Synthesis and Characterization of Novel Adsorbents Based on Functionalization of Graphene Oxide with Schiff Base and Reduced Schiff Base for Pesticide Removal
Abstract
1. Introduction
2. Materials and Methods
Adsorption Study of Pesticide on GO and Its Derivatives
3. Results and Discussion
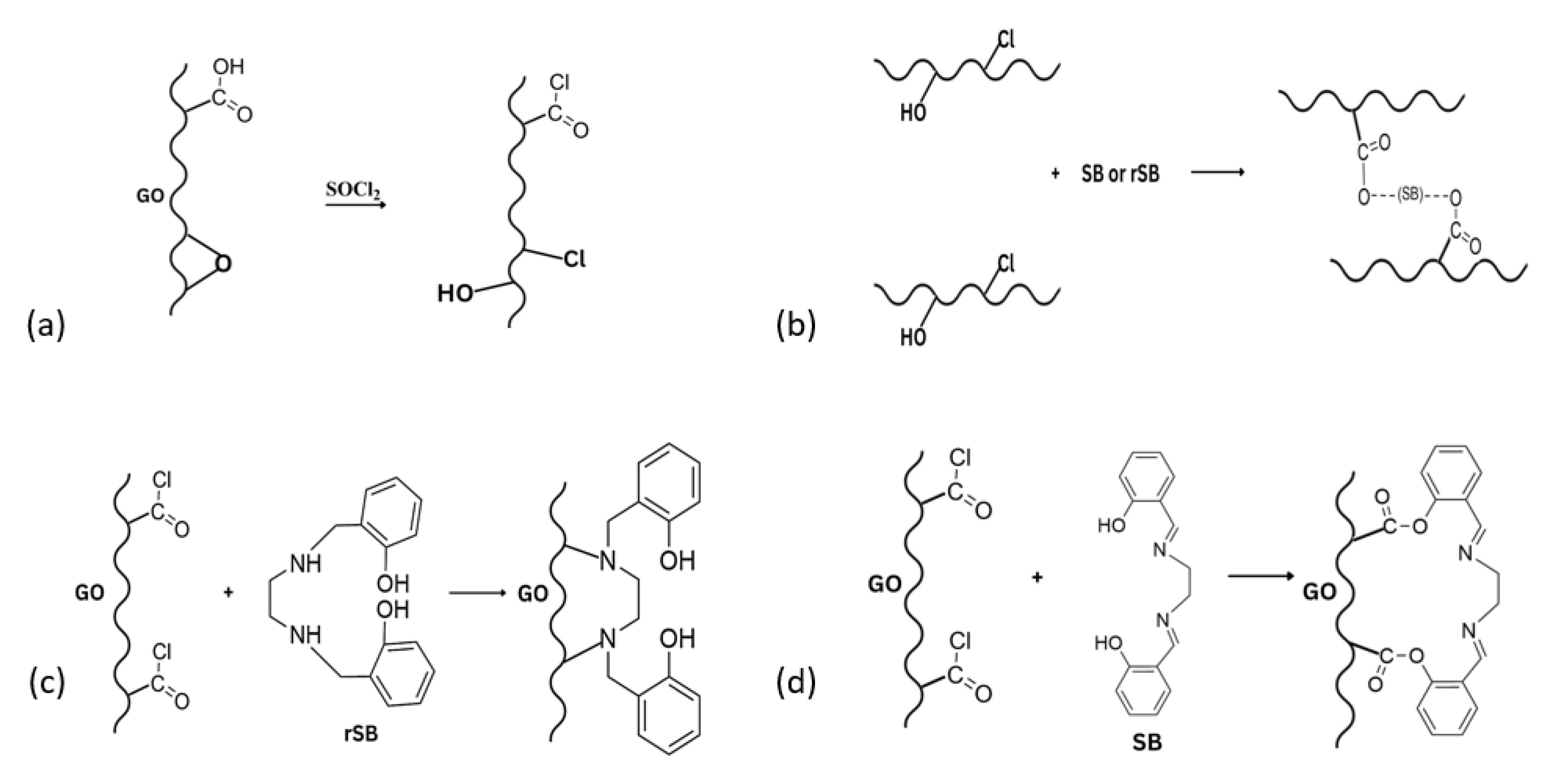
3.1. Synthesis and Functionalization of Graphene Oxide with SB and rSB
3.2. Characterization Section
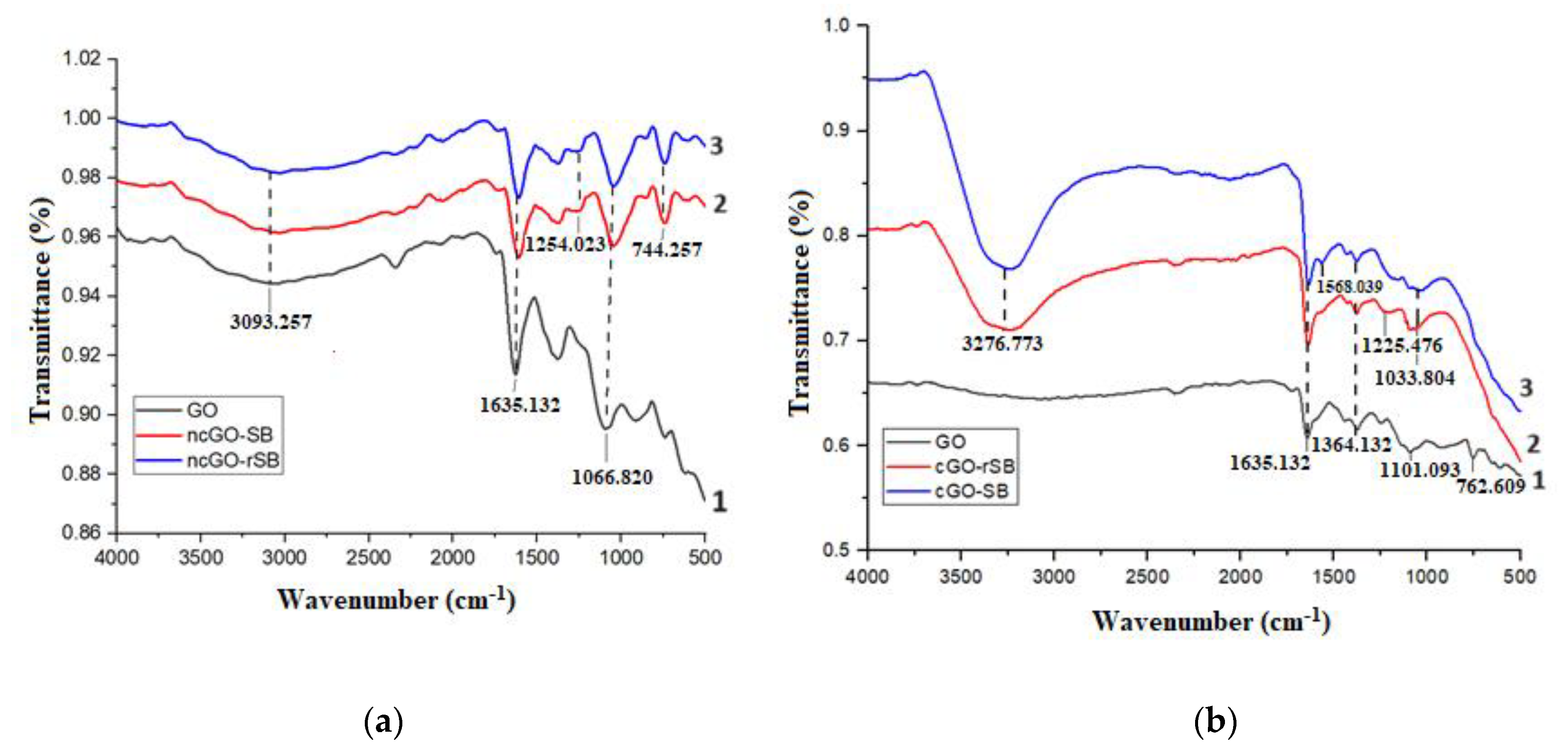
3.2.1. FTIR
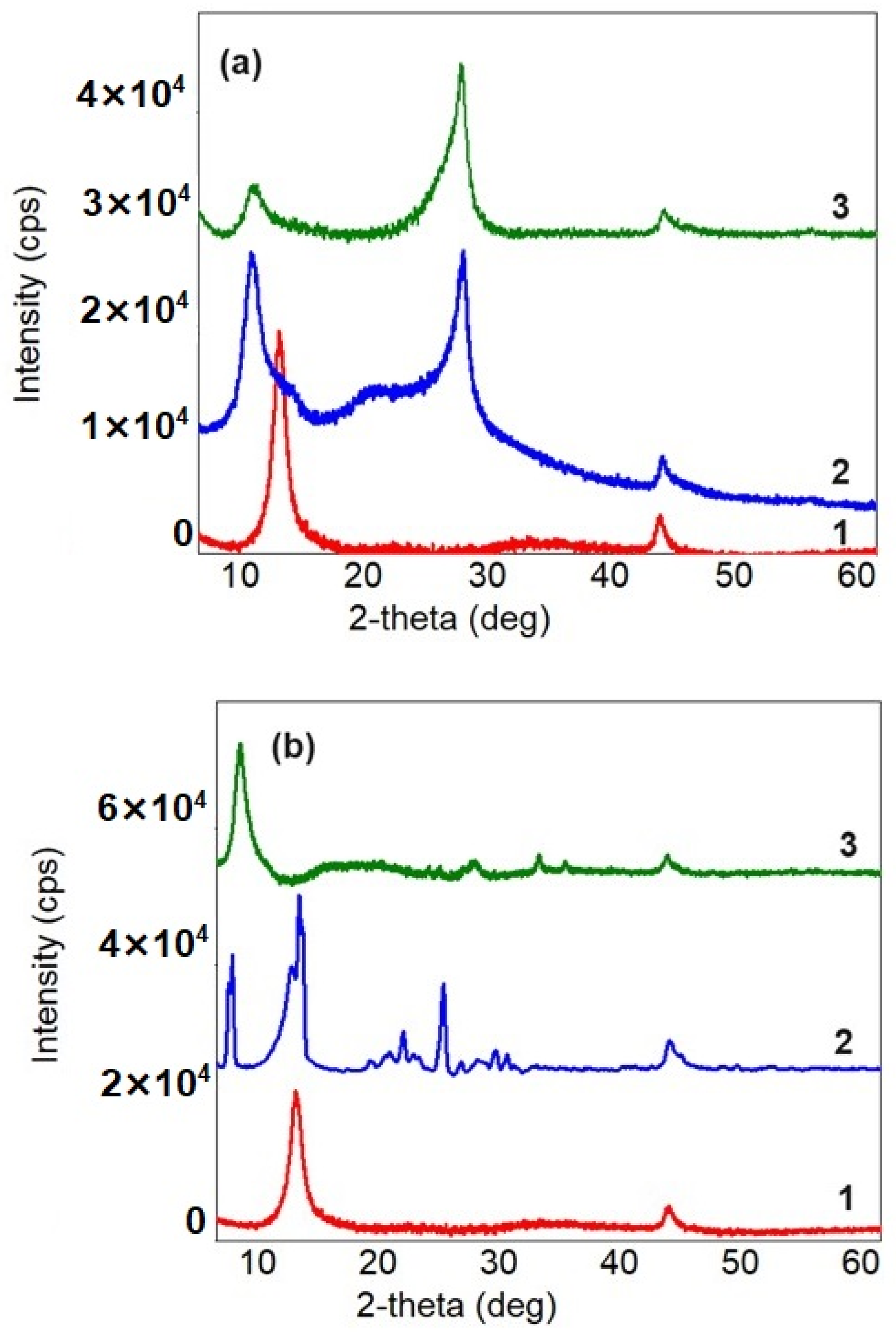
3.2.2. XRD
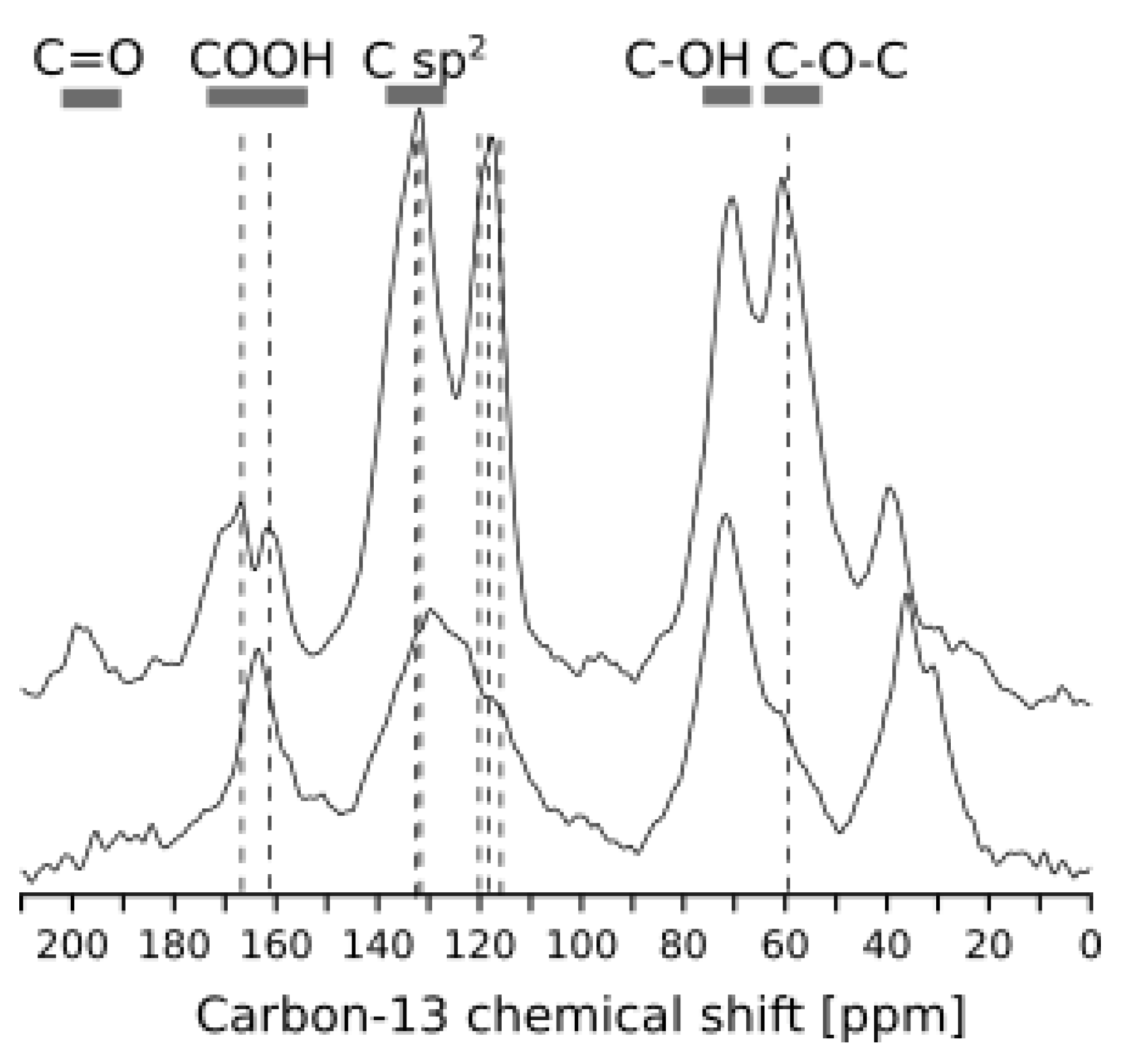
3.3. Solid-State NMR Analysis
3.3.1. SEM
3.3.2. TEM
3.3.3. TGA
3.4. GO and Its Derivatives Tested for Pesticide Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, N.; Bilal, M.; Iqbal, H.M.N. Carbon-Based Nanomaterials with Multipurpose Attributes for Water Treatment: Greening the 21st-Century Nanostructure Materials Deployment. Biomater. Polym. Horiz. 2021, 1, 48–58. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.C.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef]
- De Martino, A.; Iorio, M.; Xing, B.; Capasso, R. Removal of 4-Chloro-2-Methylphenoxyacetic Acid from Water by Sorption on Carbon Nanotubes and Metal Oxide Nanoparticles. RSC Adv. 2012, 2, 5693. [Google Scholar] [CrossRef]
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921. [Google Scholar] [CrossRef] [PubMed]
- Phatthanakittiphong, T.; Seo, G.T. Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution. Nanomaterials 2016, 6, 128. [Google Scholar] [CrossRef]
- Rajumon, R.; Anand, J.C.; Ealias, A.M.; Desai, D.S.; George, G.; Saravanakumar, M. Adsorption of Textile Dyes with Ultrasonic Assistance Using Green Reduced Graphene Oxide: An in-Depth Investigation on Sonochemical Factors. J. Environ. Chem. Eng. 2019, 7, 103479. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and Graphene Oxide-Based Composites for Removal of Organic Pollutants: A Review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Zhang, X.; Wang, Y.; Tain, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Ghadamyari, Z.; Khojastehnezhad, A.; Seyedi, S.M.; Taghavi, F.; Shiri, A. Graphene Oxide Functionalized Zn(II) Salen Complex: An Efficient and New Route for the Synthesis of 1,2,3-Triazole Derivatives. ChemistrySelect 2020, 5, 10233–10242. [Google Scholar] [CrossRef]
- Song, S.; Wan, C.; Zhang, Y. Non-Covalent Functionalization of Graphene Oxide by Pyrene-Block Copolymers for Enhancing Physical Properties of Poly(Methyl Methacrylate). RSC Adv. 2015, 5, 79947–79955. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-Dimensional Self-Assembly of Graphene Oxide and DNA into Multifunctional Hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent Attaching Protein to Graphene Oxide via Diimide-Activated Amidation. Colloids Surf. B Biointerfaces 2010, 81, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Chen, Y.; Wang, J.; Zhang, B.; Zhang, J.; Blau, W.J. Graphene Oxide Covalently Functionalized with Zinc Phthalocyanine for Broadband Optical Limiting. Carbon 2011, 49, 1900–1905. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.M.; Pourazadi, E.; Sarkar, S.; Harris, A.T.; Minett, A.I.; Yanmaz, E.; Alshehri, S.M.; Ide, Y.; Wu, K.C.-W.; et al. Boron-Functionalized Graphene Oxide-Organic Frameworks for Highly Efficient CO2 Capture. Chem. Asian J. 2017, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cheng, W.; Xu, W.; My, W.; Han, X. Functional Graphene Derivatives for Chemotherapy-Based Synergistic Tumor Therapy. Nano 2019, 14, 1930006. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Kumar, A.; Singh, R.; Kashyap, R.; Rani, S.; Kumar, D. Surface Modification of Graphene Oxide Using Esterification. Mater. Today Proc. 2019, 18, 1556–1561. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a Novel Octadecylamine Functionalized Graphene Oxide/PVDF Dual-Layer Flat Sheet Membrane for Desalination via Air Gap Membrane Distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Petrescu, S.; Avramescu, S.; Musuc, A.M.; Neatu, F.; Florea, M.; Ionita, P. Crown-Ether Functionalized Graphene Oxide for Metal Ions Sequestration. Mater. Res. Bull. 2020, 122, 110643. [Google Scholar] [CrossRef]
- Zaman Brohi, R.O.; Khuhawar, M.Y.; Mahar, R.B. Graphene Oxide Functionalized with a Schiff Base for the Removal of Pb (II) Ions from Contaminated Water: Experimental and Modeling Approach. J. Chem. Technol. Biotechnol. 2020, 95, 1694–1704. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, S.; Pathak, D.D. Synthesis and Characterization of a Recyclable Graphene Oxide-Surface- Engineered Copper (II) Schiff Base Complex: Catalytic Application in Synthesis of 1,2,3-Triazoles and 2H-Indazoles. J. Environ. Chem. Eng. 2021, 9, 105791. [Google Scholar] [CrossRef]
- Barzinmehr, H.; Mirza-Aghayan, M.; Heidarian, M. Isatin-Schiff Base Functionalized Graphene Oxide as a Highly Selective Turn-on Fluorescent Probe for the Detection of Pd (II) via Photoinduced Electron Transfer Pathway. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123673. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kin, H.-W. Carbon-Based Nanomaterials as an Emerging Platform for Theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Rajkumar, T.; Vijayakumar, C.T. Synthesis and characterization of N-[4-(chlorocarbonyl)phenyl] maleimide functionalized graphene oxide and reduced graphene oxide. J. Fuller. Nanotub. Carbon Nanostructures 2017, 257, 442–448. [Google Scholar] [CrossRef]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cai, L.; Lu, L. Magnetite/Reduced Graphene Oxide Nanocomposites: One Step Solvothermal Synthesis and Use as a Novel Platform for Removal of Dye Pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Henry, B.W.; Lawrence, B.W. The Reflection of X-rays by Crystals. Proc. R. Soc. Lond. Ser. A 1913, 88, 428–438. [Google Scholar]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of Lattice Strain in ZnO Nanoparticles: X-ray Peak Profile Analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Gul, W.; Alrobei, H. Effect of Graphene Oxide Nanoparticles on the Physical and Mechanical Properties of Medium Density Fiberboard. Polymers 2021, 13, 1818. [Google Scholar] [CrossRef] [PubMed]
- Le, G.T.; Chanlek, N.; Manyam, J.; Opaprakasit, P.; Grisdanurak, N.; Sreearunothai, P. Insight into the Ultrasonication of Graphene Oxide with Strong Changes in Its Properties and Performance for Adsorption Applications. Chem. Eng. J. 2019, 373, 1212–1222. [Google Scholar] [CrossRef]
- Zheng, W.; Tan, R.; Yin, S.; Zhang, Y.; Zhao, G.; Chen, Y.; Yin, D. Ionic Liquid-Functionalized Graphene Oxide as an Efficient Support for the Chiral Salen Mn (III) Complex in Asymmetric Epoxidation of Unfunctionalized Olefins. Catal. Sci. Technol. 2015, 5, 2092–2102. [Google Scholar] [CrossRef]
- Pavoski, G.; Maraschin, T.; Fim, F.D.C.; Balzaretti, N.M.; Galland, G.B.; Moura, C.S.; Basso, N.R.D.S. Few Layer Reduced Graphene Oxide: Evaluation of the Best Experimental Conditions for Easy Production. Mater. Res. 2016, 20, 53–61. [Google Scholar] [CrossRef]
- Bera, M.; Prabhakar, A.; Maji, P.K. Nanotailoring of Thermoplastic Polyurethane by Amine Functionalized Graphene Oxide: Effect of Different Amine Modifier on Final Properties. Compos. Part B Eng. 2020, 195, 108075. [Google Scholar] [CrossRef]
- Caliman, C.C.; Mesquita, A.F.; Cipriano, D.F.; Freitas, J.C.C.; Cotta, A.A.C.; Macedo, W.A.A.; Porto, A.O. One-Pot Synthesis of Amine-Functionalized Graphene Oxide by Microwave-Assisted Reactions: An Outstanding Alternative for Supporting Materials in Supercapacitors. RSC Adv. 2018, 8, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Daud, N.; Chieng, B.W.; Ibrahim, N.A.; Talib, Z.A.; Muhamad, E.N.; Abidin, Z.Z. Functionalizing Graphene Oxide with Alkylamine by Gamma-Ray Irradiation Method. Nanomaterials 2017, 7, 135. [Google Scholar] [CrossRef]
- Chuah, R.; Gopinath, S.C.B.; Anbu, P.; Salimi, M.N.; Yaakub, A.R.W.; Lakshmipriya, T. Synthesis and Characterization of Reduced Graphene Oxide Using the Aqueous Extract of Eclipta Prostrata. 3 Biotech 2020, 10, 364. [Google Scholar] [CrossRef]
- Tian, H.; Zeng, H.; Zha, F.; Tian, H.; Chang, Y. Synthesis of Graphene Oxide–Supported β-Cyclodextrin Adsorbent for Removal of p-Nitrophenol. Water Air Soil Pollut. 2020, 231, 495. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M. Polydopamine Functionalized Graphene Oxide as Membrane Nanofiller: Spectral and Structural Studies. Membranes 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The Chemical and Structural Analysis of Graphene Oxide with Different Degrees of Oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Xu, T.; Jiao, Y.; Su, Z.; Yin, Q.; An, L.; Tan, Y. Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites. Polymers 2023, 15, 4726. [Google Scholar] [CrossRef]
- Tian, J.; Xu, T.; Tan, Y.; Zhang, Z.; Tang, B.; Sun, Z. Effects of Non-Covalent Functionalized Graphene Oxide with Hyperbranched Polyesters on Mechanical Properties and Mechanism of Epoxy Composites. Materials 2019, 12, 3103. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Kumar, N.A.; Jeong, Y.T. Covalent Functionalization of Graphene Oxide with Polyglycerol and Their Use as Templates for Anchoring Magnetic Nanoparticles. Synth. Met. 2010, 160, 2028–2036. [Google Scholar] [CrossRef]
- Bergin, S.D.; Nicolosi, V.; Cathcart, H.; Lotya, M.; Rickard, D.; Sun, Z.; Blau, W.J.; Coleman, J.N. Large Populations of Individual Nanotubes in Surfactant-Based Dispersions without the Need for Ultracentrifugation. J. Phys. Chem. C 2008, 112, 972–977. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Shi, M.; Lin, T.; Hu, Y.; Peng, J.; Li, J.; Zhai, M. Functionalization of Graphene Oxide by Radiation Grafting Polyhedral Oligomeric Silsesquioxane with Improved Thermal Stability and Hydrophilicity. J. Mater. Sci. 2020, 55, 1489–1498. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/Aggregation of Graphene Oxide and Its Application in Cu2+ Removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; OK, Y.S.; Jiang, Y.; Gao, B. Surface Functional Groups of Carbon-Based Adsorbents and Their Roles in the Removal of Heavy Metals from Aqueous Solutions: A Critical Review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]




| SB | rSB | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2θ | β | d, nm | Scherrer’s Method D (nm) | ε | 2θ | β | d, nm | Scherrer’s Method D (nm) | ε |
| 11.396 | 0.342 | 0.78 | 24.39 | 0.01496 | 11.5464 | 0.2337 | 1.57 | 35.70 | 0.01009 |
| 17.029 | 0.271 | 0.52 | 30.97 | 0.00790 | - | - | - | - | - |
| 20.058 | 0.358 | 0.44 | 23.55 | 0.00883 | 20.875 | 0.190 | 0.43 | 44.42 | 0.00450 |
| 22.945 | 0.29 | 0.39 | 29.21 | 0.00623 | 23.313 | 0.234 | 0.38 | 36.22 | 0.00495 |
| 26.91 | 0.22 | 0.33 | 38.80 | 0.00401 | 25.840 | 0.186 | 0.34 | 45.79 | 0.00354 |
| 27.71 | 0.35 | 0.32 | 24.43 | 0.00619 | 27.55 | 0.14 | 0.32 | 61.05 | 0.00249 |
| 28.994 | 0.356 | 0.31 | 24.08 | 0.00601 | 29.311 | 0.213 | 0.30 | 40.28 | 0.00355 |
| 41.16 | 0.27 | 0.22 | 32.84 | 0.00314 | 41.5 | 0.188 | 0.22 | 47.22 | 0.00217 |
| 47.601 | 0.324 | 0.19 | 28 | 0.00321 | 47.713 | 0.255 | 0.19 | 35.59 | 0.00252 |
| Sample | (hkl) | 2θ | β | d (nm) | Scherrer’s Method D (nm) | Number of Graphene Layers (N) per Domain |
|---|---|---|---|---|---|---|
| GO | (001) | 11.608 | 1.228 | 0.76 | 6.84 | ~9 |
| ncGO-SB | (001) | 9.12 | 5.97 | 0.97 | 1.39 | ~2 |
| ncGO-rSB | (001) | 9.61 | 1.69 | 0.92 | 4.93 | ~5 |
| Structure | Name of Pesticides/Adsorbents | GO | cGO-rSB | cGO-SB |
|---|---|---|---|---|
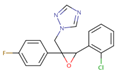 | Epoxiconazole | 65 ± 9 | 82 ± 12 | 82 ± 12 |
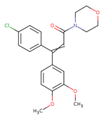 | Dimethomorph | 79 ± 11 | 82 ± 12 | 82 ± 12 |
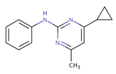 | Cyprodinil | 88 ± 13 | 91 ± 14 | 91 ± 14 |
 | Chlorothalonil | 9 ± 1 | 90 ± 13 | 90 ± 13 |
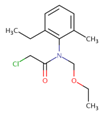 | Acetochlor | 45 ± 7 | 86 ± 13 | 94 ± 14 |
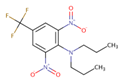 | Trifluralin | 70 ± 10 | 94 ± 14 | 96 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghiyeva, N.; Hasanova, U.; Millet, M.; Gardiennet, C.; Gakhramanova, Z.; Mirzayev, M.H.; Gahramanli, L.; Pham-Huu, C.; Aliyeva, S.; Aliyeva, G.; et al. Synthesis and Characterization of Novel Adsorbents Based on Functionalization of Graphene Oxide with Schiff Base and Reduced Schiff Base for Pesticide Removal. Materials 2024, 17, 4096. https://doi.org/10.3390/ma17164096
Taghiyeva N, Hasanova U, Millet M, Gardiennet C, Gakhramanova Z, Mirzayev MH, Gahramanli L, Pham-Huu C, Aliyeva S, Aliyeva G, et al. Synthesis and Characterization of Novel Adsorbents Based on Functionalization of Graphene Oxide with Schiff Base and Reduced Schiff Base for Pesticide Removal. Materials. 2024; 17(16):4096. https://doi.org/10.3390/ma17164096
Chicago/Turabian StyleTaghiyeva, Narinj, Ulviyya Hasanova, Maurice Millet, Carole Gardiennet, Zarema Gakhramanova, Mushfig H. Mirzayev, Lala Gahramanli, Cuong Pham-Huu, Solmaz Aliyeva, Gunel Aliyeva, and et al. 2024. "Synthesis and Characterization of Novel Adsorbents Based on Functionalization of Graphene Oxide with Schiff Base and Reduced Schiff Base for Pesticide Removal" Materials 17, no. 16: 4096. https://doi.org/10.3390/ma17164096
APA StyleTaghiyeva, N., Hasanova, U., Millet, M., Gardiennet, C., Gakhramanova, Z., Mirzayev, M. H., Gahramanli, L., Pham-Huu, C., Aliyeva, S., Aliyeva, G., Rzayev, F., Gasimov, E., Boulogne, C., & Akhundzada, H. V. (2024). Synthesis and Characterization of Novel Adsorbents Based on Functionalization of Graphene Oxide with Schiff Base and Reduced Schiff Base for Pesticide Removal. Materials, 17(16), 4096. https://doi.org/10.3390/ma17164096







