Efficient Photodegradation of Thiocyanate Ions in Mining Wastewater Using a ZnO-BiOI Heterojunction
Abstract
1. Introduction
2. Experiment
2.1. Materials
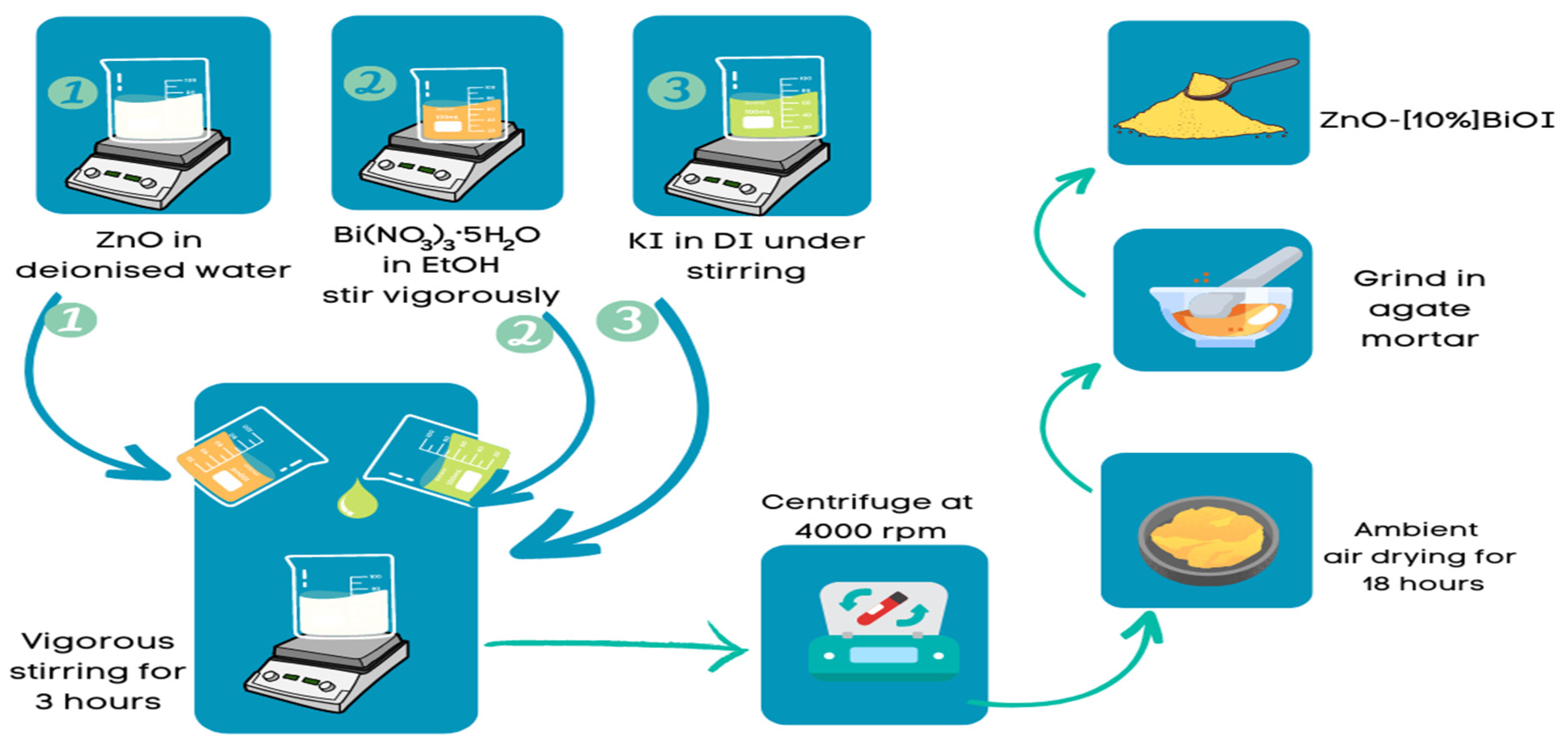
2.2. Synthesis of ZnO
2.3. Synthesis of ZnO-[10%]BiOI
2.4. Characterization
2.5. Photocatalytic Protocol
3. Results and Discussion
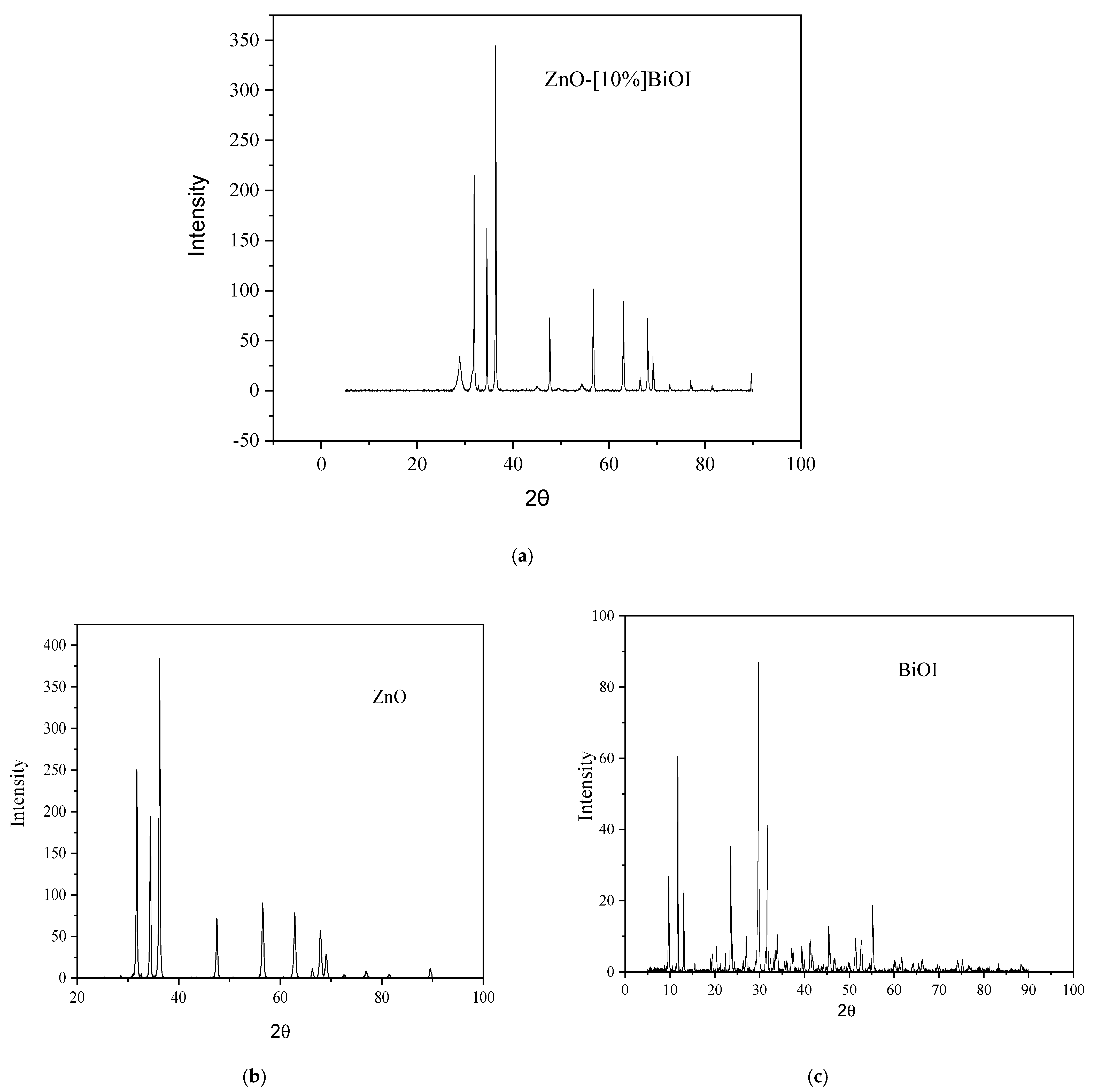
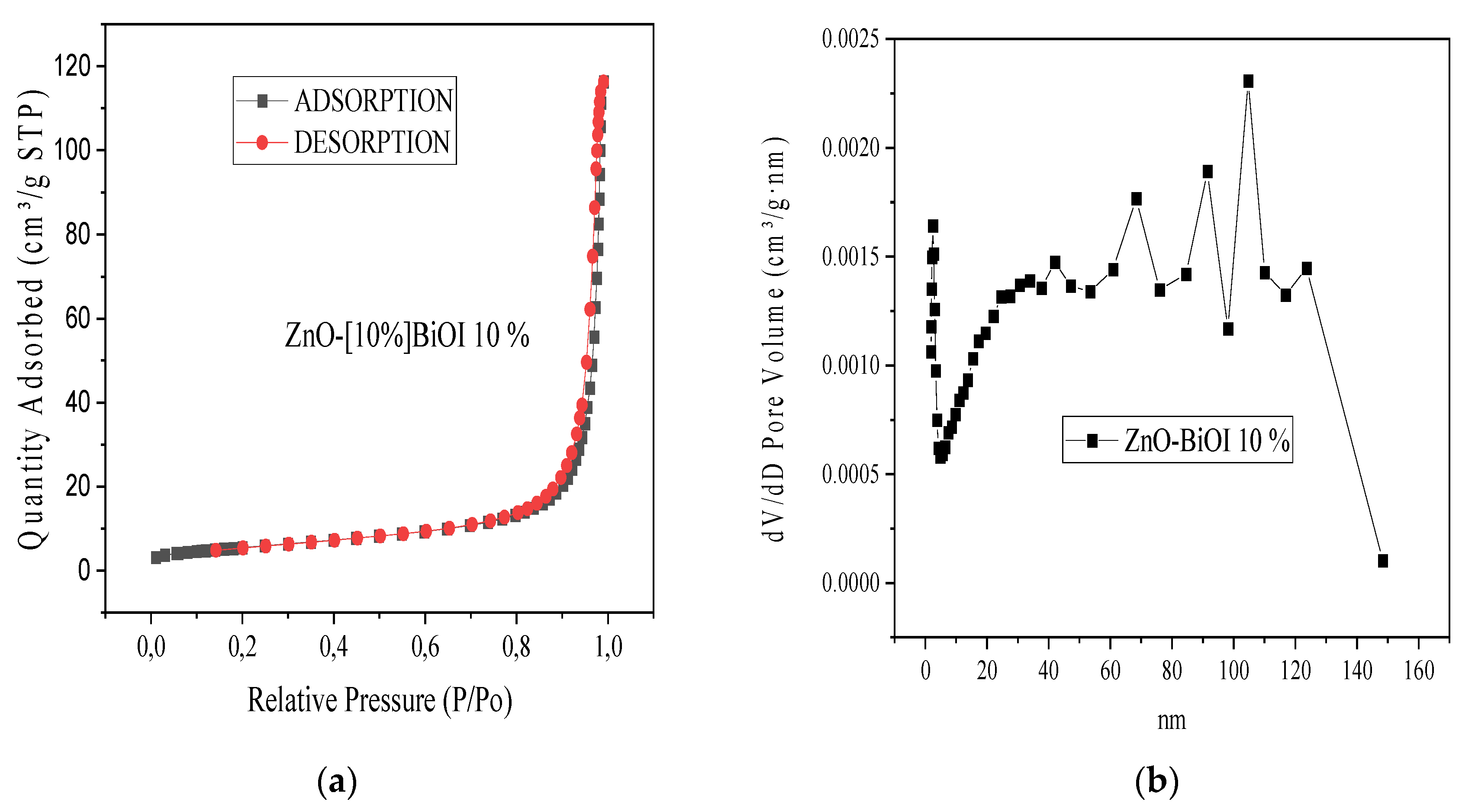
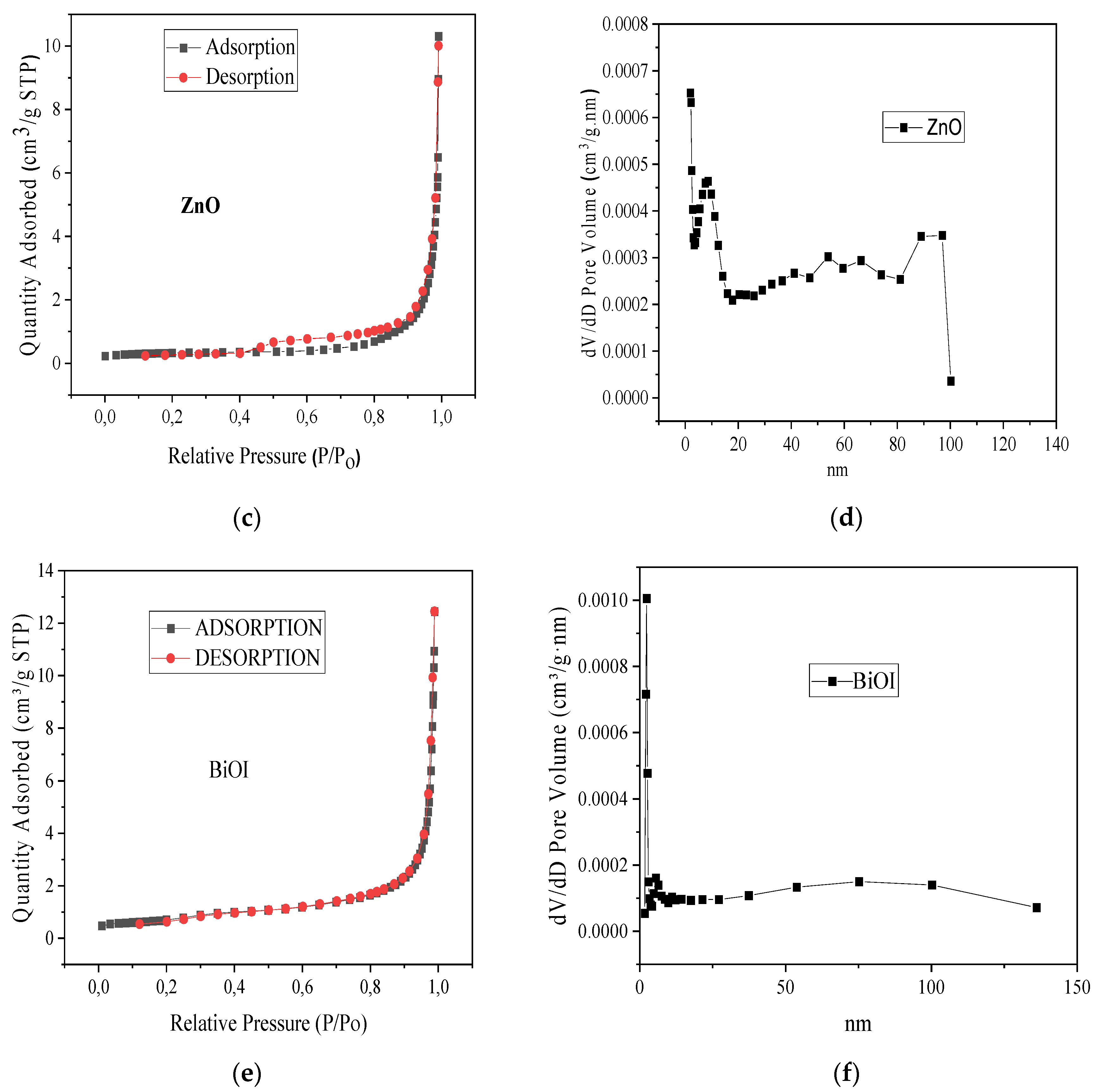
3.1. Characterization of ZnO-[10%]BiOI Heterojunction
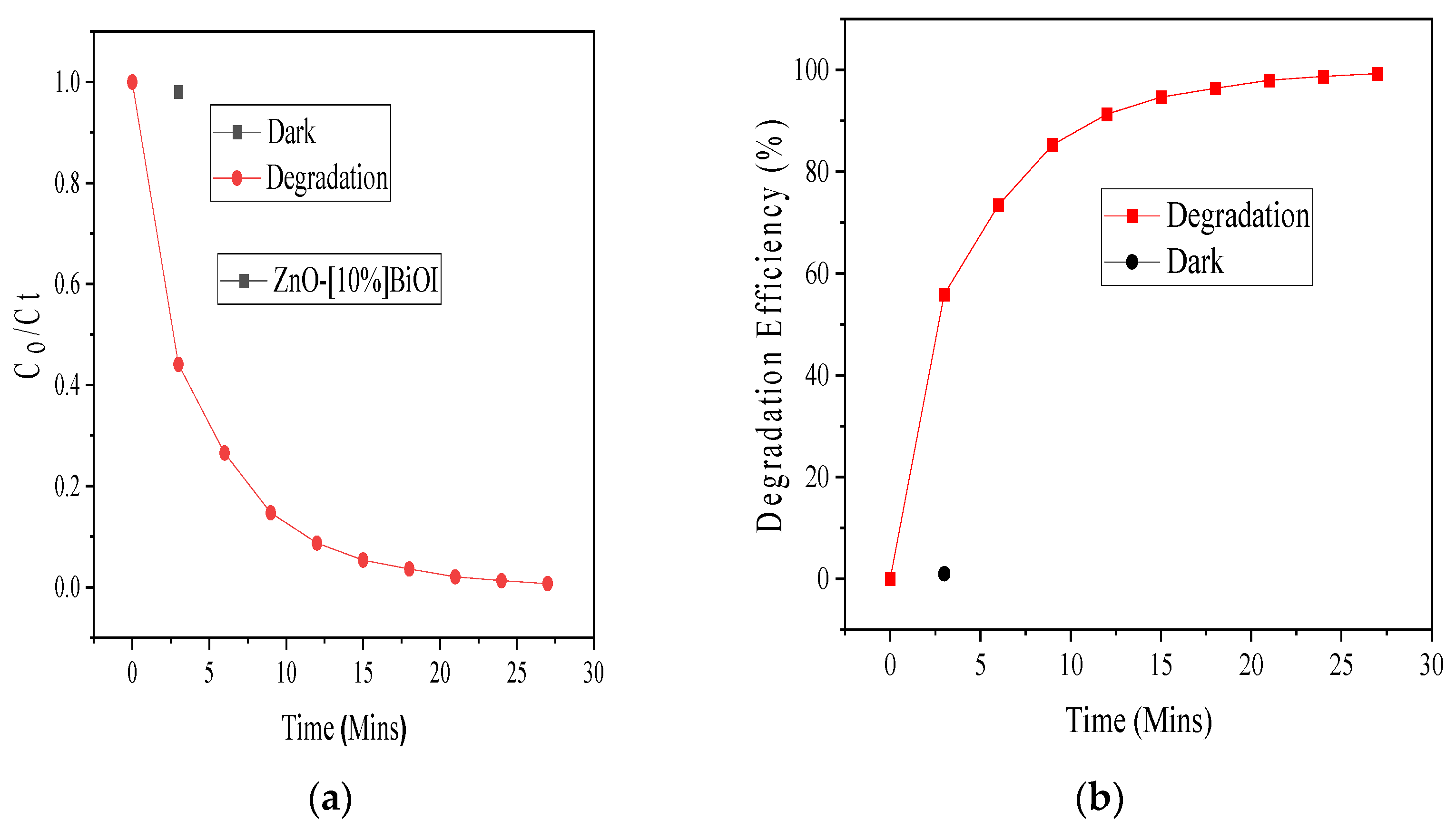
3.2. Photocatalytic Degradation Evaluation
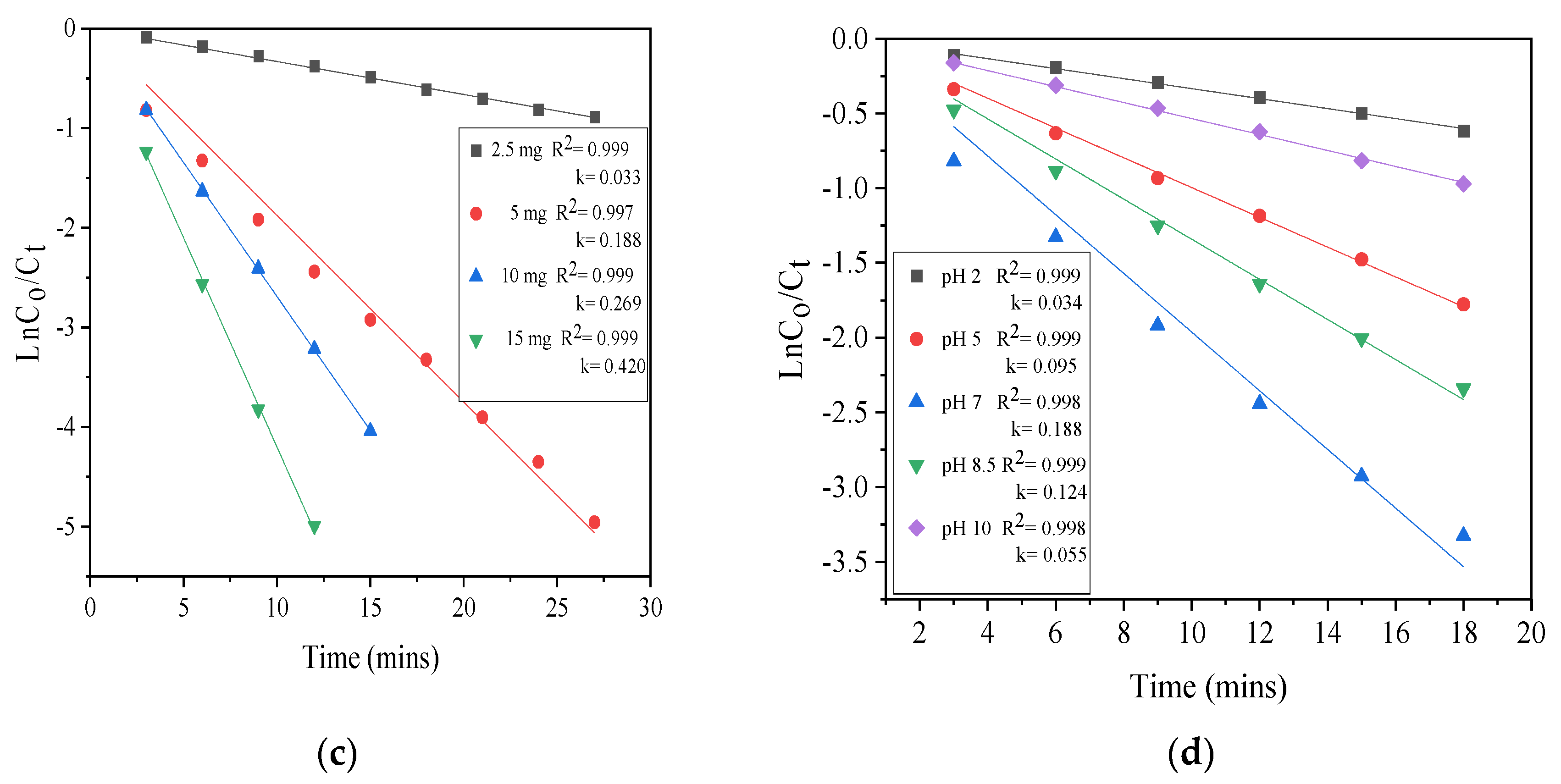
3.3. Effect of Initial Photocatalyst Loading
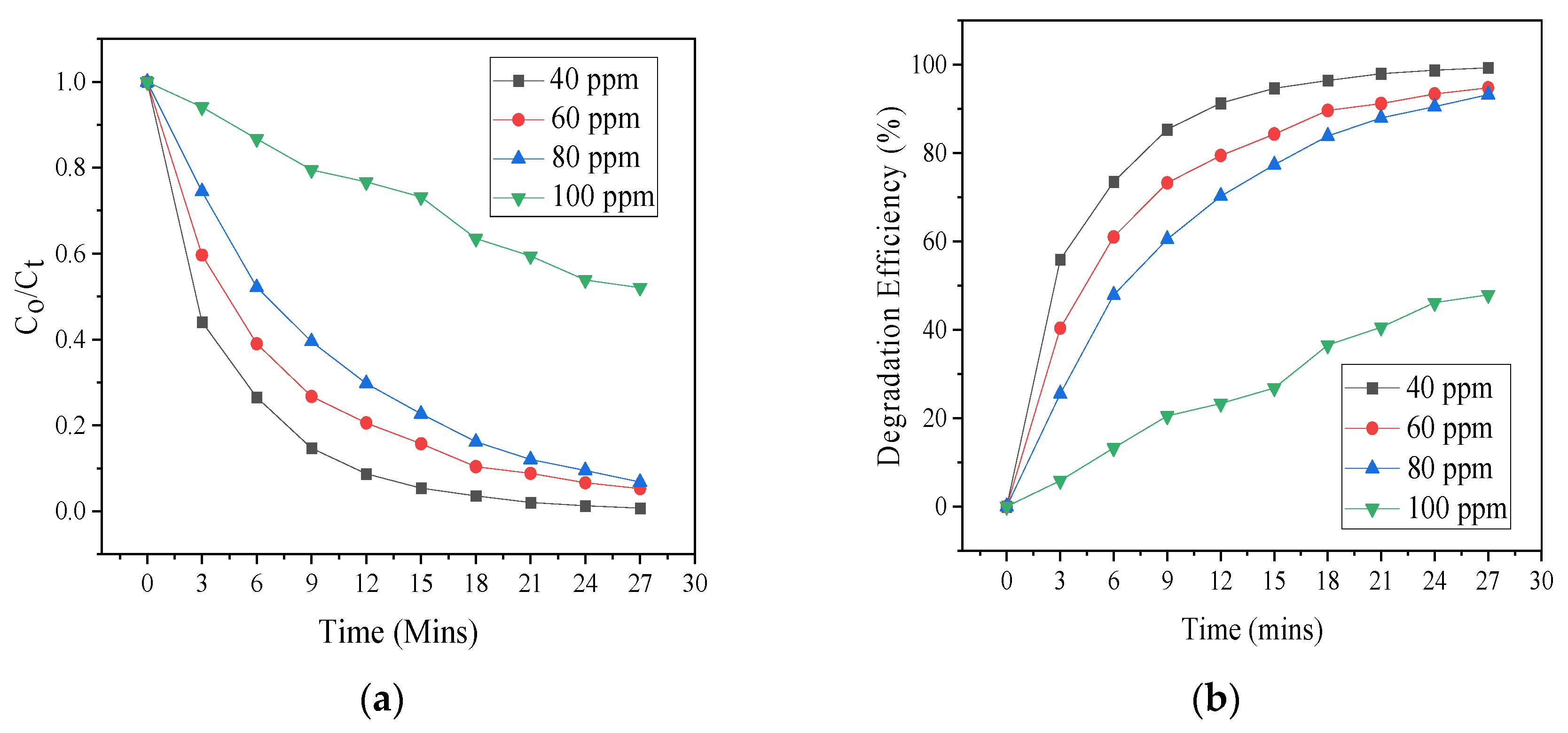
3.4. Effect of Initial SCN− Concentration
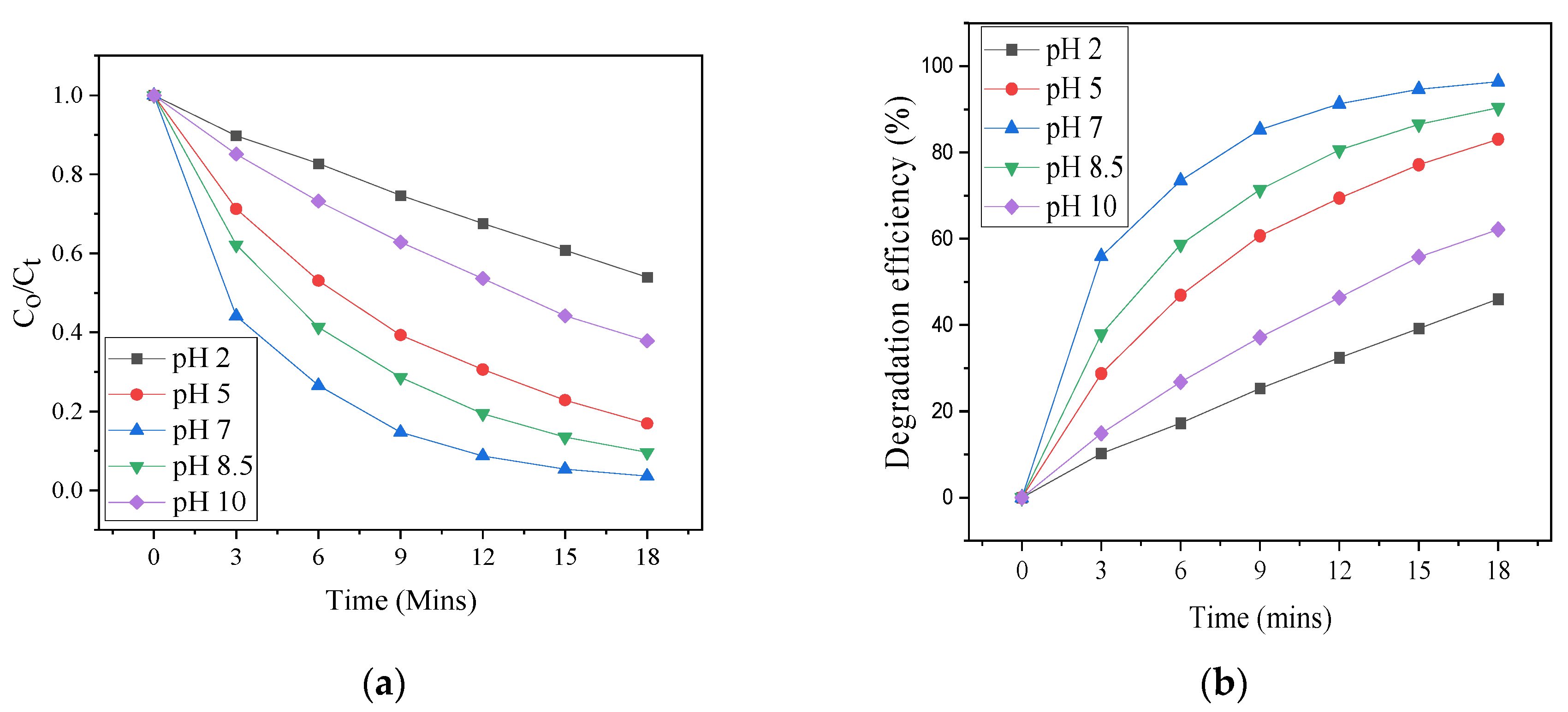
3.5. pH Effect
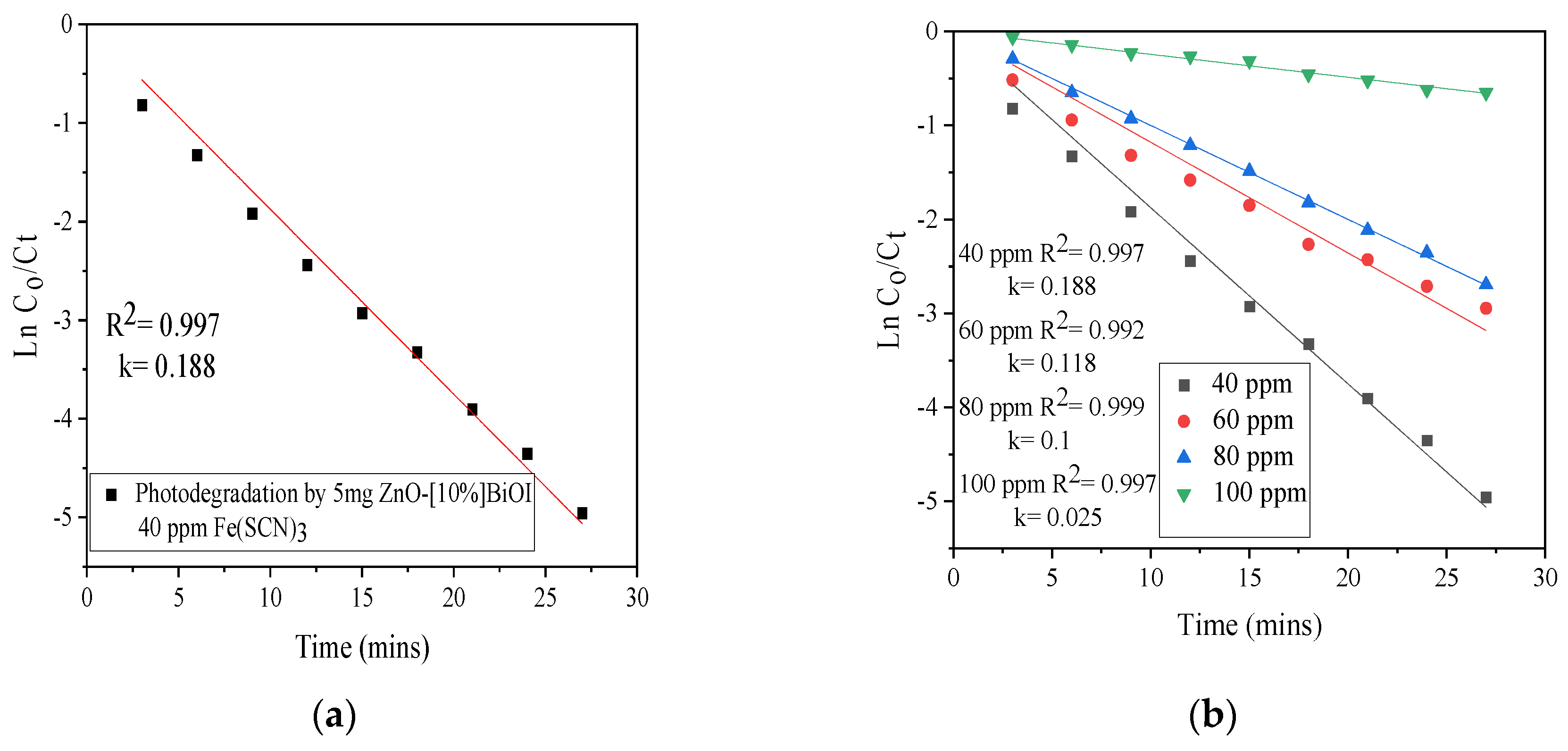
3.6. Kinetic Study
3.7. Photoluminescence Study
3.8. Proposed Photodestruction Mechanism
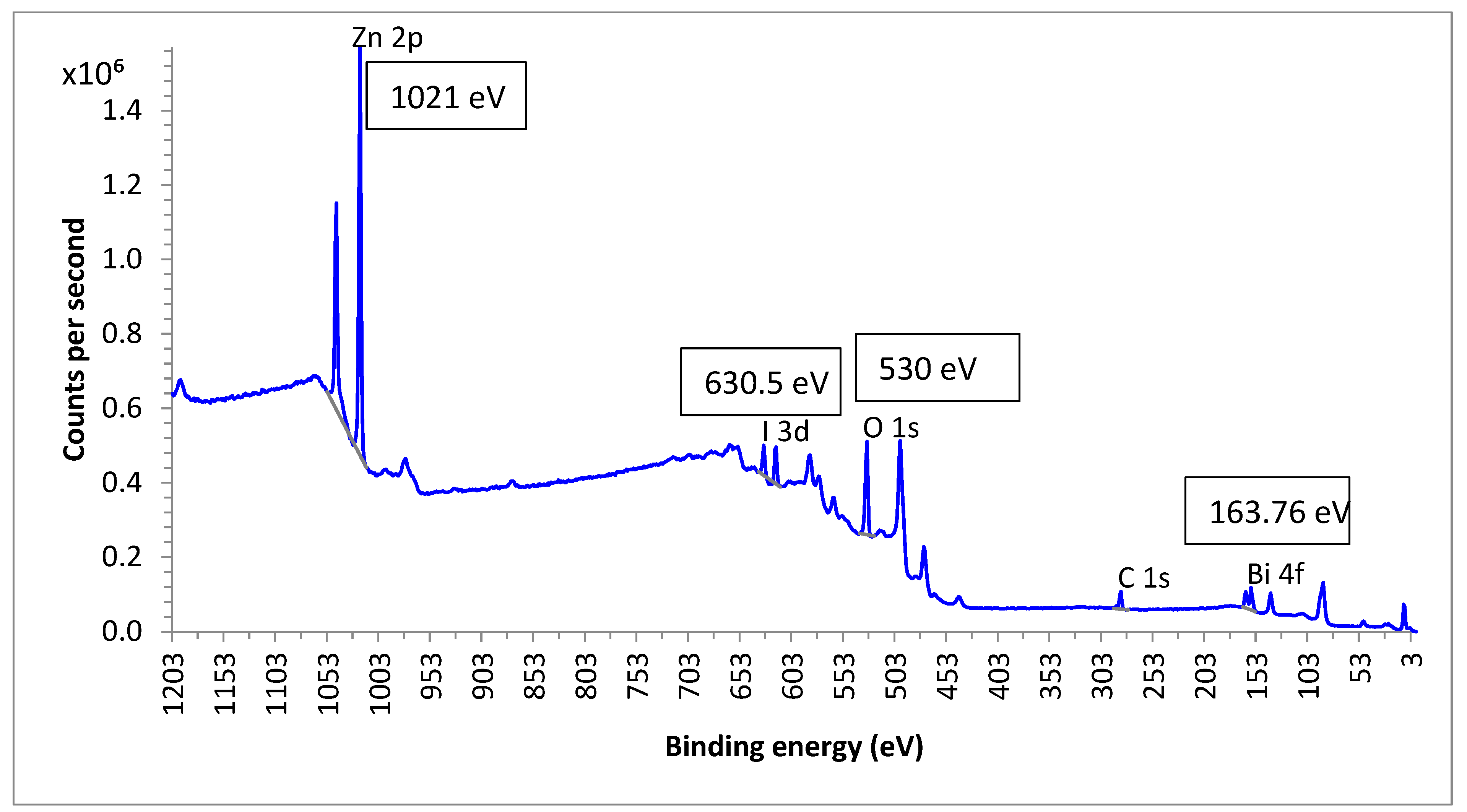
3.9. Chemical State Analysis
4. Comparison with Similar Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budaev, S.L.; Batoeva, A.A.; Tsybikova, B.A. Degradation of thiocyanate in aqueous solution by persulfate activated ferric ion. Miner. Eng. 2015, 81, 88–95. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Chen, J.; Cui, S.; Liu, K.; Han, Q. Cyclic degradation of thiocyanate in cyanide barren solution by manganese oxides. Miner. Eng. 2022, 176, 107314. [Google Scholar] [CrossRef]
- Mediavilla, J.J.V.; Perez, B.F.; De Cordoba, M.C.F.; Espina, J.A.; Ania, C.O. Photochemical degradation of cyanides and thiocyanates from an industrial wastewater. Molecules 2019, 24, 1373. [Google Scholar] [CrossRef] [PubMed]
- Azizitorghabeh, A.; Wang, J.; Ramsay, J.A.; Ghahreman, A. A review of thiocyanate gold leaching—Chemistry, thermodynamics, kinetics and processing. Miner. Eng. 2021, 160, 106689. [Google Scholar] [CrossRef]
- Cho, Y.; Xu, C.; Cattrall, R.W.; Kolev, S.D. A polymer inclusion membrane for extracting thiocyanate from weakly alkaline solutions. J. Membr. Sci. 2011, 367, 85–90. [Google Scholar] [CrossRef]
- Sharma, V.K. Destruction of cyanide and thiocyanate by ferrate [Iron (VI)]. Eur. J. Miner. Process. Environ. Prot. 2003, 3, 301–308. [Google Scholar]
- Ashiegbu, D.C.; Potgieter, H. Photocatalytic mediated destruction of 2-chlorobiphenyl by a ZnO-[10%]BiOI p-n heterojunction: Effect of some process parameters. Int. J. Environ. Sci. Technol. 2024, 21, 4119–4132. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A review on the green synthesis of nanoparticles, their biological applications, and photocatalytic efciency against environmental toxins. Environ. Sci. Pollut. Res. 2023, 30, 69796–69823. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, J.M.; Son, S.; Hwang, S.J. 2D inorganic nanosheet-based hybrid photocatalysts: Design, applications, and perspectives. J. Photochem. Photobiol. C 2019, 40, 150–190. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Talreja, N.; Ashfaq, M.; Chauhan, D.; Mera, A.C.; Rodríguez, C.A. Strategic Doping Approach of the Fe-BiOI Microstructure: An Improved Photodegradation Efficiency of Tetracycline. ACS Omega 2021, 6, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, K.; Jiang, Z.; Jiang, K.; Peng, J.; Xu, J. Flower-like ZnO/BiOI p–n heterojunction composites for enhanced photodegradation of formaldehyde and dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 23064–23074. [Google Scholar] [CrossRef]
- Sulistyarti, H.; Retnowati, R.; Sulistyo, E.; Wulandari, E.R.; Nashukha, H.L. Development of Indirect Spectrophotometric Method for Mercury Determination Based on the Formation of Iron(III)-Thiocyanate Complex. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 1–7. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, F.; Li, A.; Xu, W.; Zhang, X. Simple synthesis of BiOI/ZnO/rGO for efficient photocatalytic degradation of antibiotic chloramphenicol under visible light. J. Environ. Sci. 2023, 134, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Shirini, F.; Abedini, M.; Zamani, S.; Fallah, M.H. Introduction of W-doped ZnO nanocomposite as a new and efficient nanocatalyst for the synthesis of biscoumarins in water. J. Nanostruct. Chem. 2015, 5, 123–130. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Barjasteh-Askari, F.; Davoudi, M.; Dolatabadi, M.; Ahmadzadeh, S. Iron-modified activated carbon derived from agro-waste for enhanced dye removal from aqueous solutions. Heliyon 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Xu, M.M.; Zhao, Y.L.; Yan, Q.S. Degradation of aniline by bismuth oxyiodide (BiOI) under visible light irradiation. J. Environ. Sci. Manag. 2017, 20, 18–25. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.G.; Amin, O.; Sathish, M.; Kumar, D. Synthesis, characterization, photocatalytic effect of CuS-ZnO nanocomposite on photodegradation of Congo red and phenol pollutant. Inorg. Chem. Commun. 2022, 143, 1–10. [Google Scholar] [CrossRef]
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of doping parameters on photocatalytic degradation of methylene blue using Ag doped ZnO nanocatalyst. SN Appl. Sci. 2020, 2, 820. [Google Scholar] [CrossRef]
- Sudarsh, A.; Remya, N.; Swain, A. Recent research advancements in microwave photocatalytic treatment of aqueous solutions. Environ. Monit. Assess. 2023, 195, 142. [Google Scholar] [CrossRef] [PubMed]
- Al-Abidy, M.; Al-Nayili, A. Enhancement of photocatalytic activities of ZnFe2O4 composite by incorporating halloysite nanotubes for effective elimination of aqueous organic pollutants. Environ. Monit. Assess. 2022, 195, 190. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.Q.; Sena, S.; Mir, T.U.G.; Kumar, D.; Wani, M.; Rashid, A. Assessment of photocatalytic efficiency and antifungal activity of zinc doped copper sulfide composite embedded with graphene oxide nanosheets. J. Mol. Liq. 2024, 395, 123925. [Google Scholar] [CrossRef]
- Budaev, S.L.; Batoeva, A.A.; Tsybikova, B.A.; Khandarkhaeva, M.S.; Aseev, D.G. Photochemical degradation of thiocyanate by sulfate radical-based advanced oxidation process using UVC KrCl-excilamp. J. Environ. Chem. Eng. 2021, 9, 105584. [Google Scholar] [CrossRef]
- Noudeh, G.D.; Asdaghi, M.; Noudeh, N.D.; Dolatabadi, M.; Ahmadzadeh, S. Response surface modeling of ceftriaxone removal from hospital wastewater. Environ. Monit. Assess. 2023, 195, 217. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Kheirieh, A.; Yoosefian, M.; Ahmadzadeh, S. Hydroxyzine removal from the polluted aqueous solution using the hybrid treatment process of electrocoagulation and adsorption; optimization, and modeling. Appl. Water Sci. 2022, 12, 254. [Google Scholar] [CrossRef]
- Zargaran, M.; Zargar, B.; Rastegarzadeh, S.; Andayesh, R. Removal of Aqueous Thiocyanate Anions by Titanium Dioxide Nanoparticles. Adv. J. Chem. A 2020, 568, S551–S568. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bera, K.K.; Chatterjee, S.; Ghosh, A.; Bhattacharya, S.K. Synthesis of mesoporous BiOI flower and facile in-situ preparation of BiOI/BiOCl mixture for enhanced photocatalytic degradation of toxic dye, Rhodamine-B. J. Photochem. Photobiol. 2021, 8, 100077. [Google Scholar] [CrossRef]
- Yusoff, M.A.M.; Imam, S.S.; Shah, I.; Adnan, R. Photocatalytic activity of bismuth oxyiodide nanospheres and nanoplates in the degradation of ciprofloxacin under visible light. Mater. Res. Express 2019, 6, ab2918. [Google Scholar] [CrossRef]
- Asadzadeh, P.H.; Fattahi, M.; Khosravi-Nikou, M. Synthesis and characterization of ternary chitosan–TiO2–ZnO over graphene for photocatalytic degradation of tetracycline from pharmaceutical wastewater. Sci. Rep. 2021, 11, 24177. [Google Scholar] [CrossRef]
- Huang, H.; Feng, C.; Pan, X.; Wu, H.; Ren, Y.; Wu, C. Thiocyanate Oxidation by Coculture from a Coke Wastewater Treatment Plant. Biomater. Nanobiotechnol. 2013, 4, 37–46. [Google Scholar] [CrossRef]
- Namasivayam, C.; Prathap, K. Removal of thiocyanate by industrial solid waste Fe(III)/Cr(III) hydroxide: Kinetic and equlibrium studies. J. Environ. Eng. Manag. 2006, 16, 267–274. [Google Scholar]
- Bao, H.; Dat, N.M.; Giang, N.T.H.; Thinh, D.B.; Tai, T.L.; Trinh, D.N.; Hai, N.D.; Khoa, N.A.D.; Huong, L.M.; Nam, H.M.; et al. Behavior of ZnO-doped TiO2/rGO nanocomposite for water treatment enhancement. Surf. Interfaces 2021, 23, 100950. [Google Scholar] [CrossRef]
- Gutiérrez-Lazos, C.D.; Fundora-Cruz, A.; Solís-Pomar, F.; Pérez-Tijerina, E. Study of Structural Properties of BiOI on ZnO Thin Films and ZnO Nanorods. Adv. Mater. Sci. Eng. 2019, 9465730. [Google Scholar] [CrossRef]
- Talreja, N.; Ashfaq, M.; Chauhan, D.; Mera, A.C.; Rodríguez, C.A.; Mangalaraja, R.V. A Zn-doped BiOI microsponge-based photocatalyst material for complete photodegradation of environmental contaminants. New J. Chem. 2021, 45, 18412–18420. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Chen, Z.; Ma, X.; Liu, Y.; Wang, S.; Liu, Z.; Huang, C. Insights into the mechanism of the enhanced visible-light photocatalytic activity of a MoS2/BiOI heterostructure with interfacial coupling. Phys. Chem. Chem. Phys. 2020, 22, 22349–22356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fei, W.; Wang, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. p-n Heterojunction of BiOI/ZnO nanorod arrays for piezo-photocatalytic degradation of bisphenol A in water. J. Hazard. Mater. 2020, 399, 123109. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, J.; Yu, P.; Zhang, B.; Ma, M. Facile synthesis of a ZnO—BiOI p—n nano-heterojunction with excellent visible-light photocatalytic activity. Beilstein J. Nanotechnol. 2018, 9, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Q.; Tayyab, M.; Zhou, L.; Lei, J.; Zhang, J. Single-Atom Pt Loaded Zinc Vacancies ZnO–ZnS Induced Type-V Electron Transport for Efficiency Photocatalytic H2 Evolution. Sol. RRL 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. One-pot in-situ hydrothermal synthesis of ternary In2S3/Nb2O5/Nb2C Schottky/S-scheme integrated heterojunction for efficient photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 628, 500–512. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, H.; Chen, X.; Li, S.; Xie, T.; Wang, D.; Lin, Y. Enhanced photocatalytic degradation of phenol and photogenerated charges transfer property over BiOI-loaded ZnO composites. J. Colloid Interface Sci. 2017, 494, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, X.; Ma, L.; Xu, X. Enhanced visible-light-driven photocatalytic activity of WO3/BiOI heterojunction photocatalysts. J. Mol. Catal. A Chem. 2015, 410, 168–176. [Google Scholar] [CrossRef]
- Tong, Y.; Zheng, C.; Lang, W.; Fan, W.; Tao, W.; Luo, W.; Chen, H. ZnO-embedded BiOI hybrid nanoflakes: Synthesis, characterization, and improved photocatalytic properties. Mater. Des. 2017, 122, 90–101. [Google Scholar] [CrossRef]
- Natkritta, B.; Duangdao, C.; Burapat, I.; Chen, Z. Visible light driven BiOI/ZnO Photocatalyst films and its photodegradation of methomyl insecticide. KMUTNB Int. J. Appl. Sci. Technol. 2018, 4, 297–304. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Chen, L.; Xiao, C.; Feng, J.; Liao, L.; Wei, T.; Wang, Z. Facile Preparation and Enhanced Visible-Light Photocatalysis of ZnO Arrays@BiOI Nanosheets Heterostructures. J. Nanomater. 2019, 2019, 4508687. [Google Scholar] [CrossRef]




| Catalyst | Synthesis Method | Contaminant | Contaminant Concentration (ppm) | Efficiency (%) | Time (mins) | Reference |
|---|---|---|---|---|---|---|
| S2O82− + Fe3+ | Purchased | SCN− | 100 | 100 | 90 | [1] |
| TiO2 | Purchased | SCN− | 100 | 100 | 300 | [3] |
| UVC/PS/Fe3+ | Purchased | SCN− | 50 | 99.9 | 40 | [24] |
| TiO2 | Purchased | SCN− | 40 | 96.5 | [27] | |
| Fe(III)/Cr(III) hydroxide | Purchased | SCN− | 40 | 33 | 30 | [32] |
| ZnO-[10%]BiOI | Direct and facile | SCN− | 40 | 100 | 27 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashiegbu, D.C.; Nkhoesa, D.; Erasmus, R.; Potgieter, H.J. Efficient Photodegradation of Thiocyanate Ions in Mining Wastewater Using a ZnO-BiOI Heterojunction. Materials 2024, 17, 3832. https://doi.org/10.3390/ma17153832
Ashiegbu DC, Nkhoesa D, Erasmus R, Potgieter HJ. Efficient Photodegradation of Thiocyanate Ions in Mining Wastewater Using a ZnO-BiOI Heterojunction. Materials. 2024; 17(15):3832. https://doi.org/10.3390/ma17153832
Chicago/Turabian StyleAshiegbu, Darlington C., David Nkhoesa, Rudolph Erasmus, and Herman Johanes Potgieter. 2024. "Efficient Photodegradation of Thiocyanate Ions in Mining Wastewater Using a ZnO-BiOI Heterojunction" Materials 17, no. 15: 3832. https://doi.org/10.3390/ma17153832
APA StyleAshiegbu, D. C., Nkhoesa, D., Erasmus, R., & Potgieter, H. J. (2024). Efficient Photodegradation of Thiocyanate Ions in Mining Wastewater Using a ZnO-BiOI Heterojunction. Materials, 17(15), 3832. https://doi.org/10.3390/ma17153832










