Properties of Red-Mud-Modified Basic Magnesium Sulfate Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Strength Measuerment
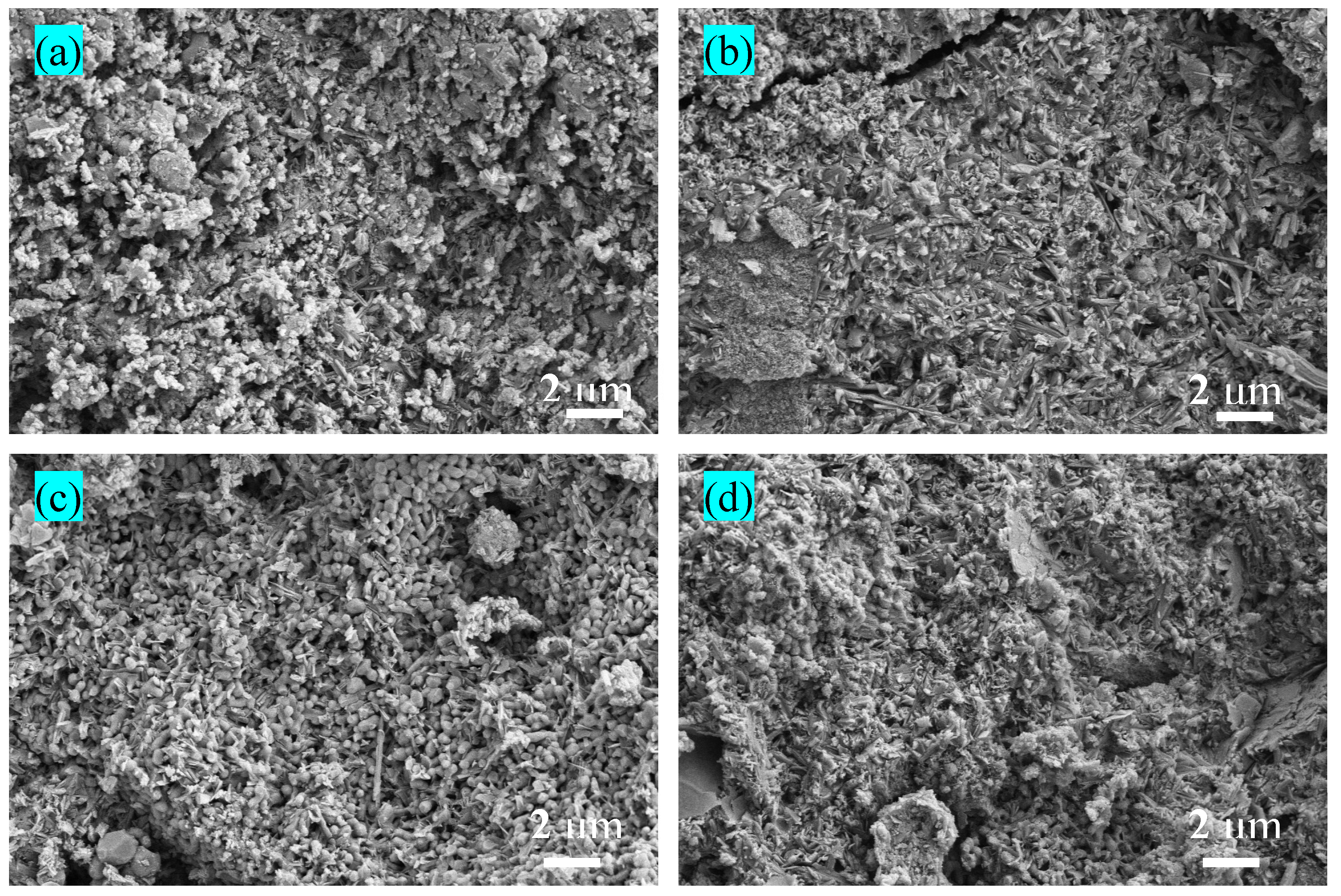
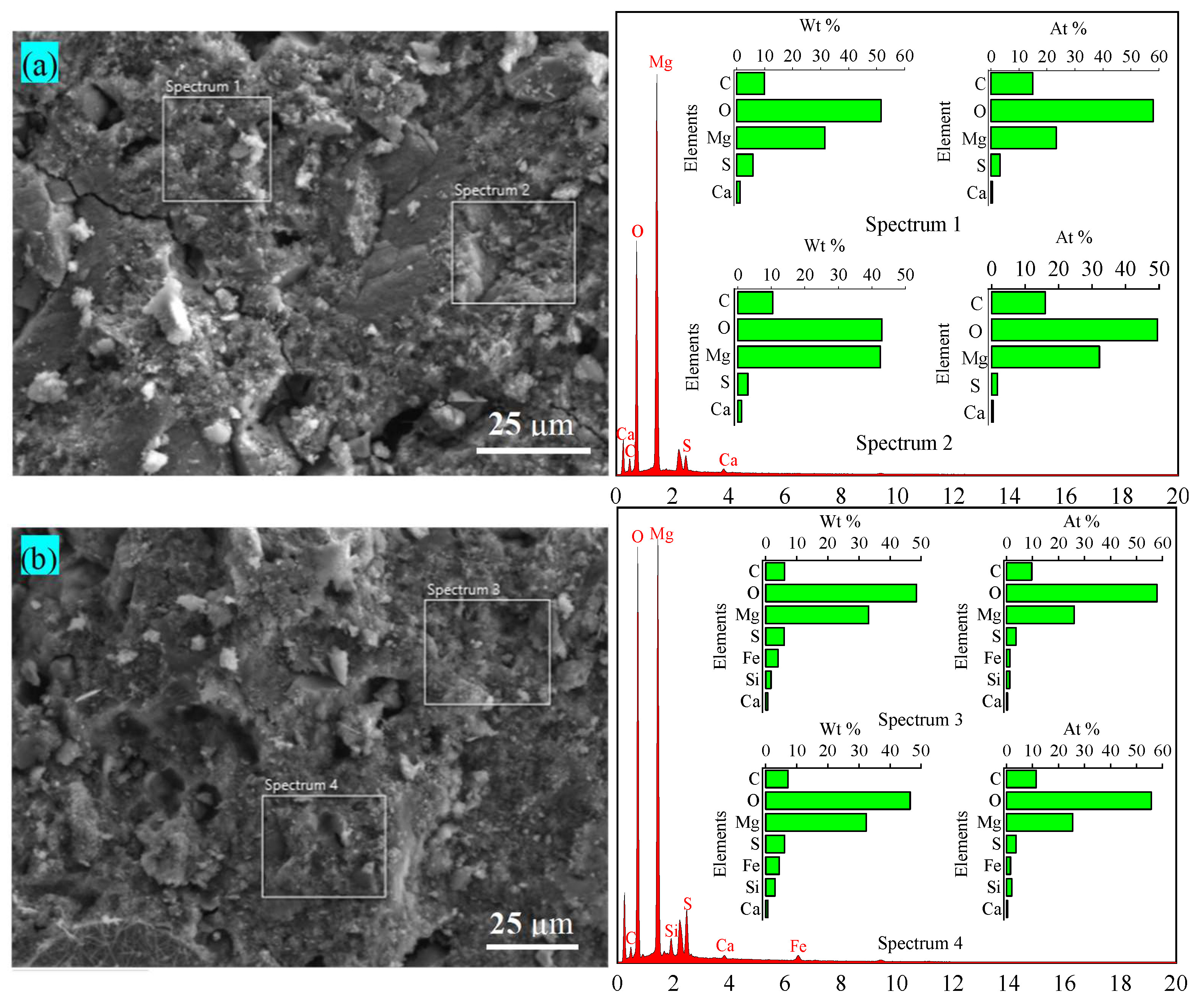
2.4. Microstructure Analysis and Mineralogical Characterization
3. Results and Analysis
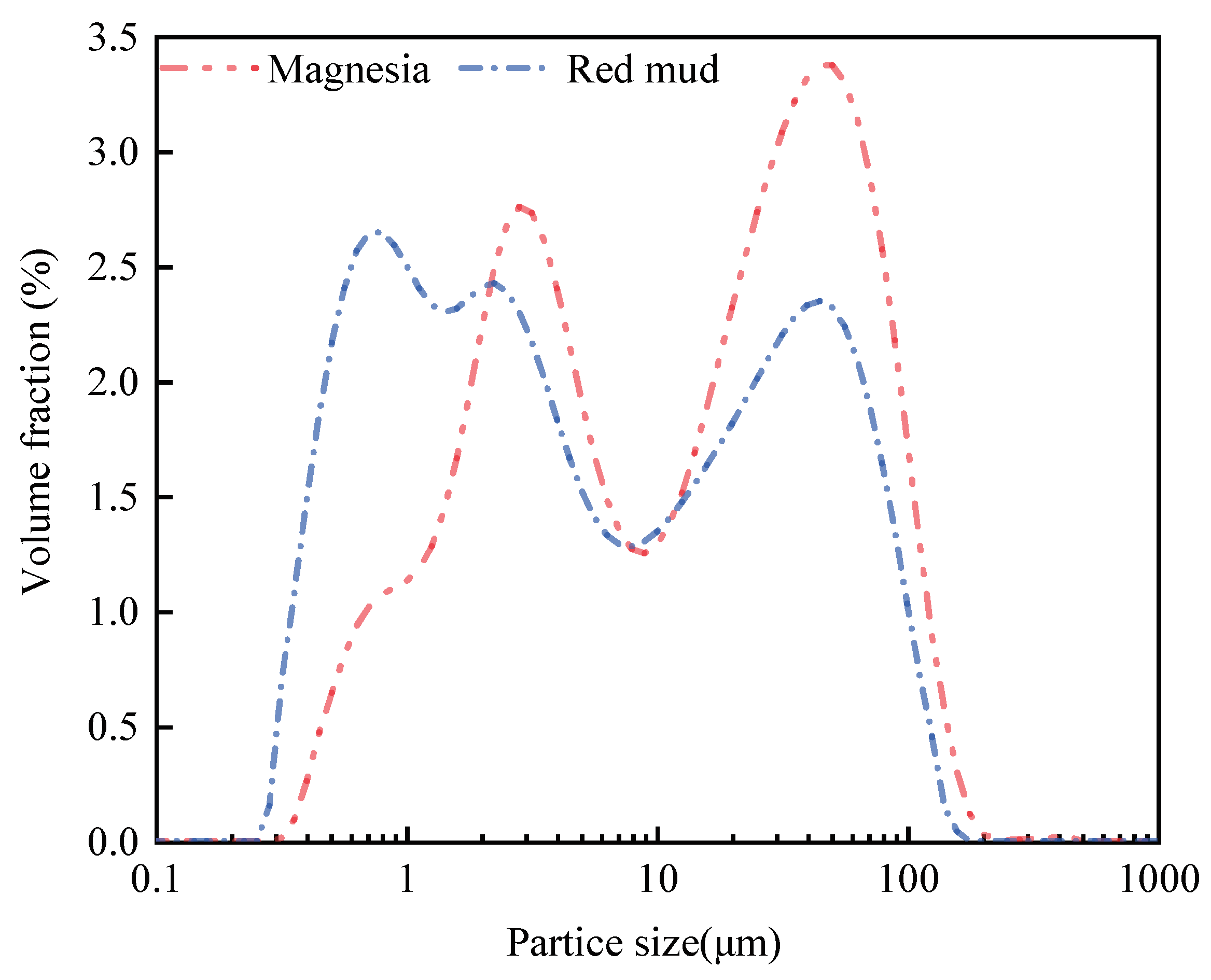
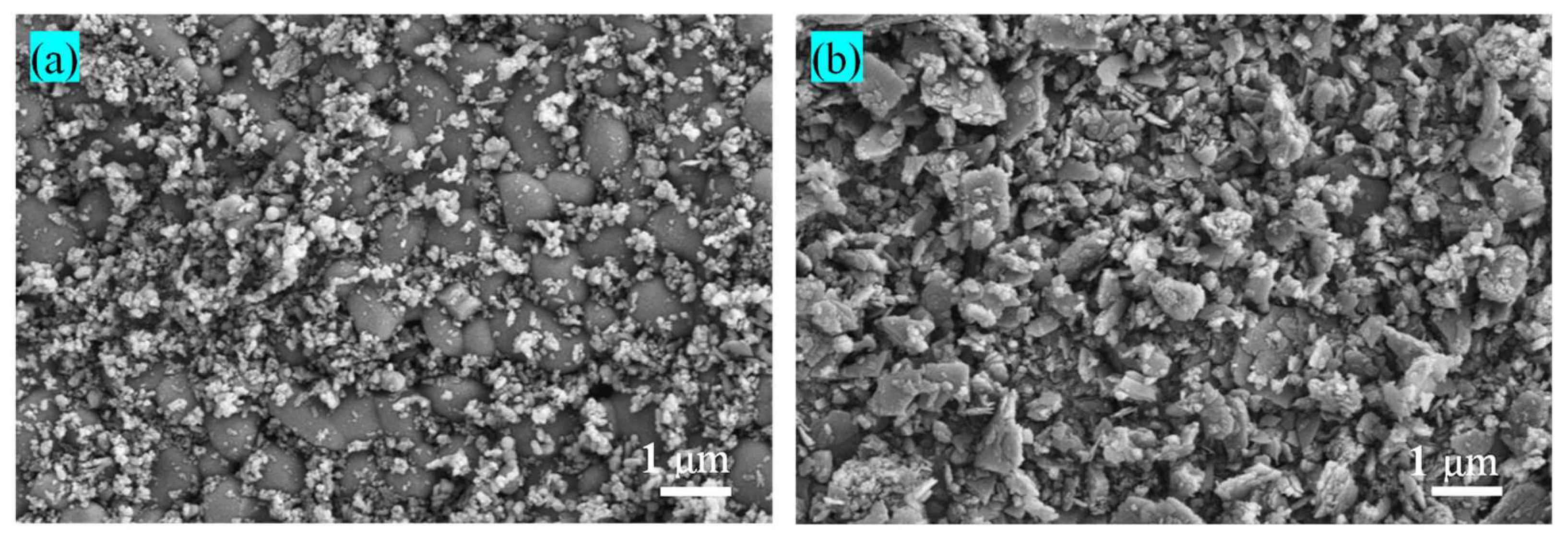
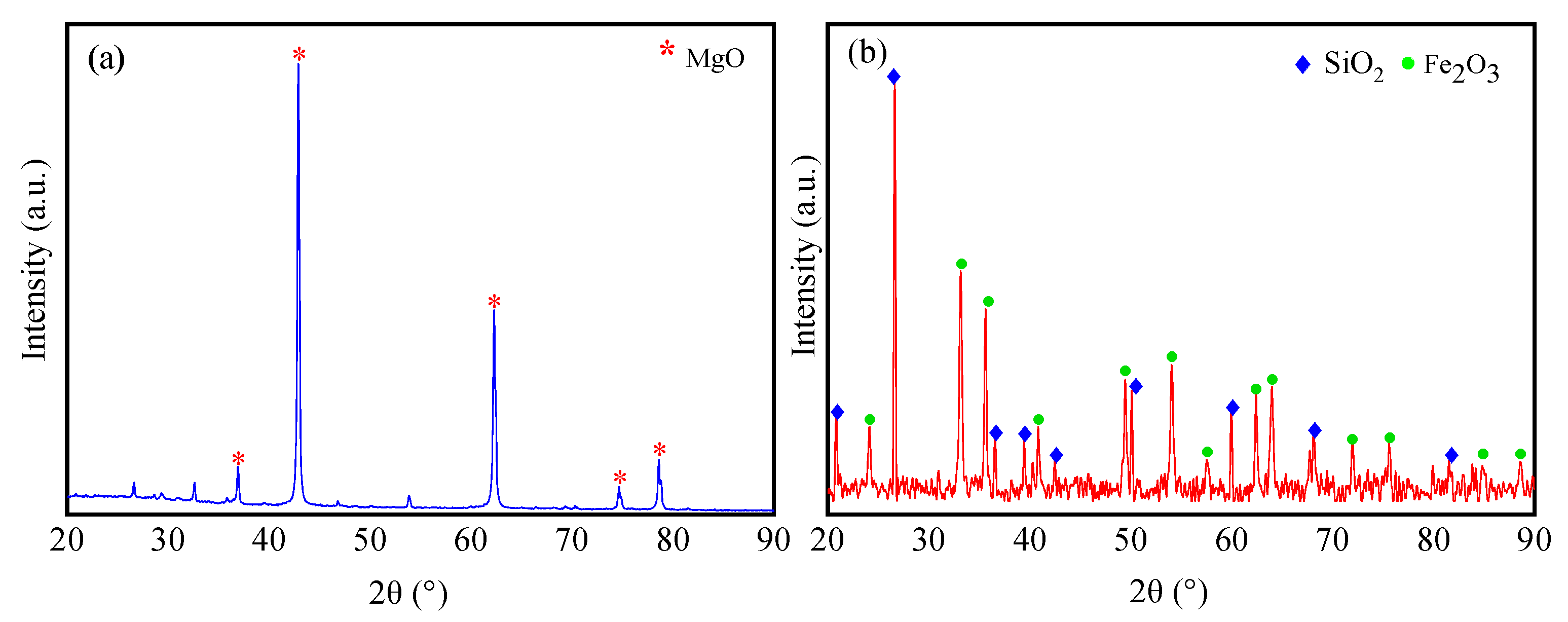
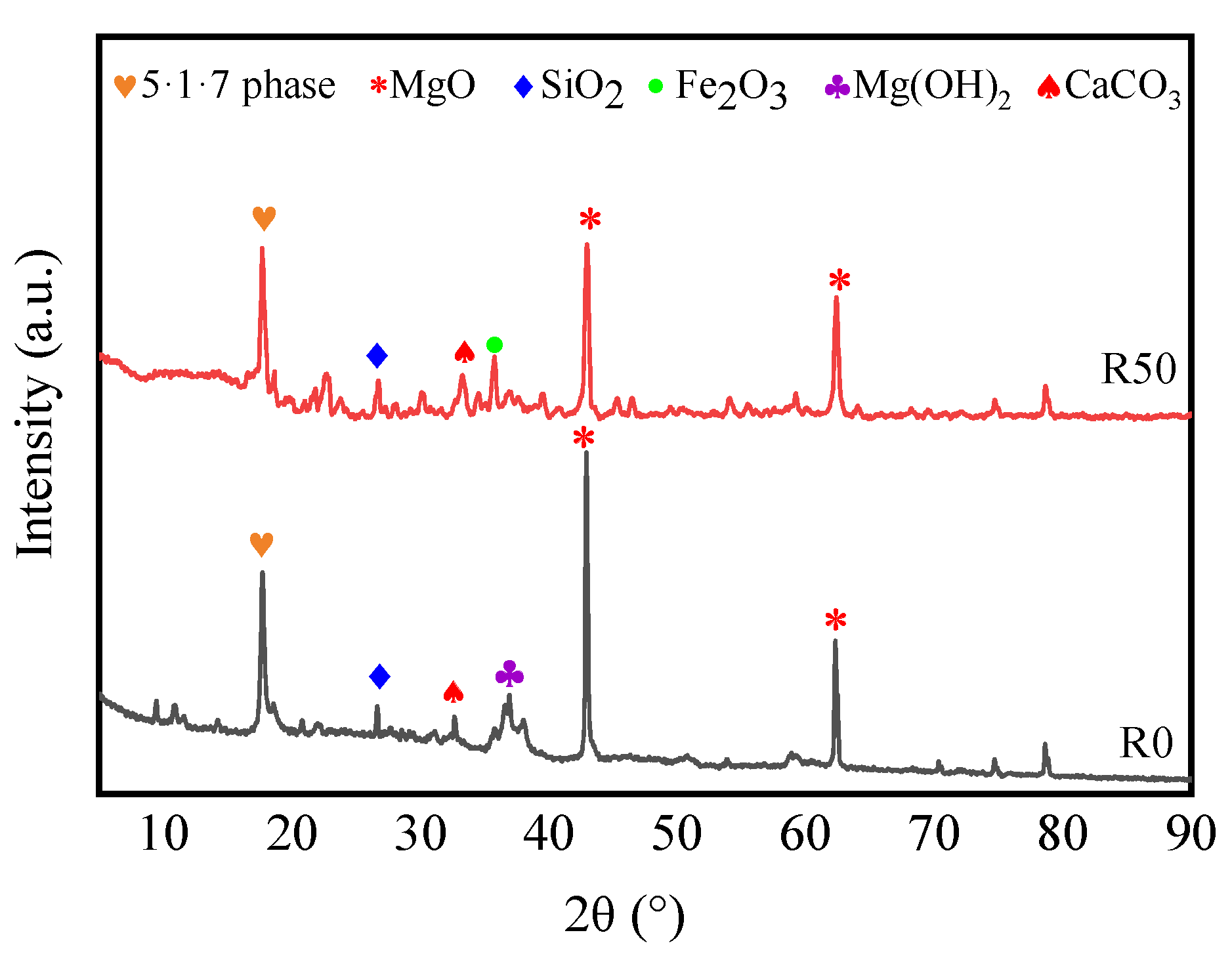
3.1. Properties of the Raw Materials
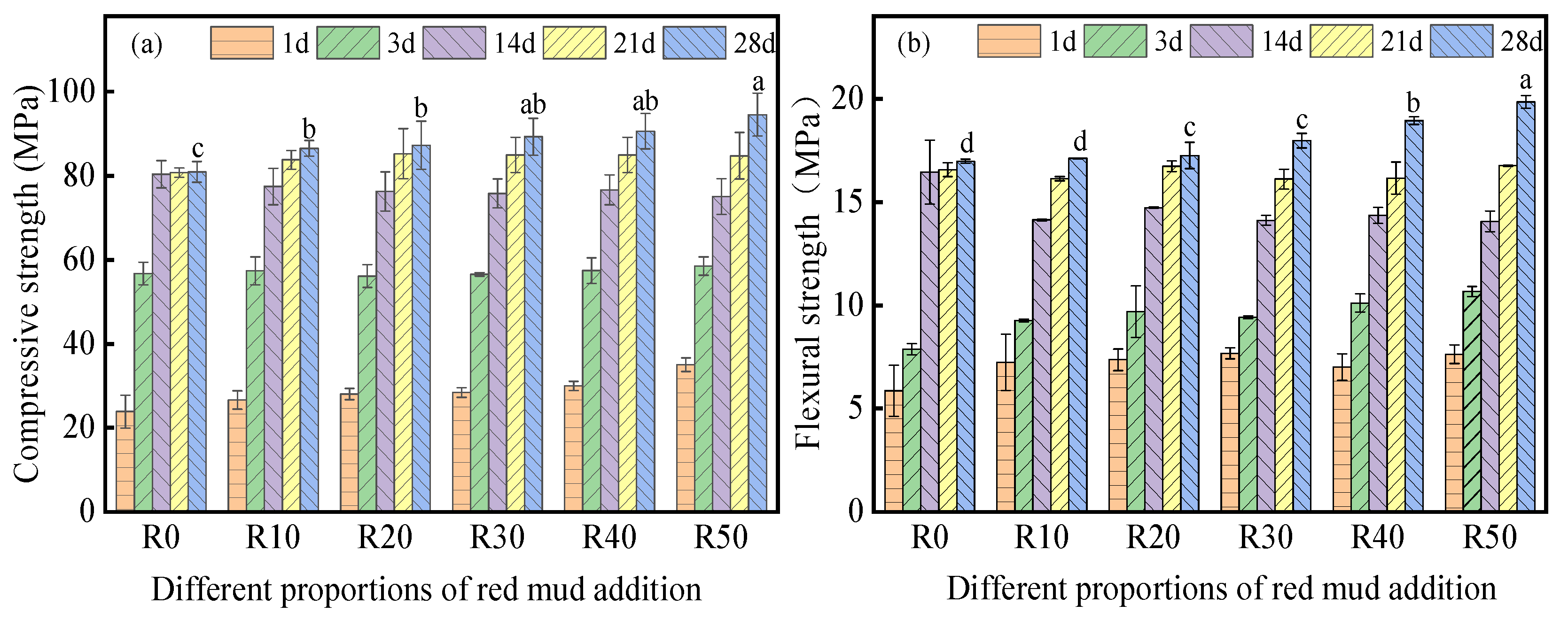
3.2. Mechanical Properties
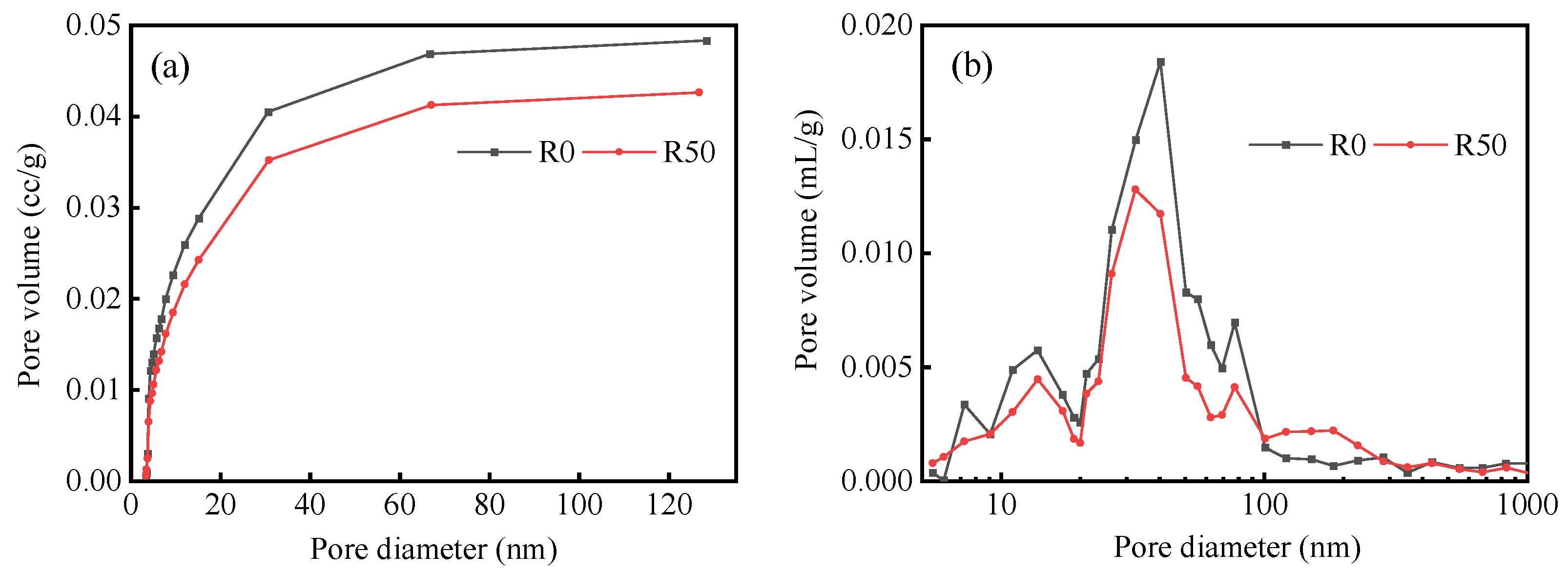
3.3. Effect of RM on the Pore Structure of BMSC
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Archambo, M.; Kawatra, S.K. Red mud: Fundamentals and new avenues for utilization. Min. Proc. Ext. Met. Rev. 2021, 42, 427–450. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Li, K.; Meng, Q.; Wang, S.; Wang, Y.; Zhu, P.; Niu, Q.; Hailong, Y.; Li, X.; et al. Red mud conserved compost nitrogen by enhancing nitrogen fixation and inhibiting denitrification revealed via metagenomic analysis. Bioresour. Technol. 2022, 346, 126654. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Wu, P.; Zha, X.; Liu, Y.; Wei, T.; Ran, W. The development and utilization of bauxite resources in the Guizhou Province and relevant challenges to the ecology and the environment. Gospod. Surowcami Min. 2022, 38, 5–32. [Google Scholar]
- Hendrik; Yuan, Y.; Fauzi, A.; Widiatmaka; Suryaningtyas, D.; Firdiyono, F.; Yao, Y. Determination of the Red Mud Industrial Cluster Sites in Indonesia Based on Sustainability Aspect and Waste Management Analysis through PROMETHEE. Energies 2022, 15, 5435. [Google Scholar] [CrossRef]
- Arroyo, F.; Luna-Galiano, Y.; Leiva, C.; Vilches, L.F. Environmental risks and mechanical evaluation of recycling red mud in bricks. Environ. Res. 2020, 186, 109537. [Google Scholar] [CrossRef]
- Svobodova-Sedlackova, A.; Calderón, A.; Fernandez, A.I.; Chimenos, J.M.; Berlanga, C.; Yücel, O.; Barreneche, C.; Rodriguez, R. Mapping the research landscape of bauxite by-products (red mud): An evolutionary perspective from 1995 to 2022. Heliyon 2024, 10, e24943. [Google Scholar] [CrossRef] [PubMed]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media, A Review. Recycling 2021, 6, 38–70. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of carbothermic microwave reduction followed by acid leaching for recovery of iron and aluminum values from Indian red mud. Miner. Eng. 2020, 159, 106653. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Zhang, Y.-X.; Wang, L.; Liu, C.-G.; Sun, W.-M.; Wang, Y.-F.; Long, S.-X.; He, X.-T.; Lin, Z.; Liang, J.-L.; et al. Rhizobacterial communities reshaped by red mud based passivators is vital for reducing soil Cd accumulation in edible amaranth. Sci. Total Environ. 2022, 826, 154002. [Google Scholar] [CrossRef]
- Bandopadhyay, A.; Giri, D. Improvement of expansive soils mixed with red mud and phosphogypsum. Arab. J. Geosci. 2023, 16, 322. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling, and utilisation of Bayer red mud. Resour. Conserv. Recy. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Antunes, M.L.P.; Conceição, F.T.; Navarro, G.R.B.; Fernandes, A.M.; Durrant, S.F. Use of red mud activated at different temperatures as a low-cost adsorbent of reactive dyes. Eng. Sanit. Ambient. 2021, 26, 805–811. [Google Scholar] [CrossRef]
- Martins, Y.J.C.; Almeida, A.C.M.; Viegas, B.M.; do Nascimento, R.A.; Ribeiro, N.F.d.P. Use of red mud from amazon region as an adsorbent for the removal of methylene blue: Process optimization, isotherm and kinetic studies. Int. J. Environ. Sci. Technol. 2020, 17, 4133–4148. [Google Scholar] [CrossRef]
- Kyrii, S.; Maletskyi, Z.; Klymenko, N.; Ratnaweera, H.; Mitchenko, T.; Dontsova, T.; Kosogina, I. Impact of modification by red mud components on the sorption properties of activated carbon. Appl. Surf. Sci. Adv. 2023, 16, 100412. [Google Scholar] [CrossRef]
- Zhen, Z.; He, C.; Wang, Y.; Ma, H. Novel Method of Synthesizing Polymeric Aluminum Ferric Sulfate Flocculant and Preparing Red Mud-Based Ceramsite. Materials 2024, 17, 1239. [Google Scholar] [CrossRef]
- Hertel, T.; van den Bulck, A.; Onisei, S.; Sivakumar, P.P.; Pontikes, Y. Boosting the use of bauxite residue (red mud) in cement–Production of an Fe-rich calcium sulfoaluminate-ferrite clinker and characterisation of the hydration. Cement Concrete Res. 2021, 145, 106463. [Google Scholar] [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; Bezerra, A.C.S.; Araújo, F.G.S. Red Mud from the Aluminium Industry: Production, Characteristics, and Alternative Applications in Construction Materials—A Review. Sustainability 2021, 13, 12741. [Google Scholar] [CrossRef]
- Babisk, M.P.; Amaral, L.F.; Ribeiro, L.D.S.; Vieira, C.M.F.; Prado, U.S.D.; Gadioli, M.C.B.; Oliveira, M.S.; da Luz, F.S.; Monteiro, S.N.; Filho, F.d.C.G. Evaluation and application of sintered red mud and its incorporated clay ceramics as materials for building construction. J. Mater. Res. Technol. 2020, 9, 2186–2195. [Google Scholar] [CrossRef]
- Vashistha, P.; Oinam, Y.; Shi, J.; Pyo, S. Application of lime mud as a sustainable alternative construction material: A comprehensive review of approaches. J. Build. Eng. 2024, 87, 109114. [Google Scholar] [CrossRef]
- Singh, V.; Bano, S.; Chauhan, V.B.; Pal, P.; Kumar, A.; Srivastava, J.B. Red mud as a sustainable road construction material: An experimental investigation. Constr. Build. Mater. 2024, 411, 134549. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. A review of comprehensive utilization of red mud. WMR 2022, 40, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Patangia, J.; Saravanan, T.J.; Kabeer, K.I.S.A.; Bisht, K. Study on the utilization of red mud (bauxite waste) as a supplementary cementitious material: Pathway to attaining sustainable development goals. Constr. Build. Mater. 2023, 375, 131005. [Google Scholar] [CrossRef]
- Luo, Z.; Hao, Y.; Mu, Y.; Tang, C.; Liu, X. Solidification/stabilization of red mud with natural radionuclides in granular blast furnace slag based geopolymers. Constr. Build. Mater. 2022, 316, 125916. [Google Scholar] [CrossRef]
- Ghalehnovi, M.; Shamsabadi, E.; Khodabakhshian, A.; Sourmeh, F.; de Brito, J. Self-compacting architectural concrete production using red mud. Constr. Build. Mater. 2019, 226, 418–427. [Google Scholar] [CrossRef]
- Kaliprasanna, S.; Sitha, R.K.; Barpanda, S.; Bhoi, B.R. Experimental Investigation of Strength Properties of Red Mud Concrete. AIP Conf. Proc. 2019, 2158, 020015. [Google Scholar]
- Liu, Y.; Qin, Z.; Chen, B. Experimental research on magnesium phosphate cements modified by red mud. Constr. Build. Mater. 2020, 231, 117131. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary reaction behaviors of red mud-based cementitious material: Hydration characteristics and Na+ utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef] [PubMed]
- Gomes Silveira, N.C.; Martins, M.L.F.; Bezerra, A.C.d.S.; Araújo, F.G.d.S. Ecological geopolymer produced with a ternary system of red mud, glass waste, and Portland cement. Clean. Eng. Technol. 2022, 6, 100379. [Google Scholar] [CrossRef]
- Wu, C.; Yu, H.; Zhang, H.; Dong, J.; Wen, J.; Tan, Y. Effects of phosphoric acid and phosphates on magnesium oxysulfate cement. Mater. Struct. 2015, 48, 907–917. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cement Concrete Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Wu, C.; Yu, H.; Dong, J.; Zheng, L. Effects of Material Ratio, Fly Ash, and Citric Acid on Magnesium Oxysulfate Cement. ACI Mater. J. 2014, 111, 291–297. [Google Scholar] [CrossRef]
- Chengyou, W.; Huifang, Z.; Hongfa, Y. Preparation and properties of modified magnesium oxysulfate cement derived from waste sulfuric acid. Adv. Cem. Res. 2016, 28, 178–188. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, H. Research on technology of performance improvement of basic magnesium sulfate cement BMS. Struct. Concr. 2023, 24, 4313–4321. [Google Scholar] [CrossRef]
- Wang, A.; Chu, Y.; Xu, H.; Liu, K.; Ma, R.; Dong, W.; Sun, D. Research Progress and Performance Improvement Technology of Basic Magnesium sulfate cement. Mater. Rep. 2020, 34, 13091–13099. [Google Scholar]
- Zhang, N.; Yu, H.; Wang, N.; Gong, W.; Tan, Y.; Wu, C. Effects of low- and high-calcium fly ash on magnesium oxysulfate cement. Constr. Build. Mater. 2019, 215, 162–170. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Duan, L. Effect of fineness and components of CFBC ash on performance of basic magnesium sulfate cement. Constr. Build. Mater. 2018, 170, 801–811. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, X.; Wei, C.; Zeng, Q.; Ma, S.; Liu, X.; Zhang, Z.; Webeck, E. Synergistic enhancement of converter steelmaking slag, blast furnace slag, Bayer red mud in cementitious materials: Strength, phase composition, and microstructure. J. Build. Eng. 2022, 60, 105177. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Zhou, J. Preparation of building materials from Bayer red mud with magnesium cement. Constr. Build. Mater. 2022, 323, 126507. [Google Scholar] [CrossRef]
- Test Method of Cement Mortar Strength (ISO Method). National Standardization Administration: Beijing, China, 2021.
- Gu, K.; Chen, B.; Cui, Q. Experimental research on properties of magnesium oxysulfate cement during high temperature exposure. Compos. B Eng. 2022, 244, 110168. [Google Scholar] [CrossRef]
- Lauermannová, A.; Lojka, M.; Jankovský, O.; Faltysová, I.; Pavlíková, M.; Pivák, A.; Záleská, M.; Pavlík, Z. High-performance magnesium oxychloride composites with silica sand and diatomite. J. Mater. Res. Technol. 2021, 11, 957–969. [Google Scholar] [CrossRef]
- Ma, H.; Xu, B.; Liu, J.; Pei, H.; Li, Z. Effects of water content, magnesia-to-phosphate molar ratio and age on pore structure, strength, and permeability of magnesium potassium phosphate cement paste. Mater. Des. 2014, 64, 497–502. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, H.; Li, Y.; Bi, W.; Yao, X. The effect of slag on the properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2016, 126, 313–320. [Google Scholar] [CrossRef]
- Yan, P.; Chen, B.; Haque, M.; Liu, T. Influence of red mud on the engineering and microstructural properties of sustainable ultra-high-performance concrete. Constr. Build. Mater. 2023, 396, 132404. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Hou, G. Utilisation of fly ash microsphere powder as a mineral admixture of cement: Effects on early hydration and microstructure at different curing temperatures. Powder Technol. 2020, 375, 262–270. [Google Scholar] [CrossRef]
- Linora Metilda, D.; Selvamony, C.; Anandakumar, T.; Seeni, A. Investigations on optimum possibility of replacing cement partially by red mud in concrete. Sci. Res. Essays 2015, 10, 137–143. [Google Scholar]
| Specimen | MgO (g) | Mud (g) | MgSO4·7H2O Solution (g) | C6H8O7 (g) |
|---|---|---|---|---|
| R0 | 1000 | 0 | 907.44 | 6 |
| R10 | 1000 | 100 | 907.44 | 6 |
| R20 | 1000 | 200 | 907.44 | 6 |
| R30 | 1000 | 300 | 907.44 | 6 |
| R40 | 1000 | 400 | 907.44 | 6 |
| R50 | 1000 | 500 | 907.44 | 6 |
| Raw Material | Mass Fraction of the Samples (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | MnO | Fe2O3 | |
| Magnesia | 93.8 | 0.36 | 3.99 | 0.03 | 0.09 | 0.02 | 1.47 | 0.02 | 0.29 |
| Red mud | 0.58 | 4.31 | 32.61 | 0.22 | 0.02 | 0.78 | 0.64 | 0.12 | 60.45 |
| Specimen | Porosity (%) | Total Pore Volume (mL/g) | Average Pore Size (nm) | Total Pore Area (m2/g) | Apparent Density (g/mL) |
|---|---|---|---|---|---|
| R0 | 22.09 | 0.13 | 32.18 | 16.50 | 2.14 |
| R50 | 18.97 | 0.10 | 31.92 | 12.90 | 2.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhen, Z. Properties of Red-Mud-Modified Basic Magnesium Sulfate Cement. Materials 2024, 17, 4085. https://doi.org/10.3390/ma17164085
Wang Y, Zhen Z. Properties of Red-Mud-Modified Basic Magnesium Sulfate Cement. Materials. 2024; 17(16):4085. https://doi.org/10.3390/ma17164085
Chicago/Turabian StyleWang, Yanrong, and Zhilei Zhen. 2024. "Properties of Red-Mud-Modified Basic Magnesium Sulfate Cement" Materials 17, no. 16: 4085. https://doi.org/10.3390/ma17164085
APA StyleWang, Y., & Zhen, Z. (2024). Properties of Red-Mud-Modified Basic Magnesium Sulfate Cement. Materials, 17(16), 4085. https://doi.org/10.3390/ma17164085





