Low-Cost and Eco-Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations
2.2. Characterizations
CaCO3 (s) → CaO (s) + CO2 (g)
Ca(CH3COO)2·H2O (s) → CaO (s) + CO2 (g) + 2CH3COO (g) + H2O (g)
3. Results and Discussion
3.1. CaO Production Results
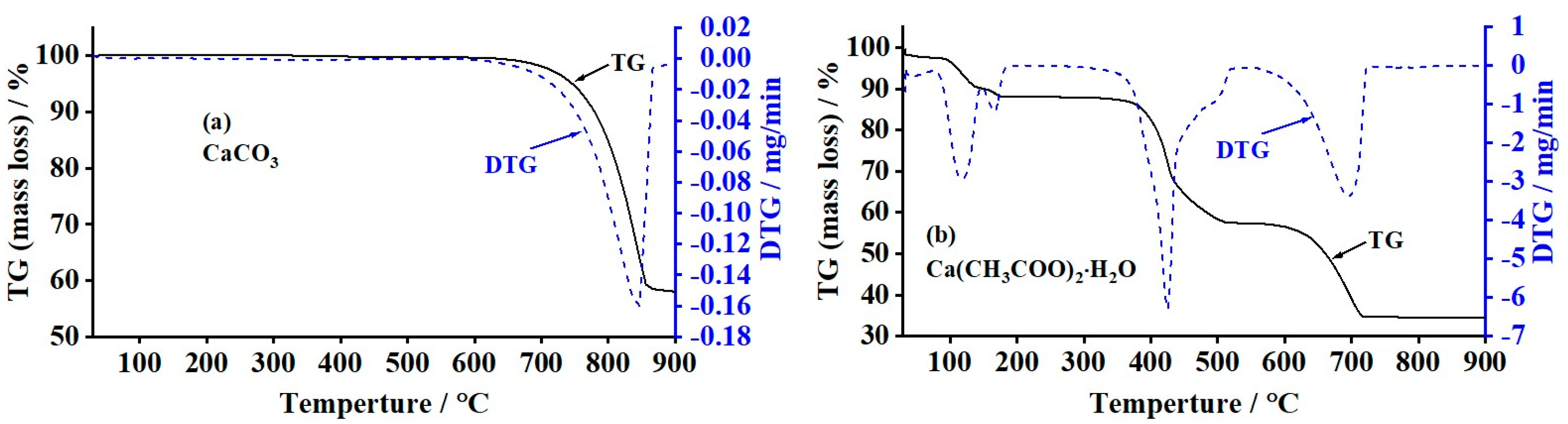
3.2. Thermogravimetric (TGA) Analysis
3.3. X-ray Fluorescence (XRF) Results
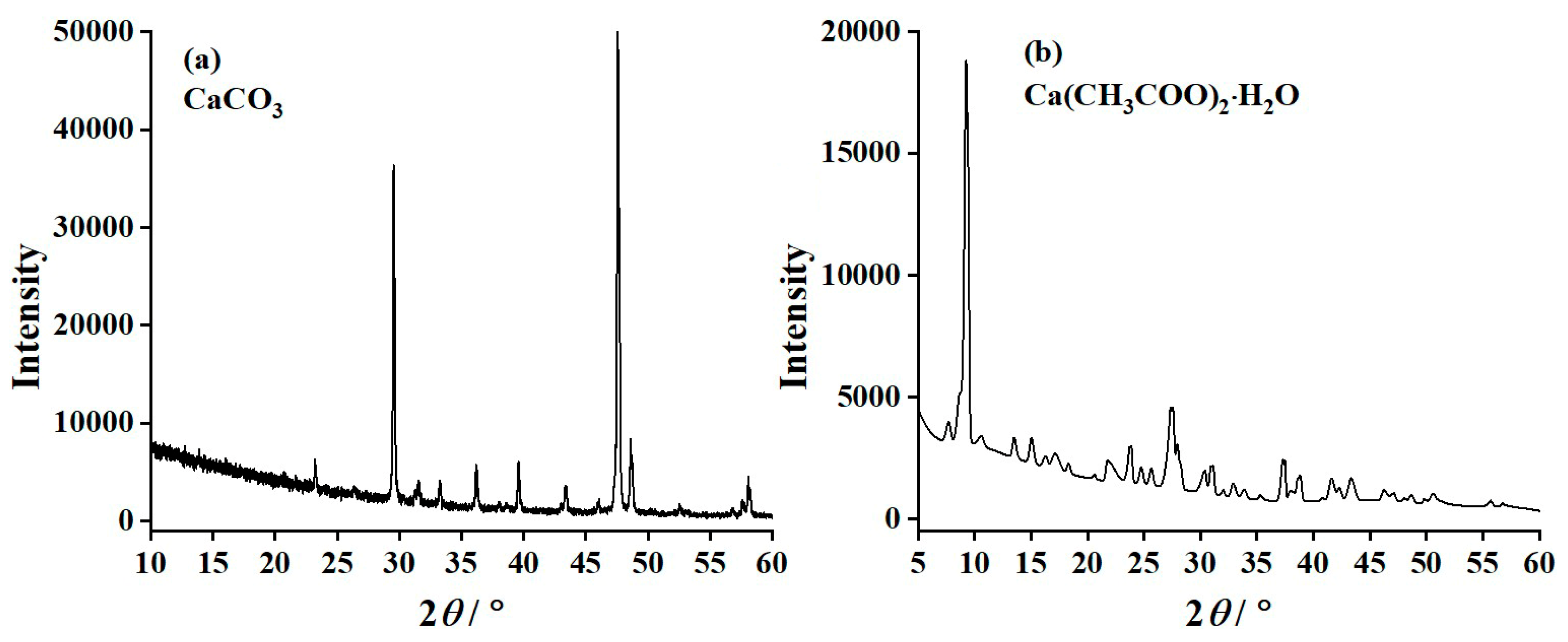
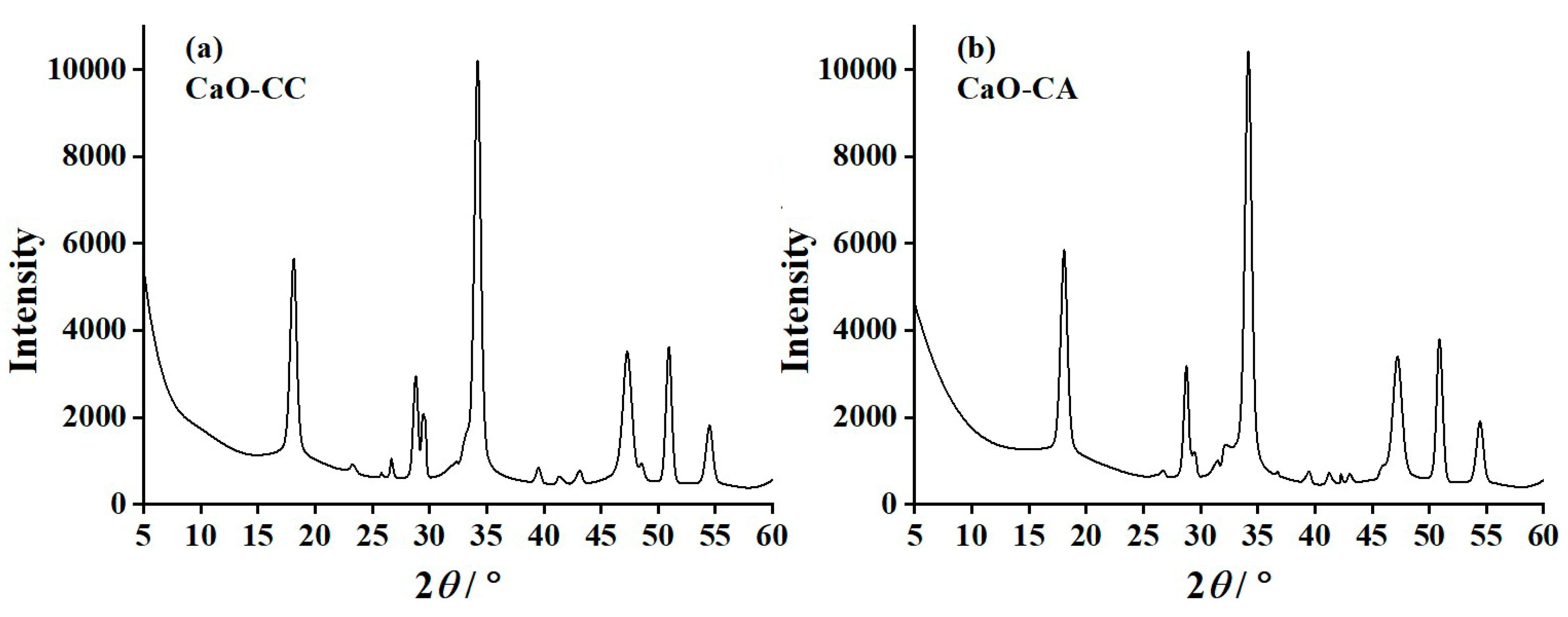
3.4. X-ray Diffraction (XRD) Results
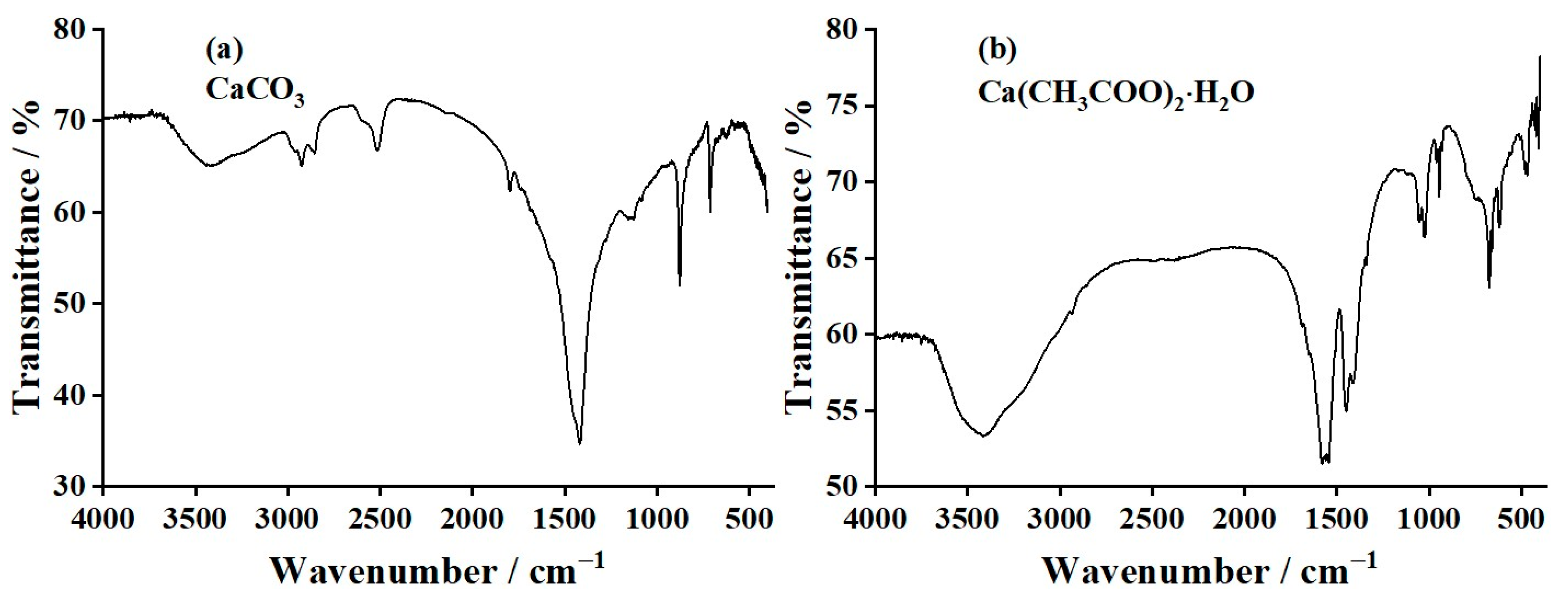
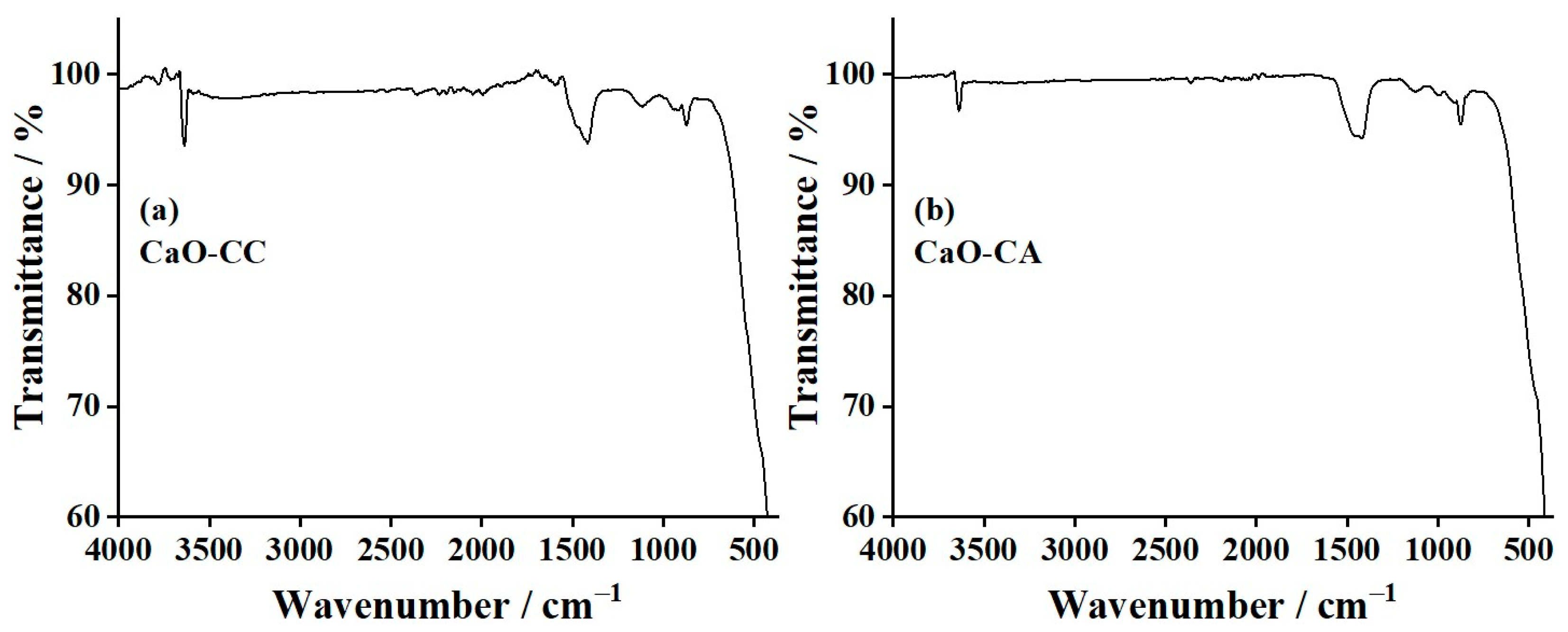
3.5. Fourier-Transform Infrared (FTIR) Results
3.6. The Scanning Electron Microscope (SEM) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fishery Statistics Group. Statistics of Marine Shellfish Culture Survey 2019; Department of Fisheries, Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2020.
- Santos, C.R.d.L.; Rodríguez, A.S.L.; Gallardo, P.S.; Rivera, M.A.H.; Pérez-Hernández, G.; Romo, M.G.G.; Trejo, J.G.F.R.; Flores, L.L.D. Effect of the thermal annealing on the phase transitions of biogenic CaCO3 nanostructures. J. Mex. Chem. Soc. 2019, 63, 1–12. [Google Scholar]
- Onoda, H.; Nakanishi, H. Preparation of calcium phosphate with oyster shells. Nat. Resour. 2012, 3, 71. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, Y.-N.; Ho, W.-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Mater. Charact. 2011, 62, 1180–1187. [Google Scholar] [CrossRef]
- Wang, T.S.; Xiao, D.C.; Huang, C.H.; Hsieh, Y.K.; Tan, C.S.; Wang, C.F. CO2 uptake performance and life cycle assessment of CaO-based sorbents prepared from waste oyster shells blended with PMMA nanosphere scaffolds. J. Hazard. Mater. 2014, 270, 92–101. [Google Scholar] [CrossRef]
- Teixeira, L.; Fernandes, V.; Maia, B.; Arcaro, S.; de Oliveira, A.N. Vitrocrystalline foams produced from glass and oyster shell wastes. Ceram. Int. 2017, 43, 6730–6737. [Google Scholar] [CrossRef]
- Ok, Y.S.; Lim, J.E.; Moon, D.H. Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ. Geochem. Health 2011, 33, 83–91. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Lv, Z.; Xie, R.; Huang, L.; Jiang, J. Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef]
- de Alvarenga, R.A.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Jung, Y.C.; Bae, S.K. A study on the properties of cement mortar with modified oyster shell powder. J. Korean Ceram. Soc. 2001, 38, 231–237. [Google Scholar]
- Choi, H.-G.; Jeong, I.-H.; Bae, G.-R.; Park, J.-Y.; Lee, J.-J.; Kim, Y.-W.; Jung, K.-S.; Kim, S.-H. A proposition for the removal of algae and phosphorus from river water using multi-purpose filtration pond. J. Korean Soc. Environ. Eng. 2013, 35, 525–531. [Google Scholar] [CrossRef]
- Lee, C.-W.; Jeon, H.-P.; Kwon, H.-B. Physical properties of pyrolized oyster shell consisting of porous CaO/CaCO3 and phosphorus removal efficiency. J. Korean Ceram. Soc. 2010, 47, 524–528. [Google Scholar] [CrossRef]
- Kwon, H.-B.; Lee, C.-W.; Jun, B.-S.; Yun, J.-D.; Weon, S.-Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, X.; Li, Z.; Zheng, S.; Wang, Z. Effects of rotation speed and media density on particle size distribution and structure of ground calcium carbonate in a planetary ball mill. Adv. Powder Technol. 2015, 26, 505–510. [Google Scholar] [CrossRef]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Lee, C.; Kwon, H.; Jeon, H.; Koopman, B. A new recycling material for removing phosphorus from water. J. Clean. Prod. 2009, 17, 683–687. [Google Scholar] [CrossRef]
- De los Santos, C.R.; Barajas, F.; Pérez, H.; Hernández, R.; Díaz, F. Adsorption of copper (II) and cadmium (II) in aqueous suspensions of biogenic nanostructured CaCO3. Bol. Soc. Esp. Ceram. Vidr. (Internet) 2019, 58, 2–13. [Google Scholar] [CrossRef]
- Chiou, I.; Chen, C.; Li, Y. Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Constr. Build. Mater. 2014, 64, 480–487. [Google Scholar] [CrossRef]
- Huh, J.-H.; Choi, Y.-H.; Ramakrishna, C.; Cheong, S.H.; Ahn, J.W. Use of calcined oyster shell powders as CO2 adsorbents in algae-containing water. J. Korean Ceram. Soc. 2016, 53, 429–434. [Google Scholar] [CrossRef]
- Khan, M.D.; Ahn, J.W.; Nam, G. Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. J. Environ. Manag. 2018, 223, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Bette, S.; Eggert, G.; Emmerling, S.; Etter, M.; Schleid, T.; Dinnebier, R.E. Crystal structure, polymorphism, and anisotropic thermal expansion of α-Ca(CH3COO)2. Cryst. Growth Des. 2020, 20, 5346–5355. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Commons, C.J.; Hudson, T.A.; Arlt, R.W.S. The elusive crystals of calcium acetate hemihydrate: Chiral rods linked by parallel hydrophilic strips. CrystEngComm 2021, 23, 707–713. [Google Scholar] [CrossRef]
- Nobre, L.C.; Santos, S.; Palavra, A.M.; Calvete, M.J.; de Castro, C.A.N.; Nobre, B.P. Supercritical antisolvent precipitation of calcium acetate from eggshells. J. Supercrit. Fluids 2020, 163, 104862. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Kim, S. Quality characteristics of calcium acetate prepared with vinegars and ash of black snail. J. Korean Soc. Food Sci. Nutr. 2004, 33, 593–597. [Google Scholar]
- Park, S.H.; Jang, S.J.; Lee, H.J.; Lee, G.-W.; Lee, J.K.; Kim, Y.J.; Kim, J.-S.; Heu, M.S. Optimization of calcium acetate preparation from littleneck clam (Ruditapes philippinarum) shell powder and its properties. Korean J. Food Sci. Technol. 2015, 47, 321–327. [Google Scholar] [CrossRef]
- Ziraman, D.U.; Doğan, Ö.M.; Uysal, B.Z. Kinetics of chemical absorption of carbon dioxide into aqueous calcium acetate solution. Int. J. Chem. Kinet. 2020, 52, 251–265. [Google Scholar] [CrossRef]
- Steciak, J.; Levendis, Y.A.; Wise, D.L. Effectiveness of calcium magnesium acetate as dual SO2-NOx emission control agent. AIChE J. 1995, 41, 712–722. [Google Scholar] [CrossRef]
- Chung, K.-H.; Jung, S.-C.; Park, B.-G. Eco-friendly deicer prepared from waste oyster shells and its deicing properties with metal corrosion. Environ. Technol. 2021, 42, 3360–3368. [Google Scholar] [CrossRef]
- Nolan, C.R.; Qunibi, W.Y. Calcium salts in the treatment of hyperphosphatemia in hemodialysis patients. Curr. Opin. Nephrol. Hypertens. 2003, 12, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Price, N.; Fei, T.; Clark, S.; Wang, T. Application of zinc and calcium acetate to precipitate milk fat globule membrane components from a dairy by-product. J. Dairy Sci. 2020, 103, 1303–1314. [Google Scholar] [CrossRef]
- Safronova, T.; Mukhin, E.; Putlyaev, V.; Knotko, A.; Evdokimov, P.; Shatalova, T.; Filippov, Y.; Sidorov, A.; Karpushkin, E. Amorphous calcium phosphate powder synthesized from calcium acetate and polyphosphoric acid for bioceramics application. Ceram. Int. 2017, 43, 1310–1317. [Google Scholar] [CrossRef]
- Abbas, M.N.; Ibrahim, S.A.; Abbas, Z.N.; Ibrahim, T.A. Eggshells as a sustainable source for acetone production. J. King Saud Univ.-Eng. Sci. 2021, 34, 381–387. [Google Scholar] [CrossRef]
- Omran, K.; Mostafa, M.; El-Sadek, M.A.; Hemeda, O.; Ubic, R. Effects of Ca doping on structural and optical properties of PZT nanopowders. Results Phys. 2020, 19, 103580. [Google Scholar] [CrossRef]
- Chen, S.; Fu, J.; He, X.; Su, Y.; Xiong, G.; Liu, Q.; Huang, Z.; Jiang, Y. Microemulsion synthesis of anhydrous calcium sulfate nanowhiskers with calcium acetate solution and its surface structure stable and crystal phase evolution after modification. J. Nanoparticle Res. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Cao, K.; Wang, L.; Xu, Y.; Shen, W.; Wang, H. The hydration and compressive strength of cement mortar prepared by calcium acetate solution. Adv. Civ. Eng. 2021, 2021, 8817725. [Google Scholar] [CrossRef]
- Borchert, R. Calcium acetate induces calcium uptake and formation of calcium-oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 1986, 168, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A. Carbonation of limestone derived CaO for thermochemical energy storage: From kinetics to process integration in concentrating solar plants. ACS Sustain. Chem. Eng. 2018, 6, 6404–6417. [Google Scholar] [CrossRef]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Sronsri, C.; Sittipol, W.; U-yen, K. Performance of CaO catalyst prepared from magnetic-derived CaCO3 for biodiesel production. Fuel 2021, 304, 121419. [Google Scholar] [CrossRef]
- Sun, H.; Wu, C.; Shen, B.; Zhang, X.; Zhang, Y.; Huang, J. Progress in the development and application of CaO-based adsorbents for CO2 capture—A review. Mater. Today Sustain. 2018, 1, 1–27. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Kunanuruksapong, R.; Sirivat, A. Preparation and properties of calcium oxide from eggshells via calcination. Mater. Sci.-Pol. 2012, 30, 313–322. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; U-yen, K. Optimization of biodiesel production using magnesium pyrophosphate. Chem. Eng. Sci. 2020, 226, 115884. [Google Scholar] [CrossRef]
- Wong-Ng, W.; McMurdie, H.F.; Hubbard, C.R.; Mighell, A.D. JCPDS-ICDD research associateship (cooperative program with NBS/NIST). J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1013. [Google Scholar] [CrossRef]
- Sronsri, C.; U-yen, K.; Sittipol, W. Application of synthetic hureaulite as a new precursor for the synthesis of lithiophilite nanoparticles. Solid State Sci. 2020, 110, 106469. [Google Scholar] [CrossRef]
- Sronsri, C.; U-yen, K.; Sittipol, W. Quantitative analysis of calcium carbonate formation in magnetized water. Mater. Chem. Phys. 2020, 245, 122735. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; Van Grieken, R. Sample preparation for X-ray fluorescence analysis. Encycl. Anal. Chem. Appl. Theory Instrum. 2006, 1–25. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; U-yen, K. Luminescence characterization of Mn-doped LiMgPO4 synthesized using different precursors. J. Solid State Chem. 2021, 297, 122083. [Google Scholar] [CrossRef]
- Aqliliriana, C.; Ernee, N.; Irmawati, R. Preparation and characterization of modified calcium oxide from natural sources and their application in the transesterification of palm oil. Int. J. Sci. Technol. Res. 2015, 4, 168–175. [Google Scholar]
- Sronsri, C.; Boonchom, B. Thermal kinetic analysis of a complex process from a solid-state reaction by deconvolution procedure from a new calculation method and related thermodynamic functions of Mn0.90Co0.05Mg0.05HPO4·3H2O. Trans. Nonferrous Met. Soc. China 2018, 28, 1887–1902. [Google Scholar] [CrossRef]
- Sronsri, C.; Boonchom, B. Determination of thermokinetic parameters and thermodynamic functions from thermoforming of LiMnPO4. J. Therm. Anal. Calorim. 2018, 134, 1575–1587. [Google Scholar] [CrossRef]
- Rice, F.; Vollrath, R. The thermal decomposition of acetone in the gaseous state. Proc. Natl. Acad. Sci. USA 1929, 15, 702–705. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, D. Study on oral ulcer powder using temperature-dependent X-ray diffraction technique. Top. Chem. Mater. Eng 2018, 1, 104–106. [Google Scholar]
- Sitepu, H.; Al-Ghamdi, R.A. Quantitative phase analysis of XRD data of sludge deposits from refineries and gas plants by use of the rietveld method. Adv. X-ray Anal. 2019, 62, 45–57. [Google Scholar]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Dionysiou, D.; Tsianou, M.; Botsaris, G. Extractive crystallization for the production of calcium acetate and magnesium acetate from carbonate sources. Ind. Eng. Chem. Res. 2000, 39, 4192–4202. [Google Scholar] [CrossRef]
- Klop, E.A.; Schouten, A.; Van der Sluis, P.; Spek, A.L. Structure of calcium acetate monohydrate, Ca(C2H3O2)2·H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, 40, 51–53. [Google Scholar] [CrossRef]
- van der Sluis, P.; Schouten, A.; Spek, A. Structure of a second polymorph of calcium acetate monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1922–1924. [Google Scholar] [CrossRef]
- Garduño-Pineda, L.; Linares-Hernández, I.; Solache-Ríos, M.J.; Teutli-Sequeira, A.; Martínez-Miranda, V. Removal of inorganic chemical species and organic matter from slaughterhouse wastewater via calcium acetate synthesized from eggshell. J. Environ. Sci. Health Part A 2019, 54, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, R.; Lin, J.; Huang, C. Phase stability study of La1.2Ca1.8Mn2O7. Mater. Res. Bull. 2001, 36, 1139–1148. [Google Scholar] [CrossRef]
- Chanda, D.K.; Khan, P.; Dey, N.; Majumder, M.; Chakraborty, A.K.; Jha, B.B.; Ghosh, J. Synthesis of calcium based nano powders for application in conservation and restoration of heritage mortar. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Seesanong, S.; Laosinwattana, C.; Boonchom, B. Microparticles of calcium carbonate CaCO3, calcium hydrogen phosphate hydrate CaHPO4·1.9H2O and tricalcium phosphate Ca3(PO4)2 prepared from golden apple snail shells (Pomacea canaliculata). Res. J. Chem. Env. 2020, 24, 1–6. [Google Scholar]
- Seesanong, S.; Laosinwattana, C.; Chaiseeda, K.; Boonchom, B. A simple and rapid transformation of golden apple snail (Pomacea canaliculata) shells to calcium carbonate, monocalcium and tricalcium phosphates. Asian J. Chem. 2019, 31, 2522–2526. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013, 460923. [Google Scholar] [CrossRef] [PubMed]
- Linggawati, A. Preparation and Characterization of Calcium Oxide Heterogeneous Catalyst Derived from Anadara granosa Shell for Biodiesel Synthesis; KnE Engineering: Singapore, 2016. [Google Scholar]

| Chemical Compositions | Chemical Contents/wt% | |||
|---|---|---|---|---|
| CaO-CC | CaO-CA | Differences | ||
| Calcium oxide | CaO | 87.80 | 91.50 | 3.70 |
| Sodium oxide | Na2O | 0.83 | 0.38 | 0.45 |
| Magnesium oxide | MgO | 2.08 | 1.46 | 0.62 |
| Aluminium oxide | Al2O3 | 1.10 | 0.75 | 0.35 |
| Silicon dioxide | SiO2 | 4.58 | 2.86 | 1.72 |
| Phosphorus oxide | P2O5 | 0.21 | 0.19 | 0.02 |
| Sulfur dioxide | SO3 | 1.10 | 0.97 | 0.13 |
| Chlorine | Cl | 0.79 | 0.61 | 0.18 |
| Potassium oxide | K2O | 0.21 | 0.16 | 0.05 |
| Titanium dioxide | TiO2 | 0.10 | 0.08 | 0.01 |
| Manganese oxide | MnO | 0.06 | 0.05 | 0.01 |
| Ferric oxide | Fe2O3 | 0.81 | 0.65 | 0.16 |
| Cupric oxide | CuO | 0.01 | 0.01 | - |
| Zinc oxide | ZnO | 0.01 | 0.01 | - |
| Bromine | Br | 0.01 | 0.01 | - |
| Strontium oxide | SrO | 0.30 | 0.31 | 0.01 |
| Total impurities | 12.1639 | 8.52 | ||
| Total | 100.00 | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Boonmee, W.; Thompho, S.; Rungrojchaipon, P. Low-Cost and Eco-Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells. Materials 2024, 17, 3875. https://doi.org/10.3390/ma17153875
Seesanong S, Seangarun C, Boonchom B, Laohavisuti N, Boonmee W, Thompho S, Rungrojchaipon P. Low-Cost and Eco-Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells. Materials. 2024; 17(15):3875. https://doi.org/10.3390/ma17153875
Chicago/Turabian StyleSeesanong, Somkiat, Chaowared Seangarun, Banjong Boonchom, Nongnuch Laohavisuti, Wimonmat Boonmee, Somphob Thompho, and Pesak Rungrojchaipon. 2024. "Low-Cost and Eco-Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells" Materials 17, no. 15: 3875. https://doi.org/10.3390/ma17153875
APA StyleSeesanong, S., Seangarun, C., Boonchom, B., Laohavisuti, N., Boonmee, W., Thompho, S., & Rungrojchaipon, P. (2024). Low-Cost and Eco-Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells. Materials, 17(15), 3875. https://doi.org/10.3390/ma17153875






