Incorporation of Nano-Zinc Oxide as a Strategy to Improve the Barrier Properties of Biopolymer–Suberinic Acid Residues Films: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blends Processing Methods
- (1)
- The PLA finishing layer was made by mixing methylene chloride (CH2Cl2) solution for PLA, 21% dry matter content, with SAR powder, 10% w/w, respectively, hereafter called “PLA/SAR”; and with nano-ZnO particles, 2% and 4%, w/w, respectively, hereafter called “PLA/SAR nano 2%” and “PLA/SAR nano 4%”. The samples with the addition of nano-ZnO but without SAR are hereafter called “PLA nano 2%” and “PLA nano 4%”. A pure PLA surface-finishing layer has also been tested (hereafter called “PLA”) as a reference. All the prepared blends were spread on Polytetrafluoroethylene (PTFE) sheets under a fume hood to evaporate the solvent and then milled to attain a powder size smaller than 0.1 mm. Such a powder was formed in a hot press (as mentioned above), as described by Gumowska et al. [50]. The prepared film was pressed in a hot press (pressing time, 75 s; temperature, 185 °C; and pressure, 0.8 MPa) onto the unfinished particleboard surface described above.
- (2)
- The PCL finishing layer was obtained from toluene (C6H5CH3) solution for PCL, 25% dry matter content. The remaining steps of the surface-finishing preparation are the same as those described above for PLA. Using this method, the subsequent samples were attained: PCL (hereafter called “PCL”; no SAR/nano-ZnO addition, reference sample) “PCL/SAR”, “PCL/SAR nano 2%”, “PCL/SAR nano 4%”, “PCL nano 2%”, “PCL nano 4%”.
2.3. Relative Hardness
2.4. Cold Liquids Resistance
2.5. Total VOC and Formaldehyde Emission
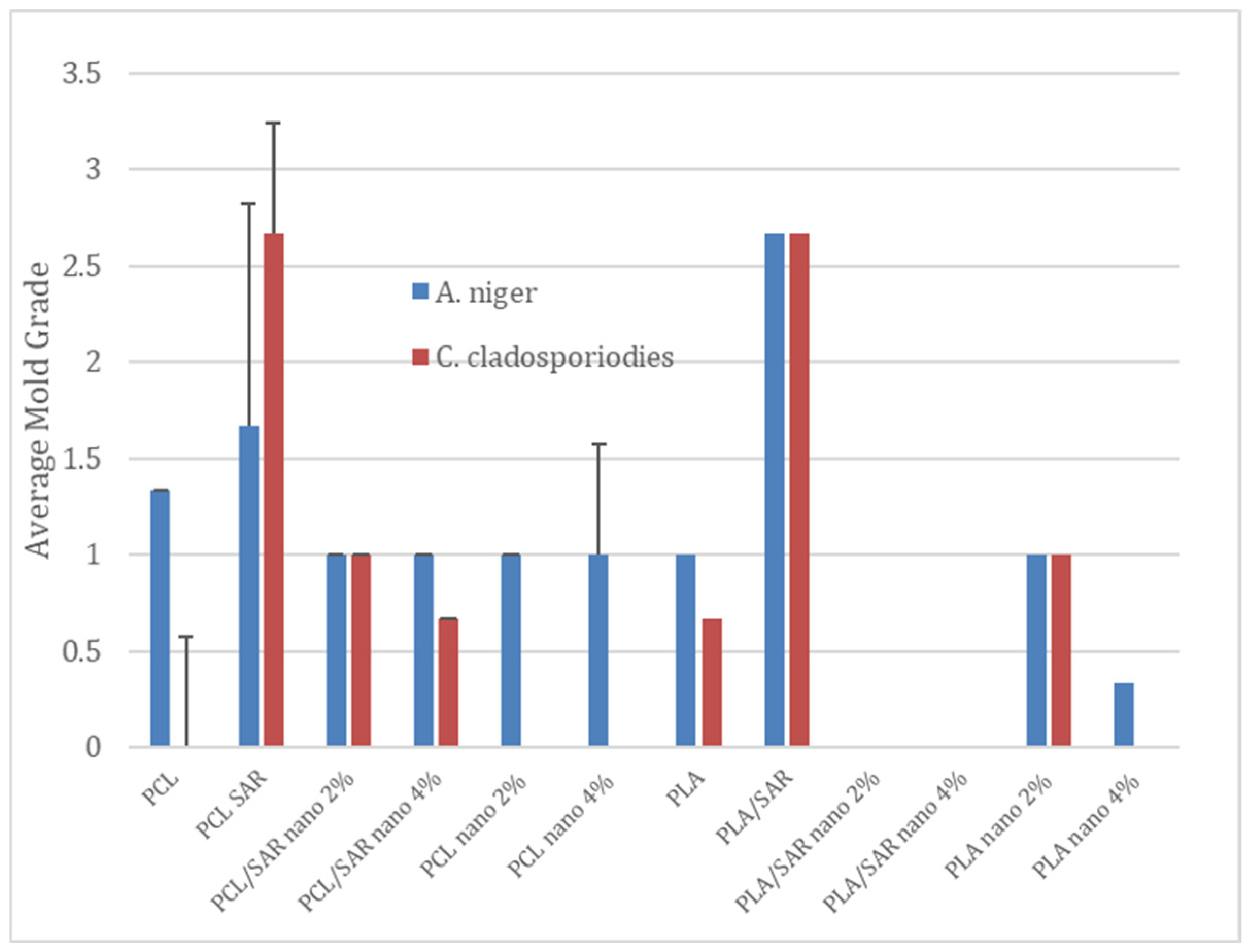
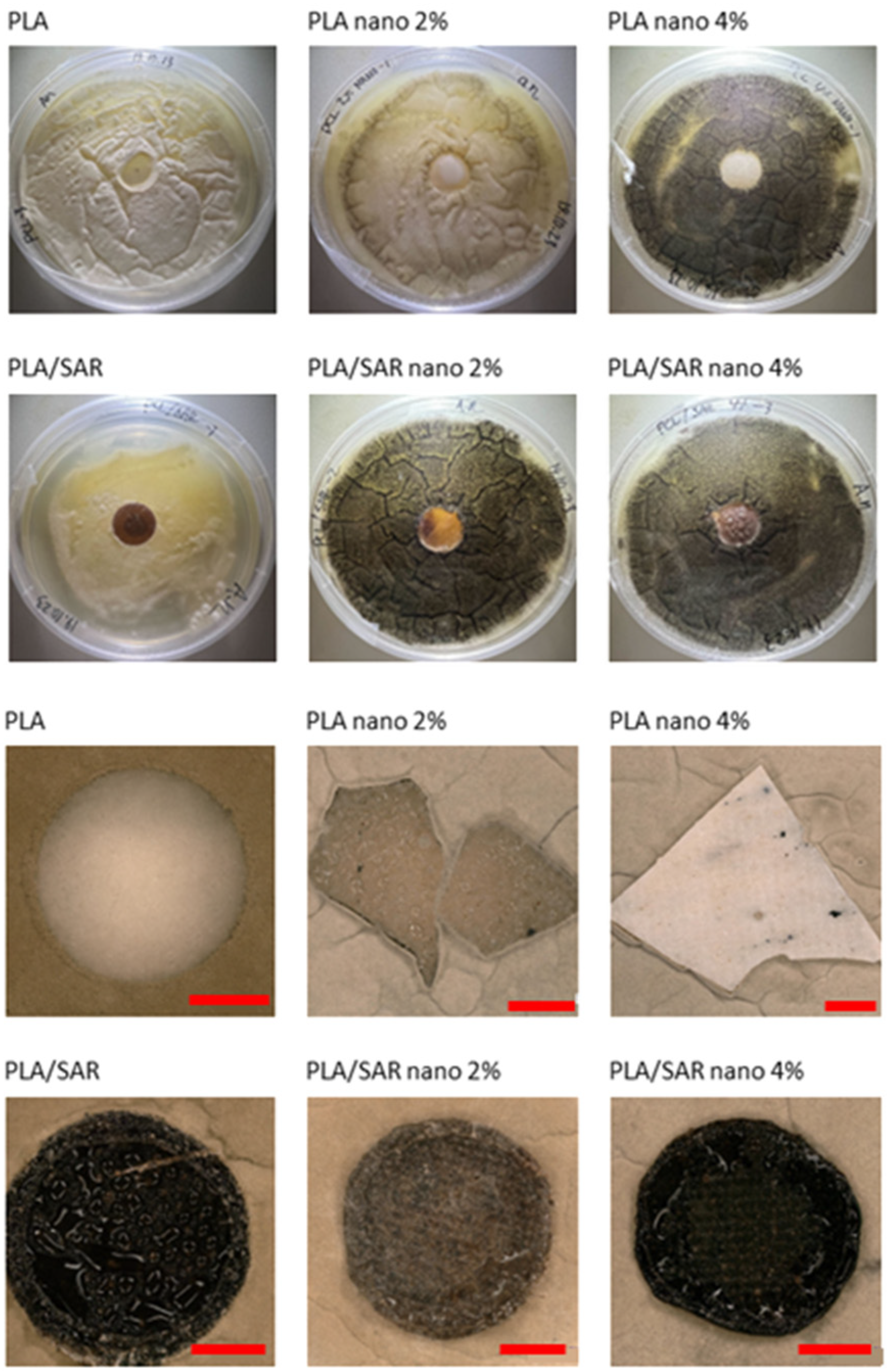
2.6. Antifungal Test
- Grade 0 = no infestation of the surface.
- Grade 1 = 1–20% infestation of the surface.
- Grade 2 = 21–40% infestation of the surface.
- Grade 3 = 1–60% infestation of the surface.
- Grade 4 = 61–80% infestation of the surface.
- Grade 5 = 81–100% infestation of the surface.
2.7. The Effect of Repeated Processing of Polymer Blends on Their Hardness
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cold Liquids Resistance
3.2. Total VOC and Formaldehyde Emission
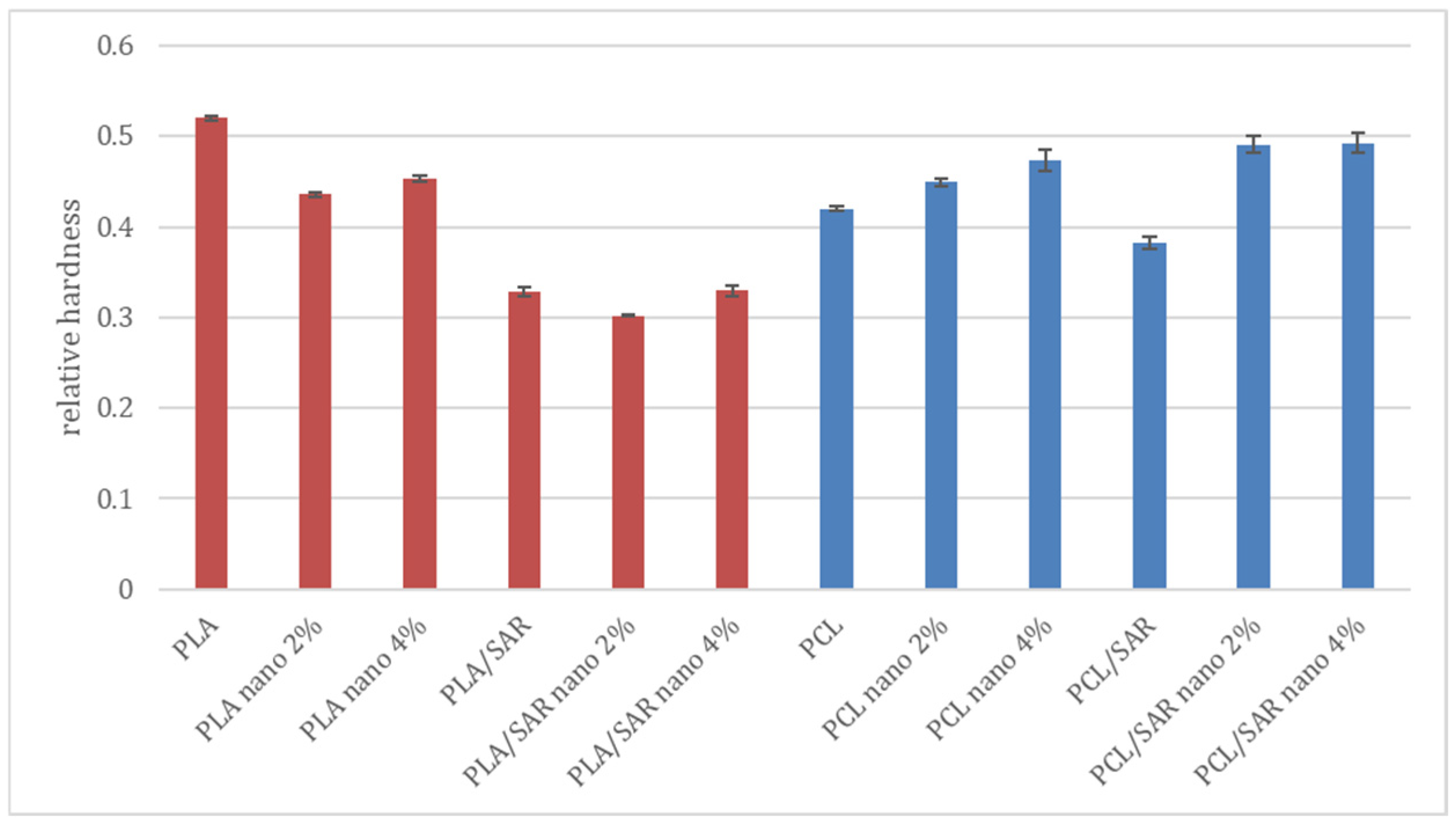
3.3. Relative Hardness
3.4. Antifungal Test
3.5. Relative Hardness after Repeated Processing of the Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Wu, Y.; Yang, F.; Wang, W. The Effect of Antibacterial and Waterproof Coating Prepared From Hexadecyltrimethoxysilane and Nano-Titanium Dioxide on Wood Properties. Front. Mater. 2021, 8, 699579. [Google Scholar] [CrossRef]
- Tracton, A.A. Chapter 83: Fluorocarbon Resins for Coatings and Inks. In Coatings Technology Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 1574446495. [Google Scholar]
- Kozlowski, R.; Wesolek, D.; Wladyka-Przybylak, M. Combustibility and toxicity of board materials used for interior fittings and decorations. Polym. Degrad. Stab. 1999, 64, 595–600. [Google Scholar] [CrossRef]
- Eric, J.; Bailey, K.I.W. Dynamics of polymer segments, polymer chains, and nanoparticles in polymer nanocomposite melts: A review. Prog. Polym. Sci. 2020, 105, 101242. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Vaidya, A.N.; Pandey, R.A.; Mudliar, S.; Kumar, M.S.; Chakrabarti, T.; Devotta, S. Production and recovery of lactic acid for polylactide—An overview. Crit. Rev. Environ. Sci. Technol. 2005, 35, 429–467. [Google Scholar] [CrossRef]
- Valerga, A.P.; Batista, M.; Fernandez-Vidal, S.R.; Gamez, A.J. Impact of chemical post-processing in fused deposition modelling (FDM) on polylactic acid (PLA) surface quality and structure. Polymers 2019, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Jin, Y.; Wan, Y.; Zhang, B.; Liu, Z. Modeling of the chemical finishing process for polylactic acid parts in fused deposition modeling and investigation of its tensile properties. J. Mater. Process. Technol. 2017, 240, 233–239. [Google Scholar] [CrossRef]
- Lv, S.; Gu, J.; Tan, H.; Zhang, Y. The morphology, rheological, and mechanical properties of wood flour/starch/poly(lactic acid) blends. J. Appl. Polym. Sci. 2017, 134, 44743. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Biodegradation behavior and modelling of soil burial effect on degradation rate of PLA blended with starch and wood flour. Colloids Surf. B Biointerfaces 2017, 159, 800–808. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S. Mechanical and thermal properties of poly(lactic acid)/sugarcane bagasse fiber green composites. J. Thermoplast. Compos. Mater. 2017, 30, 1091–1102. [Google Scholar] [CrossRef]
- Müller, K.; Van Opdenbosch, D.; Zollfrank, C. Cellulose blends with polylactic acid or polyamide 6 from solution blending: Microstructure and polymer interactions. Mater. Today Commun. 2022, 30, 103074. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Delgado-Aguilar, M.; Espinach, F.X.; Mutjé, P.; Boufi, S.; Tarrés, Q. Sustainable plastic composites by polylactic acid-starch blends and bleached kraft hardwood fibers. Compos. Part B Eng. 2022, 238, 109901. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Ghanaat, F. Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis and characterization. J. Polym. Res. 2014, 21, 355. [Google Scholar] [CrossRef]
- Jeżo, A.; Kowaluk, G. Carbon Capture and Storage through Upcycling of Suberinic Acid Residues in Wood Composites Finishing. C-J. Carbon Res. 2023, 9, 80. [Google Scholar] [CrossRef]
- Li, Y.; Shimizu, H. Toughening of polylactide by melt blending with a biodegradable poly(ether)urethane elastomer. Macromol. Biosci. 2007, 7, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Sousa, F.M.; Costa, A.R.M.; Reul, L.T.A.; Cavalcanti, F.B.; Carvalho, L.H.; Almeida, T.G.; Canedo, E.L. Rheological and thermal characterization of PCL/PBAT blends. Polym. Bull. 2019, 76, 1573–1593. [Google Scholar] [CrossRef]
- de Campos, A.; Tonoli, G.H.D.; Marconcini, J.M.; Mattoso, L.H.C.; Klamczynski, A.; Gregorski, K.S.; Wood, D.; Williams, T.; Chiou, B.-S.; Imam, S.H. TPS/PCL Composite Reinforced with Treated Sisal Fibers: Property, Biodegradation and Water-Absorption. J. Polym. Environ. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Cabedo, L.; Feijoo, J.L.; Villanueva, M.P.; Lagarón, J.M.; Giménez, E. Optimization of biodegradable nanocomposites based on aPLA/PCL blends for food packaging applications. Macromol. Symp. 2006, 233, 191–197. [Google Scholar] [CrossRef]
- Todo, M.; Park, S.D.; Takayama, T.; Arakawa, K. Fracture micromechanisms of bioabsorbable PLLA/PCL polymer blends. Eng. Fract. Mech. 2007, 74, 1872–1883. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Wu, D.; Lin, D.; Zhang, J.; Zhou, W.; Zhang, M.; Zhang, Y.; Wang, D.; Lin, B. Selective localization of nanofillers: Effect on morphology and crystallization of PLA/PCL blends. Macromol. Chem. Phys. 2011, 212, 613–626. [Google Scholar] [CrossRef]
- da Silva, W.A.; Luna, C.B.B.; de Melo, J.B.d.C.A.; Araújo, E.M.; Filho, E.A.d.S.; Duarte, R.N.C. Feasibility of Manufacturing Disposable Cups using PLA/PCL Composites Reinforced with Wood Powder. J. Polym. Environ. 2021, 29, 2932–2951. [Google Scholar] [CrossRef]
- Barreto Luna, C.B.; dos Santos Filho, E.A.; Siqueira, D.D.; de Souza, D.D.; Ramos Wellen, R.M.; Araújo, E.M. Jatobá wood flour: An alternative for the production of ecological and sustainable PCL biocomposites. J. Compos. Mater. 2022, 56, 3835–3850. [Google Scholar] [CrossRef]
- Filho, E.A.S.; Luna, C.B.B.; Silva, A.L.; Ferreira, E.S.B.; Araújo, E.M.; Costa, A.C.F.M. Effect of Kaolin Waste Annealing on the Structural and Thermal Behavior of Poly(Ε−Caprolactone). Momento 2022, 2022, 66–84. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lepot, N.; Van Bael, M.K.; Rul, H.V.D.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Influence of Incorporation of ZnO Nanoparticles and Biaxial Orientation on Mechanical and Oxygen Barrier Properties of Polypropylene Films for Food Packaging Applications. J. Appl. Polym. Sci. 2011, 120, 1616–1623. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Kaßner, L.; Nagel, K.; Grützner, R.E.; Korb, M.; Rüffer, T.; Lang, H.; Spange, S. Polyamide 6/silica hybrid materials by a coupled polymerization reaction. Polym. Chem. 2015, 6, 6297–6304. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, B.; Qi, N.; Zhang, H.F.; Zhang, L.Q. Influence of fillers on free volume and gas barrier properties in styrene-butadiene rubber studied by positrons. Polymer 2005, 46, 719–724. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; Sangroniz, A.; Etxeberria, A. Light and gas barrier properties of PLLA/metallic nanoparticles composite films. Eur. Polym. J. 2017, 91, 10–20. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Heidarian, P.; Behzad, T.; Sadeghi, M.; Bagheri, R. Effect of calcium carbonate nanoparticles on barrier properties and biodegradability of polylactic acid. Fibers Polym. 2017, 18, 2041–2048. [Google Scholar] [CrossRef]
- Kong, L.; Tu, K.; Guan, H.; Wang, X. Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency. Appl. Surf. Sci. 2017, 407, 479–484. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
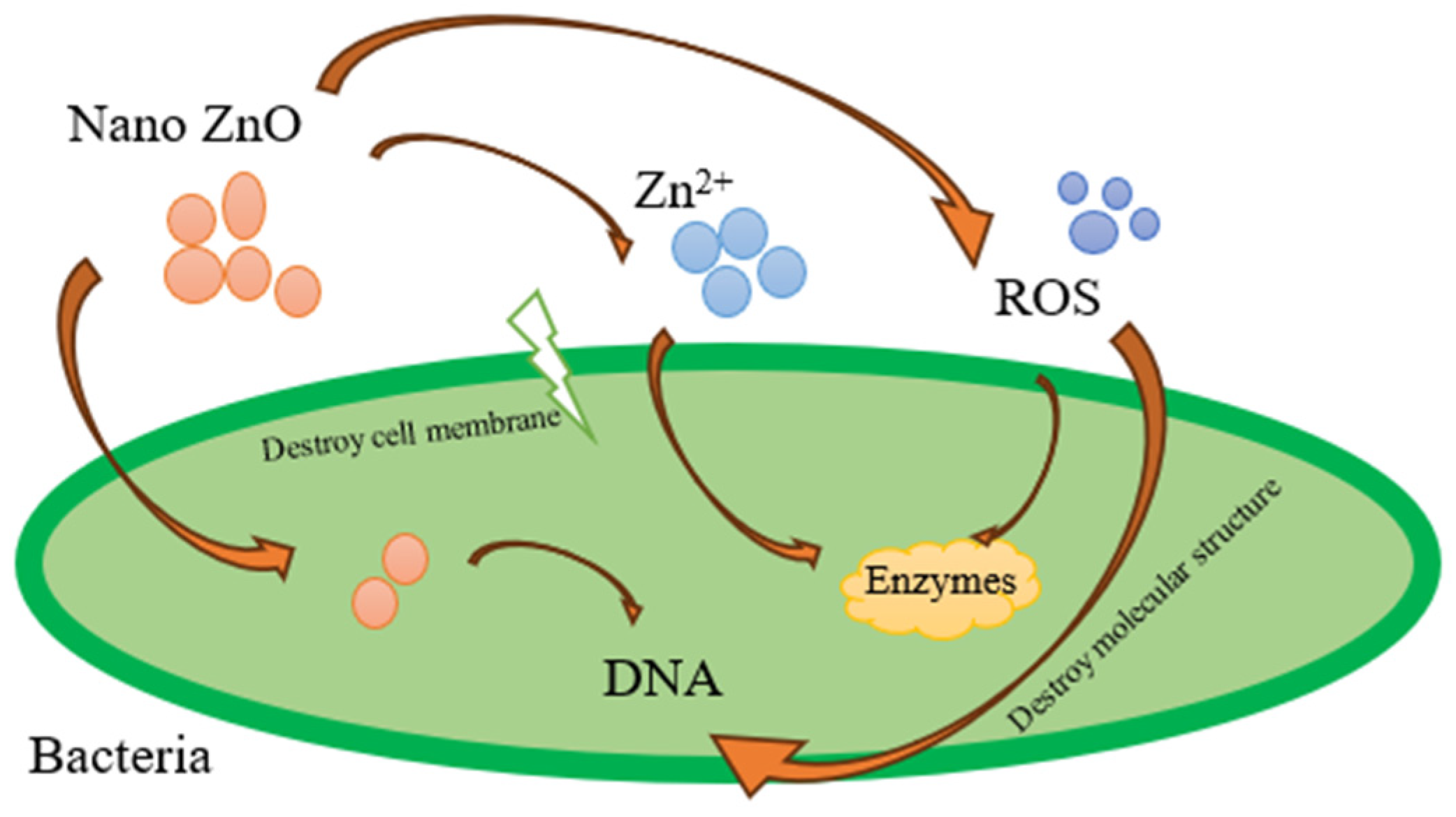
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Zhang, J. Silver-coated zinc oxide nanoantibacterial synthesis and antibacterial activity characterization. In Proceedings of the 2011 International Conference on Electronics and Optoelectronics, Dalian, China, 29–31 July 2011; IEEE: New York, NY, USA, 2011; Volume 3, pp. V3-94–V3-98. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef]
- Palomba, M.; Carotenuto, G.; Cristino, L.; Di Grazia, M.A.; Nicolais, F.; De Nicola, S. Activity of antimicrobial silver polystyrene nanocomposites. J. Nanomater. 2012, 2012, 185029. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. A versatile strategy to fabricate hydrogel-silver nanocomposites and investigation of their antimicrobial activity. J. Colloid Interface Sci. 2007, 315, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Hayat, Z.; Zhang, D.D.; Li, M.Y.; Hu, S.; Wu, Q.; Cao, Y.F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Castro, J.I.; Araujo-Rodríguez, D.G.; Valencia-Llano, C.H.; López Tenorio, D.; Saavedra, M.; Zapata, P.A.; Grande-Tovar, C.D. Biocompatibility Assessment of Polycaprolactone/Polylactic Acid/Zinc Oxide Nanoparticle Composites under In Vivo Conditions for Biomedical Applications. Pharmaceutics 2023, 15, 2196. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Paterson, T.E.; Zhang, J.; Li, Y.; Ouyang, H.; Asencio, I.O.; Hatton, P.V.; Zhao, Y.; Li, Z. Enhanced Antibacterial Ability of Electrospun PCL Scaffolds Incorporating ZnO Nanowires. Int. J. Mol. Sci. 2023, 24, 14420. [Google Scholar] [CrossRef] [PubMed]
- Makars, R.; Rizikovs, J.; Godina, D.; Paze, A.; Merijs-Meri, R. Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers 2022, 14, 2304. [Google Scholar] [CrossRef] [PubMed]
- Gumowska, A.; Kowaluk, G. Physical and Mechanical Properties of High-Density Fiberboard Bonded with Bio-Based Adhesives. Forests 2023, 14, 84. [Google Scholar] [CrossRef]
- ISO 1522; Paints and Varnishes—Pendulum Damping Test. European Committee for Standardization: Brussels, Belgium, 2022.
- EN 12720+A1; Furniture—Assessment of Surface Resistance to Cold Liquids. European Committee for Standardization: Brussels, Belgium, 2013.
- EN 717-1; Wood-Based Panels—Determination of Formaldehyde Release—Part 1: Formaldehyde Emission by the Chamber Method. European Committee for Standardization: Brussels, Belgium, 2004.
- Pavlič, M.; Petrič, M.; Žigon, J. Interactions of coating and wood flooring surface system properties. Coatings 2021, 11, 91. [Google Scholar] [CrossRef]
- Lis, B.; Krystofiak, T.P.S. Studies of the resistance upon some factors of UV acrylic lacquer coatings on MDF boards. Part I. Resistance of heat and cold liquid action. Ann. Warsaw Univ. Life Sci.—SGGW. For. Wood Technol. 2010, 71, 450–453. [Google Scholar]
- Eom, Y.-G.; Kim, J.-S.; Kim, S.; Kim, J.-A.; Kim, H.J. Reduction of Formaldehyde Emission from Particleboards by Bio Scavengers. Mokchae Konghak 2006, 34, 29–41. [Google Scholar]
- Aversa, C.; Barletta, M.; Puopolo, M.; Vesco, S. Cast extrusion of low gas permeability bioplastic sheets in PLA/PBS and PLA/PHB binary blends. Polym. Technol. Mater. 2020, 59, 231–240. [Google Scholar] [CrossRef]
- Sousa, F.M.; Cavalcanti, F.B.; Marinho, V.A.D.; Morais, D.D.S.; Almeida, T.G.; Carvalho, L.H. Effect of composition on permeability, mechanical properties and biodegradation of PBAT/PCL blends films. Polym. Bull. 2022, 79, 5327–5338. [Google Scholar] [CrossRef]
- Lykidis, C. Formaldehyde Emissions from Wood-Based Composites: Effects of Nanomaterials. In Emerging Nanomaterials: Opportunities and Challenges in Forestry Sectors; Springer International Publishing: Cham, Switzerland, 2022; pp. 337–360. [Google Scholar] [CrossRef]
- Pinchevska, O.; Lopatko, K.; Lopatko, L.; Oliynyk, R.; Sedliačik, J. The Effect of Metal Nanoparticles on Formaldehyde Emission From Wood Based Materials. Acta Fac. Xylologiae Zvolen 2023, 65, 35–43. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wu, X.; Xu, B. The influence of nano ZnO coated by phosphazene/triazine bi-group molecular on the flame retardant property and mechanical property of intumescent flame retardant poly (lactic acid) composites. Thermochim. Acta 2019, 679, 178336. [Google Scholar] [CrossRef]
- Ahmadzadeh, Y.; Babaei, A.; Goudarzi, A. Assessment of localization and degradation of ZnO nano-particles in the PLA/PCL biocompatible blend through a comprehensive rheological characterization. Polym. Degrad. Stab. 2018, 158, 136–147. [Google Scholar] [CrossRef]
- Rebholz, C.; Leyland, A.; Matthews, A.; Charitidis, C.; Logothetidis, S.; Schneider, D. Correlation of elastic modulus, hardness and density for sputtered TiAlBN thin films. Thin Solid Film. 2006, 514, 81–86. [Google Scholar] [CrossRef]
- Tien, C.-K.C.; Tzu, L. The indirect measurement of tensile strength by the deterministic grey dynamic model DGDM(1, 1, 1). Int. J. Syst. Sci. 1997, 28, 683–690. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- An, L.; Zhang, D.; Zhang, L.; Feng, G. Effect of nanoparticle size on the mechanical properties of nanoparticle assemblies. Nanoscale 2019, 11, 9563–9573. [Google Scholar] [CrossRef]
- Aydın, M.; Tozlu, H.; Kemaloglu, S.; Aytac, A.; Ozkoc, G. Effects of Alkali Treatment on the Properties of Short Flax Fiber-Poly(Lactic Acid) Eco-Composites. J. Polym. Environ. 2011, 19, 11–17. [Google Scholar] [CrossRef]
- Bužarovska, A.; Blazevska-Gilev, J.; Pérez-Martnez, B.T.; Balahura, L.R.; Pircalabioru, G.G.; Dinescu, S.; Costache, M. Poly(l-lactic acid)/alkali lignin composites: Properties, biocompatibility, cytotoxicity and antimicrobial behavior. J. Mater. Sci. 2021, 56, 13785–13800. [Google Scholar] [CrossRef]
- Gkartzou, E.; Koumoulos, E.P.; Charitidis, C.A. Production and 3D printing processing of bio-based thermoplastic filament. Manuf. Rev. 2017, 4, 1. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Kim, S.J.; Han, S.Y.; Park, J.S.; Lee, E.A.; Kwon, G.J.; Kim, Y.S.; Kim, N.H.; Lee, S.H. Effect of lignin plasticization on physico-mechanical properties of lignin/poly(lactic acid) composites. Polymers 2019, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Ya, H.H.; Hussain, P.B.; Azeem, M.; Sapuan, S.M.; Khan, R.; Ahamad, T. Experimental Investigations on the Surface Hardness of Synthesized Polystyrene/ZnO Nanocomposites. In Advances in Manufacturing Engineering; Emamian, S.S., Awang, M., Yusof, F., Eds.; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2020; pp. 345–352. [Google Scholar]
- Hadi, F.A.; Kadhim, R.G. A Study of the Effect of Nano Zinc Oxide on Cure Characteristics and Mechanical Properties of Rubber Composites. J. Phys. Conf. Ser. 2019, 1234, 012043. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do, T.V.; Ngo, T.D.; Nguyen, T.A.; Lu, L.T.; Vu, Q.T.; Thi, L.P.; Tran, D.L. Photocurable acrylate epoxy/ZnO-Ag nanocomposite coating: Fabrication, mechanical and antibacterial properties. RSC Adv. 2022, 12, 23346–23355. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, K.; Lee, Y.; Seo, J.; Seo, K.; Han, H.; Khan, S. Preparation and characterization of UV-cured polyurethane acrylate/ZnO nanocomposite films based on surface modified ZnO. Prog. Org. Coat. 2012, 74, 435–442. [Google Scholar] [CrossRef]
- González-López, M.E.; Martín del Campo, A.S.; Robledo-Ortíz, J.R.; Arellano, M.; Pérez-Fonseca, A.A. Accelerated weathering of poly(lactic acid) and its biocomposites: A review. Polym. Degrad. Stab. 2020, 179, 109290. [Google Scholar] [CrossRef]
- Soumya, S.; Kumar, S.N.; Mohamed, A.P.; Ananthakumar, S. Silanated nano ZnO hybrid embedded PMMA polymer coatings on cotton fabrics for near-IR reflective, antifungal cool-textiles. New J. Chem. 2016, 40, 7210–7221. [Google Scholar] [CrossRef]
- Gondal, M.A.; Alzahrani, A.J.; Randhawa, M.A.; Siddiqui, M.N. Morphology and antifungal effect of nano-ZnO and nano-Pd-doped nano-ZnO against Aspergillus and Candida. J. Environ. Sci. Heal.—Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1413–1418. [Google Scholar] [CrossRef]
- Sirin, H.; Tuna, B.; Ozkoc, G. The Effects of Thermomechanical Cycles on the Properties of PLA/TPS Blends. Adv. Polym. Technol. 2014, 33, 1–9. [Google Scholar] [CrossRef]
- Yarahmadi, N.; Jakubowicz, I.; Enebro, J. Polylactic acid and its blends with petroleum-based resins: Effects of reprocessing and recycling on properties. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Budin, S.; Maideen, N.C.; Koay, M.H.; Ibrahim, D.; Yusoff, H. A comparison study on mechanical properties of virgin and recycled polylactic acid (PLA). J. Phys. Conf. Ser. 2019, 1349, 012002. [Google Scholar] [CrossRef]
- Archodoulaki, V.M.; Lüftl, S.; Koch, T.; Seidler, S. Property changes in polyoxymethylene (POM) resulting from processing, ageing and recycling. Polym. Degrad. Stab. 2007, 92, 2181–2189. [Google Scholar] [CrossRef]
- Smorawska, J.; Włoch, M.; Głowińska, E. Structure–Property Relationship and Multiple Processing Studies of Novel Bio-Based Thermoplastic Polyurethane Elastomers. Materials 2023, 16, 6246. [Google Scholar] [CrossRef] [PubMed]
- da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- El Bhilat, H.; El Had, K.; Salmi, H.; Hachim, A. Thermo-mechanical characterization of post-consumer recycled high impact polystyrene from disposable cups: Influence of the number of processing cycles. J. Comput. Appl. Res. Mech. Eng. 2021, 10, 427–436. [Google Scholar] [CrossRef]




| Variant Label | Matrix | SAR Filler Content (w/w of Dry Matter) | Nano-ZnO Content (w/w of Dry Matter) |
|---|---|---|---|
| PLA | PLA | 0 | 0 |
| PLA nano 2% | 0 | 2 | |
| PLA nano 4% | 0 | 4 | |
| PLA/SAR | 10 | 0 | |
| PLA/SAR nano 2% | 10 | 2 | |
| PLA/SAR nano 4% | 10 | 4 | |
| PCL | PCL | 0 | 0 |
| PCL nano 2% | 0 | 2 | |
| PCL nano 4% | 0 | 4 | |
| PCL/SAR | 10 | 0 | |
| PCL/SAR nano 2% | 10 | 2 | |
| PCL/SAR nano 4% | 10 | 4 |
| Variant Label | Acetone | Citric Acid | Ethanol | Distilled Water |
|---|---|---|---|---|
| mg m−3 | ||||
| PLA | A | B | A | B |
| PLA nano 2% | B | C | C | B |
| PLA nano 4% | C | C | B | A |
| PLA/SAR | B | B | A | A |
| PLA/SAR nano 2% | C | C | B | C |
| PLA/SAR nano 4% | B | B | C | C |
| PCL | A | A | A | A |
| PCL nano 2% | A | A | A | A |
| PCL nano 4% | A | A | B | A |
| PCL/SAR | A | A | A | A |
| PCL/SAR nano 2% | A | A | A | A |
| PCL/SAR nano 4% | A | A | A | A |
| Variant Label | TVOC | HCHO |
|---|---|---|
| mg m−3 | ||
| REF | 0.245 | 0.044 |
| PLA | 0.220 | 0.039 |
| PLA nano 2% | 0.172 | 0.030 |
| PLA nano 4% | 0.179 | 0.031 |
| PLA/SAR | 0.222 | 0.041 |
| PLA/SAR nano 2% | 0.218 | 0.039 |
| PLA/SAR nano 4% | 0.216 | 0.040 |
| PCL | 0.264 | 0.045 |
| PCL nano 2% | 0.068 | 0.012 |
| PCL nano 4% | 0.065 | 0.012 |
| PCL/SAR | 0.080 | 0.014 |
| PCL/SAR nano 2% | 0.078 | 0.013 |
| PCL/SAR nano 4% | 0.077 | 0.016 |
| Variant Label | Hardness Decreases after the Second Pressing |
|---|---|
| PLA | 34.60% |
| PLA nano 2% | 23.72% |
| PLA nano 4% | 26.14% |
| PLA/SAR | 16.57% |
| PLA/SAR nano 2% | 24.62% |
| PLA/SAR nano 4% | 23.32% |
| PCL | −3.81% |
| PCL nano 2% | 13.28% |
| PCL nano 4% | 15.49% |
| PCL/SAR | −5.00% |
| PCL/SAR nano 2% | 24.66% |
| PCL/SAR nano 4% | 20.73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżo, A.; Poohphajai, F.; Herrera Diaz, R.; Kowaluk, G. Incorporation of Nano-Zinc Oxide as a Strategy to Improve the Barrier Properties of Biopolymer–Suberinic Acid Residues Films: A Preliminary Study. Materials 2024, 17, 3868. https://doi.org/10.3390/ma17153868
Jeżo A, Poohphajai F, Herrera Diaz R, Kowaluk G. Incorporation of Nano-Zinc Oxide as a Strategy to Improve the Barrier Properties of Biopolymer–Suberinic Acid Residues Films: A Preliminary Study. Materials. 2024; 17(15):3868. https://doi.org/10.3390/ma17153868
Chicago/Turabian StyleJeżo, Aleksandra, Faksawat Poohphajai, Rene Herrera Diaz, and Grzegorz Kowaluk. 2024. "Incorporation of Nano-Zinc Oxide as a Strategy to Improve the Barrier Properties of Biopolymer–Suberinic Acid Residues Films: A Preliminary Study" Materials 17, no. 15: 3868. https://doi.org/10.3390/ma17153868
APA StyleJeżo, A., Poohphajai, F., Herrera Diaz, R., & Kowaluk, G. (2024). Incorporation of Nano-Zinc Oxide as a Strategy to Improve the Barrier Properties of Biopolymer–Suberinic Acid Residues Films: A Preliminary Study. Materials, 17(15), 3868. https://doi.org/10.3390/ma17153868








