Trace Elements Anomalous Concentrations in Building Materials—The Impact of Secondary Mineralisation Processes
Abstract
:1. Introduction
2. Research Methodology
- Determination of the chemical composition was carried out by the atomic absorption spectroscopy (ASA) method using the PHILIPS PU 9100Xi Camera SX-100 spectrophotometer and the ICP.ULTIMA 2 HORIBA JOBIN-YVON sequential plasma spectrometer with the possibility of retrospective analysis, operating in the spectra range from 160 to 800 nm with the possibility of expanding them at any time to the range of 120–800 nm. The software compatible with the ICP spectrometer enables recording of the full spectrum in less than 200 s at the full resolution of the spectrometer. The tests were carried out at the accredited Aerospace Materials Testing Laboratory of the Rzeszów University of Technology.
- Scanning microscopy was carried out using a MIRA3 Tescan electron microscope (SEM). To determine the chemical composition, field emission and an X-ray detector (EDS) from Oxford Instruments were used. The research preparation required sputtering the samples with a layer of gold with a thickness of approximately 30–45 nm. This process was carried out on a vacuum sprayer. Sample imaging was performed at four magnifications of 2 k, 5 k, 20 k, and 50 k times. The electron acceleration voltage was selected in the range of 10 to 20 kV. Elemental mapping was performed at 1000× magnification. The average time for one mapping is approximately 10 min. X-ray detection covered the energy range from 0 to 10 keV. The surface distribution of the elements was made at an image resolution of 1024 × 1024 pixels. The time to count the signal to the spectrum from one pixel was 500 microseconds. The elemental composition was the average value of the entire map.
- An X-ray spectrometer (EDX Genesis) and backscattered electrons (BSE) detector were used for point analysis of mineral phases and obtaining microphotographs illustrating the phase differentiation of solid surfaces.
- Microscopic observations in polarised transmitted light were carried out using microscopes—Panthera TEC POL Trino equipped with a high-sensitivity Pro-S5 microscope camera with an sCMOS matrix and a Global Shutter shutter type.
- Observations and photographs of microsection were obtained using an Olympus SZX7 stereoscopic microscope, equipped with a Galilean optical system with plan apochromatic lenses, free from distortion, along with a microscope camera and software enabling image acquisition and measurements.
3. Research Stages
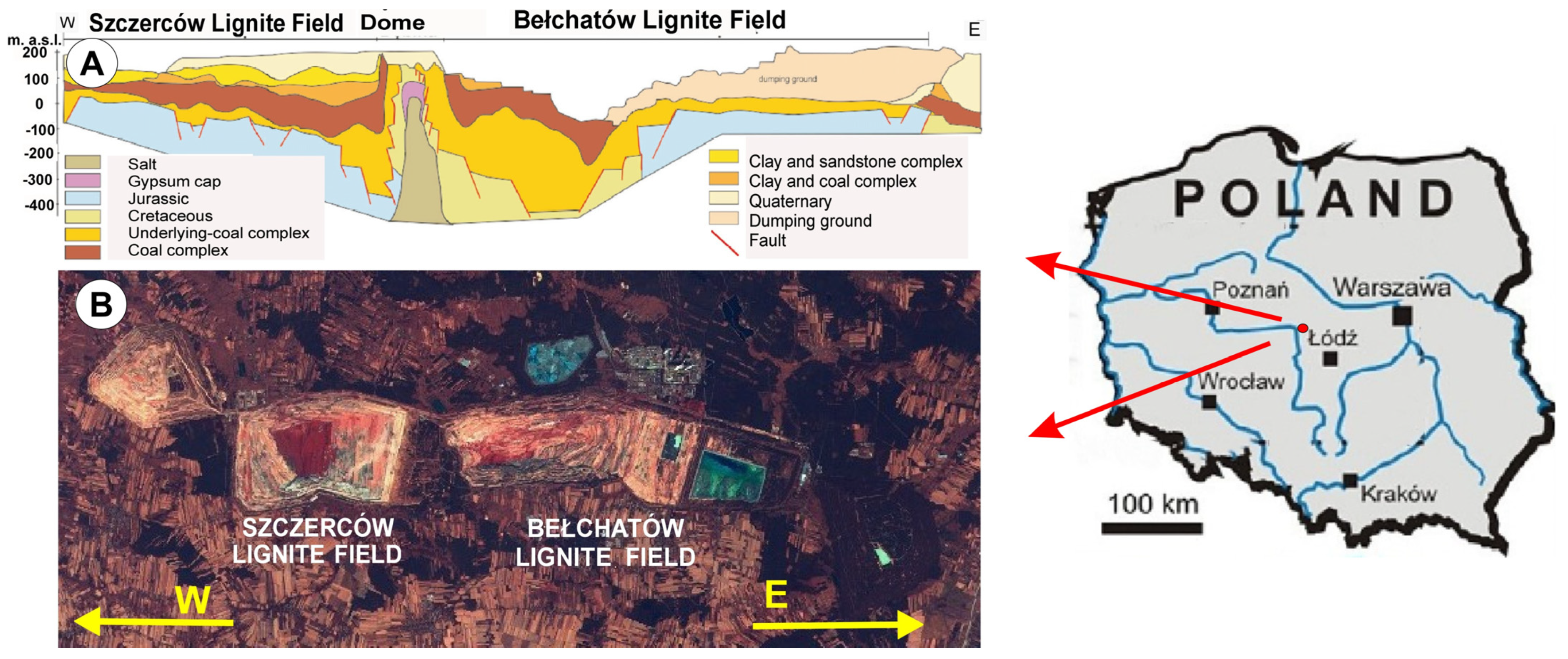
3.1. Fieldwork
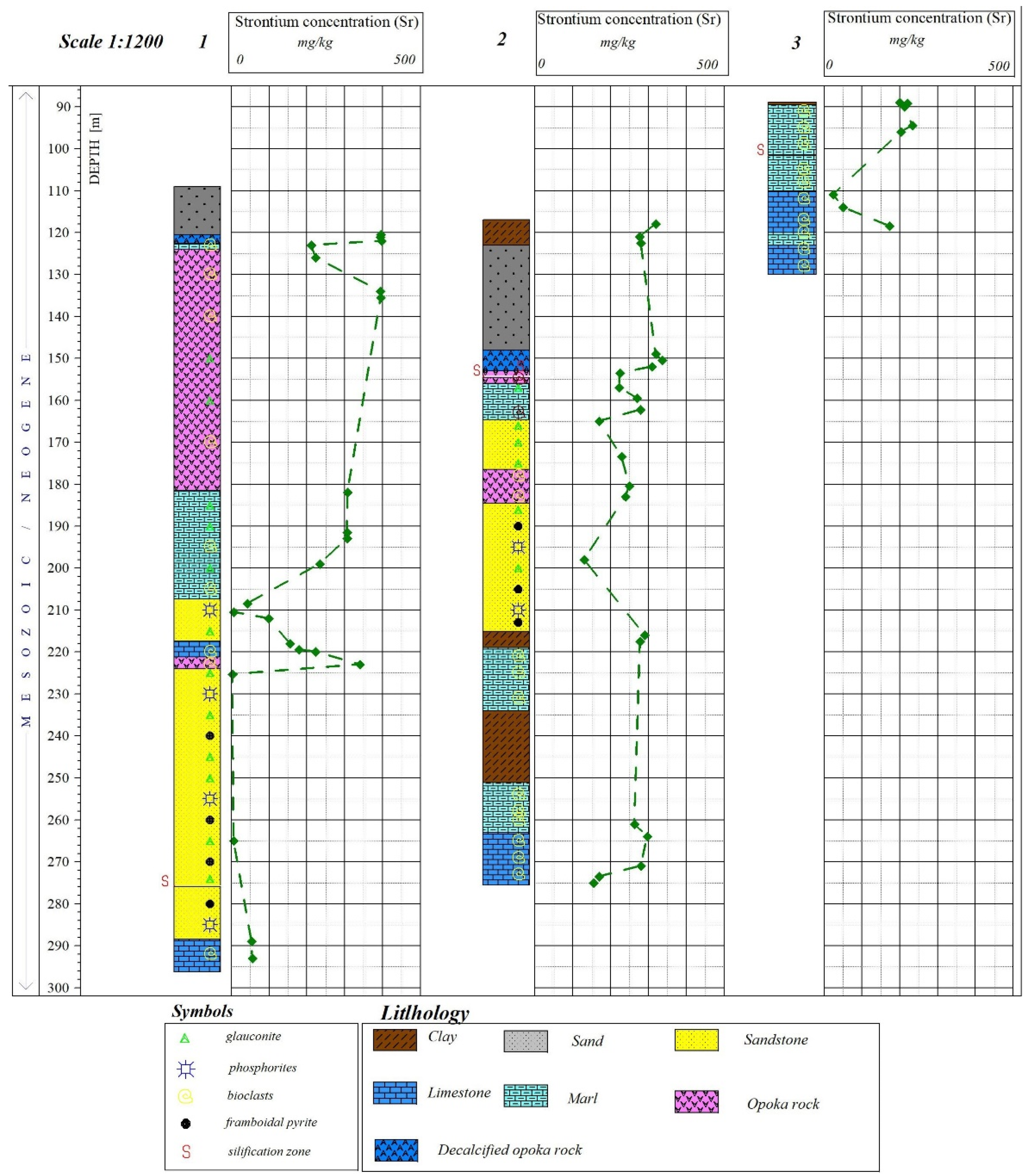
3.2. Geochemical Research
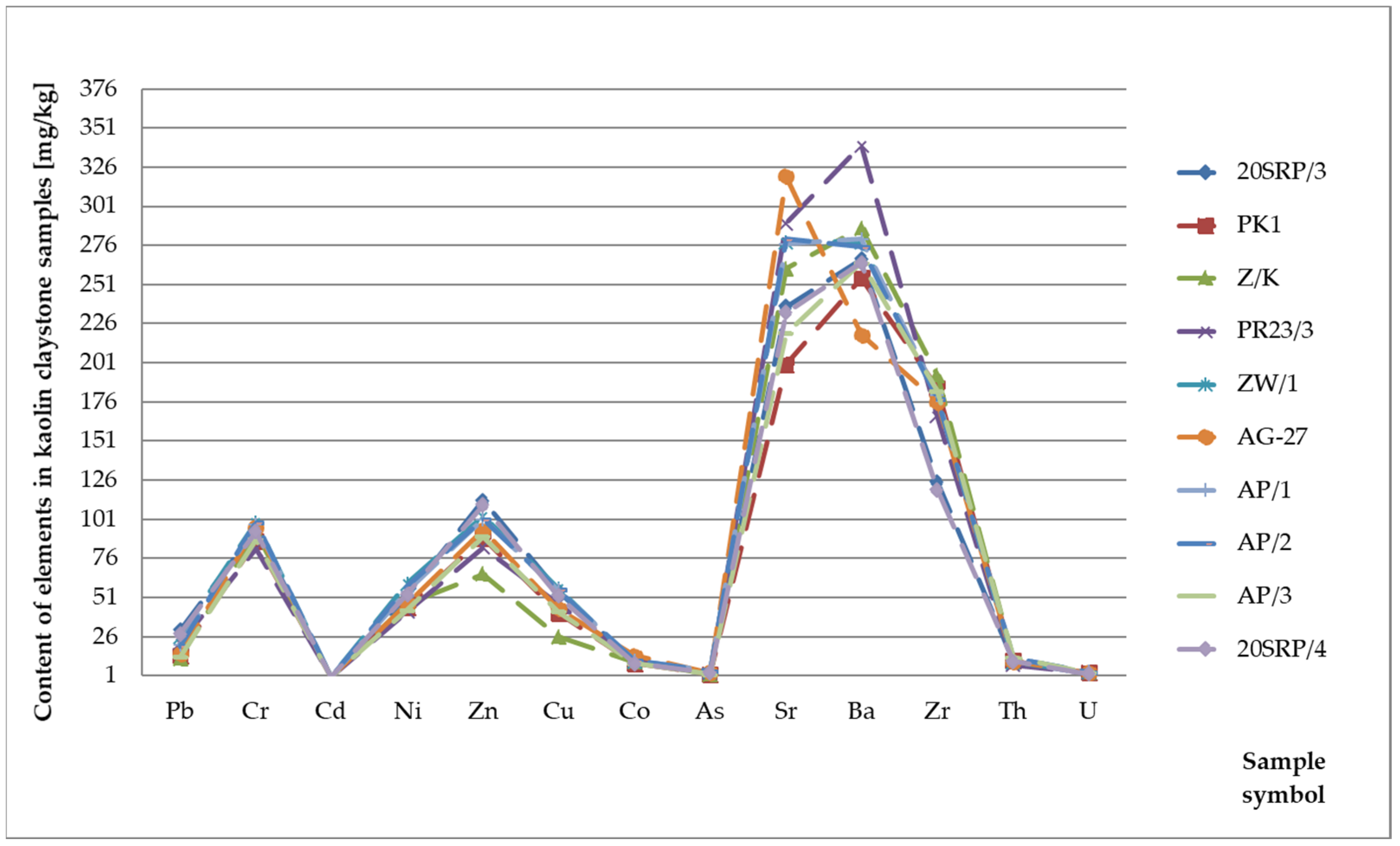
Geochemical Analysis of the Determined Elements
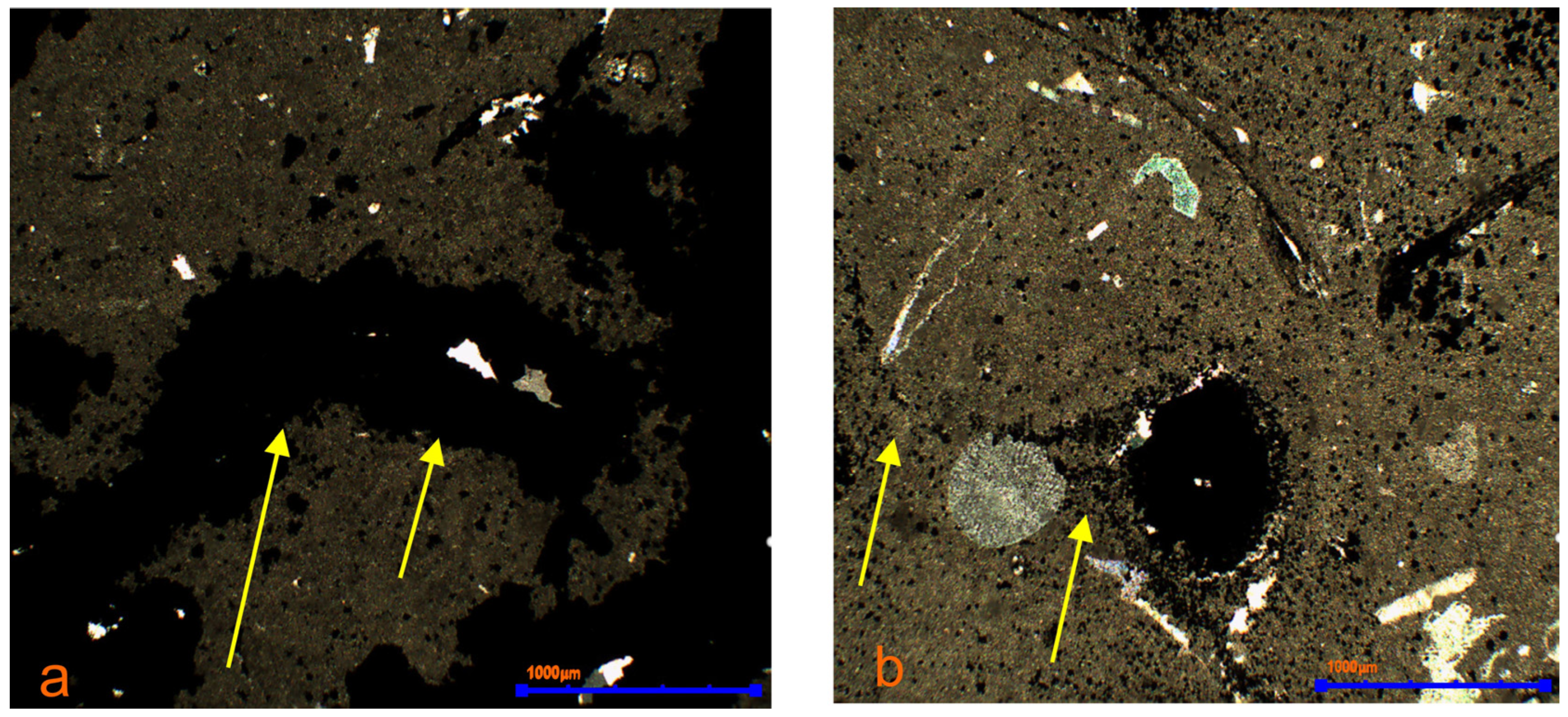
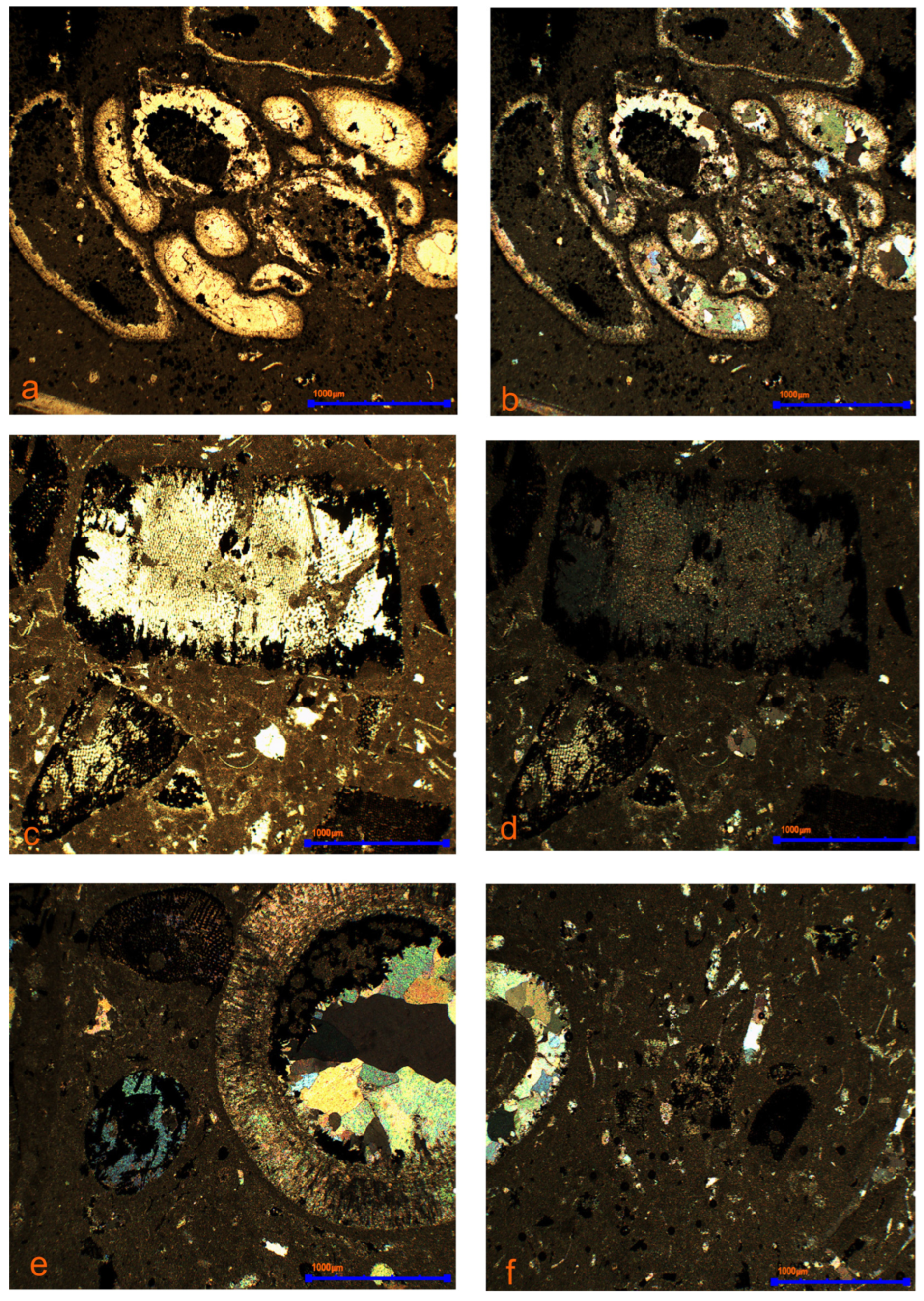
3.3. Mineralogical Research
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union. Regulation (EU) No. 305/2011 of the European Parliament and of the Council of 9 March 2011 establishing harmonized conditions for the marketing of construction products and repealing Council Directive 89/106/EEC. J. Laws 2021, 54, 1–66. [Google Scholar]
- Musiał, M.; Pękala, A. Functioning of Heat Accumulating Composites of Carbon Recyclate and Phase Change Material. Materials 2022, 15, 2331. [Google Scholar] [CrossRef] [PubMed]
- Musiał, M.; Lichołai, L.; Pękala, A. Analysis of the Thermal Performance of Isothermal Composite Heat Accumulators Containing Organic Phase-Change Material. Energies 2023, 16, 1409. [Google Scholar] [CrossRef]
- Pękala, A.; Musiał, M. Modelling the Leachability of Strontium and Barium from Stone Building Materials. Materials 2021, 14, 3403. [Google Scholar] [CrossRef] [PubMed]
- Pękala, A.; Musiał, M.; Galek, T. Pyritization in Stone-Building Materials Modeling of Geochemical Interaction. Sustainability 2022, 14, 13206. [Google Scholar] [CrossRef]
- Kuriata-Potasznik, A.; Szymczyk, S.; Skwierawski, S.; Glińska-Lewczuk, K.; Cymes, I. Metal contamination in the surface layer of bottom sediments in a flow-through lake: A case study of Lake Symsar in Northern Poland. Water 2016, 8, 358. [Google Scholar] [CrossRef]
- Kulbat, E.; Sokołowska, A. Methods of assessment of metal contamination in bottom sediments (Case study: Straszyn Lake, Poland). Arch. Environ. Contam. Toxicol. 2019, 77, 605–618. [Google Scholar] [CrossRef]
- Bartoszek, L.; Gruca-Rokosz, R.; Pękala, A.; Czarnora, J. Heavy Metal Accumulation in Sediments of Small Retention Reservoirs—Ecological Risk and the Impact of Humic Substances Distribution. Resources 2022, 11, 113. [Google Scholar] [CrossRef]
- Sakar, S.; Jnoue, K.; Mahomed, A.; Ahmed, A.A.; ElFeky, M.G.; Saleh, G.M.; Kamar, M.S.; Aroe, H.; Aono, T.; Sahoo, S.K. Distribution of natural radionuclides in NORM samples from North Abu Rusheid area, Egypt. J. Environ. Radioact. 2023, 266–267, 107240. [Google Scholar] [CrossRef]
- Turhan, S.; Metin, O.; Hancerliogullari, A.; Kunaz, A.; Duran, C. Determination of elemental concentrations of radionuclides in Turkish bentonite and calculation of radiogenic heat generation. Int. J. Environ. Anal. Chem. 2022, 102, 1–10. [Google Scholar] [CrossRef]
- Sidigue, E.; Elhaddad, M.A.; Abdelwahab, S.F.; El Hadek, H.H. Health Hazards Assessment and Geochemistry of ElSibai-Abu ElTiyur Granites, Central Eastern Desert, Egypt. Appl. Sci. 2021, 11, 12002. [Google Scholar] [CrossRef]
- Khattab, M.R. Alpha spectrometry isotopic ratios indications in the Paleozoic sedimentary rock of El Gor area, Southwestern Sinai, Egypt: Insights on uranium mobility age. J. Environ. Sci. Health Part A 2023, 58, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gezer, F.; Şeref, T.; Yüksel, U. Radiometric analysis of micas used in many industries and evaluation of radiological hazards. Radiochim. Acta 2021, 109, 643–651. [Google Scholar] [CrossRef]
- Gilarska, A.; Hycnar, E. Influence weahtering processes over mineralogy and petrographic characteristic of rocks from the Teritiary—Mezosoic zone in the Bałchatów deposit. Górnictwo Odkryw. 2007, 49, 24–29. [Google Scholar]
- Pękala, A. Silification of the mesozoic rocks accompanying the bełchatów lignite deposit, central Poland. Geosciences 2020, 10, 141. [Google Scholar] [CrossRef]
- Pękala, A. Rock raw materials from the Mesozoic–Neogene contact zone in the Bełchatów Lignite Deposit–recognition and evaluation of their utility. Miner. Resour. Manag. 2020, 36, 127–144. [Google Scholar] [CrossRef]
- Pękala, A. Thorium and uranium in the rock raw materials used for the production of building materials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022033. [Google Scholar] [CrossRef]
- Pękala, A. The Opoka-Rock from the Mesozoic/Neogene Contact Zone in the Bełchatów Lignite Deposit—Characteristics of a Petrographic Nature and as a Raw Material. J. Ecol. Eng. 2019, 20, 232–237. [Google Scholar] [CrossRef]
- Ciuk, E.; Piwocki, M. Tertiary geology in the Kleszczów fault trench and its surroundings. In Guide of the LII Congress of the Polish Geological Society; Geological Publishing: Warszawa, Poland, 1980. (In Polish) [Google Scholar]
- Available online: https://www.kwb.pl/historia_kwb.php (accessed on 10 July 2024).
- Czarnecki, L.; Frankowski, R.; Kuszneruk, J. Lithostratigraphic profile of Tertiary deposits of the Bełchatów deposit. In Symposium: “Geology of Polish Coal-Bearing Formations”; Lipiarski, I., Ed.; Publishing House of the AGH University of Science and Technology: Kraków, Poland, 1992. [Google Scholar]
- Kabata-Pendias, A.; Kabata-Pendias, H. Biogeochemistry of Trace Elements; Scientific Publishing House: Warsaw, Poland, 1999. [Google Scholar]
- Kabata-Pendias, A.; Kabata-Pendias, H. Trace Elements in Soils and Plants; CRC Press, Inc.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. Basics of Environmental Geochemistry; Scientific and Technical Publishing House: Warsaw, Poland, 2007.
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bojakowska, I.; Pasieczna, A. Arsenic and thallium in clayey and carbonate raw materials using for building material productions: Science notebooks. Min. Silesian Univ. Technol. 2008, 285, 43–55. [Google Scholar]
- Bodzioch, A. Biogeochemical Diagenesis of the Lower Muschelkalk of the Opole Region; Scientific Publishing House: Warsaw, Poland, 2005; ISBN 83-232-1572-3. [Google Scholar]
- Piga, G.; Brunetti, A.; Lasio, B.; Malfatti, L.; Galobart, À.; Vecchia, F.M.D.; Enzo, S. New insights about the presence of celestite into fossil bones from Molí del Baró 1 site. Appl. Phys. 2014, 118, 487–496. [Google Scholar] [CrossRef]
- Sánchez-Róman, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Nat. Sci. Rep. 2014, 4, 4767. [Google Scholar] [CrossRef] [PubMed]
- Pye, K.; Dickson, J.A.; Schiavon, N.; Coleman, M.L.; Cox, M. Formation of siderite-Mg-calcite-iron sulphide concretions in intertidal marsh and sandflat sediments, North Nofolk, England. Sedimentology 1990, 37, 325–343. [Google Scholar] [CrossRef]
- Bodzioch, A. Idealized Model of Mineral Infillings in Bones of Fossil Freshwater Animals, on the Example of Late Triassic Metoposaurs from Krasiejów (Poland). Austin J. Earth Sci. 2015, 2, 1008. [Google Scholar]
- Gilarska, A. Iron Sulphide Mineralization in Carbonate Rocks and Sandstones from the Tertiary-Mesozoic Contact Zone of the Bełchatów Deposit. (Szczerców Field). In Proceedings of the 11th Meeting of the Petrology Group of the Mineralogical Society of Poland, Chęciny, Poland, 24–27 October 2004; Volume 24, pp. 171–174. [Google Scholar]
- Sawłowicz, Z. Framboids—From their origin to application. Mineral. Trans. 2000, 88, 1–80. [Google Scholar]
- Szczepanik, P.; Sawłowicz, Z.; Bąk, M. Pyrite framboids in pyritized Radiolarian skeletons (Mid-Cretaceous of the Pieniny Klippen Belt, Western Carpathians, Poland). Ann. Soc. Geol. Pol. 2004, 74, 35–41. [Google Scholar]
- Nieć, M.; Saiamon, E.; Auguścik, J. Variation and utilization of zinc-lead ore resources in Poland. Inst. Gospod. Surowcani Miner. 2018, 102, 129–152. [Google Scholar]
- Fergusson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK, 1990; pp. 85–547. [Google Scholar]
- Bouška, V.; Pešek, J. Quality parameters of lignite of the north Bohemian basin in the Czech republic in comparison with the world average lignite. Int. J. Coal Geol. 1999, 40, 211–235. [Google Scholar] [CrossRef]
- Bojakowska, I. Cadmium in mineral resources of Poland and its potential emission in the environment. Prot. Environ. Nat. Resour. 2009, 40, 22–30. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N.; Ptacek, C.J.; Blowes, D.W. Negative pH and Extremely Acidic Mine Waters from Iron Mountain, California. Environ. Sci. Technol. 2000, 34, 254–258. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Frisbie, S.H.; Smith, E.; Naidu, R.; Jacks, G.; Sarkar, B. Heavy Metals in The Environment; CRC Press: Boca Raton, FL, USA, 2002; pp. 147–215. [Google Scholar]
- Bhattacharya, P.; Polya, D.A.; Jovanovic, D. Best Practice Guide on the Control of Arsenic in Drinking Water; Metals and Related Substances in Drinking Water Series; IWA Publishing: London, UK, 2017; p. 265. [Google Scholar] [CrossRef]
- Clarke, L.B.; Sloss, L. Trace Elements Emission from Coal Combustion and Gasification; IEACR/49; IEA Coal Research: London, UK, 1992. [Google Scholar]
- Welch, A.H.; Oremland, R.S.; Davis, J.A.; Watkins, S.A. Arsenic in Groundwater: A Review of Current Knowledge and Relation to the CALFED Solution Area with Recommendations for Needed Research. J. San Fr. Estuary Watershed Sci. 2006, 4, 8342704q. [Google Scholar] [CrossRef]
- Janardhana Raju, N. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef] [PubMed]
- Charlet, L.; Polya, D.A. Arsenic in Shallow, Reducing Groundwaters in Southern Asia: An Environmental Health Disaster. Elements 2006, 2, 91–96. [Google Scholar] [CrossRef]
- Morin, G.; Calas, G. Arsenic in soils, mine tailings, and former industrial sites. Elements 2006, 2, 97–101. [Google Scholar] [CrossRef]
- Yi Chen, Q.; Costa, M. Arsenic: A Global Envir Pękalaonmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef]
- Irunde, R. Arsenic in Africa: Potential sources, spatial variability, and the state of the art for arsenic removal using locally available materials. Groundw. Sustain. Dev. 2022, 18, 100746. [Google Scholar] [CrossRef]






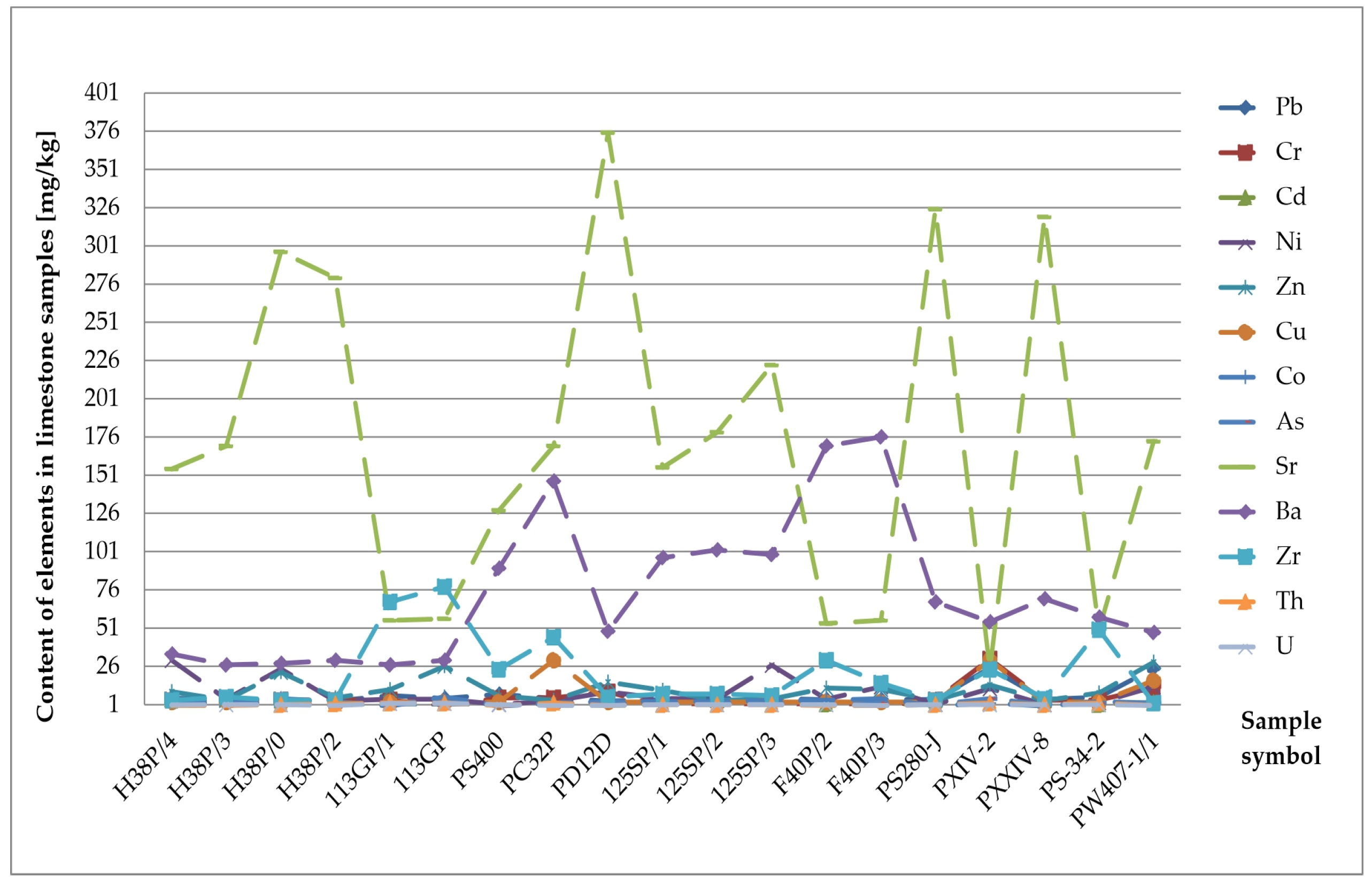
| Content of Element Min–Max (Mean) [mk/g] | Lithological Type of Rocks | |||||||
|---|---|---|---|---|---|---|---|---|
| Limestones | Sandstones | Kaolin Clays | Decalcyfied Opoka Rocks | Opoka Rocks | Diatomites | Marls | Gaizes | |
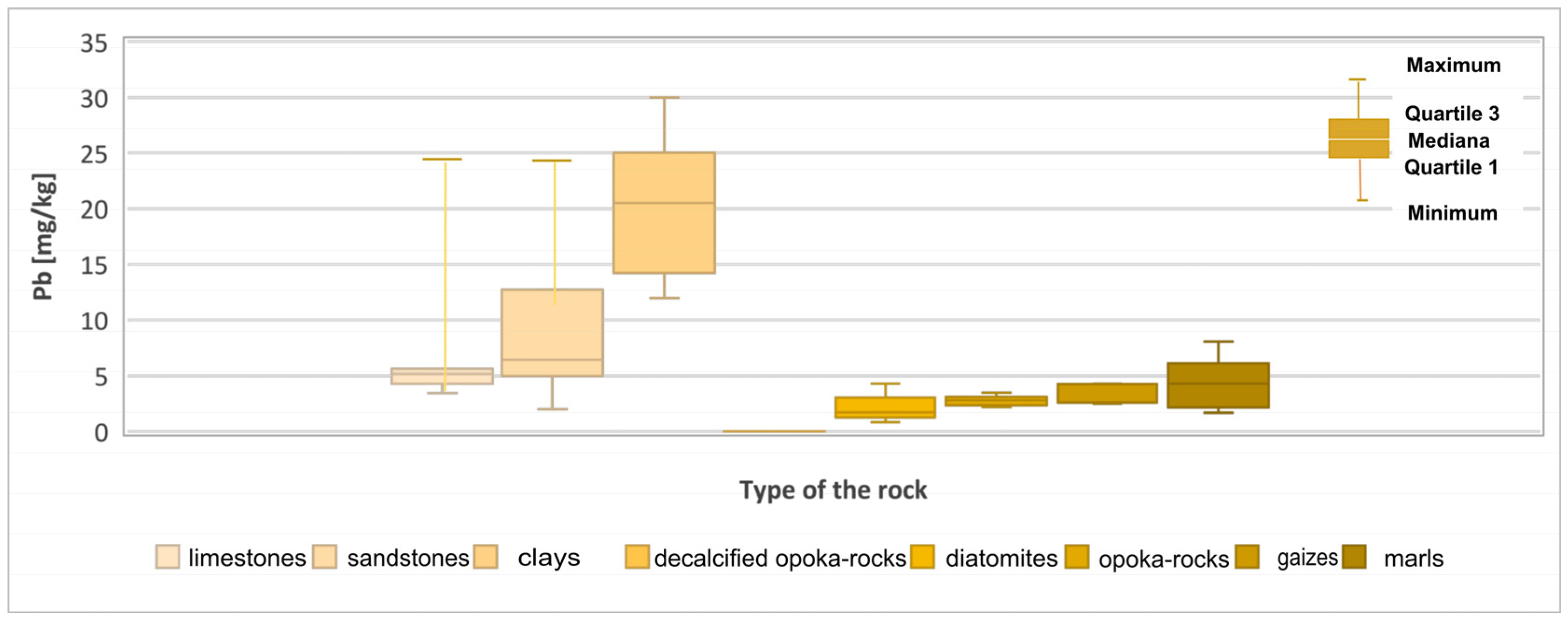
| Pb | 3.44–24.94 (7.01) | 2–24.94 (9.82) | 12–30 (20.1) | 0 | 2.2–3.3 (2.76) | 0–4.3 (2.29) | 1.72–8.06 (4.54) | 2.5–4.3 (3.24) |
| Cr | 0–30.82 (7.57) | 1.43–56 (15.82) | 82–99 (93) | 0–0.05 (0.04) | 5.36–9.40 (6.52) | 0.72–18.76 (8.37) | 0–9.3 (5.9) | 0.02–5.36 (3.13) |
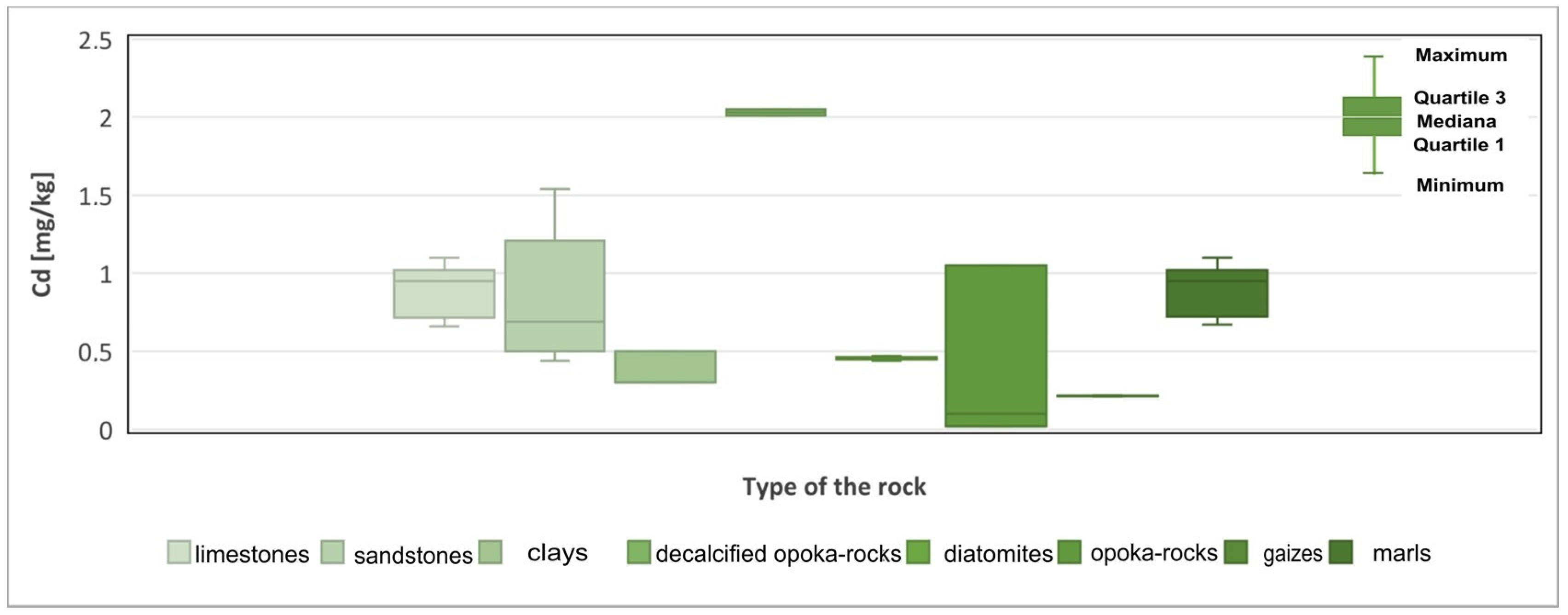
| Cd | 0–5.28 (1395) | 0–1.54 (0.86) | 0.3–0.5 (0.42) | 2.01–2.64 (2.13) | 0–1.1 (0.55) | 0–0.44 (0.44) | 0–1.1 (0.89) | 0–0.22 (0.21) |
| Ni | 1.1–30 (8.86) | 1–20 (8.99) | 42–60 (50.2) | 0 | 3.25–6 (4.54) | 0–18.56 (6.13) | 0–3.48 (2.80) | 2.22–8.12 (4.41) |
| Zn | 3.79–28.75 (10.81) | 2–87 (26.88) | 66–113 (95) | 1.2–1.58 (1.33) | 7.99–9.05 (8.63) | 4.58–77.1 (24.79) | 4.26–46.29 (19.44) | 3–13.43 (6.97) |
| Cu | 0–30 (6.6) | 1–92 (17.21) | 26–57 (47.6) | 1.5–2.8(2.35) | 1.9–9 (4.53) | 2.8–11.2 (6.74) | 2.8–30 (5.76) | 1.95–22.4 (10.29) |
| Co | 0.1–6 (2.76) | 0.1–7 (2.81) | 9–14 (10.4) | 0–001(0.01) | 0–2.21 (1.6) | 1–4 (1.9) | 0–6 (2.92) | 0–4 (3.7) |
| As | 0–5 (1.84) | 1–5 (2.67) | 2–4 (2.83) | 0 | 0–5.1 (2.44) | 0.05–9.6 (6.83) | 0–2 (2) | 1.5–4.3 (2.59) |
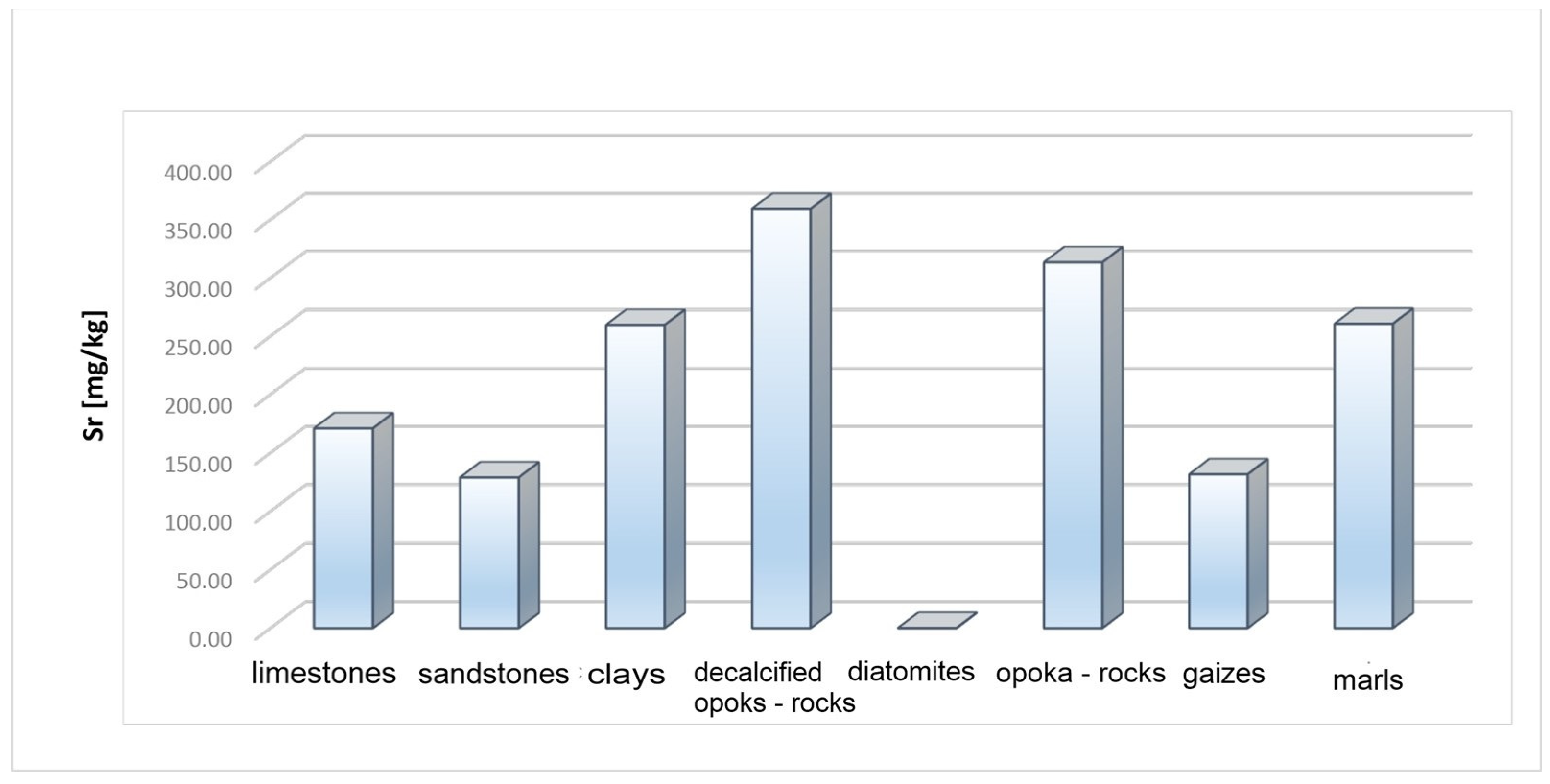
| Sr | 24–375 (170.94) | 3–325 (128.92) | 200–320 (259.6) | 320–398 (359.34) | 223–396 (313.4) | 0–0.05 (0.05) | 203–308 (260.6) | 49–222 (131.6) |
| Ba | 27–176 (73.94) | 23–145 (56.14) | 219–340 (273.1) | 37–40 (38.66) | 38–314 (131.1) | 185–210 (196) | 25–158 (60.1) | 120–190 (144) |
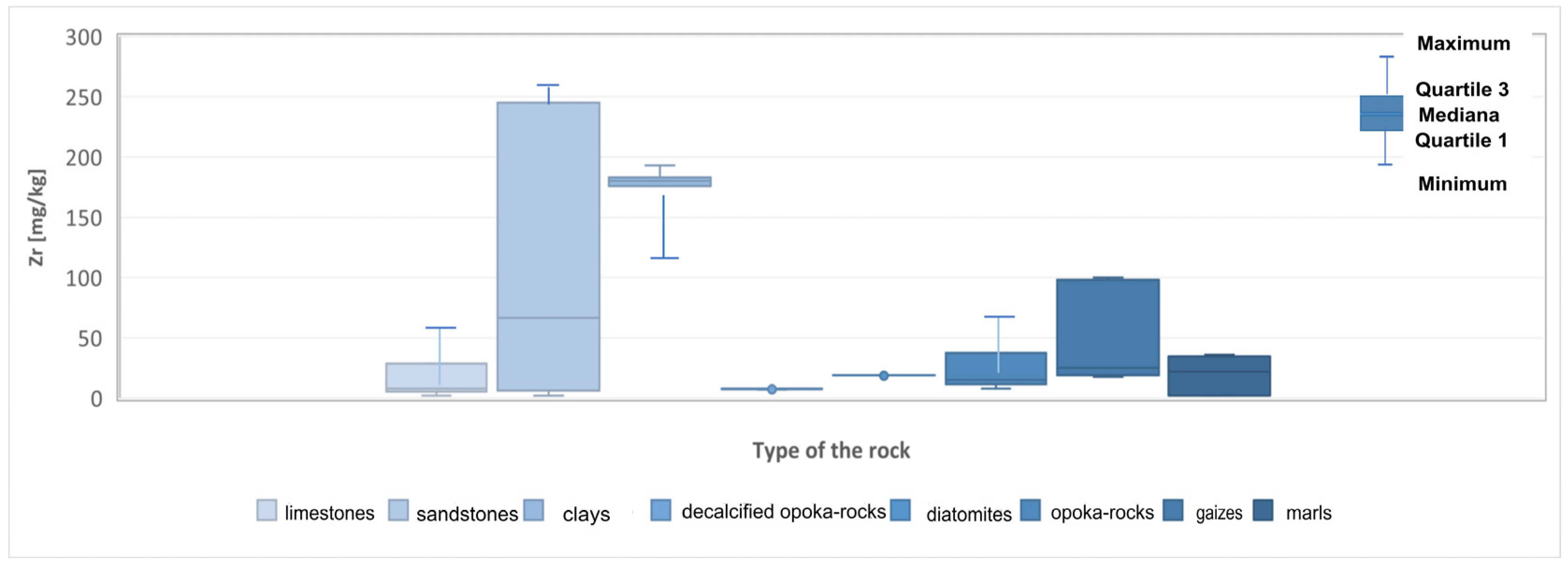
| Zr | 2–78 (20.63) | 2–698 (166) | 120–193 (168.7) | 7–10 (8) | 8–81 (28.8) | 18.5–20 (19.1) | 2–36 (19.2) | 17.5–100 (51.9) |
| Types of Rocks | Mineral Composition |
|---|---|
| Limenstones | Calcite Quartz, Feldspar, Pyrite, Apatite, Celestine, Barite, Gypsum, Siderite, Kaolinite |
| Sandstones | Quartz Feldspars, Micas, Glauconite, Pyrite, Zircon, Heavy minerals |
| Clays | Kaolinite Illite, Feldspar, Quartz |
| Decalcified opoka-rocks | Chalcedony Kaolinite, Quartz, Calcite, Zircon, Rutile, Tourmaline, Pyrite |
| Opoka-rocks | Calcite, Opal-CT Quartz, Chalcedony, Monazite |
| Diatomites | Opal-CT, Quartz Glauconite, Feldspars, Pyrite, Zeolites, Montmorillonite, Illite, Muscovite |
| Marls | Calcite, Opal, Smectite minerals Quartz, Glauconite, Biotite, Muscovite, Celestine, Pyrite |
| Gaizes | Chalcedony, Opal-CT Illit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pękala, A.; Koszelnik, P.; Musiał, M.; Galek, T. Trace Elements Anomalous Concentrations in Building Materials—The Impact of Secondary Mineralisation Processes. Materials 2024, 17, 3909. https://doi.org/10.3390/ma17163909
Pękala A, Koszelnik P, Musiał M, Galek T. Trace Elements Anomalous Concentrations in Building Materials—The Impact of Secondary Mineralisation Processes. Materials. 2024; 17(16):3909. https://doi.org/10.3390/ma17163909
Chicago/Turabian StylePękala, Agnieszka, Piotr Koszelnik, Michał Musiał, and Tomasz Galek. 2024. "Trace Elements Anomalous Concentrations in Building Materials—The Impact of Secondary Mineralisation Processes" Materials 17, no. 16: 3909. https://doi.org/10.3390/ma17163909






