Study on the Properties of TiC Coating Deposited by Spark Discharge on the Surface of AlFeCoCrNiCu High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Preparation of Matrix
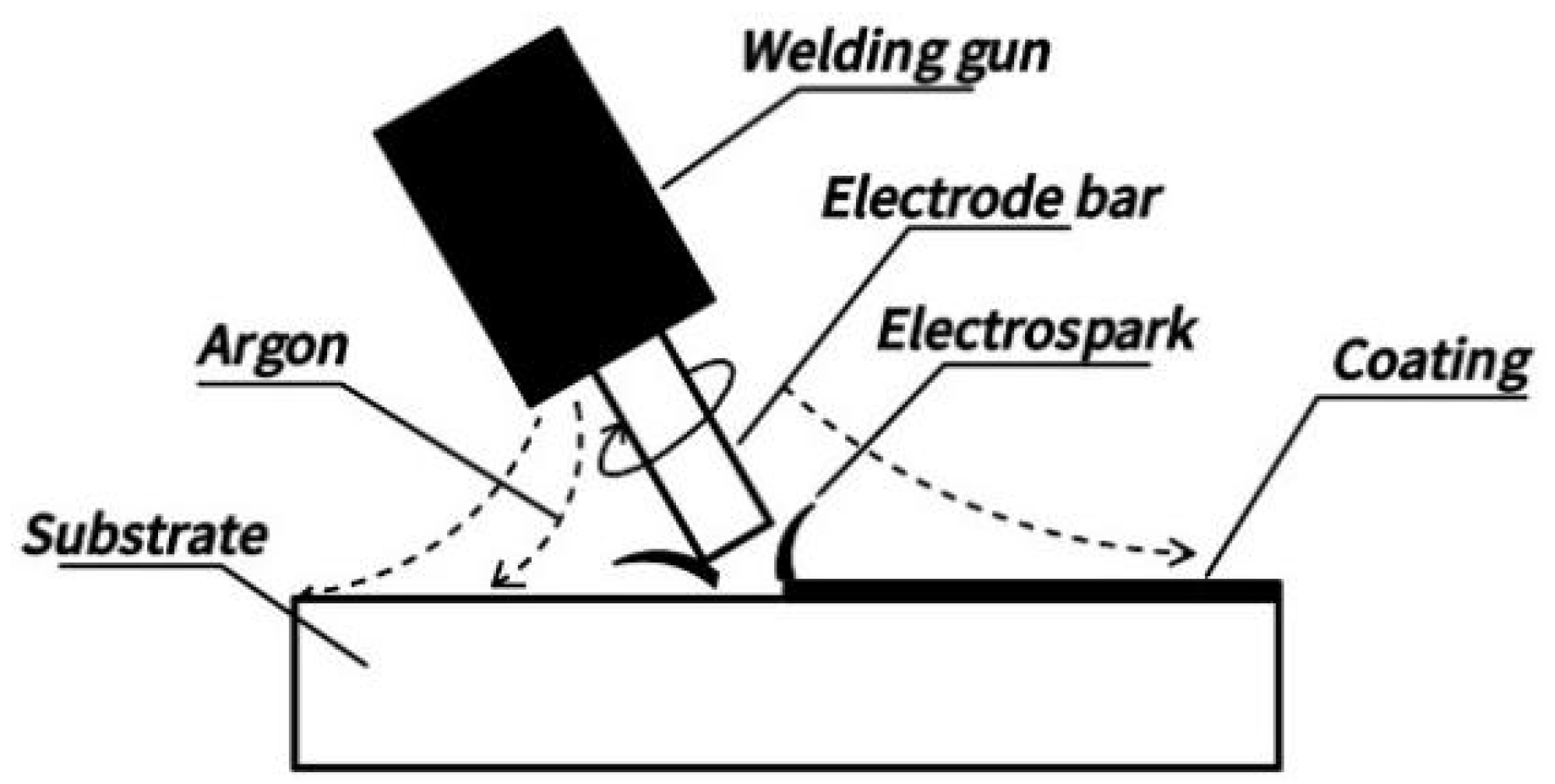
2.2. Preparation of Coatings
2.3. Characterization of Coating Microstructure
2.4. Performance Testing of TiC Coating on AlFeCoCrNiCu High-Entropy Alloy Surface
3. Results and Discussion
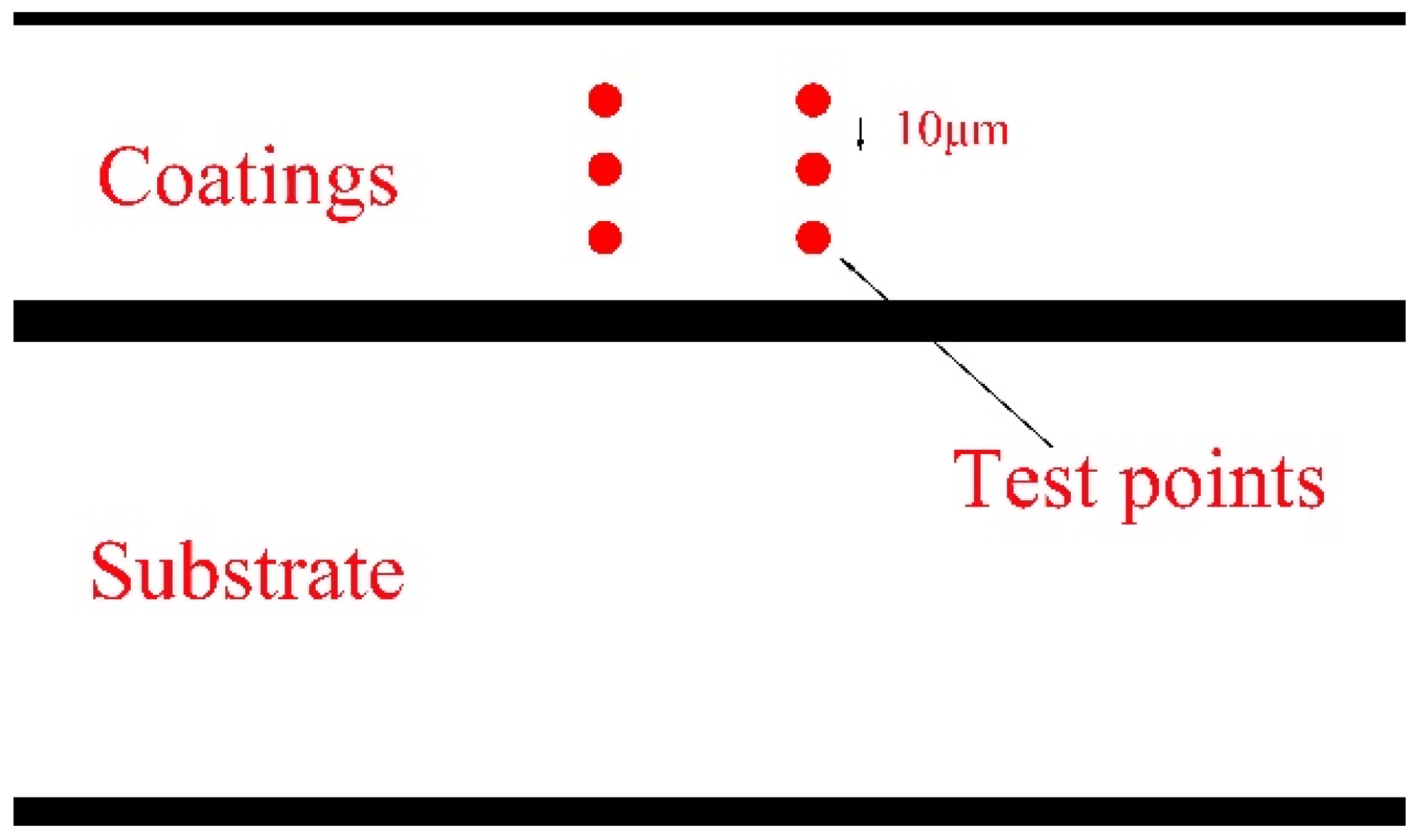
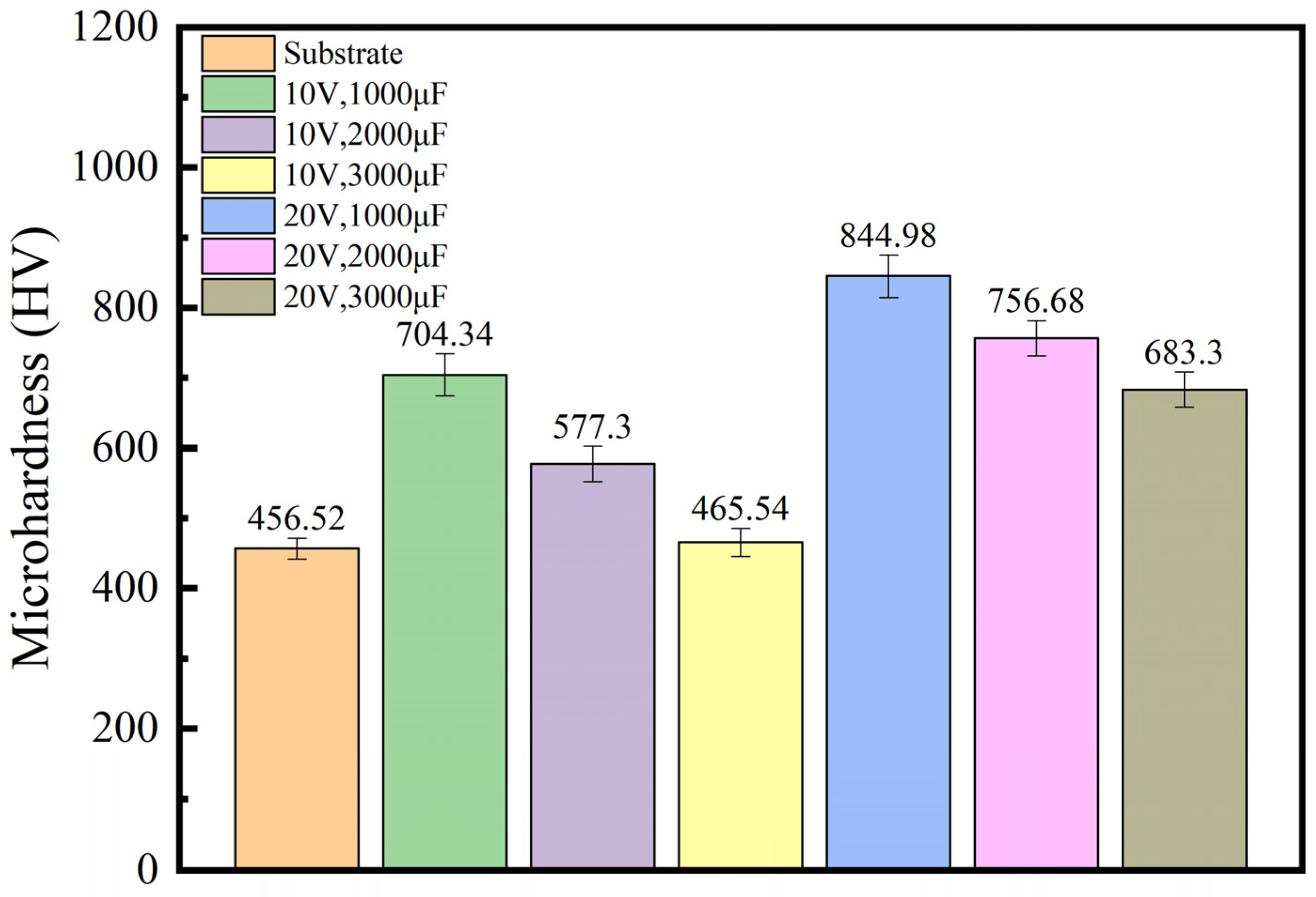
3.1. Microhardness of TiC Coating on AlFeCoCrNiCu High-Entropy Alloy
3.2. Surface Morphology Analysis of TiC Coating on AlFeCoCrNiCu High-Entropy Alloy Surface
3.3. Cross-Sectional Morphology Analysis of TiC Coating on the Surface of AlFeCoCrNiCu High-Entropy Alloy
3.4. Corrosion Behavior of TiC Coating on the Surface of AlFeCoCrNiCu High-Entropy Alloy
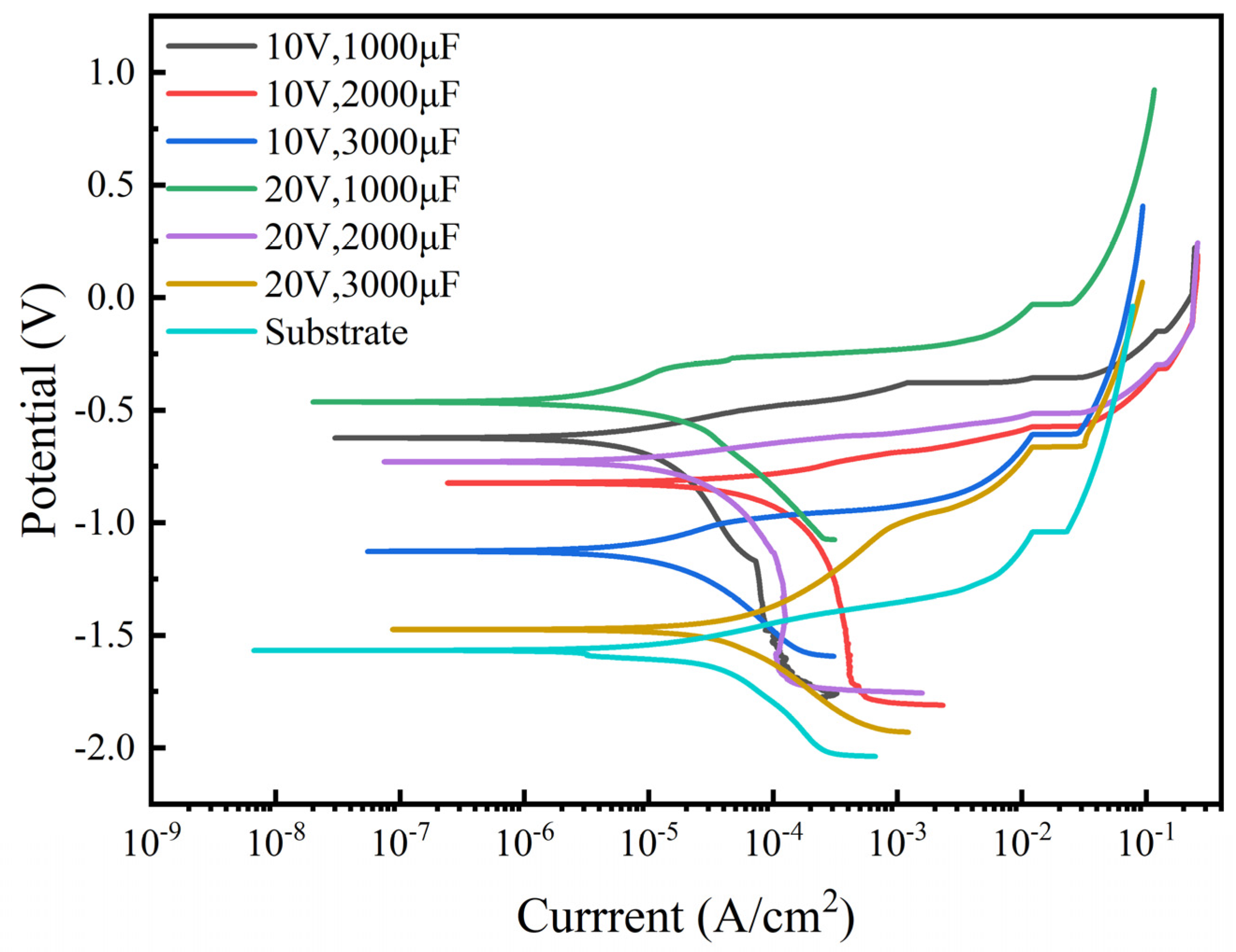
3.4.1. Analysis of Potentiodynamic Polarization Test
3.4.2. Electrochemical Impedance Testing
4. Conclusions
- The microhardness of the TiC coating is higher than that of the substrate. The microhardness of sample 4 is 844.98 HV, which is 81% higher than that of the substrate. This indicates that a TiC coating can improve the microhardness of the substrate surface and enhance its mechanical properties. Moreover, with the increase in capacitance and output voltage, the oxidation of the TiC coating intensifies, and the oxidation product TiO2 increases, which will cause a decrease in the hardness of the TiC coating.
- By comparing the surface morphology of six different coatings, it was found that samples with smaller working capacitance had fewer surface defects on the coating. This is because the working capacitance is too large, which increases the thermal stress of the coating, leading to an increase in the brittleness of the TiC coating. During the deposition process, cracks and molten metal appear in the coating, increasing coating defects. Under the action of thermal stress cycling, cracks may also appear at the interface between the coating and the substrate.
- Energy spectrum line scanning analysis was conducted at the boundary between the coating and the substrate, and it was found that the change in the content of the Al element in the coating plays a crucial role in the bonding between the coating and the substrate. This is because the Al element has a larger atomic size compared with the other elements. During the solid solution process, the significant size effect promotes the various properties of the coating.
- Action potential tests were conducted on TiC coatings and high-entropy alloy substrates prepared under six different process parameters. The corrosion current density of the sample 4 coating was the smallest (9.475 × 10−7 ± 0.06 × 10−6). After conducting electrochemical impedance testing, it was found that the maximum RP value of the coating on sample 4 was 3688 Ω·com2, and the overall trend of the dynamic potential test results was the same.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Ortega, A.; Bayón, R.; Arana, J.L.; Arredondo, A.; Igartua, A. Influence of temperature on the corrosion and tribocorrosion behaviour of High-Strength Low-Alloy steels used in offshore applications. Tribol. Int. 2018, 12, 1341–1352. [Google Scholar] [CrossRef]
- Ripeanu, R.G.; Dinita, A.; Tanase, M.; Portoaca, A.L. Experimental investigation on corrosion resistance and tribological properties of steel coated with glass-reinforced polymer. Tribol. Mater. 2024, 3, 73–80. [Google Scholar] [CrossRef]
- Xiao, L.; Pan, F.S. Microstructure and mechanical properties of directionally solidified magnesium alloy in extruded state. Trans. Mater. Sci. Eng. 2020, 38, 460–465. [Google Scholar] [CrossRef]
- Tan, M.T.; Meng, L.; Fang, S.; Lin, C.; Ke, L.S.; Yu, Z.H.; Qu, J.K.; Qi, T. Organizational Evolution during Performance Meritocracy of AlSi0.5CrxCo0.2Ni Lightweight High Entropy Alloys. Crystals 2022, 12, 1828. [Google Scholar] [CrossRef]
- Shang, Z.; Bai, W.F.; Dong, X.W.; Yang, J.G. Research progress on the characteristics and mechanical properties of high-entropy alloys. Therm. Process. Technol. 2022, 51, 11–16. [Google Scholar] [CrossRef]
- Krishna, S.A.; Noble, N.; Radhika, N.; Saleh, B. A comprehensive review on advances in high entropy alloys: Fabrication and surface modification methods, properties, applications, and future prospects. J. Manuf. Process. 2024, 109, 583–606. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Wang, Y.; Men, Z. A Review of Research Progress on Ti(C,N)-Based Cermet Binder by Intermetallic Compounds and High-Entropy Alloys. Materials 2024, 17, 675. [Google Scholar] [CrossRef]
- Zhang, H.; Yong, Y.; Wang, F.; Liang, Y.; Liu, L.; Liu, H.; Gao, Y. Progress on the Properties of Ceramic Phase-Reinforced High-Entropy Alloy Composite Coatings Produced via Laser Cladding. Coatings 2024, 14, 127. [Google Scholar] [CrossRef]
- Yu, B.; Ren, Y.; Zeng, Y.; Ma, W.; Morita, K.; Zhan, S.; Lei, Y.; Lv, G.; Li, S.; Wu, J. Recent progress in high-entropy alloys: A focused review of preparation processes and properties. J. Mater. Res. Technol. 2024, 29, 2689–2719. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Dai, S.; Zhu, L. Research Progress in Electrospark Deposition Coatings on Titanium Alloy Surfaces: A Short Review. Coatings 2023, 13, 1473. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Chang, Q.; Wang, X.M.; Zhao, Y.; Zhu, S.; Xu, A.Y.; Gao, X.W. Research status and prospects of electric spark deposition technology. Surf. Technol. 2021, 50, 150–161. [Google Scholar] [CrossRef]
- Penyashki, T.; Kostadinov, G.; Kandeva, M.; Kamburov, V.; Nikolov, A.; Dimitrova Rayna, R. Abrasive and Erosive Wear of TI6Al4V Alloy with Electrospark Deposited Coatings of Multicomponent Hard Alloys Materials Based of WC and TiB2. Coatings 2023, 13, 215. [Google Scholar] [CrossRef]
- Baysan, E.; Kayali, Y. Investigation of corrosion behavior of Aisi 304 and aisi 316l stainless steels coated with feal and nial intermetallics using electro-spark deposition method. Surf. Rev. Lett. 2023, 31, 24500264. [Google Scholar] [CrossRef]
- Wang, J.S.; Li, G.; Liu, Y.Y.; Yue, Y. Structure and Properties of Composite Coatings by Electric Spark Deposition and Laser Cladding. J. Mater. Heat Treat. 2012, 33, 155–159. [Google Scholar] [CrossRef]
- Kostadinov, G.; Penyashki, T.; Nikolov, A.; Vencl, A. Improving the Surface Quality and Tribological Characteristics of 3D-Printed Titanium Parts through Reactive Electro-Spark Deposition. Materials 2024, 17, 382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chan, H.L.; Kapat, J.S. Deposition and characterization of titanium carbide coatings using laser ablation method. Appl. Surf. Sci. 1998, 127–129, 549–552. [Google Scholar] [CrossRef]
- Masaoka, H.; Saito, T.; Kondo, K.; Okamoto, N.; Kan, T. Low Temperature TiC Coating Process by Plasma Enhanced Chemical Vapor Deposition. ECS Trans. 2013, 50, 47–51. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y. Performance of TiC coating prepared by in situ conversion of organic carbon film on 316L bipolar plate. J. Iron Steel Res. Int. 2023, 30, 1562–1573. [Google Scholar] [CrossRef]
- Cai, Q.; Li, G.; Wu, B.; Xu, S.; Wang, L.; Guo, Y. Effect of TiC content on microstructure and properties of TiC/Ni60 coatings on Ti6Al4V alloy deposited by laser cladding. Opt. Laser Technol. 2024, 168, 109854. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Zhang, W.; Xu, Z.; Zheng, J.J.; Luo, Y. Synthesis of titanium carbide coating on Ti15Mo alloy and its tribological behaviour. Biosurface Biotribology 2023, 9, 176–186. [Google Scholar] [CrossRef]
- Mertgenç, E. Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition. Open Chem. 2023, 21, 20230817. [Google Scholar] [CrossRef]
- Tkachenko, Y.G.; Yurchenko, D.Z.; Timofeeva, I.I.; Britun, V.F. Effect from the Composition of Electrodes Produced from TiC–(Fe–Cr–Al–Si) Alloys on the Formation, Phase Composition, and Properties of Wear- and Oxidation-Resistant Electrospark-Deposited Coatings on Steel. Powder Metall. Met. Ceram. 2018, 57, 465–472. [Google Scholar] [CrossRef]
- Aghajani, H.; Hadavand, E.; Peighambardoust, N.S.; Khamenehasl, S. Electro spark deposition of WC–TiC–Co–Ni cermet coatings on St52 steel. Surf. Interfaces 2020, 18, 100392. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghaffari, S.A.; Nikkhah, M.; Ghaini, F.M.; Farzan, F.; Mohammadi, S. Biocompatibility assessments of 316L stainless steel substrates coated by Fe-based bulk metallic glass through electro-spark deposition method. Colloids Surf. B Biointerfaces 2021, 198, 111469. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G. Advancements in Electrospark Deposition (ESD) Technique: A Short Review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Sun, X.Y. Corrosion and cavitation erosion behaviors of laser clad FeNiCoCr high-entropy alloy coatings with different types of TiC reinforcement. Surf. Coat. Technol. 2023, 471, 129910. [Google Scholar] [CrossRef]
- Xiao, M.; Nai, S.; Feng, C.; Guan, Z.; Huo, C.; Zhang, F.X.; Qiu, Z.G.; Li, G. Preparation, mechanical properties and wear resistance of dual-sized TiC particles reinforced high-entropy alloy cermet coating. J. Mater. Res. Technol. 2024, 28, 97–109. [Google Scholar] [CrossRef]
- Xiang, H.; Ke, F.; Tan, Y.; Wang, X.; Hua, T. Effects of process parameters on microstructure and wear resistance of TiN coatings deposited on TC11 titanium alloy by electrospark deposition. Trans. Nonferrous Met. Soc. China 2017, 27, 1767–1776. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Geng, K.; Han, T.; Manladan, S.M.; Luo, Z.; Han, J. Influence of high-temperature condition on the microstructure and properties of FeCoCrNiAl0.3 and FeCoCrNiAl0.7 high-entropy alloy coatings. Surf. Eng. 2020, 37, 1–9. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Q.; Guo, Y.; Ding, K.; Liao, T.; Wang, F. Nano-TiC reinforced [Cr–Fe4Co4Ni4]Cr3 high-entropy-alloy composite coating fabricated by laser cladding. J. Mater. Res. Technol. 2022, 21, 2076–2088. [Google Scholar] [CrossRef]
- Zhang, H.; Yong, Z.; Zengda, Z.; Chuanwei, S. Effects of CeO2 on Microstructure and Corrosion Resistance of TiC-VC Reinforced Fe-Based Laser Cladding Layers. J. Rare Earths 2014, 32, 1095–1100. [Google Scholar] [CrossRef]
- Jia, D.; Shi, W.; Zhang, H.; Wu, T.; Diao, Y.; Li, K.; Lu, C. Effects of Y2O3 Content on Wear Resistanceand Corrosion Resistance of 316L/TiC Coating Fabricated by Laser Cladding. Coatings 2023, 13, 1348. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, X.; Tang, G.; Ma, X. Microstructure and Corrosion Behavior of Cr Coating on M50 Steel Fabricated by Electrospark Deposition. J. Mater. Eng. Perform. 2019, 28, 4086–4094. [Google Scholar] [CrossRef]
- Wang, J.S. Study on Electro-spark Deposition Process and Property of ESD Coating. Surf. Technol. 2005, 4, 27–30. [Google Scholar] [CrossRef]



| Element | Al | Fe | Co | Cr | Ni | Cu |
|---|---|---|---|---|---|---|
| Content | 9.59 | 15.12 | 17.54 | 18.25 | 19.70 | 19.81 |
| Sample | Voltage/V | Capacitance/μF |
|---|---|---|
| 1 | 10 | 1000 |
| 2 | 10 | 2000 |
| 3 | 10 | 3000 |
| 4 | 20 | 1000 |
| 5 | 20 | 2000 |
| 6 | 20 | 3000 |
| Ecorr (VSHE) | Icorr (A·cm−2) | |
|---|---|---|
| Substrate | −1.567 ± 0.06 | 7.911 × 10−6 ± 0.06 × 10−6 |
| 10 V, 1000 μF | −0.623 ± 0.06 | 1.087 × 10−6 ± 0.06 × 10−6 |
| 10 V, 2000 μF | −0.837 ± 0.06 | 4.417 × 10−6 ± 0.06 × 10−6 |
| 10 V, 3000 μF | −1.126 ± 0.06 | 6.338 × 10−6 ± 0.06 × 10−6 |
| 20 V, 1000 μF | −0.445 ± 0.06 | 9.475 × 10−7 ± 0.06 × 10−7 |
| 20 V, 2000 μF | −0.730 ± 0.06 | 1.239 × 10−6 ± 0.06 × 10−6 |
| 20 V, 3000 μF | −1.465 ± 0.06 | 6.901 × 10−6 ± 0.06 × 10−6 |
| Rs/(Ω·com2) | RP/(Ω·com2) | CPE1-T | CPE1-P | |
|---|---|---|---|---|
| Substrate | 10.77 | 67.13 | 3.8405 × 10−4 | 0.73933 |
| 10 V, 1000 μF | 10.76 | 2626 | 9.2256 × 10−5 | 0.84314 |
| 10 V, 2000 μF | 13.05 | 318.7 | 4.4542 × 10−4 | 0.74324 |
| 10 V, 3000 μF | 9.22 | 253 | 5.7101 × 10−4 | 0.67879 |
| 20 V, 1000 μF | 13.02 | 3688 | 8.9813 × 10−5 | 0.85955 |
| 20 V, 2000 μF | 10.57 | 596.3 | 2.8713 × 10−4 | 0.77062 |
| 20 V, 3000 μF | 12.39 | 217.2 | 4.44667 × 10−4 | 0.73698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Nie, C.; Wang, S.; Gong, P.; Zhang, M.; Hu, Z.; Li, B. Study on the Properties of TiC Coating Deposited by Spark Discharge on the Surface of AlFeCoCrNiCu High-Entropy Alloy. Materials 2024, 17, 4110. https://doi.org/10.3390/ma17164110
Wang Y, Nie C, Wang S, Gong P, Zhang M, Hu Z, Li B. Study on the Properties of TiC Coating Deposited by Spark Discharge on the Surface of AlFeCoCrNiCu High-Entropy Alloy. Materials. 2024; 17(16):4110. https://doi.org/10.3390/ma17164110
Chicago/Turabian StyleWang, Ying, Cheng Nie, Shengding Wang, Pan Gong, Mao Zhang, Zhigang Hu, and Bin Li. 2024. "Study on the Properties of TiC Coating Deposited by Spark Discharge on the Surface of AlFeCoCrNiCu High-Entropy Alloy" Materials 17, no. 16: 4110. https://doi.org/10.3390/ma17164110





