In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
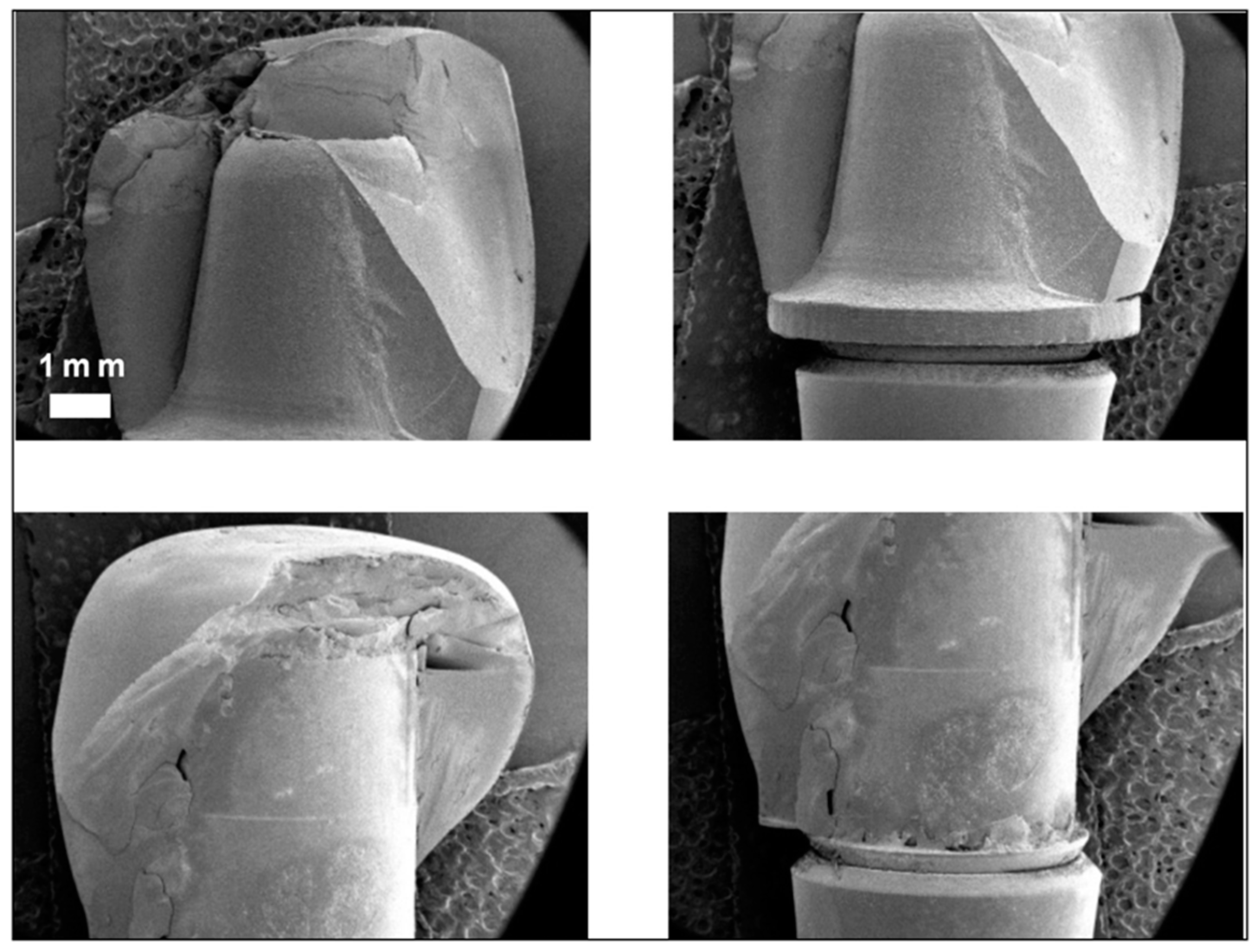
2.3. Observation by Field Emission Scanning Electron Microscope
2.4. Determination of the Maximum Compression Strength
2.5. Determination of S-N Curve
2.6. Characterization of Hardness and Fracture Toughness
2.7. Statistical Analysis
3. Results
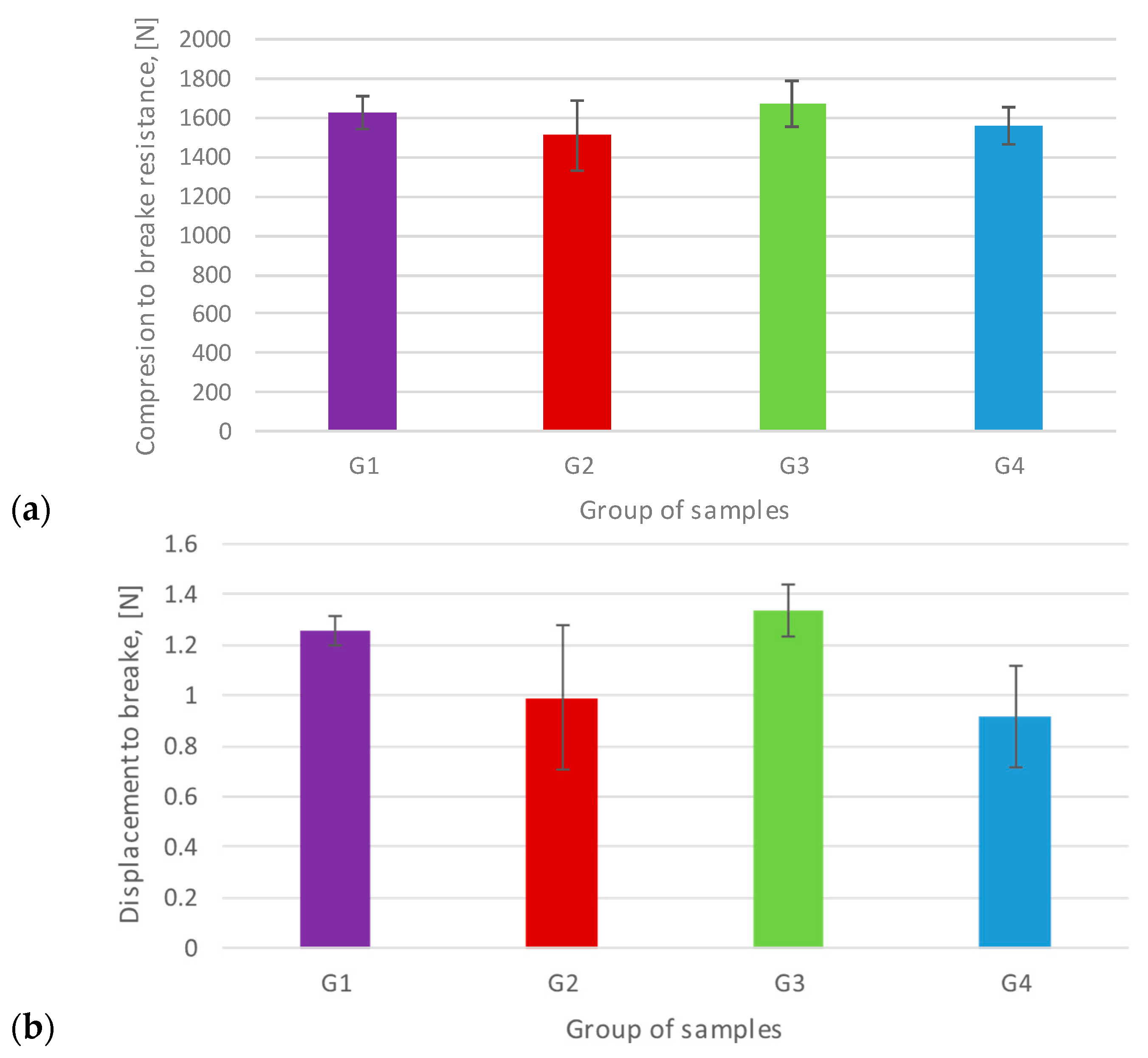
3.1. Uniaxial Flex-Compression Resistance
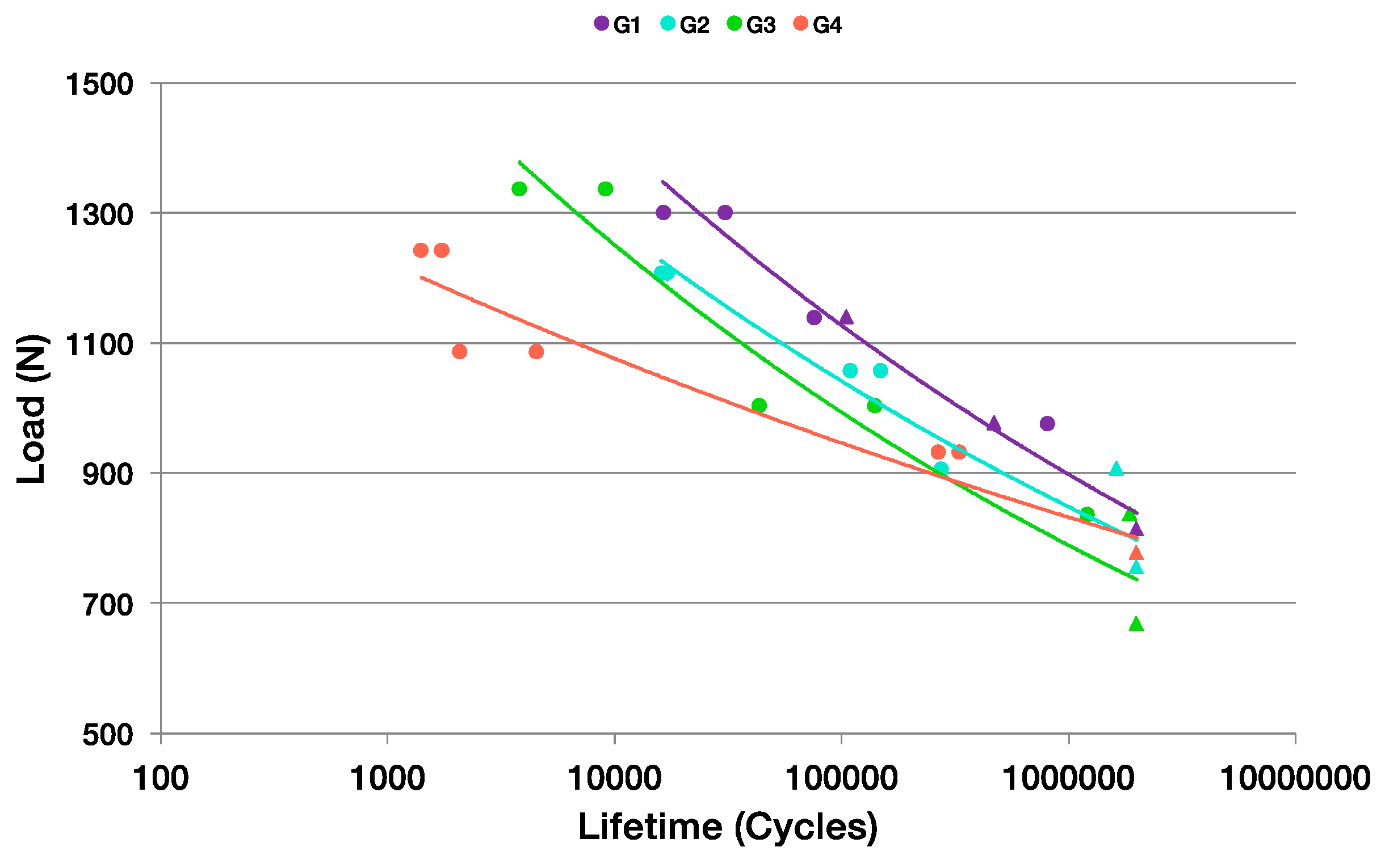
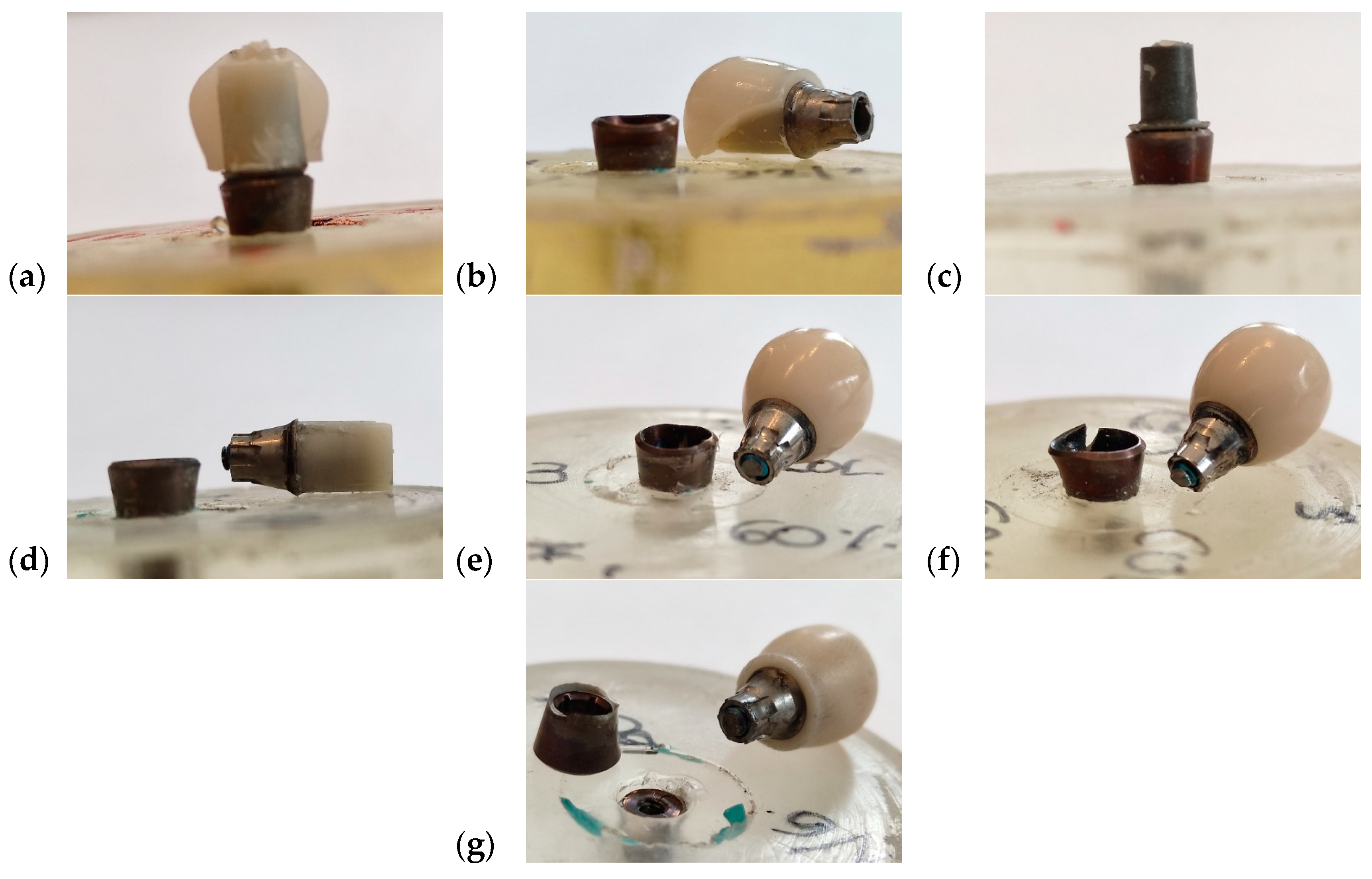
3.2. Uniaxial Cyclic Fatigue Test
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, H.H.; Yeh, C.L.; Wang, Y.L.; Huang, Y.C.; Tsai, S.J.; Li, Y.T.; Yang, J.H.; Lin, C.P. Differences in the biomechanical behaviors of natural teeth and dental implants. Dent. Mater. 2021, 37, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Igarashi, K.; Fuse, M.; Kitagawa, T.; Igarashi, M.; Uchibori, S.; Komine, C.; Gotouda, H.; Okada, H.; Kawai, Y. Risk factors for abutment and implant fracture after loading. J. Oral Sci. 2021, 63, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Heo, S.J.; Koak, J.Y.; Kim, S.K. Mechanical complications of implant-supported restorations with internal conical connection implants: A 14-year retrospective study. J. Prosthet. Dent. 2023, 129, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Sailer, I.; Latyshev, A.; Rabel, K.; Kohal, R.J.; Karasan, D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral Implants Res. 2021, 32 (Suppl. S21), 254–288. [Google Scholar] [CrossRef] [PubMed]
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A New Classification System for All-Ceramic and Ceramic-like Restorative Materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Resin composite blocks via high-pressure high-temperature polymerization. Dent. Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Mainjot, A.K.; Dupont, N.M.; Oudkerk, J.C.; Dewael, T.Y.; Sadoun, M.J. From Artisanal to CAD-CAM Blocks: State of the Art of Indirect Composites. J. Dent. Res. 2016, 95, 487–495. [Google Scholar] [CrossRef]
- Mourouzis, P.; Andreasidou, E.; Samanidou, V.; Tolidis, K. Short-term and long-term release of monomers from newly developed resin-modified ceramics and composite resin CAD-CAM blocks. J. Prosthet. Dent. 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Hampe, R.; Theelke, B.; Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Fracture toughness analysis of ceramic and resin composite CAD/CAM material. Oper. Dent. 2019, 44, E190–E201. [Google Scholar] [CrossRef]
- Naffah, N.; Ounsi, H.; Ozcan, M.; Bassal, H.; Salameh, Z. Evaluation of the adaptation and fracture resistance of three CAD-CAM resin ceramics: An in vitro study. J. Contemp. Dent. Pract. 2019, 20, 571–576. [Google Scholar]
- Lauvahutanon, S.; Takahashi, H.; Shiozawa, M.; Iwasaki, N.; Asakawa, Y.; Oki, M.; Finger, W.J.; Arksornnukit, M. Mechanical properties of composite resin blocks for CAD/CAM. Dent. Mater. J. 2014, 33, 705–710. [Google Scholar] [CrossRef]
- Awada, A.; Nathanson, D.; Coldea, A.; Swain, M.V.; Thiel, N.; Della Bona, A. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J. Prosthet. Dent. 2015, 114, 587–593. [Google Scholar] [CrossRef]
- Chavali, R.; Nejat, A.H.; Lawson, N.C. Machinability of CAD-CAM materials. J. Prosthet. Dent. 2017, 118, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lauvahutanon, S.; Takahashi, H.; Oki, M.; Arkornnukit, M.; Kanehira, M.; Finger, W.J. In vitro evaluation of the wear resistance of composite resin blocks for CAD/CAM. Dent. Mater. J. 2015, 34, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zaim, B.; Kalay, T.S.; Purcek, G. Friction, and wear behavior of chairside CAD-CAM materials against different types of antagonists: An in vitro study. J. Prosthet. Dent. 2022, 128, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Liebermann, A.; Eichberger, M.; Güth, J.F. Evaluation of mechanical and optical behavior of current esthetic dental restorative CAD/CAM composites. J. Mech. Behav. Biomed. Mater. 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Özcan, M.; Trottmann, A.; Schmutz, F.; Roos, M.; Hämmerle, C. Two-body wear rate of CAD/CAM resin blocks and their enamel antagonists. J. Prosthet. Dent. 2013, 109, 325–332. [Google Scholar] [CrossRef]
- Gracis, S.E.; Nicholls, J.I.; Chalupnik, J.D.; Yuodelis, R.A. Shock-absorbing behavior of five restorative materials used on implants. Int. J. Prosthodont. 1991, 4, 282–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niem, T.; Youssef, N.; Wöstmann, B. Energy dissipation capacities of CAD-CAM restorative materials: A comparative evaluation of resilience and toughness. J. Prosthet. Dent. 2019, 121, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Schneider-Feyrer, S.; Behr, M.; Preis, V. In Vitro Shock Absorption Tests on Implant-Supported Crowns: Influence of Crown Materials and Luting Agents. Int. J. Oral Maxillofac. Implants 2018, 33, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Silva, M.; Oderich, E.; Boff, L.L.; Enciso, R. Damping behavior of implant-supported restorations. Clin. Oral Implants Res. 2013, 24, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Conserva, E.; Tealdo, T.; Bevilacqua, M.; Pera, F.; Signori, A.; Pera, P. Shock Absorption Capacity of Restorative Materials for Dental Implant Prostheses: An In Vitro Study. Int. J. Prosthodont. 2013, 26, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, J.Y.; Hou, J.X.; Zhou, G.; Wang, C.; Fan, Y.B. A histological and biomechanical study of bone stress and bone remodeling around immediately loaded implants. Sci. China Life Sci. 2014, 57, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Wolff’s Law and bone’s structural adaptation to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [PubMed]
- Fu, J.H.; Hsu, Y.T.; Wang, H.L. Identifying occlusal overload and how to deal with it to avoid marginal bone loss around implants. Eur. J. Oral Implantol. 2012, 5, S91–S103. [Google Scholar]
- Rossenberg, E.S.; Torosian, J.P.; Slots, J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin. Oral Implants Res. 1991, 2, 135–144. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Effects of occlusal forces on the peri-implant-bone interface stability. Periodontol. 2000 2019, 81, 179–193. [Google Scholar] [CrossRef]
- Mish, C.E. Contemporary Implant Dentistry, 3rd ed.; Elsevier: St. Louis, MO, USA, 2008. [Google Scholar]
- Korabi, R.; Shemtov-Yona, K.; Dorogoy, A.; Rittel, D. The Failure Envelope Concept Applied to the Bone-Dental Implant System. Sci. Rep. 2017, 7, 2051. [Google Scholar] [CrossRef]
- Atkinson, S.R. Balance-the magic word. Am. J. Orthod. 1964, 50, 189–202. [Google Scholar] [CrossRef]
- Taha, D.; Cesar, P.F.; Sabet, A. Influence of different combinations of CAD-CAM crown and customized abutment materials on the force absorption capacity in implant supported restorations—In Vitro study. Dent. Mater. 2022, 38, e10–e18. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Yazigi, C.; Kern, M.; Chaar, M.S. Mechanical behavior of nano-hybrid composite in comparison to lithium disilicate as posterior cement-retained implant-supported crowns restoring different abutments. Dent. Mater. 2021, 37, e435–e442. [Google Scholar] [CrossRef]
- Mascarenhas, F.; Yilmaz, B.; McGlumphy, E.; Clelland, N.; Seidt, J. Load to failure of different zirconia implant abutments with titanium components. J. Prosthet. Dent. 2017, 117, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, N.M.; Chung, K.H.; Flinn, B.D.; Zheng, C.; Raigrodski, A.J. Assessment of reliability of CAD/CAM tooth-colored implant custom abutments. J. Prosthet. Dent. 2016, 116, 206–213. [Google Scholar] [CrossRef]
- Moris, I.C.M.; Chen, Y.C.; Faria, A.C.L.; Ribeiro, R.F.; Fok, A.S.L.; Rodrigues, R.C.S. Fracture loads and failure modes of customized and non-customized zirconia abutments. Dent. Mater. 2018, 34, e197–e204. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kaysser, W.A.; Rabin, B.H.; Kawasaki, A.; Ford, R.G. Functionally Graded Materials; Design, Processing and Applications; Springer Science + Business Media: New York, NY, USA, 1999; pp. 2–9. [Google Scholar]
- Cui, C.; Sun, J. Optimizing the design of bio-inspired functionally graded material (FGM) layer in all-ceramic dental restorations. Dent. Mater. J. 2014, 33, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Ferrante, M.; Su, B. Fabrication and characterization of biomimetic ceramic/polymer composite materials for dental restoration. Dent. Mater. 2013, 29, 375–381. [Google Scholar] [CrossRef]
- Chen, Y.C.; Fok, A. Stress distributions in human teeth modeled with a natural graded material distribution. Dent. Mater. 2014, 30, e337–e348. [Google Scholar] [CrossRef]
- He, L.H.; Yin, Z.H.; Van Vuuren, L.J.; Carter, E.A.; Liang, X.W. A natural functionally graded biocomposite coating-human enamel. Acta Biomater. 2013, 9, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Enamel—A “metallic-like” deformable biocomposite. J. Dent. 2007, 35, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Arola, D. Fatigue testing of biomaterials and their interfaces. Dent. Mater. 2017, 33, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Xu, W.; Gong, X.; Zhang, F. Mapping the mechanical gradient of human dentin-enamel-junction at different intratooth locations. Dent. Mater. 2018, 34, 376–388. [Google Scholar] [CrossRef]
- Joda, T.; Huber, S.; Bürki, A.; Zysset, P.; Brägger, U. Influence of Abutment Design on Stiffness, Strength, and Failure of Implant-Supported Monolithic Resin Nano Ceramic (RNC) Crowns. Clin. Implant. Dent. Relat. Res. 2015, 17, 1200–1207. [Google Scholar] [CrossRef]
- ISO 14801-2016; Dentistry—Implants—Dynamic Loading Test for Endosseous Dental Implants. International Organization for Standardization: Geneva, Switzerland, 2016.
- ASTM-E-384-17; Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Anderson, T.L. Fracture Mechanics: Fundamentals and Applications; Taylor & Francis: Abingdon, UK, 2005; ISBN 978-0-8493-1656-2. [Google Scholar]
- Marshall, D.B.; Lawn, B.R.; Evans, A.G. Elastic/plastic indentation damage in ceramics: The lateral crack system. J. Am. Ceram. 1980, 574. [Google Scholar] [CrossRef]
- Laugier, M.T. Load bearing capacity of TiN coated WC–Co cemented carbides. J. Mater. Sci. Lett. 1983, 2, 419–421. [Google Scholar] [CrossRef]
- Dukino, R.D.; Swain, M.V. Comparative measurement of indentation fracture toughness with Berkovich and Vickers indenters. J. Am. Ceram. Soc. 1992, 75, 3299–3304. [Google Scholar] [CrossRef]
- Casellas, D.; Caro, J.; Molas, S.; Prado, J.M.; Valls, I. Fracture toughness of carbides in tool steels evaluated by nanoindentation. Acta Mater. 2007, 55, 4277–4286. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of KIc of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. 1982, 1, 13–16. [Google Scholar]
- Mautsch, C.; Wolfard, S.; Mautsch, W.; Rittich, A. Long-term outcome of the IMZ implant system: A retrospective clinical study with a follow-up between 23 and 34 years. Int. J. Implant. Dent. 2022, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Chena, Y.Y.; Chena, W.P.; Chang, H.H.; Huang, S.H.; Lin, C.P. A novel dental implant abutment with micro-motion capability—Development and biomechanical evaluations. Dent. Mater. 2014, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Schepke, U.; Meijer, H.J.; Vermeulen, K.M.; Raghoebar, G.M.; Cune, M.S. Clinical Bonding of Resin Nano Ceramic Restorations to Zirconia Abutments: A Case Series within a Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2016, 18, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Krejci, I.; Daher, R. Stress distribution difference between Lava Ultimate full crowns and IPS e. max CAD full crowns on a natural tooth and on tooth-shaped implant abutments. Odontology 2017, 105, 254–256. [Google Scholar] [PubMed]
- Lohbauer, U.; Belli, R.; Cune, M.S.; Schepke, U. Fractography of clinically fractured, implant-supported dental computer- aided design and computer-aided manufacturing crowns. SAGE Open Med. Case Rep. 2017, 5, 2050313X17741015. [Google Scholar] [CrossRef]
- Reymus, M.; Roos, M.; Eichberger, M.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Bonding to new CAD/CAM resin composites: Influence of air abrasion and conditioning agents as pretreatment strategy. Clin. Oral Investig. 2019, 23, 529–538. [Google Scholar] [CrossRef]
- Emsermann, I.; Eggmann, F.; Krastl, G.; Weiger, R.; Amato, J. Influence of Pretreatment Methods on the Adhesion of Composite and Polymer Infiltrated Ceramic CAD-CAM Blocks. J. Adhes. Dent. 2019, 21, 433–443. [Google Scholar]
- Ferrario, V.F.; Sforza, C.; Serrao, G.; Dellavia, C.; Tartaglia, G.M. Single tooth bite forces in healthy young adults. J. Oral Rehabil. 2004, 31, 18–22. [Google Scholar] [CrossRef]
- Varga, S.; Spalj, S.; Lapter Varga, M.; Anic Milosevic, S.; Mestrovic, S.; Slaj, M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur. J. Orthod. 2011, 33, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Padma, S.; Umesh, S.; Asokan, S.; Srinivas, T. Bite Force Measurement Based on Fiber Bragg Grating Sensor. J. Biomed. Opt. 2017, 22, 107002. [Google Scholar] [CrossRef]
- Ramalho, I.S.; Bergamo, E.T.P.; Witek, L.; Coelho, P.G.; Lopes, A.C.O.; Bonfante, E.A. Implant-abutment fit influences the mechanical performance of single-crown prostheses. J. Mech. Behav. Biomed. Mater. 2020, 102, 103506. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, R.; Bartolomé, J.F.; Ferreiroa, A.; Salido, M.P.; Pradíes, G. Original vs. non-original abutments for screw-retained single implant crowns: An in vitro evaluation of internal fit, mechanical behaviour and screw loosening. Clin. Oral Implants Res. 2018, 29, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Doering, H.; Nonhoff, J. Performance of conical abutment (Morse Taper) connection implants: A systematic review. J. Biomed. Mater. Res. A 2014, 102, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Südbeck, S.; Hoffmann, M.; Reymus, M.; Buser, R.; Edelhoff, D.; Stawarczyk, B. Bending moment of implants restored with CAD/CAM polymer-based restoration materials with or without a titanium base before and after artificial aging. Dent. Mater. 2022, 38, e245–e255. [Google Scholar] [CrossRef] [PubMed]

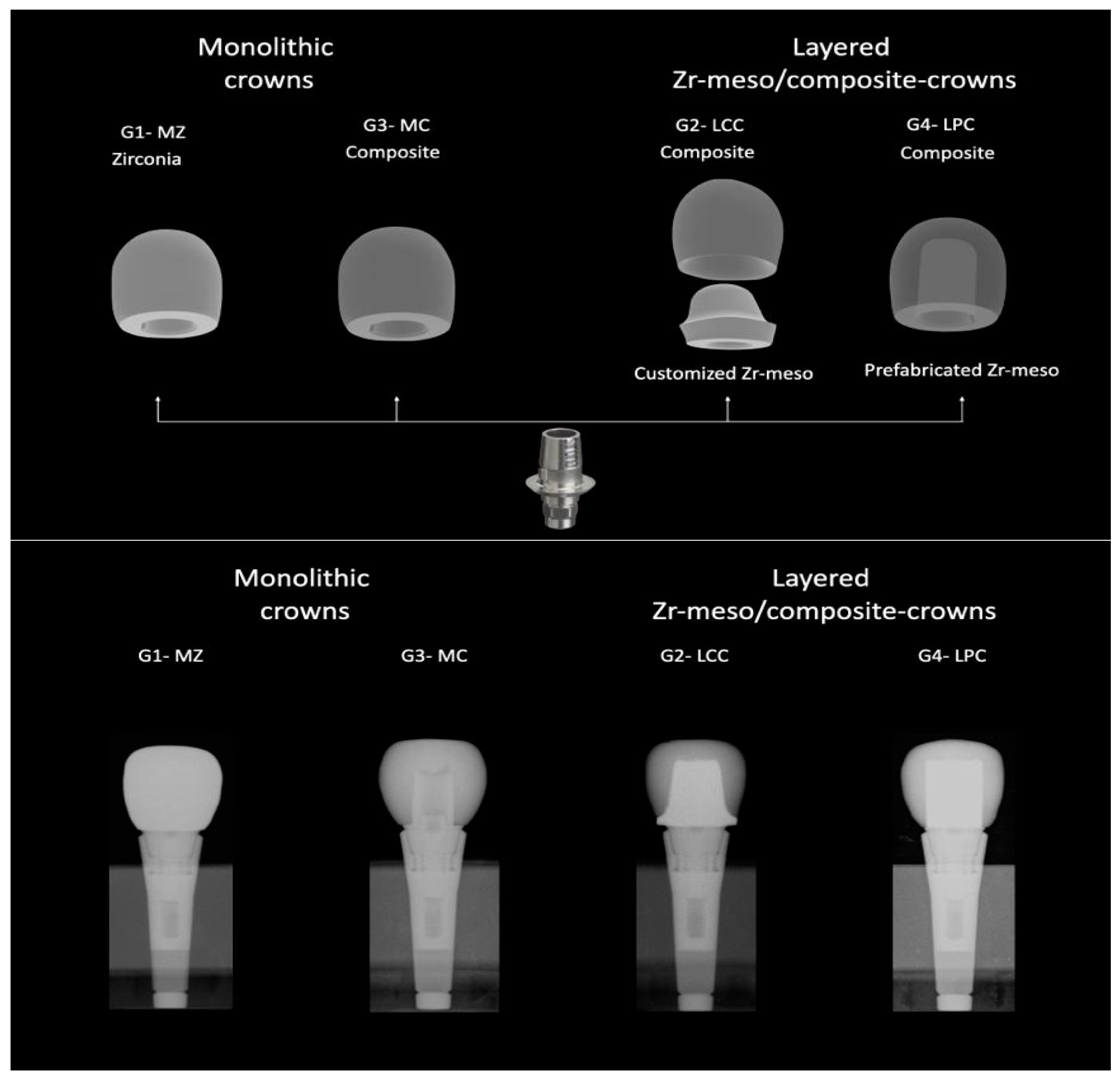

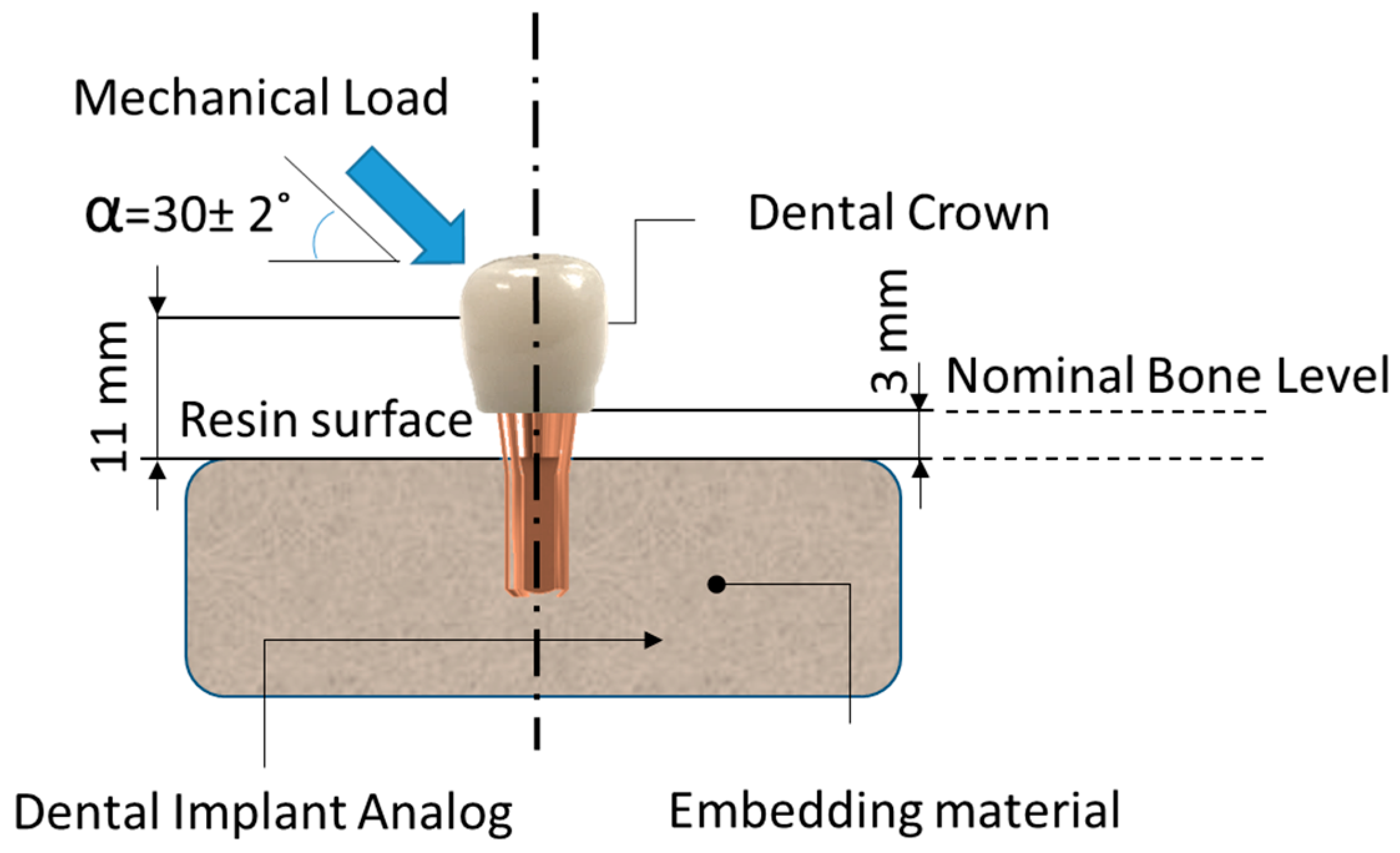
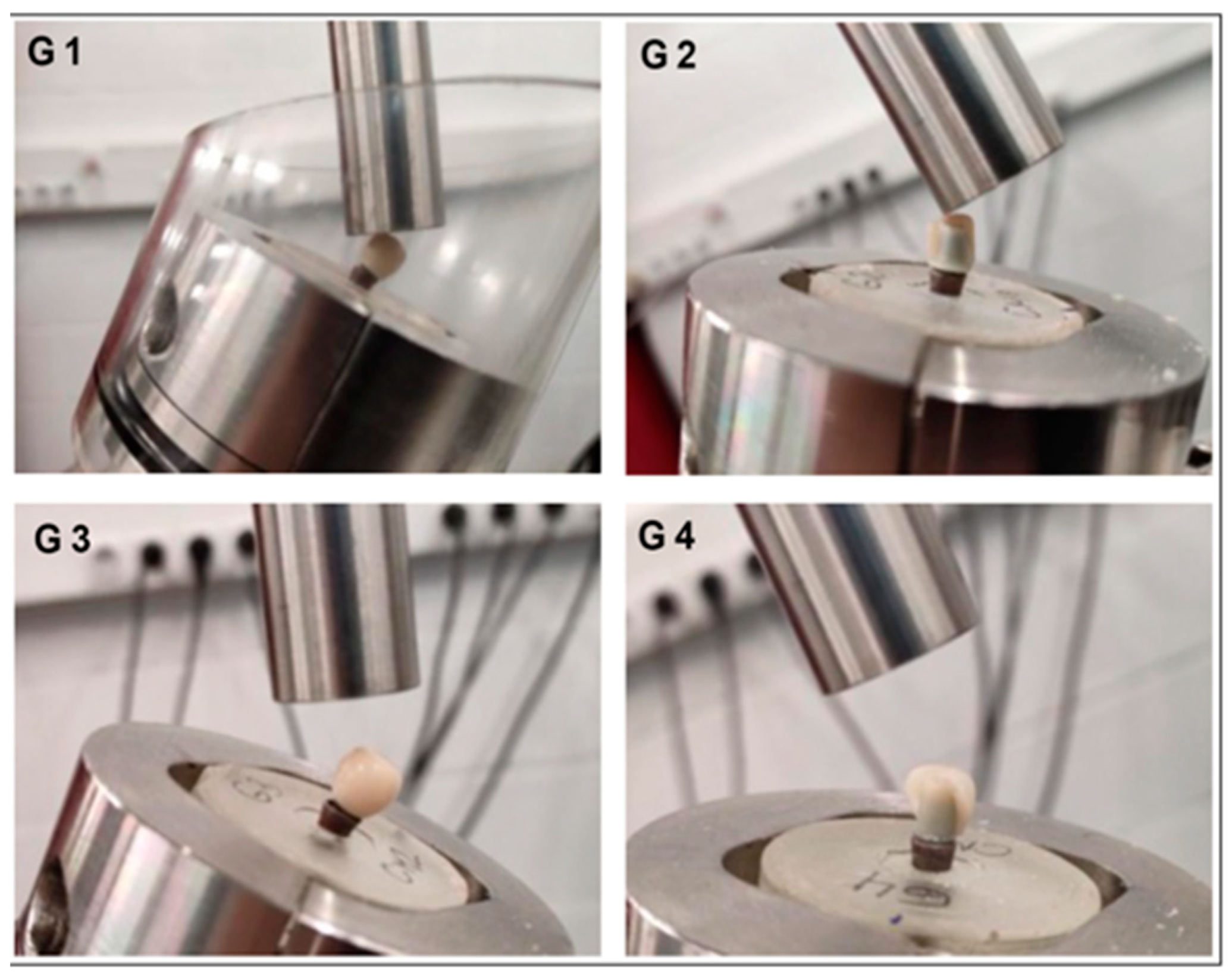
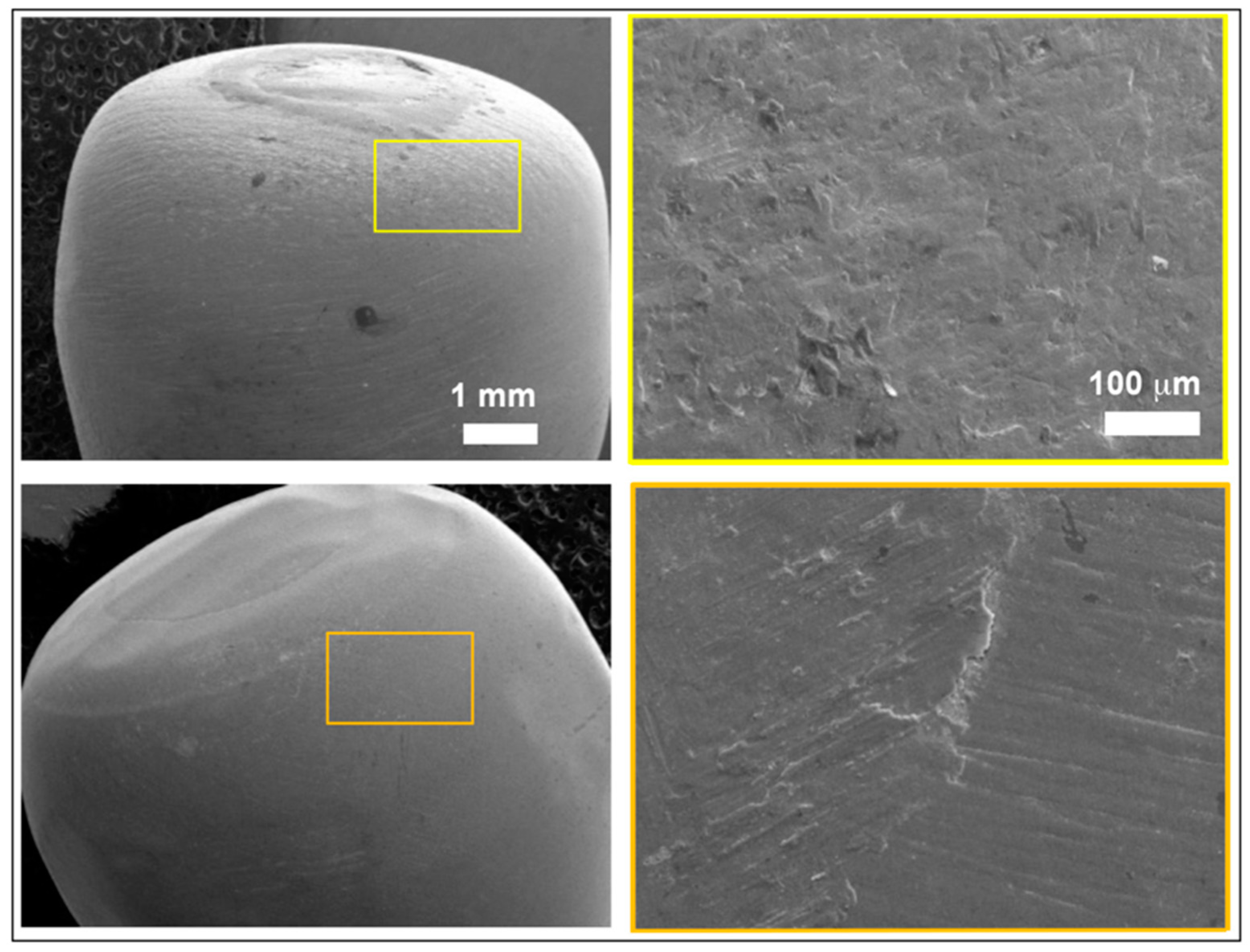
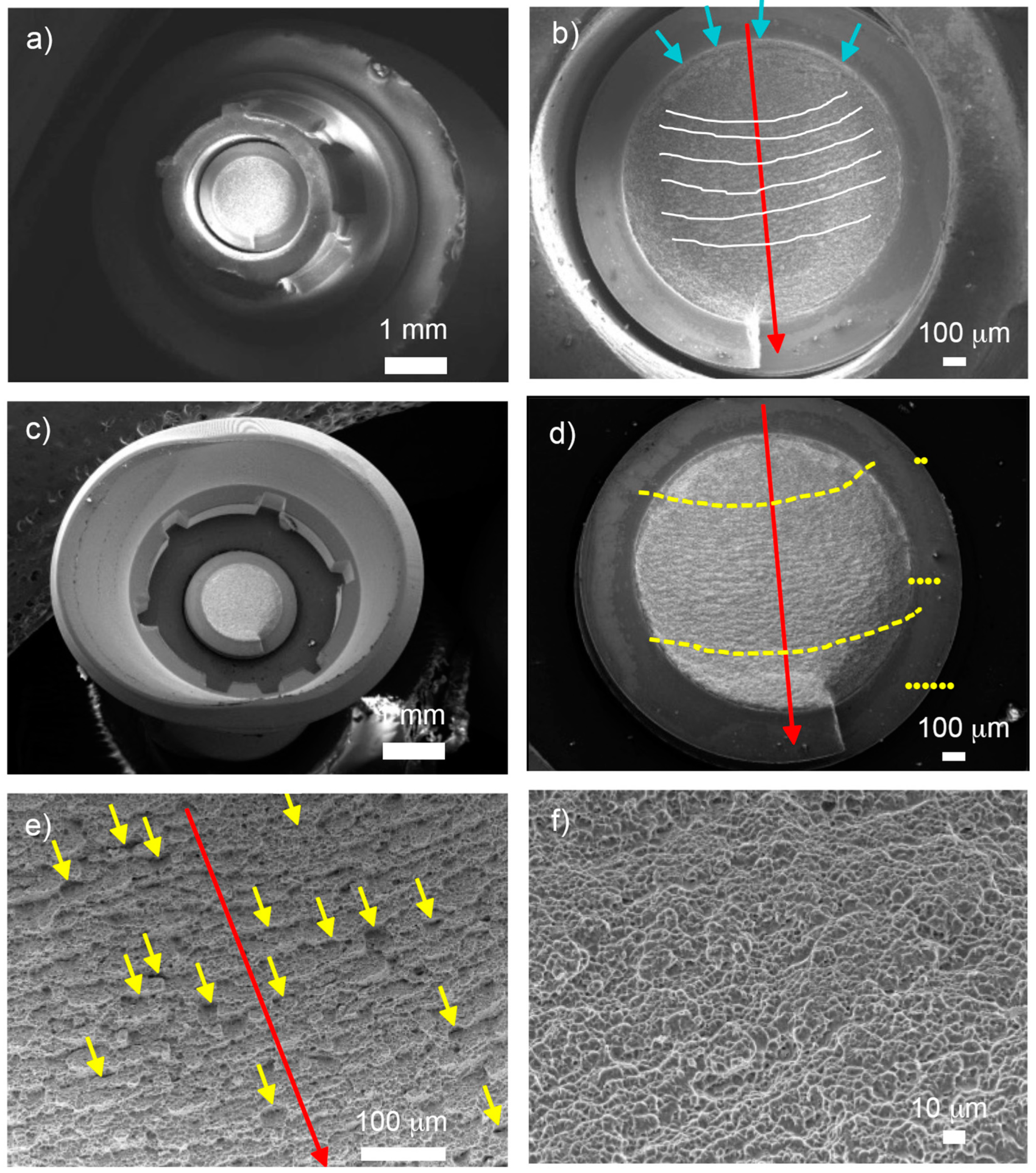

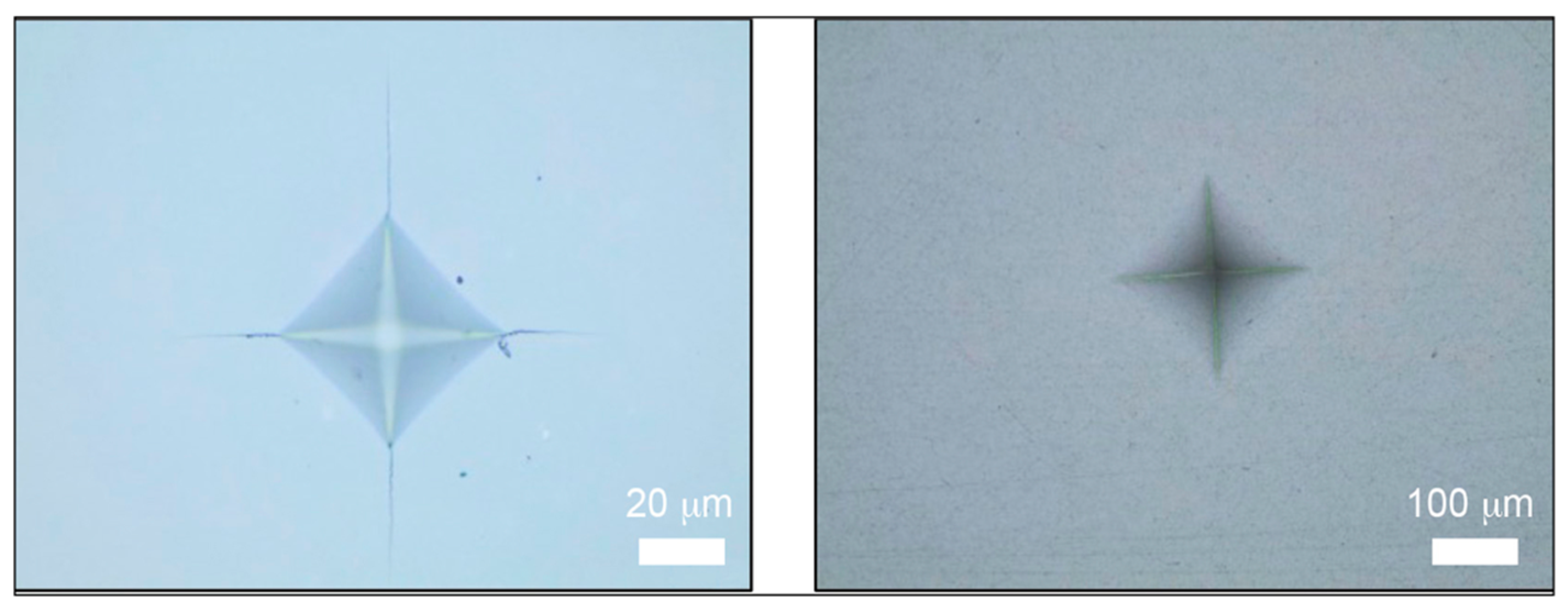
| Materials | Manufacturers | Composition | Flexural Strength | Fracture Toughness | Modulus of Elasticity |
|---|---|---|---|---|---|
| Zirconia crown: CEREC Zirconia meso L A2 LOT 2017484418 | Dentsply Sirona® | Yttria stabilized zirconia | >900 MPa | 7.1 MPa1/2 | 210 GPa |
| Composite crown: BRILLIANT Crios Disc A2 LT H18 LOT J52869 | Coltene® | Barium glass < 1.0 µm, amorphous silica SiO2 < 20 nm, cross linked methacrylic matrix and inorganic pigments | 198 MPa | 2.0 MPa m0.5 | 10.3 GPa |
| Customized meso structure: inCoris ZI meso F2 L LOT 3314000426 | Dentsply Sirona® | Dentsply Sirona® | >900 MPa | 5.8 MPa1/2 | 210 GPa |
| Zirconia insert | Coltene® | Yttria stabilized zirconia (3 mol% Y2O3) | 500–1000 MPa | 5.8–10.5 MPa m0.5 | 210 GPa |
| TiBase CEREC/inLab AT EV 4,8 GH 1 “L” LOT B200003054 | Dentsply Sirona® | Ti6Al4V, medical grade 5, ASTM 136 | n.a | n.a | 105–117 GPa |
| Implant Replica EV 5.4 LOT 456009 | Dentsply Sirona® | Ti6Al4V Grade 5 ASTM F136 | n.a | n.a | 105–117 GPa |
| Cement: Solocem® LOT J64901 | Coltene® | UDMA, TEGDMA, HEMA Methacrylate, zinc oxide, dental glass, MDP and 4-MET (A) monomers | 120 MPa | n.a | 7.2 GPa |
| Bonding: OneCoat 7 Universal® LOT J69945 | Coltene® | Methacrylates including 10-MDP photoinitiators, ethanol, water | n.a | n.a | n.a |
| Surface Pre-Treatment Group | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| Ti-base | Sand-blasting 2.5 bar, Al2O3, particles, 50 µm | |||
| Monolithic zirconia crown | Sand-blasting, 1 bar, Al2O3 particles, 50 µm, | |||
| Composite crown | Sand-blasting, 2 bar, Al2O3 particles, 50 µm, | |||
| Zirconia mesostructure | Sand-blasting, 1 bar, Al2O3 particles, 50 µm, | |||
| Zirconia insert | Sand-blasting, 1 bar, Al2O3 particles, 50 µm, | |||
| Primer/Cement | OneCoat 7 universal and Solocem by Coltene® | |||
| Properties/Group of Samples | N° | G1 | G1 | G3 | G4 |
|---|---|---|---|---|---|
| Fmax, N | 1 | 1706.10 | 1699.50 | 1720.10 | 1572.20 |
| 2 | 1578.60 | 1397.70 | 1717.10 | 1701.40 | |
| 3 | 1737.10 | 1439.70 | 1818.10 | 1487.40 | |
| 4 | 1565.00 | 1696.50 | 1523.00 | 1549.60 | |
| 5 | 1546.10 | 1317.60 | 1577.60 | 1457.10 | |
| 1626.58 | 1510.20 | 1671.18 | 1553.54 | ||
| D. Std | 88.19 | 176.96 | 119.17 | 94.74 | |
| Elongation at break, mm | 1 | 1.25 | 0.95 | 1.32 | 0.67 |
| 2 | 1.29 | 0.60 | 1.23 | 0.88 | |
| 3 | 1.17 | 1.06 | 1.28 | 1.16 | |
| 4 | 1.26 | 1.40 | 1.50 | 1.07 | |
| 5 | 1.31 | 0.94 | 1.35 | 0.80 | |
| 1.26 | 0.99 | 1.34 | 0.92 | ||
| D. Std | 0.05 | 0.29 | 0.10 | 0.20 |
| Property/Group of Samples | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| FL, N | 813 | 755 | 668 | 777 |
| Group of Samples | % Fmax | Fmax (N) | Fmin (N) | N° Cycles to Break | Failure Mode |
|---|---|---|---|---|---|
| G1 | 80 | 1301 | 130 | 16,314 | T2 |
| 80 | 1301 | 130 | 30,475 | T4 | |
| 70 | 1139 | 114 | 105,522 | T6 | |
| 70 | 1139 | 114 | 75,315 | T5 | |
| 60 | 976 | 98 | 470,741 | T7 | |
| 60 | 976 | 98 | 805,596 | T8 | |
| 50 | 813 | 81 | 2,000,000 | Run out | |
| 50 | 813 | 81 | 2,000,000 | Run out | |
| G2 | 80 | 1208 | 121 | 16,028 | T5 |
| 80 | 1208 | 121 | 16,930 | T5 | |
| 70 | 1057 | 106 | 109,106 | T5 | |
| 70 | 1057 | 106 | 148,110 | T6 | |
| 60 | 906 | 91 | 1,631,627 | T5 | |
| 60 | 906 | 91 | 273,531 | T6 | |
| 50 | 755 | 76 | 2,000,000 | Run out | |
| 50 | 755 | 76 | 2,000,000 | Run out | |
| G3 | 80 | 1337 | 134 | 3797 | T5 |
| 80 | 1337 | 134 | 9108 | T6 | |
| 60 | 1003 | 100 | 43,309 | T5 | |
| 60 | 1003 | 100 | 140,250 | T6 | |
| 50 | 836 | 84 | 1,197,943 | T5 | |
| 50 | 836 | 84 | 1,864,478 | T6 | |
| 40 | 668 | 67 | 2,000,000 | Run out | |
| 40 | 668 | 67 | 2,000,000 | Run out | |
| G4 | 80 | 1243 | 124 | 1724 | T1 |
| 80 | 1243 | 124 | 1396 | T5 | |
| 70 | 1087 | 109 | 2063 | T3 | |
| 70 | 1087 | 109 | 4518 | T2 | |
| 60 | 932 | 93 | 265,460 | T1 | |
| 60 | 932 | 93 | 328,837 | T4 | |
| 50 | 777 | 78 | 2,000,000 | Run out | |
| 50 | 777 | 78 | 2,000,000 | Run out |
| Sample Groups | Region | HV5 (GPa) | KIC (MPa·m1/2) |
|---|---|---|---|
| G1 | Zirconia | 13.7 ± 0.3 | 5.0 ± 0.3 |
| G2 | Zirconia (internal region) | 13.6 ± 0.2 | 4.6 ± 0.4 |
| Composite (external region) | 0.7 ± 0.1 | - | |
| G3 | Composite | 0.7 ± 0.1 | - |
| G4 | Zirconia (internal region) | 13.8 ± 0.3 | 4.6 ± 0.4 |
| Composite (external region) | 0.7 ± 0.1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Robledo, N.; Punset Fuste, M.; Rodríguez-Contreras, A.; García Marro, F.; Manero Planella, J.M.; Figueras-Álvarez, O.; Roig Cayón, M. In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert. Materials 2024, 17, 3815. https://doi.org/10.3390/ma17153815
Gutiérrez Robledo N, Punset Fuste M, Rodríguez-Contreras A, García Marro F, Manero Planella JM, Figueras-Álvarez O, Roig Cayón M. In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert. Materials. 2024; 17(15):3815. https://doi.org/10.3390/ma17153815
Chicago/Turabian StyleGutiérrez Robledo, Nicolás, Miquel Punset Fuste, Alejandra Rodríguez-Contreras, Fernando García Marro, José María Manero Planella, Oscar Figueras-Álvarez, and Miguel Roig Cayón. 2024. "In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert" Materials 17, no. 15: 3815. https://doi.org/10.3390/ma17153815
APA StyleGutiérrez Robledo, N., Punset Fuste, M., Rodríguez-Contreras, A., García Marro, F., Manero Planella, J. M., Figueras-Álvarez, O., & Roig Cayón, M. (2024). In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert. Materials, 17(15), 3815. https://doi.org/10.3390/ma17153815







