Evolution of the Corrosion Products around MnS Embedded in AISI 304 Stainless Steel in NaCl Solution
Abstract
1. Introduction
2. Experimental
2.1. Material, Specimen Preparation and Solution
2.2. Quasi-In-Situ Immersion Tests
2.3. Surface Analyses
3. Results
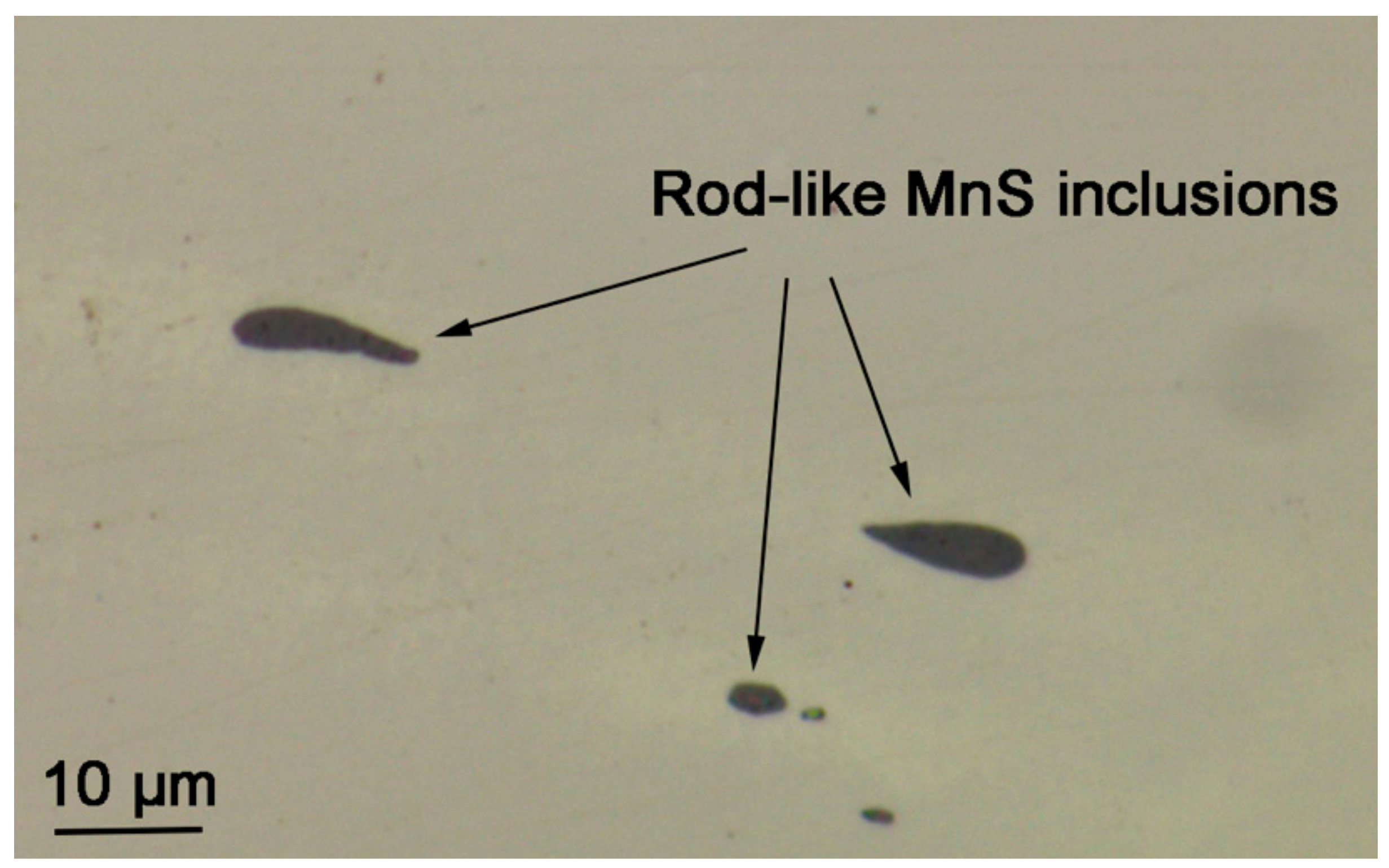
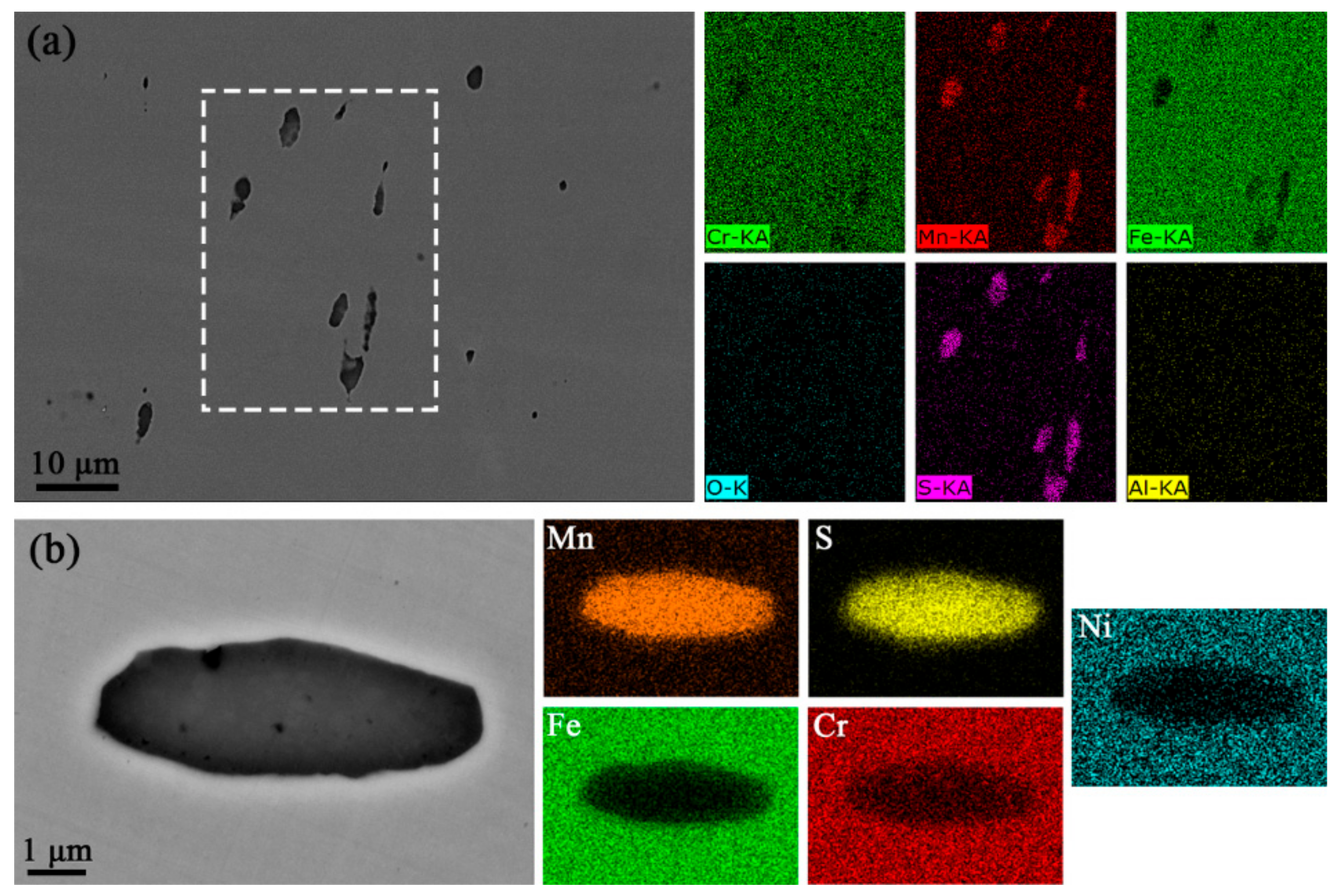
3.1. Inclusion Identification
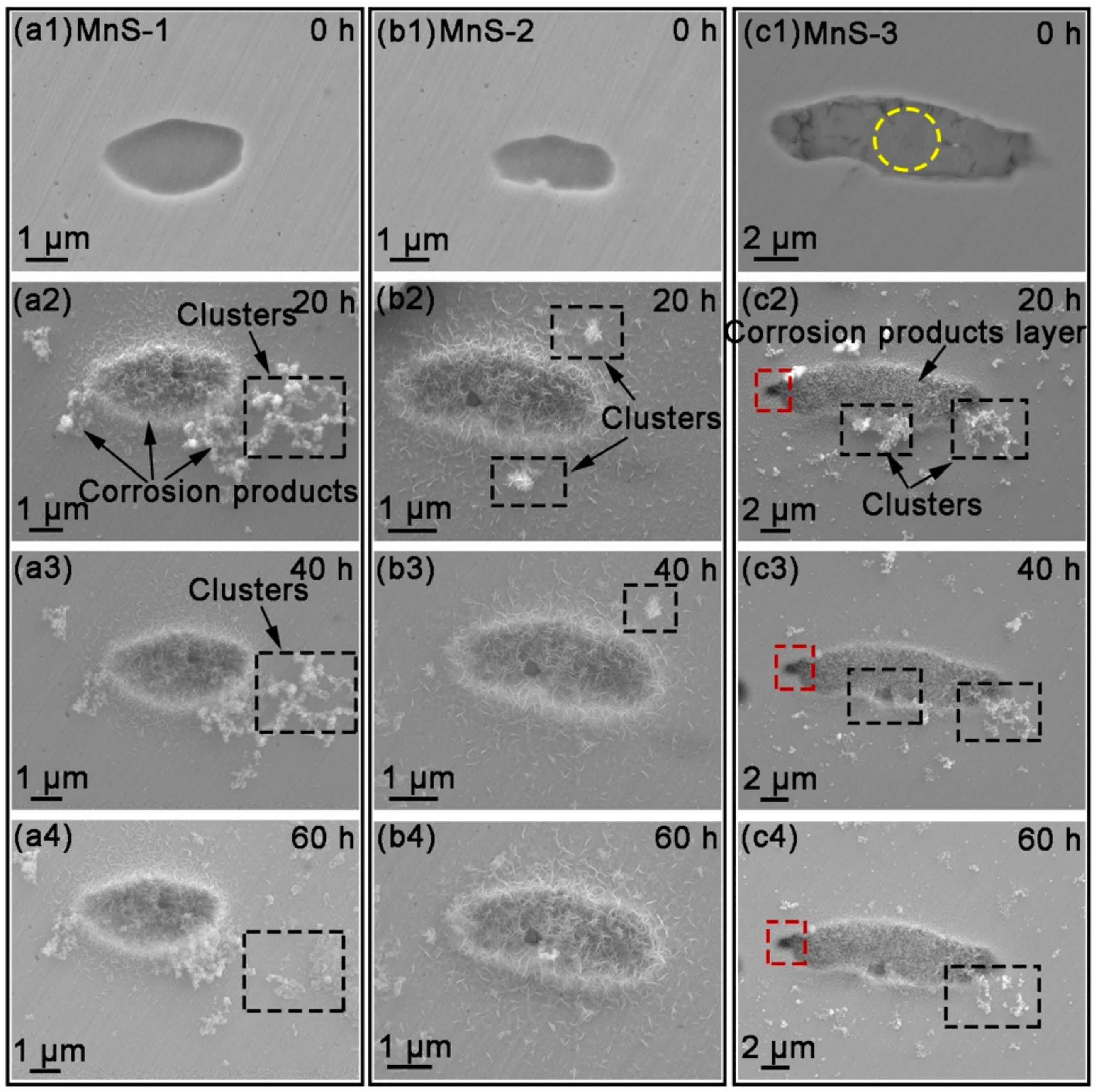
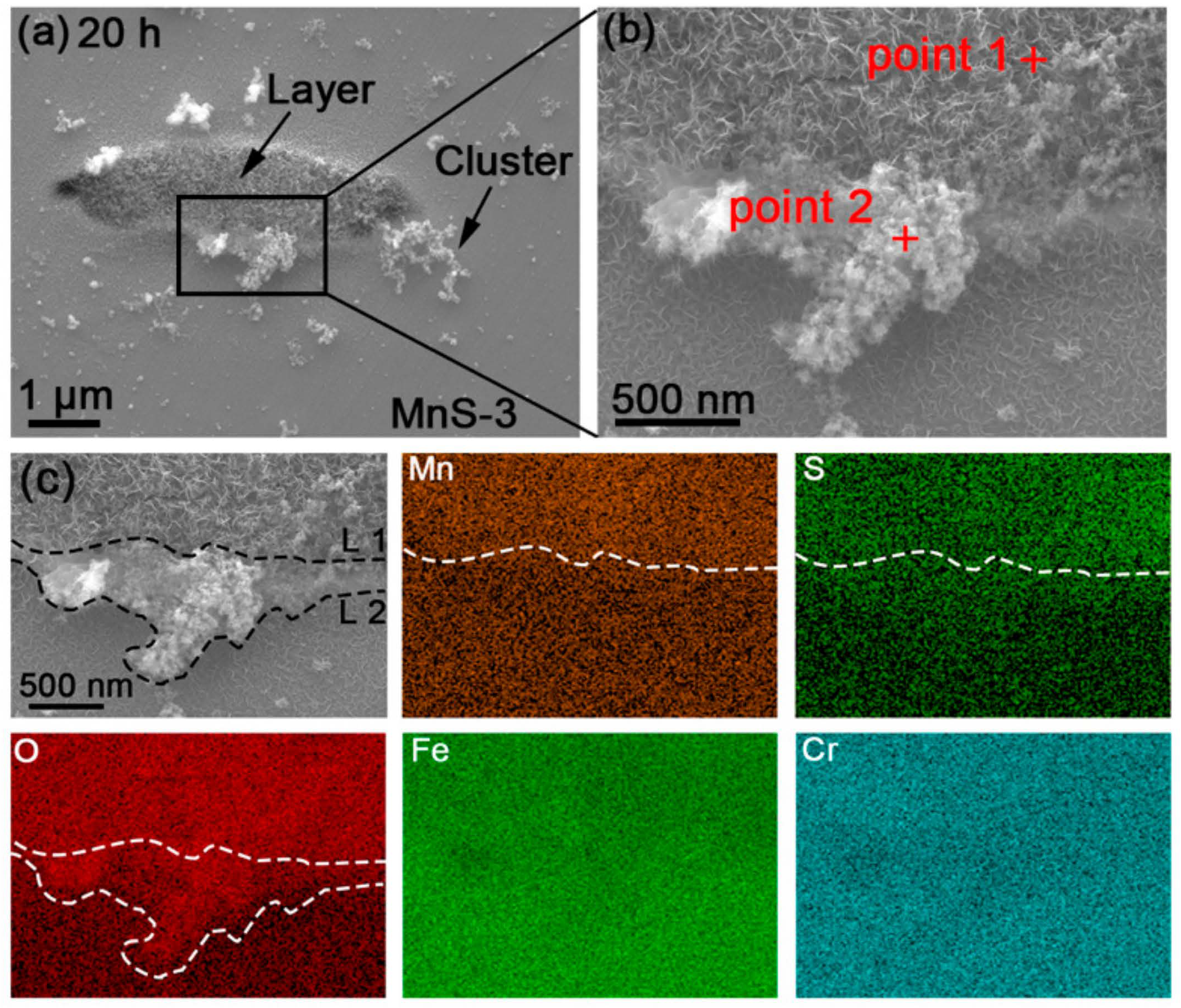
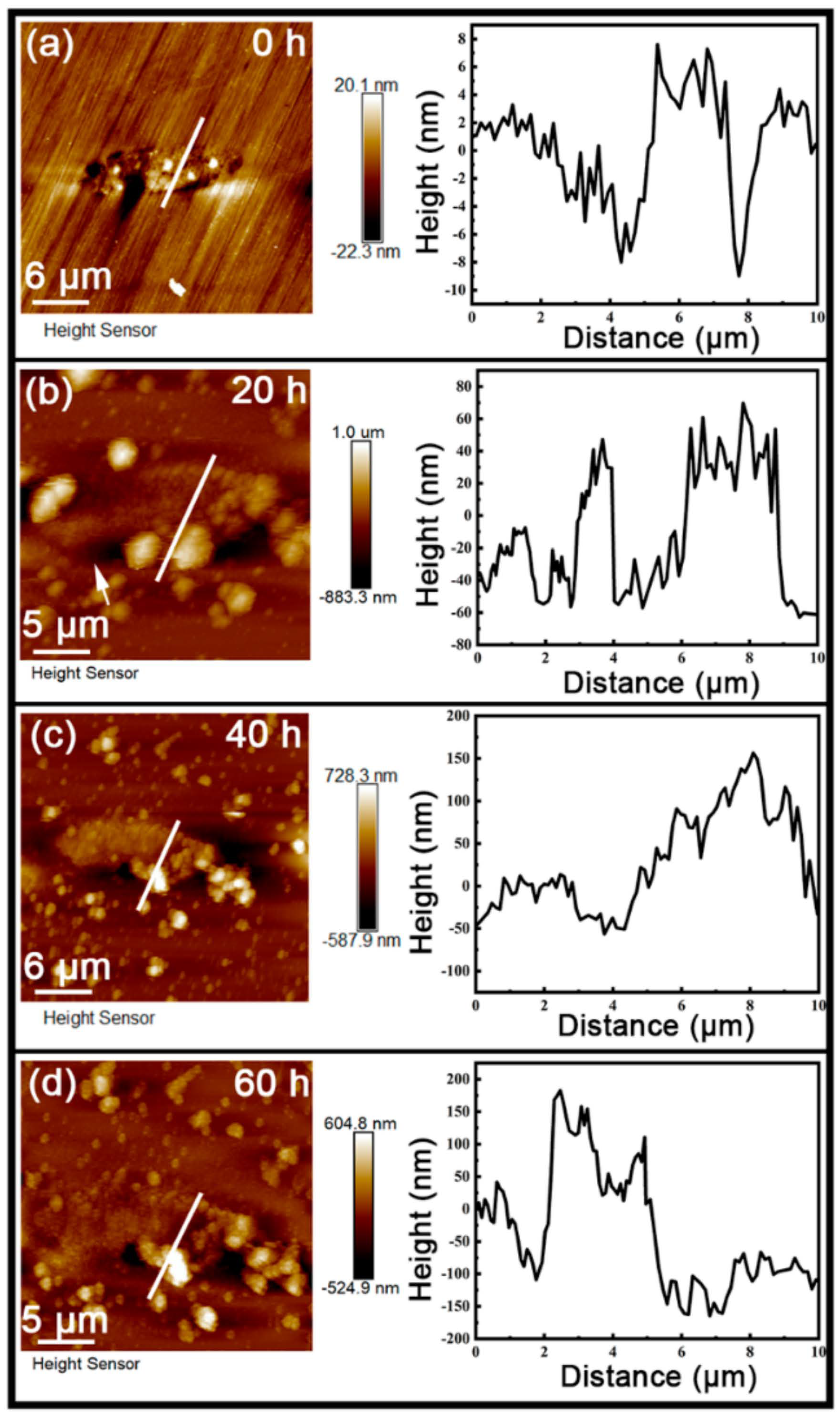
3.2. Quasi-In-Situ FE-SEM Observation of Corrosion Product Deposition
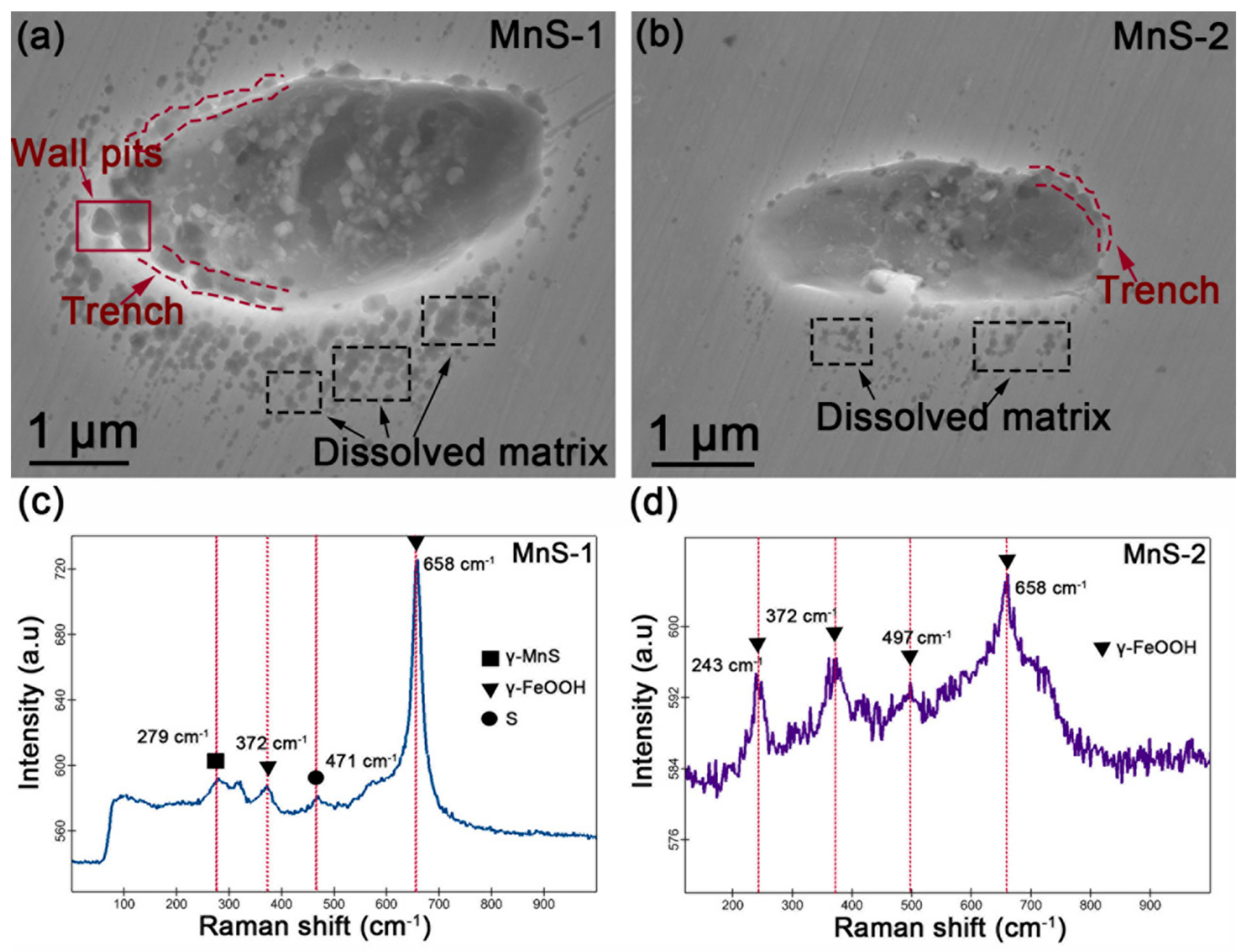
3.3. EDS and Raman Detection of Corrosion Product Composition
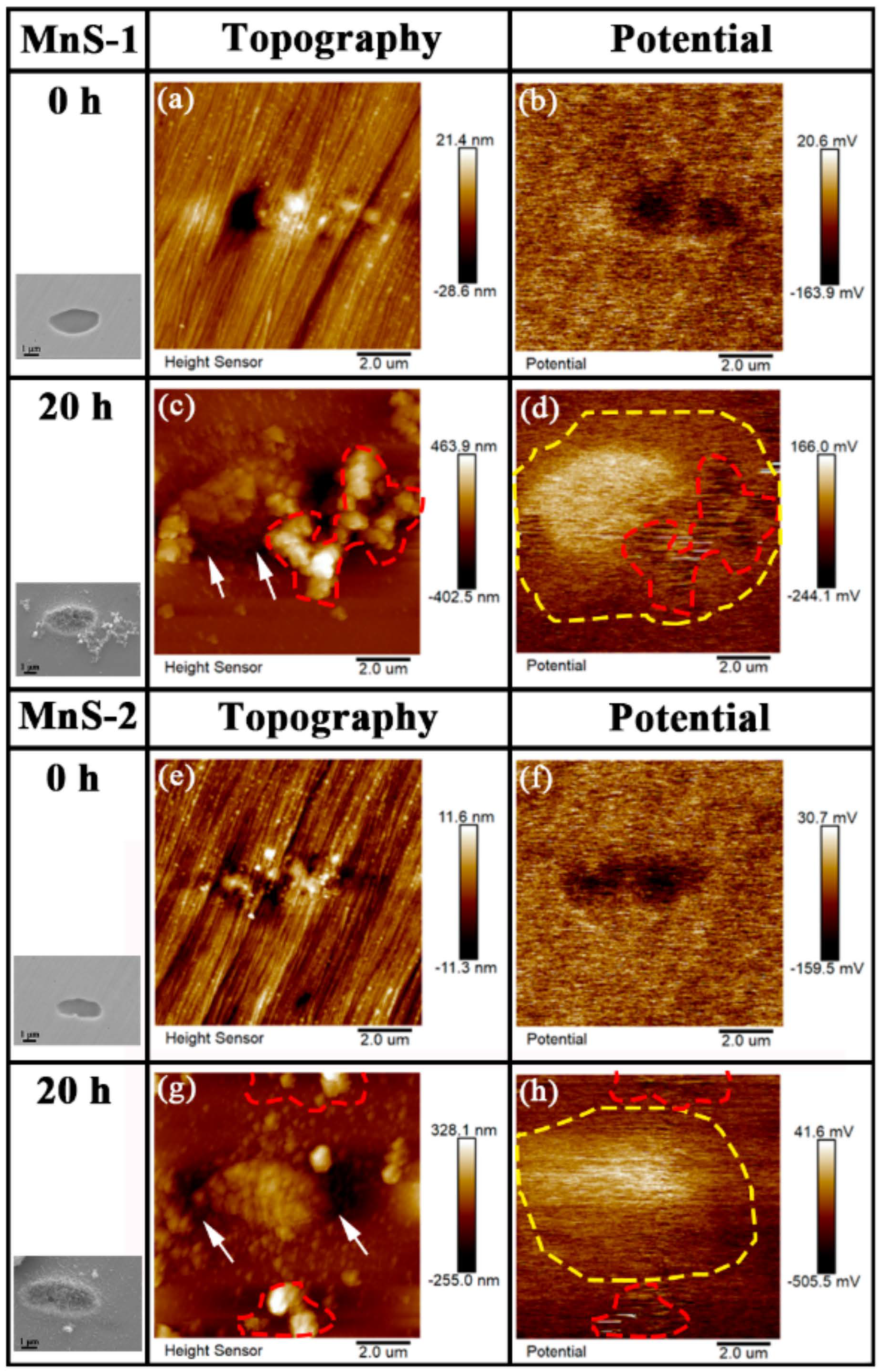
3.4. AFM/SKPFM Measurement of Topography and Surface Volta Potential
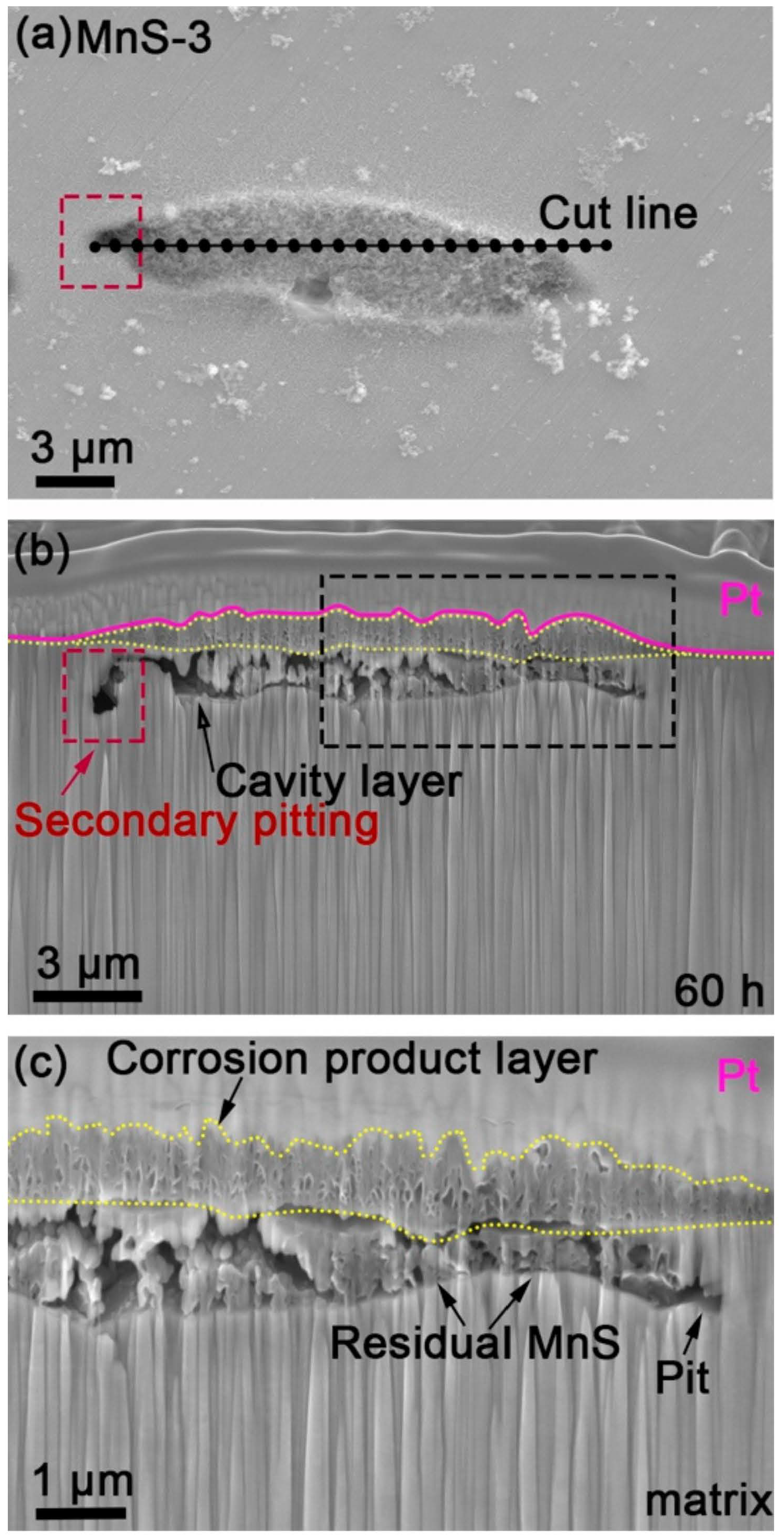
3.5. FIB-FESEM Observation of Cross-Sectional Morphology
3.6. FE-SEM Morphology after Removal of Corrosion Product
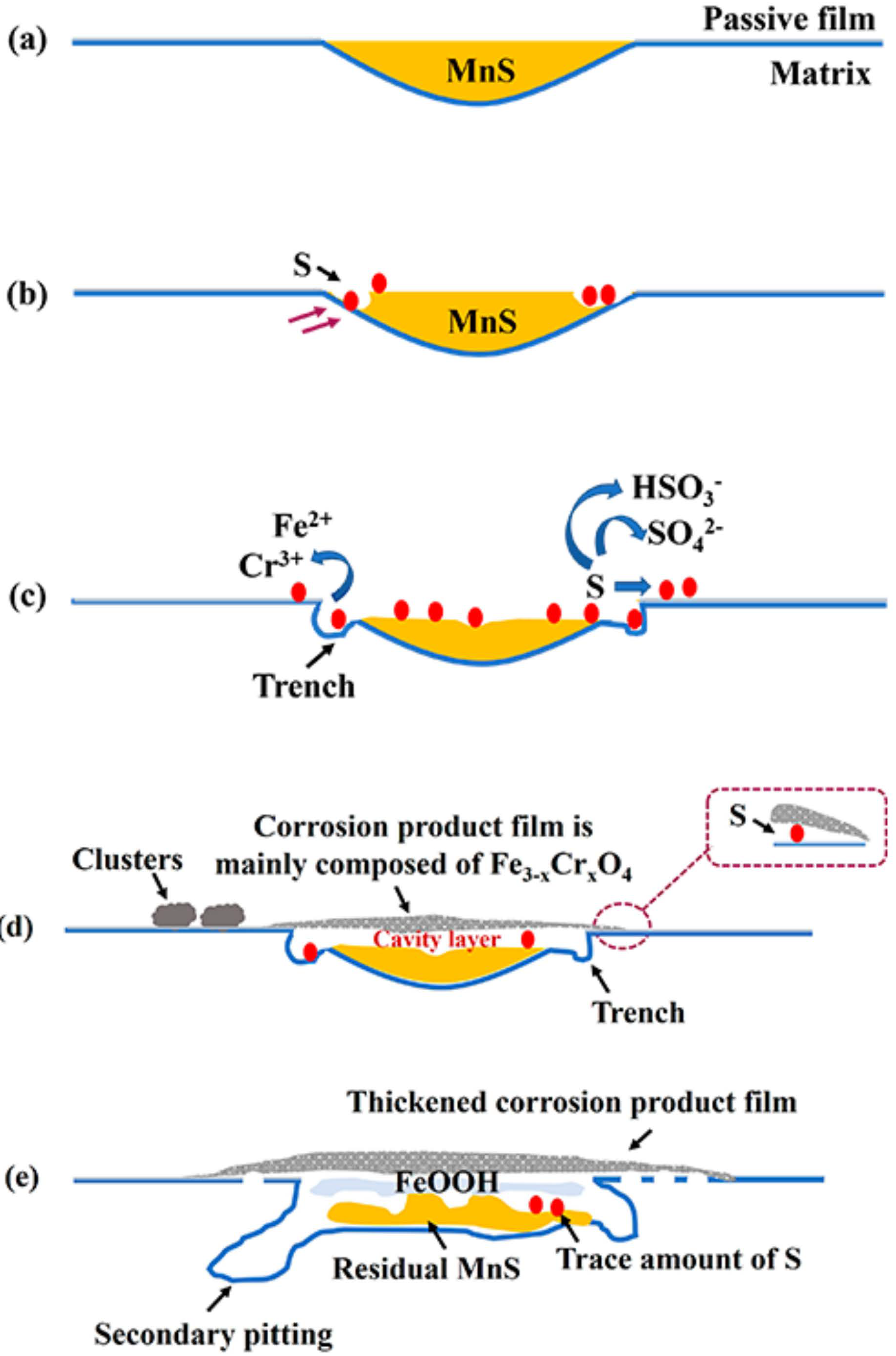
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Meng, Q.; Frankel, G.S.; Colijn, H.O.; Goss, S.H. Stainless-steel corrosion and MnS inclusions. Nature 2003, 424, 389–390. [Google Scholar] [CrossRef]
- Park, J.; Kang, Y. Inclusions in stainless steels—A review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D.E. The initiation of pitting corrosion on austenitic stainless steel on the role and importance of sulfide inclusions. Corros. Sci. 1992, 33, 457–474. [Google Scholar] [CrossRef]
- Burstein, G.T.; Pistorius, P.C.; Mattin, S.P. The nucleation and growth of corrosion pits on stainless steel. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Webb, E.G.; Alkire, R.C. Pit Initiation at Single Sulfide Inclusions in Stainless Steel: II. Detection of Local pH, Sulfide, and Thiosulfate. J. Electrochem. Soc. 2002, 149, B280. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Webb, E.G.; Alkire, R.C. Pit Initiation at Single Sulfide Inclusions in Stainless Steel III. Mathematical Model. J. Electrochem. Soc. 2002, 149, B286–B295. [Google Scholar] [CrossRef]
- Webb, E.G.; Alkire, R.C. Pit Initiation at Single Sulfide Inclusions in Stainless Steel I. Electrochemical Microcell Measurements. J. Electrochem. Soc. 2002, 149, B272–B279. [Google Scholar] [CrossRef]
- Newman, R.C.; Wong, W.P.; Ezuber, H.; Garne, A. Pitting of Stainless Steels by Thiosulfate Ions. Corros. Sci. 1989, 45, 282–287. [Google Scholar] [CrossRef]
- Lott, S.E.; Alkire, R.C. The Role of Inclusions on Initiation of Crevice Corrosion of Stainless Steel. J. Electrochem. Soc. 1989, 136, 973–979. [Google Scholar] [CrossRef]
- Williams, D.E.; Kilburn, M.R.; Cliff, J.; Waterhouse, G.I.N. Composition changes around sulphide inclusions in stainless steels, and implications for the initiation of pitting corrosion. Corros. Sci. 2010, 52, 3702–3716. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chate, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Schmuki, P.; Hildebrand, H.; Friedrich, A.; Virtanen, S. The composition of the boundary region of MnS inclusions in stainless steel and its relevance in triggering pitting corrosion. Corros. Sci. 2005, 47, 1239–1250. [Google Scholar] [CrossRef]
- Castle, J.E.; Ke, R. Studies by auger spectroscopy of pit initiation at the site of inclusions in stainless steel. Corros. Sci. 1990, 30, 409–428. [Google Scholar] [CrossRef]
- Suter, T.; Webb, E.G.; Böhni, H.; Alkire, R.C. Pit initiation on stainless steel in 1 M NaCl with and without mechanical stress. J. Electrochem. Soc. 2001, 148, B174–B185. [Google Scholar] [CrossRef]
- Böhni, H.; Suter, T.; Schreyer, A. Micro- and nanotechniques to study localized corrosion. Electrochim. Acta 1995, 40, 1361–1368. [Google Scholar] [CrossRef]
- Parvathavarthini, N.; Gupta, R.K.; Kumar, A.V.; Ramya, S.; Kamachi Mudali, U. Interpretation of Electrochemical Potentiokinetic Reactivation data in the presence of sulphide/oxysulphide inclusions in 316LN stainless steel. Corros. Sci. 2011, 53, 3202–3214. [Google Scholar] [CrossRef]
- Chiba, A.; Muto, I.; Sugawara, Y.; Hara, N. Direct Observation of Pit Initiation Process on Type 304 Stainless Steel. Mater. Trans. 2014, 55, 857–860. [Google Scholar] [CrossRef]
- Muto, I.; Ito, D.; Hara, N. Microelectrochemical Investigation on Pit Initiation at Sulfide and Oxide Inclusions in Type 304 Stainless Steel. J. Electrochem. Soc. 2009, 156, C55. [Google Scholar] [CrossRef]
- Muto, I.; Izumiyama, Y.; Hara, N. Microelectrochemical Measurements of Dissolution of MnS Inclusions and Morphological Observation of Metastable and Stable Pitting on Stainless Steel. J. Electrochem. Soc. 2007, 154, C439–C444. [Google Scholar] [CrossRef]
- Chiba, A.; Muto, I.; Sugawara, Y.; Hara, N. Pit Initiation Mechanism at MnS Inclusions in Stainless Steel: Synergistic Effect of Elemental Sulfur and Chloride Ions. J. Electrochem. Soc. 2013, 160, C511–C520. [Google Scholar] [CrossRef]
- Chiba, A.; Muto, I.; Sugawara, Y.; Hara, N. A Microelectrochemical System for In Situ High-Resolution Optical Microscopy: Morphological Characteristics of Pitting at MnS Inclusion in Stainless Steel. J. Electrochem. Soc. 2012, 159, C341–C350. [Google Scholar] [CrossRef]
- Chiba, A.; Shibukawa, S.; Muto, I.; Doi, T.; Kawano, K.; Sugawara, Y.; Hara, N. Microelectrochemical Aspects of Interstitial Carbon in Type 304 Stainless Steel: Improving Pitting Resistance at MnS Inclusion. J. Electrochem. Soc. 2015, 162, C270–C278. [Google Scholar] [CrossRef]
- Laycocka, N.J.; White, S.P. Computer simulation of single pit propagation in stainless steel under potentiostatic control. J. Electrochem. Soc. 2001, 148, B264. [Google Scholar] [CrossRef]
- Sun, W.; Liu, G.; Wang, L.; Wu, T.; Liu, Y. An arbitrary Lagrangian–Eulerian model for studying the influences of corrosion product deposition on bimetallic corrosion. J. Solid State Electr. 2012, 17, 829–840. [Google Scholar] [CrossRef]
- Li, W.C.; Li, D.; Yu, Z.G.; Xie, Y.; Liu, F.H.; Jin, Y. A FEM model for simulating trenching process around a MnS inclusion embedded in stainless steel. J. Electroanal. Chem. 2021, 882, 114977. [Google Scholar] [CrossRef]
- Li, D.; Huang, F.F.; Lei, X.; Jin, Y. Localized corrosion of 304 stainless steel triggered by embedded MnS. Corros. Sci. 2023, 221, 110860. [Google Scholar] [CrossRef]
- Örnek, C.; Engelberg, D.L. SKPFM measured Volta potential correlated with strain localisation in microstructure to understand corrosion susceptibility of cold-rolled grade 2205 duplex stainless steel. Corros. Sci. 2015, 99, 164–171. [Google Scholar] [CrossRef]
- Gyakwaa, F.; Aula, M.; Alatarvas, T.; Shu, Q.; Huttula, M.; Fabritius, T. Quantification of Synthetic Nonmetallic Inclusion Multiphase Mixtures from a CaO–Al2O3–MgO–CaS System Using Raman Spectroscopy. Steel Res. Int. 2020, 92, 2000322. [Google Scholar] [CrossRef]
- Katayanagi, Y.; Shimizu, T.; Hashimasa, Y.; Matsushita, N.; Yamazaki, Y.; Yamaguchi, T. Cross-sectional observation of nanostructured catalyst layer of polymer electrolyte fuel cell using FIB-SEM. J. Power Sources 2015, 280, 210–216. [Google Scholar] [CrossRef]
- Chiba, A.; Muto, I.; Sugawara, Y.; Hara, N. Effect of atmospheric aging on dissolution of MnS inclusions and pitting initiation process in type 304 stainless steel. Corros. Sci. 2016, 106, 25–34. [Google Scholar] [CrossRef]
- Li, G.X.; Wang, L.W.; Wu, H.L.; Liu, C.; Wang, X.; Cui, Z.Y. Dissolution kinetics of the sulfide-oxide complex inclusion and resulting localized corrosion mechanism of X70 steel in deaerated acidic environment. Corros. Sci. 2020, 174, 108815. [Google Scholar] [CrossRef]
- Guo, M.X.; Tang, J.R.; Gu, T.Z.; Peng, C.; Li, Q.X.; Pan, C.; Wang, Z.Y. Corrosion behavior of 316L stainless steels exposed to salt lake atmosphere of western China for 8 years. Acta Metall. Sin. 2021, 34, 555–564. [Google Scholar] [CrossRef]
- Dhandayuthapani, T.; Sivakumar, R.; Sanjeeviraja, C.; Gopalakrishnan, C.; Arumugam, S. Microstructure, optical and magnetic properties of micro-crystalline γ-MnS film prepared by chemical bath deposition method. Mater. Sci. Semicond. Process. 2017, 72, 67–71. [Google Scholar] [CrossRef]
- Lughi, V.; Lenaz, D.; Bonifacio, A.; Princivalle, F.; Sergo, V.; Parisi, F. A Raman spectroscopy study of the oxidation processes in synthetic chromite FeCr2O4. Ceram. Int. 2020, 46, 29382–29387. [Google Scholar] [CrossRef]
- Nabizadeh, M.; Boissy, C.; Baert, K.; Goderis, S.; Ottevaere, H.; Terryn, H.; Hauffman, T. The mechanism of thermal oxide film formation on low Cr martensitic stainless steel and its behavior in fluoride-based pickling solution in conversion treatment. Corros. Sci. 2021, 181, 109206. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Mccarty, K.F.; Boehme, D.R. A raman study of the systems Fe3−xCrxO4 and Fe2−xCrxO3. J. Solid. State. Chem. 1989, 79, 19–27. [Google Scholar] [CrossRef]
- Eklund, G.S. Initiation of Pitting at Sulfide Inclusions in Stainless Steel. J. Electrochem. Soc. 1974, 121, 467–473. [Google Scholar] [CrossRef]
- Ke, R.; Alkire, R. Initiation of Corrosion Pits at Inclusions on 304 Stainless Steel. J. Electrochem. Soc. 1995, 142, 4056–4062. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, X.L.; Li, M.C. Effect of vacuum pressure on the initiation and propagation of pitting corrosion of 2205 duplex stainless steel in concentrated seawater. Acta Metall. Sin. 2022, 35, 1023–1033. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Coronado, J.J.; Rodríguez, S.A. Mechanical properties and phases determination of low carbon steel oxide scales formed at 1200 °C in air. Surf. Coat. Technol. 2015, 282, 155–162. [Google Scholar] [CrossRef]
- Shibagaki, S.; Koga, A.; Shirakawa, Y.; Onishi, H.; Yokokawa, H.; Tanaka, J. Chemical reaction path for thin film oxidation of stainless steel. Thin Solid Films 1997, 303, 101–106. [Google Scholar] [CrossRef]
- Ramya, S.; Krishna, D.N.G.; Kamachi, M.U. In-situ Raman and X-ray photoelectron spectroscopic studies on the pitting corrosion of modified 9Cr-1Mo steel in neutral chloride solution. Appl. Surf. Sci. 2018, 428, 1106–1118. [Google Scholar]
- Ostwald, C.; Grabke, H.J. Initial oxidation and chromium diffusion. I. Effects of surface working on 9–20% Cr steels. Corros. Sci. 2004, 46, 1113–1127. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Heintz, O.; Oltra, R. Influence of the dissolution of MnS inclusions under free corrosion and potentiostatic conditions on the composition of passive films and the electrochemical behaviour of stainless steels. Electrochim. Acta 2006, 51, 3235–3243. [Google Scholar] [CrossRef]
- Luo, Y.Z.; Zhang, J.M.; Liu, Z.M.; Xiao, C.; Wu, S.Z. In situ observation and thermodynamic calculation of MnS in 49MnVS3 non-quenched and tempered steel. Acta Metall. Sin. 2011, 24, 326–334. [Google Scholar]
- Ma, H.; Wang, Z.X.; Liu, Y.; Wang, Y.X.; Wang, T.F.; Zhang, Q.P.; Cui, Z.Y. pH-dependent corrosion initiation behavior induced by inclusions of low alloy steel in simulated marine environments. J. Iron Steel Res. Int. 2022, 122, 2067–2079. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | P | S | Ni | Cr | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Re-sulfurized SS 304 | 0.073 | 0.72 | 2.81 | 0.022 | 0.043 | 8.17 | 18.1 | 0.24 | 0.013 | balance |
| Substance | Raman Shift (cm−1) | Literature |
|---|---|---|
| MnS | Around 283 cm−1 | [35] |
| Fe3−xCrxO4 | Between 542–531, around 636 cm−1 | [37] |
| FeCr2O4 | 630 cm−1 | [36] |
| Fe2−xCrxO3 | 303, 424 cm−1 | [37] |
| Between 413–424, 515, 537 cm−1 | [38] | |
| FeOOH | 243, 372, 497 and 658 cm−1 | [39] |
| Cr2O3 | 353, 529, 553 cm−1 | [37] |
| 353, 616 cm−1 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Hao, H.; Wang, Z.; Nyakilla, E.E. Evolution of the Corrosion Products around MnS Embedded in AISI 304 Stainless Steel in NaCl Solution. Materials 2024, 17, 4050. https://doi.org/10.3390/ma17164050
Li D, Hao H, Wang Z, Nyakilla EE. Evolution of the Corrosion Products around MnS Embedded in AISI 304 Stainless Steel in NaCl Solution. Materials. 2024; 17(16):4050. https://doi.org/10.3390/ma17164050
Chicago/Turabian StyleLi, Dan, Hongliang Hao, Zhichao Wang, and Edwin Ernest Nyakilla. 2024. "Evolution of the Corrosion Products around MnS Embedded in AISI 304 Stainless Steel in NaCl Solution" Materials 17, no. 16: 4050. https://doi.org/10.3390/ma17164050
APA StyleLi, D., Hao, H., Wang, Z., & Nyakilla, E. E. (2024). Evolution of the Corrosion Products around MnS Embedded in AISI 304 Stainless Steel in NaCl Solution. Materials, 17(16), 4050. https://doi.org/10.3390/ma17164050







