Sustainable Alkali Activation: The Role of Water- and Alkali-Treated Sisal Leaf Wastewaters in Solid- Waste-Based Composite Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Binder Materials
2.1.2. Fine Aggregate
2.1.3. Sisal Leaf Wastewater
2.1.4. Alkali Solution
2.2. Preparation of Alkali-Activated Samples
2.2.1. Effect of Wastewater Obtained from Water-Treated Sisal Leaves
2.2.2. Effect of Wastewater Obtained from Alkali-Treated Sisal Leaves
2.3. Experimental Program
2.3.1. Characterization of Fresh Properties
2.3.2. Assessment of Hardened Properties
- (1)
- Determination of drying shrinkage and density
- (2)
- Evaluation of flexural and compressive strengths
2.3.3. Analysis of Microstructural Features
- (1)
- Surface morphology and microstructure examination
- (2)
- Identification of mineral phases and products
3. Results and Discussion
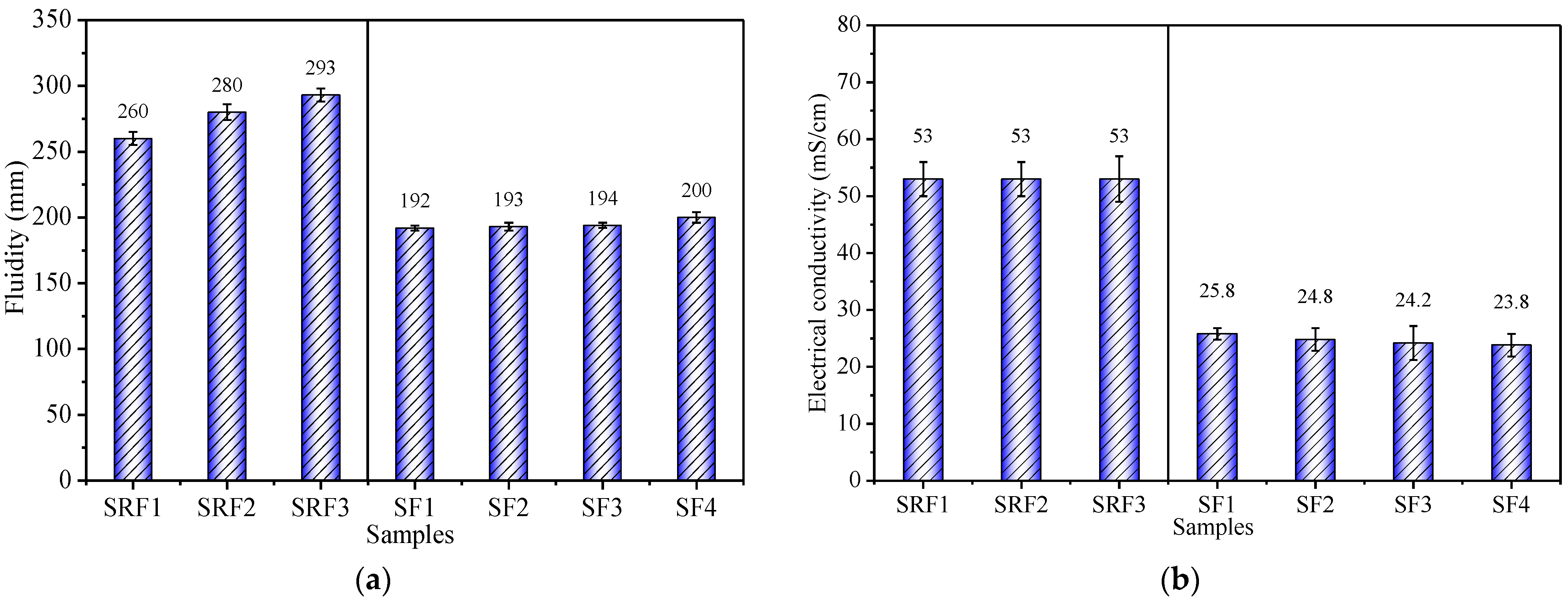
3.1. Effects of Sisal Leaf Wastewater on Electrical Conductivity and Fluidity
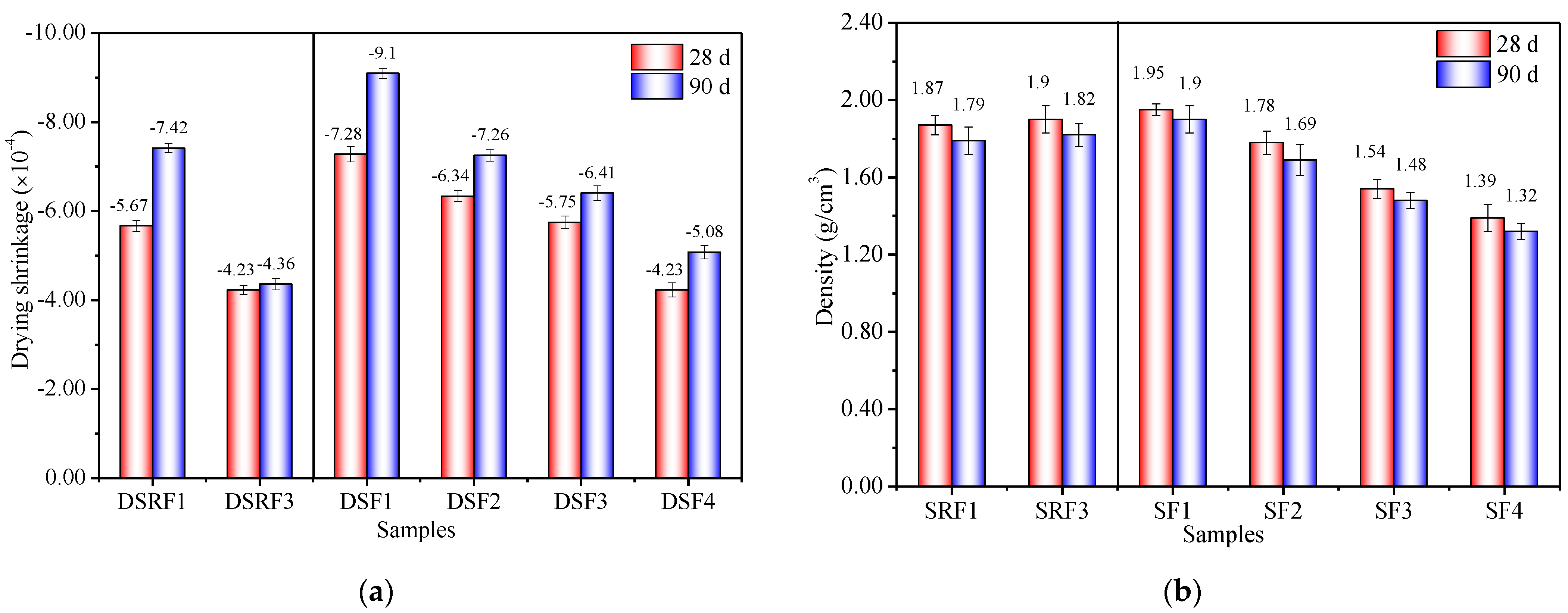
3.2. Effects of Sisal Leaf Wastewater on Drying Shrinkage and Density
3.3. Effects of Sisal Leaf Wastewater on Flexural and Compressive Strengths
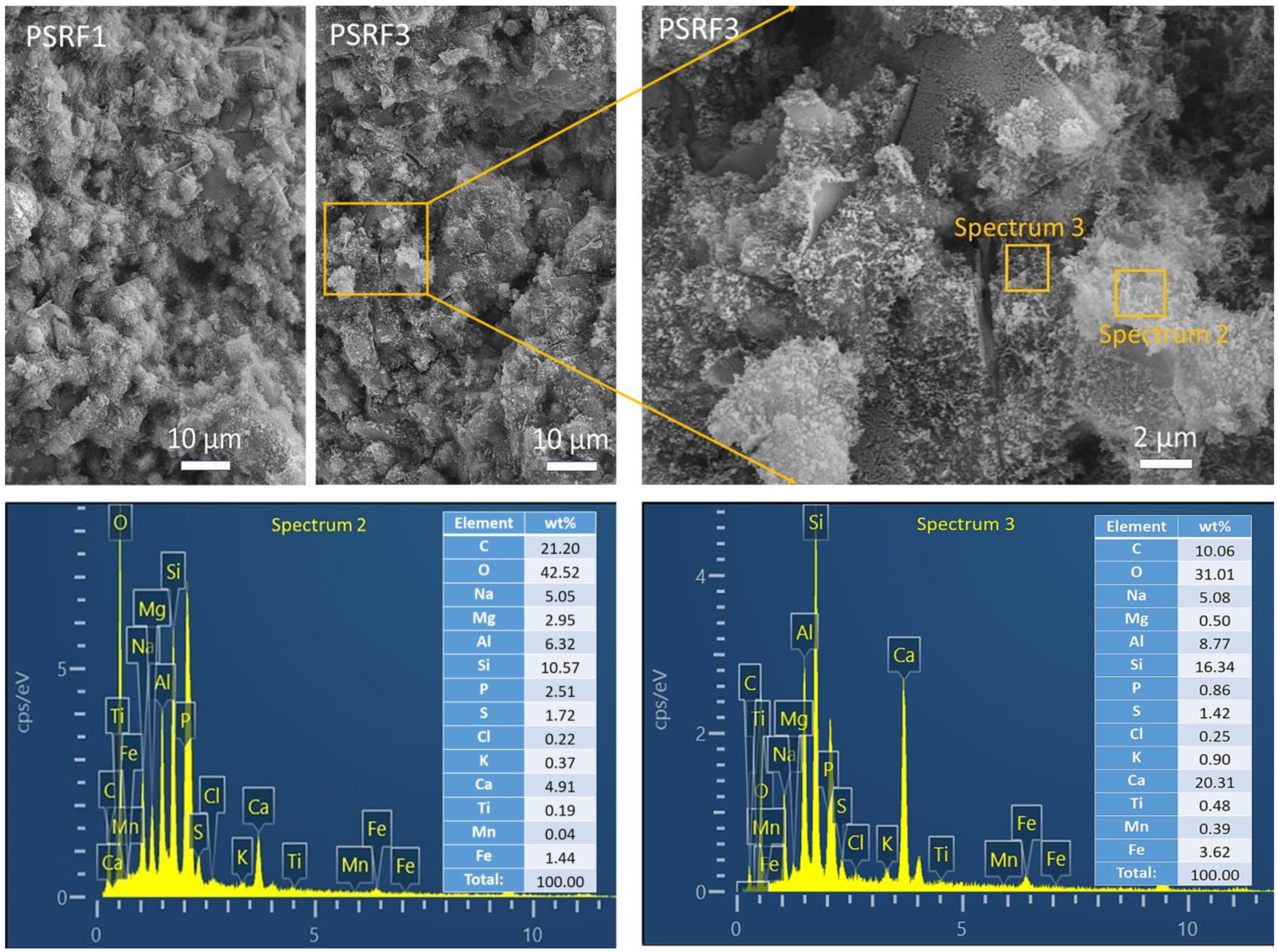
3.4. Effects of Sisal Leaf Wastewater on Morphology and Microstructure
3.5. Effects of Sisal Leaf Wastewater on Mineral Compositions
3.6. Effects of Sisal Leaf Wastewater on Chemical Bonds and Gel Products
3.7. Discussion
4. Conclusions
- (1)
- The substitution of 14% water-treated sisal leaf wastewater (WTSLW) for the alkali activator (NaOH solution) in the synthesis of alkali-activated solid-waste-based composites, although delaying the early strength of the composite, was found to enhance the later-term flexural and compressive strengths and eliminate the strength regression with age.
- (2)
- Mature sisal leaves were soaked in a 10 wt% NaOH solution for 8 ± 0.2 h to obtain alkali-treated sisal leaf wastewater (ATSLW). The use of ATSLW as a substitute for the alkali activator in the synthesis of alkali-activated solid-waste-based composites resulted in the production of porous composites (with a porosity of 43.6% and a compressive strength of 5.2 MPa after 90 days). This indicates that ATSLW possesses the capacity to activate binder materials and increase porosity.
- (3)
- The substitution of both WTSLW and ATSLW for the alkali activator in the synthesis of alkali-activated solid-waste-based composites not only increases fluidity but also reduces the drying shrinkage. The DSRF3, with 14% WTSLW, exhibited a 41.24% reduction in drying shrinkage at 90 days compared to DSRF1 (without WTSLW). Similarly, DSF4, with ATSLW, resulted in a 44.18% reduction in drying shrinkage at 90 days compared to DSF1 (without ATSLW).
- (4)
- At a 90-day curing age, excluding similar carbonation, the substitution of 14% WTSLW does not affect the composition and structure of the products of the SP-RM-FA-based alkali-activated material. The composite still consisted of amorphous N-A-S-H, C-S-H, and C-A-S-H gels coexisting with crystalline phases to produce cementation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamopoulos, I.P.; Frantzana, A.A.; Syrou, N.F. Epidemiological surveillance and environmental hygiene, SARS-CoV-2 infection in the community, urban wastewater control in Cyprus, and water reuse. J. Contemp. Stud. Epidemiol. Public Health 2023, 4, ep23003. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Bello, O.S.; Oluyori, A.P.; Inyinbor, H.E.; Fadiji, A.E. Wastewater conservation and reuse in quality vegetable cultivation: Overview, challenges and future prospects. Food Control 2019, 98, 489–500. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Gao, H.; Yuan, T.; Liu, X.; Zhang, W. Effects of alkali-treated plant wastewater on the properties and microstructures of alkali-activated composites. Ceram. Int. 2023, 49, 8583–8597. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Synthesis of porous alkali-activated materials for high-acidic wastewater treatment. J. Water Process Eng. 2020, 33, 101118. [Google Scholar] [CrossRef]
- Amoah, I.D.; Kumari, S.; Bux, F. A probabilistic assessment of microbial infection risks due to occupational exposure to wastewater in a conventional activated sludge wastewater treatment plant. Sci. Total Environ. 2022, 843, 156849. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The promising way to treat wastewater by microalgae: Approaches, mechanisms, applications and challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Zhang, M.; Hao, X.; Liu, J. Review on the fate and recovery of cellulose in wastewater treatment. Resour. Conserv. Recycl. 2022, 184, 106354. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Ma, G.; Zhao, X.; Zhao, X. Preparation and properties of bio-geopolymer composites with waste cotton stalk materials. J. Clean. Prod. 2020, 245, 118842. [Google Scholar] [CrossRef]
- Belhadj, B.; Bederina, M.; Montrelay, N.; Houessou, J.; Quéneudec, M. Effect of substitution of wood shavings by barley straws on the physico-mechanical properties of lightweight sand concrete. Constr. Build. Mater. 2014, 66, 247–258. [Google Scholar] [CrossRef]
- Al-Akhras, N.M.; Abu-Alfoul, B.A. Effect of wheat straw ash on mechanical properties of autoclaved mortar. Cem. Concr. Res. 2002, 32, 859–863. [Google Scholar] [CrossRef]
- Adesanya, D.A.; Raheem, A.A. Development of corn cob ash blended cement. Constr. Build. Mater. 2009, 23, 347–352. [Google Scholar] [CrossRef]
- Silva, F.d.A.; Filho, R.D.T.; Filho, J.d.A.M.; Fairbairn, E.d.M.R. Physical and mechanical properties of durable sisal fiber–cement composites. Constr. Build. Mater. 2010, 24, 777–785. [Google Scholar] [CrossRef]
- Bahja, B.; Elouafi, A.; Tizliouine, A.; Omari, L.H. Morphological and structural analysis of treated sisal fibers and their impact on mechanical properties in cementitious composites. J. Build. Eng. 2021, 34, 102025. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Luhar, I. Incorporation of natural waste from agricultural and aquacultural farming as supplementary materials with green concrete: A review. Compos. Part B Eng. 2019, 175, 107076. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Aravinth, K.; Ramakrishnan, T.; Tamilarasan, V.D.; Veeramanikandan, K. A brief review on plant fibres composites: Extraction, chemical treatment and fibre orientation. Mater. Today Proc. 2022, 62, 2005–2009. [Google Scholar] [CrossRef]
- Sathish, S.; Karthi, N.; Prabhu, L.; Gokulkumar, S.; Balaji, D.; Vigneshkumar, N.; Ajeem Farhan, T.S.; AkilKumar, A.; Dinesh, V.P. A review of natural fiber composites: Extraction methods, chemical treatments and applications. Mater. Today Proc. 2021, 45, 8017–8023. [Google Scholar] [CrossRef]
- Sahu, P.; Gupta, M.K. Eco-friendly treatment and coating for improving the performance of sisal composites. Polym. Test. 2021, 93, 106923. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Siengchin, S.; Parameswaranpillai, J.; Jawaid, M.; Pruncu, C.I.; Khan, A. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar]
- Hossain, M.K.; Karim, M.R.; Chowdhury, M.R.; Imam, M.A.; Hosur, M.; Jeelani, S.; Farag, R. Comparative mechanical and thermal study of chemically treated and untreated single sugarcane fiber bundle. Ind. Crops Prod. 2014, 58, 78–90. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Zakka, W.P.; Lim, N.H.A.S.; Khun, M.C. A scientometric review of geopolymer concrete. J. Clean. Prod. 2021, 280, 124353. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Longhi, M.A.; Zhang, Z.; Rodríguez, E.D.; Kirchheim, A.P.; Wang, H. Efflorescence of Alkali-Activated Cements (Geopolymers) and the Impacts on Material Structures: A Critical Analysis. Front. Mater. 2019, 6, 89. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Zhang, B.; Fang, C.; Li, L. Physicochemical properties of alkali activated GGBS and fly ash geopolymeric recycled concrete. Constr. Build. Mater. 2019, 204, 384–398. [Google Scholar] [CrossRef]
- Neupane, K. Fly ash and GGBFS based powder-activated geopolymer binders: A viable sustainable alternative of portland cement in concrete industry. Mech. Mater. 2016, 103, 110–122. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Chinnaraju, K. Durability of ambient cured alumina silicate concrete based on slag/fly ash blends against sulfate environment. Constr. Build. Mater. 2019, 204, 70–83. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, V.C. Treatment of highly acidic wastewater containing high energetic compounds using dimensionally stable anode. Chem. Eng. J. 2017, 325, 289–299. [Google Scholar] [CrossRef]
- Silva, G.; Kim, S.; Aguilar, R.; Nakamatsu, J. Natural fibers as reinforcement additives for geopolymers—A review of potential eco-friendly applications to the construction industry. Sustain. Mater. Technol. 2020, 23, e00132. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S.J. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.K.; Huang, B.S.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength (Idt ISO 679: 2021). China National Standardization Administration Committee: Beijing, China, 2021. (In Chinese)
- Kamide, K.; Saito, M.; Kowsaka, K. Temperature Dependence of Limiting Viscosity Number and Radius of Gyration for Cellulose Dissolved in Aqueous 8% Sodium Hydroxide Solution. Polym. J. 1987, 19, 1173–1181. [Google Scholar] [CrossRef]
- Bekele, A.E.; Lemu, H.G.; Jiru, M.G. Experimental study of physical, chemical and mechanical properties of enset and sisal fibers. Polym. Test. 2022, 106, 107453. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Li, Y.; Waterhouse, G.I.N. Effect of alkali treatment on interfacial bonding in abaca fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 589–597. [Google Scholar] [CrossRef]
- Dawit, J.B.; Regassa, Y.; Lemu, H.G. Property characterization of acacia tortilis for natural fiber reinforced polymer composite. Results Mater. 2020, 5, 100054. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Drying Shrinkage of Slag Blended Fly Ash Geopolymer Concrete Cured at Room Temperature. Procedia Eng. 2015, 125, 594–600. [Google Scholar] [CrossRef]
- Yusriah, L.; Sapuan, S.M.; Zainudin, E.S.; Mariatti, M. Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fibre. J. Clean. Prod. 2014, 72, 174–180. [Google Scholar] [CrossRef]
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. China National Standardization Administration Committee: Beijing, China, 2005. (In Chinese)
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. China National Standardization Administration Committee: Beijing, China, 2004. (In Chinese)
- Ma, G.; Zhou, B.; Zhang, M.; Sanjayan, J. Understanding and eliminating of expansion caused by recycled glass fiber reinforced plastic powder in concrete. Constr. Build. Mater. 2022, 347, 128542. [Google Scholar] [CrossRef]
- Abbas, A.-G.N.; Aziz, F.N.A.A.; Abdan, K.; Nasir, N.A.M.; Huseien, G.F. A state-of-the-art review on fibre-reinforced geopolymer composites. Constr. Build. Mater. 2022, 330, 127187. [Google Scholar] [CrossRef]
- Sethi, S.; Rathore, D.K.; Ray, B.C. Effects of temperature and loading speed on interface-dominated strength in fibre/polymer composites: An evaluation for in-situ environment. Mater. Des. (1980–2015) 2015, 65, 617–626. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Hu, X. Reaction mechanism of sulfate attack on alkali-activated slag/fly ash cements. Constr. Build. Mater. 2022, 318, 126052. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Effect of synthesis parameters on compressive strength of fly ash-slag blended geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Guo, X.; Pan, X. Mechanical properties and mechanisms of fiber reinforced fly ash–steel slag based geopolymer mortar. Constr. Build. Mater. 2018, 179, 633–641. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Jiang, L.; Meng, L.; Zhou, B.; Zhang, J. Long-Term Physical and Mechanical Properties and Microstructures of Fly-Ash-Based Geopolymer Composite Incorporating Carbide Slag. Materials 2021, 14, 6692. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.Y.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Reeb, C.; Pierlot, C.; Davy, C.; Lambertin, D. Incorporation of organic liquids into geopolymer materials—A review of processing, properties and applications. Ceram. Int. 2021, 47, 7369–7385. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Zhao, X.-E. Sustainable activation of sisal fiber-reinforced slag composites: Mechanical strength and microstructural insights through recycling of alkali-treated wastewaters. Constr. Build. Mater. 2024, 424, 135971. [Google Scholar] [CrossRef]
- Novo, C.C.; Senff, L.; Seabra, M.P.; Novais, R.M.; Labrincha, J.A. The Role of an Industrial Alkaline Wastewater in the Alkali Activation of Biomass Fly Ash. Appl. Sci. 2022, 12, 3612. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernandez-Jimenez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, D. Study on the effect of emulsiffers on the pore structures of geopolymer prepared by emulsion templating. Mater. Res. Express 2020, 7, 055508. [Google Scholar] [CrossRef]
- Haffz, M.M.A.; Ridzuan, A.R.M.; Faiza, A.M.; Arshad, M.F.; Nurliza, J. Morphology of formulated used cooking oil foam (FUSCOF) created for lightweight greencrete. Adv. Mater. Res. 2013, 701, 270–274. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of Calcium Additions on N-A-S-H Cementitious Gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Gao, H.; Yuan, T.; Zhang, X. The effects of salt-loss soda residue and oxalate acid on property and structure of fly ash-based geopolymer. Constr. Build. Mater. 2023, 366, 130214. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Xu, Z.; Zhang, Z.; Zhang, C.; Bai, Y.; Zhao, Q. Mechanical performance and microstructure improvement of soda residue–carbide slag–ground granulated blast furnace slag binder by optimizing its preparation process and curing method. Constr. Build. Mater. 2021, 302, 124403. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.-y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-ash based geopolymer mortar for high-temperature application—Effect of slag addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, T.; Su, L.; Zhou, B.; Lin, Y.; Mohan, S. Determination of Gel Products in Alkali-Activated Fly Ash-Based Composites Incorporating Inorganic Calcium Additives. Adv. Mater. Sci. Eng. 2022, 2022, 7476671. [Google Scholar] [CrossRef]
- Arthanarieswaran, V.P.; Kumaravel, A.; Saravanakumar, S.S. Physico-Chemical Properties of Alkali-Treated Acacia leucophloea Fibers. Int. J. Polym. Anal. Charact. 2015, 20, 704–713. [Google Scholar] [CrossRef]
- Jabli, M.; Tka, N.; Ramzi, K.; Saleh, T.A. Physicochemical characteristics and dyeing properties of lignin-cellulosic fibers derived from Nerium oleander. J. Mol. Liq. 2018, 249, 1138–1144. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H.; Dong, W.; Liu, X. All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef]
- Becerra, F.Y.G.; Acosta, E.J.; Allen, D.G. Alkaline extraction of wastewater activated sludge biosolids. Bioresour. Technol. 2010, 101, 6972–6980. [Google Scholar] [CrossRef]
- Castoldi, R.d.S.; de Souza, L.M.S.; Souto, F.; Liebscher, M.; Mechtcherine, V.; de Andrade Silva, F. Effect of alkali treatment on physical–chemical properties of sisal fibers and adhesion towards cement-based matrices. Constr. Build. Mater. 2022, 345, 128363. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Maheswari, C.U.; Reddy, D.J.P.; Rajulu, A.V. Thermal properties of Napier grass fibers. Mater. Lett. 2009, 63, 2390–2392. [Google Scholar] [CrossRef]
- Rajkumar, R.; Manikandan, A.; Saravanakumar, S.S. Physicochemical properties of alkali-treated new cellulosic fiber from cotton shell. Int. J. Polym. Anal. Charact. 2016, 21, 359–364. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Moorthy, I.G. Investigation of Physico-Chemical Properties of Alkali-Treated Fibers. Int. J. Polym. Anal. Charact. 2014, 19, 309–317. [Google Scholar] [CrossRef]
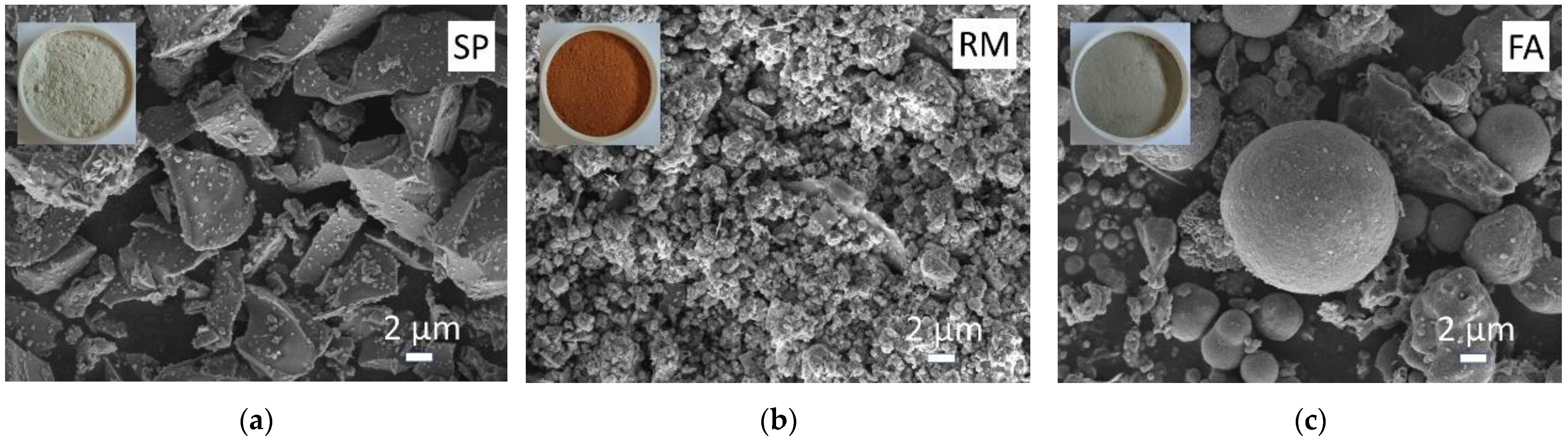



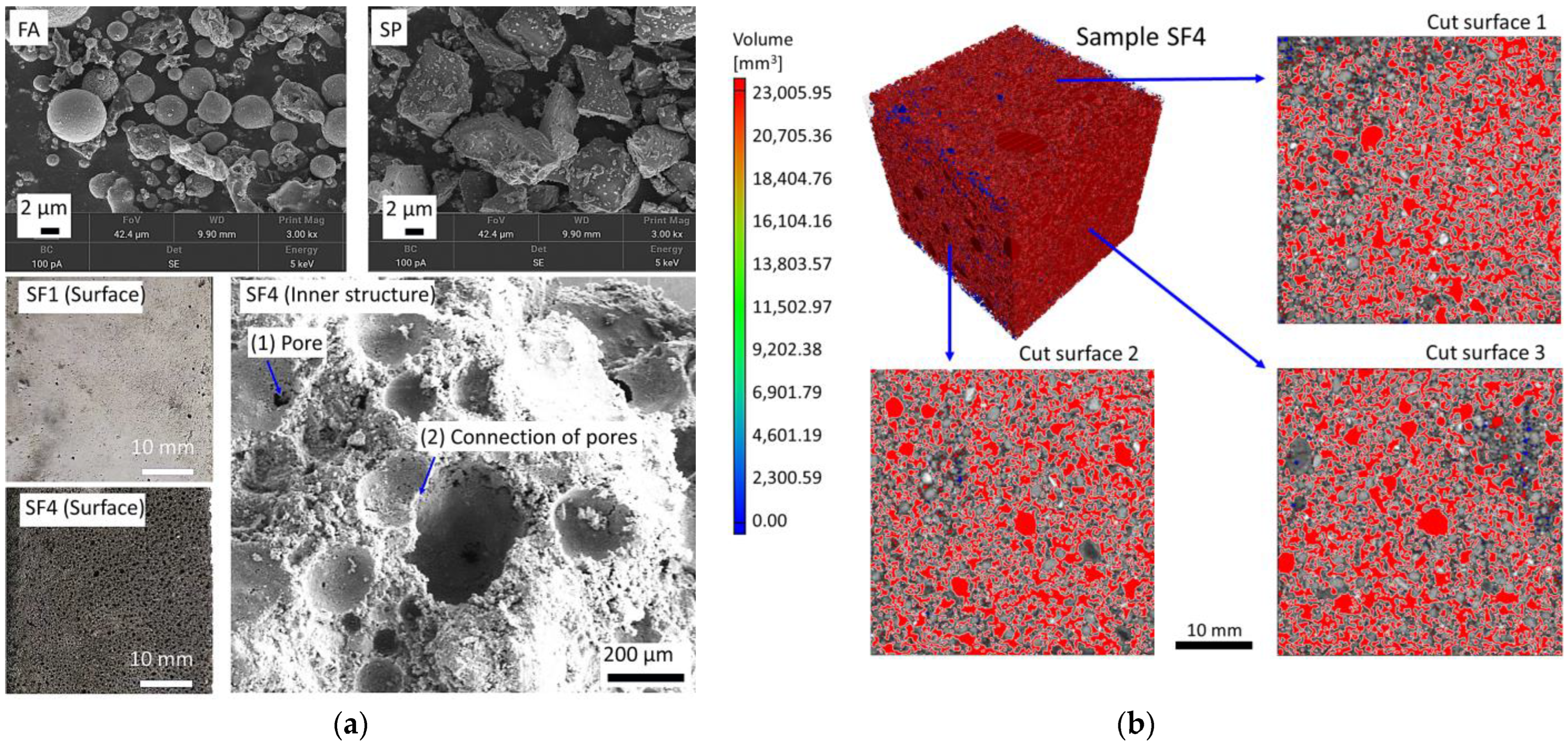
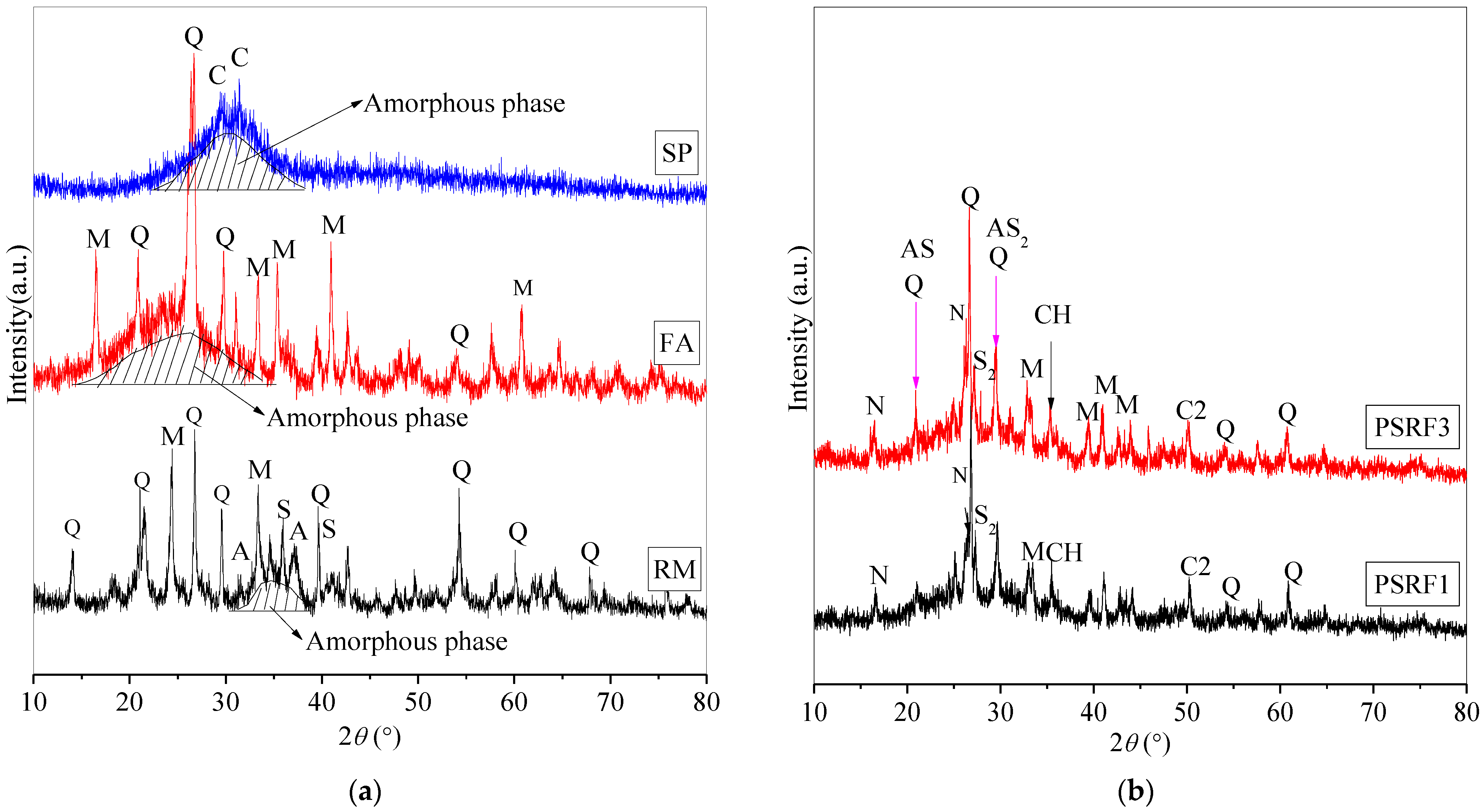

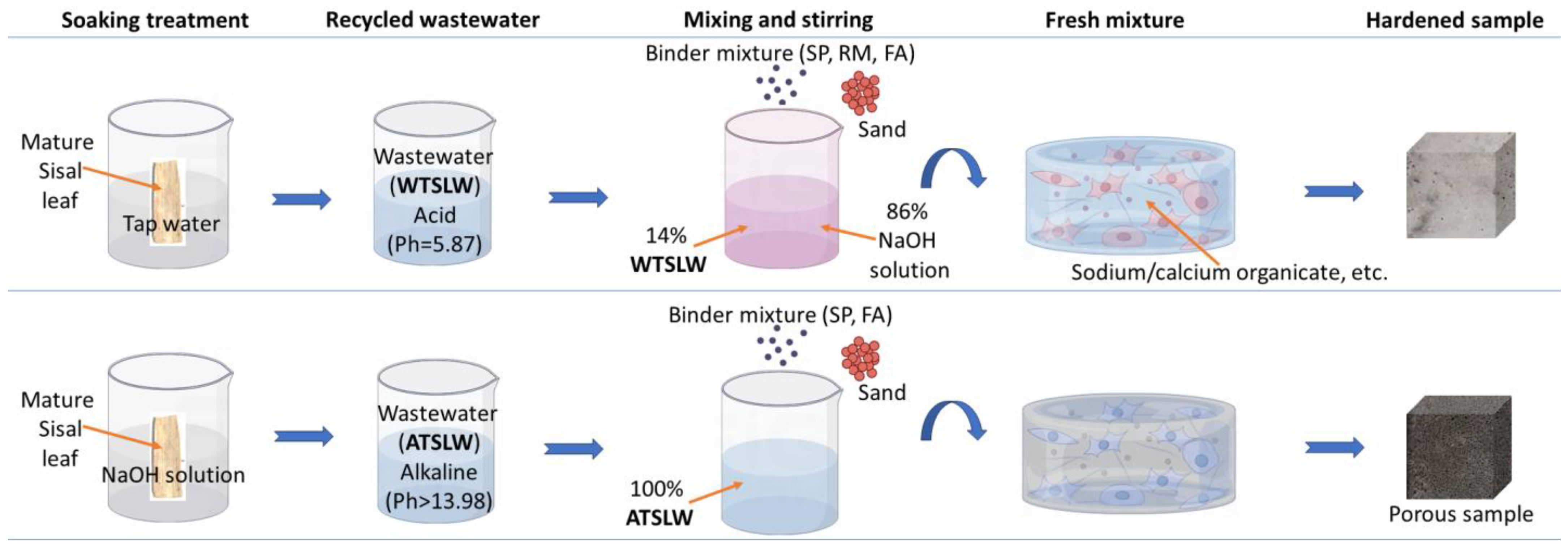
| Components | Slag Powder (SP) | Red Mud (RM) | Fly Ash (FA) |
|---|---|---|---|
| SiO2 | 35.1 | 27.5 | 46.1 |
| Al2O3 | 16.2 | 28.4 | 23.2 |
| CaO | 33.6 | 2.5 | 5.4 |
| MgO | 11.1 | 0.2 | 2.6 |
| Fe2O3 | Non. | 25.8 | 8.0 |
| K2O | Non. | 0.1 | Non. |
| SO3 | Non. | 0.8 | 0.8 |
| Na2O | Non. | 14.7 | Non. |
| P2O5 | Non. | Non. | 0.6 |
| TiO2 | Non. | Non. | 0.3 |
| Others | 4.1 | Non. | 1.5 |
| Loss on ignition (1000 °C) | Non. | Non. | 9.1 |
| Sample No. | Binder Materials (g) | 2 mol/L NaOH Solution (g) | WTSLW (g)—(mass%) | ATSLW (g) | Liquid– Binder Ratio | Binder– Sand Ratio | Soaking Duration (h) | Influencing Factors |
|---|---|---|---|---|---|---|---|---|
| SRF1 | Mixture 1 | 570.0 | 0.0—(0%) | — | 1:1.3 | 1:1.8 | — | (WTSLW) Substitution |
| SRF2 | Mixture 1 | 535.0 | 35.0—(7%) | — | 1:1.3 | 1:1.8 | — | |
| SRF3 | Mixture 1 | 500.0 | 70.0—(14%) | — | 1:1.3 | 1:1.8 | — | |
| PSRF1 | Mixture 1 | 540.0 | 0.0—(0%) | — | 1:1.4 | — | — | (WTSLW) Substitution |
| PSRF3 | Mixture 1 | 464.4 | 75.6—(14%) | — | 1:1.4 | — | — | |
| DSRF1 | Mixture 1 | 480.0 | 0.0—(0%) | — | 1:1.6 | 1:2.0 | — | (WTSLW) Substitution |
| DSRF3 | Mixture 1 | 412.8 | 67.2—(14%) | — | 1:1.6 | 1:2.0 | — | |
| SF1 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:3.0 | 0 | (ATSLW) Soaking duration |
| SF2 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:3.0 | 1 ± 0.2 | |
| SF3 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:3.0 | 5 ± 0.2 | |
| SF4 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:3.0 | 8 ± 0.2 | |
| DSF1 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:2.0 | 0.0 | (ATSLW) Soaking duration |
| DSF2 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:2.0 | 1 ± 0.2 | |
| DSF3 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:2.0 | 5 ± 0.2 | |
| DSF4 | Mixture 2 | — | — | 172.5 | 1:4.3 | 1:2.0 | 8 ± 0.2 |
| Abbreviations | Mineral Phases | pdf Card Number |
|---|---|---|
| Q | Quartz, SiO2 | pdf# 97-003-9830 |
| C | Calcite, CaCO3 | pdf# 97-001-8166 |
| M | Mullite, Si-Al-O | pdf# 00-029-1487 |
| A | Calcium aluminate hydrated, C-A-H | pdf# 97-041-8966 |
| S | Calcium silicate hydrated, C-S-H | pdf# 97-024-0406 |
| S2 | Calcium silicate hydrated, C-S-H | pdf# 97-034-0002 |
| AS | Calcium aluminosilicate hydrated, C-A-S-H | pdf# 00-015-0179 |
| AS2 | Calcium aluminosilicate hydrated, C-A-S-H | pdf# 99-000-4228 |
| C2 | Calcium carbonate, CaCO3 | pdf# 99-000-4172 |
| N | Sodium aluminosilicate hydrated, N-A-S-H | pdf# 00-056-0499 |
| CH | Calcium hydroxide, Ca(OH)2 | pdf# 00-003-0865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yang, H.; Zhao, X.; Wang, H.; Zhao, R. Sustainable Alkali Activation: The Role of Water- and Alkali-Treated Sisal Leaf Wastewaters in Solid- Waste-Based Composite Synthesis. Materials 2024, 17, 3838. https://doi.org/10.3390/ma17153838
Li L, Yang H, Zhao X, Wang H, Zhao R. Sustainable Alkali Activation: The Role of Water- and Alkali-Treated Sisal Leaf Wastewaters in Solid- Waste-Based Composite Synthesis. Materials. 2024; 17(15):3838. https://doi.org/10.3390/ma17153838
Chicago/Turabian StyleLi, Liang, Hongqi Yang, Xianhui Zhao, Haoyu Wang, and Renlong Zhao. 2024. "Sustainable Alkali Activation: The Role of Water- and Alkali-Treated Sisal Leaf Wastewaters in Solid- Waste-Based Composite Synthesis" Materials 17, no. 15: 3838. https://doi.org/10.3390/ma17153838







