Hybrid Nanoparticles Based on Mesoporous Silica and Functionalized Biopolymers as Drug Carriers for Chemotherapeutic Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of MSNs
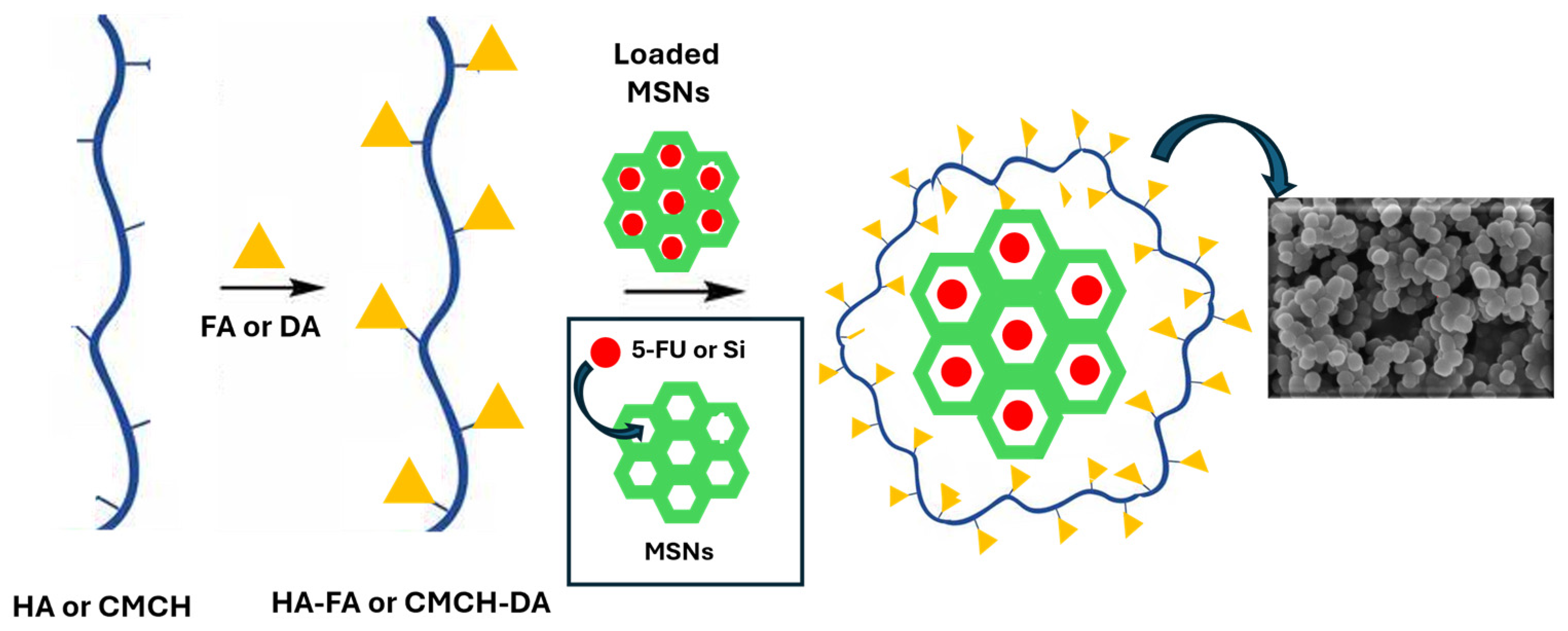
2.4. Synthesis and Coating of MSNs with Active Substances
2.5. Characterization of Nanoparticles
2.6. Drug Entrapping Profile
2.7. In Vitro Release Profile
2.8. Antitumor Activity
2.8.1. Cell Culture
2.8.2. MTT Assay
2.9. Statistical Analysis
3. Results
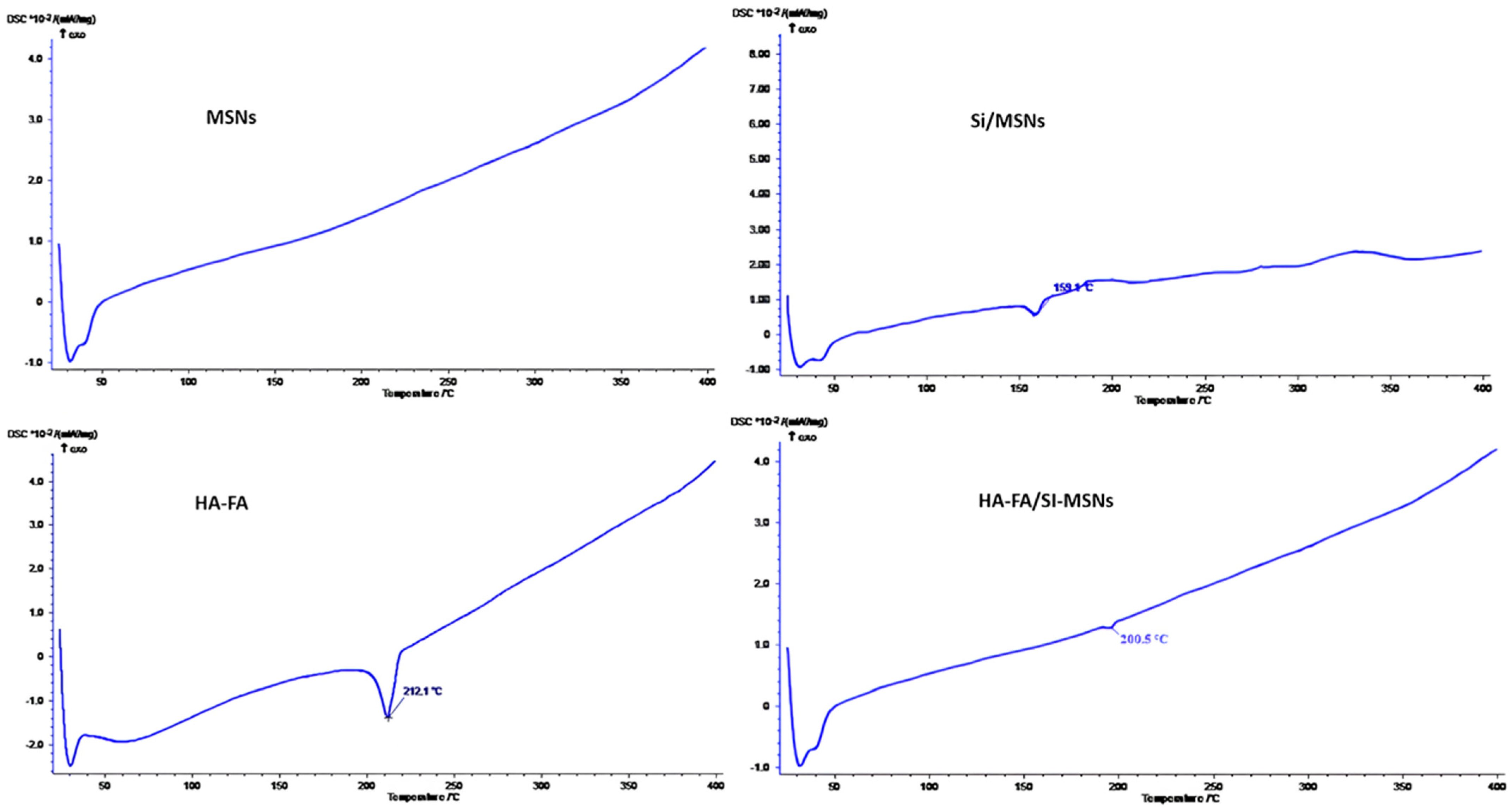
3.1. Preparation and Characterization of Coated MSNs
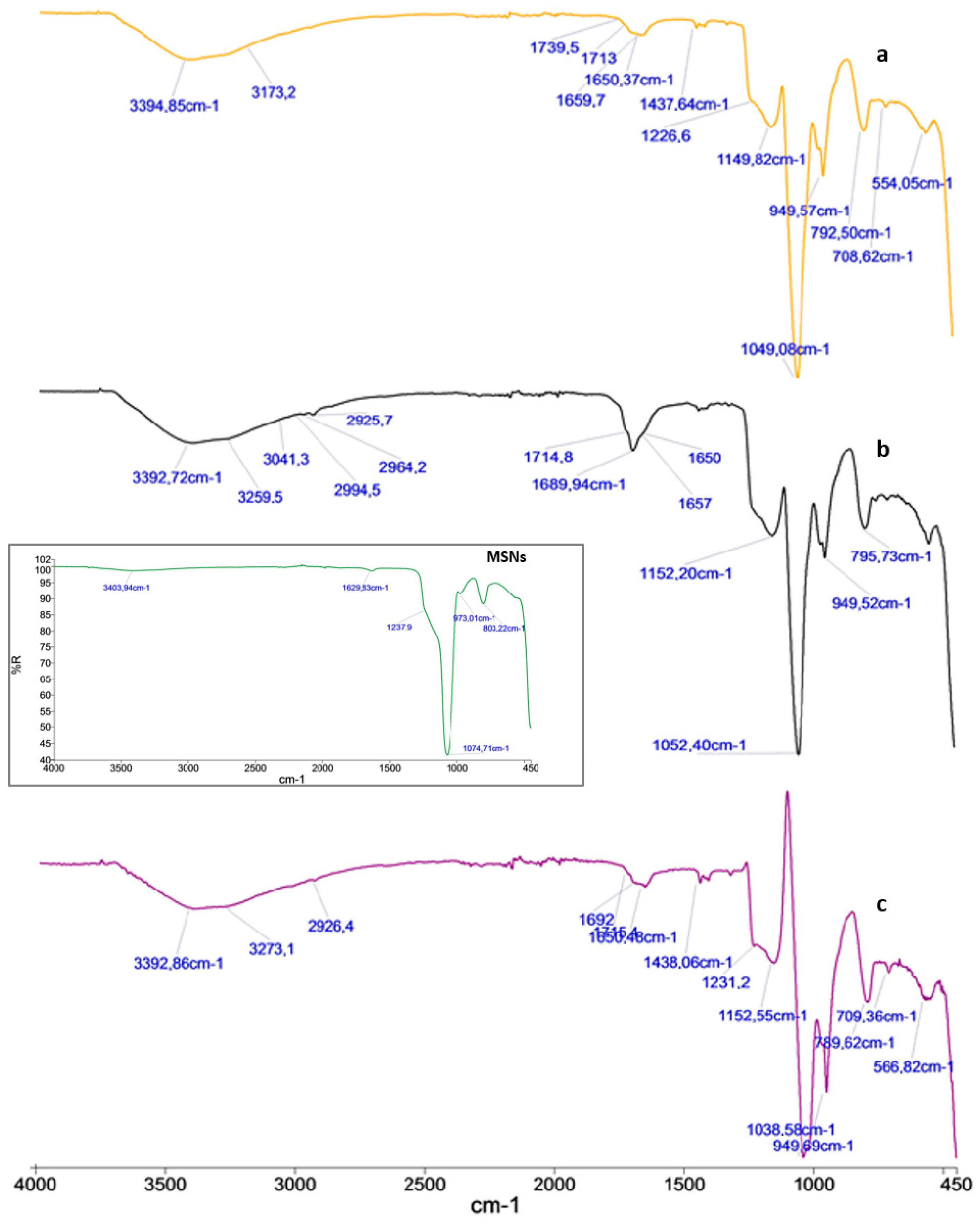
3.2. FT-IR Spectra
3.3. Morphological Analysis of MSNs
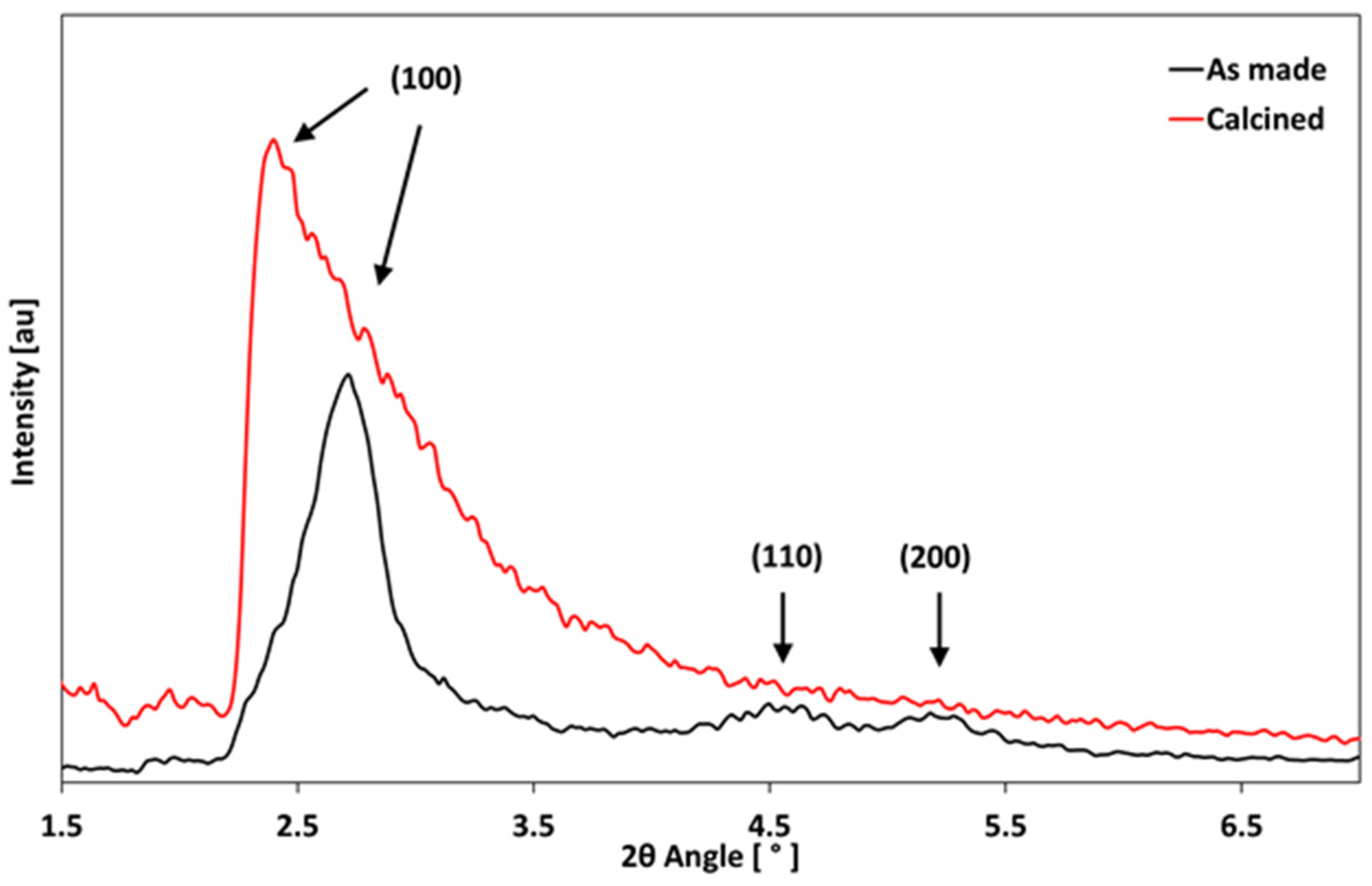
3.4. XRD Analysis
3.5. Drug Entrapping Profile
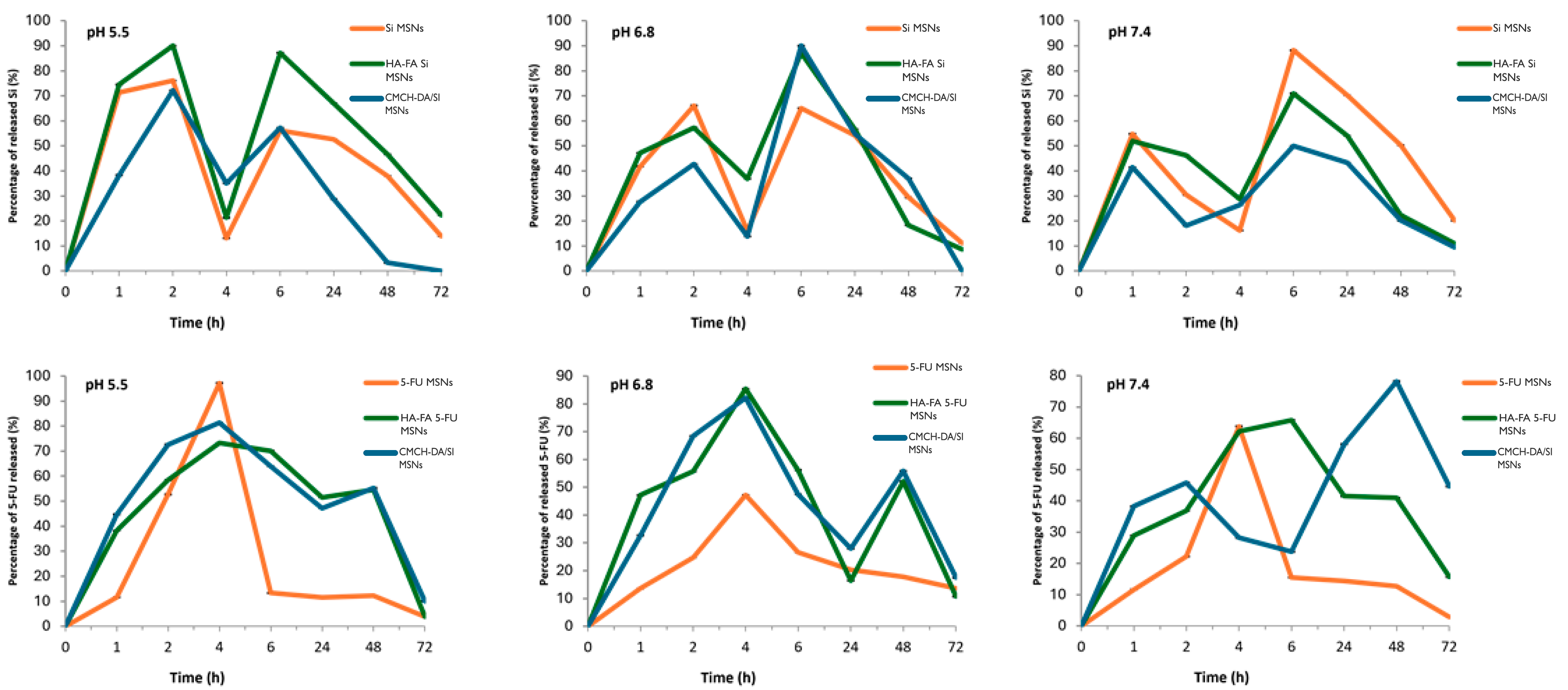
3.6. In Vitro Drug Release
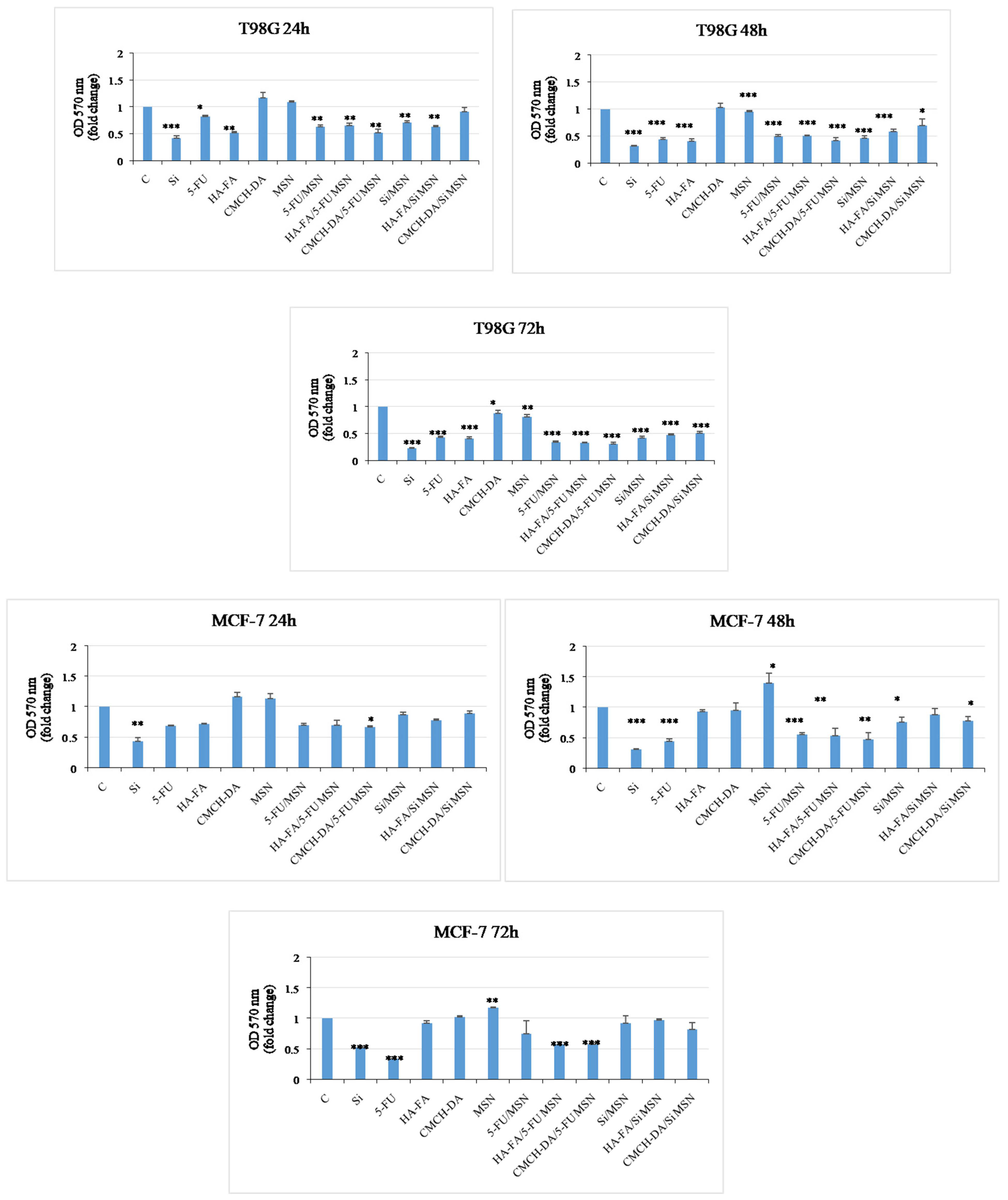
3.7. Antitumor Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kankala, R.K.; Han, Y.H.; Xia, H.Y.; Wang, S.B.; Chen, A.Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; McNally, L.R.; et al. Mesoporous silica nanoparticles: Properties and strategies for enhancing clinical effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Zhang, Z.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Downing, M.A.; Jain, P.K. Mesoporous silica nanoparticles: Synthesis, properties, and biomedical applications. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–281. [Google Scholar]
- Chen, L.; Liu, M.; Zhou, Q.; Li, X. Recent developments of mesoporous silica nanoparticles in biomedicine. Emergent Mater. 2020, 3, 381–405. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.W.; Guan, Y.Y.; Jiang, Y.X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.H.; Na, J.; Lee, C.H.; Sun, Z.; Wang, S.B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Wu, K.C.W.; et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Abdo, G.G.; Zagho, M.M.; Khalil, A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent Mater. 2020, 3, 407–425. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, Q.; Wang, Y.; Hu, P.; Zhong, W.; Huang, C.B.; Yu, Z.-Q.; Ding, C.-D.; Liu, H.; Fu, J. Chemically engineered mesoporous silica nanoparticles-based intelligent delivery systems for theranostic applications in multiple cancerous/non-cancerous diseases. Coord. Chem. Rev. 2022, 452, 214309. [Google Scholar] [CrossRef]
- Guo, F.; Li, G.; Zhou, H.; Ma, S.; Guo, L.; Liu, X. Temperature and H2O2-operated nano-valves on mesoporous silica nanoparticles for controlled drug release and kinetics. Colloids Surf. B Biointerfaces 2020, 187, 110643. [Google Scholar] [CrossRef]
- Wagner, J.; Gößl, D.; Ustyanovska, N.; Xiong, M.; Hauser, D.; Zhuzhgova, O.; Hocevar, S.; Taskoparan, B.; Poller, L.; Bourquin, C.; et al. Mesoporous silica nanoparticles as pH-responsive carrier for the immune-activating drug resiquimod enhance the local immune response in mice. ACS Nano 2021, 15, 4450–4466. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Redox-responsive mesoporous silica nanoparticles for cancer treatment: Recent updates. Nanomaterials 2021, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Moodley, T.; Singh, M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of mesoporous silica nanoparticles in cancer therapy and delivery of repurposed anthelmintics for cancer therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased Dopamine And Its Receptor Dopamine Receptor D1 Promote Tumor Growth In Human Hepatocellular Carcinoma. Cancer Commun. 2020, 12, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Al-Serwi, R.H.; Eladl, M.A.; El-Sherbiny, M.; Saleh, M.A.; Othman, G.; Alshahrani, S.M.; Alnefaie, R.; Jan, A.M.; Alnasser, S.M.; Menaa, F.; et al. Targeted drug administration onto cancer cells using hyaluronic acid–quercetin-conjugated silver nanoparticles. Molecules 2023, 28, 4146. [Google Scholar] [CrossRef]
- Altememy, D.; Khoobi, M.; Javar, H.A.; Alsamarrai, S.; Khosravian, P. Synthesis and Characterization of Silk Fibroin-Coated Mesoporous Silica Nanoparticles for Tioguanine Targeting to Leukemia. Int. J. Pharm. Res. 2020, 12, 1181. [Google Scholar]
- Cassano, R.; Trapani, A.; Di Gioia, M.L.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di Gioia, S.; Conese, M. Synthesis and characterization of novel chitosan-dopamine or chitosan-tyrosine conjugates for potential nose-to-brain delivery. Int. J. Pharm. 2020, 589, 119829. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Cellamare, S.; Bolognino, I.; Hossain, M.N.; Sanna, E.; Conese, M.; et al. Nose-to-brain delivery: A comparative study between carboxymethyl carboxymethylchitosanbased conjugates of dopamine. Int. J. Pharm. 2021, 599, 120453. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: Antineoplastic effect in human ovarian cancer cells in vitro. Int. J. Pharm. 2021, 606, 120899. [Google Scholar] [CrossRef]
- Cassano, R.; Trombino, S.; Curcio, F.; Sole, R.; Calviello, G.; Serini, S. ROS-Responsive PLGA-NPs for Co-Delivery of DTX and DHA for Colon Cancer Treatment. Int. J. Transl. Med. 2024, 4, 262–277. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of glioblastoma multiforme–literature review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.N.; Samling, B.A.; Tong, W.Y.; Chear, N.J.Y.; Yusof, S.R.; Lim, J.W.; Tchamgoue, J.; Leong, C.R.; Ramanathan, S. Chitosan-Based Nanoencapsulated Essential Oils: Potential Leads against Breast Cancer Cells in Preclinical Studies. Polymers 2024, 16, 478. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Gozali, D.; Shamsuddin, S.; Muchtaridi, M. Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics 2023, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Blakely, B.; Shin, S.; Jin, K. Overview of the therapeutic strategies for ER positive breast cancer. Biochem. Pharmacol. 2023, 212, 115552–115583. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, T.; Foekens, J.A.; Umar, A.; Martens, J.W. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov. Today 2016, 21, 1181–1188. [Google Scholar] [CrossRef]
- Harrelson, J.P.; Lee, M.W. Expanding the view of breast cancer metabolism: Promising molecular targets and therapeutic opportunities. Pharmacol. Ther. 2016, 167, 60–73. [Google Scholar] [CrossRef]


| Sample | MSN | 5-Fluoruracil | Sylimarin | HA-FA | CMCH-DA |
|---|---|---|---|---|---|
| 5-FU/MSNs | 30 mg | 30 mg | - | - | - |
| HA-FA/5-FU-MSNs | 30 mg | 30 mg | - | 2% | - |
| CMCH-DA/5-FU-MSNs | 30 mg | 30 mg | - | - | 2% |
| SI/MSNs | 30 mg | - | 30 mg | - | - |
| HA-FA/SI-MSNs | 30 mg | - | 30 mg | 2% | - |
| CMCH-DA/SI-MSNs | 30 mg | - | 30 mg | - | 2% |
| Formulation | Size (nm) | Polydispersion Index (PDI) |
|---|---|---|
| MSNs | 185.2 ± 1.3 | 0.183 |
| 5-FU/MSNs | 200.8 ± 0.3 | 0.225 |
| HA-FA/5-FU-MSNs | 227.2 ± 1.2 | 0.251 |
| CMCH-DA/5-FU-MSNs | 238 ± 2.1 | 0.214 |
| SI/MSNs | 202.5 ± 0.7 | 0.246 |
| HA-FA/SI-MSNs | 232.2 ± 0.5 | 0.239 |
| CMCH-DA/SI-MSNs | 241.1 ± 1.4 | 0.278 |
| Formulation | Loading Efficiency (%) |
|---|---|
| 5-FU/MSNs | 73.3% |
| HA-FA/5-FU-MSNs | 89% |
| CMCH-DA/5-FU-MSNs | 71.6% |
| SI/MSNs | 66.6% |
| HA-FA/SI-MSNs | 56.6% |
| CMCH-DA/SI-MSNs | 72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, F.; Sanguedolce, M.; Filice, L.; Testa, F.; Catapano, G.; Giordano, F.; Trombino, S.; Cassano, R. Hybrid Nanoparticles Based on Mesoporous Silica and Functionalized Biopolymers as Drug Carriers for Chemotherapeutic Agents. Materials 2024, 17, 3877. https://doi.org/10.3390/ma17153877
Curcio F, Sanguedolce M, Filice L, Testa F, Catapano G, Giordano F, Trombino S, Cassano R. Hybrid Nanoparticles Based on Mesoporous Silica and Functionalized Biopolymers as Drug Carriers for Chemotherapeutic Agents. Materials. 2024; 17(15):3877. https://doi.org/10.3390/ma17153877
Chicago/Turabian StyleCurcio, Federica, Michela Sanguedolce, Luigino Filice, Flaviano Testa, Gerardo Catapano, Francesca Giordano, Sonia Trombino, and Roberta Cassano. 2024. "Hybrid Nanoparticles Based on Mesoporous Silica and Functionalized Biopolymers as Drug Carriers for Chemotherapeutic Agents" Materials 17, no. 15: 3877. https://doi.org/10.3390/ma17153877
APA StyleCurcio, F., Sanguedolce, M., Filice, L., Testa, F., Catapano, G., Giordano, F., Trombino, S., & Cassano, R. (2024). Hybrid Nanoparticles Based on Mesoporous Silica and Functionalized Biopolymers as Drug Carriers for Chemotherapeutic Agents. Materials, 17(15), 3877. https://doi.org/10.3390/ma17153877











