Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases
Abstract
:1. Introduction
2. Experimental Method
2.1. Material
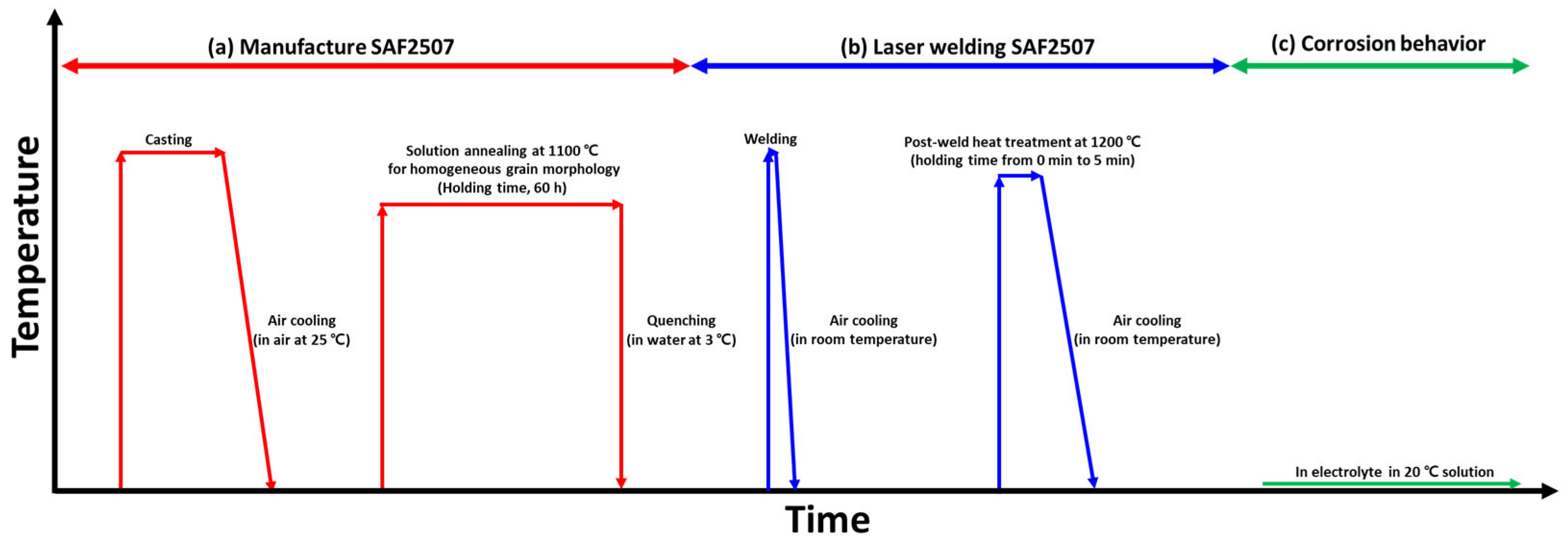
2.2. Manufacturing Process
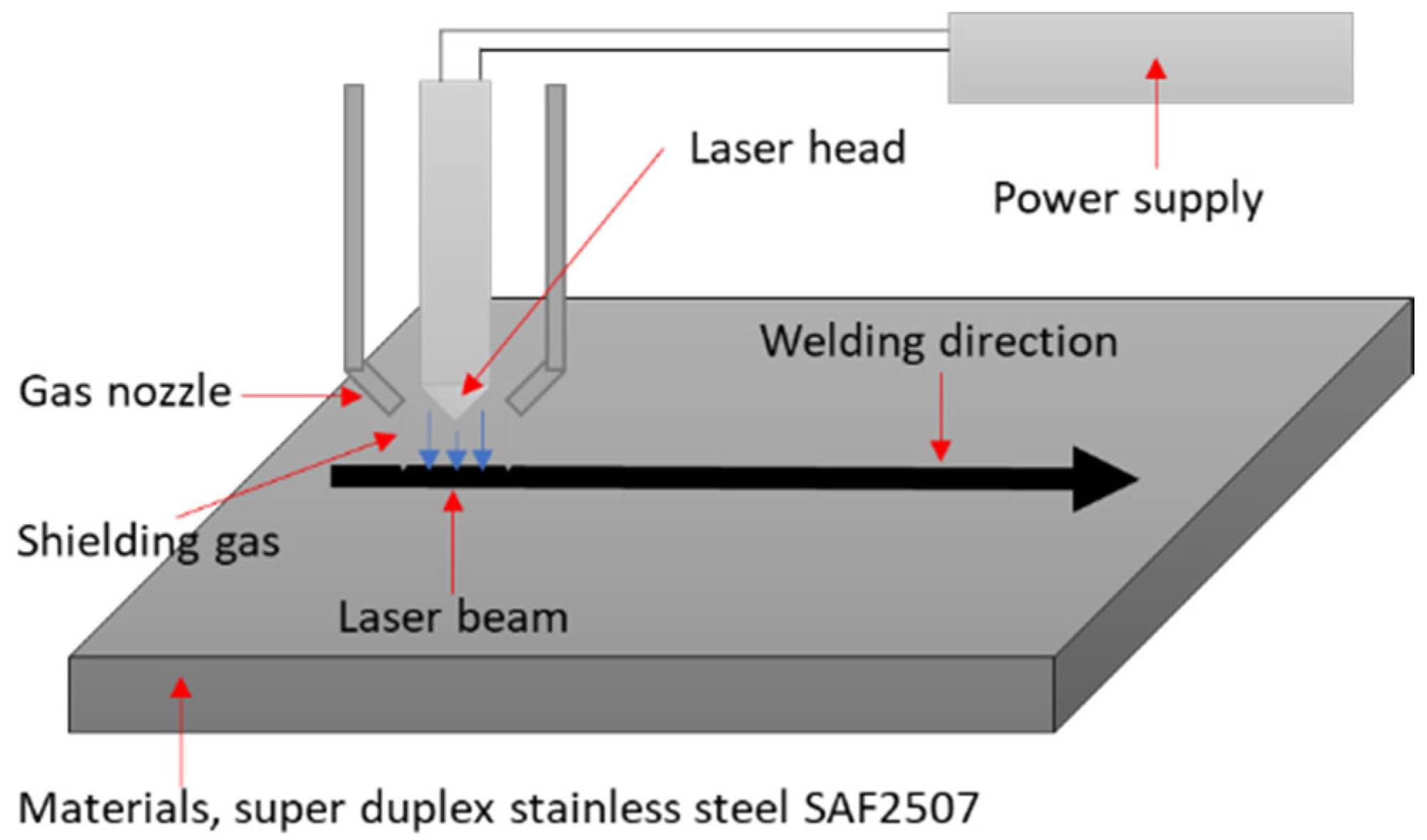
2.3. Laser Welding
2.4. Post-Weld Heat Treatment
2.5. Corrosion Behavior
3. Results
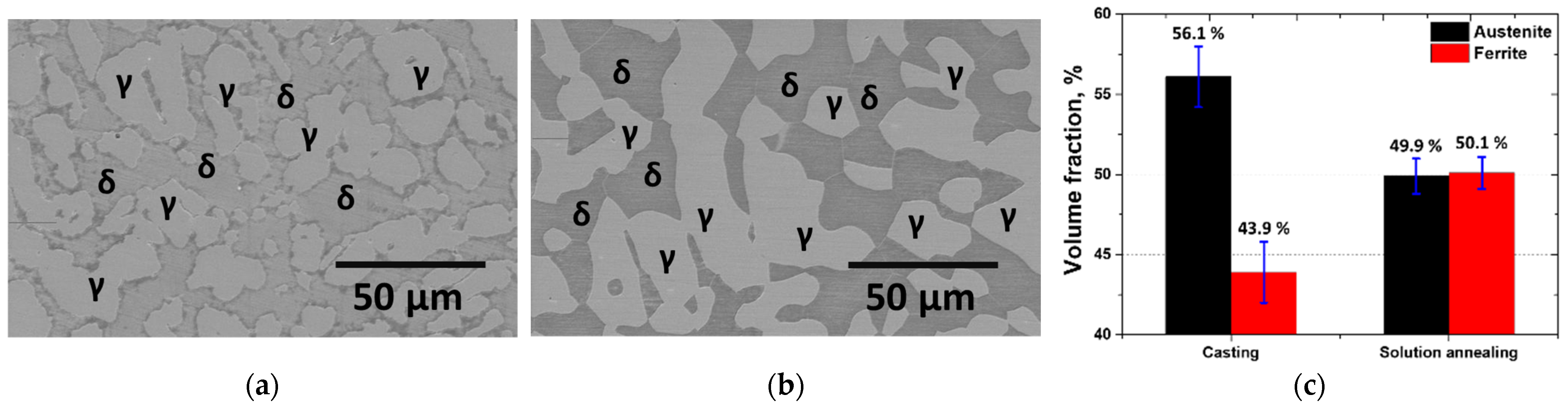
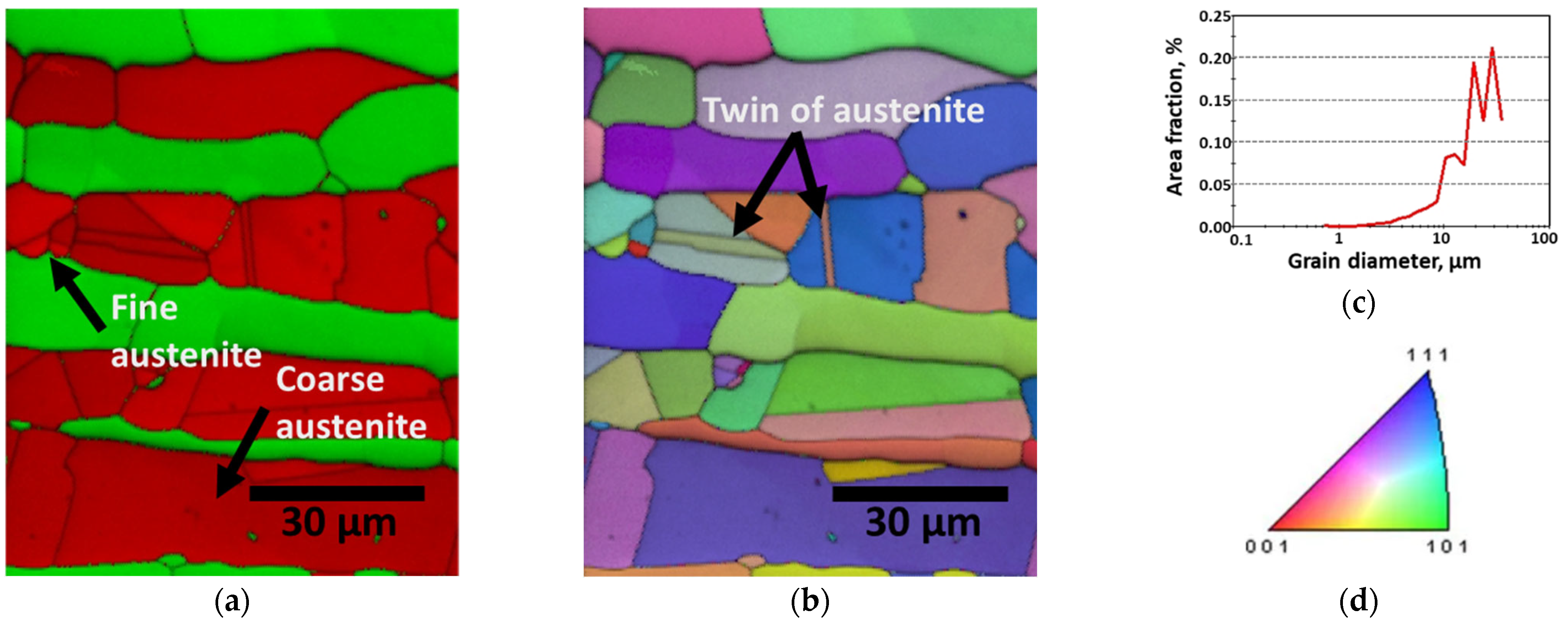
3.1. Manufacture of SAF 2507
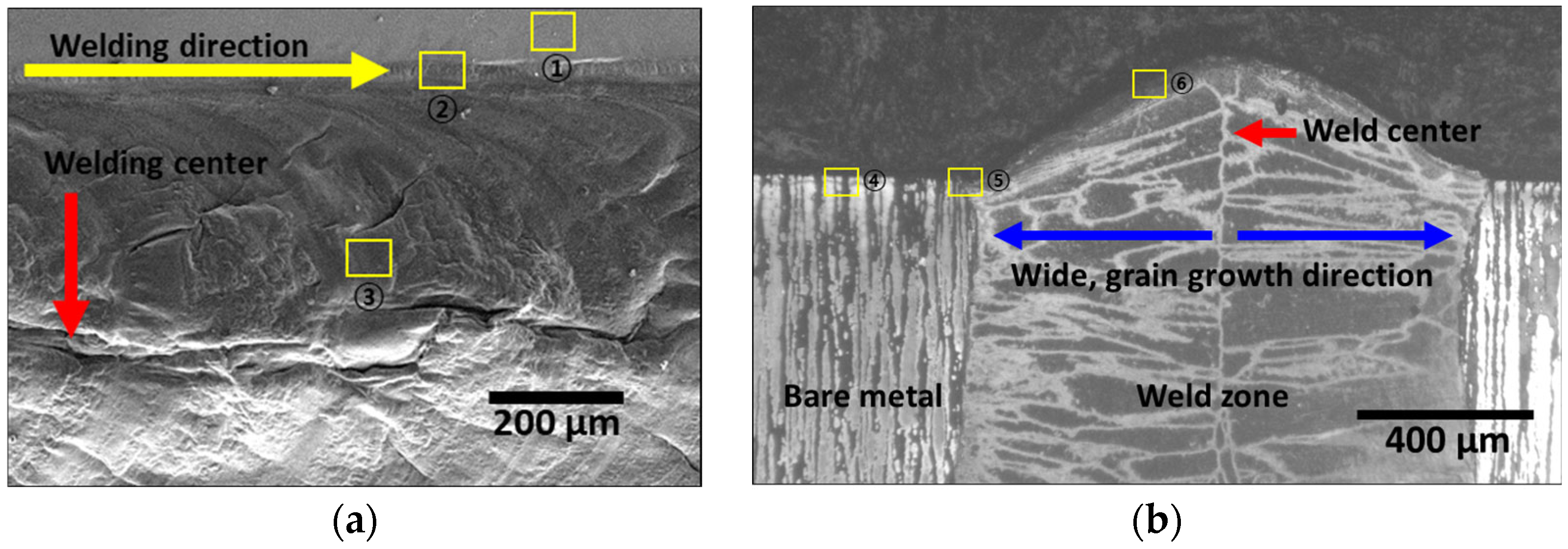
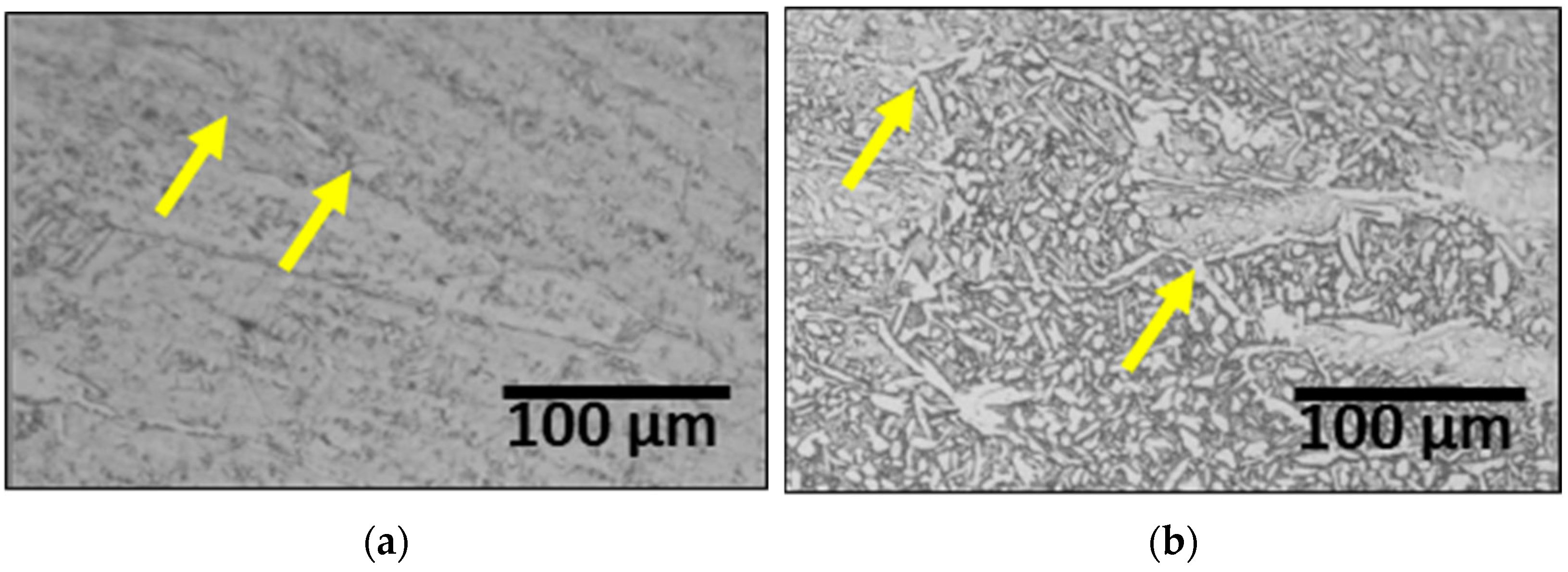
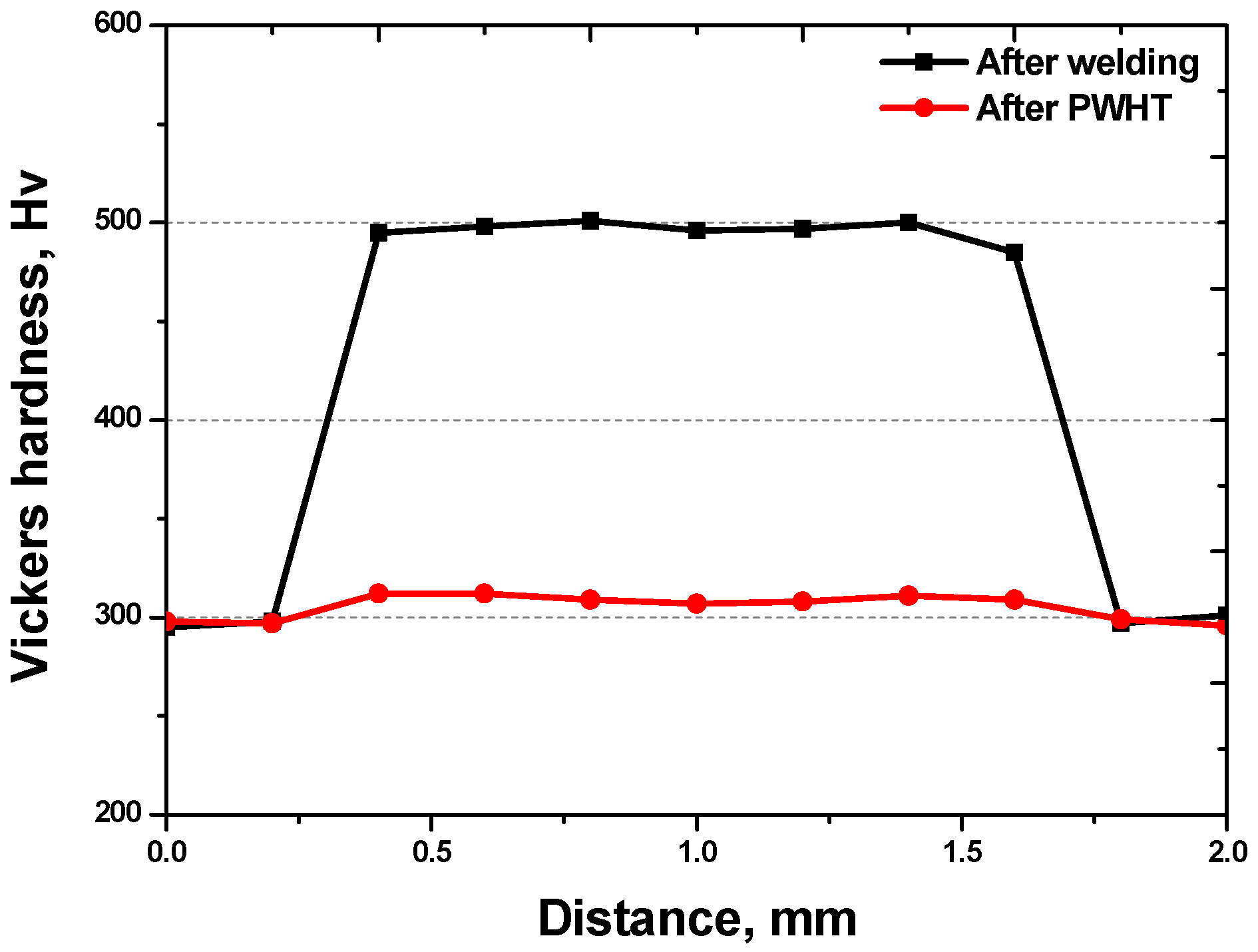
3.2. Effect of Laser Welding
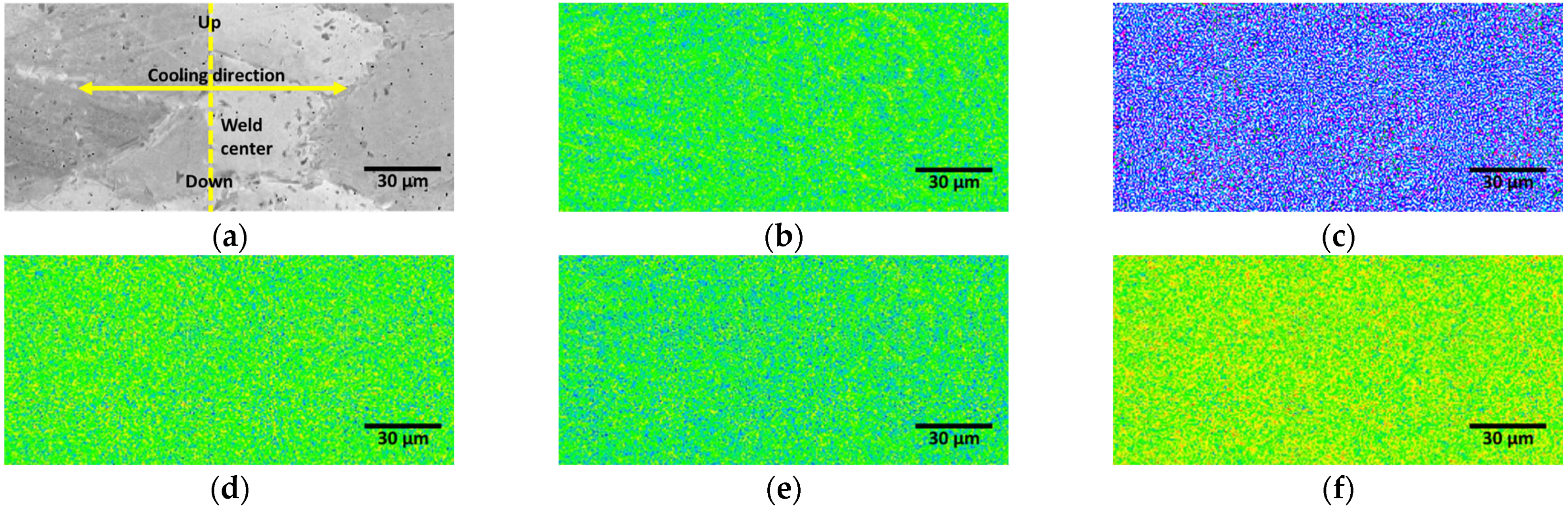
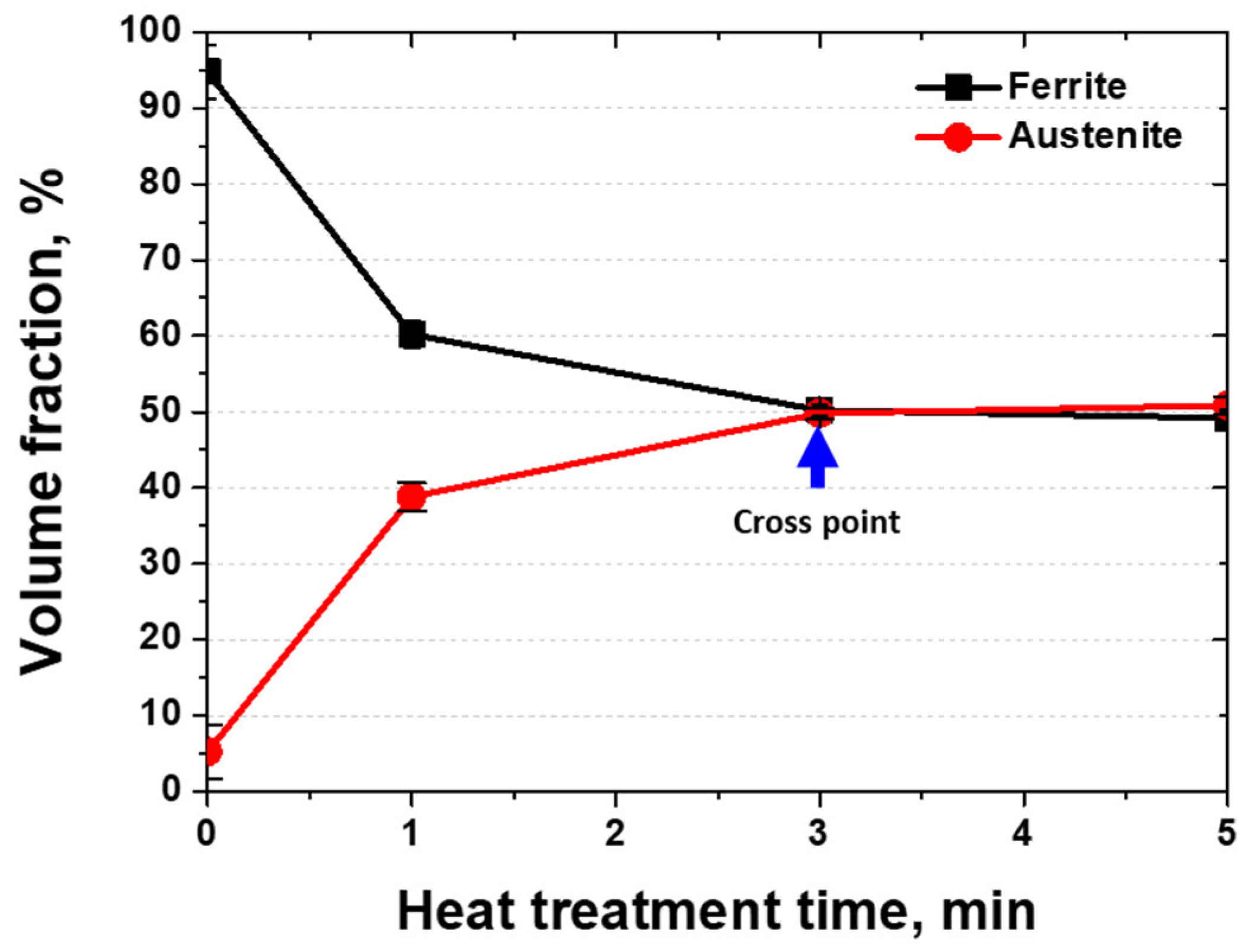
3.3. Effect of Post-Weld Heat Treatment
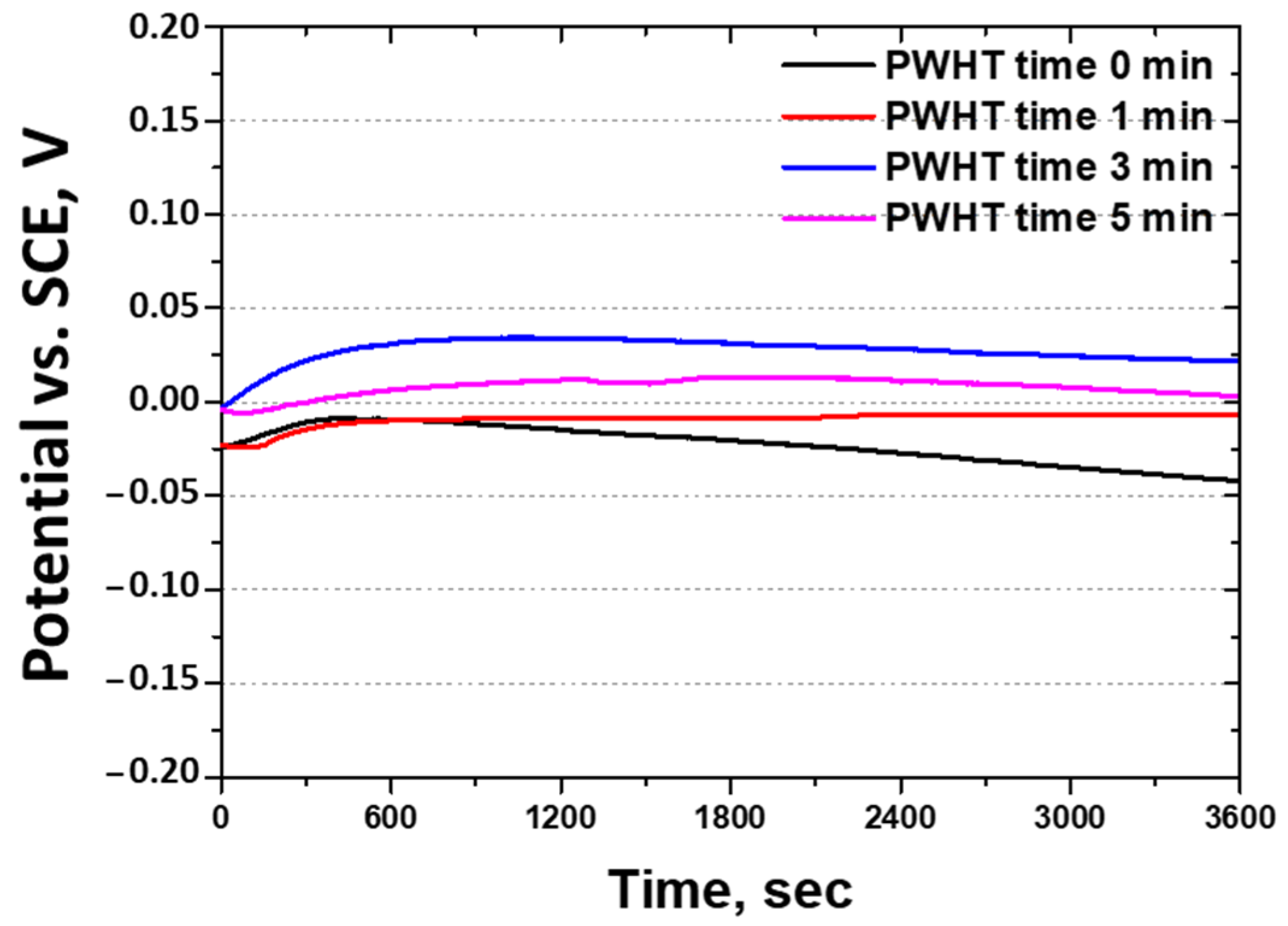
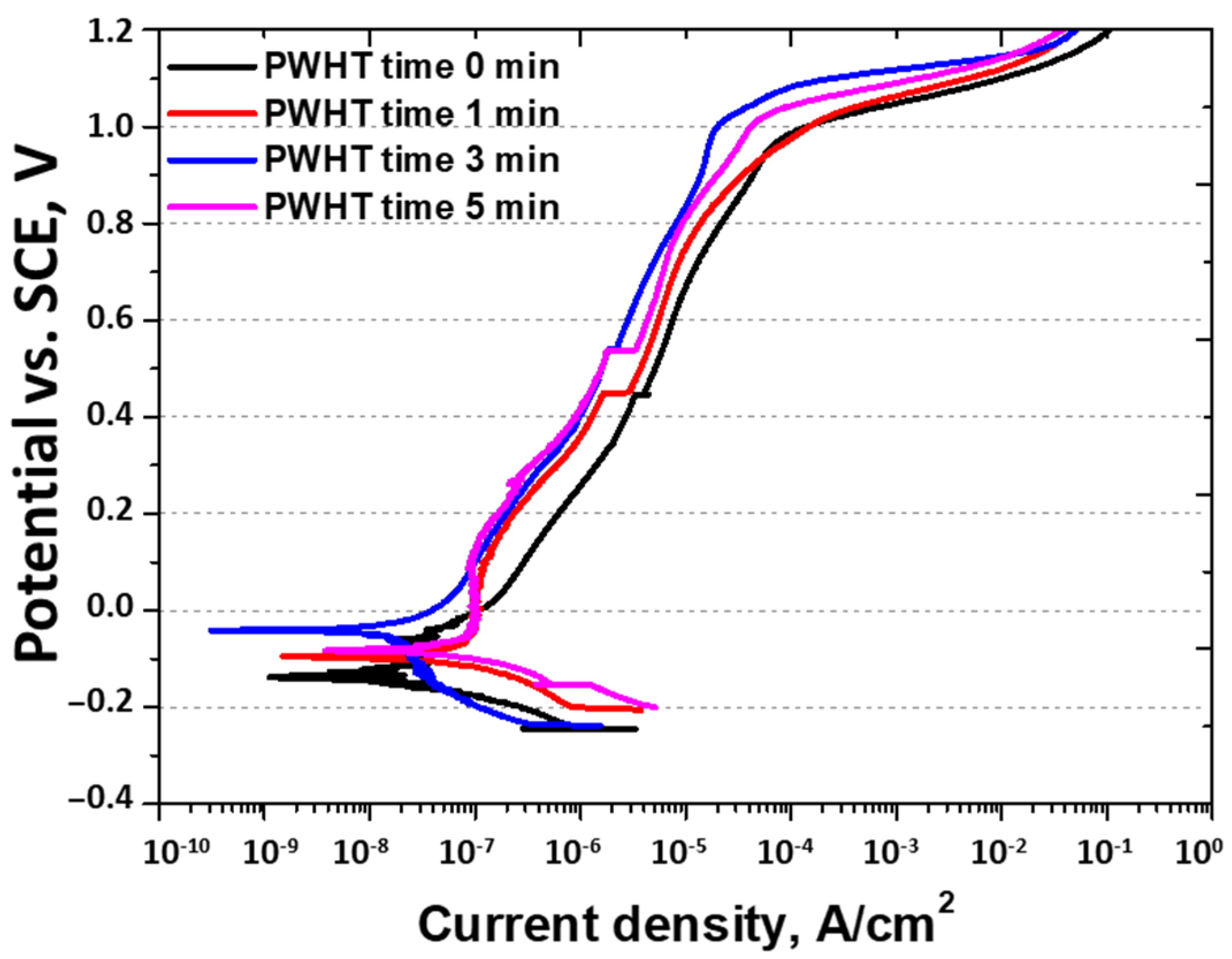
3.4. Effect of PWHT on Corrosion Behavior
4. Conclusions
- (1)
- The as-cast microstructure of SDSS SAF 2507 was heterogeneous, but solution heat treatment resulted in uniform austenite grains of 20–30 μm. Solution annealing at 1100 °C produced equal volume fractions and PRENs, leading to a decrease in the galvanic corrosion and improved corrosion resistance. Laser welding produced a narrow weld width of 1200 μm and a low bead height of 160 μm. However, rapid cooling induced a ferritic microstructure in the weld, with ferrite growing in the direction of the cooling (toward the base metal), comprising 95% of the weld. Despite the use of a shielding gas to prevent oxidation, the high heat during cooling resulted in a thick oxide layer on the weld surface.
- (2)
- The PWHT increased the proportion of austenite. The austenite growth was characterized by coarse austenite along the ferrite grain boundaries and fine austenite within the ferrite grains. After 3 min of PWHT, the volume fractions of austenite and ferrite intersected at 50%, as did their PREN values. During the 3 min of PWHT at 1200 °C, the high volume fraction of ferrite (95%, metastable state) facilitated a phase transformation from ferrite and austenite.
- (3)
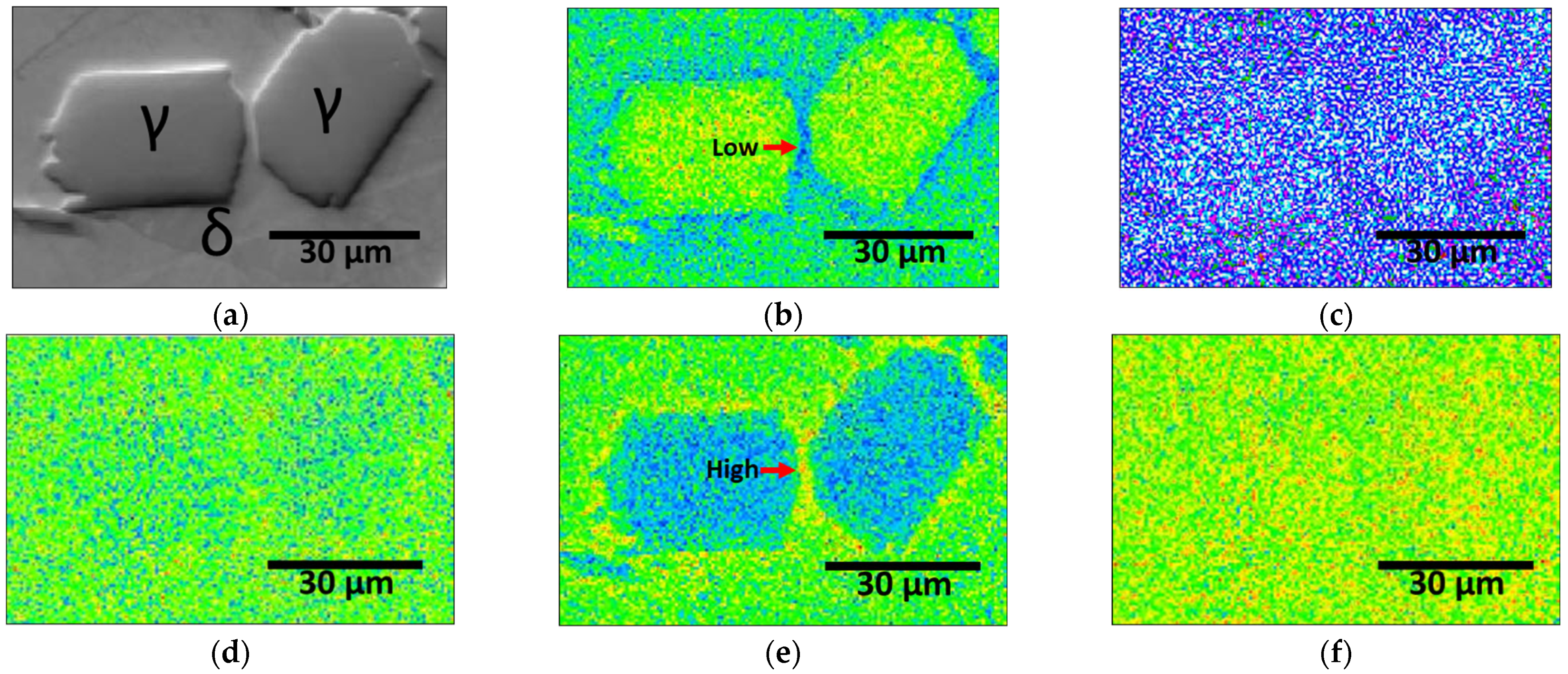
- The corrosion resistance after the PWHT was measured using the OCP (−0.21 V to +0.03 V), potentiodynamic polarization tests, and CPT (56 °C to 73 °C). All the corrosion tests showed that PWHT improved the corrosion resistance, although it remained lower than that of solution-heat-treated SAF 2507. The decreased corrosion resistance was attributed to grain refinement, heterogeneity, increased twinning, and the segregation of Mo and Ni. Consequently, although the PWHT restored the corrosion resistance of SAF 2507, it did not reach the level of the base metal. This reduction in corrosion resistance is a critical consideration for applications such as batteries. Therefore, although PWHT can enhance the corrosion resistance of SAF 2507, the residual differences compared with solution-heat-treated materials must be considered in practical applications.
- (4)
- This study performed welding and PWHT on SDSS SAF 2507 for use as a material for Li-ion battery cases and analyzed resulting the corrosion resistance and alloy segregation. To use it for Li-ion batteries, plating technology to enhance electrical conductivity inside the case is necessary. However, research on this aspect has not been conducted, indicating a need for future studies on the plating behavior of welds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A New Energy Storage Anode Material for Li-Ion Batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar]
- Bizeray, A.M.; Howey, D.A.; Monroe, C.W. Resolving a Discrepancy in Diffusion Potentials, with a Case Study for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, E223. [Google Scholar] [CrossRef]
- Sutopo, W.; Astuti, R.W.; Purwanto, A.; Nizam, M. Commercialization Model of New Technology Lithium Ion Battery: A Case Study for Smart Electrical Vehicle. In Proceedings of the 2013 Joint International Conference on Rural Information & Communication Technology and Electric-Vehicle Technology (rICT & ICeV-T), Bandung, Indonesia, 26–28 November 2013; IEEE: New York, NY, USA, 2013; pp. 1–5. [Google Scholar]
- Tudoroiu, N.; Zaheeruddin, M.; Tudoroiu, R.-E.; Radu, M.S.; Chammas, H. Investigations on Using Intelligent Learning Techniques for Anomaly Detection and Diagnosis in Sensors Signals in Li-Ion Battery—Case Study. Inventions 2023, 8, 74. [Google Scholar] [CrossRef]
- Trinh, L.N.; Lee, D. The Characteristics of Laser Welding of a Thin Aluminum Tab and Steel Battery Case for Lithium-Ion Battery. Metals 2020, 10, 842. [Google Scholar] [CrossRef]
- Koleva, M.; Shi, Y.; McKenna, K.; Craig, M.; Nagarajan, A. Optimal Strategies for Hybrid Battery Storage Systems Design. Energy Technol. 2023, 11, 2300115. [Google Scholar]
- Hoosain, S.E.; Tshabalala, L.C.; Skhosana, S.; Freemantle, C.; Mndebele, N. Investigation of the Properties of Direct Energy Deposition Additive Manufactured 304 Stainless Steel. S. Afr. J. Ind. Eng. 2021, 32, 258–263. [Google Scholar] [CrossRef]
- Yoo, Y.-R.; Choi, S.-H.; Kim, Y.-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials 2023, 16, 804. [Google Scholar] [CrossRef] [PubMed]
- Speidel, M.O. Nitrogen Containing Austenitic Stainless Steels. In Materialwissenschaft und Werkstofftechnik: Entwicklung, Fertigung, Prüfung, Eigenschaften und Anwendungen technischer Werkstoffe; Wiley: Hoboken, NJ, USA, 2006; Volume 37, pp. 875–880. [Google Scholar]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, W.G.; Kim, Y.H.; Jang, H. Surface Roughness and the Corrosion Resistance of 21Cr Ferritic Stainless Steel. Corros. Sci. 2012, 63, 404–409. [Google Scholar] [CrossRef]
- Seo, M.; Hultquist, G.; Leygraf, C.; Sato, N. The Influence of Minor Alloying Elements (Nb, Ti and Cu) on the Corrosion Resistivity of Ferritic Stainless Steel in Sulfuric Acid Solution. Corros. Sci. 1986, 26, 949–960. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar]
- Makhdoom, M.A.; Ahmad, A.; Kamran, M.; Abid, K.; Haider, W. Microstructural and Electrochemical Behavior of 2205 Duplex Stainless Steel Weldments. Surf. Interfaces 2017, 9, 189–195. [Google Scholar] [CrossRef]
- Saeid, T.; Abdollah-Zadeh, A.; Shibayanagi, T.; Ikeuchi, K.; Assadi, H. EBSD Investigation of Friction Stir Welded Duplex Stainless Steel. World Acad. Sci. Eng. Technol. 2010, 61, 376–379. [Google Scholar]
- Tehovnik, F.; Arzensek, B.; Arh, B.; Skobir, D.; Pirnar, B.; Zuzek, B. Microstructure Evolution in SAF 2507 Super Duplex Stainless Steel. Mater. Technol 2011, 45, 339–345. [Google Scholar]
- Fande, A.W.; Taiwade, R.V. Welding of Super Duplex Stainless Steel and Austenitic Stainless Steel:# Xd; Influence and Role of Bicomponent Fluxes. Mater. Manuf. Process. 2023, 38, 434–448. [Google Scholar]
- Kannan, A.R.; Shanmugam, N.S.; Rajkumar, V.; Vishnukumar, M. Insight into the Microstructural Features and Corrosion Properties of Wire Arc Additive Manufactured Super Duplex Stainless Steel (ER2594). Mater. Lett. 2020, 270, 127680. [Google Scholar]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2021, 16, 210813. [Google Scholar] [CrossRef]
- Linton, V.M.; Laycock, N.J.; Thomsen, S.J.; Klumpers, A. Failure of a Super Duplex Stainless Steel Reaction Vessel. Eng. Fail. Anal. 2004, 11, 243–256. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Fréchard, S.; Martin, F.; Clément, C.; Cousty, J. AFM and EBSD Combined Studies of Plastic Deformation in a Duplex Stainless Steel. Mater. Sci. Eng. A 2006, 418, 312–319. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Ishikawa, Y. Current and Potential Transients during Localized Corrosion of Stainless Steel. J. Electrochem. Soc. 1985, 132, 1288. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.H. Review of the Potential of the Ni/Cu Plating Technique for Crystalline Silicon Solar Cells. Materials 2014, 7, 1318–1341. [Google Scholar] [CrossRef]
- Martins, M.; Casteletti, L.C. Sigma Phase Morphologies in Cast and Aged Super Duplex Stainless Steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Gutiérrez-Vargas, G.; Ruiz, A.; López-Morelos, V.H.; Kim, J.-Y.; González-Sánchez, J.; Medina-Flores, A. Evaluation of 475 C Embrittlement in UNS S32750 Super Duplex Stainless Steel Using Four-Point Electric Conductivity Measurements. Nucl. Eng. Technol. 2021, 53, 2982–2989. [Google Scholar]
- Vignal, V.; Delrue, O.; Heintz, O.; Peultier, J. Influence of the Passive Film Properties and Residual Stresses on the Micro-Electrochemical Behavior of Duplex Stainless Steels. Electrochim. Acta 2010, 55, 7118–7125. [Google Scholar] [CrossRef]
- Valiente Bermejo, M.A.; Thalavai Pandian, K.; Axelsson, B.; Harati, E.; Kisielewicz, A.; Karlsson, L. Microstructure of Laser Metal Deposited Duplex Stainless Steel: Influence of Shielding Gas and Heat Treatment. Weld. World 2021, 65, 525–541. [Google Scholar]
- Beziou, O.; Hamdi, I.; Boumerzoug, Z.; Brisset, F.; Baudin, T. Effect of Heat Treatment on the Welded Joint of X70 Steel Joined to Duplex Stainless Steel by Gas Tungsten Arc Welding. Int. J. Adv. Manuf. Technol. 2023, 127, 2799–2814. [Google Scholar]
- Amatsuka, S.; Nishimoto, M.; Muto, I.; Kawamori, M.; Takara, Y.; Sugawara, Y. Micro-Electrochemical Insights into Pit Initiation Site on Aged UNS S32750 Super Duplex Stainless Steel. Npj Mater. Degrad. 2023, 7, 15. [Google Scholar]
- Shin, B.-H.; Kim, S.; Park, J.; Ok, J.-W.; Kim, D.; Yoon, J.-H. Effect of Secondary Phase on Passivation Layer of Super Duplex Stainless Steel UNS S 32750: Advanced Safety of Li-Ion Battery Case Materials. Materials 2024, 17, 2760. [Google Scholar] [CrossRef]
- Shin, B.-H.; Kim, S.; Park, J.; Ok, J.-W.; Kim, D.-I.; Kim, D.; Yoon, J.-H. Effect of Secondary Phase on Electroless Ni Plating Behaviour of Super Duplex Stainless Steel SAF 2507 for Advanced Li-Ion Battery Case. Materials 2024, 17, 1441. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, F.N.U.M.; Shakil, S.I.; Shaha, S.K.; Kacher, J.; Nasiri, A.; Haghshenas, M.; Hadadzadeh, A. Study of Direct Aging Heat Treatment of Additively Manufactured PH13–8Mo Stainless Steel: Role of the Manufacturing Process, Phase Transformation Kinetics, and Microstructure Evolution. J. Mater. Res. Technol. 2023, 24, 3772–3787. [Google Scholar]
- Acharyya, S.G.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface Working of 304L Stainless Steel: Impact on Microstructure, Electrochemical Behavior and SCC Resistance. Mater. Charact. 2012, 72, 68–76. [Google Scholar]
- Skrotzki, W.; Pukenas, A.; Odor, E.; Joni, B.; Ungar, T.; Völker, B.; Hohenwarter, A.; Pippan, R.; George, E.P. Microstructure, Texture, and Strength Development during High-Pressure Torsion of CrMnFeCoNi High-Entropy Alloy. Crystals 2020, 10, 336. [Google Scholar] [CrossRef]
- Friel, J.J. ASTM Standards in Microscopy and Microanalysis. In Proceedings of the Conference Series-Institute of Physics, Kailua-Kona, HI, USA, 9–13 July 2000; Institute of Physics: College Park, MD, USA, 2000; Volume 165, pp. 399–400. [Google Scholar]
- Vander Voort, G.F. Measuring Inclusion Content by ASTM E 1245. Clean Steel 1997, 5, 58–67. [Google Scholar]
- ASTM E1245; Standard Test Method for Determining the Inclusion or Second-Phase Constituent Content of Metals by Automatic Image Analysis. ASTM International: West Conshohocken, PA, USA, 2017.
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Electrochemical Behavior of Stainless Steel in Aerated NaCl Solutions by Electrochemical Impedance and Rotating Disk Electrode Methods. Russ. J. Electrochem. 2006, 42, 370–374. [Google Scholar] [CrossRef]
- Guerrini, E.; Cristiani, P.; Grattieri, M.; Santoro, C.; Li, B.; Trasatti, S. Electrochemical Behavior of Stainless Steel Anodes in Membraneless Microbial Fuel Cells. J. Electrochem. Soc. 2013, 161, H62. [Google Scholar]
- Shin, B.-H.; Kim, S.; Park, J.; Ok, J.-W.; Kim, D.; Yoon, J.-H. Study of Precipitated Secondary Phase at 700 °C on the Electrochemical Properties of Super Duplex Stainless Steel AISI2507: Advanced High-Temperature Safety of a Lithium-Ion Battery Case. Materials 2024, 17, 2009. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.; Park, J.; Kim, D.-I.; Shin, B.-H.; Yoon, J.-H. Effects of Passivation with Cu and W on the Corrosion Properties of Super Duplex Stainless Steel PRE 42. Metals 2024, 14, 284. [Google Scholar] [CrossRef]
- ASTM G61:2018; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices. ASTM International: West Conshohocken, PA, USA, 2019.
- Barbosa, C.A.; Sokolowski, A. Development of UNS S 32760 Super-Duplex Stainless Steel Produced in Large Diameter Rolled Bars. Rem Rev. Esc. Minas 2013, 66, 201–208. [Google Scholar]
- ASTM G150-99; Standard Test Method for Electrochemical Critical Pitting Temperature Testing of Stainless Steels. ASTM International: West Conshohocken, PA, USA, 1999.
- Nilsson, J.O.; Wilson, A. Influence of Isothermal Phase Transformations on Toughness and Pitting Corrosion of Super Duplex Stainless Steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar]
- Large, D.; Scandella, F.; Robineau, A.; Dupoiron, F.; Peultier, J.; Fanica, A.; Petit, B.; Thulin, A.; Pettersson, R.; Weisang-Hoinard, F. Welding of Lean Duplex Stainless Steel Grades: Microstructure, Corrosion Resistance and Mechanical Properties. In Proceedings of the NACE Corrosion, Salt Lake City, UT, USA, 11–15 March 2012; NACE: Pleasant Grove, UT, USA, 2012; p. NACE-2012. [Google Scholar]
- Soria, L.; Herrera, E.J. A Reliable Technique to Determine Pitting Potentials of Austenitic Stainless Steels by Potentiodynamic Methods. Weld. Int. 1992, 6, 959–964. [Google Scholar] [CrossRef]
- Bogdan, D.; Grosu, I.-G.; Filip, C. How Thick, Uniform and Smooth Are the Polydopamine Coating Layers Obtained under Different Oxidation Conditions? An in-Depth AFM Study. Appl. Surf. Sci. 2022, 597, 153680. [Google Scholar]
- Ha, H.-Y.; Jang, M.-H.; Lee, T.-H.; Moon, J. Interpretation of the Relation between Ferrite Fraction and Pitting Corrosion Resistance of Commercial 2205 Duplex Stainless Steel. Corros. Sci. 2014, 89, 154–162. [Google Scholar] [CrossRef]
- Rajesh, D.; Sunandana, C.S. XRD, Optical and AFM Studies on Pristine and Partially Iodized Ag Thin Film. Results Phys. 2012, 2, 22–25. [Google Scholar] [CrossRef]
- Sen, S.K.; Paul, T.C.; Dutta, S.; Hossain, M.N.; Mia, M.N.H. XRD Peak Profile and Optical Properties Analysis of Ag-Doped h-MoO 3 Nanorods Synthesized via Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2020, 31, 1768–1786. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y. Review―Understanding and Controlling the Electrochemical Properties of Sulfide Inclusions for Improving the Pitting Corrosion Resistance of Stainless Steels. Mater. Trans. 2023, 64, 2051–2058. [Google Scholar] [CrossRef]



| Element | C | N | Mn | Ni | Cr | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| Chemical composition | 0.01 | 0.27 | 0.8 | 6.8 | 25.0 | 3.8 | 0.2 | Bal. |
| Phase | Cr | Mo | Ni | N | Fe | PREN | |
|---|---|---|---|---|---|---|---|
| Casting | Austenite | 23.2 ± 2.1 | 3.1 ± 0.6 | 7.8 ± 1.4 | 0.49 | Bal. | 41.3 |
| Ferrite | 26.7 ± 2.3 | 5.0 ± 0.8 | 5.8 ± 1.2 | 0.05 | Bal. | 43.9 | |
| Solution annealing | Austenite | 23.4 ± 1.9 | 3.3 ± 0.7 | 7.9 ± 1.3 | 0.51 | Bal. | 42.5 |
| Ferrite | 26.5 ± 1.8 | 4.6 ± 0.8 | 5.7 ± 1.2 | 0.05 | Bal. | 42.4 |
| Site | Cr | Ni | Mo | N | O | Fe | PRE |
|---|---|---|---|---|---|---|---|
| 1 | 24.7 | 6.5 | 3.7 | 0.27 | 3.3 | Bal. | 41.2 |
| 2 | 23.7 | 6.1 | 3.1 | 0.27 | 8.5 | Bal. | 38.3 |
| 3 | 23.1 | 5.8 | 3.0 | 0.27 | 16.1 | Bal. | 37.3 |
| 4 | 25.0 | 6.7 | 3.7 | 0.27 | 2.2 | Bal. | 41.5 |
| 5 | 24.4 | 6.4 | 3.5 | 0.27 | 6.5 | Bal. | 40.3 |
| 6 | 23.6 | 6.2 | 3.2 | 0.27 | 13.5 | Bal. | 38.5 |
| Temperature | Phase | Cr | Mo | Ni | N | Fe | PREN |
|---|---|---|---|---|---|---|---|
| 0 min | Austenite | 25.0 ± 0.3 | 3.6 ± 0.1 | 12.5 ± 0.1 | 2.05 | Bal. | 69.7 |
| Ferrite | 25.3 ± 0.4 | 3.8 ± 0.1 | 6.1 ± 0.2 | 0.05 | Bal. | 38.6 | |
| 1 min | Austenite | 24.4 ± 0.2 | 3.3 ± 0.1 | 7.4 ± 0.3 | 0.54 | Bal. | 43.9 |
| Ferrite | 26.0 ± 0.4 | 4.1 ± 0.1 | 6.3 ± 0.2 | 0.05 | Bal. | 40.3 | |
| 3 min | Austenite | 24.1 ± 0.2 | 3.1 ± 0.1 | 7.2 ± 0.1 | 0.47 | Bal. | 41.9 |
| Ferrite | 26.3 ± 0.3 | 4.5 ± 0.1 | 6.4 ± 0.2 | 0.05 | Bal. | 42.0 | |
| 5 min | Austenite | 24.1 ± 0.3 | 2.7 ± 0.1 | 7.2 ± 0.1 | 0.47 | Bal. | 40.5 |
| Ferrite | 26.4 ± 0.3 | 4.8 ± 0.1 | 6.5 ± 0.2 | 0.05 | Bal. | 43.0 |
| PWHT Time | OCP Potential | Potentiodynamic Polarization Curve | ||
|---|---|---|---|---|
| Ecorr | Icorr | Epit | ||
| 0 min | −0.21 V | −0.16 V | 2 × 10−8 A/cm2 | 1.00 V |
| 1 min | −0.13 V | −0.10 V | 8 × 10−8 A/cm2 | 1.02 V |
| 3 min | 0.03 V | 0.05 V | 1 × 10−7 A/cm2 | 1.11 V |
| 5 min | −0.02 V | 0.00 V | 1 × 10−7 A/cm2 | 1.09 V |
| PWHT Time | OCP | Epit | CPT |
|---|---|---|---|
| Al 1005 | −1.07 V | −0.65 V | Under 1 °C |
| SAF 30400 | −0.10 V | 0.20 V | Under 1 °C |
| SAF 2507 after PWHT | 0.03 V | 1.11 V | 73 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Park, J.; Ok, J.-W.; Kim, S.; Shin, B.-H.; Yoon, J.-H. Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases. Materials 2024, 17, 4107. https://doi.org/10.3390/ma17164107
Lee Y-S, Park J, Ok J-W, Kim S, Shin B-H, Yoon J-H. Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases. Materials. 2024; 17(16):4107. https://doi.org/10.3390/ma17164107
Chicago/Turabian StyleLee, Yoon-Seok, Jinyong Park, Jung-Woo Ok, Seongjun Kim, Byung-Hyun Shin, and Jang-Hee Yoon. 2024. "Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases" Materials 17, no. 16: 4107. https://doi.org/10.3390/ma17164107






