Properties of Polymer-Modified Cement–Water-Glass Slurry
Abstract
1. Introduction
1.1. Research Background
1.2. Research Content
2. Test Materials and Instruments
2.1. Test Materials
- Retarder
- 2.
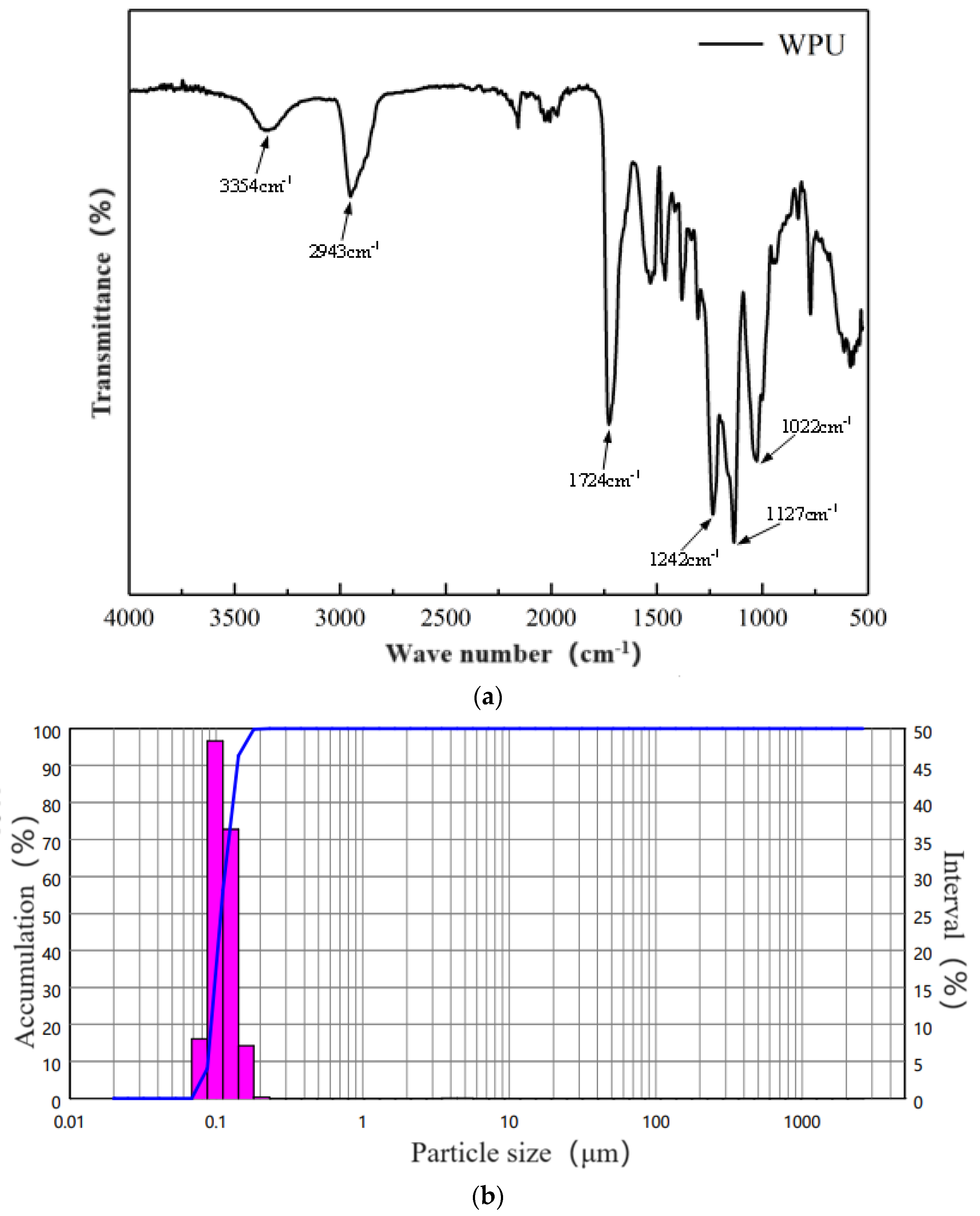
- Waterborne polyurethane
- 3.
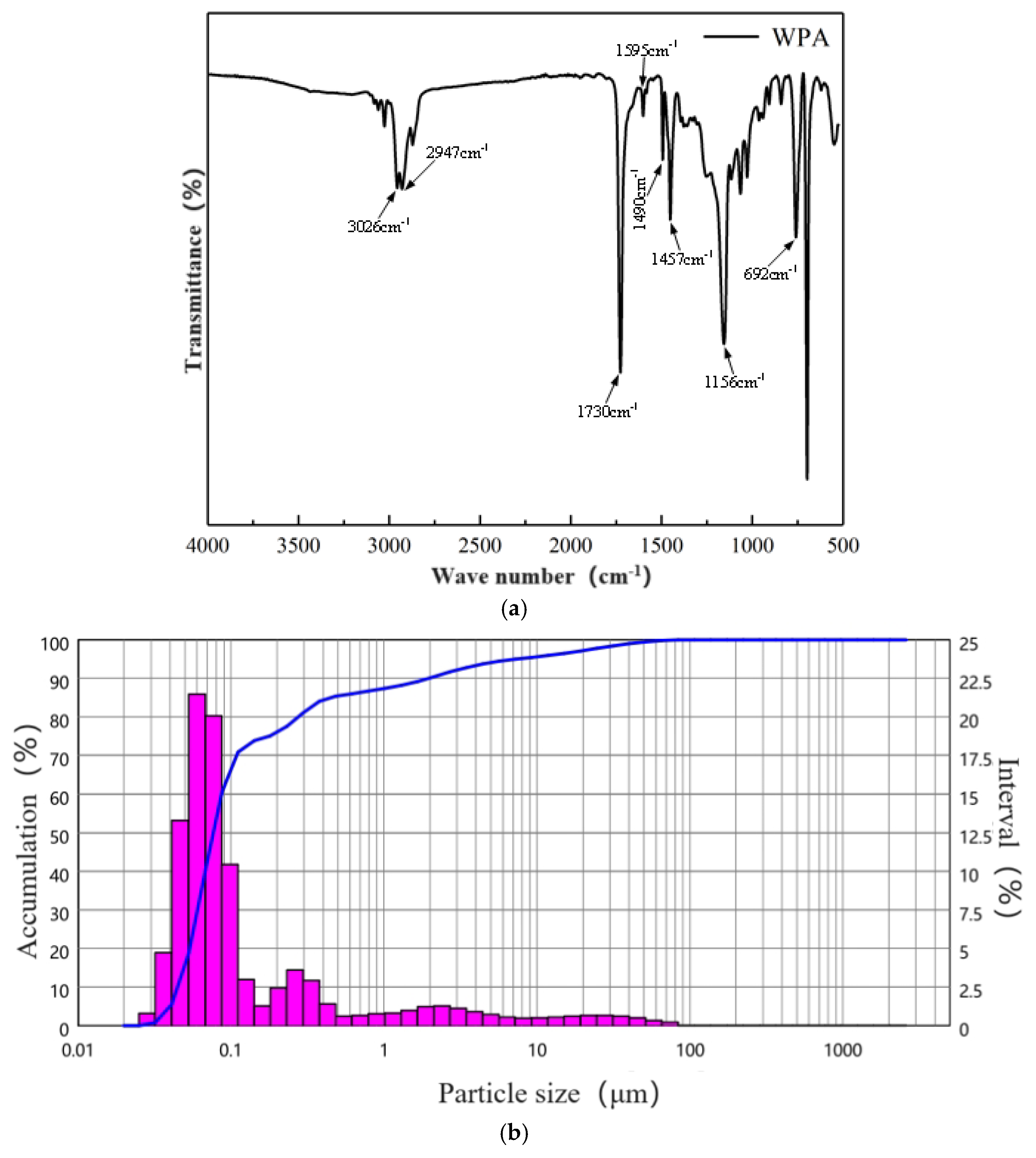
- Waterborne acrylic acid
- 4.
- Defoamer
- 5.
- Silane coupling agent
2.2. Test Methods
2.2.1. Basic Performance Test
- Pour the retarder into the water and stir for one minute to mix the water and retarder evenly. Then, pour the mixture into cement and stir until the two are evenly mixed.
- Pour the silane coupling agent into the water-based lotion and stir for 1 min to mix the water-based lotion and silane coupling agent evenly.
- Mix the above materials and add a defoamer, followed by stirring for three minutes.
- Add the water-glass solution and stir quickly for 15 s.
2.2.2. XRD Testing
2.2.3. Microstructure Testing
2.2.4. Pore-S
3. Comparative Analysis of Data
3.1. Fluidity
3.2. Gelation Time
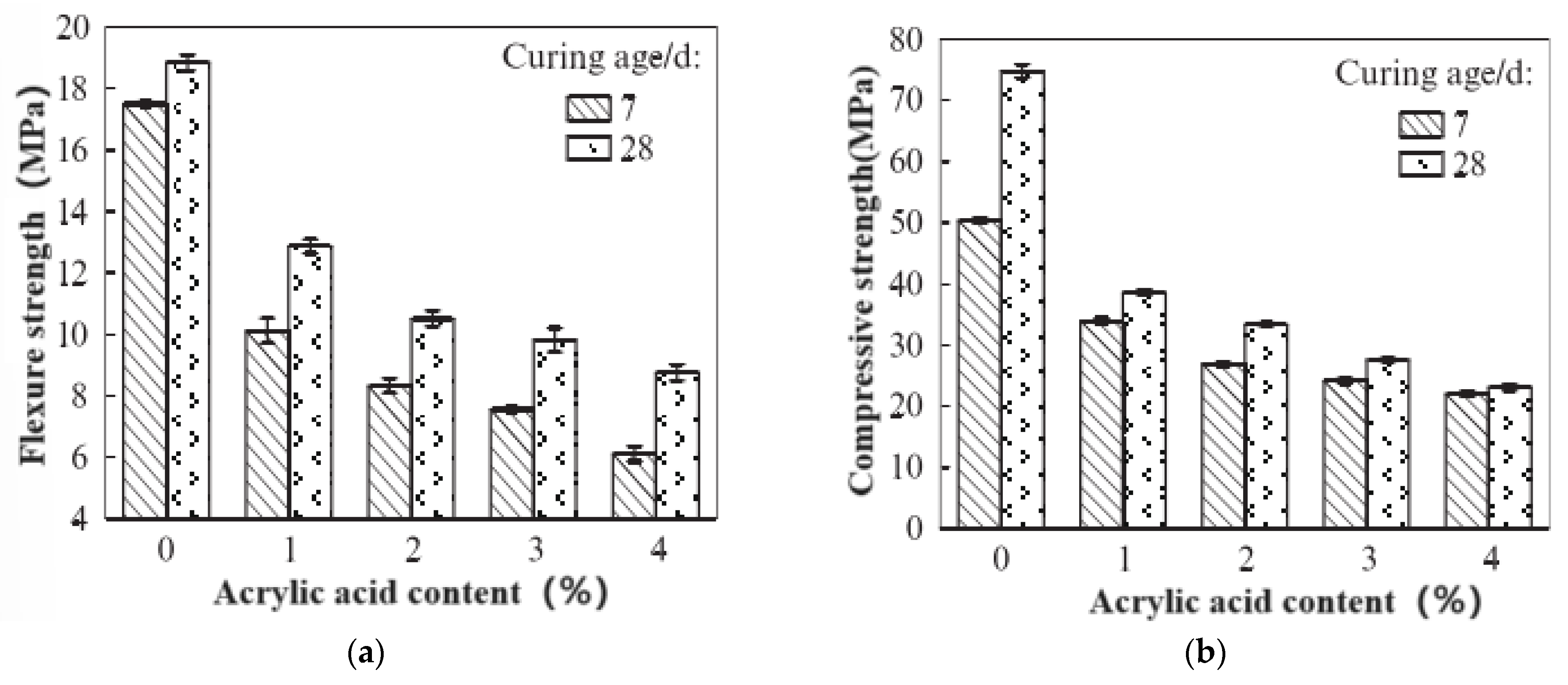
3.3. Physical Properties of Stones
- R—compression ratio, accurate to 0.1;
- Rf—flexural strength, MPa;
- Rc—compressive strength, MPa.

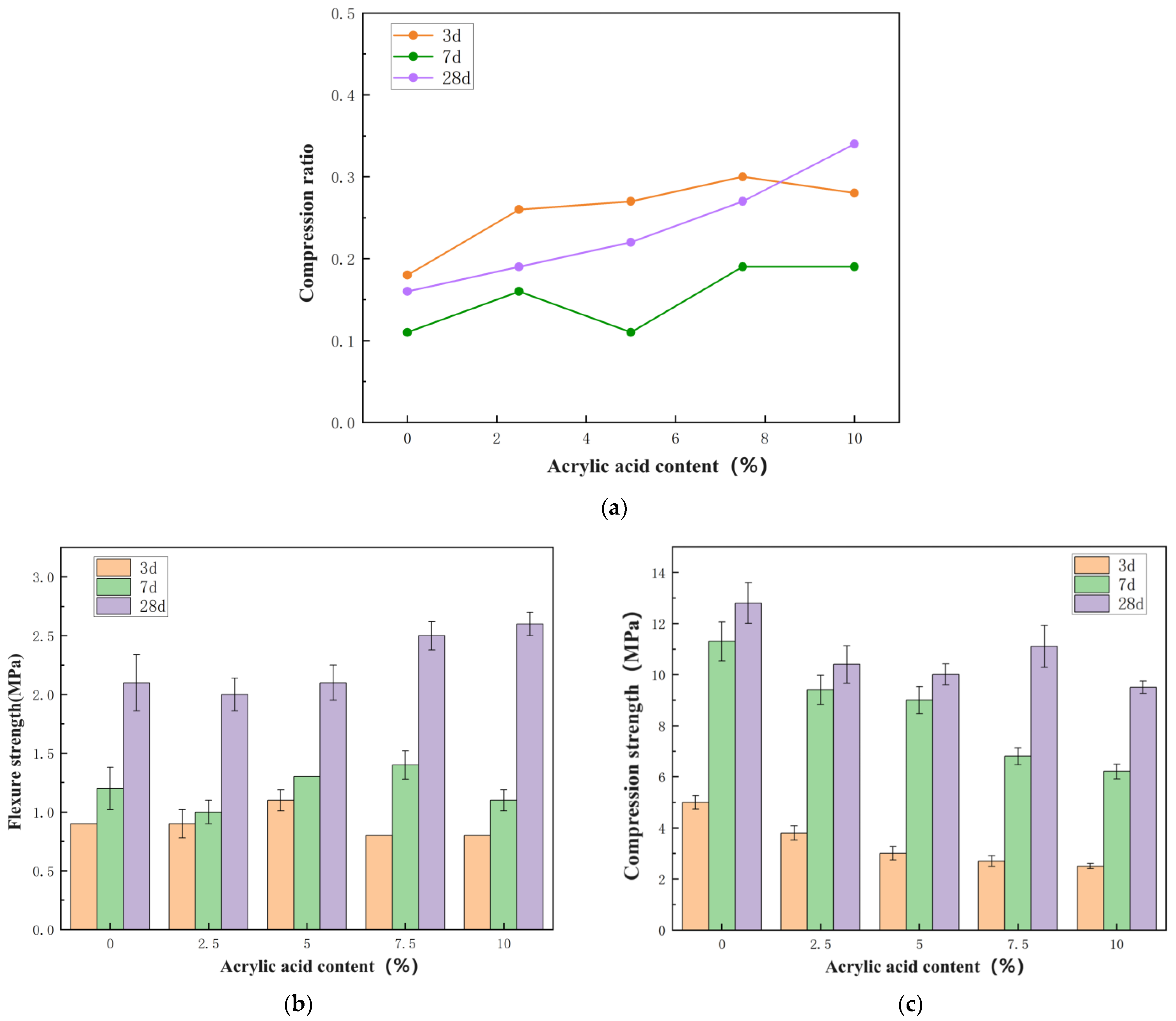
| Number | Flexural Strength (MPa) | Compressive Strength (MPa) | Compression Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |
| P0 | 0.9 | 1.2 | 2.1 | 5.0 | 11.3 | 12.8 | 0.18 | 0.11 | 0.16 |
| PU1 | 0.9 | 1.0 | 2.0 | 2.6 | 9.0 | 9.7 | 0.35 | 0.11 | 0.21 |
| PU2 | 1.1 | 1.3 | 2.1 | 2.6 | 8.3 | 9.3 | 0.42 | 0.16 | 0.23 |
| PU3 | 0.8 | 1.4 | 2.5 | 2.2 | 5.5 | 8.3 | 0.36 | 0.25 | 0.30 |
| PU4 | 0.8 | 1.1 | 2.6 | 1.9 | 3.8 | 6.7 | 0.42 | 0.29 | 0.39 |
| Number | Flexural Strength (MPa) | Compressive Strength (MPa) | Compression Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |
| P0 | 0.9 | 1.2 | 2.1 | 5.0 | 11.3 | 12.8 | 0.18 | 0.11 | 0.16 |
| PA1 | 1.0 | 1.5 | 2.0 | 3.8 | 9.4 | 10.4 | 0.26 | 0.16 | 0.19 |
| PA2 | 0.8 | 1.0 | 2.2 | 3.0 | 9.0 | 10.0 | 0.27 | 0.11 | 0.22 |
| PA3 | 0.8 | 1.3 | 3 | 2.7 | 6.8 | 11.1 | 0.30 | 0.19 | 0.27 |
| PA4 | 0.7 | 1.2 | 3.2 | 2.5 | 6.2 | 9.5 | 0.28 | 0.19 | 0.34 |
4. Characterization Analyses
4.1. XRD
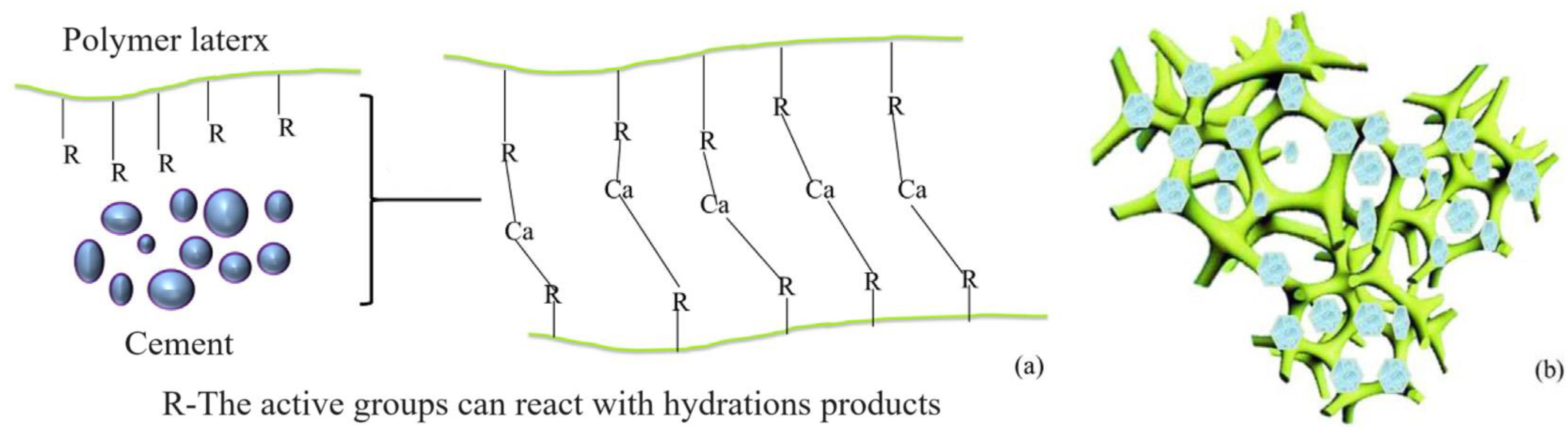
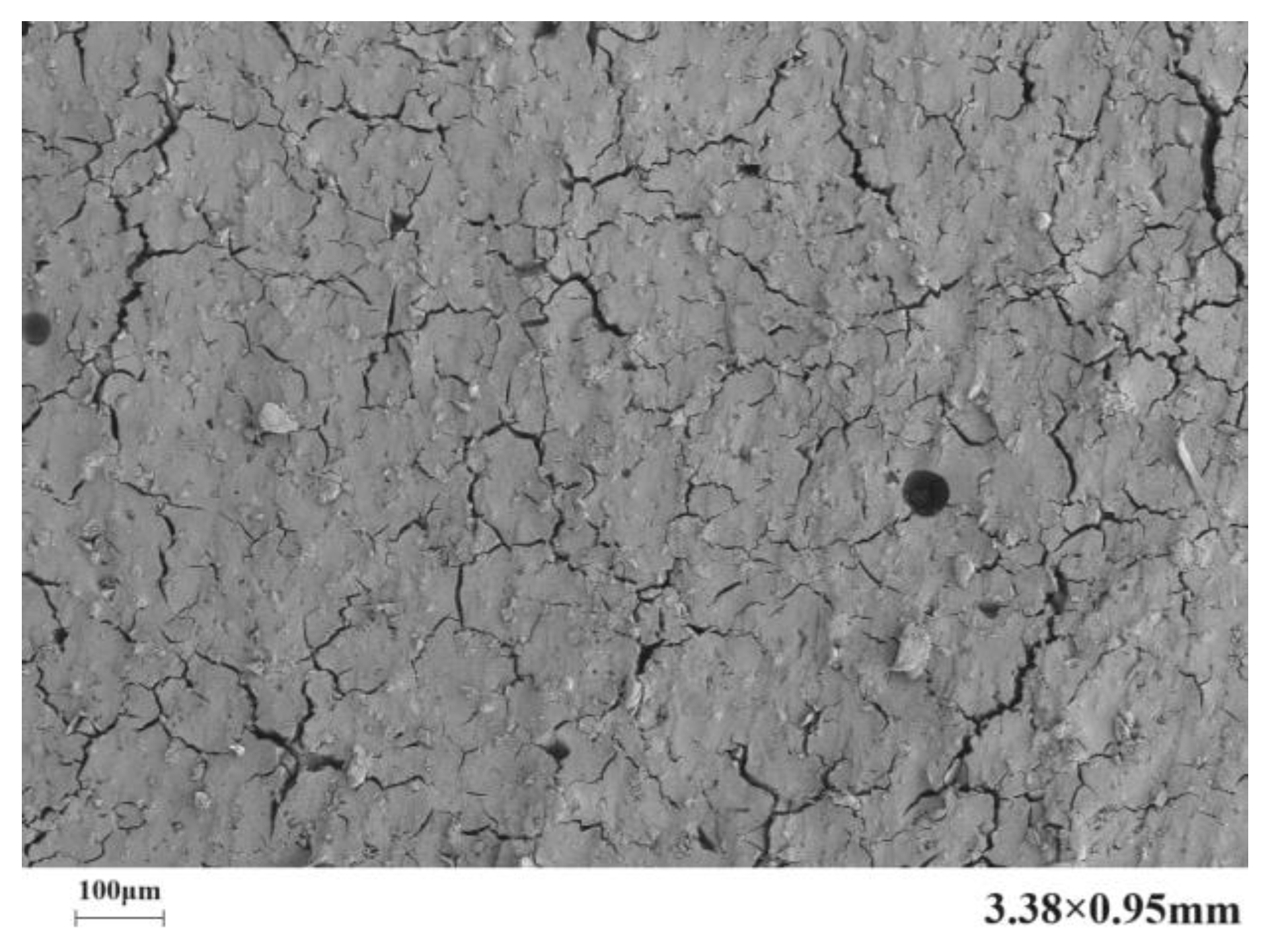
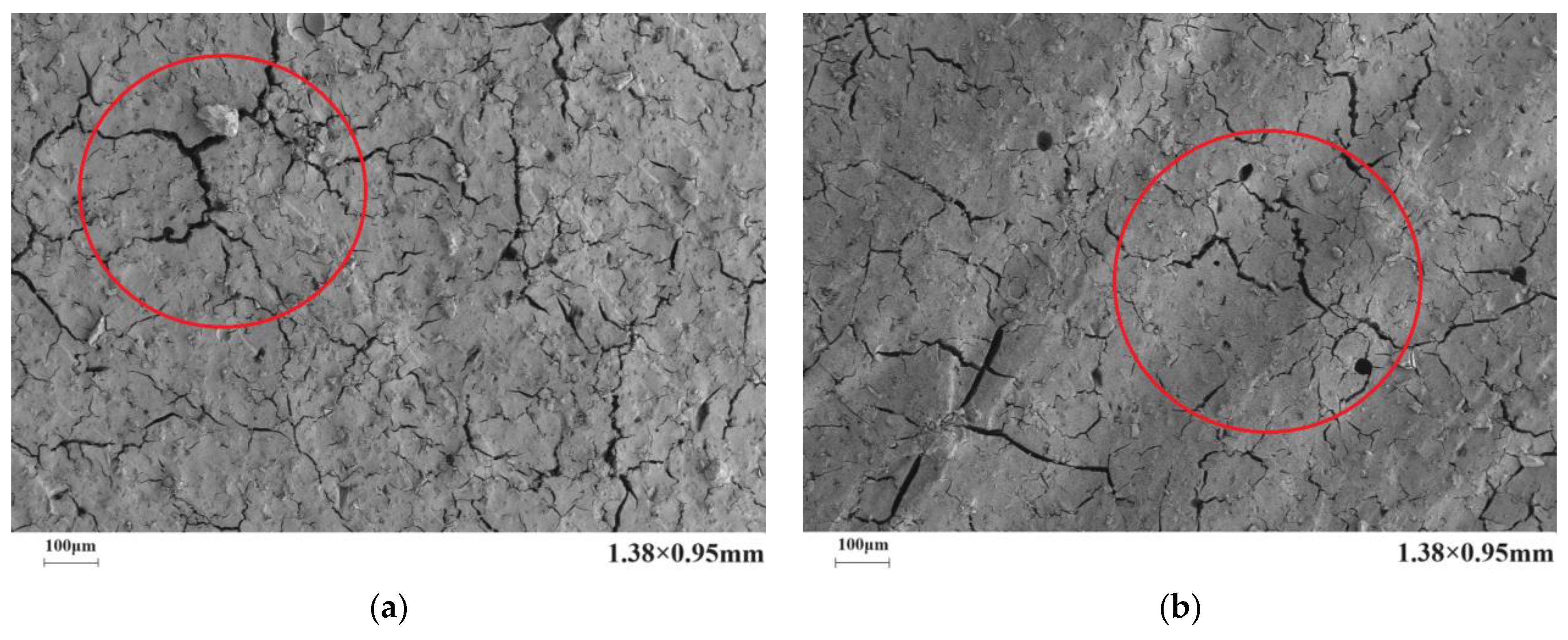
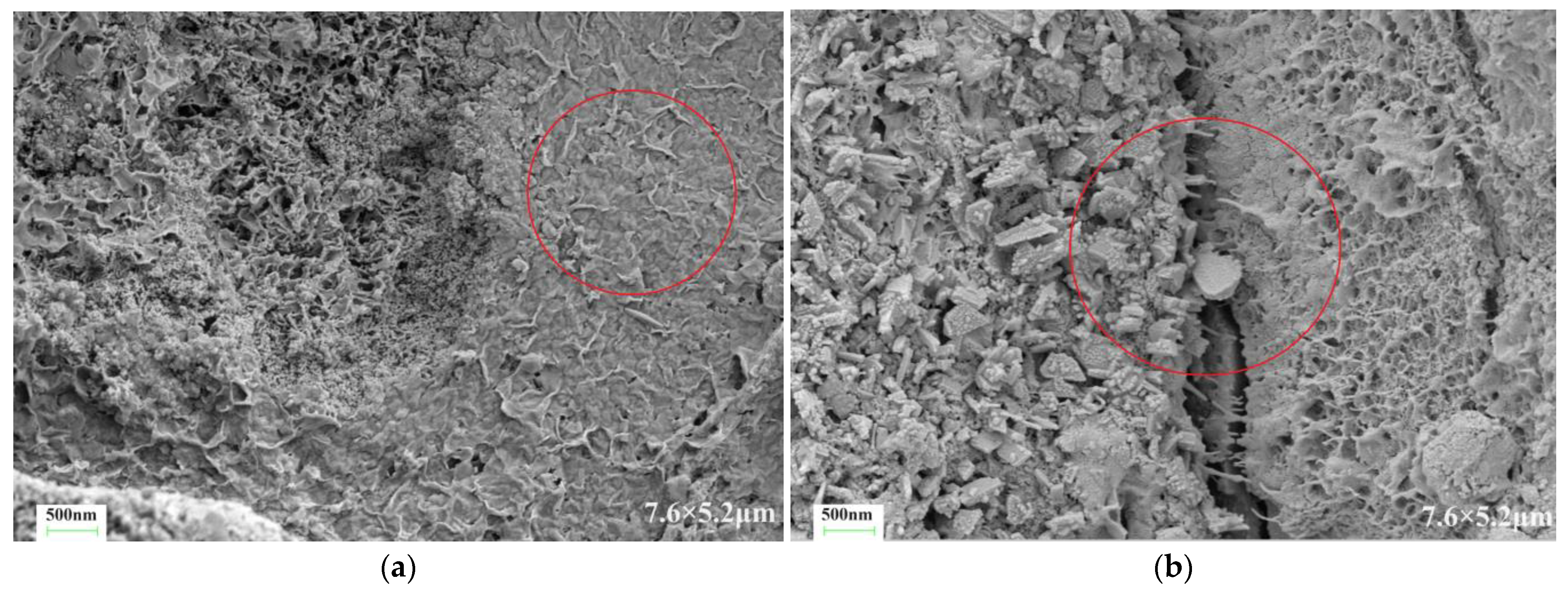
4.2. Microstructure
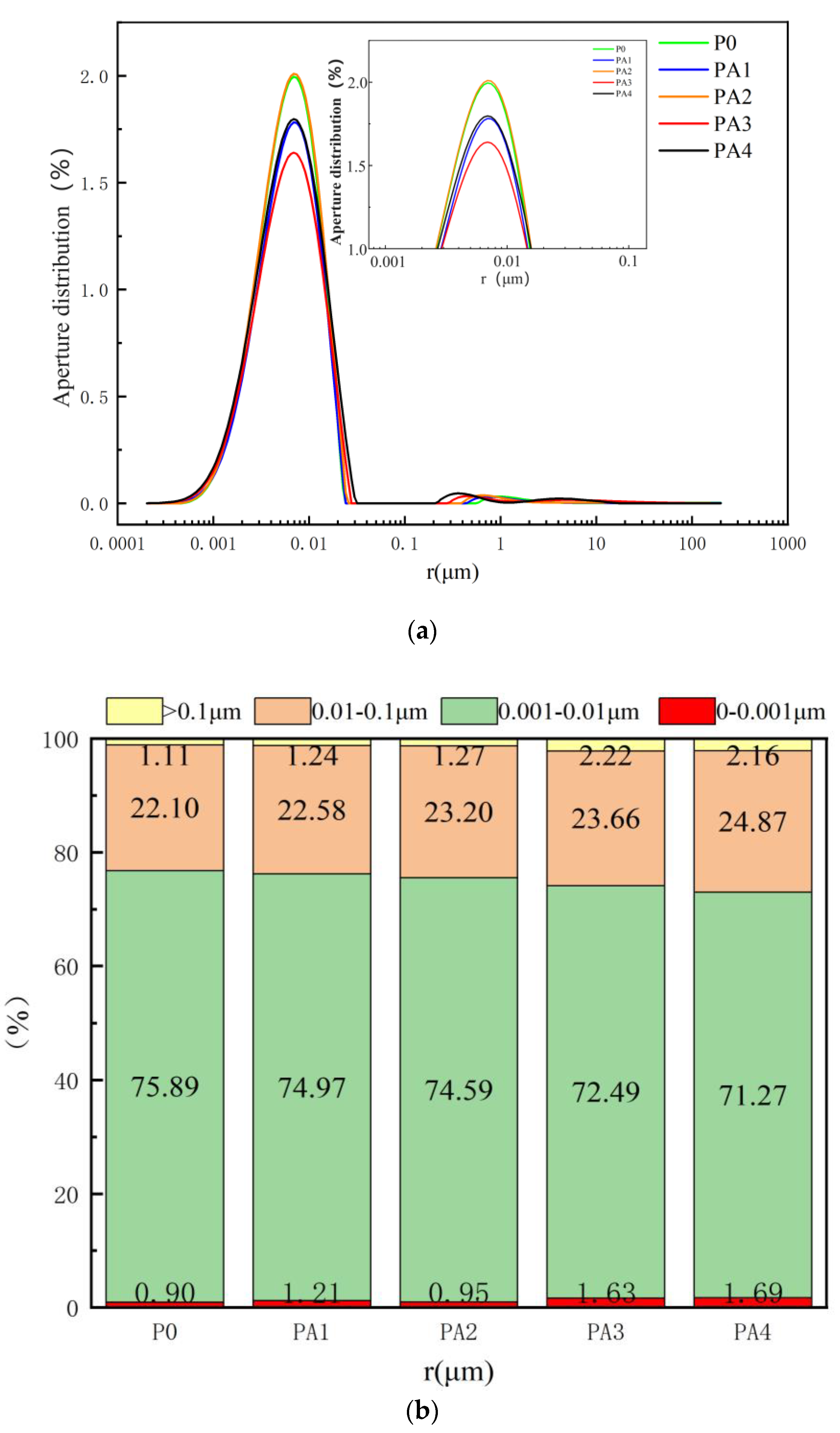
4.3. Pore Distribution
5. Conclusions
- The aqueous polyurethane lotion reduced the fluidity of the dual-liquid slurry, while the waterborne acrylic lotion improved the fluidity of the liquid, increasing the fluidity of the dual-liquid slurry.
- The higher the content of waterborne polyurethane lotion, the shorter the gelation time of the dual-liquid slurry. The higher the content of waterborne acrylic lotion, the longer the gelation time of the dual-liquid slurry.
- Waterborne polyurethane lotion and waterborne acrylic lotion provided a small increase in the flexural strength of a C-S dual-liquid slurry at a certain amount, but the compressive strength of all dual-liquid slurry stones mixed with polyurethane and acrylic acid significantly decreased. The flexural compression ratio demonstrated a certain increase.
- The main chemical substance of the dual-liquid slurry stone body was hydrated calcium silicate. Waterborne acrylic acid and waterborne polyurethane did not change the types of hydration products.
- The introduction of polyurethane and acrylic acid during the dehydration process effectively reduced the number, width, and length of cracks in the microstructure of a stone body. The improvement effect of polyurethane on the microstructure of a stone body was significantly better than that of acrylic acid.
- The introduction of both polyurethane and acrylic acid increased the proportion of pores > 0.01 μm generated in a stone body, but the degree of influence varied. The influence of acrylic acid on the pore distribution of the stone body was lower than that of polyurethane. Although polyurethane significantly improved the crack resistance of stone bodies during the dehydration process and the resulting porosity was low when the dosage was high, polyurethane had adverse effects on the flowability and gelation-time performance of the dual-liquid slurry. The flowability and gelation-time performance of the dual-liquid slurry were excellent when the acrylic acid dosage was 7.5%. The compressive strength of the stone body slightly decreased, the flexural strength improved to a certain extent, the crack resistance of the stone body was also enhanced during the dehydration process, and the porosity of the stone body was low. Therefore, the optimal dosage was determined to be 7.5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Jiang, G.; Yuan, D.; Polk, J. Evolution of major environmental geological problems in karst areas of Southwestern China. Environ. Earth Sci. 2012, 69, 2427–2435. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, Y.; Hu, W.; Cao, M.; Mao, Y. A review of the effects of tunnel excavation on the hydrology, ecology, and environment in karst areas: Current status, challenges, and perspectives. J. Hydrol. 2020, 586, 542–549. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wu, J.; Zhang, X.; Zhang, L.; Li, Z. Grouting sealing mechanism of water gushing in karst pipelines and engineering application. Constr. Build. Mater. 2020, 254, 119250. [Google Scholar] [CrossRef]
- Liu, D.; Xie, W.J.; Gao, J.L.; Hu, S.; Chen, M.; Li, Y.; Li, L. Study on the Construction Method and Effects of Ipsilateral, Multi-Nozzle, High-Pressure Jet Grouting Cut-Off Wall. Sustainability 2022, 14.16, 10383. [Google Scholar] [CrossRef]
- Bohloli, B.; Skjolsvold, O.; Justnes, H.; Olsson, R.; Grøv, E.; Aarset, A. Cements for tunnel grouting—Rheology and flow properties tested at different temperatures. Tunn. Undergr. Space Technol. 2019, 91, 103011.1–103011.10. [Google Scholar] [CrossRef]
- Xu, Z.G.; Wang, Y.Z.; Xiong, W.; Chai, J.; Cao, C. Experimental investigation on the sealing of flowing water by cement-sodium silicate slurry in rough single fracture. Bull. Eng. Geol. Environ. 2023, 82, 361. [Google Scholar] [CrossRef]
- Zhu, M.T.; Sui, H.T.; Yang, H.T. The differences between soil grouting with cement slurry and cement-water glass slurry. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022013. [Google Scholar] [CrossRef]
- Zhang, X.; Du, M.; Fang, H.; Shi, M.; Zhang, C.; Wang, F. Polymer-modified cement mortars: Their enhanced properties, applications, prospects, and challenges. Constr. Build. Mater. 2021, 299, 124290. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, Y.; Liu, G. Study on the effect of waterborne epoxy resins on the performance and microstructure of cement paste. Constr. Build. Mater. 2018, 167, 831–845. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.A.; Sapidis, G.; Papastergiadis, E. Fundamental properties of epoxy resin-modified cement grouts. Constr.Build. Mater. 2016, 125, 184–195. [Google Scholar] [CrossRef]
- Fan, L.; Xu, F.; Wang, S.; Yu, Y.; Zhang, J.; Guo, J. A review on the modification mechanism of polymer on cement-based materials. J. Mater. Res. Technol. 2023, 26, 5816–5837. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Wang, Z.; Du, C.; Jiang, H. Research on the mechanism of polymer latex modified cement. Constr. Build. Mater. 2016, 111, 710–718. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Microstructures and mechanical properties of polymer modified mortars under distinct mechanisms. Constr. Build. Mater. 2013, 47, 579–587. [Google Scholar] [CrossRef]
- Wang, R.; Yao, L.; Wang, P. Mechanism analysis and effect of styrene-acrylate copolymer powder on cement hydrates. Constr. Build. Mater. 2013, 41, 538–544. [Google Scholar] [CrossRef]
- Chen, B.; Qiao, G.; Hou, D.; Wang, M.; Li, Z. Cement-based material modified by in-situ polymerization: From experiments to molecular dynamics investigation. Compos. Part. B Eng. 2020, 194, 108036. [Google Scholar] [CrossRef]
- GB50119-2013; Technical Specification for the Application of Concrete Admixtures. China National Standards: Beijing, China, 2013.
- Li, G.; Fang, Y.H.; Wu, C.D.; Shao, Y.Z.; Guo, Y.Q.; Ma, X.X. Study on the Development and Performance of a New Concrete Antifoaming Agent. Key Eng. Mater. 2021, 871, 357–362. [Google Scholar] [CrossRef]
- He, Z.; Li, Q.; Wang, J.; Yin, N.; Jiang, S.; Kang, M. Effect of silane treatment on the mechanical properties of polyurethane/water glass grouting materials. Constr. Build. Mater. 2016, 116, 110–120. [Google Scholar] [CrossRef]
- GB/T8077-2012; Test Method for Uniformity of Concrete Admixtures. China National Standards: Beijing, China, 2012.
- GB/T 17671-2021; Test Method for Strength of Cement Mortar (ISO Method). China National Standards: Beijing, China, 2021.
- Sun, Z.P.; Ye, D.M.; Fu, L.F.; Zheng, B.C.; Feng, Z.J.; Xie, H.D. Influence of three kinds of acrylic latex on properties of cement mortar. In Proceedings of the 4th National Academic Exchange Conference on Commercial Mortar, Shanghai, China, 23 November 2011; pp. 86–94. [Google Scholar]
- Su, F.; He, T.; He, Z.; Yu, Q.; Wang, H. Mechanism of Acrylate Emulsion-Modified Cement-Based Materials. Molecules 2024, 29, 1260. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Yu, C.; Wang, R. Influence of cationic polyurethane on mechanical properties of cement based materials and its hydration mechanism. Constr. Build. Mater. 2017, 137, 494–504. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Song, Z. Influence of styrene-acrylic copolymer latex on the mechanical properties and microstructure of Portland cement/Calcium aluminate cement/Gypsum cementitious mortar. Constr. Build. Mater. 2019, 227, 116666. [Google Scholar] [CrossRef]
- Kong, X.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene-acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Li, X.Q.; Bian, L.B.; Ding, L.N.; Zhang, L.; Wei, X. Properties of Acrylic Emulsion Modified Magnesium Oxychloride Cement and Its Mechanism. J. Build. Mater. 2024, 27, 479–486. [Google Scholar]
- He, Y.J.; Zhao, X.G.; Lu, L.N.; Leslie, J.; Hu, S.G. Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 770–773. [Google Scholar] [CrossRef]
- Okada, Y.; Ishida, H.; Mitsuda, T. 2 9Si NMR spectroscopy of silicate anions in hydrothermally formed C-S-H. J. Am. Ceram. Soc. 1994, 77, 765–768. [Google Scholar] [CrossRef]
- Kalina, L.; Bílek, V., Jr.; Bradová, L.; Topolář, L. Blastfurnace Hybrid Cement with Waste Water Glass Activator: Alkali–Silica Reaction Study. Materials 2020, 13, 3646. [Google Scholar] [CrossRef]
- Chen, J.H.; Zeng, X.H.; Yang, J.F.; Long, G.C.; Xie, Y.J. Enhanced damping properties of cement paste containing polyurethane and its improvement mechanism. Constr. Build. Mater. 2022, 364, 129986. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.H.; Sun, G.W.; Li, Y.; Zhang, Y. Research on damping properties and microscopic mechanism of polyurethane cement-based composites. Constr. Build. Mater. 2023, 365, 130137. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Wang, Q.; Han, L.; Han, G. Film-formation processes of polymer emulsions in polymer-cement waterproof coatings and their effect on coatings’ macroscopic properties. Constr. Build. Mater. 2024, 438, 137137. [Google Scholar] [CrossRef]
- Zheng, H.P.; Pang, B.; Jin, Z.Q.; Liu, S.; Zhang, Y.; Bi, J.; Chang, H.; Liu, Y.; Wang, F. Mechanical properties and microstructure of waterborne polyurethane-modified cement composites as concrete repair mortar. J. Build. Eng. 2024, 84, 108394. [Google Scholar] [CrossRef]
- Li, B.; Tia, Y.; Zhao, R.Y.; Duan, A.; Li, Z.; Ma, H. Microstructure and modification mechanism of polyacrylate emulsion modified mortar. J. Zhejiang Univ. (Eng. Sci.) 2014, 48, 1345–1352. [Google Scholar]







| Type | Appearance | Solid Content | PH Value |
|---|---|---|---|
| Anionic | Transparent liquid | 35% | 7–9 |
| Type | Appearance | Solid Content | PH Value |
|---|---|---|---|
| Anionic | Transparent liquid | 48% | 5–6 |
| Number | Water–Cement Ratio | Polyurethane (%) | Coupling Agent (%) | Defoamer (%) | Original C:S Volume Ratio |
|---|---|---|---|---|---|
| P0 | 0.8 | 0 | 0 | 0 | 1:0.6 |
| PU1 | 0.8 | 2.5 | 1% | 0.5 | 1:0.6 |
| PU2 | 0.8 | 5 | 1% | 0.5 | 1:0.6 |
| PU3 | 0.8 | 7.5 | 1% | 0.5 | 1:0.6 |
| PU4 | 0.8 | 10 | 1% | 0.5 | 1:0.6 |
| Number | Water–Cement Ratio | Acrylic Acid (%) | Coupling Agent (%) | Defoamer (%) | Original C:S Volume Ratio |
|---|---|---|---|---|---|
| P0 | 0.8 | 0 | 0 | 0 | 1:0.6 |
| PA1 | 0.8 | 2.5 | 1 | 0.5 | 1:0.6 |
| PA2 | 0.8 | 5 | 1 | 0.5 | 1:0.6 |
| PA3 | 0.8 | 7.5 | 1 | 0.5 | 1:0.6 |
| PA4 | 0.8 | 10 | 1 | 0.5 | 1:0.6 |
| Polyurethane Dosage (%) | Fluidity (mm) |
|---|---|
| 0 | 323 |
| 2.5 | 325 |
| 5 | 305 |
| 7.5 | 278 |
| 10 | 240 |
| Acrylic Acid Content (%) | Fluidity (mm) |
|---|---|
| 0 | 323 |
| 2.5 | 320 |
| 5 | 325 |
| 7.5 | 330 |
| 10 | 326 |
| Dosage (%) | Gelation Time after Adding Polyurethane (s) | Gelation Time after Adding |
|---|---|---|
| Acrylic Acid (s) | ||
| 0 | 58 | 58 |
| 2.5 | 57 | 60 |
| 5 | 51 | 61 |
| 7.5 | 47 | 62 |
| 10 | — | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Xu, J.; Ju, Y.; Lu, D.; Meng, W.; Wu, J.; Zhang, X. Properties of Polymer-Modified Cement–Water-Glass Slurry. Materials 2024, 17, 3888. https://doi.org/10.3390/ma17163888
Yang Q, Xu J, Ju Y, Lu D, Meng W, Wu J, Zhang X. Properties of Polymer-Modified Cement–Water-Glass Slurry. Materials. 2024; 17(16):3888. https://doi.org/10.3390/ma17163888
Chicago/Turabian StyleYang, Qian, Junxiang Xu, Yiheng Ju, Dewang Lu, Wei Meng, Jing Wu, and Xuefu Zhang. 2024. "Properties of Polymer-Modified Cement–Water-Glass Slurry" Materials 17, no. 16: 3888. https://doi.org/10.3390/ma17163888
APA StyleYang, Q., Xu, J., Ju, Y., Lu, D., Meng, W., Wu, J., & Zhang, X. (2024). Properties of Polymer-Modified Cement–Water-Glass Slurry. Materials, 17(16), 3888. https://doi.org/10.3390/ma17163888





