Calcium Sulfate Disks for Sustained-Release of Amoxicillin and Moxifloxacin for the Treatment of Osteomyelitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Disk Production for Antibiotic Release Analysis
2.2. In Vitro Degradation Rate Analysis
2.3. CS Surface Characterization
2.4. Antibacterial Activity by Agar Diffusion Assay
2.5. Statistical Analysis
3. Results
3.1. In Vitro Degradation Rate Analysis
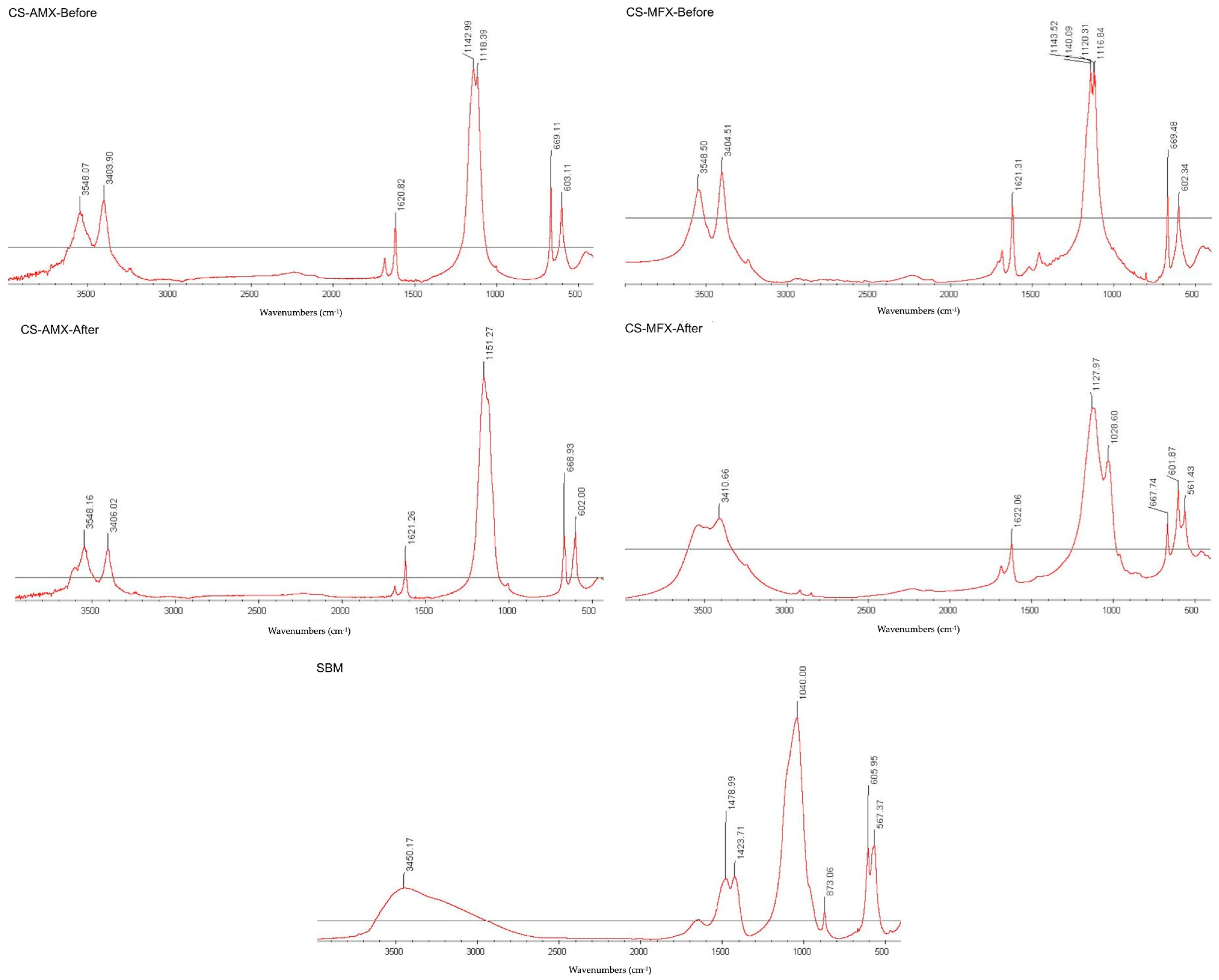
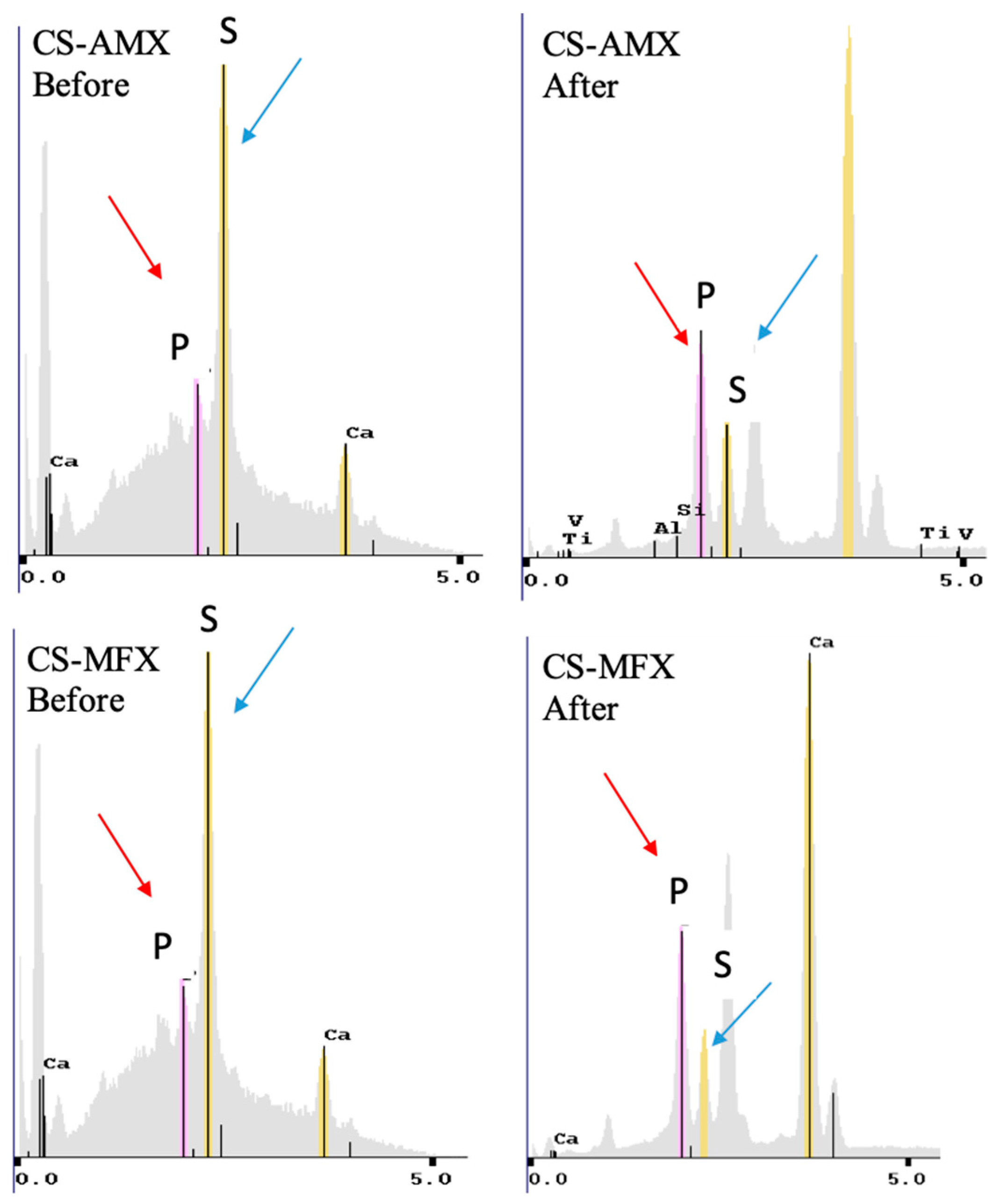
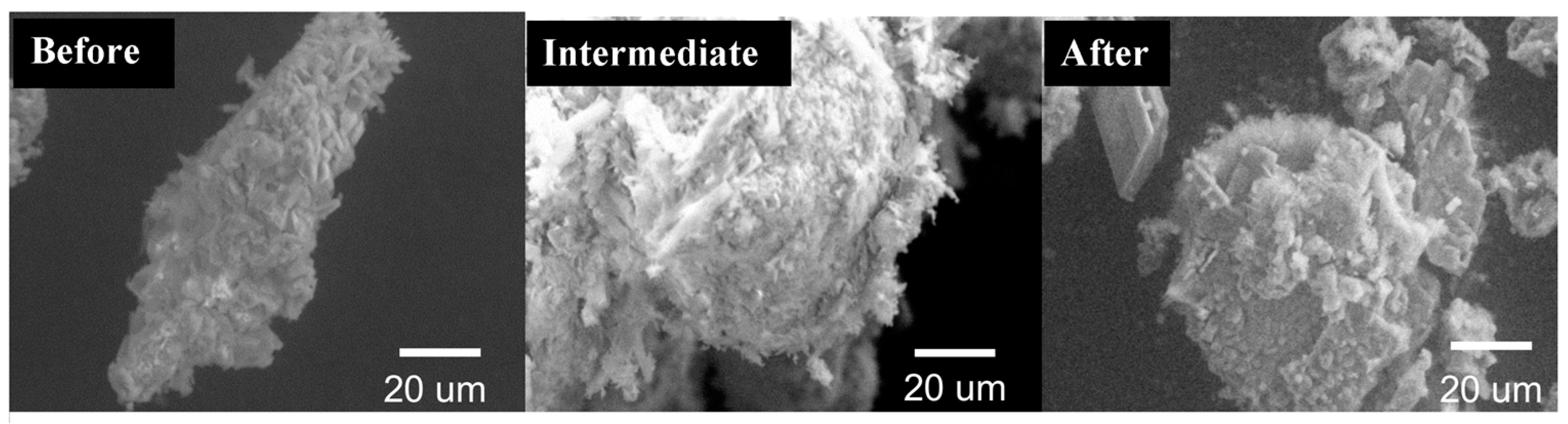
3.2. CS Surface Characterization
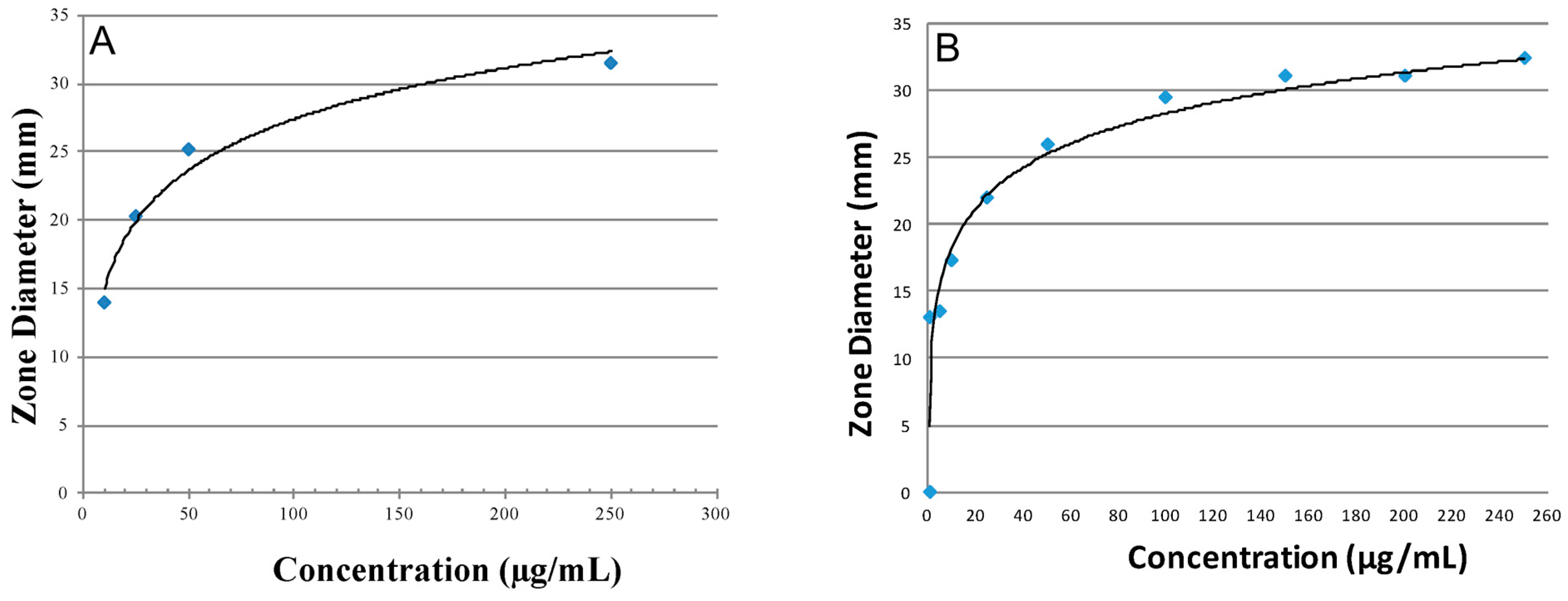
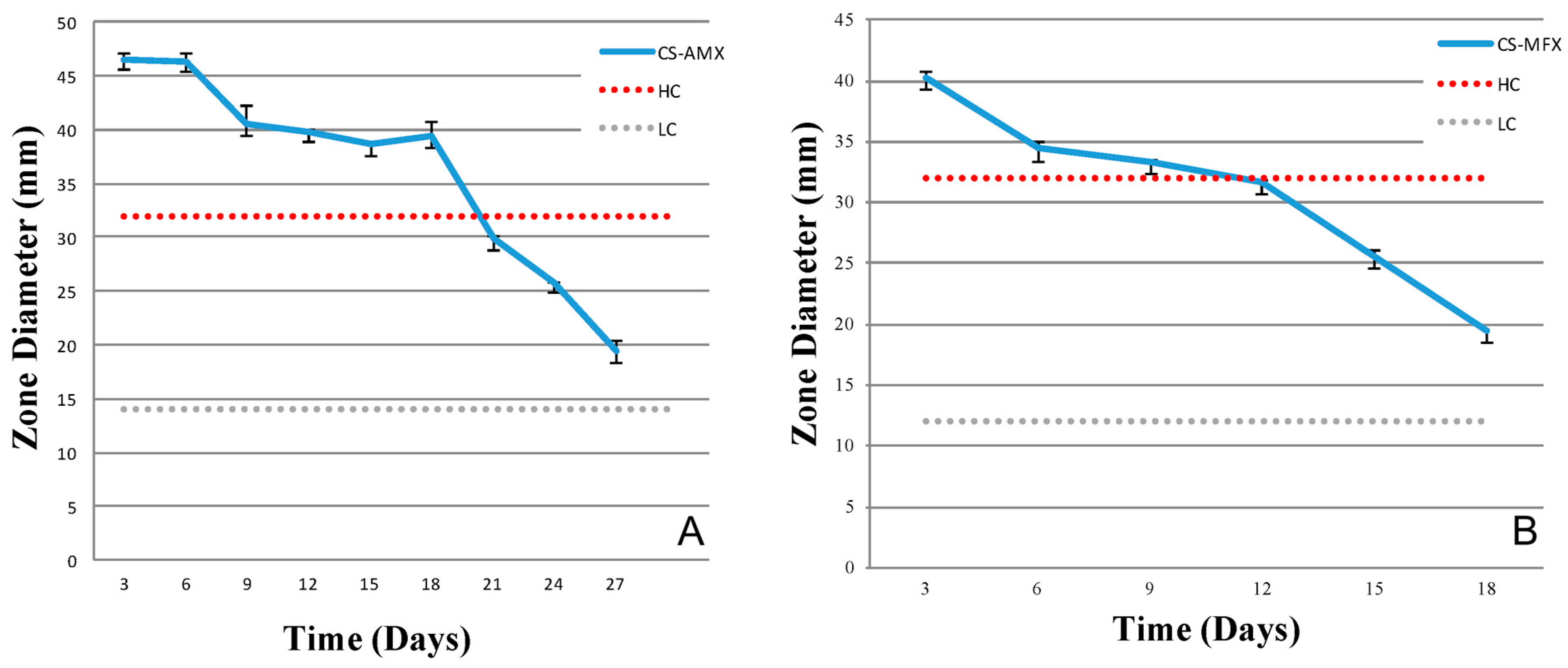
3.3. Antibiotic Activity and Release Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baltensperger, M.; Gratz, K.; Bruder, E.; Lebeda, R.; Makek, M.; Eyrich, G. Is primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J. Cranio-Maxillo-Facial Surg. 2004, 32, 43–50. [Google Scholar] [CrossRef]
- Bevin, C.R.; Inwards, C.Y.; Keller, E.E. Surgical management of primary chronic osteomyelitis: A long-term retrospective analysis. J. Oral Maxillofac. Surg. 2008, 66, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Julien Saint Amand, M.; Sigaux, N.; Gleizal, A.; Bouletreau, P.; Breton, P. Chronic osteomyelitis of the mandible: A comparative study of 10 cases with primary chronic osteomyelitis and 12 cases with secondary chronic osteomyelitis. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 342–348. [Google Scholar] [CrossRef]
- Andre, C.V.; Khonsari, R.H.; Ernenwein, D.; Goudot, P.; Ruhin, B. Osteomyelitis of the jaws: A retrospective series of 40 patients. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 261–264. [Google Scholar] [CrossRef]
- Coviello, V.; Stevens, M.R. Contemporary concepts in the treatment of chronic osteomyelitis. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef]
- Sanders, J.; Mauffrey, C. Long bone osteomyelitis in adults: Fundamental concepts and current techniques. Orthopedics 2013, 36, 368–375. [Google Scholar] [CrossRef]
- Ciampolini, J.; Harding, K.G. Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgrad. Med. J. 2000, 76, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Lata, J.; Pansotra, N. Osteomyelitis of Maxilla: A Rare Presentation Yet Not So Rare. J. Maxillofac. Oral Surg. 2022, 21, 1023–1031. [Google Scholar] [CrossRef]
- Akshayaa, L.; Ramani, P. Prevalence of Osteomyelitis in Jaws among the Patients Visiting Private Dental Hospital: An Institutional Study. J. Pharm. Res. Int. 2021, 33, 425–432. [Google Scholar] [CrossRef]
- Sukumaran, G.; Ramani, P.; Ramasubramanian, A.; Karunagaran, M.; Ravikumar, H. Implantation dermoid cyst. J. Evol. Med. Dent. Sci. 2019, 8, 4023–4025. [Google Scholar] [CrossRef]
- Lieblich, S.E.; Piecuch, J.F. Infections of the jaws, including infected fractures, osteomyelitis, and osteoradionecrosis. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2000, 8, 121–132. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.J.; Ueng, S.W.; Chan, E.C. The release of cefazolin and gentamicin from biodegradable PLA/PGA beads. Int. J. Pharm. 2004, 273, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Doty, H.A.; Leedy, M.R.; Courtney, H.S.; Haggard, W.O.; Bumgardner, J.D. Composite chitosan and calcium sulfate scaffold for dual delivery of vancomycin and recombinant human bone morphogenetic protein-2. J. Mater. Sci. Mater. Med. 2014, 25, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jiang, T.; Yang, Y.; Yang, X.; Zhao, J. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet. Disord. 2016, 17, 502. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Terai, H.; Toyoda, H.; Namikawa, T.; Yokota, Y.; Tsunoda, T.; Takaoka, K. A biodegradable delivery system for antibiotics and recombinant human bone morphogenetic protein-2: A potential treatment for infected bone defects. J. Orthop. Res. 2006, 24, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Dikmen, G.; Fragomen, A.; Rozbruch, S.R. Antibiotic-coated nail for fusion of infected charcot ankles. Foot Ankle Int. 2013, 34, 80–84. [Google Scholar] [CrossRef]
- Ueng, S.W.; Yuan, L.J.; Lee, N.; Lin, S.S.; Chan, E.C.; Weng, J.H. In vivo study of biodegradable alginate antibiotic beads in rabbits. J. Orthop. Res. 2004, 22, 592–599. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Maus, U.; Andereya, S.; Gravius, S.; Ohnsorge, J.A.; Niedhart, C.; Siebert, C.H. BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J. Biomater. Appl. 2008, 22, 559–576. [Google Scholar] [CrossRef]
- Butini, M.E.; Cabric, S.; Trampuz, A.; Di Luca, M. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf. B Biointerfaces 2018, 161, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Nakkeeran, K.P.; Saravanan, K.; Babu, P.; John, R.R. Evaluation of bone regeneration in periapical osseous defects with and without platelet rich plasma, combined calcium sulfate and autologous bone graft—A comparative study. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, Y.K.; Chang, W.J.; Wu, Y.C.; Wang, C.C.; Yang, K.C. Limitation of the antibiotic-eluting bone graft substitute: An example of gentamycin-impregnated calcium sulfate. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.M.; Anderson, J.M.; Marchant, R.E. Targeted delivery of vancomycin to Staphylococcus epidermidis biofilms using a fibrinogen-derived peptide. J. Biomed. Mater. Res. A 2012, 100, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.R.; Behera, B.; Maiti, T.K.; Mohapatra, S. Multifunctional magnetic calcium phosphate nanoparticles for targeted platin delivery. Dalton Trans. 2012, 41, 10777–10783. [Google Scholar] [CrossRef] [PubMed]
- Peltier, L.F. The use of plaster of Paris to fill large defects in bone: A preliminary report. Clin. Orthop. Relat. Res. 2001, 382, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Yeo, H.H.; Kim, Y.K. Grafting of large defects of the jaws with a particulate dentin-plaster of paris combination. Oral Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1999, 88, 22–25. [Google Scholar] [CrossRef]
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50. [Google Scholar] [CrossRef]
- al Ruhaimi, K.A. Effect of adding resorbable calcium sulfate to grafting materials on early bone regeneration in osseous defects in rabbits. Int. J. Oral. Maxillofac. Implant. 2000, 15, 859–864. [Google Scholar]
- Karthikeyan, R.; Amaechi, B.T.; Rawls, H.R.; Lee, V.A. Antimicrobial activity of nanoemulsion on cariogenic Streptococcus mutans. Arch. Oral Biol. 2011, 56, 437–445. [Google Scholar] [CrossRef]
- Ullman, R.F.; Strampfer, M.J.; Cunha, B.A. Streptococcus mutans vertebral osteomyelitis. Heart Lung 1988, 17, 319–321. [Google Scholar] [PubMed]
- Chang, W.; Colangeli, M.; Colangeli, S.; Di Bella, C.; Gozzi, E.; Donati, D. Adult osteomyelitis: Debridement versus debridement plus Osteoset T pellets. Acta Orthop. Belg. 2007, 73, 238–243. [Google Scholar]
- Kent, M.E.; Rapp, R.P.; Smith, K.M. Antibiotic beads and osteomyelitis: Here today, what’s coming tomorrow? Orthopedics 2006, 29, 599–603. [Google Scholar] [PubMed]
- Slosarczyk, A.; Paluszkiewicz, C.; Gawlicki, M.; Paszkiewicz, Z. The FTIR spectroscopy and QXRD studies of calcium phosphate based materials produced from the powder precursors with different Ca/P ratios. Ceram. Int. 1997, 23, 297–304. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Marco, F.; Amero, Y.; Garcia de la Maria, C.; Amat, E.; Almela, M.; Moreno, A.; Claramonte, X.; Perez, N.; Mestres, C.A.; Gatell, J.M.; et al. Trends in antimicrobial susceptibilities of viridans group streptococci isolated in patients with infective endocarditis from 1990 to 2003. In Proceedings of the 15th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 2–5 April 2005. [Google Scholar]
- Koo, H.; Gomes, B.P.; Rosalen, P.L.; Ambrosano, G.M.; Park, Y.K.; Cury, J.A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Park, Y.B.; Mohan, K.; Al-Sanousi, A.; Almaghrabi, B.; Genco, R.J.; Swihart, M.T.; Dziak, R. Synthesis and characterization of nanocrystalline calcium sulfate for use in osseous regeneration. Biomed. Mater. 2011, 6, 055007. [Google Scholar] [CrossRef]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cell Mater. 2010, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Marx, R. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Harwood, A.; McGurk, M. Osteomyelitis presenting in two patients: A challenging disease to manage. Brit Dent. J. 2010, 209, 393–396. [Google Scholar] [CrossRef]
- Fleisher, K.; Pham, S.; Raad, R.; Friedman, K.; Ghesani, M.; Chan, K.; Amintavaleoli, N.; Janal, M.; Levine, J.; Glickman, R. Does fluorodeoxyglucose positron emission tomography with computed tomography facilitate treatment of medication-related osteonecrosis of hte jaw? J. Oral Maxillofac. Surg. 2016, 74, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, G.; Baltensperger, M.; Bruder, E.; Graetz, K. Primary chronic osteomyelitis in childhood and adolescence: A retrospective analysis of 11 cases and review of hte literature. J. Oral Maxilofac. Surg. 2003, 61, 561–573. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nemati, R. Cephalexin-loaded injectable macroporous calcium phosphate bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Tatebayashi, S.; Imai, Y.; Kirita, T. Efficacy of vancomycin-impregnated cement beads for the treatment of MRSA infection of failed graft tissue at the mandible. J. Oral Maxillofac. Surg. 2005, 63, 1234–1238. [Google Scholar] [CrossRef]
- Beenken, K.E.; Bradney, L.; Bellamy, W.; Skinner, R.A.; McLaren, S.G.; Gruenwald, M.J.; Spencer, H.J.; Smith, J.K.; Haggard, W.O.; Smeltzer, M.S. Use of xylitol to enhance the therapeutic efficacy of polymethylmethacrylate-based antibiotic therapy in treatment of chronic osteomyelitis. Antimicrob. Agents Chemother. 2012, 56, 5839–5844. [Google Scholar] [CrossRef]
- Hamada, S.; Koga, T.; Ooshima, T. Virulence factors of Streptococcus mutans and dental caries prevention. J. Dent. Res. 1984, 63, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Aberg, J.; Eriksson, O.; Spens, E.; Nordblom, J.; Mattsson, P.; Sjodahl, J.; Svensson, M.; Engqvist, H. Calcium sulfate spinal cord scaffold: A study on degradation and fibroblast growth factor 1 loading and release. J. Biomater. Appl. 2012, 26, 667–685. [Google Scholar] [CrossRef]
- Zhang, F.; Chang, J.; Lin, K.; Lu, J. Preparation, mechanical properties and in vitro degradability of wollastonite/tricalcium phosphate macroporous scaffolds from nanocomposite powders. J. Mater. Sci. Mater. Med. 2008, 19, 167–173. [Google Scholar] [CrossRef]
- Heughebaert, M.; LeGeros, R.Z.; Gineste, M.; Guilhem, A.; Bonel, G. Physicochemical characterization of deposits associated with HA ceramics implanted in nonosseous sites. J. Biomed. Mater. Res. 1988, 22, 257–268. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar]
- LeGeros, R.Z.; Ol Gregoire, M.; Daculsi, G. Substrate Surface dissolution and Interfacial Biological Mineralization. In The Bone-Biomaterial Interface; University of Toronto Press: Toronto, ON, Canada, 1991; Chapter 7; pp. 76–89. [Google Scholar]
- Tay, B.K.; Patel, V.V.; Bradford, D.S. Calcium sulfate- and calcium phosphate-based bone substitutes. Mimicry of the mineral phase of bone. Orthop. Clin. N. Am. 1999, 30, 615–623. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef]
- Thomsen, V.F. Correlation of the plate-dilution method to the agar diffusion method (disc and tablet methods) with a special view to the importance of pre-diffusion. Acta Pathol. Microbiol. Scand. 1962, 54, 107–120. [Google Scholar] [CrossRef]
- James, P.A. Comparison of four methods for the determination of MIC and MBC of penicillin for viridans streptococci and the implications for penicillin tolerance. J. Antimicrob. Chemother. 1990, 25, 209–216. [Google Scholar] [CrossRef]
- van Merkesteyn, J.P.; Groot, R.H.; van den Akker, H.P.; Bakker, D.J.; Borgmeijer-Hoelen, A.M. Treatment of chronic suppurative osteomyelitis of the mandible. Int. J. Oral Maxillofac. Surg. 1997, 26, 450–454. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Kinzig, M.; Bulitta, J.B.; Hennig, F.F.; Holzgrabe, U.; Sorgel, F.; Gusinde, J. Bone penetration of amoxicillin and clavulanic acid evaluated by population pharmacokinetics and Monte Carlo simulation. Antimicrob. Agents Chemother. 2009, 53, 2569–2578. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangolli, R.; Pushalkar, S.; Beutel, B.G.; Danna, N.; Duarte, S.; Ricci, J.L.; Fleisher, K.; Saxena, D.; Coelho, P.G.; Witek, L.; et al. Calcium Sulfate Disks for Sustained-Release of Amoxicillin and Moxifloxacin for the Treatment of Osteomyelitis. Materials 2024, 17, 4086. https://doi.org/10.3390/ma17164086
Gangolli R, Pushalkar S, Beutel BG, Danna N, Duarte S, Ricci JL, Fleisher K, Saxena D, Coelho PG, Witek L, et al. Calcium Sulfate Disks for Sustained-Release of Amoxicillin and Moxifloxacin for the Treatment of Osteomyelitis. Materials. 2024; 17(16):4086. https://doi.org/10.3390/ma17164086
Chicago/Turabian StyleGangolli, Riddhi, Smruti Pushalkar, Bryan G. Beutel, Natalie Danna, Simone Duarte, John L. Ricci, Kenneth Fleisher, Deepak Saxena, Paulo G. Coelho, Lukasz Witek, and et al. 2024. "Calcium Sulfate Disks for Sustained-Release of Amoxicillin and Moxifloxacin for the Treatment of Osteomyelitis" Materials 17, no. 16: 4086. https://doi.org/10.3390/ma17164086
APA StyleGangolli, R., Pushalkar, S., Beutel, B. G., Danna, N., Duarte, S., Ricci, J. L., Fleisher, K., Saxena, D., Coelho, P. G., Witek, L., & Tovar, N. (2024). Calcium Sulfate Disks for Sustained-Release of Amoxicillin and Moxifloxacin for the Treatment of Osteomyelitis. Materials, 17(16), 4086. https://doi.org/10.3390/ma17164086







