| Mk = 2.6 | 1186.00 | 1351.00 |
| Mk = 3.2 | 1186.00 | 1372.00 |
| Mk = 3.8 | 1186.00 | 1380.00 |
| Mk = 4.4 | 1186.00 | 1382.00 |
| Mk = 5.0 | 1213.00 | 1384.00 |
| Diameter/μm | Cross-Sectional Area/m2 | Tensile Strength/MPa | Young’s Modulus/GPa |
|---|---|---|---|
| 45.6 | 1.63 × 10−9 | 533 | 59.4 |
| 62.4 | 3.06 × 10−9 | 663 | 66.3 |
| 58.5 | 2.69 × 10−9 | 386 | 55.2 |
| 53.6 | 2.26 × 10−9 | 686 | 62.4 |
| 64.5 | 3.27 × 10−9 | 494 | 61.8 |
| 84.2 | 5.56 × 10−9 | 469 | 74.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhou, T.; Li, Z.; Ding, Y.; Song, Q.; Zhang, M.; Hu, N.; Yang, H. Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash. Materials 2024, 17, 3800. https://doi.org/10.3390/ma17153800
Wang Q, Zhou T, Li Z, Ding Y, Song Q, Zhang M, Hu N, Yang H. Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash. Materials. 2024; 17(15):3800. https://doi.org/10.3390/ma17153800
Chicago/Turabian StyleWang, Qingjia, Tuo Zhou, Zhiao Li, Yi Ding, Qiang Song, Man Zhang, Nan Hu, and Hairui Yang. 2024. "Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash" Materials 17, no. 15: 3800. https://doi.org/10.3390/ma17153800
APA StyleWang, Q., Zhou, T., Li, Z., Ding, Y., Song, Q., Zhang, M., Hu, N., & Yang, H. (2024). Experimental Study on Preparation of Inorganic Fibers from Circulating Fluidized Bed Boilers Ash. Materials, 17(15), 3800. https://doi.org/10.3390/ma17153800









