Seawater Mixed with One Part Alkali Activated Material: An Environmental and Cost Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Strength Measurement
2.4. XRD Measurement and Data Analysis
2.5. Equivalent CO2 Emission
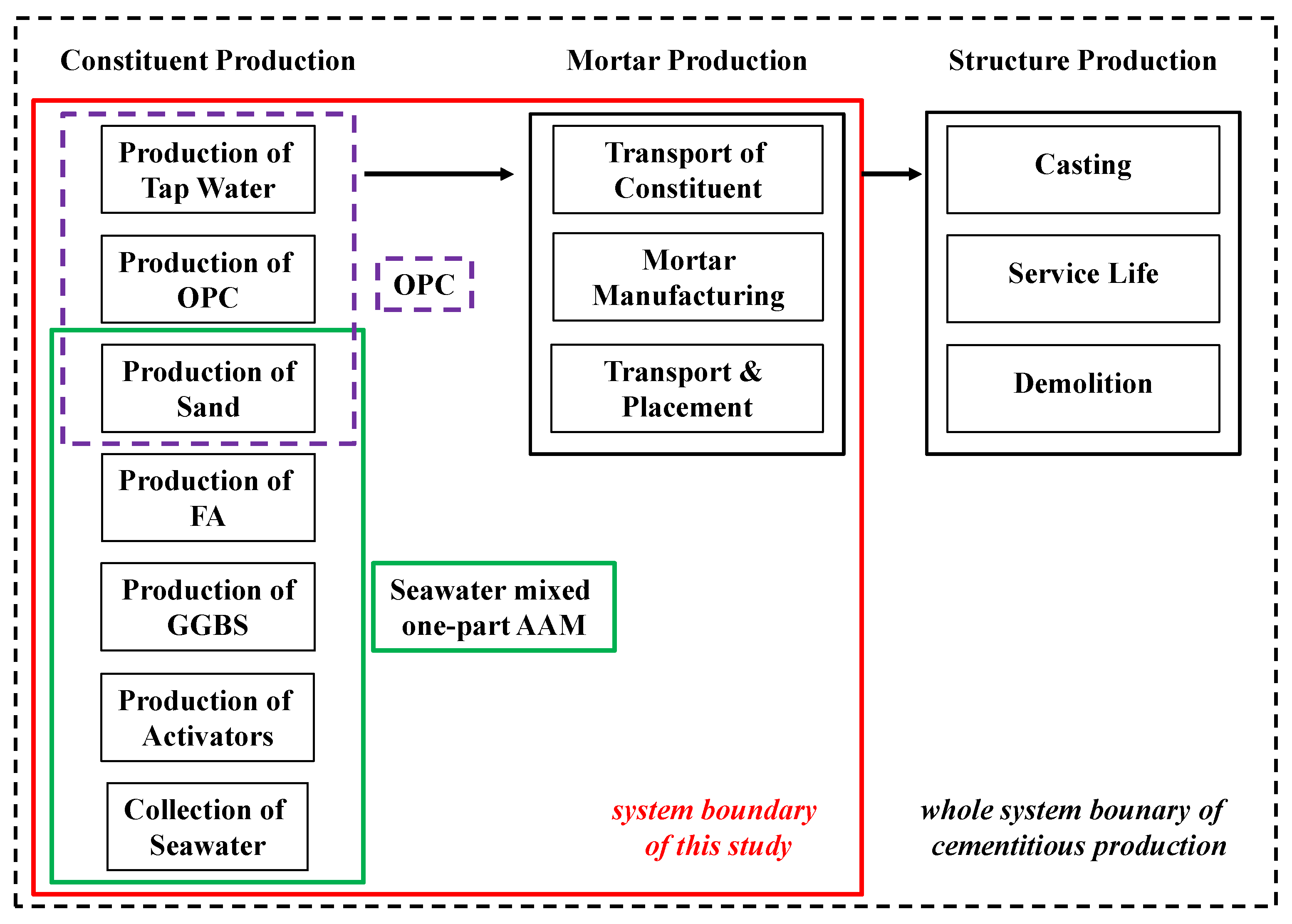
2.5.1. Goal and Scope
2.5.2. Inventory Analysis
2.5.3. Calculation and Interpretation
2.6. Cost Evaluation
3. Results and Discussion
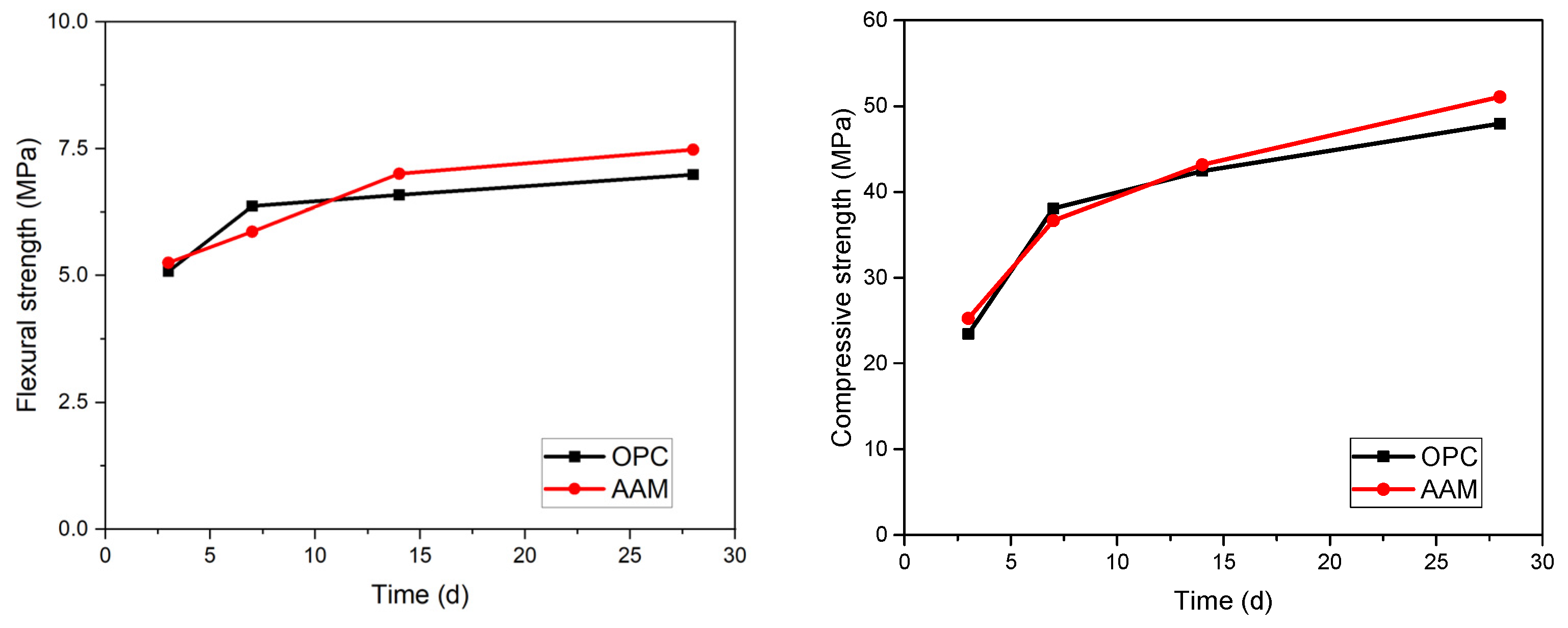
3.1. Flexural and Compressive Strengths
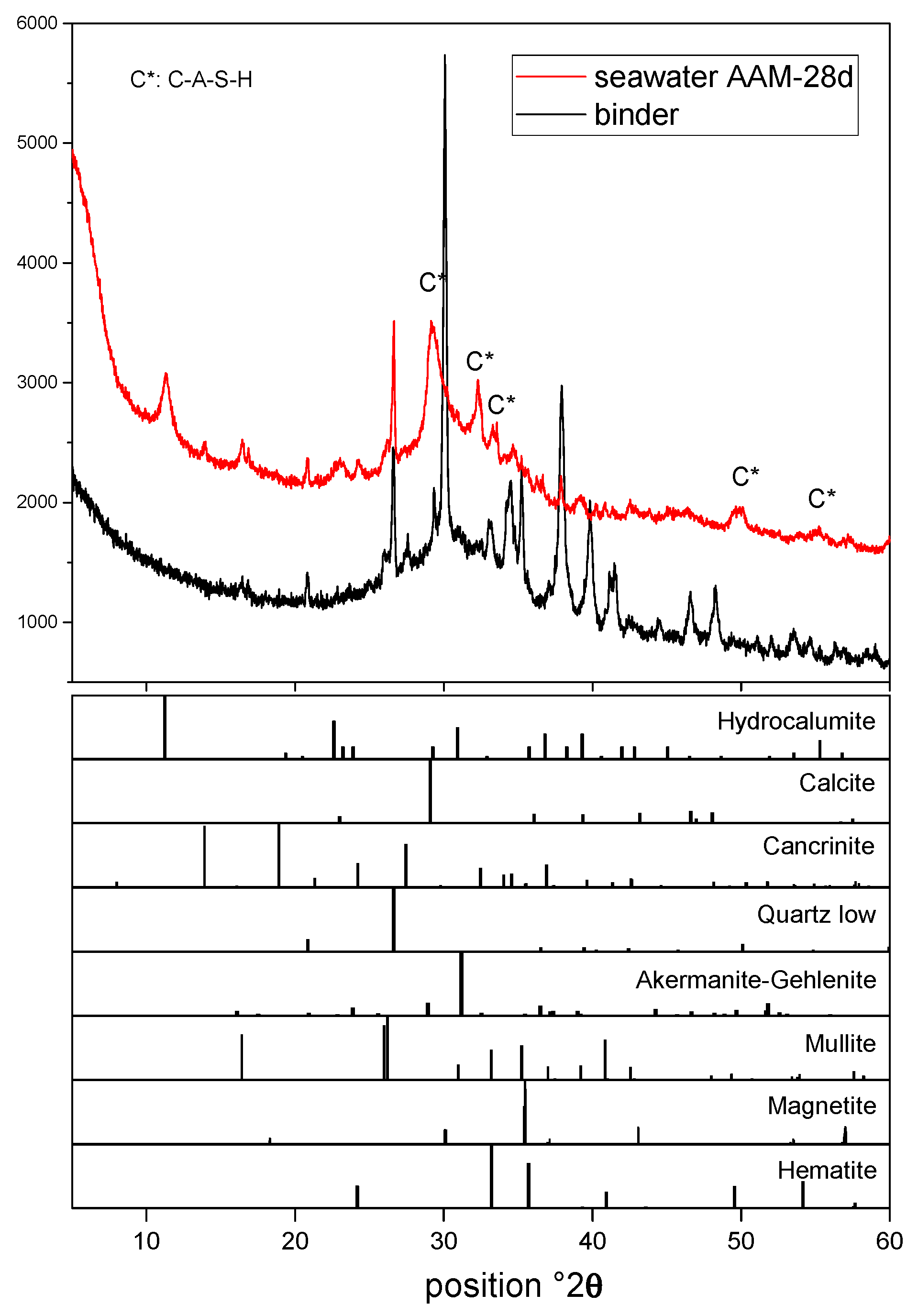
3.2. Reaction Product
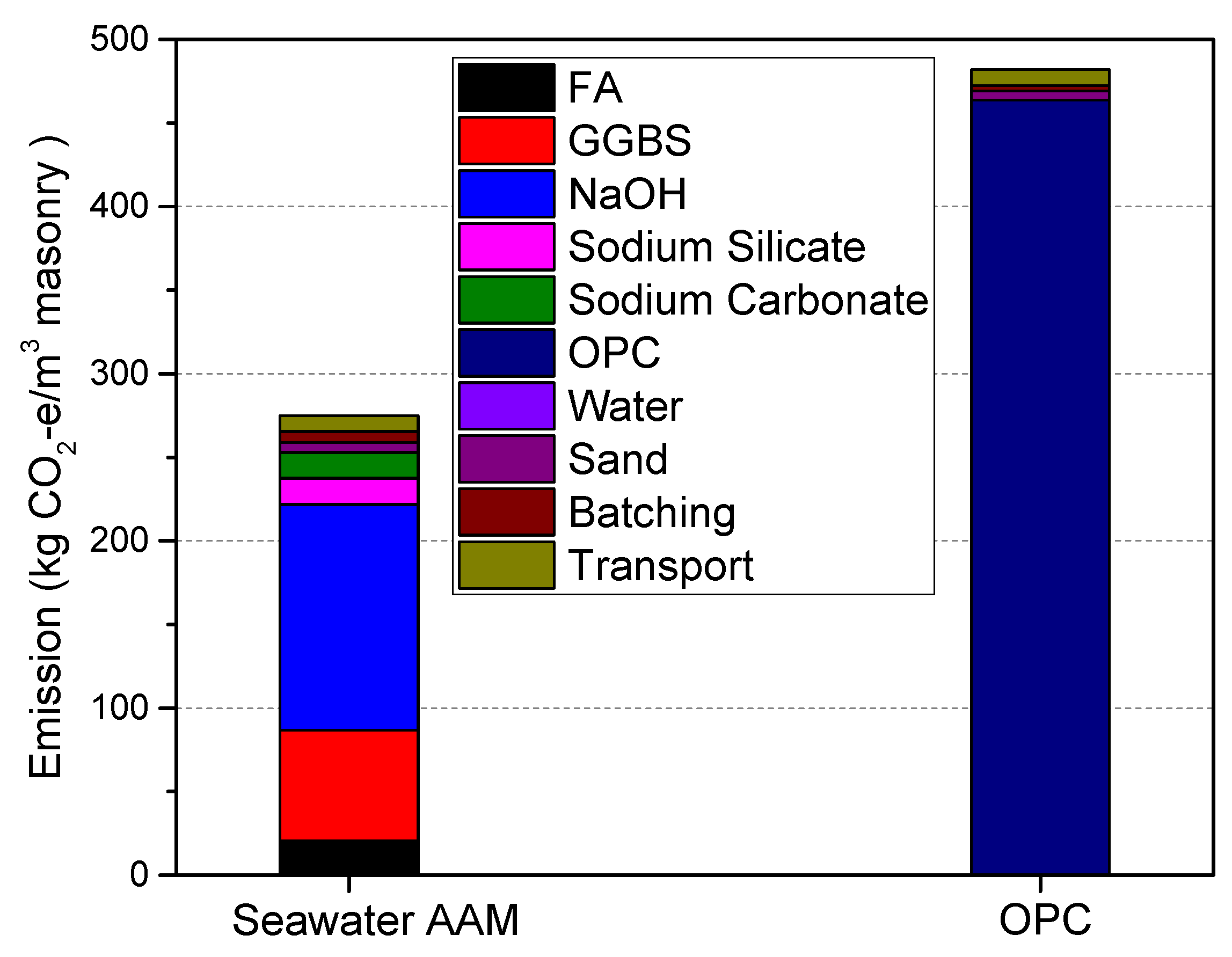
3.3. Equivalent CO2 Emissions
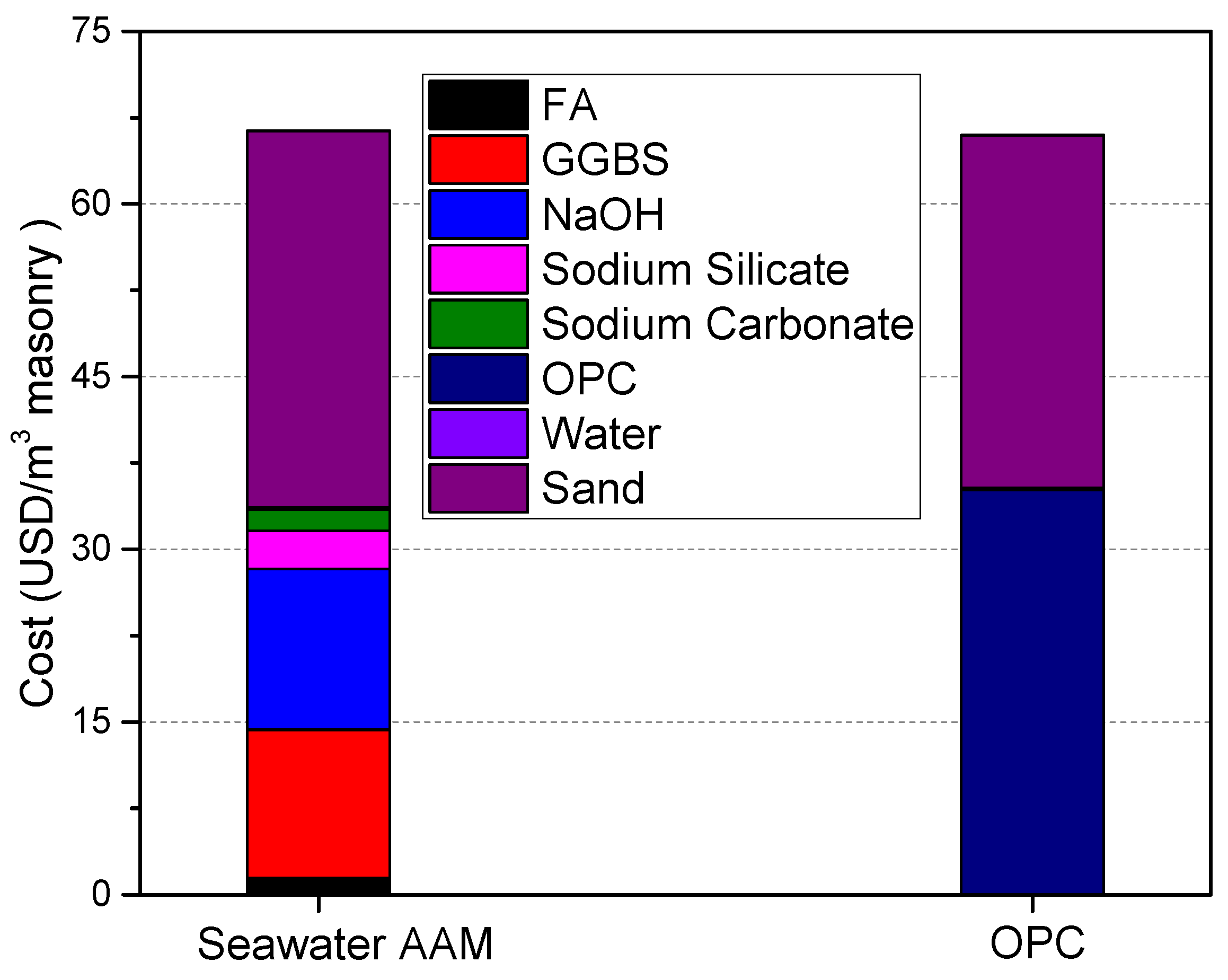
3.4. Cost Evaluation
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of booming concrete production on water resources worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Mclellan, B.C.; Williams, R.P.; Lay, J.; Riessen, A.V.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of limestone calcined clay cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Karen, S.; Fernando, M.; Shashank, B.; Soumen, M. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar]
- Berriel, S.S.; Favier, A.; Domínguez, E.R.; Machado, I.S.; Heierli, U.; Scrivener, K.; Hernández, F.M.; Habert, G. Assessing the environmental and economic potential of limestone calcined clay cement in cuba. J. Clean. Prod. 2016, 124, 361–369. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali-Activated Materials, State-of-the-Art Report; RILEM TC 224-AAM; Springer: Berlin, Germany, 2014. [Google Scholar]
- Davidovits, J. Mineral Polymers and Methods of Making Them. U.S. Patent US4349386A, 14 September 1982. [Google Scholar]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; Van Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2015, 48, 517–529. [Google Scholar] [CrossRef]
- Fernandez-Pereira, C.; Luna, Y.; Querol, X.; Antenucci, D.; Vale, J. Waste stabilization/solidification of an electric arc furnace dust using fly ash-based geopolymers. Fuel 2009, 88, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; Van Deventer, J.S.J. The role of sulfide in the immobilization of Cr(VI) in fly ash geopolymers. Cem. Concr. Res. 2008, 38, 681–688. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E.; Lachowski, E.E. Fixing arsenic in alkali-activated cementitious matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Blackford, M.G.; Hanna, J.V.; Pike, K.J.; Vance, E.R.; Perera, D.S. Transmission electron microscopy and nuclear magnetic resonance studies of geopolymers for radioactive waste immobilization. J. Am. Ceram. Soc. 2007, 90, 1193–1199. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Zhai, J. Immobilization of simulated radionuclide 133cs(+) by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262C, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Majersy, D.; Sekely, S.; Breza, M. Verified possibilities of specific and historical waste solidification. In Proceedings of the IAEA RCM on Behaviour of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste Meeting, Moscow, Russia, 10–14 September 2007. [Google Scholar]
- Witze, A. Rare mineral is the key to long-lasting ancient concrete. Nature 2017. [Google Scholar] [CrossRef]
- Eamon, C.; Jensen, E.; Grace, N.; Shi, X. Life-Cycle Cost Analysis of Alternative Reinforcement Materials for Bridge Superstructures Considering Cost and Maintenance Uncertainties. J. Mater. Civ. Eng. 2012, 24, 373–380. [Google Scholar] [CrossRef]
- El-Hassan, H.; El-Maaddawy, T.; Al-Sallamin, A.; Al-Saidy, A. Performance evaluation and microstructural characterization of gfrp bars in seawater-contaminated concrete. Constr. Build. Mater. 2017, 147, 66–78. [Google Scholar] [CrossRef]
- Robert, M.; Benmokrane, B. Combined effects of saline solution and moist concrete on long-term durability of gfrp reinforcing bars. Constr. Build. Mater. 2013, 38, 274–284. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Production of seawater mixed one-part alkali activated material. Cem. Concr. Compos. 2019. under review. [Google Scholar]
- Yang, Z.; Zhan, X.; Zhu, H.; Zhang, B.; Feng, P.; Li, H.; Kua, H.W. Performance-based alkali-activated seawater sea-sand concrete: Mixture optimization for mechanical, environmental, and economical objectives. Constr. Build. Mater. 2023, 409, 134156. [Google Scholar] [CrossRef]
- Jun, Y.; Han, S.H.; Bae, Y.H.; Kim, J.H. Seawater-mixed alkali-activated materials: A comparative investigation of metal slag suitability. J. Sustain. Cem.-Based Mater. 2023, 12, 907–923. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Feiz, R.; Ammenberg, J.; Baas, L.; Eklund, M.; Helgstrand, A.; Marshall, R. Improving the CO2 performance of cement, part I: Utilizing life-cycle assessment and key performance indicators to assess development within the cement industry. J. Clean. Prod. 2015, 98, 272–281. [Google Scholar] [CrossRef]
- Habert, G. 1–environmental impact of portland cement production. In Eco-Efficient Concrete; Woodhead Publishing: Sawston, UK, 2013; Volume 179, pp. 3–25. [Google Scholar]
- Li, C.; Nie, Z.; Cui, S.; Gong, X.; Wang, Z.; Meng, X. The life cycle inventory study of cement manufacture in China. J. Clean. Prod. 2014, 72, 204–211. [Google Scholar] [CrossRef]
- Hong, J.; Chen, W.; Wang, Y.; Xu, C.; Xu, X. Life cycle assessment of caustic soda production: A case study in China. J. Clean. Prod. 2014, 66, 113–120. [Google Scholar] [CrossRef]
- European Commission. Integrated Pollution Prevention and Control Reference Document on Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals—Solids and Others Industry; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- European Commission. Integrated Pollution Prevention and Control Reference Document on Best Available Techniques Reference Document for the Production of Chlor-Alkali; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized structural description of calcium–sodium aluminosilicate hydrate gels: The cross-linked substituted tobermorite model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Saout, G.L.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part ii: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, H.; Fujita, T.; Ohnishi, S.; Li, H.; Fujii, M.; Dong, H. Environmental and economic gains of industrial symbiosis for Chinese iron/steel industry: Kawasaki’s experience and practice in liuzhou and jinan. J. Clean. Prod. 2013, 59, 226–238. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of chloride and carbonate by Mg-Al and Ca-Al layered double hydroxides in simulated pore solutions of alkali-activated slag cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Chen, Z.; Lee, H.; Lim, S. Chloride-Binding Capacity of Portland Cement Paste Blended with Synthesized CA2 (CaO·2Al2O3). Adv. Mater. Sci. Eng. 2018, 2018, 5418930. [Google Scholar] [CrossRef]
- Heede, P.V.D.; Belie, N.D. Environmental impact and life cycle assessment (lca) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and opc cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Teh, S.H.; Wiedmann, T.; Castel, A.; De Burgh, J. Hybrid life cycle assessment of greenhouse gas emissions from cement, concrete and geopolymer concrete in Australia. J. Clean. Prod. 2017, 152, 312–320. [Google Scholar] [CrossRef]
- Lin, B.; Li, X. The effect of carbon tax on per capita CO2 emissions. Energy Policy 2011, 39, 5137–5146. [Google Scholar] [CrossRef]
- United Nations. World Water Development Report 2019. Available online: https://www.unwater.org/publications/world-water-development-report-2019/ (accessed on 7 December 2023).
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, 1500323. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Younis, A.; Ebead, U.; Suraneni, P.; Nanni, A. Fresh and hardened properties of seawater-mixed concrete. Constr. Build. Mater. 2018, 190, 276–286. [Google Scholar] [CrossRef]
| Parameter/Compound | FA | GGBS |
|---|---|---|
| wt.% | wt.% | |
| Al2O3 | 21.67 | 10.53 |
| SiO2 | 52.72 | 40.28 |
| CaO | 4.85 | 34.54 |
| Fe2O3 | 9.55 | 0.39 |
| MgO | 1.89 | 8.63 |
| P2O5 | 0.48 | 0.15 |
| TiO2 | 0.90 | 0.40 |
| MnO | 0.08 | 1.14 |
| Na2O | 1.11 | 0.59 |
| K2O | 2.27 | 1.62 |
| Compound | Al2O3 | SiO2 | CaO | Fe2O3 | MgO | Na2O | K2O |
| Content (wt.%) | 21.67 | 52.72 | 4.85 | 9.55 | 1.89 | 1.11 | 2.27 |
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | Br− | HCO3− | |
|---|---|---|---|---|---|---|---|---|
| Seawater | 11.87 | 0.38 | 0.39 | 1.33 | 20.95 | 2.97 | 0.11 | 0.14 |
| Material | Market Price (USD/t) |
|---|---|
| FA | 7.3–21.8 |
| GGBS | 30.2–35.1 |
| NaOH | 421.4–501.7 |
| Sodium silicate | 316.1–372.7 |
| Sodium carbonate | 206.3–247.1 |
| OPC | 56.4–71.6 |
| Sand | 14.3–22.9 |
| Water | 0.52–1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, H.; Xing, J.; Gan, M.; Ji, Z.; Fan, X.; Sun, Z. Seawater Mixed with One Part Alkali Activated Material: An Environmental and Cost Evaluation. Materials 2024, 17, 4113. https://doi.org/10.3390/ma17164113
Li X, Liu H, Xing J, Gan M, Ji Z, Fan X, Sun Z. Seawater Mixed with One Part Alkali Activated Material: An Environmental and Cost Evaluation. Materials. 2024; 17(16):4113. https://doi.org/10.3390/ma17164113
Chicago/Turabian StyleLi, Xiaoyu, Huiyang Liu, Jinxin Xing, Min Gan, Zhiyun Ji, Xiaohui Fan, and Zengqing Sun. 2024. "Seawater Mixed with One Part Alkali Activated Material: An Environmental and Cost Evaluation" Materials 17, no. 16: 4113. https://doi.org/10.3390/ma17164113
APA StyleLi, X., Liu, H., Xing, J., Gan, M., Ji, Z., Fan, X., & Sun, Z. (2024). Seawater Mixed with One Part Alkali Activated Material: An Environmental and Cost Evaluation. Materials, 17(16), 4113. https://doi.org/10.3390/ma17164113





