Impact of Ground Granulated Blast Furnace Slag on Calcium Leaching of Low-Heat Portland Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Test Methods
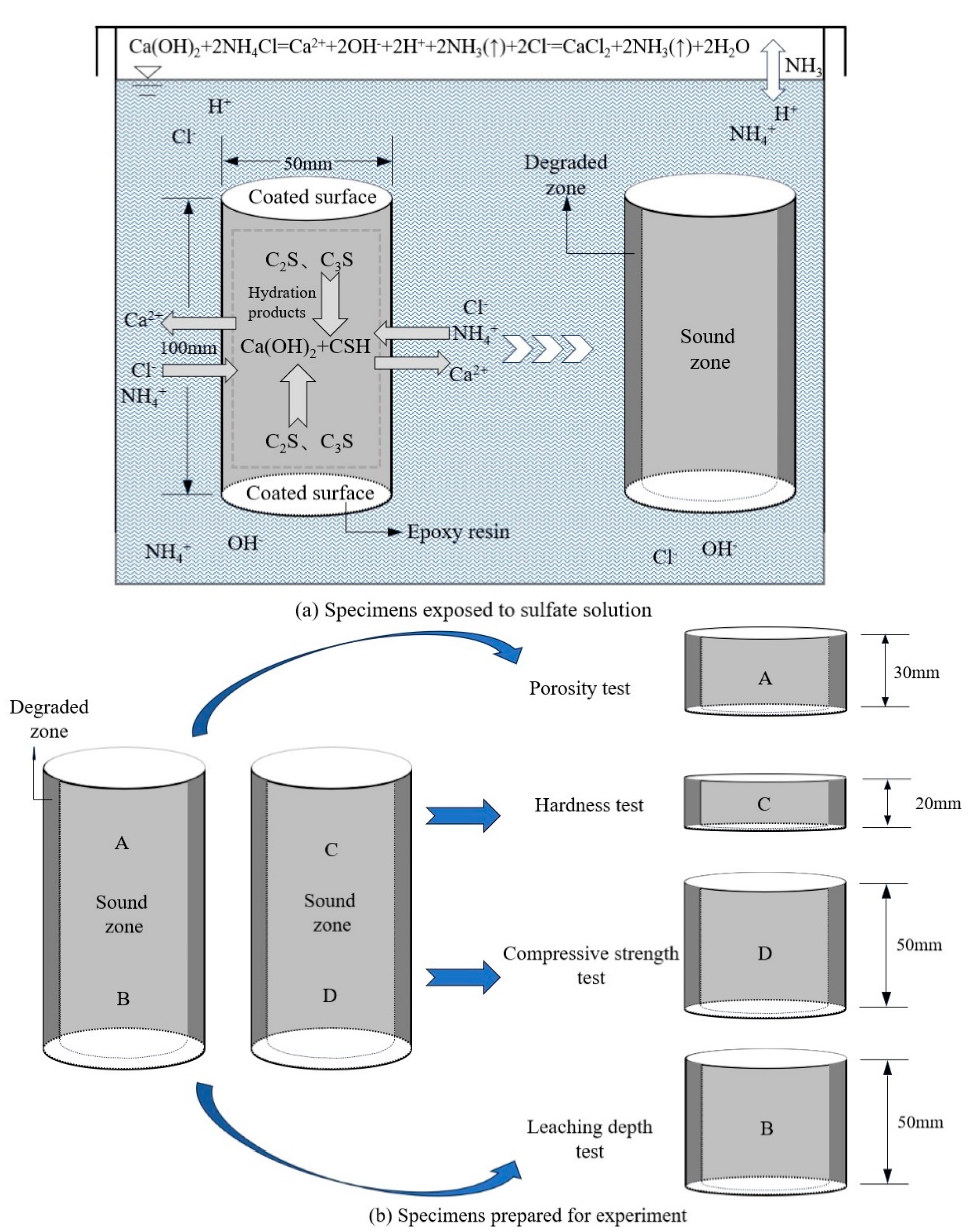
2.2.1. Mass Loss and Porosity
2.2.2. Leaching Depth
2.2.3. Compressive Strength
2.2.4. Vickers Hardness
2.2.5. Mercury Intrusion Porosimetry
2.2.6. X-ray Diffraction
2.2.7. Scanning Electron Microscope
3. Results and Analysis
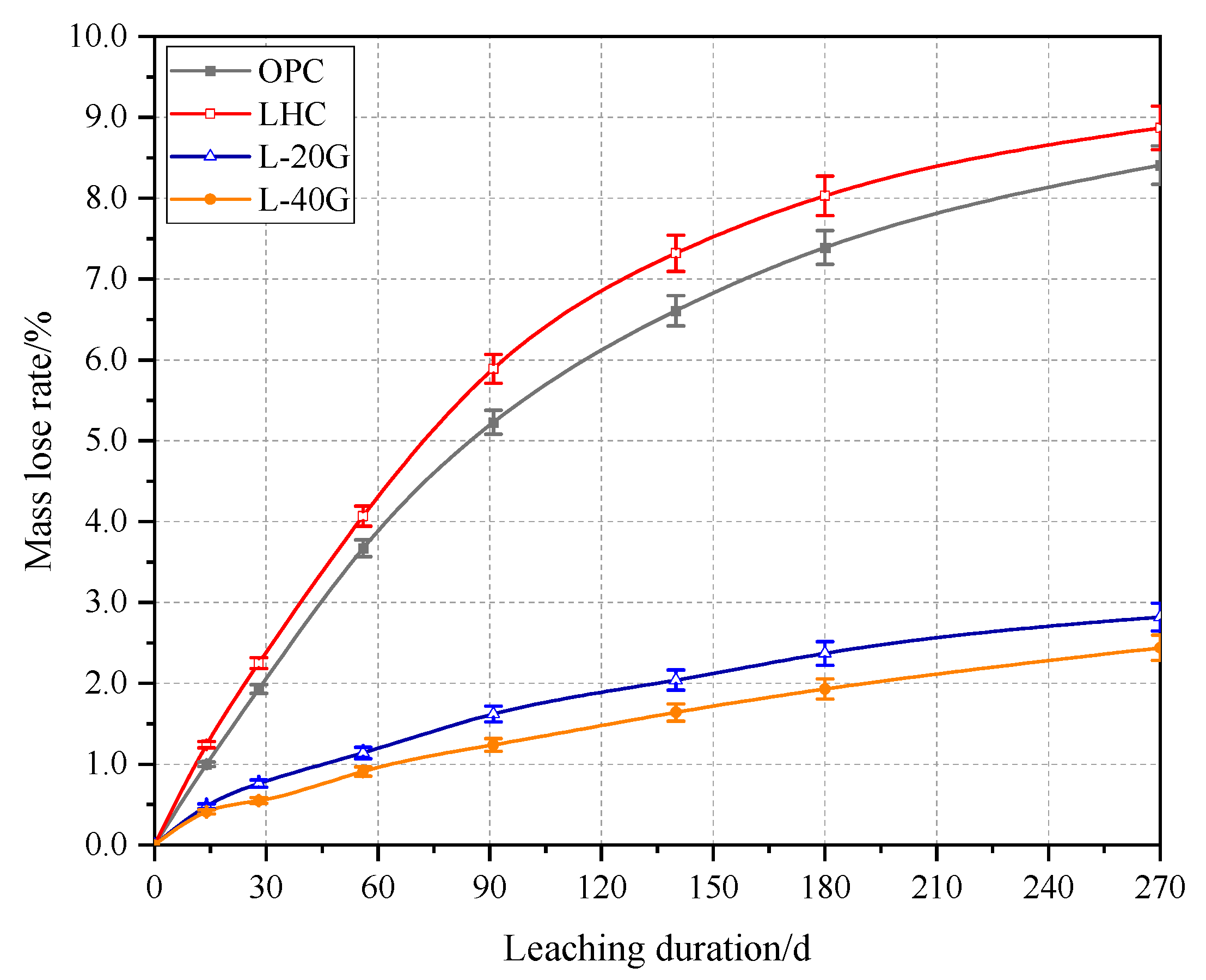
3.1. Mass Loss
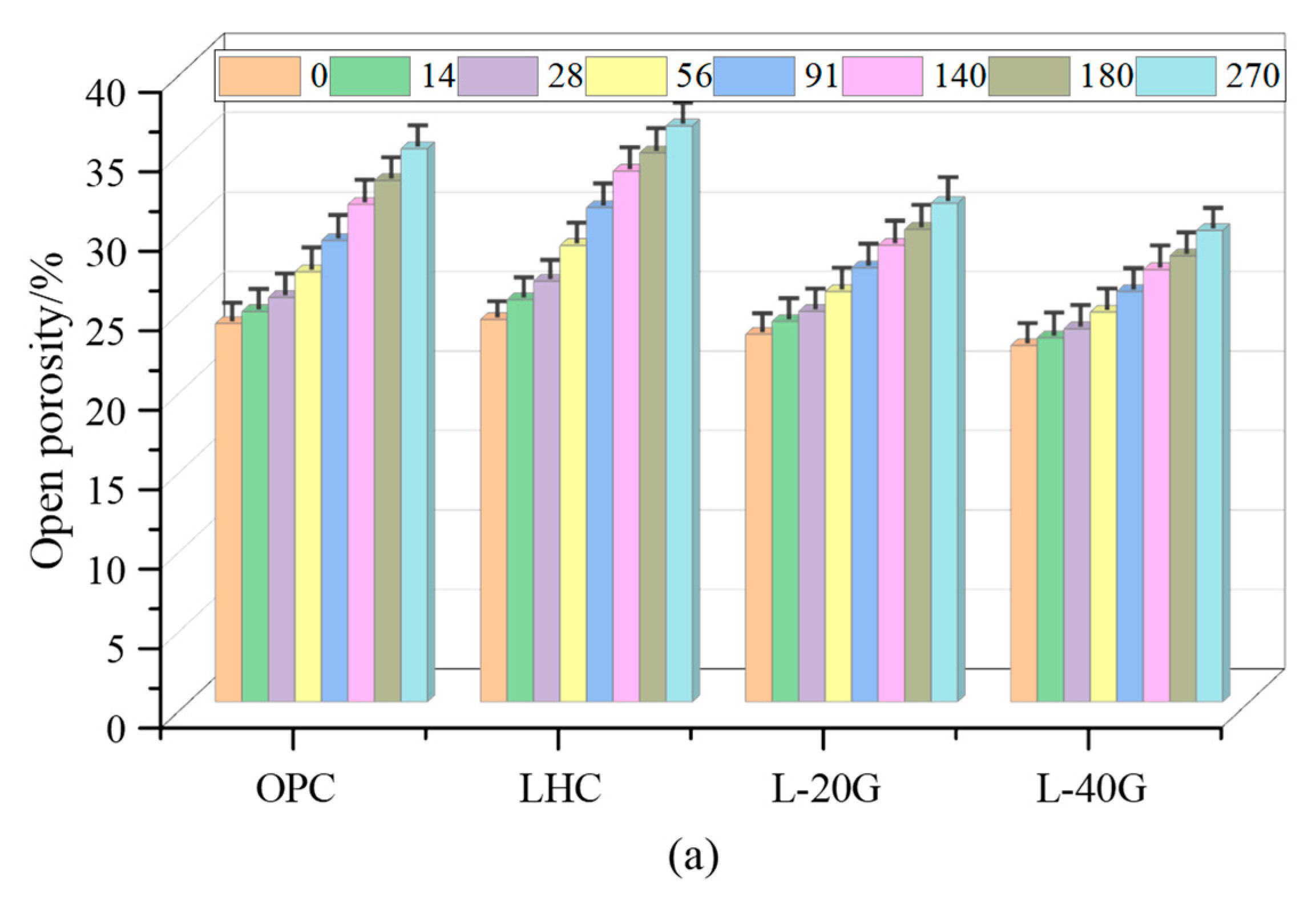
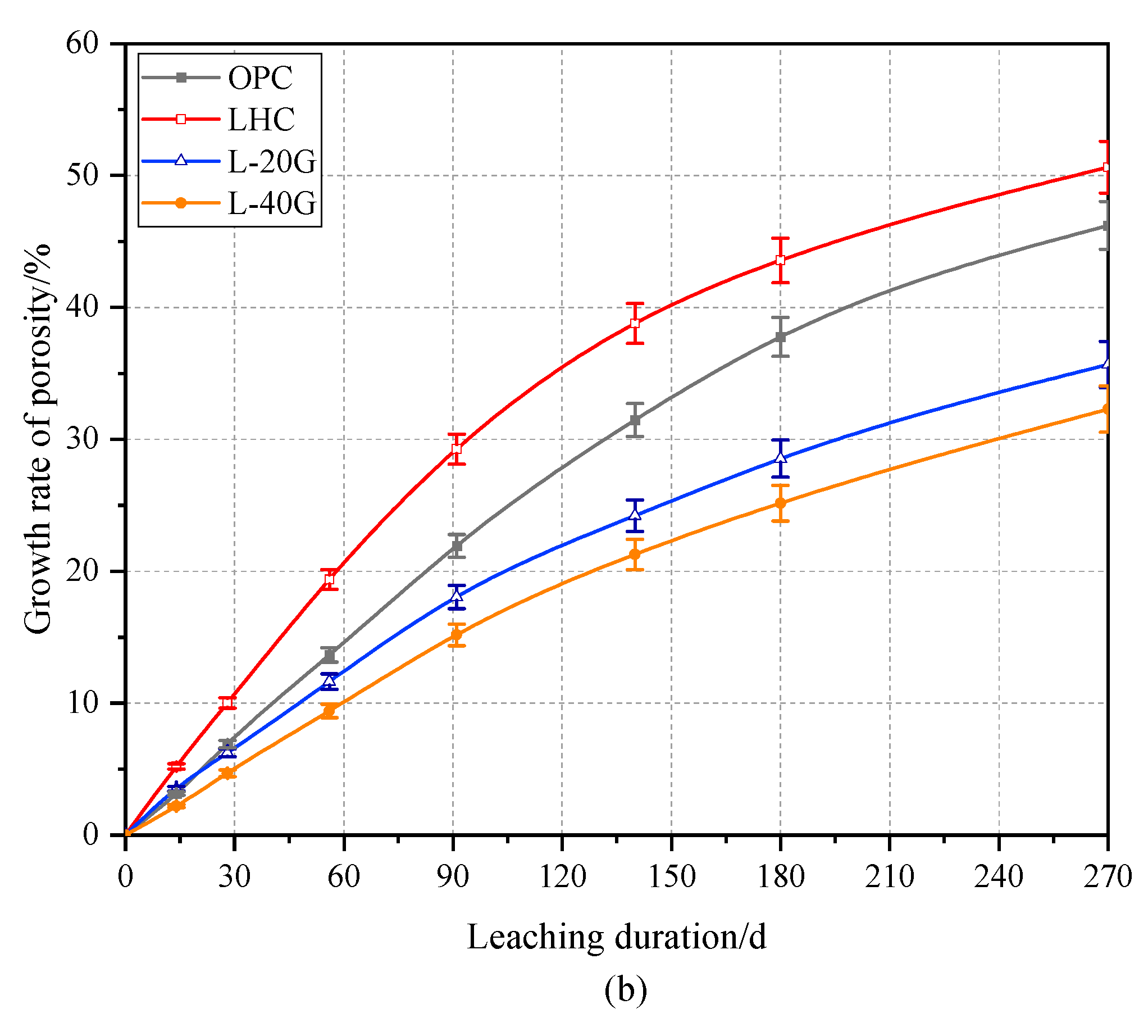
3.2. Porosity
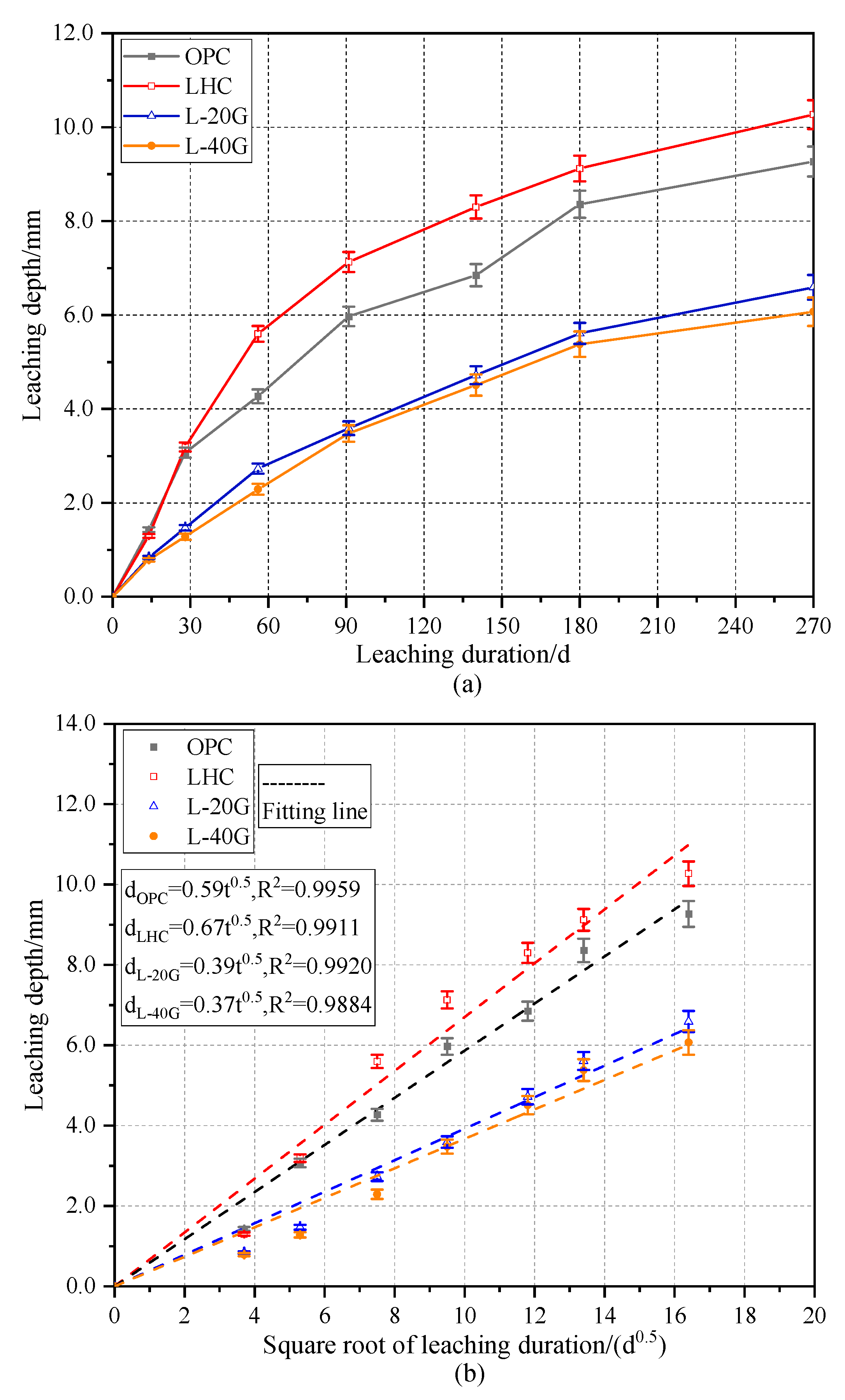
3.3. Leaching Depth
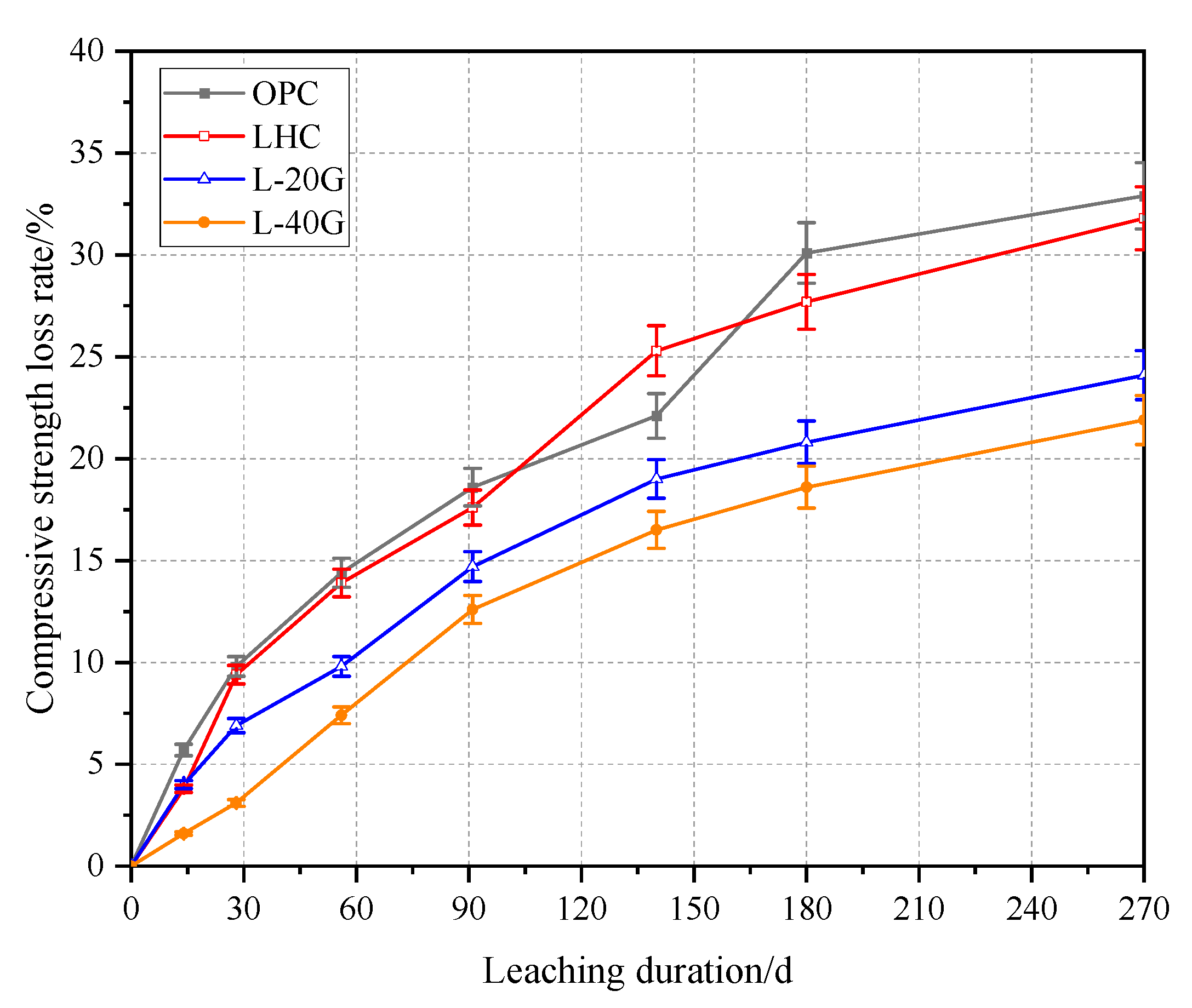
3.4. Compressive Strength
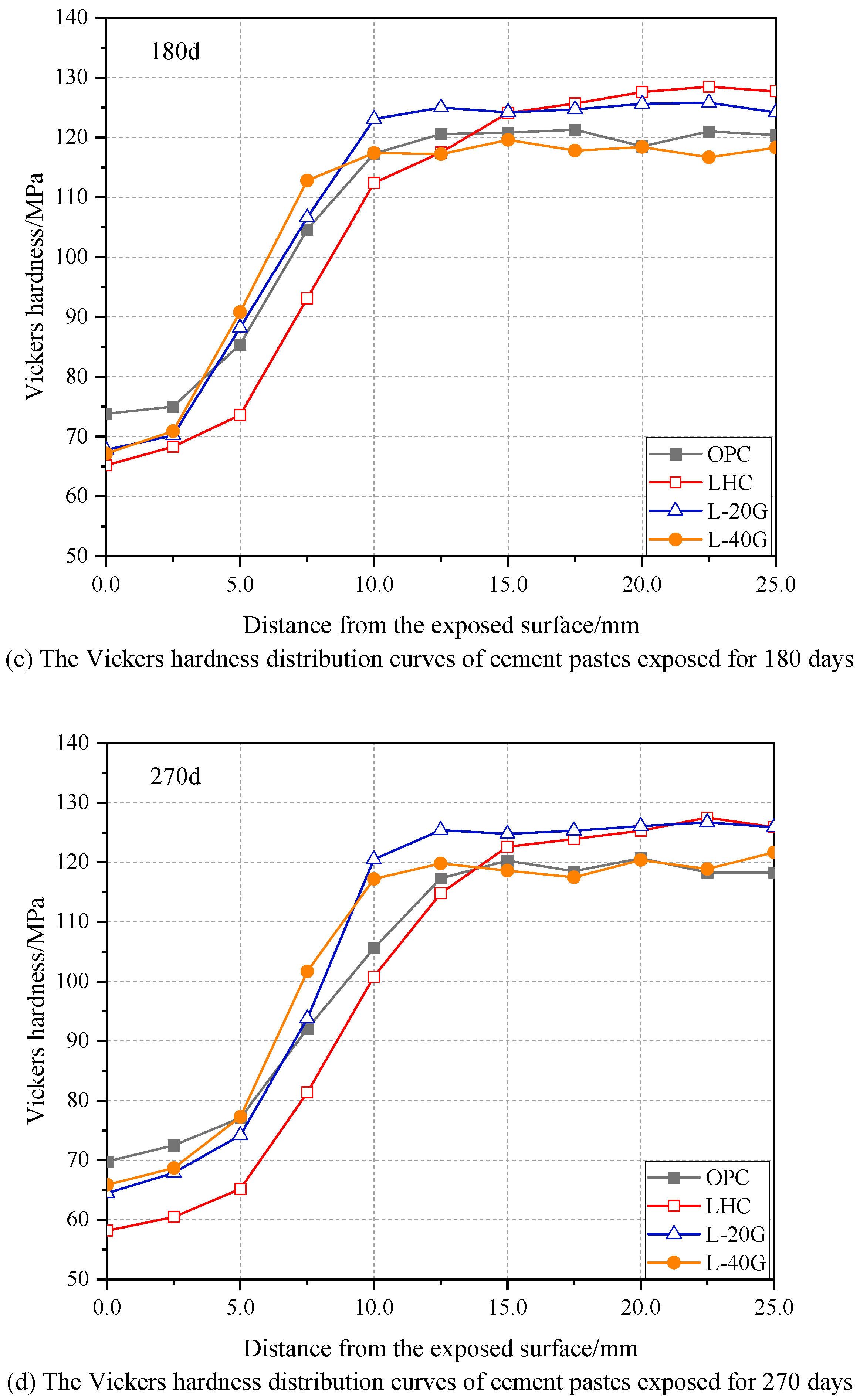
3.5. Vickers Hardness
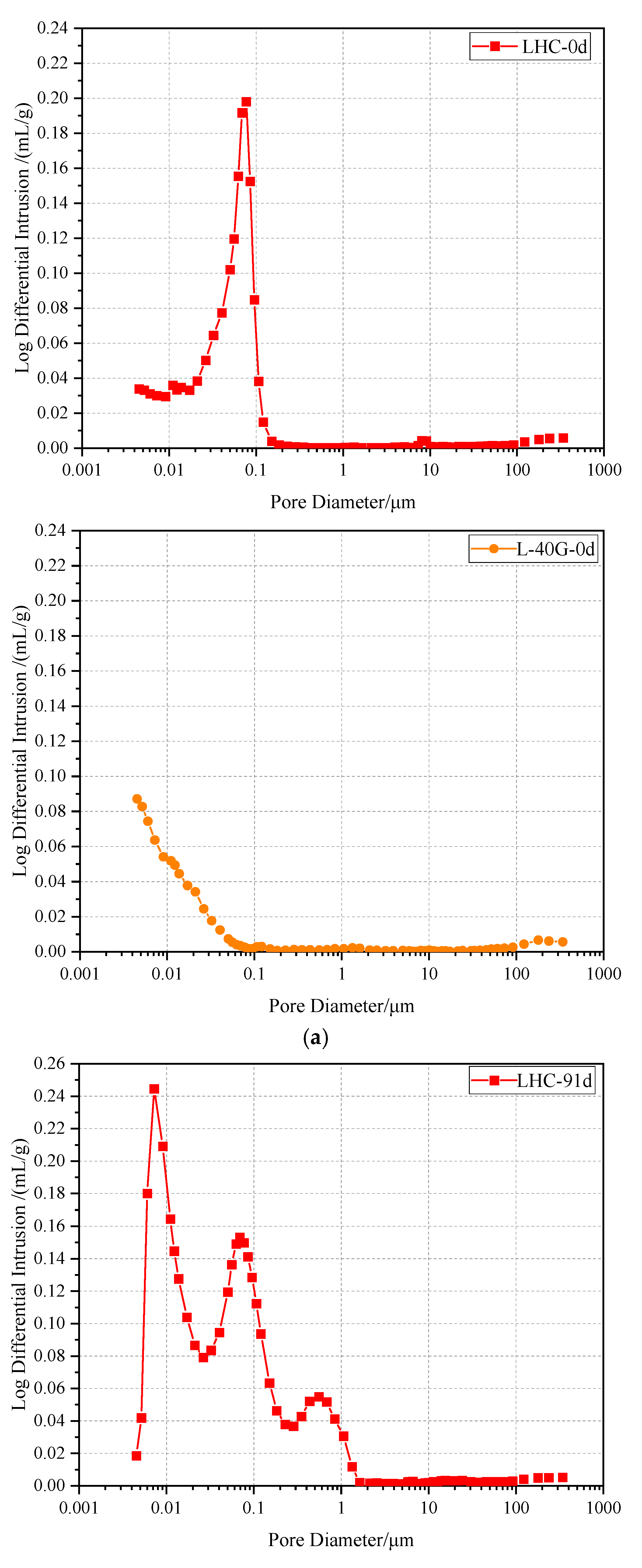
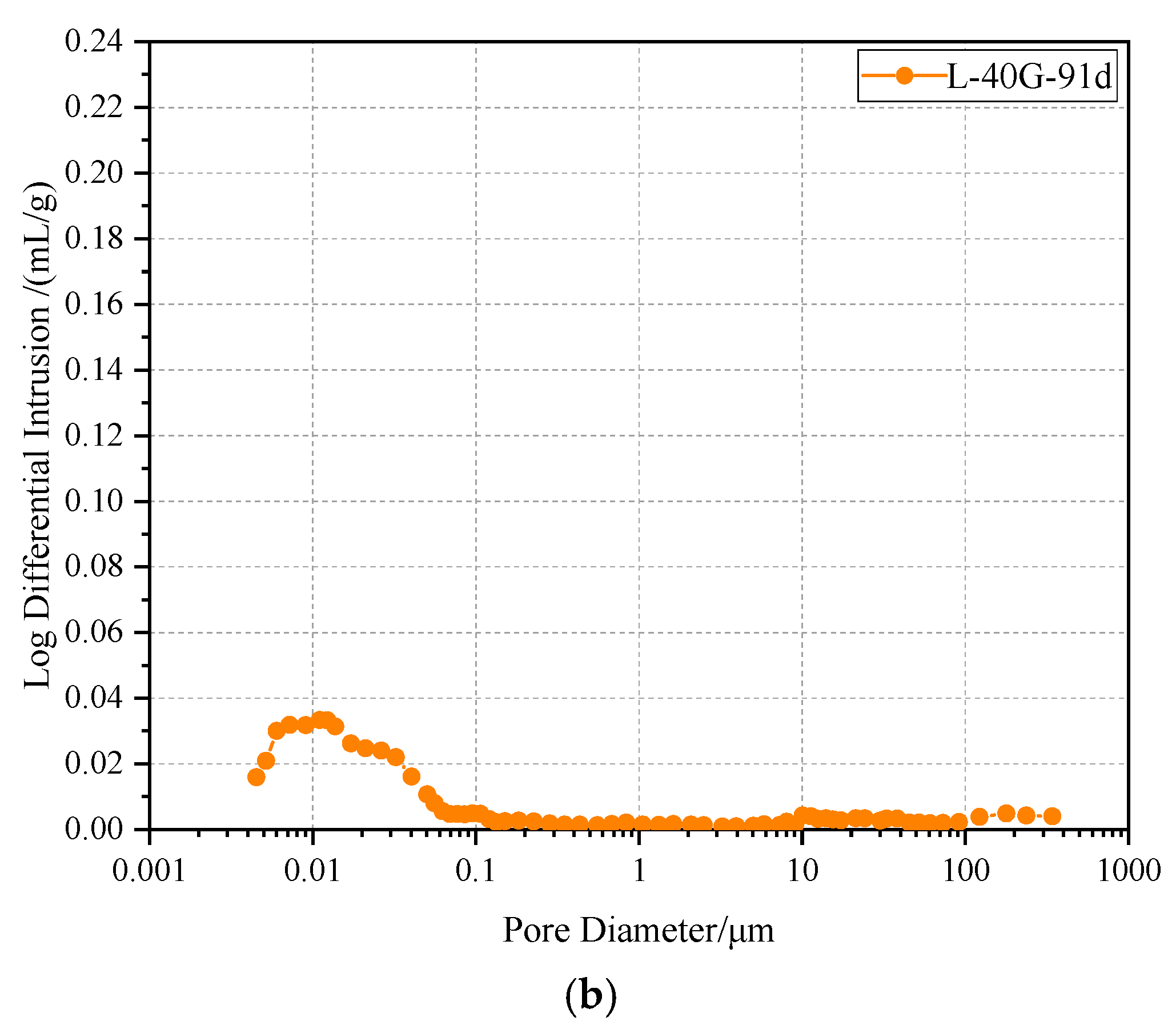
3.6. Mercury Intrusion Porosimetry
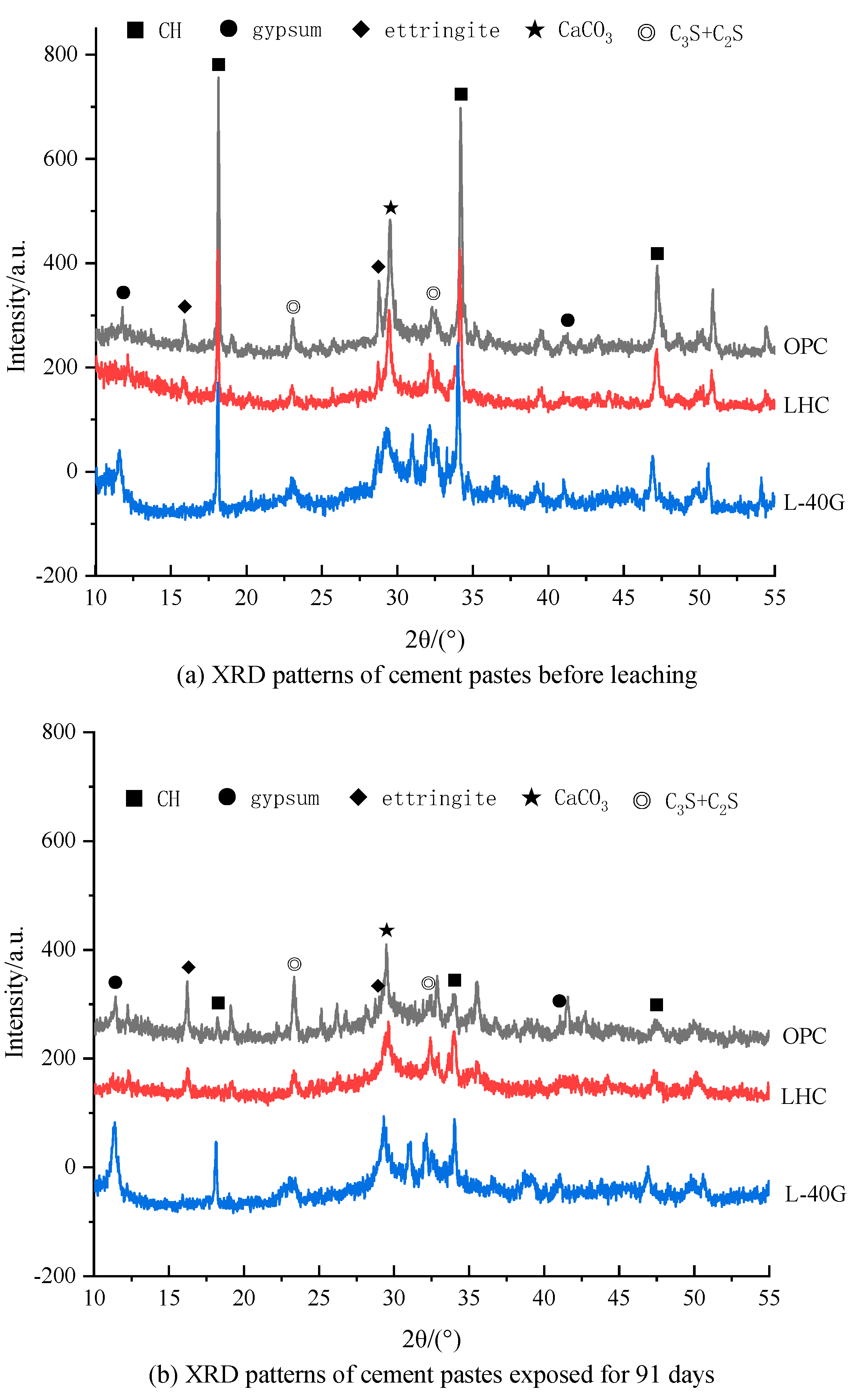
3.7. X-ray Diffraction
3.8. Scanning Electron Microscope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawin, D.; Pesavento, F.; Schrefler, B.A. Modeling deterioration of cementitious materials exposed to calcium leaching in non-isothermal conditions. Comput. Methods Appl. Mech. Eng. 2009, 198, 3051–3083. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Colina, H.; Torrenti, J.M.; Boulay, C.; Nedjar, B. Chemo-mechanical coupling behaviour of leached concrete: Part I: Experimental results. Nucl. Eng. Des. 2007, 237, 2083–2089. [Google Scholar] [CrossRef]
- Cuesta, A.; Ayuela, A.; Aranda, M.A.G. Belite cements and their activation. Cem. Concr. Res. 2021, 140, 106319. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Z.; Zhang, X.; Hu, X.; Shi, C. Trends and developments in low-heat portland cement and concrete: A review. Constr. Build. Mater. 2023, 392, 131535. [Google Scholar] [CrossRef]
- Lin, F.; Meyer, C. Hydration kinetics modeling of Portland cement considering the effects of curing temperature and applied pressure. Cem. Concr. Res. 2009, 39, 255–265. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration mechanism and leaching behavior of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.W.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- Shen, W. Cement Technology; Wuhan University of Technology Press: Wuhan, China, 1991. [Google Scholar]
- Codina, M.; Cau-dit-Coumes, C.; Le Bescop, P.; Verdier, J.; Ollivier, J.P. Design and characterization of low-heat and low-alkalinity cements. Cem. Concr. Res. 2008, 38, 437–448. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, L.; Tang, X.; Gong, J.; Chu, H. Impact of calcium leaching on mechanical and physical behaviors of high belite cement pastes. Constr. Build. Mater. 2021, 286, 122983. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, L.; Tang, X.; Chu, H.; Jiang, L. Deterioration process of high belite cement paste exposed to sulfate attack, calcium leaching and the dual actions. J. Mater. Res. Technol. 2021, 15, 2982–2992. [Google Scholar] [CrossRef]
- Wang, X.; Xu, K.; Li, Y.; Guo, S. Dissolution and leaching mechanisms of calcium ions in cement based materials. Constr. Build. Mater. 2018, 180, 103–108. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhou, S.H.; Chen, E.; Tang, S.W. Energy saving benefit, mechanical performance, volume stabilities, hydration properties and products of low heat cement-based materials. Energy Build. 2018, 170, 157–169. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.Q.; Zhou, S.H.; Chen, E.; Tang, S.W. Mechanical properties, long-term hydration heat, shinkage behavior and crack resistance of dam concrete designed with low heat Portland (LHP) cement and fly ash. Constr. Build. Mater. 2018, 187, 1073–1091. [Google Scholar] [CrossRef]
- Saoût, G.L.; Haha, M.B.; Winnefeld, F.; Lothenbach, B. Hydration degree of alkali-activated slags: A 29Si NMR study. J. Am. Ceram. Soc. 2011, 94, 4541–4547. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Enhancing microstructure and durability of concrete from ground granulated blast furnace slag and metakaolin as cement replacement materials. J. Mater. Res. Technol. 2013, 2, 52–59. [Google Scholar] [CrossRef]
- Niu, Q.; Feng, N.; Yang, J.; Zheng, X. Effect of superfine slag powder on cement properties. Cem. Concr. Res. 2002, 32, 615–621. [Google Scholar] [CrossRef]
- Dong, W.; Qin, L. Comparative Study of Slag Powder and Fly Ash through Microscopic Analysis. Coal Ash 2001, 13, 17–19. (In Chinese) [Google Scholar]
- Tang, Y.J.; Zuo, X.B.; He, S.L.; Ayinde, O.; Yin, G.J. Influence of slag content and water-binder ratio on leaching behavior of cement pastes. Constr. Build. Mater. 2016, 129, 61–69. [Google Scholar] [CrossRef]
- He, S. Experimental Study on Corrosion Behavior of Mineral Admixture Concrete in Soft Water. Doctoral Dissertation, Nanjing University of Science and Technology, Nanjing, China, 2018; pp. 60–72. (In Chinese). [Google Scholar]
- GB/T 200-2017; Moderate-Heat Portland Cement, Low-Heat Portland Cement. China Standards Press: Beijing, China, 2017.
- GB 175-2007; Common Portland Cement. China Standards Press: Beijing, China, 2007.
- Haga, K.; Sutou, S.; Hironaga, M.; Tanaka, S.; Nagasaki, S. Effects of porosity on leaching of Ca from hardened ordinary Portland cement paste. Cem. Concr. Res. 2005, 35, 1764–1775. [Google Scholar] [CrossRef]
- Sumer, M. Compressive strength and sulfate resistance properties of concretes containing Class F and Class C fly ashes. Constr. Build. Mater. 2012, 34, 531–536. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Abell, A.B.; Willis, K.L.; Lange, D.A. Mercury intrusion porosimetry and image analysis of cement-based materials. J. Colloid Interface Sci. 1999, 211, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Epp, J. X-ray diffraction (XRD) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Woodhead Publishing: Sawston, UK, 2016; pp. 81–124. [Google Scholar]
- Faucon, P.; Adenot, F.; Jacquinot, J.F.; Petit, J.C.; Cabrillac, R.; Jorda, M. Long-term behaviour of cement pastes used for nuclear waste disposal: Review of physico-chemical mechanisms of water degradation. Cem. Concr. Res. 1998, 28, 847–857. [Google Scholar] [CrossRef]


| Material | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | R2O * | Mineral Composition | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C3S | C2S | C3A | C4AF | ||||||||
| LHC | 58.74 | 22.82 | 3.55 | 4.28 | 4.99 | 2.43 | 0.39 | 28.7 | 43.9 | 2.1 | 13.0 |
| OPC | 62.83 | 20.50 | 5.61 | 3.84 | 1.70 | 3.07 | 1.05 | 48.0 | 22.9 | 8.4 | 11.7 |
| GGBS | 38.36 | 29.03 | 15.17 | 0.37 | 10.7 | 2.90 | 1.21 | - | - | - | - |
| Sample Codes | P.LH/% | P.O/% | GGBS/% |
|---|---|---|---|
| OPC | 0 | 100 | 0 |
| LHC | 100 | 0 | 0 |
| L-20G | 80 | 0 | 20 |
| L-40G | 60 | 0 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Xia, L.; Li, S.; Li, X.; Chen, Y.; Liu, J. Impact of Ground Granulated Blast Furnace Slag on Calcium Leaching of Low-Heat Portland Cement Paste. Materials 2024, 17, 3857. https://doi.org/10.3390/ma17153857
Jiang C, Xia L, Li S, Li X, Chen Y, Liu J. Impact of Ground Granulated Blast Furnace Slag on Calcium Leaching of Low-Heat Portland Cement Paste. Materials. 2024; 17(15):3857. https://doi.org/10.3390/ma17153857
Chicago/Turabian StyleJiang, Chunmeng, Li Xia, Shuangxi Li, Xiaoqing Li, Yingjie Chen, and Jian Liu. 2024. "Impact of Ground Granulated Blast Furnace Slag on Calcium Leaching of Low-Heat Portland Cement Paste" Materials 17, no. 15: 3857. https://doi.org/10.3390/ma17153857





