Reactivity Control of Oxidative CL-20@PVDF Composite Microspheres by Using Carbon Nanomaterials as Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
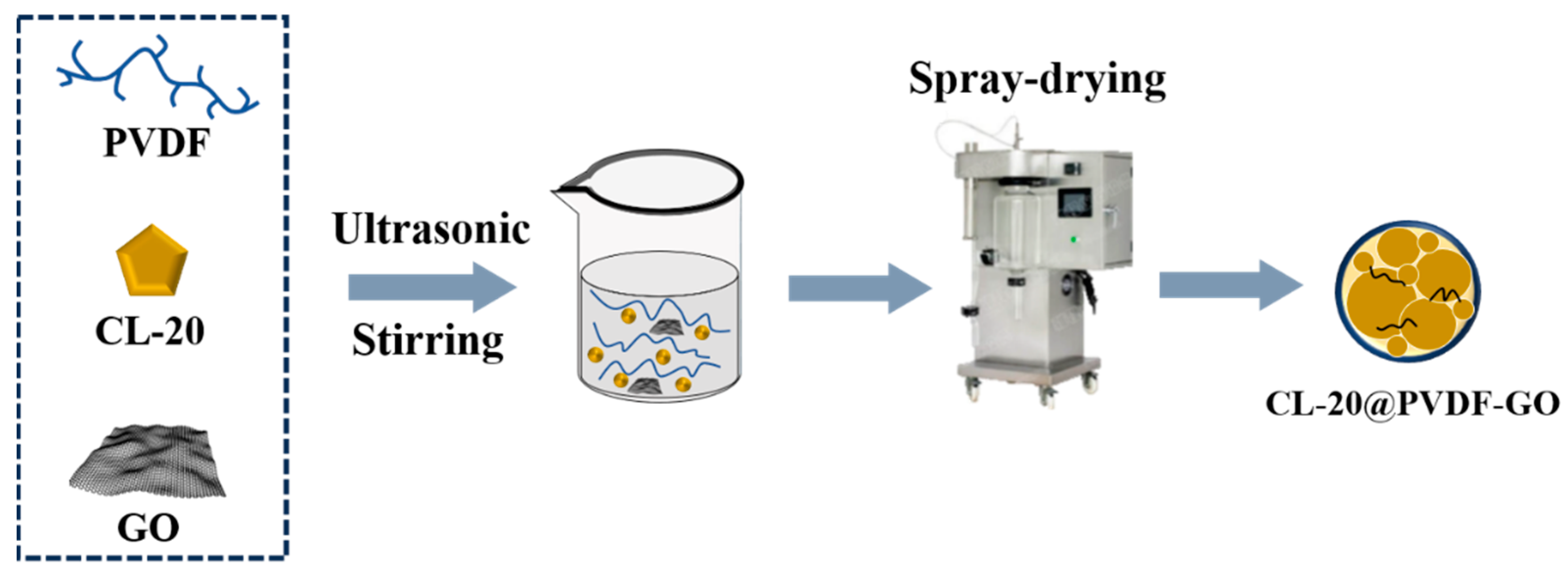
2.2. Preparation of CL-20@PVDF and CL-20@PVDF@B Microspheres
2.3. Characterization Techniques
3. Results
3.1. Morphology Characterization of Samples
3.2. Thermal Stability of Samples
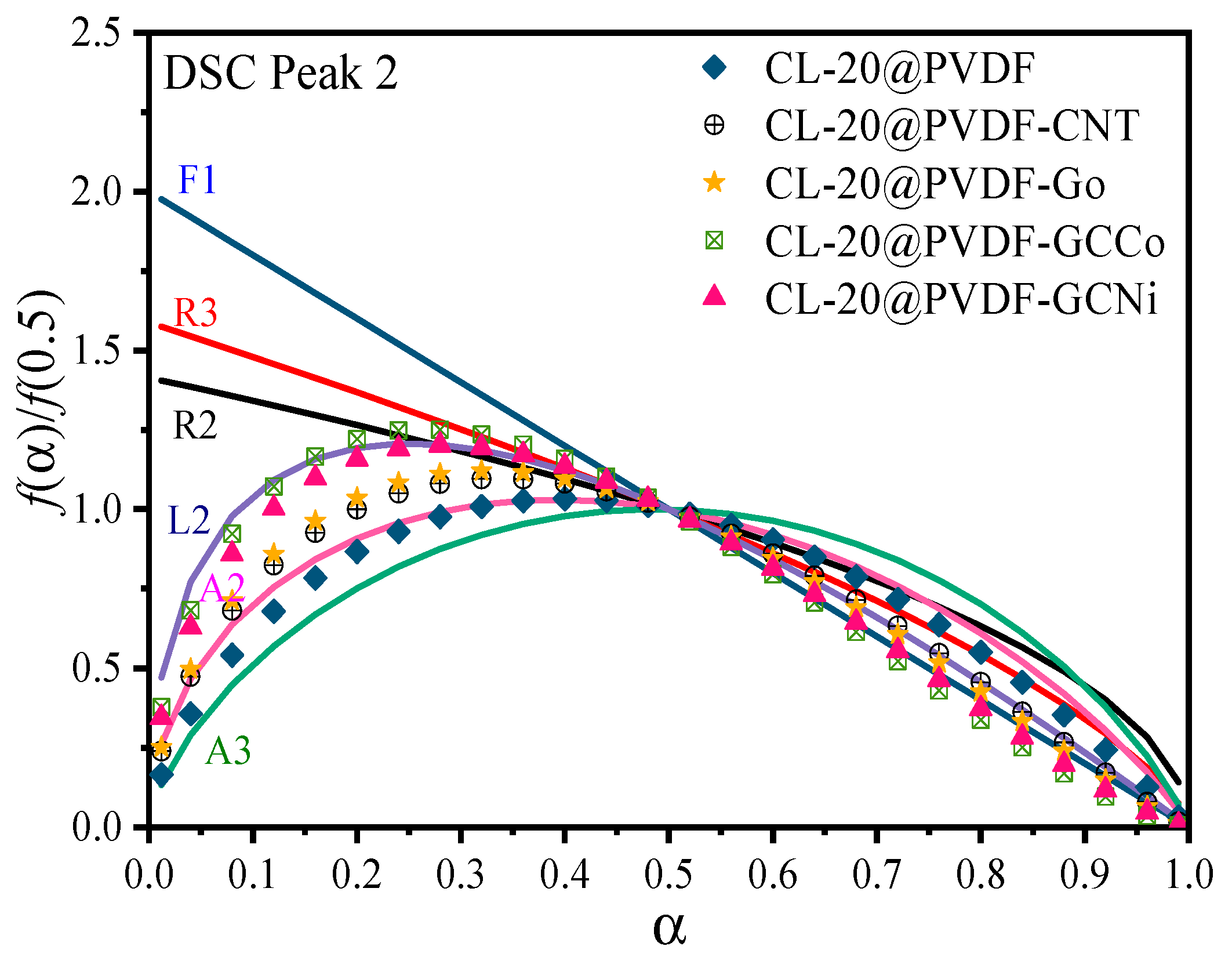
3.3. Kinetics and Mechanisms of CL-20@PVDF Microspheres
3.4. Impact Sensitivity of CL-20@PVDF Microspheres
3.5. Burn Rate and Flame Temperature of CL-20@PVDF@B Microspheres
4. Conclusions
- (1)
- For CL-20@PVDF dual-oxidant particles, PVDF can promote the degradation of CL-20. Four different types of nanocarbon materials all show an accelerated effect on the decomposition of CL-20@PVDF microspheres. Among all the additives, GO shows a more obvious catalytic effect on CL-20 thermal decomposition, and GCNi presents the maximum acceleration effect on the decomposition of PVDF.
- (2)
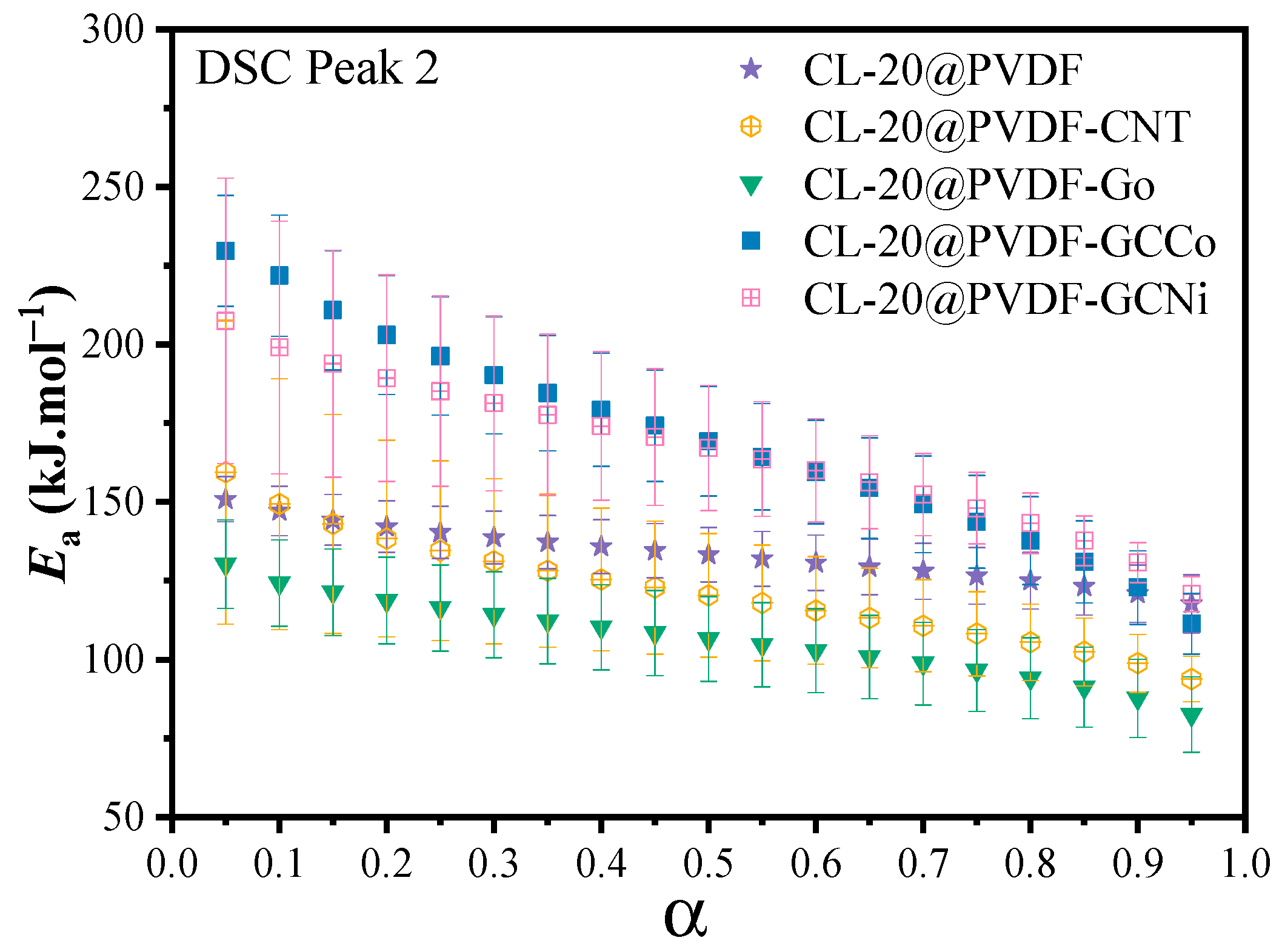
- In the Kissinger method, the doping of nanocarbon causes the increase of activation energy for CL-20@PVDF. For the Friedman method, the Ea value of involved CL-20@PVDF particles decreases as the conversion rate increases, which suggests that the autocatalytic reaction occurred. By using the combined kinetic method, the additive of CNTs and GO did not change the decomposition model of CL-20@PVDF, all their kinetic models remained A2 and then L2 model. However, CL-20@PVDF-GCCo and CL-20@PVDF-GCNi followed L2 and then the F1 model.
- (3)
- Since nanocarbon additives have superior thermal conductivity and deformation resistance, they reduce the impact sensitivity of CL-20@PVDF particles, wherein GO could reduce the impact sensitivity by 100%.
- (4)
- The additive of nanocarbon materials would increase the burn rate of CL-20@PVDF@B, since they have excellent thermal conductivity. Moreover, graphene-based carbohydrazide complexes could increase the flame temperature and control the burn rate with their synergetic effect. It shows that the introduction of graphene-based energetic complexes could lead to higher combustion efficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayat, Y.; Taheripouya, G.; Zeynali, V.; Azizkhani, V. Methods and strategies to achieve hexanitrohexaazaisowurtzitane (HNIW or CL-20): A comprehensive overview. J. Energetic Mater. 2023, 1–35. [Google Scholar] [CrossRef]
- Nair, U.R.; Sivabalan, R.; Gore, G.M.; Geetha, M.; Asthana, S.N.; Singh, H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-based formulations. Combust. Explos. Shock Waves 2005, 41, 121–132. [Google Scholar] [CrossRef]
- Xing, J.; Wang, W.; Yao, T.; Dong, J.; Miao, R.; Xu, W. Preparation and Characterization of Spherical Submicron CL-20 by Siphon Spray Refinement. J. Nanomater. 2020, 2020, 2394698. [Google Scholar] [CrossRef]
- Chen, L.; Meng, D.; Zhang, J.; Cao, X.; Nan, F.; Liao, X.; He, W. Bio-inspired designing strategy and properties of energetic crystals@ (CNFs@PDA) composites. Cellulose 2023, 30, 7729–7743. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Q.; Jin, B.; Peng, R. Electrostatic self-assembly desensitization of CL-20 by enhanced interface interaction. J. Alloys Compd. 2022, 900, 163504. [Google Scholar] [CrossRef]
- Duan, B.; Lu, X.; Mo, H.; Tan, B.; Wang, B.; Liu, N. Fabrication of CL-20/HMX Cocrystal@Melamine-Formaldehyde Resin Core-Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization. Int. J. Mol. Sci. 2022, 23, 6710. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Jing, J.; Cheng, W.; Song, H.; Li, S.; Zhang, Z.; Wang, J.; An, C. Molecular dynamics simulation of bilayer core-shell structure of CL-20 surface-modified by polydopamine coated with polymer binder. Mater. Today Commun. 2023, 37, 107099. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, Y.; Wang, K.-X.; Wang, T.-P.; Li, Y.; Zhu, S.-G.; Zhang, L.; Yi, Z.-X.; Guan, H.; Zhu, C.-G. Tuning the Energetic Performance of CL-20 by Surface Modification Using Tannic Acid and Energetic Coordination Polymers. ACS Omega 2022, 7, 10469–10475. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Han, J.; Yan, Z. MOF as the rigid shell to improve the mechanical sensitivity of nitramine explosives. Mater. Lett. 2022, 306, 130940. [Google Scholar] [CrossRef]
- Atamanov, M.; Lyu, J.-Y.; Chen, S.; Yan, Q.-L. Preparation of CNTs Coated with Polydopamine-Ni Complexes and Their Catalytic Effects on the Decomposition of CL-20. ACS Omega 2021, 6, 22866–22875. [Google Scholar] [CrossRef]
- Lv, J.; Wu, Q.; Zhou, Z.; Zhang, L.; Chen, Z.; Cao, H.; Zheng, W.; Tan, L. Bionic functional layer strategy to construct synergistic effect-based high-safety CL-20@PDA@GO core-shell-shell structural composites. J. Alloys Compd. 2022, 924, 166494. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Hussain, I.; Shen, R.; Yang, G.; Zhang, K. Core-Shell Structured Nanoenergetic Materials: Preparation and Fundamental Properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef] [PubMed]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E. Fluorine-containing oxidizers for metal fuels in energetic formulations. Def. Technol. 2019, 15, 1–22. [Google Scholar] [CrossRef]
- Chen, S.; Tang, D.-Y.; Zhang, X.-X.; Lyu, J.-Y.; He, W.; Liu, P.; Yan, Q.-L. Enhancing the Combustion Performance of Metastable Al@AP/PVDF Nanocomposites by Doping with Graphene Oxide. Engineering 2020, 6, 1019–1027. [Google Scholar] [CrossRef]
- Örnek, M.; Uhlenhake, K.E.; Zhou, Y.; Zhang, B.; Kalaswad, M.; Collard, D.N.; Wang, H.; Wang, Q.; Son, S.F. Preparation and characterization of multifunctional piezoenergetic polyvinylidene fluoride/aluminum nanocomposite films. J. Appl. Phys. 2022, 131, 1–14. [Google Scholar] [CrossRef]
- Zuo, B.; Wang, S.-Z.; Yang, S.; Liu, P.; Yan, Q.-L. Thermal decomposition and combustion behavior of solid propellant containing Si-based composites. Combust. Flame 2022, 240, 111959. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Huang, B.; Chen, S.; Liu, P.-J.; Li, W.; Yan, Q.-L. High energy core-shell Al@PVDF/AP composites with enhanced combustion efficiency by doping of graphene-based carbohydrazide complexes as catalysts. Fuel 2022, 330, 125592. [Google Scholar] [CrossRef]
- Jing, X. Technology of Research CL-20 Based Explosives of Low Energy Detonating. Ph.D. Thesis, North University of China, Taiyuan, China, 2014. [Google Scholar]
- Huang, Y.; Guo, Q.; Gou, R.; Zhu, S.; Zhang, S.; Yuan, X.; Chen, Y. Theoretical investigation on interface interaction and properties of 3-nitro-1,2,4-triazol-5-one (NTO)/fluoropolymer polymer-bonded explosives (PBXs). Theor. Chem. Acc. Theory Comput. Model. 2022, 141, 74. [Google Scholar] [CrossRef]
- Abdelhafiz, M.; Yehia, M.; Mostafa, H.E.; Wafy, T.Z. Catalytic action of carbon nanotubes on ammonium perchlorate thermal behavior. React. Kinet. Mech. Catal. 2020, 131, 353–366. [Google Scholar] [CrossRef]
- Hussein, A.K.; Zaki, M.G.; Elbeih, A. Influence of a novel nano-thermite colloid based on CuO coated CNTs on the thermo-analytical characteristics of 1,3,5-trinitro-1,3,5-triazinane. Combust. Sci. Technol. 2023, 195, 2523–2535. [Google Scholar] [CrossRef]
- Li, H.; Ren, H.; Jiao, Q.; Yu, L.; Du, S. Fabrication and Properties of Insensitive CNT/HMX Energetic Nanocomposites as Ignition Ingredients. Propellants Explos. Pyrotech. 2016, 41, 126–135. [Google Scholar] [CrossRef]
- Shi, X.; Tian, L.; Shen, J.; Pei, C.; Jia, Y.; Chen, L. Tuning the Laser Ignition Properties of Nitrocellulose-Nitroglycerine-Hexogen Propellants via Incorporation of Carbon Nanotubes. Cent. Eur. J. Energetic Mater. 2021, 1, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, J.P.; Yang, G.C.; Wang, J.; Hua, C. Preparation and characterization of insensitive HMX/graphene oxide composites. Propellants Explos. Pyrotech. 2013, 38, 798–804. [Google Scholar] [CrossRef]
- Wang, S.; An, C.; Wang, J.; Ye, B. Reduce the Sensitivity of CL-20 by Improving Thermal Conductivity Through Carbon Nanomaterials. Nanoscale Res. Lett. 2018, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, L.; Xu, K.; Song, J.; Zhao, F. Graphene oxide-enveloped Bi2WO6 composites as a highly efficient catalyst for the thermal decomposition of cyclotrimethylenetrinitramine. RSC Adv. 2016, 6, 42428–42434. [Google Scholar] [CrossRef]
- He, W.; Guo, J.-H.; Cao, C.-K.; Liu, X.-K.; Lv, J.-Y.; Chen, S.-W.; Liu, P.-J.; Yan, Q.-L. Catalytic Reactivity of Graphene Oxide Stabilized Transition Metal Complexes of Triaminoguanidine on Thermolysis of RDX. J. Phys. Chem.-Part C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Liang, T.; Yang, X.; Liu, B.; Song, R.; Xiao, F.; Yang, Y.; Wang, D.; Dong, M.; Ren, J.; Xu, B.B.; et al. Ammonium perchlorate@graphene oxide/Cu-MOF composites for efficiently catalyzing the thermal decomposition of ammonium perchlorate. Adv. Compos. Hybrid Mater. 2023, 6, 67. [Google Scholar] [CrossRef]
- Feng, C.; Ye, B.; An, C.; Zhang, F.; Hong, Z.; Wang, J. Preparation of functionalized GO coordination compound and its catalytic performance for thermal decomposition of ammonium perchlorate. J. Mater. Sci. 2021, 56, 19599–19613. [Google Scholar] [CrossRef]
- Huang, B.; Wang, S.-Z.; Zhao, X.; Yang, Z.-J.; Yan, Q.-L. Largely desensitized and stabilized CL-20 crystals obtained through reinforcement with cross-linked graphene oxide. CrystEngComm 2022, 24, 8070–8081. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Liu, P.-J.; He, A.-F.; Zhang, J.-K.; Ma, Y.; Hao, H.-X.; Zhao, F.-Q.; Gozin, M. Photosensitive but mechanically insensitive graphene oxide-carbohydrazide-metal hybrid crystalline energetic nanomaterials. Chem. Eng. J. 2018, 338, 240–247. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.-Z.; Yang, S.-L.; Fu, X.; Yan, Q.-L. Thermal behavior and thermolysis mechanisms of graphene oxide-intercalated energetic complexes of carbohydrazide. Chem. Thermodyn. Therm. Anal. 2023, 9, 100106. [Google Scholar] [CrossRef]
- GJB 772A-97; Explosive Test Method. National Defense Science and Technology Committee: Beijing, China, 1997.
- Gołofit, T.; Zyśk, K. Thermal decomposition properties and compatibility of CL-20 with binders HTPB, PBAN, GAP and polyNIMMO. J. Therm. Anal. Calorim. An Int. Forum Therm. Stud. 2015, 119, 1931–1939. [Google Scholar] [CrossRef]
- Nedelko, V.V.; Chukanov, N.V.; Raevskii, A.V.; Korsounskii, B.L.; Larikova, T.S.; Kolesova, O.I.; Volk, F. Comparative investigation of thermal decomposition of various modifications of hexanitrohexaazaisowurtzitane (CL-20). Propellants Explos. Pyrotech. 2000, 25, 255–259. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Li, J.; Luo, Y. Effect of Carbon Nanotubes on Thermal Decomposition of Aminonitrobenzodifuroxan. J. Combust. Sci. Technol. 2004, 1, 92–95. [Google Scholar]
- Xue, Z.-H.; Zhang, X.-X.; Huang, B.-B.; Bai, X.; Zhu, L.-Y.; Chen, S.; Yan, Q.-L. The structural diversity of hybrid qy-HMX crystals with constraint of 2D dopants and the resulted changes in thermal reactivit. Chem. Eng. J. 2020, 390, 124565. [Google Scholar] [CrossRef]
- Xu, R.; Xue, Z.; Yang, S.; Xu, J.; Nie, H.; Yan, Q.-L. Enhancing the reaction efficiency and ignition performance of core-shell Al@HMX composites by precise catalysis of graphene-based carbohydrazide complexes. Fuel 2023, 347, 128442. [Google Scholar] [CrossRef]
- Yuan, Z.-F.; Yang, Y.-J.; Zhao, F.-Q.; Song, X.-D.; Gao, H.-X.; Xu, S.-Y.; Zhang, J.-Q. Effects of Different Content of Nanomaterials on the Combustion Performance of RDX-CMDB Propellants. Huozhayao Xuebao/Chin. J. Explos. Propellants 2019, 42, 566–570, 582. [Google Scholar]
- Vyazovkin, S. Computational aspects of kinetic analysis. Part C. The ICTAC Kinetics Project-The light at the end of the tunnel. Thermochim. Acta 2000, 355, 155–163. [Google Scholar] [CrossRef]
- Jaques, N.G.; William de Lima Souza, J.; Popp, M.; Kolbe, J.; Lia Fook, M.V.; Ramos Wellen, R.M. Kinetic investigation of eggshell powders as biobased epoxy catalyzer. Compos. Part B 2020, 183, 107651. [Google Scholar] [CrossRef]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics and Gaseous Products of Thermal Decomposition of Polymers. J. Macromol. Sci. Part A-Chem. 1967, 1, 57–79. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Zeman, S.; Svoboda, R.; Elbeih, A.; Málek, J. The effect of crystal structure on the thermal reactivity of CL-20 and its C4-bonded explosives: Part II. Models for overlapped reactions and thermal stability. J. Therm. Anal. Calorim. Int. Forum Therm. Stud. 2013, 112, 837–849. [Google Scholar] [CrossRef]
- Chen, S.; He, W.; Luo, C.-J.; An, T.; Chen, J.; Yang, Y.; Liu, P.-J.; Yan, Q.-L. Thermal behavior of graphene oxide and its stabilization effects on transition metal complexes of triaminoguanidine. J. Hazard. Mater. 2019, 368, 404. [Google Scholar] [CrossRef] [PubMed]

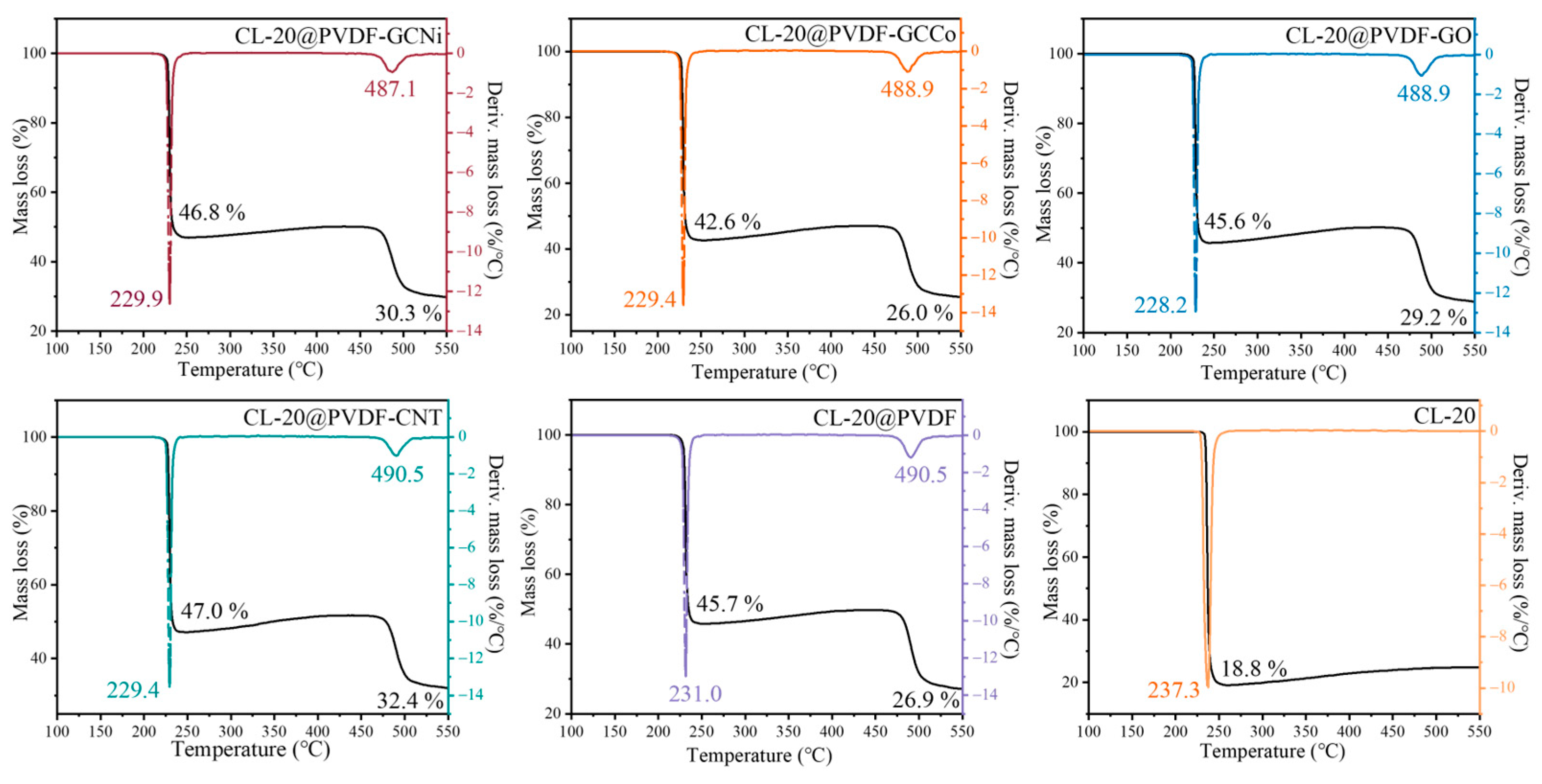
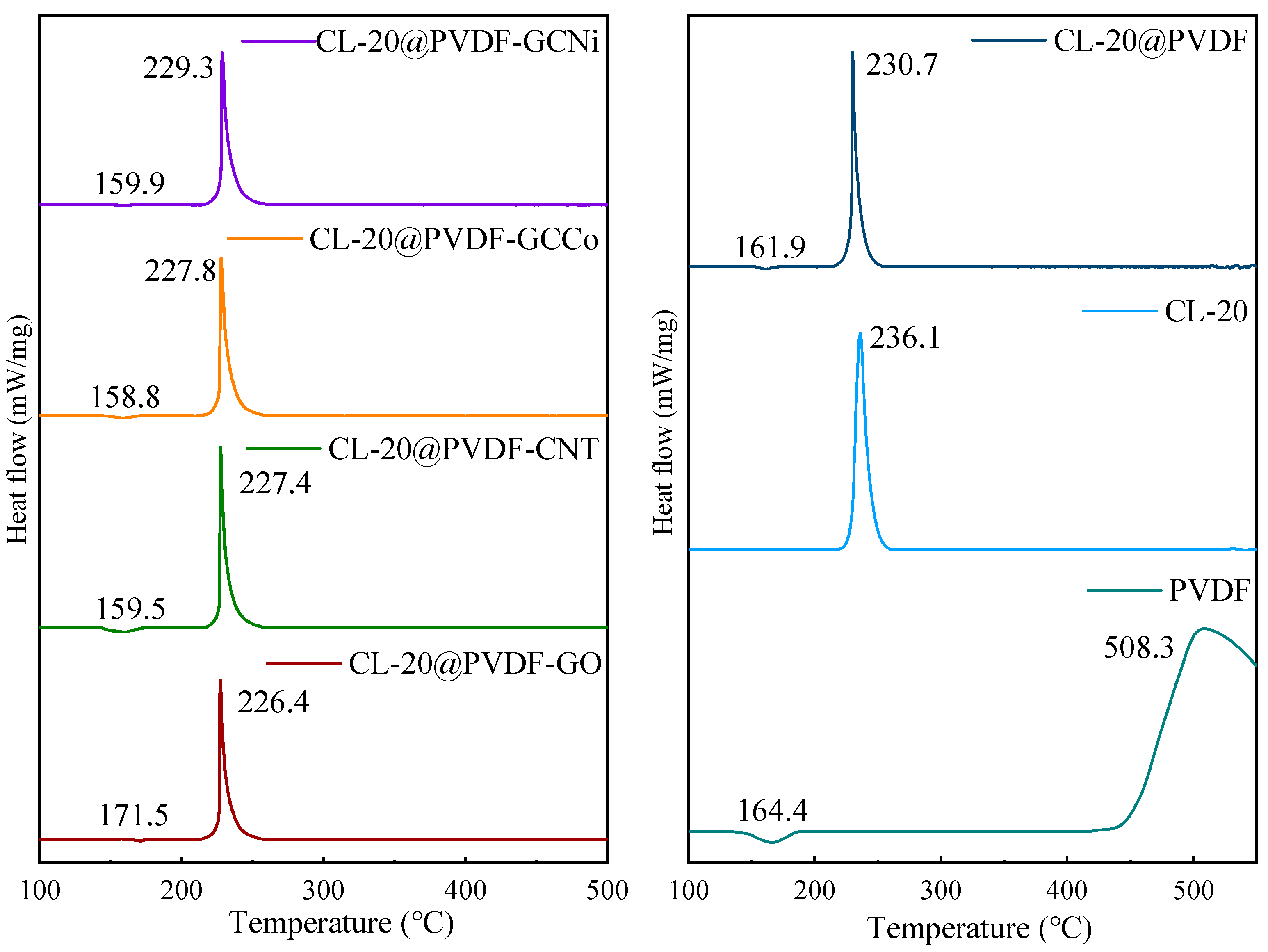

| Samples | TG Curves | DTG Peaks | |||
|---|---|---|---|---|---|
| Ti/°C | Mass Loss/% | Lmax %/°C | Tp/°C | Ti/°C | |
| CL-20 | 227.6 | 81.2 | 15.6 | 237.3 | 233.8 |
| CL-20@PVDF | 213.3/463.3 | 54.3/18.8 | 10.7/0.87 | 231.0/490.5 | 228.9/482.5 |
| CL-20@PVDF-CNT | 213.7/463.3 | 53.0/14.6 | 10.4/0.72 | 229.4/490.5 | 226.5/480.0 |
| CL-20@PVDF-GO | 209.2/462.1 | 54.4/16.4 | 11.1/0.67 | 228.2/488.9 | 226.1/476.9 |
| CL-20@PVDF-GCCo | 214.4/463.3 | 57.4/16.6 | 11.3/0.82 | 229.4/488.9 | 226.5/475.9 |
| CL-20@PVDF-GCNi | 213.2/459.8 | 53.2/16.5 | 11.0/0.75 | 229.9/487.1 | 227.5/474.8 |
| Samples | Exothermic Peaks (10 K min−1) | |||
|---|---|---|---|---|
| To/°C | Tp/°C | Te/°C | ∆T | |
| CL-20 | 216.4 | 236.1 | 261.6 | 45.2 |
| CL-20@PVDF | 153.4/210.4 | 161.9/230.7 | 169.5/254.8 | 16.1/44.4 |
| CL-20@PVDF-CNT | 149.2/212.1 | 159.5/227.4 | 169.3/256.6 | 20.1/44.5 |
| CL-20@PVDF-GO | 165.7/209.8 | 171.5/226.4 | 174.8/255.9 | 9.1/46.1 |
| CL-20@PVDF-GCCo | 153.0/208.9 | 158.8/227.8 | 168.7/259.4 | 15.7/50.5 |
| CL-20@PVDF-GCNi | 154.7/214.2 | 159.9/229.3 | 164.8/259.3 | 10.1/45.1 |
| Samples | Combined Kinetic Method | Friedman Method | Kissinger Method | ||||||
|---|---|---|---|---|---|---|---|---|---|
| m | n | Ea(1) | A/min−1 | Ea(2) | r | Ea(3) | log A | r | |
| CL-20@PVDF | 0.665 | 0.993 | 130.9 ± 1.2 | 4.6 × 1014 | 133.3 | 0.9958 | 167.3 | 11.6 | 0.9916 |
| CL-20@PVDF-CNT | 0.586 | 1.163 | 135.2 ± 3.5 | 1.3 × 1015 | 120.5 | 0.9766 | 211.2 | 16.2 | 0.9896 |
| CL-20@PVDF-GO | 0.597 | 1.238 | 116.1 ± 3.8 | 1.1 × 1013 | 106.6 | 0.9734 | 233.9 | 18.6 | 0.9881 |
| CL-20@PVDF-GCCo | 0.527 | 1.453 | 171.5 ± 5.1 | 7.5 × 1018 | 169.4 | 0.9671 | 270.6 | 22.5 | 0.9740 |
| CL-20@PVDF-GCNi | 0.532 | 1.350 | 161.1 ± 2.2 | 4.9 × 1017 | 167.1 | 0.9875 | 267.5 | 22.1 | 0.9777 |
| Samples | Impact Sensitivity (Im, J) |
|---|---|
| CL-20@PVDF | 3.0 |
| CL-20@PVDF-CNT | 4.0 |
| CL-20@PVDF-GO | 6.0 |
| CL-20@PVDF-GCCo | 4.0 |
| CL-20@PVDF-GCNi | 3.6 |
| Samples | CPB | CPB-CNTs | CPB-GO | CPB-GCCo | CPB-GCNi |
|---|---|---|---|---|---|
| Burn time (s) | 11.85 | 12.06 | 13.35 | 13.55 | 13.04 |
| Burn rate (mg s−1) | 19.89 | 24.44 | 21.85 | 20.30 | 21.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yu, M.; Xue, Z.-H.; Ding, Y.; Zhang, C.; Yan, Q.-L. Reactivity Control of Oxidative CL-20@PVDF Composite Microspheres by Using Carbon Nanomaterials as Catalysts. Materials 2024, 17, 3805. https://doi.org/10.3390/ma17153805
Chen S, Yu M, Xue Z-H, Ding Y, Zhang C, Yan Q-L. Reactivity Control of Oxidative CL-20@PVDF Composite Microspheres by Using Carbon Nanomaterials as Catalysts. Materials. 2024; 17(15):3805. https://doi.org/10.3390/ma17153805
Chicago/Turabian StyleChen, Shuwen, Minghui Yu, Zhi-Hua Xue, Yibing Ding, Chao Zhang, and Qi-Long Yan. 2024. "Reactivity Control of Oxidative CL-20@PVDF Composite Microspheres by Using Carbon Nanomaterials as Catalysts" Materials 17, no. 15: 3805. https://doi.org/10.3390/ma17153805





