Preparation and Properties of Lightweight Aggregates from Discarded Al2O3-ZrO2-C Refractories
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Processing
2.3. Characterization
3. Results and Analysis
3.1. Determination of Calcination Temperature
3.1.1. Phase Composition
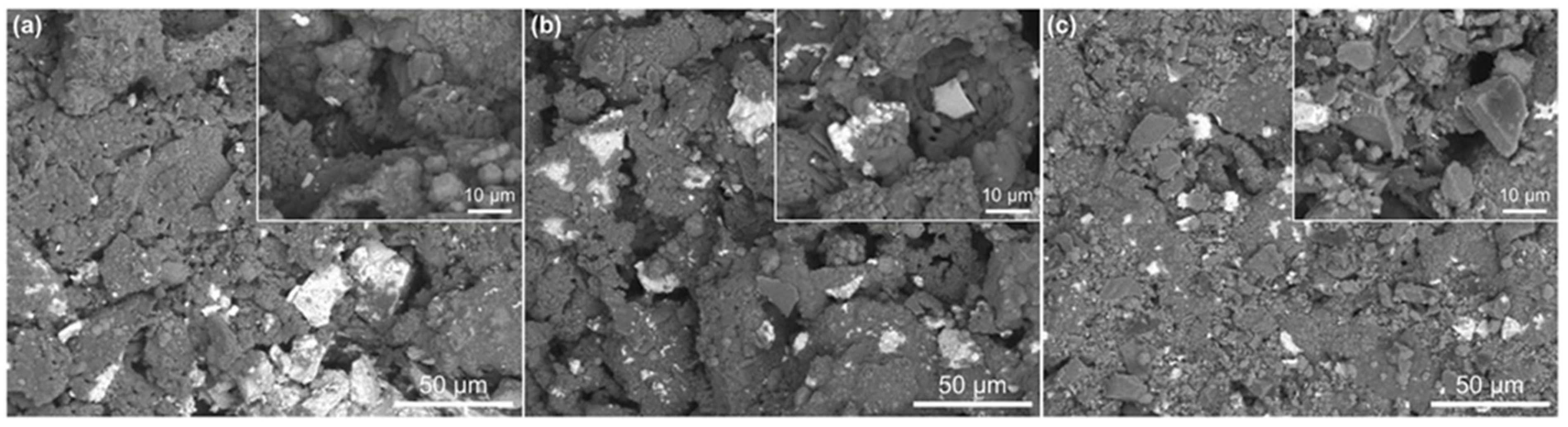
3.1.2. Microstructure Evolution
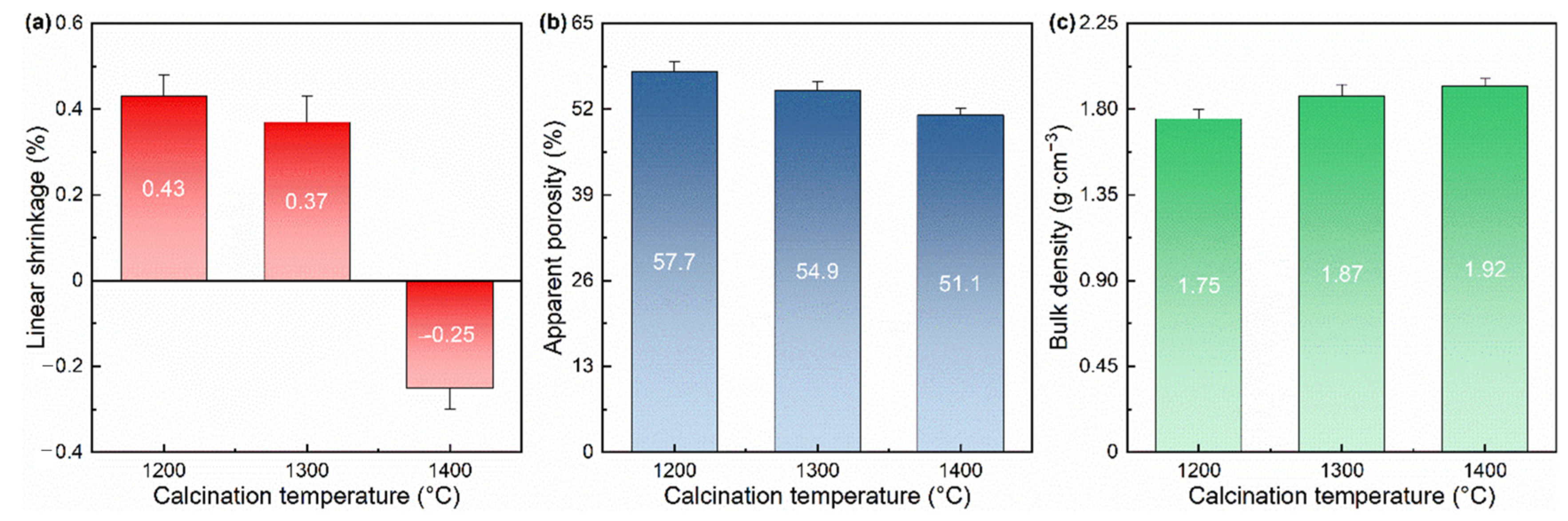
3.1.3. Porosity and Density
3.1.4. Cold Compressive Strength and Thermal Shock Resistance
3.2. Effect of Light Calcined Magnesia Addition
3.2.1. Phase Composition
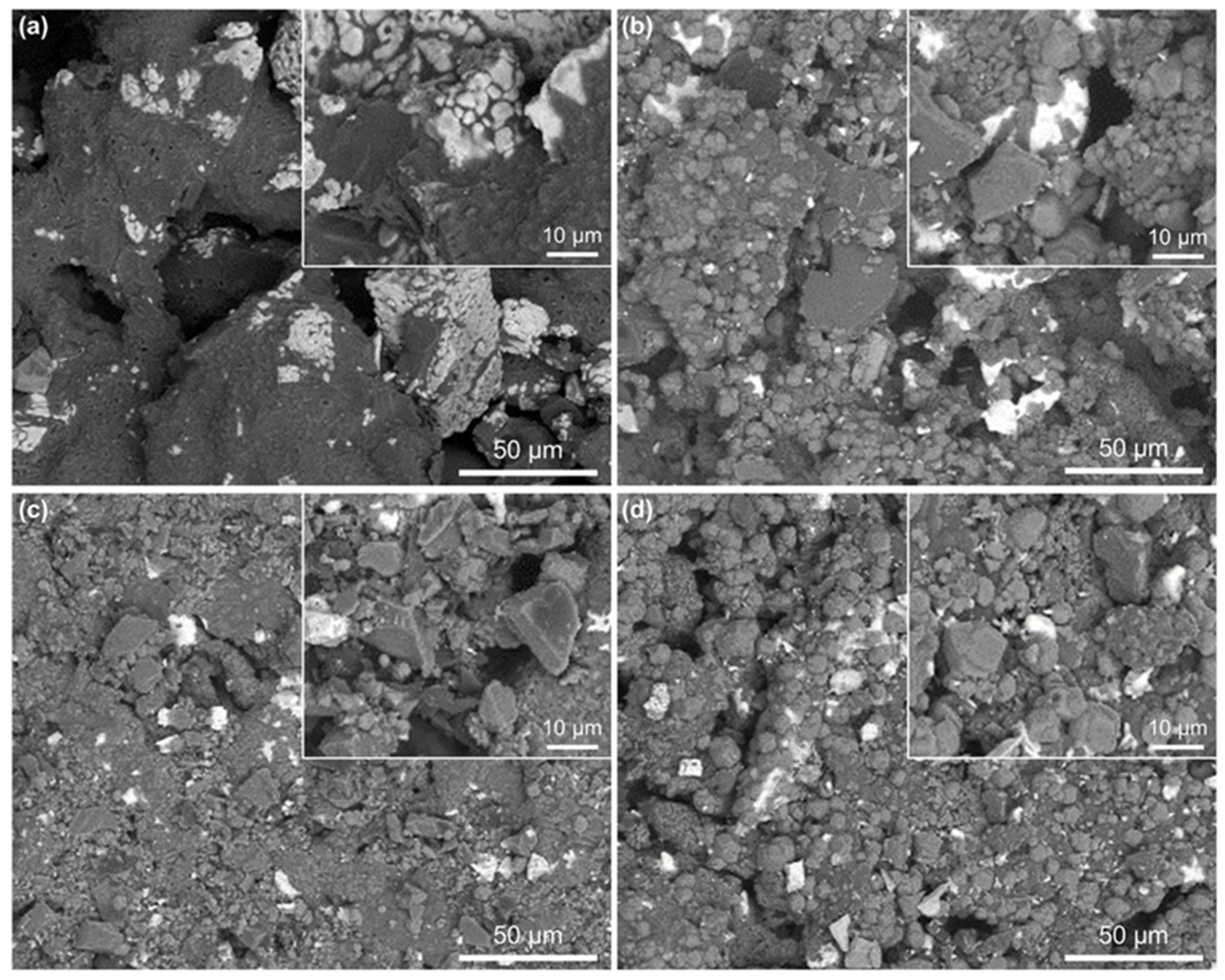
3.2.2. Microstructure Evolution
3.2.3. Porosity and Density
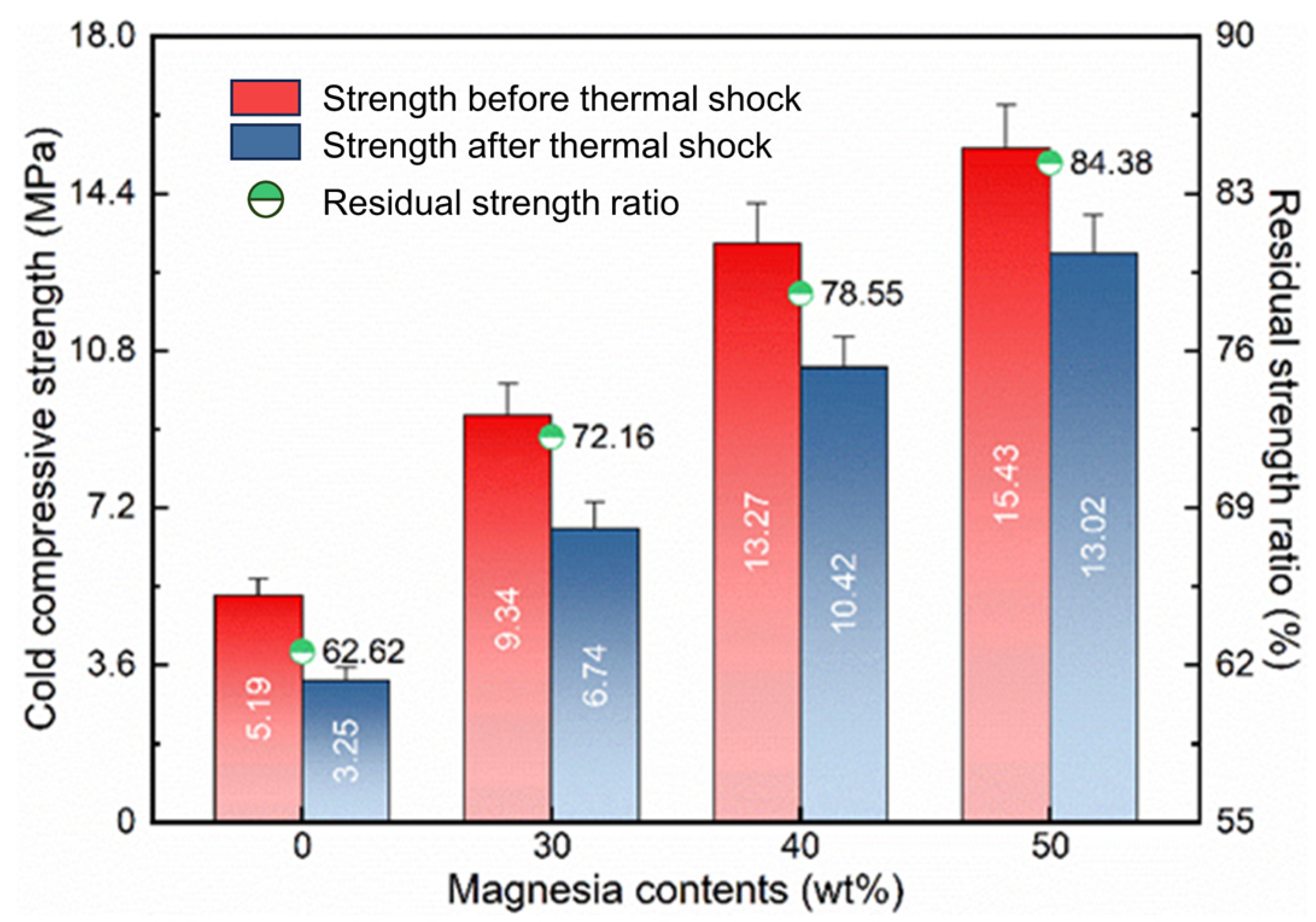
3.2.4. Cold Compressive Strength and Thermal Shock Resistance
3.3. Analysis of Lightweight Aggregate Formation
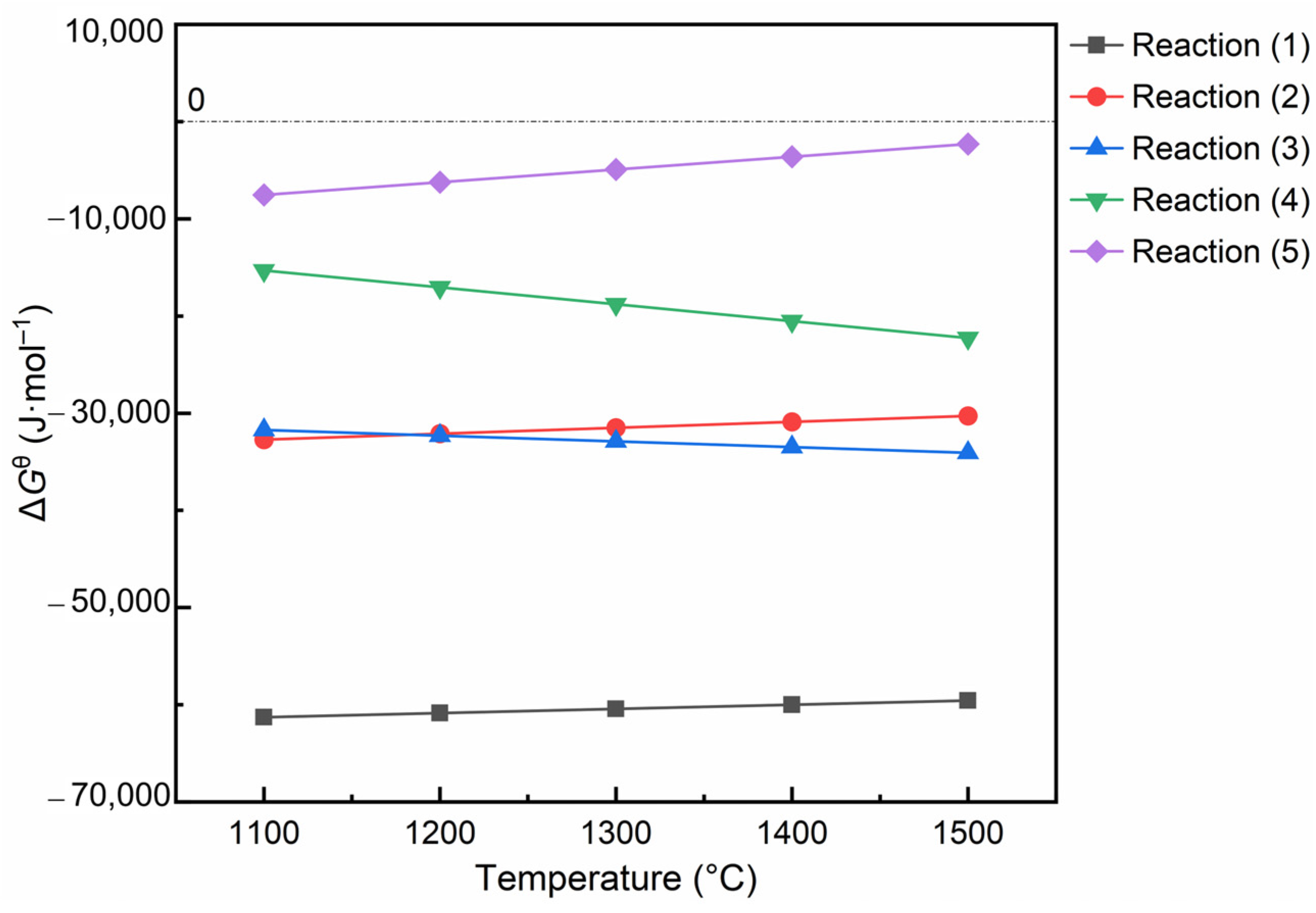
3.3.1. Thermodynamics
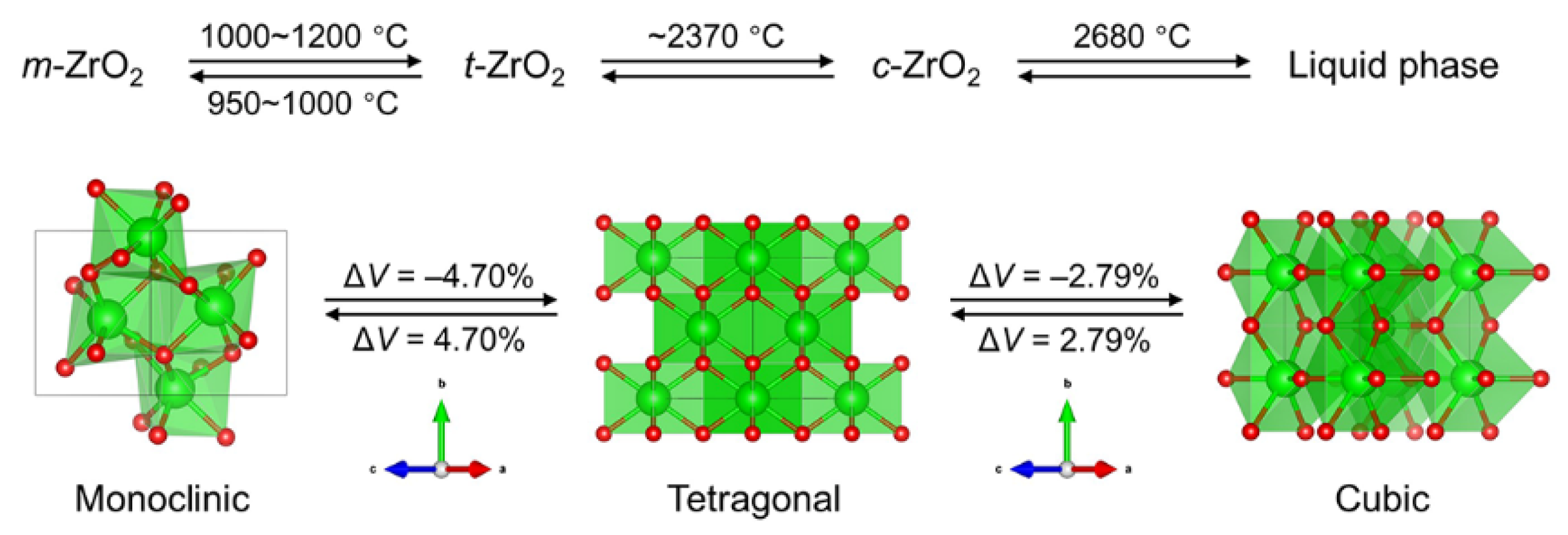
3.3.2. Lightweight Mechanism
4. Conclusions
- (1)
- As the calcination temperature rises, the apparent porosity of the prepared lightweight aggregates decreases, and the cold compressive strength and thermal shock resistance increase. The comprehensive performance of the sample fired at 1400 °C is relatively balanced: the linear shrinkage is −0.25%; the apparent porosity is 51.1%; the bulk density is 1.92 g·cm−3; the cold compressive strength is 13.27 MPa; and the residual strength ratio (thermal shock resistance) is 78.55%.
- (2)
- With the introduction of light calcined magnesia, the apparent porosity, cold compressive strength, and thermal shock resistance of the prepared lightweight aggregates are effectively improved. This can be attributed to the formation of the magnesia-alumina spinel and the phase transformation toughening mechanism. However, for applications in high-temperature insulation environments, the effects of properties such as thermal conductivity need to be further considered to determine the optimal addition amount.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.T.; Tan, H.B.; Du, C.; Wang, J.; Deng, X.F.; Zheng, Z.Q.; He, X.Y. Enhancement of ultra-fine slag on compressive strength of solid waste-based cementitious materials: Towards low carbon emissions. J. Build. Eng. 2022, 63, 105475. [Google Scholar] [CrossRef]
- Fu, C.; Liang, J.W.; Yang, G.; Dagestani, A.; Liu, W.; Luo, X.D.; Zeng, B.B.; Wu, H.D.; Huang, M.P.; Lin, L.F.; et al. Recycling of waste glass as raw materials for the preparation of self-cleaning, light-weight and high-strength porous ceramics. J. Clean. Prod. 2021, 317, 128395. [Google Scholar] [CrossRef]
- Li, M.J.; Zheng, F.; Wang, J.; Jia, D.H.; Mao, X.D.; Li, P.; Yuan, Q.; Zhen, Q.; Yu, Y. Energy-saving production of high value-added foamed glass ceramic from blast furnace slag and hazardous wastes containing heavy metal ions. J. Clean. Prod. 2023, 383, 135544. [Google Scholar] [CrossRef]
- Horckmans, L.; Nielsen, P.; Dierckx, P.; Ducastel, A. Recycling of refractory bricks used in basic steelmaking: A review. Resour. Conserv. Recycl. 2019, 140, 297–304. [Google Scholar] [CrossRef]
- Muñoz, I.; Soto, A.; Maza, D.; Bayón, F. Life cycle assessment of refractory waste management in a Spanish steel works. Waste Manag. 2020, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.; Oliveira, K.S.; Fernandes, L.; Tiba, P.; Prado, U.S. Porous refractory ceramics for high-temperature thermal insulation—Part 1: The science behind energy saving. Interceram 2021, 70, 38–45. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Kozlovskiy, A.A.; Konuhova, M.; Popov, A.I.; Shlimas, D.D.; Borgekov, D.B. Determination of gamma radiation shielding efficiency by radiation-resistant composite ZrO2–Al2O3–TiO2–WO3–Nb2O5 ceramics. Opt. Mater. 2024, 154, 115752. [Google Scholar] [CrossRef]
- Agravat, D.; Patel, S.K.; Almawgani, A.H.M.; Irfan, M.; Armghan, A.; Taya, S.A. Graphite-based surface plasmon resonance structure using Al2O3-TiO2-ZrO2 materials for solar thermal absorption. Plasmonics 2024, 19, 227–238. [Google Scholar] [CrossRef]
- Li, X.X.; Yan, L.W.; Zhang, Y.B.; Yang, X.K.; Guo, A.R.; Du, H.Y.; Hou, F.; Liu, J.C. Lightweight porous silica ceramics with ultra-low thermal conductivity and enhanced compressive strength. Ceram. Int. 2022, 48, 9788–9796. [Google Scholar] [CrossRef]
- Yan, W.; Wu, G.Y.; Ma, S.B.; Schafföner, S.; Dai, Y.J.; Chen, Z.; Qi, J.T.; Li, N. Energy efficient lightweight periclase-magnesium alumina spinel castables containing porous aggregates for the working lining of steel ladles. J. Eur. Ceram. Soc. 2018, 38, 4276–4282. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, N.Y.; Ye, Y.C.; Fu, F.H.; Lv, Y.F.; Jia, J.P.; Chen, D.X.; Ou, Z.B.; Li, J.L. A novel route for the fabrication of melilite-spinel porous ceramics with ultralow thermal conductivity and sufficient strength. Ceram. Int. 2022, 48, 37488–37491. [Google Scholar] [CrossRef]
- Pan, M.B.; Li, X.; Wu, X.P.; Zhao, F.; Ma, C.L. Preparation of thermal insulation materials based on granite waste using a high-temperature micro-foaming method. J. Asian Ceram. Soc. 2022, 10, 223–229. [Google Scholar] [CrossRef]
- Fan, Y.B.; Li, S.J.; Yin, B.; Li, Y.B.; Tu, Z.; Cai, Z. Preparation and microstructure evolution of novel ultra-low thermal conductivity calcium silicate-based ceramic foams. Ceram. Int. 2022, 48, 21602–21611. [Google Scholar] [CrossRef]
- Pilli, V.; Priyadarshini, S.; Sarkar, R. Nanocarbon containing low carbon Al2O3-C refractories: Comparison between N220 and N990 nanocarbons. Int. J. Appl. Ceram. Technol. 2022, 19, 2761–2779. [Google Scholar] [CrossRef]
- Wei, X.W.; Yehorov, A.; Storti, E.; Dudczig, S.; Fabrichnaya, O.; Aneziris, C.G.; Volkova, O. Phenomenon of whiskers formation in Al2O3−C refractories. Adv. Eng. Mater. 2022, 24, 2100718. [Google Scholar] [CrossRef]
- Feng, C.Z.; Xiao, G.Q.; Ding, D.H.; Lei, C.K.; Lv, L.H.; Chong, X.C.; Feng, Y.; Zou, C. Enhanced thermal shock resistance of low-carbon Al2O3-C refractories via CNTs/MgAl2O4 whiskers composite reinforcement. J. Am. Ceram. Soc. 2024, 107, 3881–3894. [Google Scholar] [CrossRef]
- Ren, X.M.; Ma, B.Y.; Tian, J.L.; Jiang, Z.H. The effects of pre-sintering temperature and La2O3 addition on sinterability and corrosion resistance of stoichiometric magnesium aluminate spinel. Ceram. Int. 2022, 48, 32470–32478. [Google Scholar] [CrossRef]
- Ren, X.M.; Wang, Z.H.; Ji, W.; Hou, S.Y.; Sun, S.L.; Fu, G.F.; Deng, C.J.; Ma, B.Y. Porous MgO-based ceramics synthesized by the volume expansion effect: Designing and performance. J. Mater. Res. Technol. 2024, 28, 4370–4381. [Google Scholar] [CrossRef]
- Mandal, S.; Hemrick, J.G.; Mahapatra, M.K. Zinc aluminate (ZnAl2O4) refractory aggregates: Dilatometric sintering studies and thermal expansion coefficient. J. Eur. Ceram. Soc. 2022, 42, 6244–6254. [Google Scholar] [CrossRef]
- Gu, Q.; Liu, G.Q.; Li, H.X.; Jia, Q.L.; Zhao, F.; Liu, X.H. Synthesis of MgO–MgAl2O4 refractory aggregates for application in MgO–C slide plate. Ceram. Int. 2019, 45, 24768–24776. [Google Scholar] [CrossRef]
- Brito-Chaparro, J.A.; Aguilar-Elguezabal, A.; Echeberria, J.; Bocanegra-Bernal, M.H. Using high-purity MgO nanopowder as a stabilizer in two different particle size monoclinic ZrO2: Its influence on the fracture toughness. Mater. Chem. Phys. 2009, 114, 407–414. [Google Scholar] [CrossRef]
- Ren, X.M.; Ma, B.Y.; Tang, J.H.; Li, Y.W.; Yu, J.K. Fabrication of energy-saving MgO with large grain size and low thermal conductivity: Towards a new type of magnesia for high-temperature furnaces. Constr. Build. Mater. 2022, 342, 128097. [Google Scholar] [CrossRef]
- Hofer, A.-K.; Kraleva, I.; Prötsch, T.; Vratanar, A.; Wratschko, M.; Bermejo, R. Effect of second phase addition of zirconia on the mechanical response of textured alumina ceramics. J. Eur. Ceram. Soc. 2023, 43, 2935–2942. [Google Scholar] [CrossRef]
- Hasselman, D.P.H. Unified theory of thermal shock fracture initiation and crack propagation in brittle ceramics. J. Am. Ceram. Soc. 1969, 52, 600–604. [Google Scholar] [CrossRef]
- Hasselman, D.P.H. Griffith criterion and thermal shock resistance of single-phase versus multiphase brittle ceramics. J. Am. Ceram. Soc. 1969, 52, 288–289. [Google Scholar] [CrossRef]
- Hasselman, D.P.H. Elastic energy at fracture and surface energy as design criteria for thermal shock. J. Am. Ceram. Soc. 1963, 46, 535–540. [Google Scholar] [CrossRef]




| Al2O3 | CaO | C | MgO | SiO2 | ZrO2 | Other | |
|---|---|---|---|---|---|---|---|
| Used Al2O3-ZrO2-C refractories | 65.83 | - | 22.1 | 0.29 | 3.83 | 7.55 | 0.4 |
| Light calcined magnesia | - | 1.09 | - | 95.52 | 2.69 | - | 0.7 |
| Chemical Equation | Gibbs Free Energy | Serial Number |
|---|---|---|
| 2MgO(s) + SiO2(s) = Mg2SiO4(s) | −68,200 + 4.31T (T < 2171.15 K) | (1) |
| MgO(s) + SiO2(s) = MgSiO3(s) | −41,100 + 6.1T (T < 1850.15 K) | (2) |
| MgO(s) + Al2O3(s) = MgAl2O4(s) | −23,604 − 5.91T (T < 1973.15 K) | (3) |
| 3Al2O3(s) + 2SiO2(s) = 3Al2O3·2SiO2(s) | 8600 − 17.41T (T < 2123.15 K) | (4) |
| ZrO2(s) + SiO2(s) = ZrSiO4(s) | −25,496 + 13.08T (T < 1949.15 K) | (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Qu, J.; Sun, M.; Ren, X.; Gong, C.; Mu, X.; Zan, W.; Zhou, Z.; Deng, C.; Ma, B. Preparation and Properties of Lightweight Aggregates from Discarded Al2O3-ZrO2-C Refractories. Materials 2024, 17, 3968. https://doi.org/10.3390/ma17163968
Sun S, Qu J, Sun M, Ren X, Gong C, Mu X, Zan W, Zhou Z, Deng C, Ma B. Preparation and Properties of Lightweight Aggregates from Discarded Al2O3-ZrO2-C Refractories. Materials. 2024; 17(16):3968. https://doi.org/10.3390/ma17163968
Chicago/Turabian StyleSun, Shuli, Junfeng Qu, Mengyong Sun, Xinming Ren, Cheng Gong, Xin Mu, Wenyu Zan, Zhangyan Zhou, Chengji Deng, and Beiyue Ma. 2024. "Preparation and Properties of Lightweight Aggregates from Discarded Al2O3-ZrO2-C Refractories" Materials 17, no. 16: 3968. https://doi.org/10.3390/ma17163968





