The Influence of Pore-Forming Diluents on Porous Structure, Thermal and Sorption Properties of the Divinylbenzene and Glycidyl Methacrylate Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polymeric Microspheres
2.3. Measurements
3. Results and Discussion
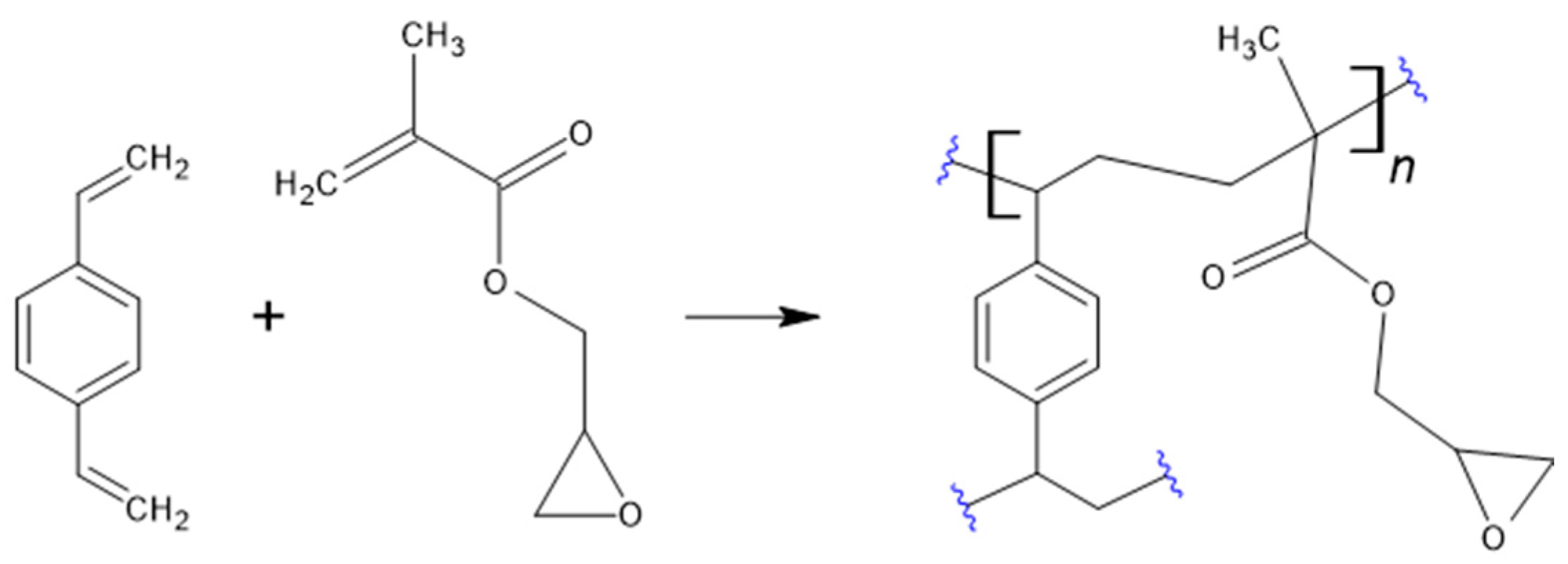
3.1. Chemical Structure Characterization
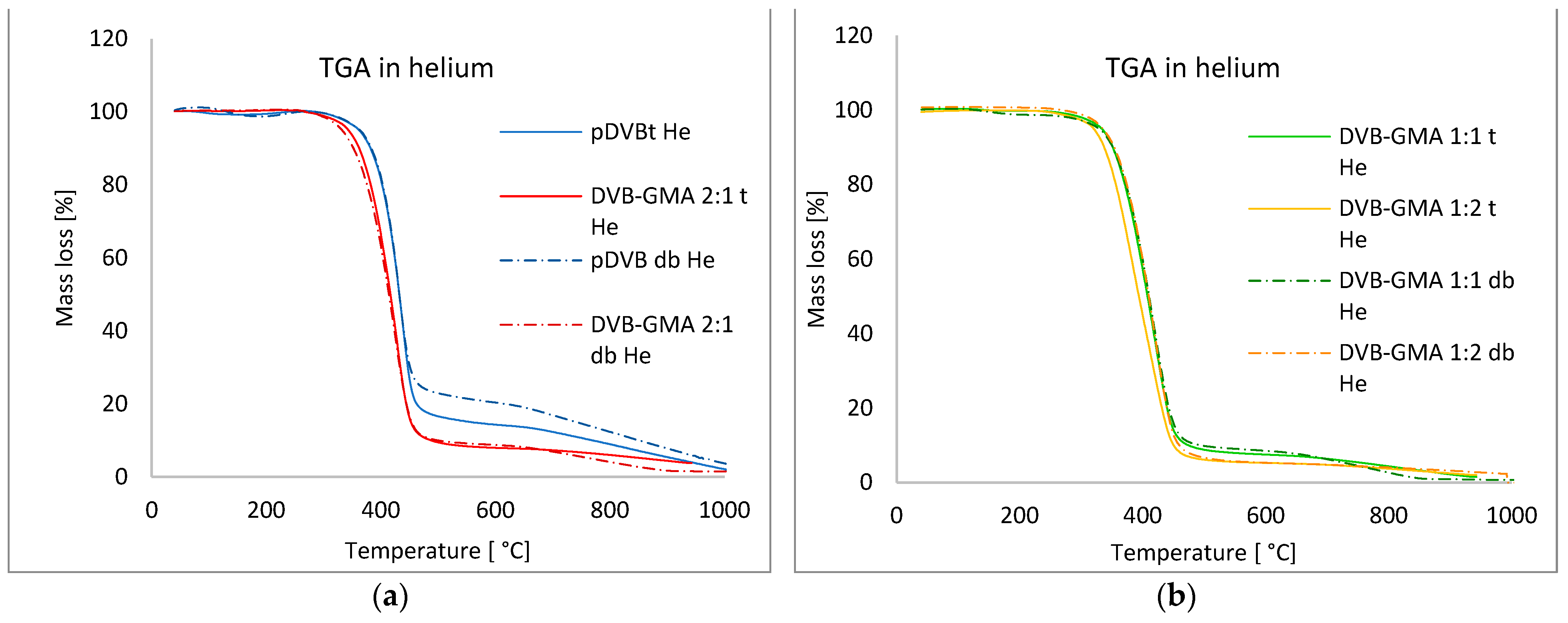
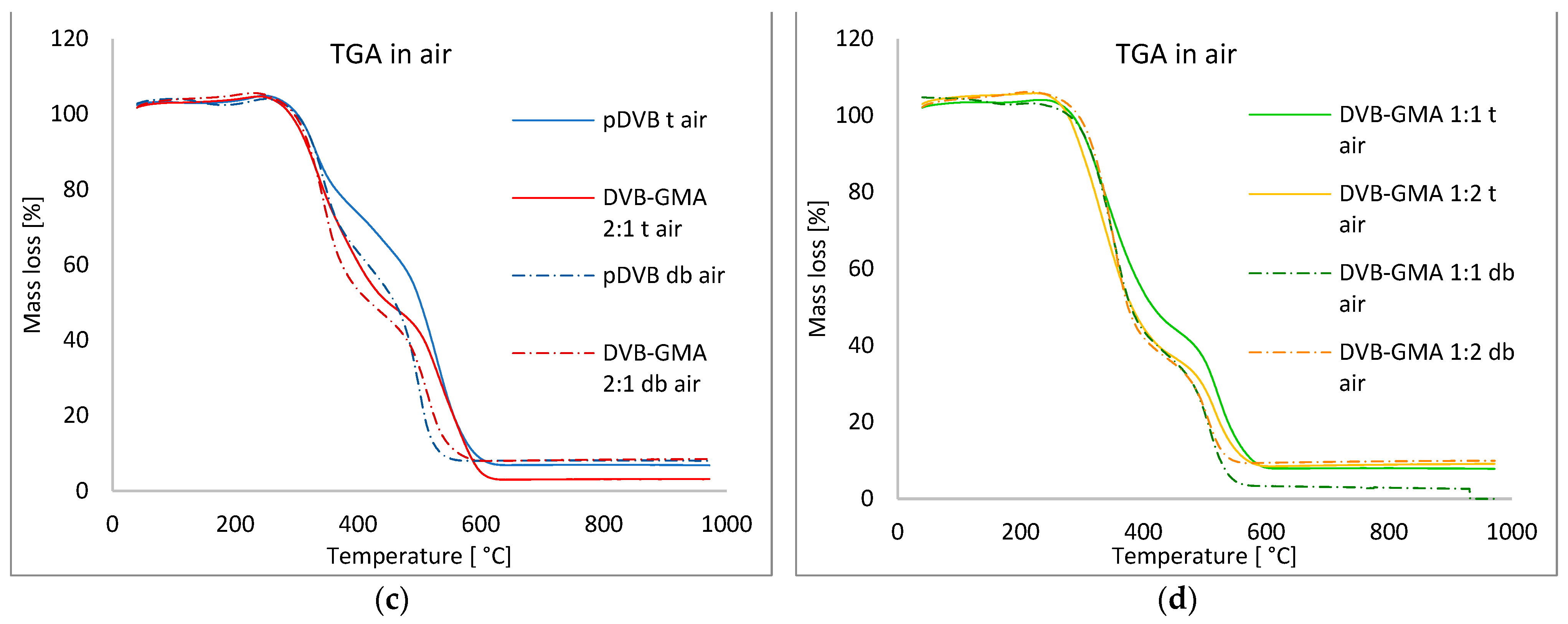
3.2. Thermal Properties of the Studied Polymers
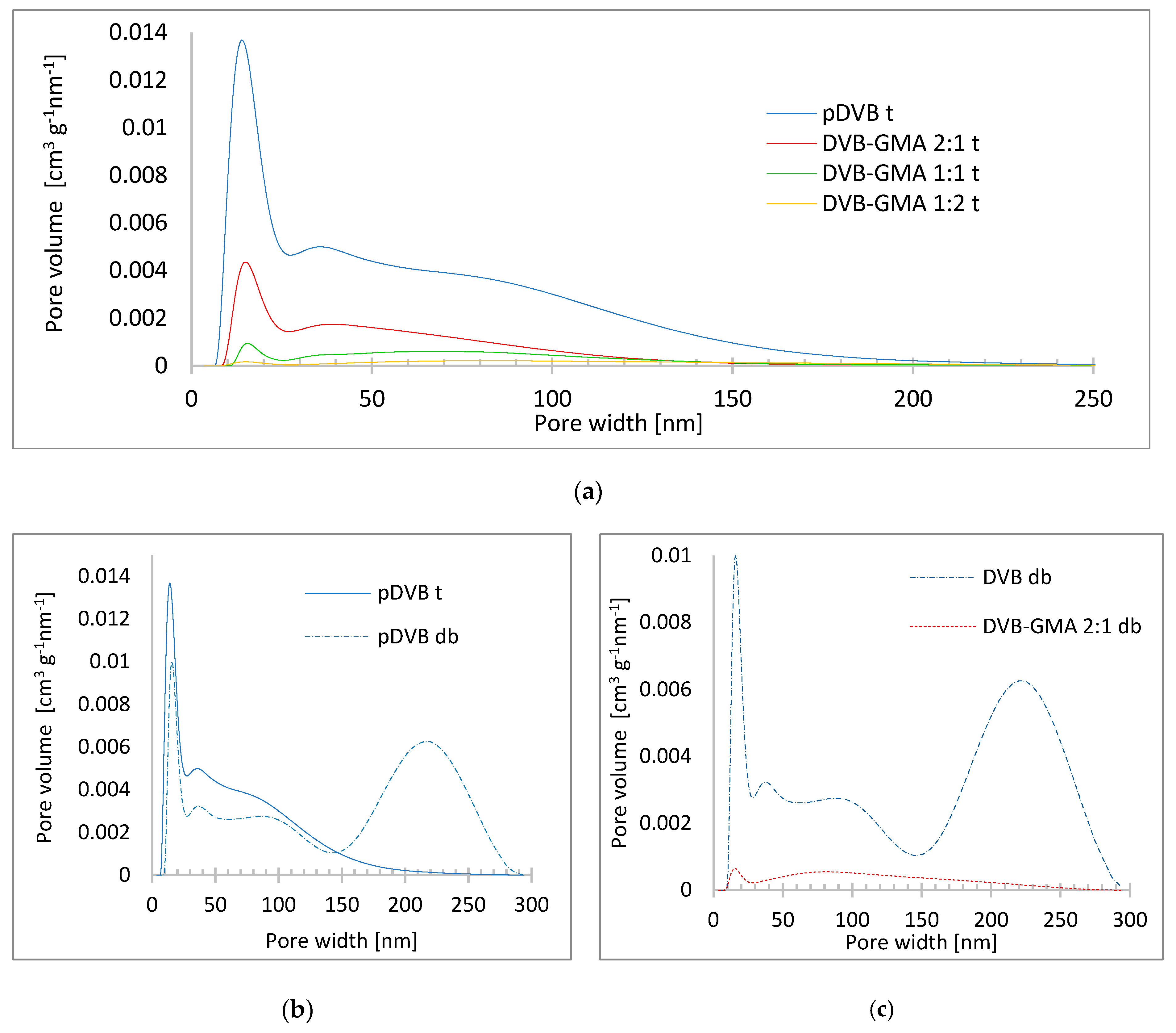
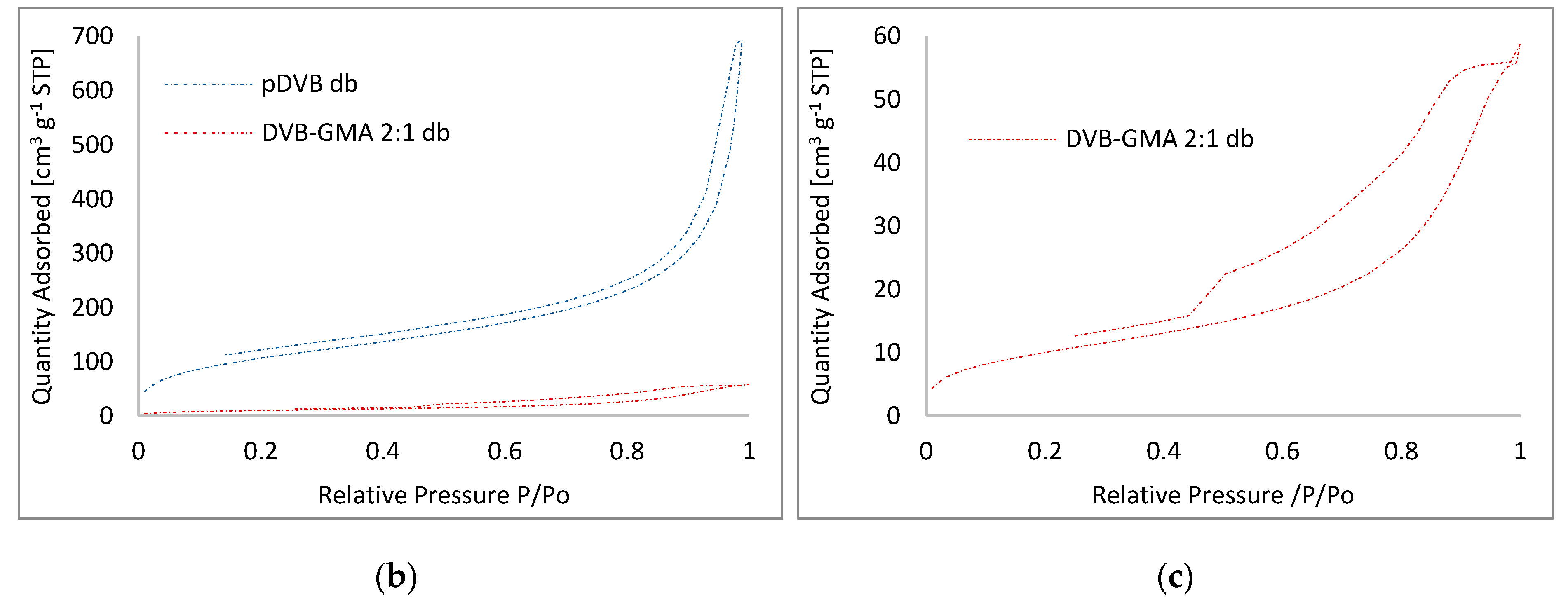
3.3. Morphology of Studied Polymers
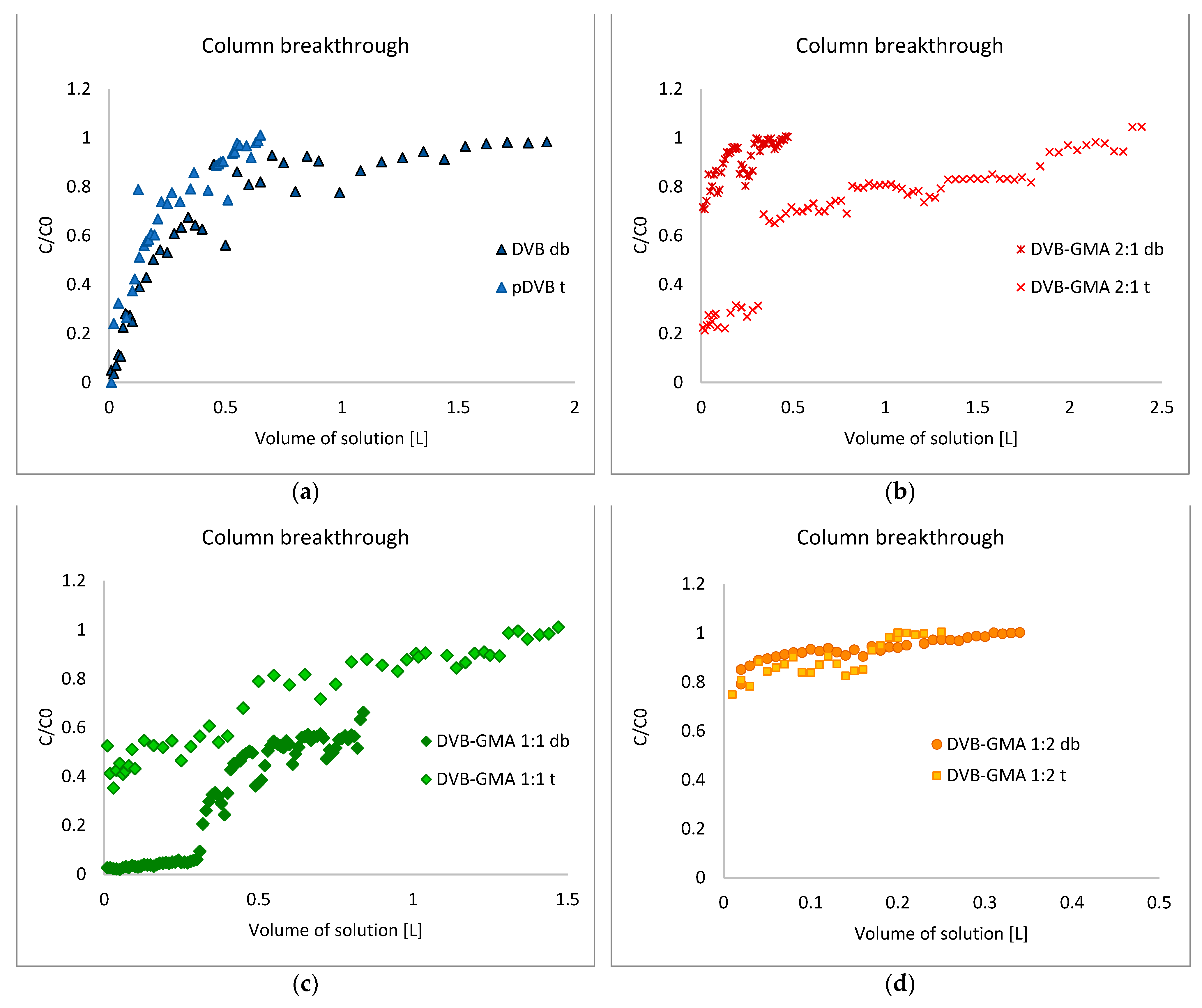
3.4. Sorption Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Alipoori, S.; Rouhi, H.; Linn, E.; Stumpfl, H.; Mokarizadeh, H.; Esfahani, M.R.; Koh, A.; Weinman, S.T.; Wujcik, E.K. Polymer-Based Devices and Remediation Strategies for Emerging Contaminants in Water. ACS Appl. Polym. Mater. 2021, 3, 549–577. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sharma, S.; Soni, V. Classification and Impact of Synthetic Textile Dyes on Aquatic Flora: A Review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- El Nemr, A.; Shoaib, A.G.M.; El Sikaily, A.; Mohamed, A.E.-D.A.; Hassan, A.F. Evaluation of Cationic Methylene Blue Dye Removal by High Surface Area Mesoporous Activated Carbon Derived from Ulva Lactuca. Environ. Process. 2021, 8, 311–332. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Xie, R.; El-Sheekh, M.M.; Sun, J. Construction of a New Lipase- and Xylanase-Producing Oleaginous Yeast Consortium Capable of Reactive Azo Dye Degradation and Detoxification. Bioresour. Technol. 2020, 313, 123631. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Samoylova, N.A.; Potanina, D.V.; Popov, V.N. The Effect of Methylene Blue and Its Metabolite—Azure I—On Bioenergetic Parameters of Intact Mouse Brain Mitochondria. Biochem. Suppl. Ser. B Biomed. Chem. 2022, 16, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kayabaşı, Y. Methylene Blue and Its Importance in Medicine. Demiroglu Sci. Univ. Florence Nightingale J. Med. 2020, 6, 136–145. [Google Scholar] [CrossRef]
- Li, H.; Zhang, R.; Tang, L.; Zhang, J.; Mao, Z. Evaluation of Bacillus sp. MZS10 for Decolorizing Azure B Dye and Its Decolorization Mechanism. J. Environ. Sci. 2014, 26, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Shitu, A.; Ibrahim, A. Removal of Methylene Blue Using Low Cost Adsorbent: A Review. Res. J. Chem. Sci. 2014, 4, 91–102. [Google Scholar]
- Moradi, O.; Sharma, G. Emerging Novel Polymeric Adsorbents for Removing Dyes from Wastewater: A Comprehensive Review and Comparison with Other Adsorbents. Environ. Res. 2021, 201, 111534. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional Microspheres for Tissue Regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Cong, H.; Shen, Y.; Yu, B. Preparation of Functional Polymer Microspheres with Large Pores and Their Application in Liquid Chromatography. SSRN Electron. J. 2023, 1–17. [Google Scholar] [CrossRef]
- Song, T.; Zhou, M.; Liu, W.; Bian, G.; Qi, Y.; Bai, F.; Yang, X. Preparation of Polymer Microspheres with Reactive Epoxy Group and Amino Groups as Stabilizers for Gold Nanocolloids with Recoverable Catalysis. Colloid Polym. Sci. 2015, 293, 187–197. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.; Jiang, H.-X.; Chen, Y.; Tang, A.-N.; Kong, D.-M. Controllable Synthesis of Uniform Large-Sized Spherical Covalent Organic Frameworks for Facile Sample Pretreatment and as Naked-Eye Indicator. Talanta 2022, 236, 122829. [Google Scholar] [CrossRef]
- Maciejewska, M. Special Issue: “Structural and Thermal Properties of Polymeric Microspheres”. Materials 2022, 15, 8017. [Google Scholar] [CrossRef]
- Sobiesiak, M. Analysis of Structure and Properties of DVB–GMA Based Porous Polymers. Adsorption 2019, 25, 257–266. [Google Scholar] [CrossRef]
- Simpson, N.J.K. Solid-Phase Extraction: Principles, Techniques, and Applications; CRC Press: New York, NY, USA, 2000; ISBN 1420056247. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470093072. [Google Scholar]
- Sobiesiak, M.; Banaszek, P. Low Cross-Linked Terpenes-Based Porous Polymers with Reduced Content of Divinylbenzene: Synthesis, Physicochemical Properties and Sorption Abilities. Adsorption 2024, 30, 235–249. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, J. Thermal, Physical and Chemical Stability of Porous Polystyrene-Type Beads with Different Degrees of Crosslinking. Polym. Degrad. Stab. 2001, 73, 163–167. [Google Scholar] [CrossRef]
- Li, L.; Song, H.; Chen, X. Enhancement of Thermal Stability of Poly(Divinylbenzene) Microspheres. Mater. Lett. 2008, 62, 179–182. [Google Scholar] [CrossRef]
- Simister, C.; Caron, F.; Gedye, R. Determination of the Thermal Degradation Rate of Polystyrene-Divinyl Benzene Ion Exchange Resins in Ultra-Pure Water at Ambient and Service Temperature. J. Radioanal. Nucl. Chem. 2004, 261, 523–531. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, J. The Thermal Properties of Porous Polydivinylbenzene Beads. React. Funct. Polym. 2002, 50, 57–65. [Google Scholar] [CrossRef]
- Wilkie, C.A. TGA/FTIR: An Extremely Useful Technique for Studying Polymer Degradation. Polym. Degrad. Stab. 1999, 66, 301–306. [Google Scholar] [CrossRef]
- Gałka, P.; Kowalonek, J.; Kaczmarek, H. Thermogravimetric Analysis of Thermal Stability of Poly(Methyl Methacrylate) Films Modified with Photoinitiators. J. Therm. Anal. Calorim. 2014, 115, 1387–1394. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-Y. The Effect of End Groups on the Thermal Degradation of Poly(Methyl Methacrylate). Polym. Degrad. Stab. 2003, 82, 81–88. [Google Scholar] [CrossRef]
- Philippe, D.; Vieille, B.; Barbe, F.; Philippe, D.; Vieille, B.; Barbe, F. Experimental Characterization and Numerical Modeling of the Porosity Formation Mechanisms in Thermoplastic Laminates at High Temperature; HAL Open Science; HAL: Lyon, France, 2024. [Google Scholar]
- Podkościelna, B.; Gawdzik, B. Influence of Diluent Compositions on the Porous Structure of Methacrylate Derivatives of Aromatic Diols and Divinylbenzene. Appl. Surf. Sci. 2010, 256, 2462–2467. [Google Scholar] [CrossRef]
- Sobiesiak, M. New Bio-Based Polymer Sorbents out of Terpene Compounds or Vegetable Oils: Synthesis, Properties, Analysis of Sorption Processes. Polymers 2022, 14, 5389. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Du Prez, F.E. Porous Polymer Particles—A Comprehensive Guide to Synthesis, Characterization, Functionalization and Applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Xiao, A. Research Progress in Macroporous Styrene-Divinylbenzene Co-Polymer Microspheres. Des. Monomers Polym. 2007, 10, 405–423. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Aminzadeh, S.; Pylypchuk, I.V.; Sternik, D.; Tertykh, V.A.; Lindström, M.E.; Sevastyanova, O. Methylene Blue Dye Sorption by Hybrid Materials from Technical Lignins. J. Environ. Chem. Eng. 2018, 6, 4997–5007. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High Adsorption Capacity of Phenol and Methylene Blue Using Activated Carbon Derived from Lignocellulosic Agriculture Wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef] [PubMed]
- Holliday, M.C.; Parsons, D.R.; Zein, S.H. Agricultural Pea Waste as a Low-Cost Pollutant Biosorbent for Methylene Blue Removal: Adsorption Kinetics, Isotherm And Thermodynamic Studies. Biomass Convers. Biorefinery 2024, 14, 6671–6685. [Google Scholar] [CrossRef]
- Arias, M.; López, E.; Nuñez, A.; Rubinos, D.; Soto, B.; Barral, M.T.; Díaz-Fierros, F. Adsorption of Methylene Blue by Red Mud, An Oxide- Rich Byproduct of Bauxite Refining. In Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments; Berthelin, J., Huang, P.M., Bollag, J.-M., Andreux, F., Eds.; Springer: Boston, MA, USA, 1999; pp. 361–365. ISBN 978-1-4615-4683-2. [Google Scholar]
- Ataei-Germi, T.; Nematollahzadeh, A. Bimodal Porous Silica Microspheres Decorated with Polydopamine Nano-Particles for the Adsorption of Methylene Blue in Fixed-Bed Columns. J. Colloid Interface Sci. 2016, 470, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, J.; Liu, W.; Xu, Y.; Cao, Y.; Chen, B.; Xu, M. Preparation of Hyper-Cross-Linked Hydroxylated Polystyrene for Adsorptive Removal of Methylene Blue. RSC Adv. 2021, 11, 25551–25560. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-L.; Zhang, Y.-L.; Jiang, Y.; Zeng, G.-M.; Cui, Z.-H.; Liu, K.; Deng, C.-H.; Niu, Q.-Y.; Deng, J.-H.; Huan, S.-Y. Continuous Adsorption of Pb(II) and Methylene Blue by Engineered Graphite Oxide Coated Sand in Fixed-Bed Column. Appl. Surf. Sci. 2015, 330, 148–157. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption Performance of Continuous Fixed Bed Column for the Removal of Methylene Blue (MB) Dye Using Eucalyptus Sheathiana Bark Biomass. Res. Chem. Intermed. 2016, 42, 2343–2364. [Google Scholar] [CrossRef]
- Tamez Uddin, M.; Rukanuzzaman, M.; Maksudur Rahman Khan, M.; Akhtarul Islam, M. Adsorption of Methylene Blue from Aqueous Solution by Jackfruit (Artocarpus heteropyllus) Leaf Powder: A Fixed-Bed Column Study. J. Environ. Manag. 2009, 90, 3443–3450. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Zou, W.; Wang, Y.; Shi, J. Comparison of Linear and Nonlinear Analysis in Estimating the Thomas Model Parameters for Methylene Blue Adsorption onto Natural Zeolite in Fixed-Bed Column. J. Hazard. Mater. 2007, 145, 331–335. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Yu, W.; Zou, W.; Shi, J.; Liu, H. Biosorption of Methylene Blue from Aqueous Solution by Rice Husk in a Fixed-Bed Column. J. Hazard. Mater. 2007, 141, 713–718. [Google Scholar] [CrossRef]
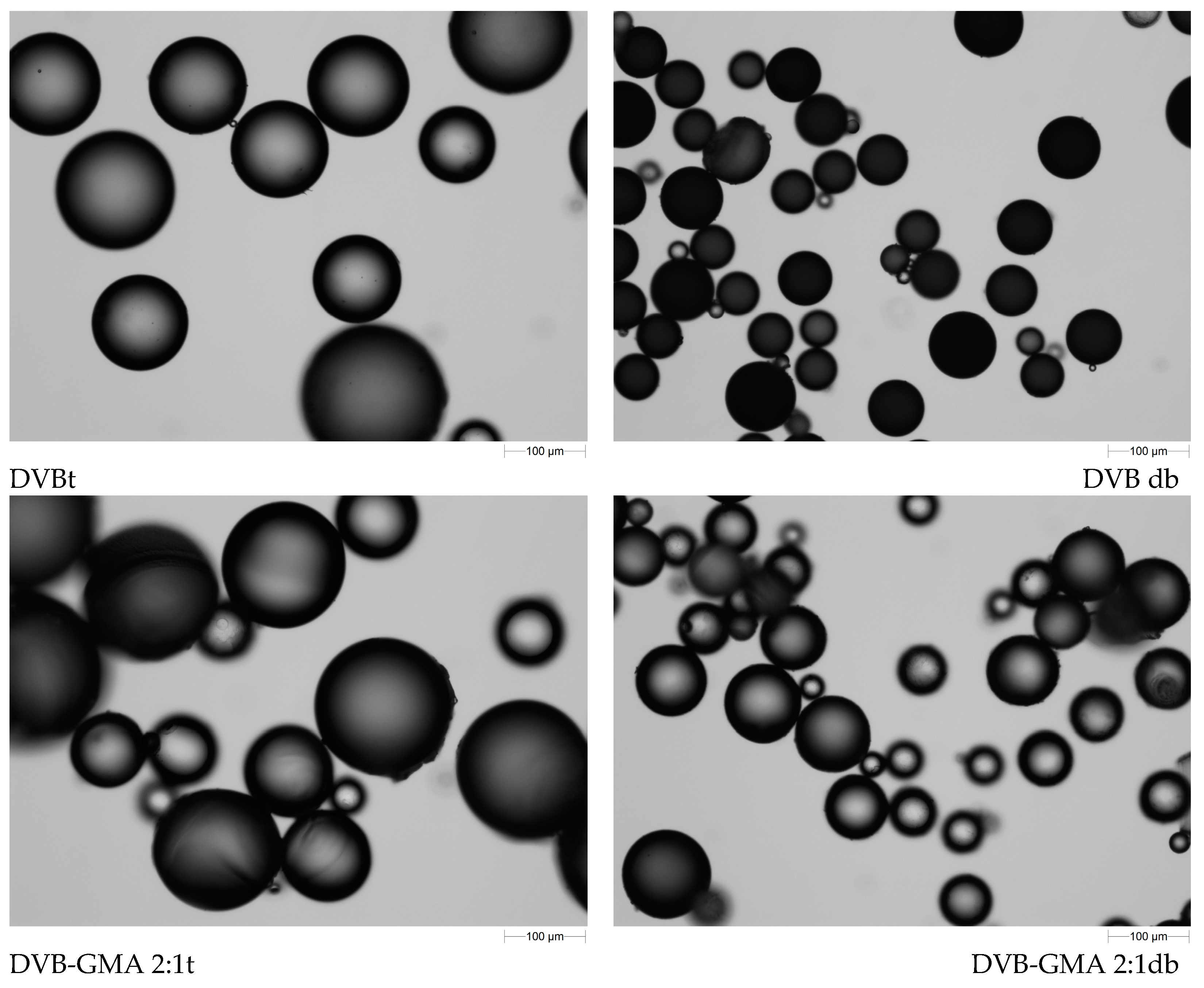




| Polymer | DVB [mL] | GMA [mL] | t [mL] | d [mL] | b [mL] |
|---|---|---|---|---|---|
| pDVB t | 30 | - | 45 | - | - |
| DVB-GMA 2:1 t | 20 | 10 | 45 | - | - |
| DVB-GMA 1:1 t | 15 | 15 | 45 | - | - |
| DVB-GMA1:2 t | 10 | 20 | 45 | - | - |
| pDVB db | 30 | - | - | 10 | 35 |
| DVB-GMA 2:1 db | 20 | 10 | - | 10 | 35 |
| DVB-GMA 1:1 db | 15 | 15 | - | 10 | 35 |
| DVB-GMA 1:2 db | 10 | 20 | - | 10 | 35 |
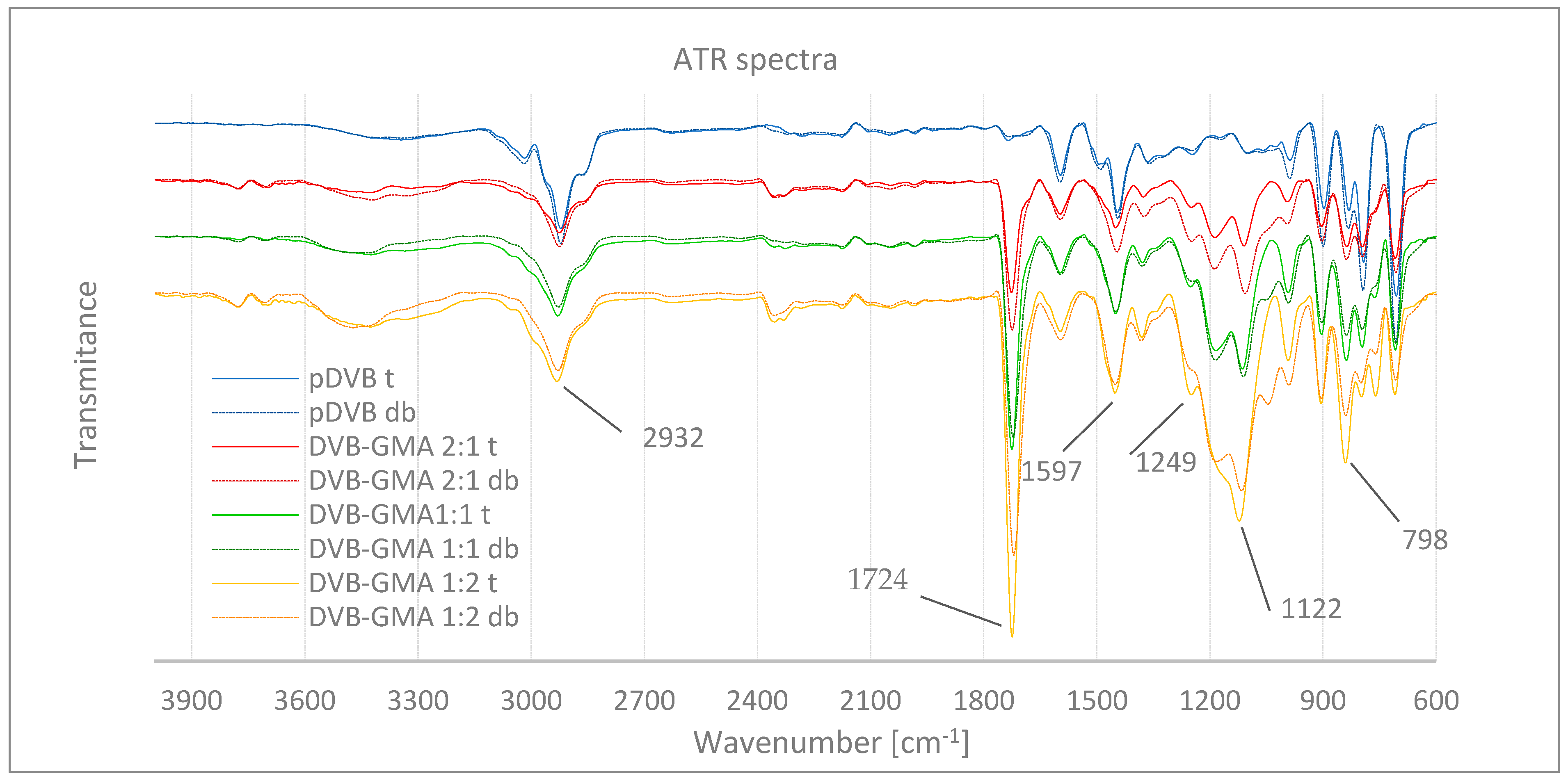
| Pore-Forming Diluent | Copolymer | Stretching Vibrations [cm−1] | |||
|---|---|---|---|---|---|
 | -CH2- |  |  | ||
| toluene | pDVB t | 1596; 793; 706 | 2922 | - | - |
| DVB-GMA 2:1 t | 1597; 796; 708 | 2926 | 1249; 905; 837 | 1726; 1187; 1110 | |
| DVB-GMA 1:1 t | 1597; 797; 709 | 2929 | 1251; 905; 840 | 1725; 1184; 1114 | |
| DVB-GMA1:2 t | 1597; 798; 710 | 2932 | 1249; 905; 841 | 1724; - ; 1122 | |
| decanol and benzyl alcohol | pDVB db | 1598; 794; 707 | 2922 | - | - |
| DVB-GMA 2:1 db | 1596; 796; 707 | 2826 | 1248; 903; 837 | 1724; 1189; 1106 | |
| DVB-GMA 1:1 db | 1596; 796; 706 | 2928 | 1248; 903; 838 | 1722; 1187; 1110 | |
| DVB-GMA 1:2 db | 1596; 798; 707 | 2929 | - ; 904; 839 | 1720; 1183; 1119 | |
| pDVB | DVB-GMA 2:1 | DVB-GMA 1:1 | DVB-GMA 1:2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t | db | theor. | t | db | theor. | t | db | theor. | t | db | theor. | |
| C | 89.6 | 90.3 | 92.3 | 79.3 | 76.6 | 80.6 | 75.3 | 75.8 | 75.0 | 70.15 | 70.6 | 69.5 |
| H | 8.0 | 7.8 | 7.7 | 7.7 | 7.7 | 7.5 | 7.4 | 7.5 | 7.4 | 7.3 | 7.1 | 7.3 |
| O | 2.0 | 1.6 | 0 | 12.6 | 15.3 | 11.9 | 17.0 | 16.4 | 17.6 | 22.2 | 22.0 | 23.2 |
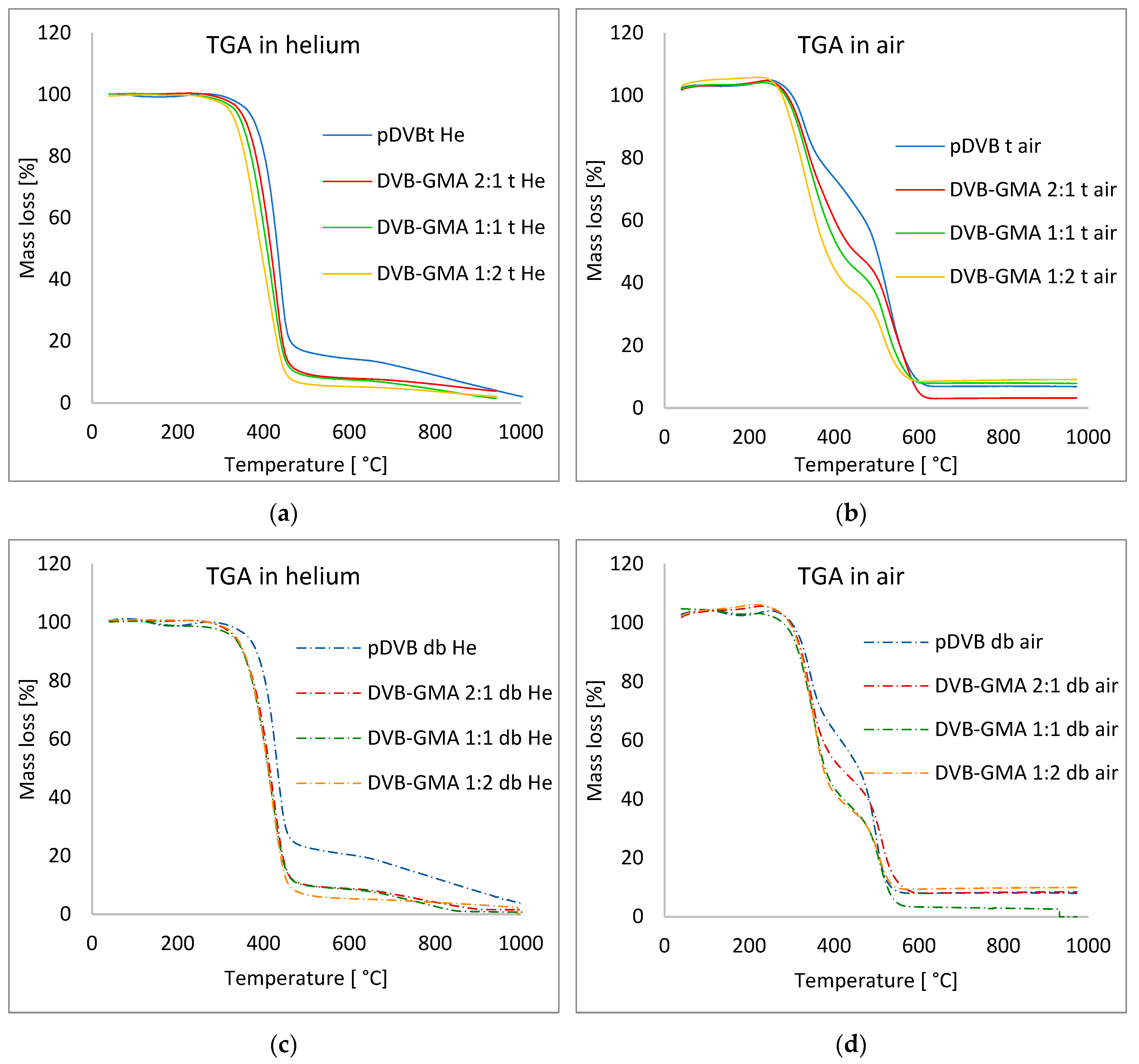
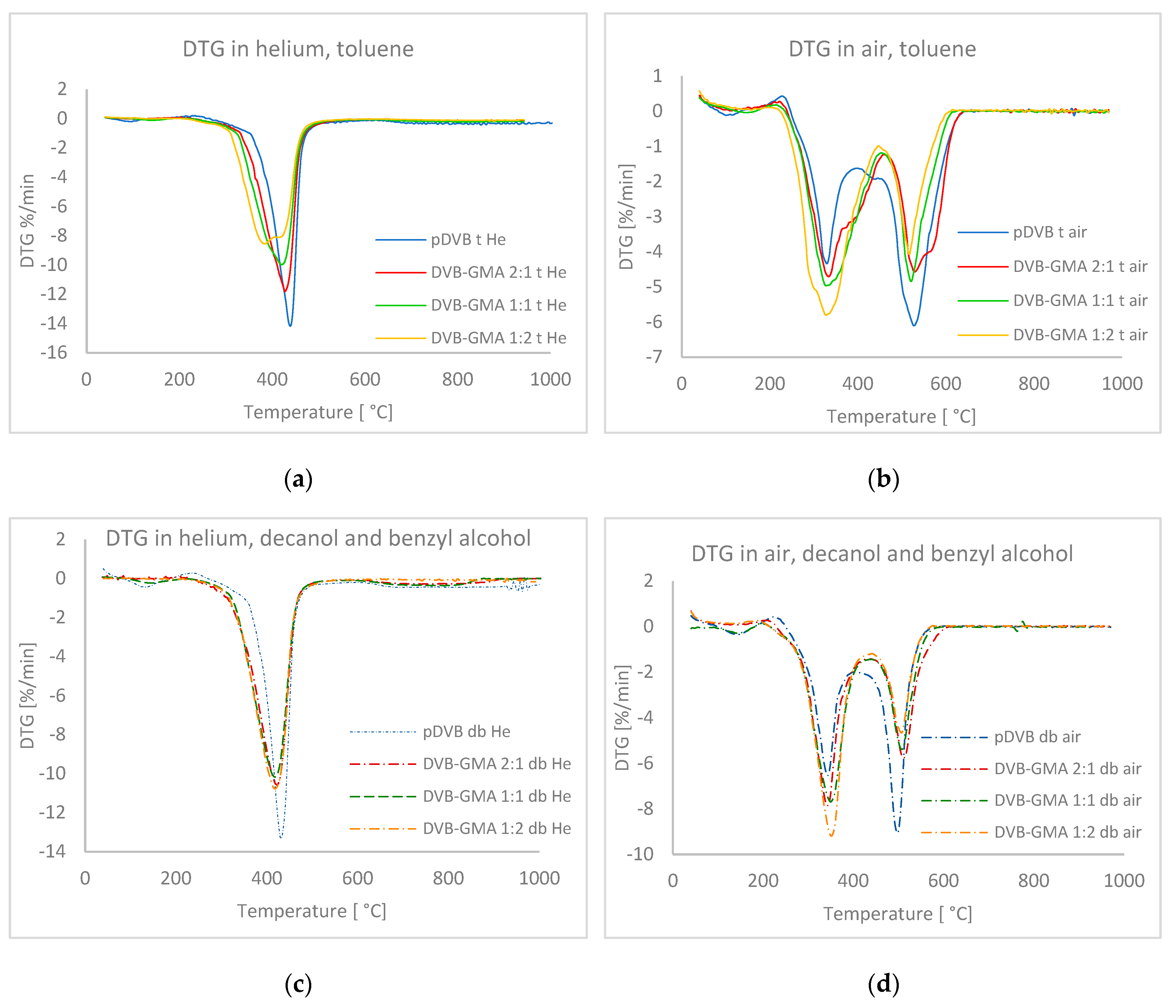
| Polymer | T2% [°C] | Trange [°C] | Tmax [°C] | DR * [°C·min−1] | Mass Loss [%] | Rm ** [%] | ||
|---|---|---|---|---|---|---|---|---|
| Atmosphere: helium | toluene | pDVB t | 331.8 | 395–458 | 439.1 | −14.18 | 82.96 | 6.0 |
| DVB-GMA 2:1 t | 316.0 | 373–453 | 428.1 | −11.80 | 87.42 | 4.30 | ||
| DVB-GMA 1:1 t | 300.7 | 357–450 | 420.8 | −9.98 | 88.38 | 4.46 | ||
| DVB-GMA 1:2 t | 288.2 | 337–446 | 384.2 | −8.57 | 90.08 | 4.38 | ||
| Mix of alcohols | pDVB db | 333.6 | 393–452 | 431.3 | −13.35 | 75.00 | 6.89 | |
| DVB-GMA 2:1 db | 307.1 | 365–451 | 423.5 | −10.58 | 86.72 | 1.62 | ||
| DVB-GMA 1:1 db | 279.4 | 361–450 | 418.0 | −10.17 | 85.73 | 0.79 | ||
| DVB-GMA 1:2 db | 311.7 | 360–451 | 418.2 | −10.78 | 93.52 | 2.42 | ||
| Atmosphere: air | toluene | pDVB t | 309.3 | 299–575 | 329.5; 527.4 | −4.34; −6.11 | 35.83; 62.16 | 4.50 |
| DVB-GMA 2:1 t | 297.8 | 290–593 | 333.3; 528.8 | −4.71; −4.57 | 53.69; 45.55 | 1.39 | ||
| DVB-GMA 1:1 t | 293.2 | 285–564 | 327.9; 520.8 | −4.97; −4.85 | 60.21; 35.87 | 4.12 | ||
| DVB-GMA 1:2 t | 283.4 | 275–577 | 327.5; 516.8 | −5.81; −4.08 | 68.60; 28.49 | 3.28 | ||
| Mix of alcohols | pDVB db | 308.5 | 299–526 | 343.0; 497.8 | −6.54; −9.07 | 44.33; 51.63 | 5.28 | |
| DVB-GMA 2:1 db | 303.8 | 303–543 | 342.9; 511.7 | −7.86; −5.77 | 57.98; 39.54 | 2.86 | ||
| DVB-GMA 1:1 db | 229.6 | 302–536 | 349.9; 507.6 | −7.69; −5.39 | 64.92; 34.88 | 0 | ||
| DVB-GMA 1:2 db | 301.9 | 307–531 | 351.0; 506.8 | −9.20; −4.66 | 69.12; 27.42 | 3.78 |
| Pore-Forming Diluent | Polymer | SBET [m2∙g−1] | Vtot [cm3∙g−1] | W [nm] |
|---|---|---|---|---|
| toluene | pDVB t | 534 | 0.56 | 5.5 |
| DVB-GMA 2:1 t | 155 | 0.16 | 5 | |
| DVB-GMA 1:1 t | 38 | 0.07 | 7 | |
| DVB-GMA1:2 t | 12 | 0.03 | 11.6 | |
| decanol and benzyl alcohol | pDVB db | 396 | 1.05 | 12.3 |
| DVB-GMA 2:1 db | 37 | 0.09 | 9.7 | |
| DVB-GMA 1:1 db | 0.2 | 0.002 | 22.1 | |
| DVB-GMA 1:2 db | 0.2 | 0.001 | 17.5 |
| Pore-Forming Diluent | Polymer | qa [mg/g] | Total Mass Adsorbed [%] | Mass Desorbed [%] | Mass of MB Retained on the Bed [%] |
|---|---|---|---|---|---|
| toluene | pDVB t | 2.02 | 69.43 | 6.53 | 93.47 |
| DVB-GMA 2:1 t | 2.25 | 69.53 | 2.04 | 97.07 | |
| DVB-GMA 1:1 t | 1.37 | 72.11 | 4.18 | 95.82 | |
| DVB-GMA 1:2 t | 0.26 | 89.61 | 0.79 | 99.21 | |
| Decanol and benzyl alcohol | pDVB db | 1.51 | 63.08 | 42.53 | 57.47 |
| DVB-GMA 2:1 db | 0.39 | 91.71 | 1.16 | 98.84 | |
| DVB-GMA 1:1 db | 5.73 | 31.77 | 32.02 | 67.98 | |
| DVB-GMA 1:2 db | 0.2 | 94.01 | 0.35 | 99.65 |
| Adsorbents | SBET [m2g−1] | C0 [mg/L] | qa [mg/g] | Recovery [%] | Reference |
|---|---|---|---|---|---|
| Silica microspheres decorated with polydopamine | 612.3 | 200 | 83.8 | 81 0.1 M HCl * | [39] |
| Hyper-cross-linked hydroxylated polystyrene (HCPS-OH 4) | 69 | 482 | 293.9 | 92.2 1 M HCl * | [40] |
| Graphite oxide coated sand | Not reported | 50 | 0.4 | Not reported | [41] |
| Eucalyptus sheathiana bark biomass | 6.55 | 50 | 30.7 | 46 0.1 M HCl/NaOH * | [42] |
| Jackfruit (Artocarpus heteropyllus) leaf powder | Not reported | 100 | 245 | Not reported | [43] |
| Zeolite | Not reported | 30 | 4.5 | Not reported | [44] |
| Rise husk | Not reported | 50 | 6.63 | Not reported | [45] |
| DVB-GMA 1:1 db | 0–500 | 1 | 0.2–5.73 | 0–42 methanol * | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobiesiak, M.; Parcheta, M. The Influence of Pore-Forming Diluents on Porous Structure, Thermal and Sorption Properties of the Divinylbenzene and Glycidyl Methacrylate Copolymers. Materials 2024, 17, 4114. https://doi.org/10.3390/ma17164114
Sobiesiak M, Parcheta M. The Influence of Pore-Forming Diluents on Porous Structure, Thermal and Sorption Properties of the Divinylbenzene and Glycidyl Methacrylate Copolymers. Materials. 2024; 17(16):4114. https://doi.org/10.3390/ma17164114
Chicago/Turabian StyleSobiesiak, Magdalena, and Monika Parcheta. 2024. "The Influence of Pore-Forming Diluents on Porous Structure, Thermal and Sorption Properties of the Divinylbenzene and Glycidyl Methacrylate Copolymers" Materials 17, no. 16: 4114. https://doi.org/10.3390/ma17164114






