Antifungal Effect of Poly(methyl methacrylate) with Farnesol and Undecylenic Acid against Candida albicans Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physico-Chemical Characterization of PMMA_UDA+FAR Composites
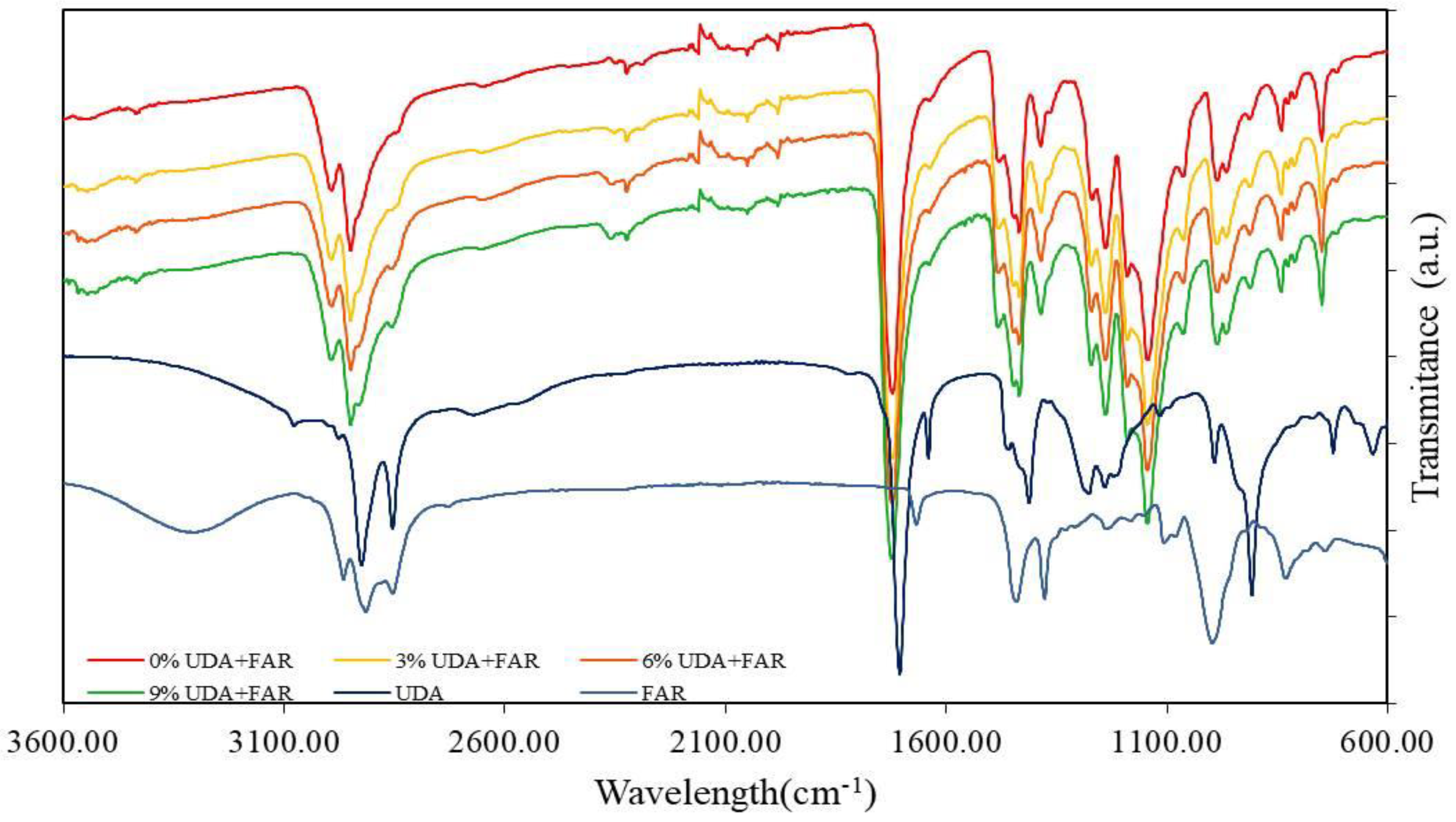
2.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.2. Contact Angle Measurements
2.3. Antifungal Characterization of Composites
2.3.1. Candida Strain and Culture Conditions
2.3.2. Biofilm Formation on PMMA_UDA+FAR Composites and the XTT Test
2.4. Antifungal Susceptibility Test
2.4.1. Determination of the Minimal Inhibitory Concentration (MIC)
2.4.2. Embedded Filamentation Test (EFT)
2.5. Cytotoxicity Study
2.6. Statistic
3. Results and Discussion
3.1. Physico-Chemical Characterization of PMMA_UDA+FAR Composites
3.1.1. FTIR (Chemical Characterization of PMMA_UDA+FAR Composite Surfaces)
3.1.2. Contact Angle Measurement (Physical Characterization of PMMA_UDA+FAR Composite Surfaces)
3.2. Antifungal Characterization of Composites
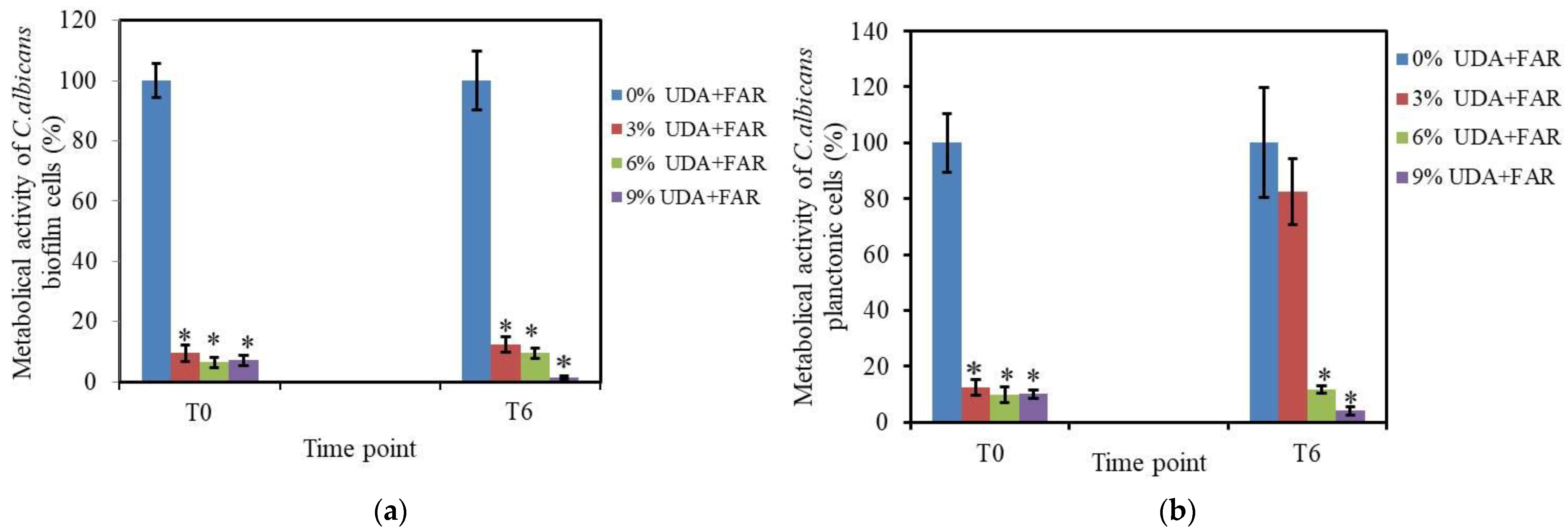
3.2.1. Biofilm Formation and XTT Assay on Biofilm Cells
3.2.2. XTT Assay on Planktonic Cells
3.3. Antifungal Characterization of UDA+FAR
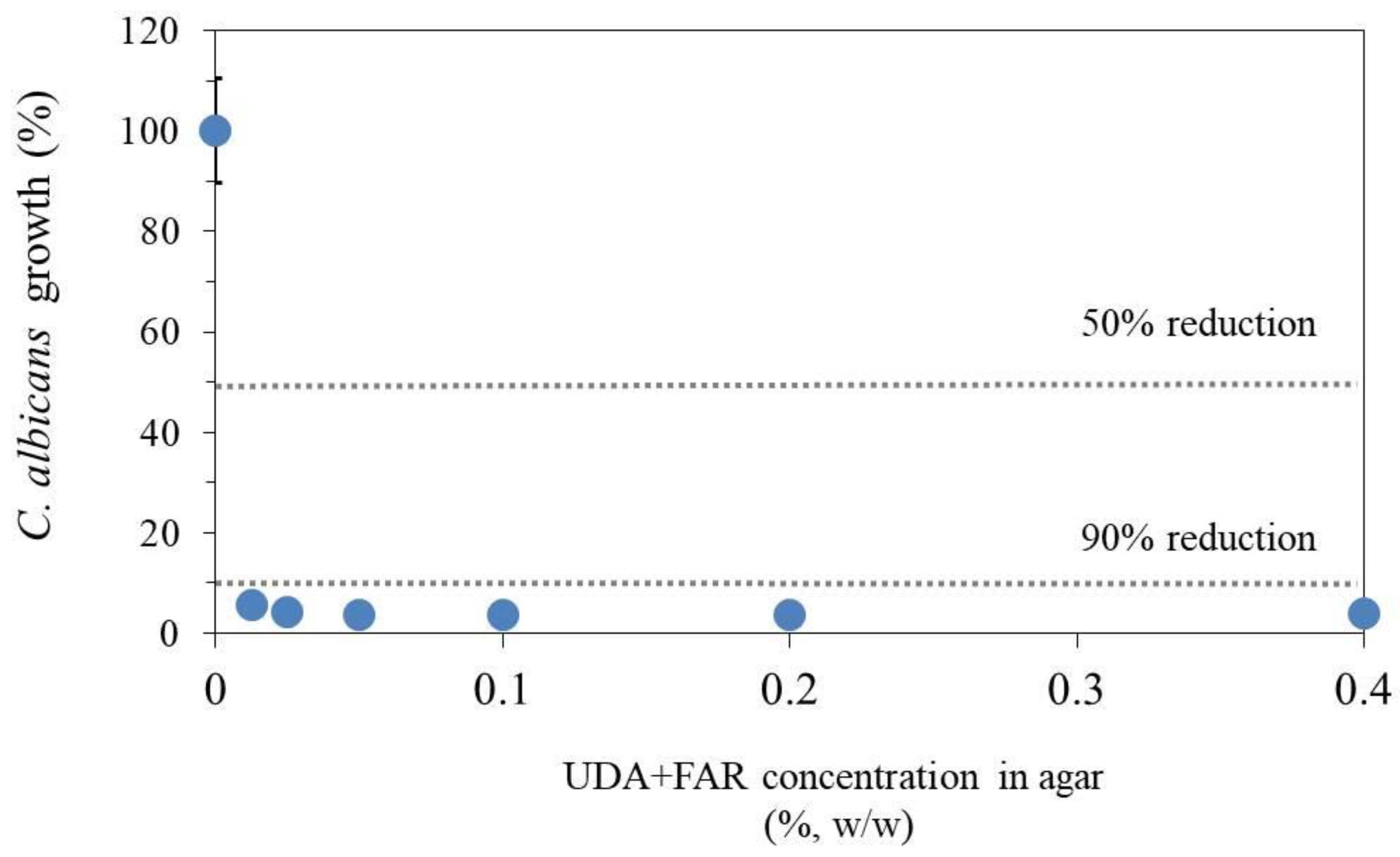
3.3.1. Antifungal Susceptibility Test—Determining Minimal Inhibitory Concentration of UDA+FAR
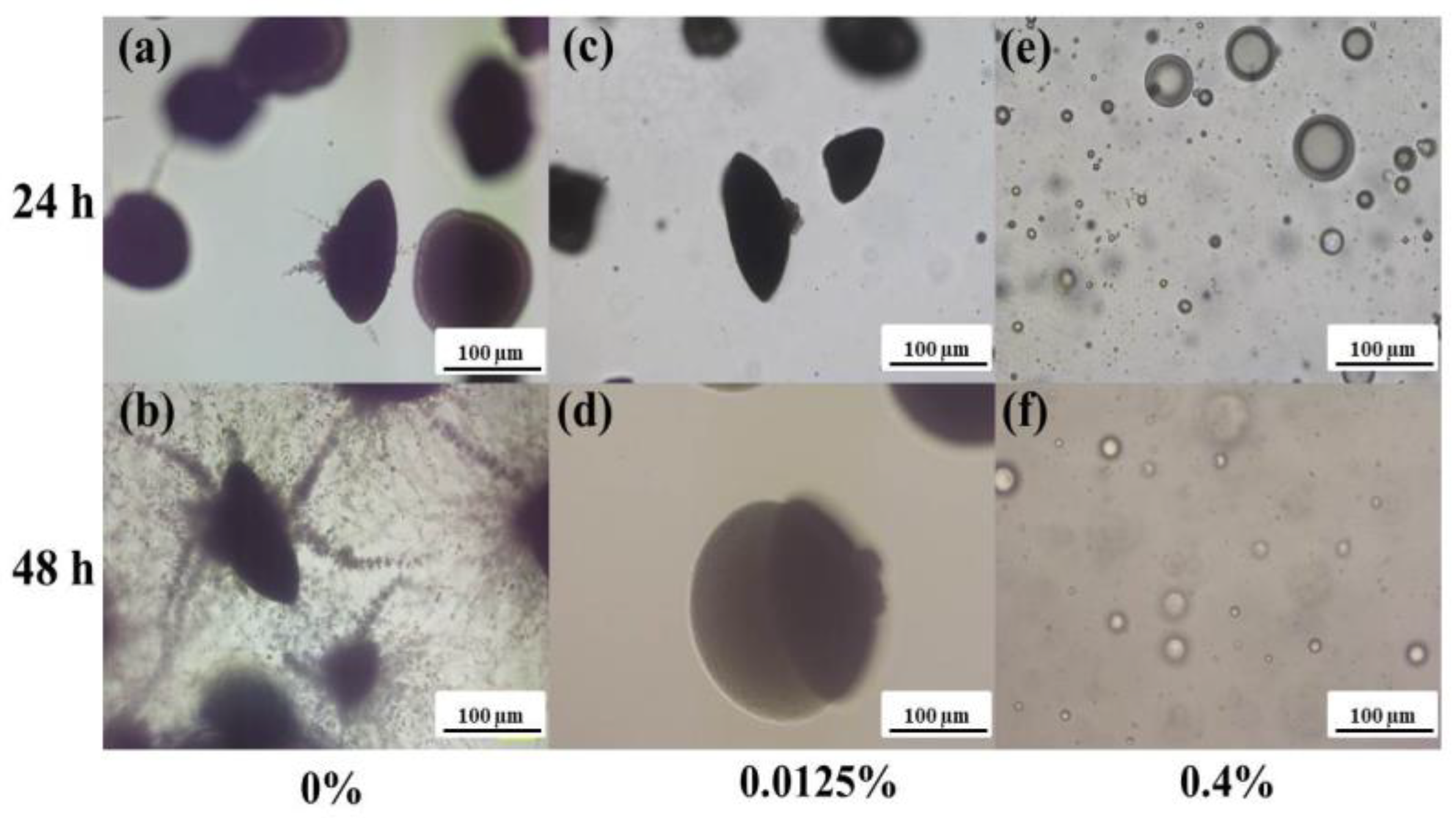
3.3.2. Embedded Filamentation Test
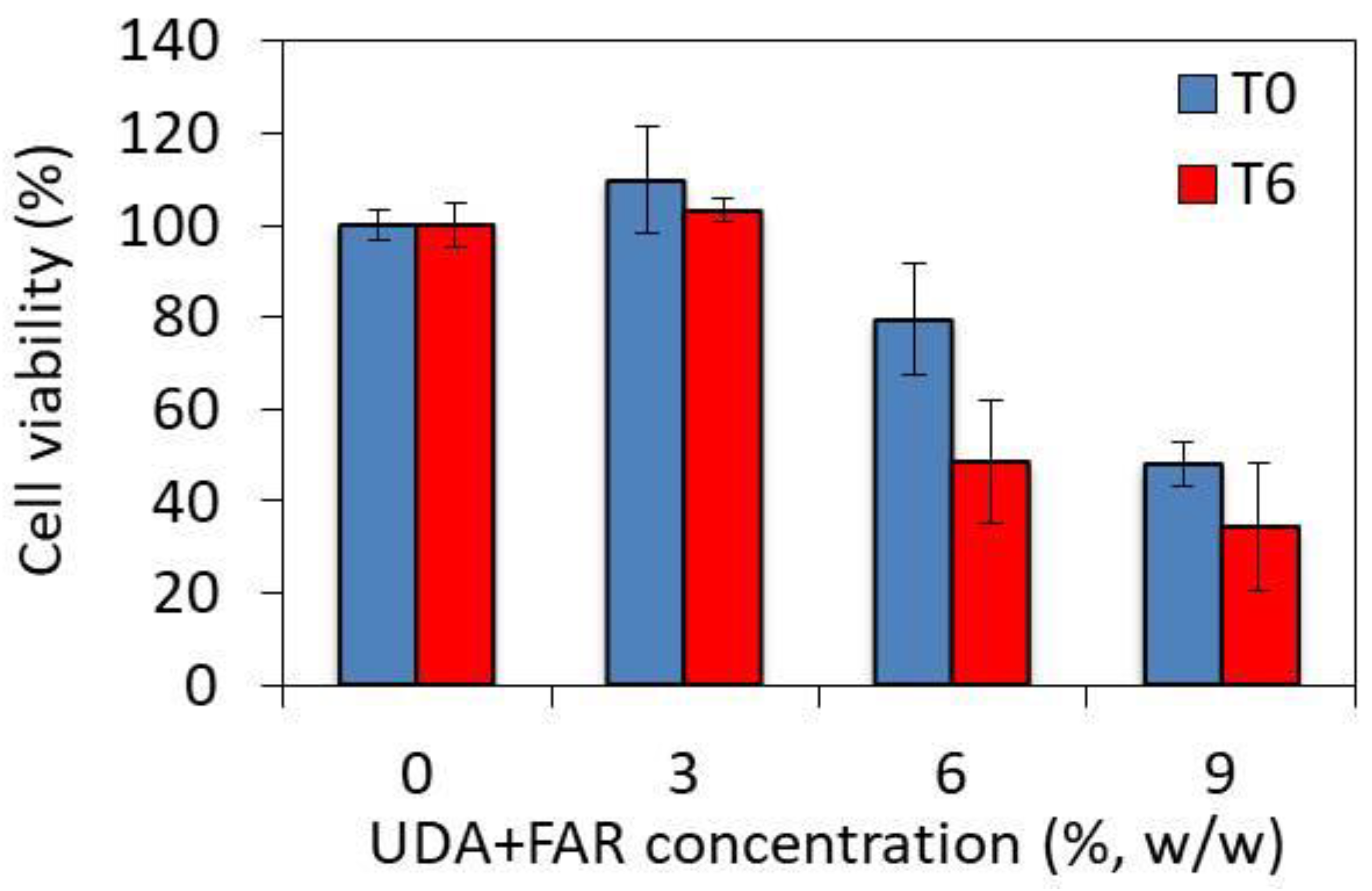
3.4. Toxicity of PMMA_UDA+FAR Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranđelović, M.; Dimitrijević, M.; Otašević, S.; Stanojević, L.; Išljamović, M.; Ignjatović, A.; Arsić-Arsenijević, V.; Stojanović-Radić, Z. Antifungal Activity and Type of Interaction of Melissa officinalis Essential Oil with Antimycotics against Biofilms of Multidrug-Resistant Candida Isolates from Vulvovaginal Mucosa. J. Fungi 2023, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture Stomatitis: Causes, Cures and Prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, D.E.; Moorthy, A.; Moneley, J.O.; Jabra-Rizk, M.A.; Sultan, A.S. Denture Stomatitis-An Interdisciplinary Clinical Review. J. Prosthodont. 2023, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- de Barros, P.P.; Rossoni, R.D.; de Souza, C.M.; Scorzoni, L.; Fenley, J.D.C.; Junqueira, J.C. Candida Biofilms: An Update on Developmental Mechanisms and Therapeutic Challenges. Mycopathologia 2020, 185, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Ajetunmobi, O.H.; Badali, H.; Romo, J.A.; Ramage, G.; Lopez-Ribot, J.L. Antifungal Therapy of Candida Biofilms: Past, Present and Future. Biofilm 2023, 5, 100126. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials 2021, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.Z.; Khan, S.B. Antimicrobial Efficacy of Silver Nanoparticles against Candida albicans. Materials 2022, 15, 5666. [Google Scholar] [CrossRef] [PubMed]
- Bruzual, I.; Riggle, P.; Hadley, S.; Kumamoto, C.A. Biofilm Formation by Fluconazole-Resistant Candida albicans Strains Is Inhibited by Fluconazole. J. Antimicrob. Chemother. 2007, 59, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M. Current Perspectives and the Future of Candida albicans-Associated Denture Stomatitis Treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Alharbi, F.A.; Suresh, C.S. Effectiveness of Coating Acrylic Resin Dentures on Preventing Candida Adhesion. J. Prosthodont. 2013, 22, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Yodmongkol, S.; Chantarachindawong, R.; Thaweboon, S.; Thaweboon, B.; Amornsakchai, T.; Srikhirin, T. The Effects of Silane-SiO2 Nanocomposite Films on Candida albicans Adhesion and the Surface and Physical Properties of Acrylic Resin Denture Base Material. J. Prosthet. Dent. 2014, 112, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Lazarin, A.A.; Zamperini, C.A.; Vergani, C.E.; Wady, A.F.; Giampaolo, E.T.; Machado, A.L. Candida albicans Adherence to an Acrylic Resin Modified by Experimental Photopolymerised Coatings: An In Vitro Study. Gerodontology 2014, 31, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Fouad, H.; Hashem, M.; Niazy, A.A.; AlBadah, A. Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials 2018, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Partida, E.; Valdez-Salas, B.; Curiel-Álvarez, M.; Castillo-Uribe, S.; Escamilla, A.; Nedev, N. Enhanced Antifungal Activity by Disinfected Titanium Dioxide Nanotubes via Reduced Nano-Adhesion Bonds. Mater. Sci. Eng. C 2017, 76, 59–65. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Ghazali, M.J.; Muchtar, A.; Azhari, C.H. Studies on the Effects of Titanate and Silane Coupling Agents on the Performance of Poly (Methyl Methacrylate)/Barium Titanate Denture Base Nanocomposites. J. Dent. 2017, 56, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Kim, M.-J.; Oh, S.-H.; Kwon, J.-S. Novel Dental Poly (Methyl Methacrylate) Containing Phytoncide for Antifungal Effect and Inhibition of Oral Multispecies Biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Kalamarz, I.; Pakieła, W.; Barszczewska-Rybarek, I.; Czuba, Z.; Mertas, A. A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties. Materials 2024, 17, 902. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In Vitro Interactions between Farnesol and Fluconazole, Amphotericin B or Micafungin against Candida albicans Biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Bonvin, D.; Hofmann, H.; Mionić Ebersold, M. Fungicidal PMMA-Undecylenic Acid Composites. Int. J. Mol. Sci. 2018, 19, 184. [Google Scholar] [CrossRef]
- Nikoomanesh, F.; Falahatinejad, M.; Černáková, L.; dos Santos, A.L.S.; Mohammadi, S.R.; Rafiee, M.; Rodrigues, C.F.; Roudbary, M. Combination of Farnesol with Common Antifungal Drugs: Inhibitory Effect against Candida Species Isolated from Women with RVVC. Medicina 2023, 59, 743. [Google Scholar] [CrossRef]
- Petrovic, M.; Bonvin, D.; Todic, J.; Zivkovic, R.; Randjelovic, M.; Arsenijevic, V.A.; Ebersold, M.M.; Otasevic, S. Surface Modification of Poly(Methyl-Methacrylate) with Farnesol to Prevent Candida Biofilm Formation. Lett. Appl. Microbiol. 2022, 75, 982–990. [Google Scholar] [CrossRef]
- Petrović, M.; Randjelović, M.; Igić, M.; Randjelović, M.; Arsić Arsenijević, V.; Mionić Ebersold, M.; Otašević, S.; Milošević, I. Poly(Methyl Methacrylate) with Oleic Acid as an Efficient Candida albicans Biofilm Repellent. Materials 2022, 15, 3750. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Finckbeiner, S.; Yu, J.S.; Wiemer, D.F.; Eisner, T.; Attygalle, A.B. Characterization of (E,E)-Farnesol and Its Fatty Acid Esters from Anal Scent Glands of Nutria (Myocastor coypus) by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Infrared Spectrometry. J. Chromatogr. A 2007, 1165, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Horta, S.; Santos, M.; Rocha, S.M.; Trindade, T. Release Behavior of Trans,Trans-Farnesol Entrapped in Amorphous Silica Capsules. Results Pharma Sci. 2012, 2, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-X.; Xu, X.; Wu, C.D. In Vitro Synergism between Berberine and Miconazole against Planktonic and Biofilm Candida Cultures. Arch. Oral. Biol. 2011, 56, 565–572. [Google Scholar] [CrossRef]
- Li, L.; Finnegan, M.B.; Özkan, S.; Kim, Y.; Lillehoj, P.B.; Ho, C.-M.; Lux, R.; Mito, R.; Loewy, Z.; Shi, W. In Vitro Study of Biofilm Formation and Effectiveness of Antimicrobial Treatment on Various Dental Material Surfaces. Mol. Oral. Microbiol. 2010, 25, 384–390. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, F.; Yeh, C.-K.; Sun, Y. Controlling Fungal Biofilms with Functional Drug Delivery Denture Biomaterials. Colloids Surf. B Biointerfaces 2016, 140, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Guo, Z. Bioinspired Surfaces with Wettability: Biomolecule Adhesion Behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, G.; Pan, J.; Ye, G.; Sun, K.; Zhang, J.; Wang, J. Cold Plasma-Induced Surface Modification of Heat-Polymerized Acrylic Resin and Prevention of Early Adherence of Candida albicans. Dent. Mater. J. 2015, 34, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Drutz, D.J.; Zajic, J.E. Factors Governing Adherence of Candida Species to Plastic Surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Akiba, N.; Minakuchi, S. Hydrophilic Surface Modification of Acrylic Denture Base Material by Silica Coating and Its Influence on Candida albicans Adherence. J. Med. Dent. Sci. 2012, 59, 1–7. [Google Scholar] [PubMed]
- Amirabad, L.M.; Tahriri, M.; Zarrintaj, P.; Ghaffari, R.; Tayebi, L. Preparation and Characterization of TiO2-Coated Polymerization of Methyl Methacrylate (PMMA) for Biomedical Applications: In Vitro Study. Asia Pac. J. Chem. Eng. 2022, 17, e2761. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Tsutsumi-Arai, C.; Takakusaki, K.; Oya, T.; Fueki, K.; Wakabayashi, N. Superhydrophilic Co-Polymer Coatings on Denture Surfaces Reduce Candida albicans Adhesion-An In Vitro Study. Arch. Oral. Biol. 2018, 87, 143–150. [Google Scholar] [CrossRef]
- Puri, G.; Berzins, D.W.; Dhuru, V.B.; Raj, P.A.; Rambhia, S.K.; Dhir, G.; Dentino, A.R. Effect of Phosphate Group Addition on the Properties of Denture Base Resins. J. Prosthet. Dent. 2008, 100, 302–308. [Google Scholar] [CrossRef] [PubMed]
- McLain, N.; Ascanio, R.; Baker, C.; Strohaver, R.A.; Dolan, J.W. Undecylenic Acid Inhibits Morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhao, Y.; Yan, H.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Qiu, Y.; Li, D.; Liu, W. Antifungal Effects of Undecylenic Acid on the Biofilm Formation of Candida albicans. Int. J. Clin. Pharmacol. Ther. 2016, 54, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Mekala, S.; Chaffin, W.L. Farnesol-Mediated Inhibition of Candida albicans Yeast Growth and Rescue by a Diacylglycerol Analogue. Yeast 2007, 24, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; López-Ribot, J.L. Inhibition of Candida albicans Biofilm Formation by Farnesol, a Quorum-Sensing Molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Kowalewska, A.; Kręgiel, D. Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies. Surfaces 2020, 3, 197–210. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A Simple and Reproducible 96-Well Plate-Based Method for the Formation of Fungal Biofilms and Its Application to Antifungal Susceptibility Testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Išljamović, M.; Bonvin, D.; Milojević, M.; Stojanović, S.; Spasić, M.; Stojković, B.; Janošević, P.; Otašević, S.; Ebersold, M.M. Antifungal Effect of Poly(methyl methacrylate) with Farnesol and Undecylenic Acid against Candida albicans Biofilm Formation. Materials 2024, 17, 3936. https://doi.org/10.3390/ma17163936
Išljamović M, Bonvin D, Milojević M, Stojanović S, Spasić M, Stojković B, Janošević P, Otašević S, Ebersold MM. Antifungal Effect of Poly(methyl methacrylate) with Farnesol and Undecylenic Acid against Candida albicans Biofilm Formation. Materials. 2024; 17(16):3936. https://doi.org/10.3390/ma17163936
Chicago/Turabian StyleIšljamović, Milica, Debora Bonvin, Milena Milojević, Simona Stojanović, Milan Spasić, Branislava Stojković, Predrag Janošević, Suzana Otašević, and Marijana Mionić Ebersold. 2024. "Antifungal Effect of Poly(methyl methacrylate) with Farnesol and Undecylenic Acid against Candida albicans Biofilm Formation" Materials 17, no. 16: 3936. https://doi.org/10.3390/ma17163936





