Effect of Microwave Irradiation on Lead Adsorption Properties of Vermiculite with Different Particle Sizes
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Sample Preparation
2.2. Adsorption Experiment
2.3. Measurement and Characterization Techniques
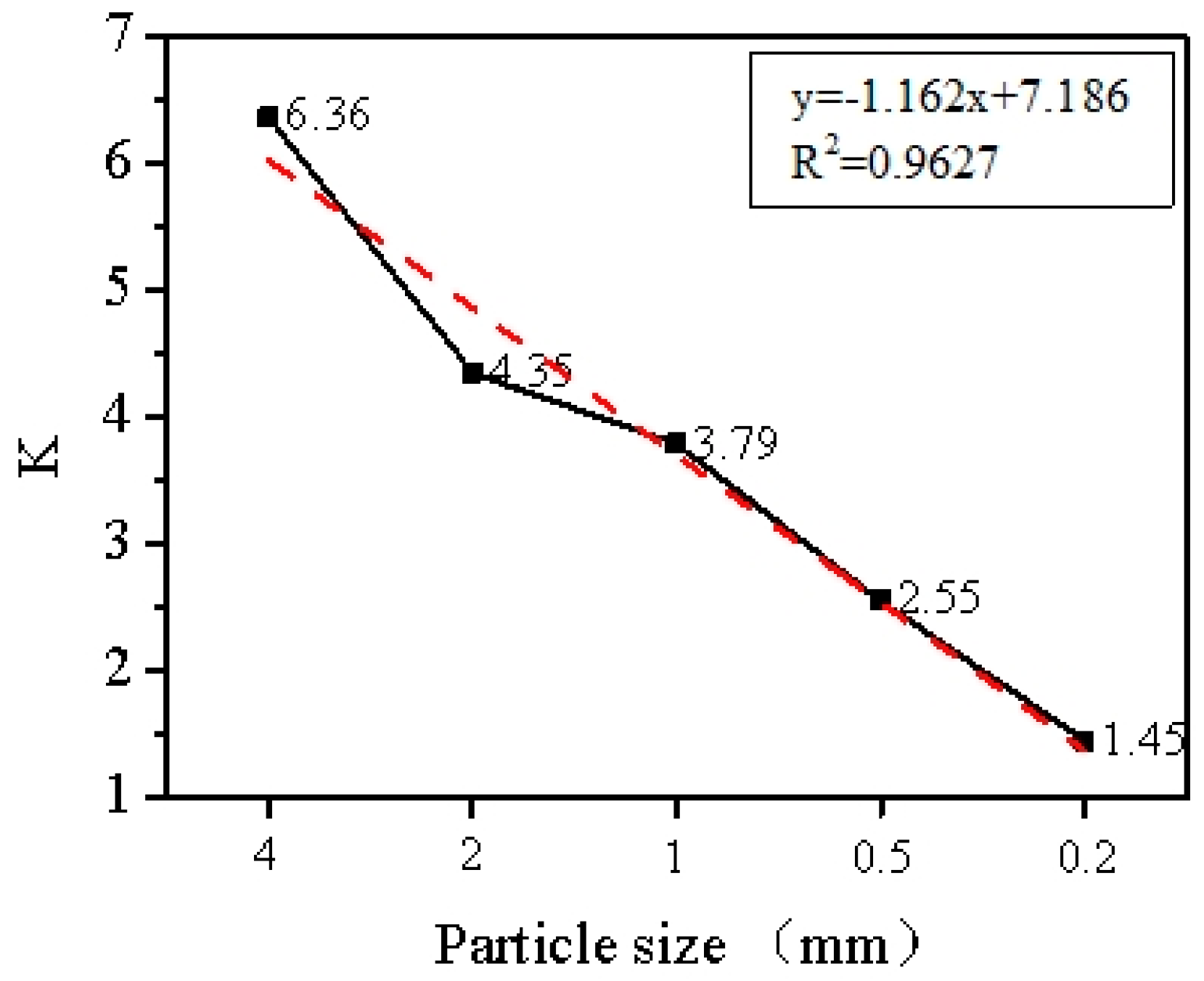
3. Results and Discussion
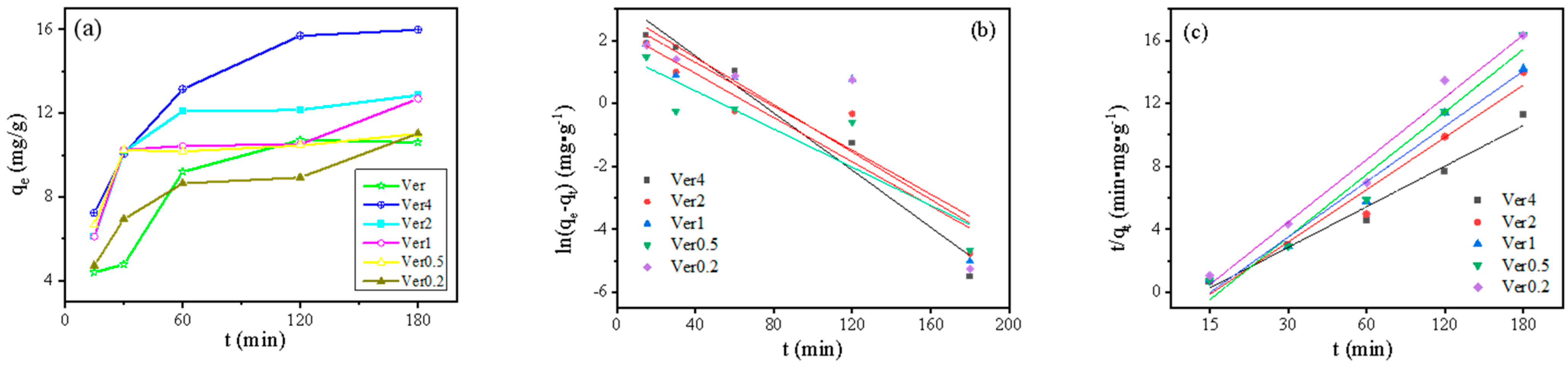
3.1. Adsorption Kinetics
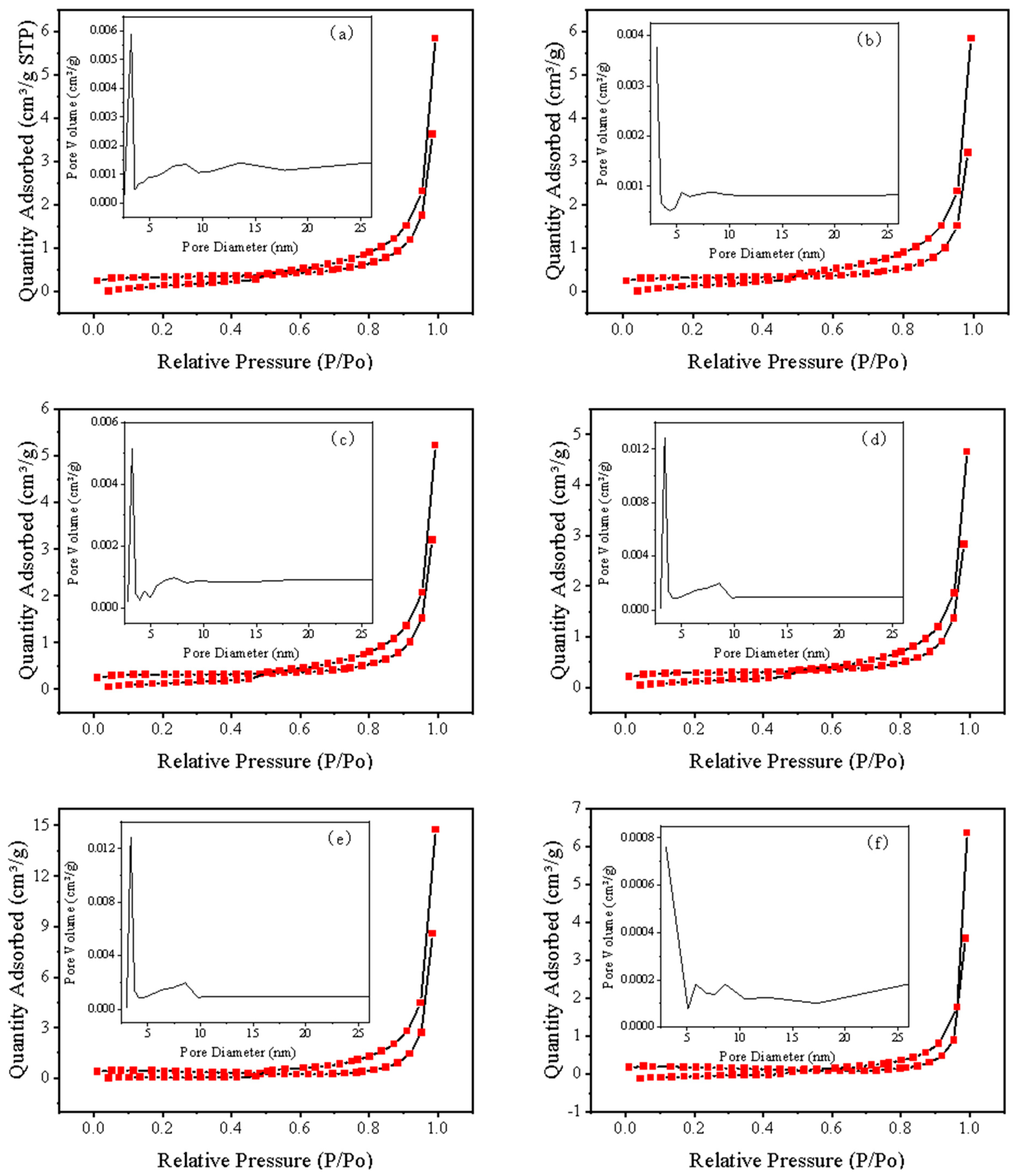
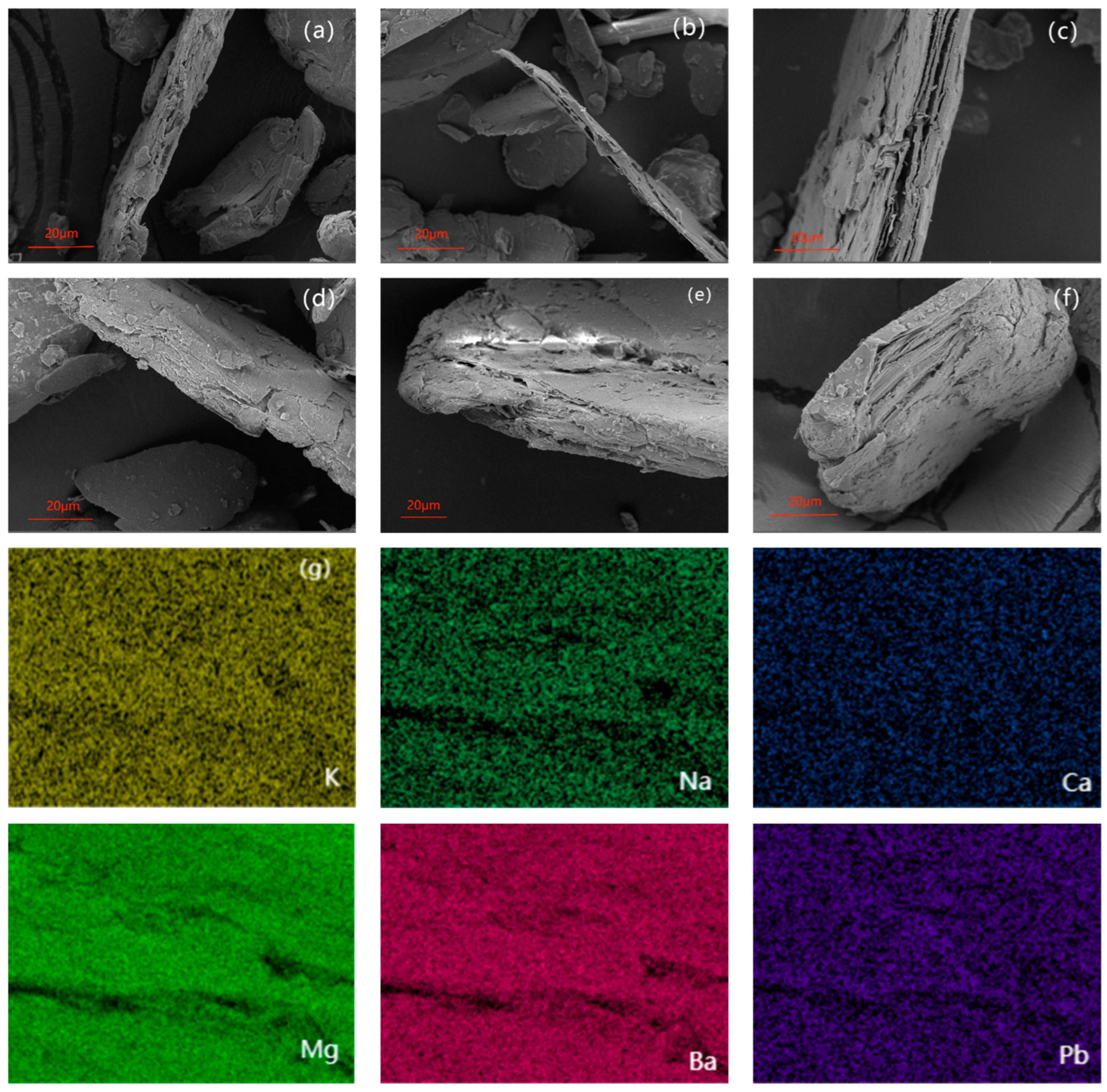
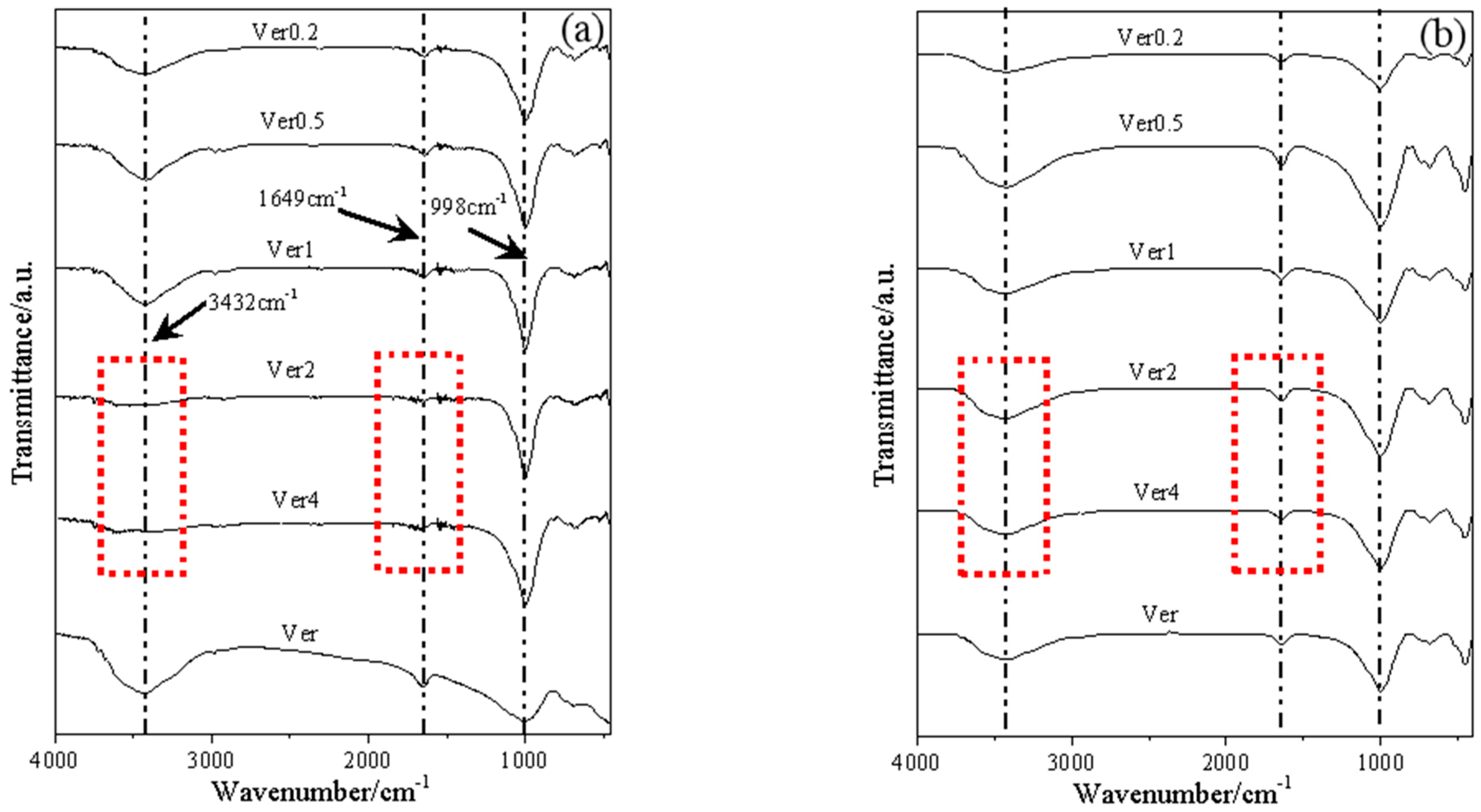
3.2. Characterization of Materials
3.3. Adsorption Mechanisms Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Alsaman, A.S.; Maher, H.; Ghazy, M.; Ali, E.S.; Askalany, A.A.; Saha, B.B. 2D materials for adsorption desalination applications: A state of the art. Therm. Sci. Eng. Prog. 2024, 49, 102455. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Węgrzyn, A.; Tsurtsumia, A.; Witkowski, S.; Freitas, O.; Figueiredo, S.; Cybińska, J.; Stawiński, W. Vermiculite as a potential functional additive for water treatment bioreactors inhibiting toxic action of heavy metal cations upsetting the microbial balance. J. Hazard. Mater. 2022, 433, 128812. [Google Scholar] [CrossRef]
- de Vargas Brião, G.; Franco, D.S.P.; da Silva, F.V.; da Silva, M.G.C.; Vieira, M.G.A. Critical rare earth metal adsorption onto expanded vermiculite: Accurate modeling through response surface methodology and machine learning techniques. Sustain. Chem. Pharm. 2023, 31, 100938. [Google Scholar] [CrossRef]
- Hou, L.; Xing, B.; Guo, H.; Zeng, H.; Cheng, S.; Meng, M.; Qu, X.; López, A.V.; Zhang, C.; Cao, Y. Effect of mineralogical characteristics evolution of vermiculite upon thermal and chemical expansions on its adsorption behavior for aqueous Pb (II) removal. Powder Technol. 2023, 430, 119040. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Expansion behaviour of commercial vermiculites at 1000 °C. Appl. Clay Sci. 2010, 48, 492–498. [Google Scholar] [CrossRef]
- Obut, A.; Girgin, I.; Yorukoglu, A. Microwave exfoliation of vermiculite and phlogopite. Clays Clay Miner. 2003, 51, 452–456. [Google Scholar] [CrossRef]
- Obut, A.; Girgin, İ. Hydrogen peroxide exfoliation of vermiculite and phlogopite. Miner. Eng. 2002, 15, 683–687. [Google Scholar] [CrossRef]
- He, Y.; Qian, Y.; Gao, H.; Wu, Y.; Ren, Z.; Zhu, X. Effects of Phsical Treatment on the Expansion Characteristics and Brittleness of Vermiculite. Bull. Chin. Ceram. Soc. 2017, 36, 564–569. [Google Scholar]
- Ritsuko, N.; Kazuhiko, T. Encouragements for the Use of Microwaves in Industrial Chemistry. Chem. Rec. 2019, 19, 51–64. [Google Scholar]
- Sun, H.; Peng, T.; Liang, Z.; Zhao, S. Study on Preparation of Organic-Montmorillonite by Microwave Irradiation. Non-Met. Mines 2006, 2, 9–11. [Google Scholar]
- Marcos, C.; Rodríguez, I. Expansibility of vermiculites irradiated with microwaves. Appl. Clay Sci. 2011, 51, 33–37. [Google Scholar] [CrossRef]
- Liu, M.; Feng, J.; Mo, W.; Su, X.; Fu, L. A novel quantitative analysis method of microwave energy absorption during vermiculite expansion process. Thermochim. Acta 2020, 691, 178718. [Google Scholar] [CrossRef]
- Huo, X.; Wu, L.; Liao, L.; Xia, Z.; Wang, L. The effect of interlayer cations on the expansion of vermiculite. Powder Technol. 2012, 224, 241–246. [Google Scholar] [CrossRef]
- Ruiz-Conde, A.; Ruiz-Amil, A.; Perez-Rodriguez, J.L.; Sánchez-Soto, P.J. Dehydration-rehydration in magnesium vermiculite: Conversion from two-one and one-two water layer hydration states through the formation of interstratified phases. J. Mater. Chem. 1996, 6, 1557–1566. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 2010, 158, 1134–1146. [Google Scholar] [CrossRef]
- JC/T 441-2009; Expanded Vermiculite. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2009.
- Peng, T.; Liu, F. Exchangeable cations and exchange capacity of interstratified minerals with vermiculite layers. Acta Petrol. Et Mineral. 2003, 22, 391–396. [Google Scholar]
- Zhao, S.; Meng, Z.; Fan, X.; Jing, R.; Yang, J.; Shao, Y.; Liu, X.; Wu, M.; Zhang, Q.; Liu, A. Removal of heavy metals from soil by vermiculite supported layered double hydroxides with three-dimensional hierarchical structure. Chem. Eng. J. 2020, 390, 124554. [Google Scholar] [CrossRef]
- Subramanian, N.; Nielsen Lammers, L. Thermodynamics of ion exchange coupled with swelling reactions in hydrated clay minerals. J. Colloid Interface Sci. 2022, 608, 692–701. [Google Scholar] [CrossRef]
- Mathieson, A.M.; Walker, G.F. Crystal structure of magnesium–vermiculite. Am. Mineral. 1954, 3, 231–255. [Google Scholar]
- Du, Y.; Chen, C.; Fan, Y.; Wang, J. A New Method Study on Preparation of Vermiculite with High Expansion Rate. Conserv. Util. Miner. Resour. 2008, 5, 13–17. [Google Scholar]
- Ghanem, N.; Mihoubi, D.; Kechaou, N.; Mihoubi, N.B. Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crops Prod. 2012, 40, 167–177. [Google Scholar] [CrossRef]
- Yan, B.; Chen, T.; Tao, Y.; Zhang, N.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Conformation and hydration property of low-acetyl gellan gum under microwave irradiation: Experiments and molecular dynamics simulations. Food Hydrocoll. 2023, 145, 109140. [Google Scholar] [CrossRef]
- Suquet, H.; Franck, R.; Lambert, J.F.; Elsass, F.; Marcilly, C.; Chevalier, S. Catalytic properties of two pre-cracking matrices: A leached vermiculite and a Al-pillared saponite. Appl. Clay Ence 1994, 8, 349–364. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, P.; Zhu, L.; Hua, M.; Huang, Y.; Chao, Y.; Wu, P.; Qiu, Z.; Zhu, W. Construction of hydrophilic hydroxyl-rich porous organic polymers for efficient removal of heavy metal ions. Inorg. Chem. Commun. 2023, 153, 110821. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zhang, Y.; Huang, H.; Ou, H.; Zhang, Y. In-situ adsorption-conversion recovery of heavy metal cadmium by natural clay mineral for multi-functional photocatalysis. Sep. Purif. Technol. 2023, 319, 124058. [Google Scholar] [CrossRef]


| Component | SiO2 | MgO | Al2O3 | Fe2O3 | K2O | Na2O | TiO2 | CaO | F | BaO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 38.32 | 20.18 | 12.34 | 7.36 | 4.86 | 1.50 | 1.39 | 1.26 | 0.16 | 0.10 | 12.53 |
| qe/ (mg·g−1) | Pseudo First Order Model | Pseudo Second Order Model | |||||
|---|---|---|---|---|---|---|---|
| qe,cal1/ (mg·−1) | K1/min−1 | R2 | qe,cal2/ (mg·−1) | K2/(g·mg−1·min−1) | R2 | ||
| Ver4 | 15.98 | 23.54 | −1.84 | 0.84 | 15.42 | −9.06 × 10−3 | 0.98 |
| Ver2 | 12.87 | 18.49 | −1.47 | 0.82 | 13.31 | −1.35 × 10−2 | 0.96 |
| Ver1 | 12.68 | 20.91 | −1.39 | 0.63 | 13.19 | −1.39 × 10−2 | 0.97 |
| Ver0.5 | 11.01 | 18.99 | −1.26 | 0.76 | 11.32 | −1.74 × 10−2 | 0.97 |
| Ver0.2 | 11.02 | 29.37 | −1.49 | 0.64 | 11.36 | −1.36 × 10−2 | 0.96 |
| Samples | K+ | Na+ | Ca2+ | Mg2+ | Ba2+ | CEC (1) | CEC (2) |
|---|---|---|---|---|---|---|---|
| Ver | 0.7734 | 31.64 | 1.427 | 0.1017 | 38.93 | 73.00 | 74.20 |
| Ver4 | 0.8361 | 22.35 | 0.9300 | 0.0688 | 31.13 | 55.47 | 56.40 |
| Ver2 | 0.9146 | 26.53 | 1.100 | 0.0791 | 31.79 | 60.57 | 64.60 |
| Ver1 | 0.9893 | 27.18 | 1.114 | 0.0730 | 33.62 | 63.57 | 66.60 |
| Ver0.5 | 0.8425 | 31.29 | 1.057 | 0.0807 | 35.56 | 68.98 | 67.40 |
| Ver0.2 | 0.9087 | 27.27 | 0.9717 | 0.0865 | 41.55 | 70.94 | 73.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, H.; Peng, T.; Ding, W.; Yin, H. Effect of Microwave Irradiation on Lead Adsorption Properties of Vermiculite with Different Particle Sizes. Materials 2024, 17, 4152. https://doi.org/10.3390/ma17164152
Chen Y, Sun H, Peng T, Ding W, Yin H. Effect of Microwave Irradiation on Lead Adsorption Properties of Vermiculite with Different Particle Sizes. Materials. 2024; 17(16):4152. https://doi.org/10.3390/ma17164152
Chicago/Turabian StyleChen, Yunzhu, Hongjuan Sun, Tongjiang Peng, Wenjin Ding, and Hongmei Yin. 2024. "Effect of Microwave Irradiation on Lead Adsorption Properties of Vermiculite with Different Particle Sizes" Materials 17, no. 16: 4152. https://doi.org/10.3390/ma17164152





