In-Situ Sulfuration of CoAl Metal–Organic Framework for Enhanced Supercapacitor Properties
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Materials Synthesis
2.3. Characterizations and Electrochemical Measurements
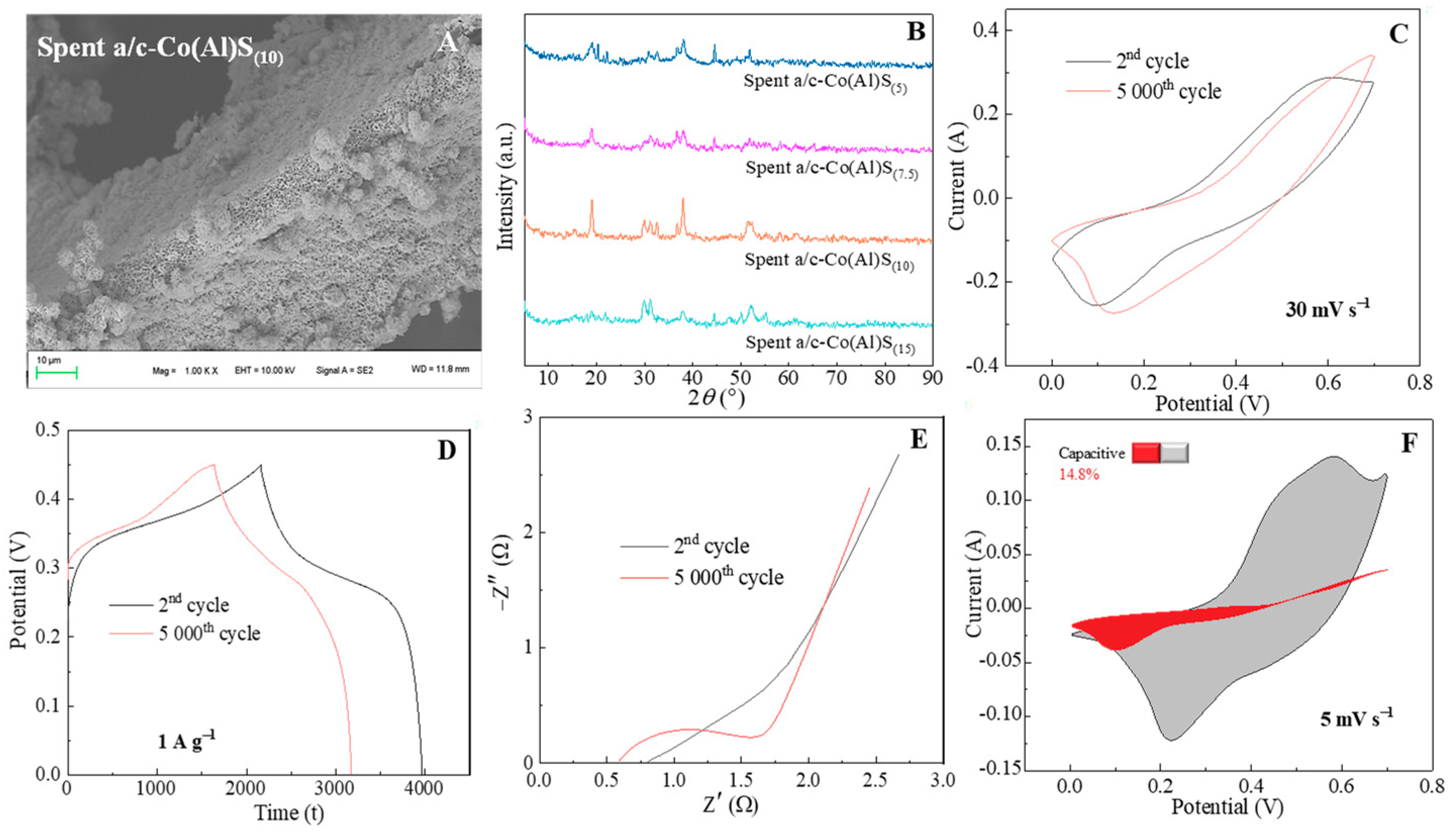
3. Result and Discussion
3.1. Synthesis of Materials
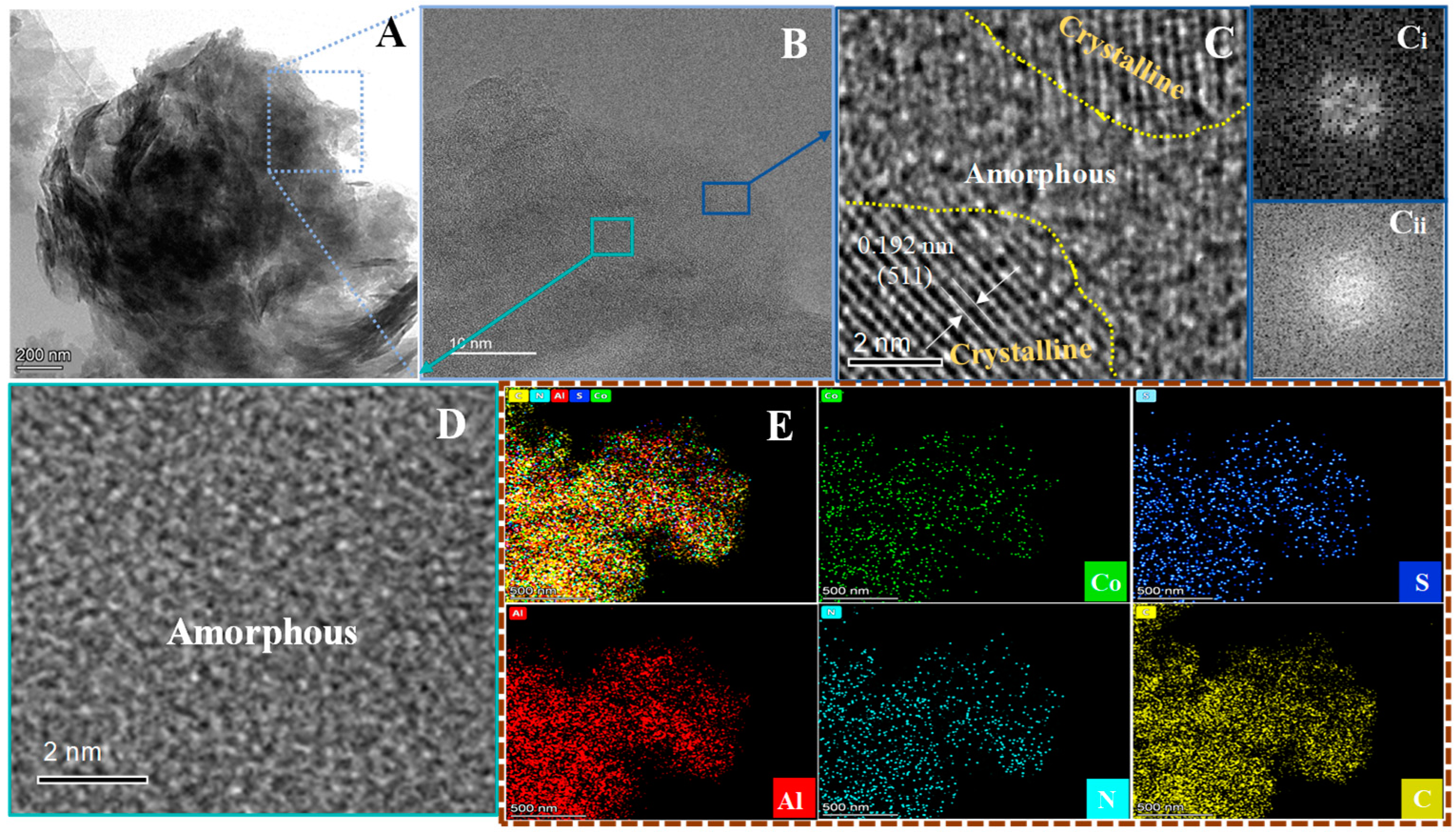
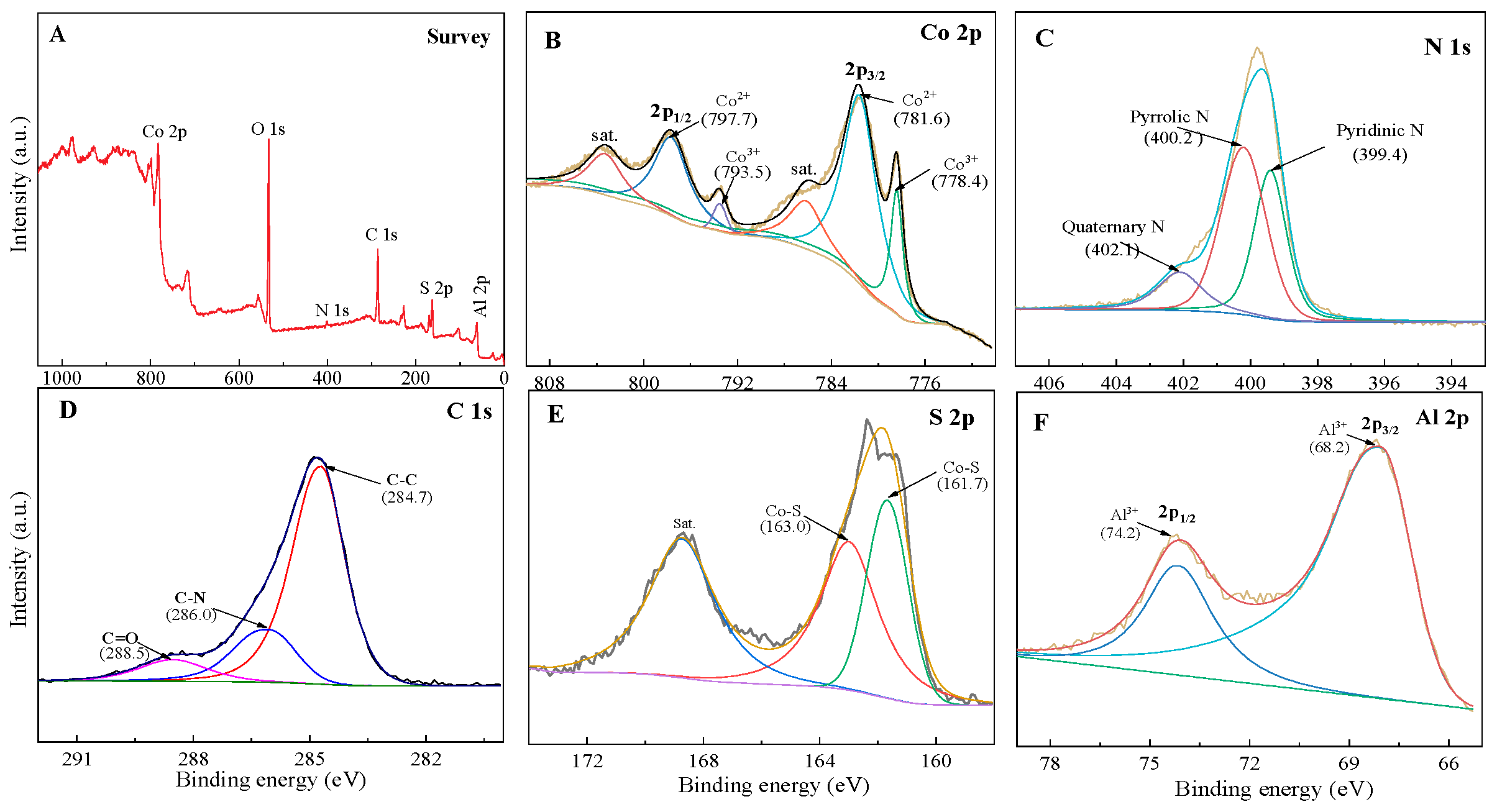
3.2. Structural Characterization of Materials
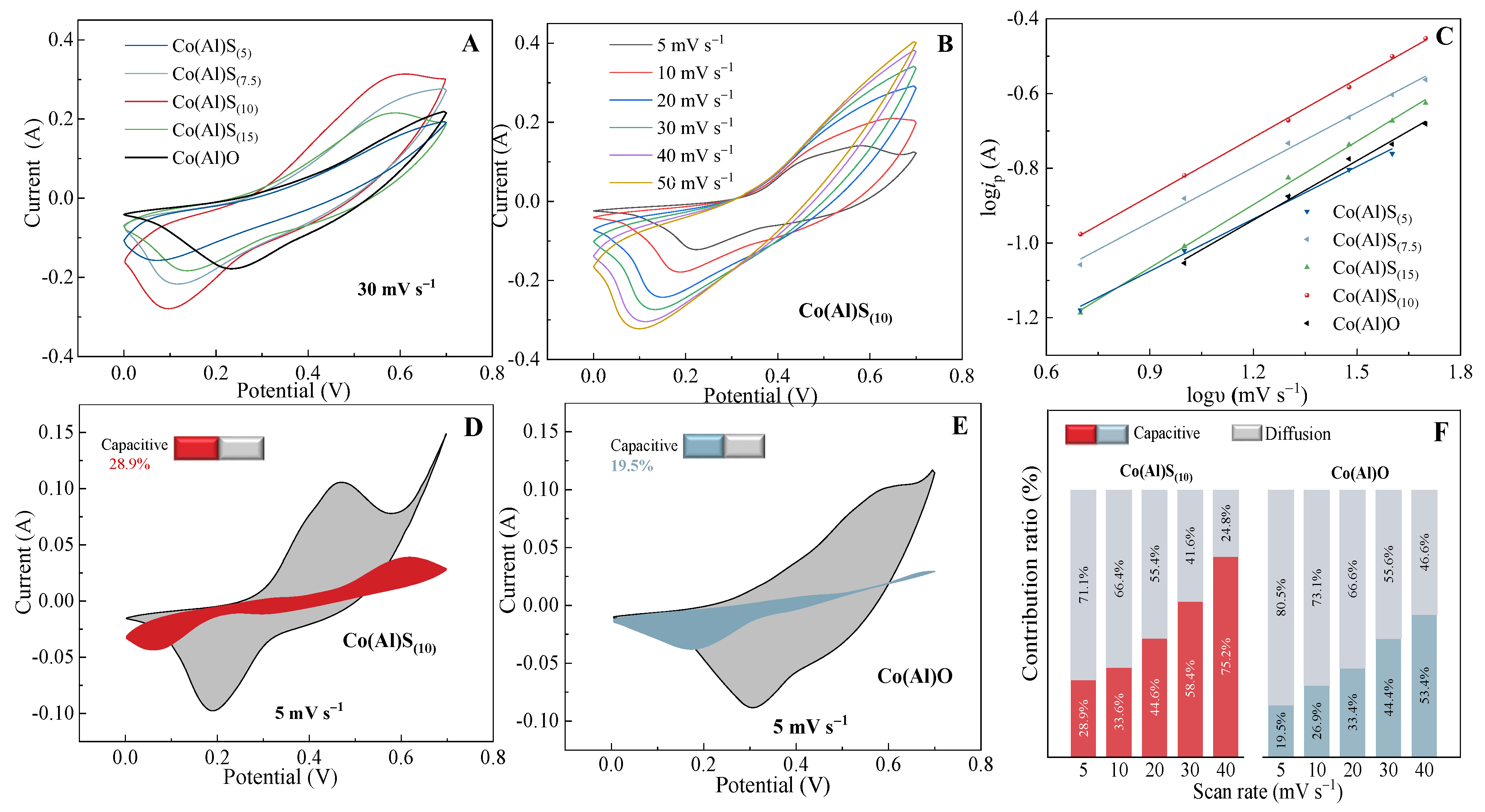
3.3. Electrochemical Performance of Materials
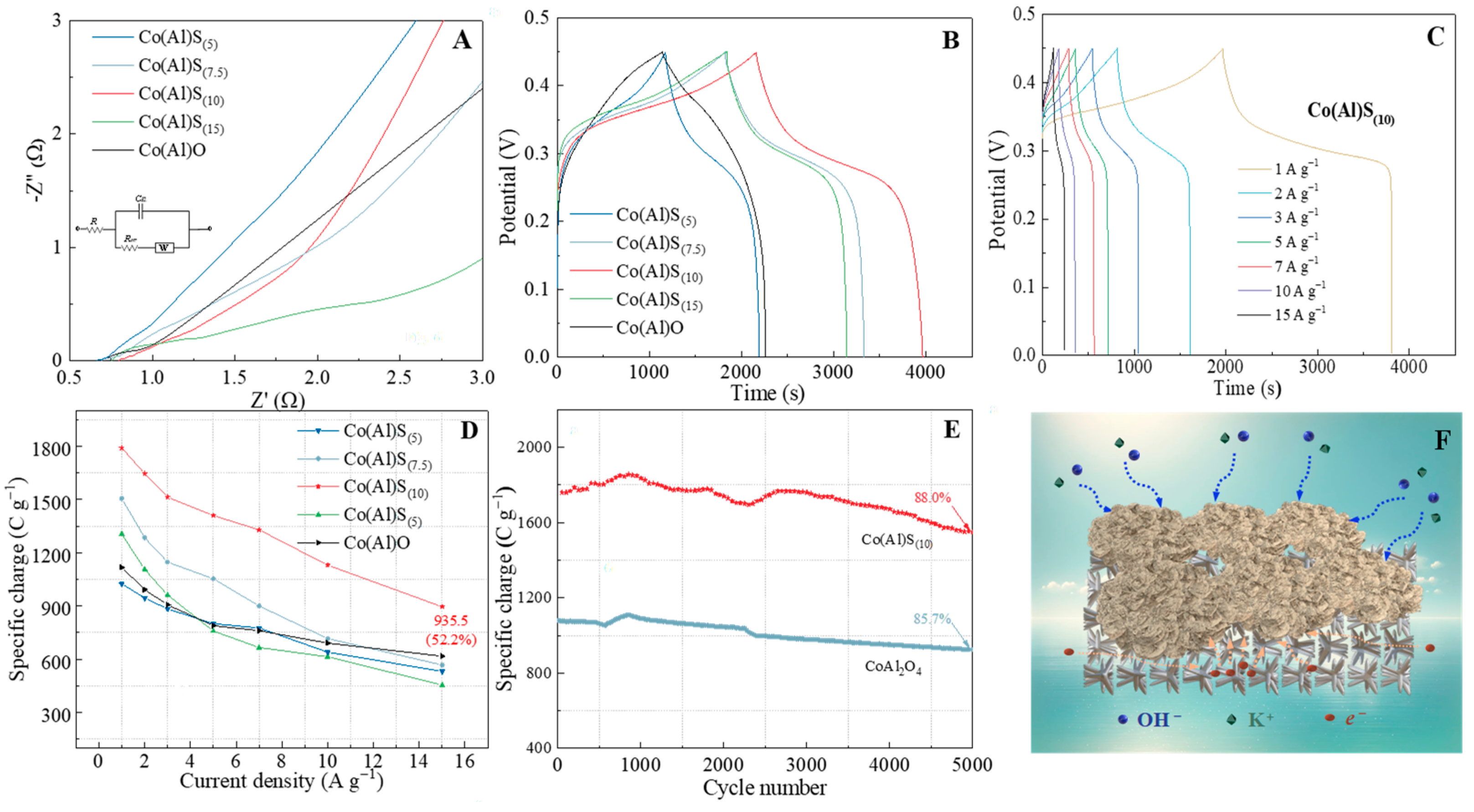
3.4. Performance of the Co(Al)S//AC Hybrid Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.; Iqbal, M.Z.; Hegazy, H.H. Recent advancements in redox-active transition metal sulfides as battery-grade electrode materials for hybrid supercapacitors. J. Energy Storage 2023, 73, 108857. [Google Scholar] [CrossRef]
- Park, H.W.; Roh, K.C. Recent advances in and perspectives on pseudocapacitive materials for supercapacitors—A review. J. Power Sources 2023, 557, 232558. [Google Scholar] [CrossRef]
- Dong, W.; Xie, M.; Zhao, S.; Qin, Q.; Huang, F. Materials design and preparation for high energy density and high power density electrochemical supercapacitors. Mater. Sci. Eng. R 2023, 152, 100713. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Shao, Z. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Mustaqeem, M.; Naikoo, G.A.; Yarmohammadi, M.; Pedram, M.Z.; Pourfarzad, H.; Dar, R.A.; Taha, S.A.; Hassan, I.U.; Bhat, M.Y.; Chen, Y.F. Rational design of metal oxide-based electrode materials for high performance supercapacitors—A review. J. Energy Storage 2022, 55, 105419. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Sridhar, D.; Xu, B.B.; Algadi, H.; El-Bahy, Z.M.; Ma, Y.; Li, T.; Guo, Z. Progress of layered double hydroxide-based materials for supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar] [CrossRef]
- Tang, G.; Liang, J.; Wu, W. Transition metal selenides for supercapacitors. Adv. Funct. Mater. 2024, 34, 2310399. [Google Scholar] [CrossRef]
- Öztürk, O.; Gür, E. Layered transition metal sulfides for supercapacitor applications. ChemElectroChem 2024, 11, e202300575. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Dahiya, Y.; Hariram, M.; Kumar, M.; Jain, A.; Sarkar, D. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coordin. Chem. Rev. 2022, 451, 214265. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar] [CrossRef]
- Hao, C.; Ni, C.; Wang, X.; Pan, Y.; Wu, Q.; Wu, J.; Wang, X. Fabrication of three-dimensional CuS2@CoNi2S4 core-shell rod-like structures as cathode and thistle-derived carbon as anode for hybrid supercapacitors. Chem. Eng. J. 2023, 465, 143024. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, B.; Guo, X.; Li, W.; Ma, W.; Wu, X.; Pang, H. MOF-derived metal sulfides for electrochemical energy applications. Energy Storage Mater. 2022, 51, 840–872. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.L.; Lin, J.N.; Zou, M.Y.; Gu, S.T.; Hong, X.J.; Si, L.P.; Cai, Y.P. MOF-derived Ni3S4 encapsulated in 3D conductive network for high-performance supercapacitor. Inorg. Chem. 2020, 59, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Bai, Y.; Liu, C.; Cao, S.; Kong, Q.; Pang, H. Applications of metal-organic framework-derived N, P, S doped materials in electrochemical energy conversion and storage. Coordin. Chem. Rev. 2022, 2466, 214602. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sekhar, S.C.; Ramulu, B.; Yu, J.S. High-performance hybrid supercapacitors based on MOF-derived hollow ternary chalcogenides. Energy Storage Mater. 2021, 35, 750–760. [Google Scholar] [CrossRef]
- Kang, C.; Ma, L.; Chen, Y.; Fu, L.; Hu, Q.; Zhou, C.; Liu, Q. Metal-organic framework derived hollow rod-like NiCoMn ternary metal sulfide for high-performance asymmetric supercapacitors. Chem. Eng. J. 2022, 427, 131003. [Google Scholar] [CrossRef]
- Huang, C.; Gao, A.; Yi, F.; Wang, Y.; Shu, D.; Liang, Y.; Zhu, Z.; Ling, J.; Hao, J. Metal organic framework derived hollow NiS@C with S-vacancies to boost high-performance supercapacitors. Chem. Eng. J. 2021, 419, 129643. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Kong, W.; Yu, Q.; Long, Y.; Zhou, T.; Hu, C.; Dai, Y.; Gong, J.; Pu, L.; et al. N-doped bimetallic sulfides hollow spheres derived from MOF as battery-type electrode for asymmetric supercapacitors. J. Energy Storage 2023, 73, 109164. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, F.; Wang, D.; Li, Z.; Wang, X.; Wang, C.; Zhang, K.; Du, Y. Amorphous/crystalline heterostructure transition-metal-based catalysts for high-performance water splitting. Coordin. Chem. Rev. 2023, 475, 214916. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, J.; Lu, Y.; Zhao, Y.; Qian, X.; Xu, H.; He, G.; Chen, H. Hollow nanospheres comprising amorphous NiMoS4 and crystalline NiS2 for all-solid-state supercapacitors. Chem. Eng. J. 2022, 436, 135231. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Song, L.; Zhang, M. Research advances in amorphous-crystalline heterostructures toward efficient electrochemical applications. Small 2023, 19, e2206081. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Pan, Y.; Wei, J.; Han, D.; Xu, P.; Wei, Y.; Mao, L.; Yin, X. Interfacial engineering in amorphous/crystalline heterogeneous nanostructures as a highly effective battery-type electrode for hybrid supercapacitors. J. Mater. Chem. A 2022, 10, 11186–11195. [Google Scholar] [CrossRef]
- Chao, D.; DeBlock, R.; Lai, C.H.; Wei, Q.; Dunn, B.; Fan, H.J. Amorphous VO2: A pseudocapacitive platform for high-rate symmetric batteries. Adv. Mater. 2021, 33, 2103736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, Z.; Zhao, S.; Chen, P.; Wang, Y.; Guo, T.; Wei, L.; Cui, X.; Ouyang, X.; Wang, X.; et al. Construction of triple-shelled hollow nanostructure by confining amorphous Ni-Co-S/crystalline MnS on/in hollow carbon nanospheres for all-solid-state hybrid supercapacitors. Chem. Eng. J. 2021, 416, 129500. [Google Scholar] [CrossRef]
- Xue, W.L.; Deng, W.H.; Chen, H.; Liu, R.H.; Taylor, J.M.; Li, Y.K.; Wang, L.; Deng, Y.H.; Li, W.H.; Wen, Y.Y.; et al. MOF-directed synthesis of crystalline ionic liquids with enhanced proton conduction. Angew. Chem. Int. Ed. 2021, 60, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, J.; Zhang, D.; Wang, B.; Xia, X.; Meng, X.; Yang, B.; Chen, J.; He, Y.; Han, Z. In-situ construction of carbon cloth-supported amorphous/crystalline hybrid NiCo-sulfide with permeable concrete-like morphology for high-performance solid-state hybrid supercapacitors. Chem. Eng. J. 2023, 452, 139429. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Wang, J.; Lin, Y.; Meng, J.; Cui, W.; Liu, X.X. Vanadium oxides with amorphous-crystalline heterointerface network for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 135, e202216290. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, S.; Rehman, S.; Li, Z.; Zhang, P. Highly improved adsorption performance of metal-organic frameworks CAU-1 for trace toluene in humid air via sequential internal and external surface modification. Chem. Eng. J. 2020, 389, 123424. [Google Scholar] [CrossRef]
- Gumber, N.; Pai, R.V.; Bahadur, J.; Sengupta, S.; Das, D.; Goutam, U.K. γ-Resistant microporous CAU-1 MOF for selective remediation of thorium. ACS Omega 2023, 8, 12268–12282. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, Q.; Wei, Y.; Wei, L.; Ju, Y.; Zou, B.; Gao, Y.; Chen, G. Co9S8/Co as a high-performance anode for sodium-ion batteries with an ether-based electrolyte. ChemSusChem 2017, 10, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yang, Z.; Li, Y.; Wang, J.; Wang, J.; Zhang, G. Amorphous/crystalline Cu1.5Mn1.5O4 with rich oxygen vacancies for efficiently photothermocatalytic mineralization of toluene. Chem. Eng. J. 2023, 471, 144295. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Ye, M.; Zhuo, K.; Fang, Z. In situ formation of Co9S8/N-C hollow nanospheres by pyrolysis and sulfurization of ZIF-67 for high-performance lithium-ion batteries. Chem. Eur. J. 2017, 23, 9517–9524. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Docio, C.M.; Reinosa, J.J.; Del Campo, A.; Fernández, J.F. Investigation of thermal stability of 2D and 3D CoAl2O4 particles in core-shell nanostructures by Raman spectroscopy. J. Alloy. Compd. 2019, 779, 244–254. [Google Scholar] [CrossRef]
- Mujtaba, J.; He, L.; Zhu, H.; Xiao, Z.; Huang, G.; Solovev, A.A.; Mei, Y. Co9S8 nanoparticles for hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 1776–1785. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Zhao, M.; Zhu, H.; Ma, X.; Li, Z.; Lu, W.; Chen, X.; Ying, L.; Lin, R.; et al. Oxygen-vacancy induced structural changes of Co species in CoAl2O4 spinels for CO2 hydrogenation. Appl. Catal. B Environ. Energ. 2024, 347, 123824. [Google Scholar] [CrossRef]
- Zou, J.; Lin, Y.; Wu, S.; Zhong, Y.; Yang, C. Molybdenum dioxide nanoparticles anchored on nitrogen-doped carbon nanotubes as oxidative desulfurization catalysts: Role of electron transfer in activity and reusability. Adv. Funct. Mater. 2021, 31, 2100442. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Tay, B.K.; Lau, S.P.; Shi, X.; Qiao, X.L.; Sun, Z.H.; Chen, J.G.; Wu, Y.P.; Xie, C.S. Influence of nitrogen ion energy on the Raman spectroscopy of carbon nitride films. Diam. Relat. Mater. 2001, 10, 2137–2144. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, H.Y.; Wang, M.X.; Li, H.B.; Yan, W.; Wang, H.B.; Tang, Z.H. 3D hierarchical core-shell structural NiCoMoS@NiCoAl hydrotalcite for high-performance supercapacitors. J. Mater. Chem. A 2022, 10, 11213–11224. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, M.; Chen, X.; Wang, B.; Fan, W.; Yang, C.; Yang, X.; Zhang, Z.; Yang, X.; Li, C.; et al. A bismuth-based zeolitic organic framework with coordination-linked metal cages for efficient electrocatalytic CO2 reduction to HCOOH. Angew. Chem. Int. Ed. 2023, 62, e202311223. [Google Scholar] [CrossRef]
- Yan, W.; Zeng, H.Y.; Zhang, K.; Long, Y.W.; Wang, M.X. Ni-Co-Mn hydrotalcite-derived hierarchically porous sulfide for hybrid supercapacitors. J. Colloid Interf. Sci. 2023, 635, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jeong, D.I.; Sarwar, N.; Dutta, S.; Chauhan, N.; Han, S.A.; Kim, H.J.; Yoon, D.H. Cobalt supported nitrogen-doped carbon nanotube as efficient catalyst for hydrogen evolution reaction and reduction of 4-nitrophenol. Appl. Surf. Sci. 2022, 572, 151450. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Wu, H.T.; Kong, F.J.; Yin, W.Y.; Cheng, H.J.; Tang, X.Y.; Qian, B.; Tao, S.; Yi, J.; et al. MOFs derived Co1-xS nanoparticles embedded in N-doped carbon nanosheets with improved electrochemical performance for lithium ion batteries. Appl. Surf. Sci. 2019, 479, 693–699. [Google Scholar] [CrossRef]
- Gu, S.; Wang, H.; Wu, C.; Bai, Y.; Li, H.; Wu, F. Confirming reversible Al3+ storage mechanism through intercalation of Al3+ into V2O5 nanowires in a rechargeable aluminum battery. Energy Storage Mater. 2017, 6, 9–17. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zeng, H.Y.; Zhang, K.; Yue, H.L.; Tang, L.Q.; Lv, S.B.; Wang, H.B. Engineering core-shell NiC2O4@C/N-direct-doped NiCoZn-LDH for supercapacitors. Chem. Eng. Sci. 2024, 289, 119865. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Yuan, M.; Chen, Y.; Huang, Y.; Lian, J.; Yang, S.; Li, H.; Wu, L. Amorphous MoSx nanoparticles grown on cobalt-iron-based needle-like array for high-performance flexible asymmetric supercapacitor. Chem. Eng. J. 2021, 417, 127927. [Google Scholar] [CrossRef]
- Guo, J.; He, B.; Gong, W.; Xu, S.; Xue, P.; Li, C.; Sun, Y.; Wang, C.; Wei, L.; Zhang, Q.; et al. Emerging amorphous to crystalline conversion chemistry in Ca-doped VO2 cathodes for high-capacity and long-term wearable aqueous zinc-ion batteries. Adv. Mater. 2024, 36, 2303906. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, Y.; Zhang, T.; Ma, L.; Luo, X.; Huang, C.; Ning, J.; Zhong, Y.; Hu, Y. Synergistic effects of Fe and Mn dual-doping in Co3S4 ultrathin nanosheets for high-performance hybrid supercapacitors. J. Colloid Interf. Sci. 2021, 590, 226–237. [Google Scholar] [CrossRef]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Ferrah, D.; Haines, A.R.; Galhenage, R.P.; Bruce, J.P.; Babore, A.D.; Hunt, A.; Waluyo, I.; Hemminger, J.C. Wet chemical growth and thermocatalytic activity of Cu-based nanoparticles supported on TiO2 nanoparticles/HOPG: In situ ambient pressure XPS study of the CO2 hydrogenation reaction. ACS Catal. 2019, 9, 6783–6802. [Google Scholar] [CrossRef]
- Burghaus, U. Surface chemistry of CO2-Adsorption of carbon dioxide on clean surfaces at ultrahigh vacuum. Prog. Surf. Sci. 2014, 89, 161–217. [Google Scholar] [CrossRef]




| KOH Electrolyte (mol L−1) | Working Voltage (V) | Energy Density (Wh kg−1) | Power Density (W kg−1) | Cycling Stability | Ref. | |
|---|---|---|---|---|---|---|
| Ni-Ni3S4/CNTs//CNTs * | 3 | 1.5 | 39.8 | 749.8 | 82.8% (15,000 cycles at 1 A g−1) | [14] |
| NCM-S@HCNS//N-rGO * | 2 | 1.6 | 23.5 | 800.4 | 87.7% (10,000 cycles at 10 A g−1) | [25] |
| NiCoS//porous carbon | 6 | 1.6 | 59.7 | 710 | 92.3% (10,000 cycles at 5 A g−1) | [27] |
| NiCoMoS@NiCoAl-LDH//AC | 3 | 1.6 | 41.9 | 800 | 89.3% (10,000 cycles at 5 A g−1) | [39] |
| NiCoMnS//AC | 3 | 1.5 | 47.5 | 750.0 | 85.2% (10,000 cycles at 5 A g−1) | [41] |
| FC@MS-EG *//AC | 6 | 1.5 | 88.1 | 750.0 | 84.7% (6000 cycles at 8 A g−1) | [46] |
| FM-Co3S4 *//AC | 6 | 1.5 | 55.0 | 752.0 | 86.1% (5000 cycles at 10 A g−1) | [48] |
| F-CuCo2S4//AC | 1 | 1.6 | 49.8 | 897.4 | 89.2% (10,000 cycles at 20 A g−1) | [49] |
| Co(Al)S//AC | 3 | 1.6 | 72.3 | 750 | 87.5% (10,000 cycles at 1 A g−1) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Zhang, K.; Luo, C.; Wu, G.; Zeng, H. In-Situ Sulfuration of CoAl Metal–Organic Framework for Enhanced Supercapacitor Properties. Materials 2024, 17, 4030. https://doi.org/10.3390/ma17164030
Liao M, Zhang K, Luo C, Wu G, Zeng H. In-Situ Sulfuration of CoAl Metal–Organic Framework for Enhanced Supercapacitor Properties. Materials. 2024; 17(16):4030. https://doi.org/10.3390/ma17164030
Chicago/Turabian StyleLiao, Mengchen, Kai Zhang, Chaowei Luo, Guozhong Wu, and Hongyan Zeng. 2024. "In-Situ Sulfuration of CoAl Metal–Organic Framework for Enhanced Supercapacitor Properties" Materials 17, no. 16: 4030. https://doi.org/10.3390/ma17164030






