Enhanced Supercapacitor and Cycle-Life Performance: Self-Supported Nanohybrid Electrodes of Hydrothermally Grown MnO2 Nanorods on Carbon Nanotubes in Neutral Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Materials Analysis
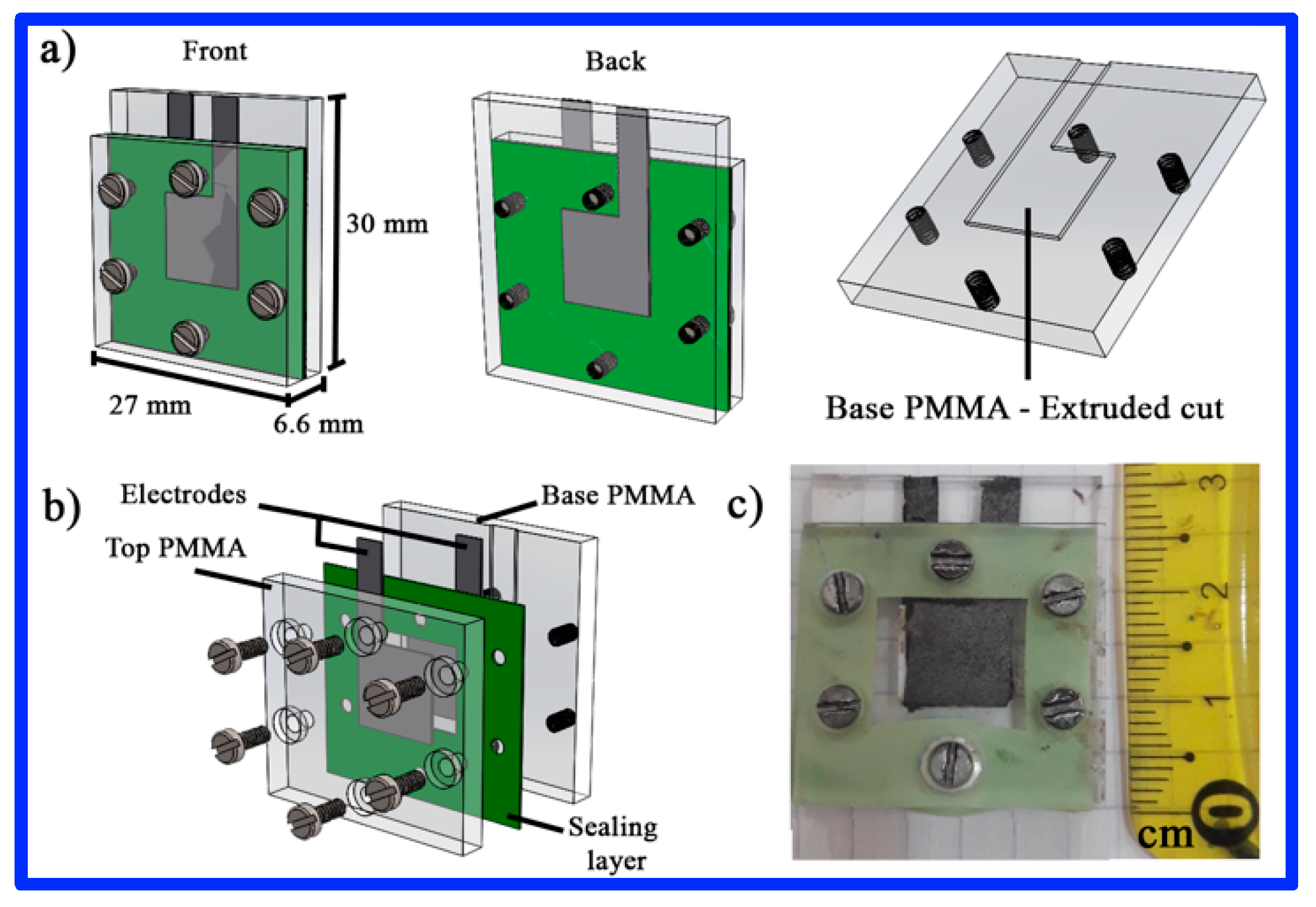
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Materials Characterization
3.2. Electrochemical Performance
3.3. Symmetric Supercapacitor Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: New York, NY, USA, 1999. [Google Scholar]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Gyulavári, T.; Tóth, Z.-R.; Pápa, Z.; Budai, J.; Hernadi, K. The effect of various substrates and catalyst layer deposition on the incorporation of nitrogen into carbon nanotube forest structures. Thin Solid Films 2020, 709, 138194. [Google Scholar] [CrossRef]
- Pramitha, A.; Raviprakash, Y. Recent developments and viable approaches for high-performance supercapacitors using transition metal-based electrode materials. J. Energy Storage 2022, 49, 104120. [Google Scholar] [CrossRef]
- Sakib, M.N.; Ahmed, S.; Rahat, S.M.S.M.; Shuchi, S.B. A review of recent advances in manganese-based supercapacitors. J. Energy Storage 2021, 44, 103322. [Google Scholar] [CrossRef]
- Yang, D. Pulsed laser deposition of manganese oxide thin films for supercapacitor applications. J. Power Sources 2011, 196, 8843–8849. [Google Scholar] [CrossRef]
- Qiu, G.; Huang, H.; Dharmarathna, S.; Benbow, E.; Stafford, L.; Suib, S.L. Hydrothermal Synthesis of Manganese Oxide Nanomaterials and Their Catalytic and Electrochemical Properties. Chem. Mater. 2011, 23, 3892–3901. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Liu, C.-S.; Huang, C.-L.; Fang, H.-C.; Hung, K.-Y.; Su, C.-A.; Li, Y.-Y. MnO2-based carbon nanofiber cable for supercapacitor applications. J. Energy Storage 2021, 33, 102130. [Google Scholar] [CrossRef]
- Merritt, A.R.; Rajagopalan, R.; Carter, J.D. Synthesis of electro-active manganese oxide thin films by plasma enhanced chemical vapor deposition. Thin Solid Films 2014, 556, 28–34. [Google Scholar] [CrossRef]
- Garten, L.M.; Selvarasu, P.; Perkins, J.; Ginley, D.; Zakutayev, A. Phase formation of manganese oxide thin films using pulsed laser deposition. Mater. Adv. 2021, 2, 303–309. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, X. Pulsed laser deposition of large-area manganese oxide nanosheet arrays for high-rate supercapacitors. New J. Chem. 2013, 37, 869–872. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Khomenko, V.; Frackowiak, E.; Béguin, F. Performance of Manganese Oxide/CNTs Composites as Electrode Materials for Electrochemical Capacitors. J. Electrochem. Soc. 2005, 152, A229–A235. [Google Scholar] [CrossRef]
- Liew, K.B.; Wan Daud, W.R.; Ghasemi, M.; Loh, K.S.; Ismail, M.; Lim, S.S.; Leong, J.X. Manganese oxide/functionalised carbon nanotubes nanocomposite as catalyst for oxygen reduction reaction in microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 11625–11632. [Google Scholar] [CrossRef]
- Zhou, Y.K.; He, B.L.; Zhang, F.B.; Li, H.L. Hydrous manganese oxide/carbon nanotube composite electrodes for electrochemical capacitors. J. Solid State Electrochem. 2004, 8, 482–487. [Google Scholar] [CrossRef]
- Lee, C.Y.; Tsai, H.M.; Chuang, H.J.; Li, S.Y.; Lin, P.; Tseng, T.Y. Characteristics and Electrochemical Performance of Supercapacitors with Manganese Oxide-Carbon Nanotube Nanocomposite Electrodes. J. Electrochem. Soc. 2005, 152, A716–A720. [Google Scholar] [CrossRef]
- Han, Z.J.; Seo, D.H.; Yick, S.; Chen, J.H.; Ostrikov, K. MnOx/carbon nanotube/reduced graphene oxide nanohybrids as high-performance supercapacitor electrodes. NPG Asia Mater. 2014, 6, e140. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Lin, J.; Lu, L. Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors. Nanoscale Res. Lett. 2012, 7, 33. [Google Scholar] [CrossRef]
- Ko, J.M.; Kim, K.M. Electrochemical properties of MnO2/activated carbon nanotube composite as an electrode material for supercapacitor. Mater. Chem. Phys. 2009, 114, 837–841. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Cheng, J.; Shao, B.; Wang, K.; Song, L.; Zhang, M. Carbon nanotube/MnO2 composites synthesized by microwave-assisted method for supercapacitors with high power and energy densities. J. Power Sources 2009, 194, 1202–1207. [Google Scholar] [CrossRef]
- Gerard, O.; Numan, A.; Krishnan, S.; Khalid, M.; Subramaniam, R.; Kasi, R. A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J. Energy Storage 2022, 50, 104283. [Google Scholar] [CrossRef]
- Zheng, X.; Mohammadi, N.; Moreno Zuria, A.; Mohamedi, M. Advanced Zinc–Air Batteries with Free-Standing Hierarchical Nanostructures of the Air Cathode for Portable Applications. ACS Appl. Mater. Interfaces 2021, 13, 61374–61385. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, K.; Vix-Guterl, C.; Frackowiak, E.; Saadallah, S.; Reda, M.; Parmentier, J.; Patarin, J.; Béguin, F. Capacitance properties of ordered porous carbon materials prepared by a templating procedure. J. Phys. Chem. Solids 2004, 65, 287–293. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sources 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Javed, M.S.; Chen, J.; Chen, L.; Xi, Y.; Zhang, C.; Wan, B.; Hu, C. Flexible full-solid state supercapacitors based on zinc sulfide spheres growing on carbon textile with superior charge storage. J. Mater. Chem. A 2016, 4, 667–674. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Huang, C.; Wu, Y.; Hou, J.; Situ, Y.; Huang, H. Electrochemical charge/discharge cycling and morphological effects in MnO2/PANC nanostructures for supercapacitors. Electrochim. Acta 2022, 428, 140929. [Google Scholar] [CrossRef]
- Lee, H.-J.; Noor, N.; Gumeci, C.; Dale, N.; Parrondo, J.; Higgins, D.C. Understanding the Impact of the Morphology, Phase Structure, and Mass Fraction of MnO2 within MnO2/Reduced Graphene Oxide Composites for Supercapacitor Applications. J. Phys. Chem. C 2022, 126, 13004–13014. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Lu, Y.; Wang, W.; Peng, T.; Zhang, Y.; Guo, Y.; Wang, Y.; Huo, K.; Kim, J.-K.; et al. Cable-like double-carbon layers for fast ion and electron transport: An example of CNT@NCT@MnO2 3D nanostructure for high-performance supercapacitors. Carbon 2019, 143, 335–342. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L. 15—Should low carbon energy technologies be envisaged in the context of sustainable energy systems? In Low Carbon Energy Technologies in Sustainable Energy Systems; Kyriakopoulos, G.L., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 357–389. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouachma, S.; Zheng, X.; Moreno Zuria, A.; Kechouane, M.; Gabouze, N.; Mohamedi, M. Enhanced Supercapacitor and Cycle-Life Performance: Self-Supported Nanohybrid Electrodes of Hydrothermally Grown MnO2 Nanorods on Carbon Nanotubes in Neutral Electrolyte. Materials 2024, 17, 4079. https://doi.org/10.3390/ma17164079
Bouachma S, Zheng X, Moreno Zuria A, Kechouane M, Gabouze N, Mohamedi M. Enhanced Supercapacitor and Cycle-Life Performance: Self-Supported Nanohybrid Electrodes of Hydrothermally Grown MnO2 Nanorods on Carbon Nanotubes in Neutral Electrolyte. Materials. 2024; 17(16):4079. https://doi.org/10.3390/ma17164079
Chicago/Turabian StyleBouachma, Soraya, Xiaoying Zheng, Alonso Moreno Zuria, Mohamed Kechouane, Noureddine Gabouze, and Mohamed Mohamedi. 2024. "Enhanced Supercapacitor and Cycle-Life Performance: Self-Supported Nanohybrid Electrodes of Hydrothermally Grown MnO2 Nanorods on Carbon Nanotubes in Neutral Electrolyte" Materials 17, no. 16: 4079. https://doi.org/10.3390/ma17164079
APA StyleBouachma, S., Zheng, X., Moreno Zuria, A., Kechouane, M., Gabouze, N., & Mohamedi, M. (2024). Enhanced Supercapacitor and Cycle-Life Performance: Self-Supported Nanohybrid Electrodes of Hydrothermally Grown MnO2 Nanorods on Carbon Nanotubes in Neutral Electrolyte. Materials, 17(16), 4079. https://doi.org/10.3390/ma17164079







