Graphene–Liquid Crystal Synergy: Advancing Sensor Technologies across Multiple Domains
Abstract
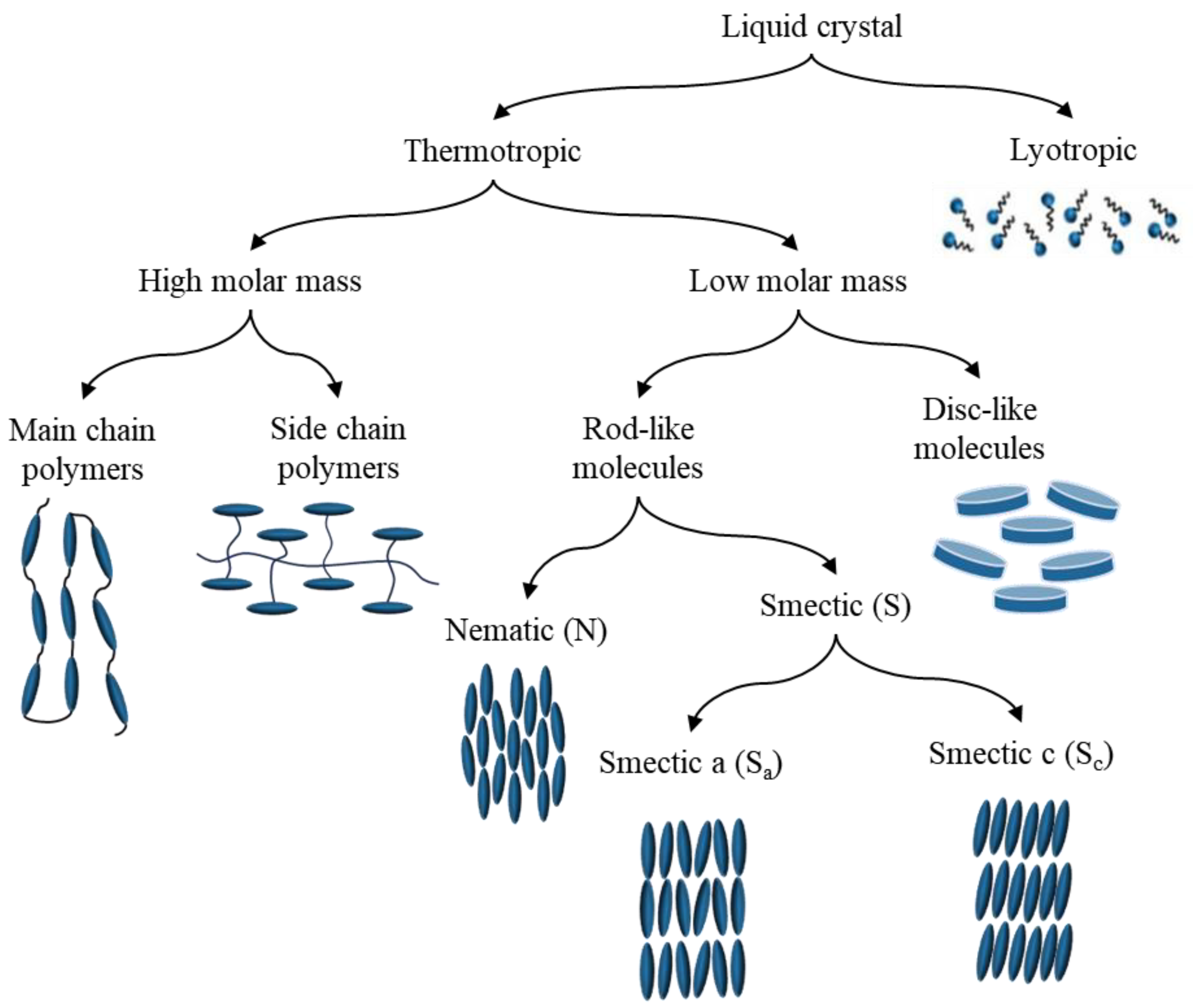
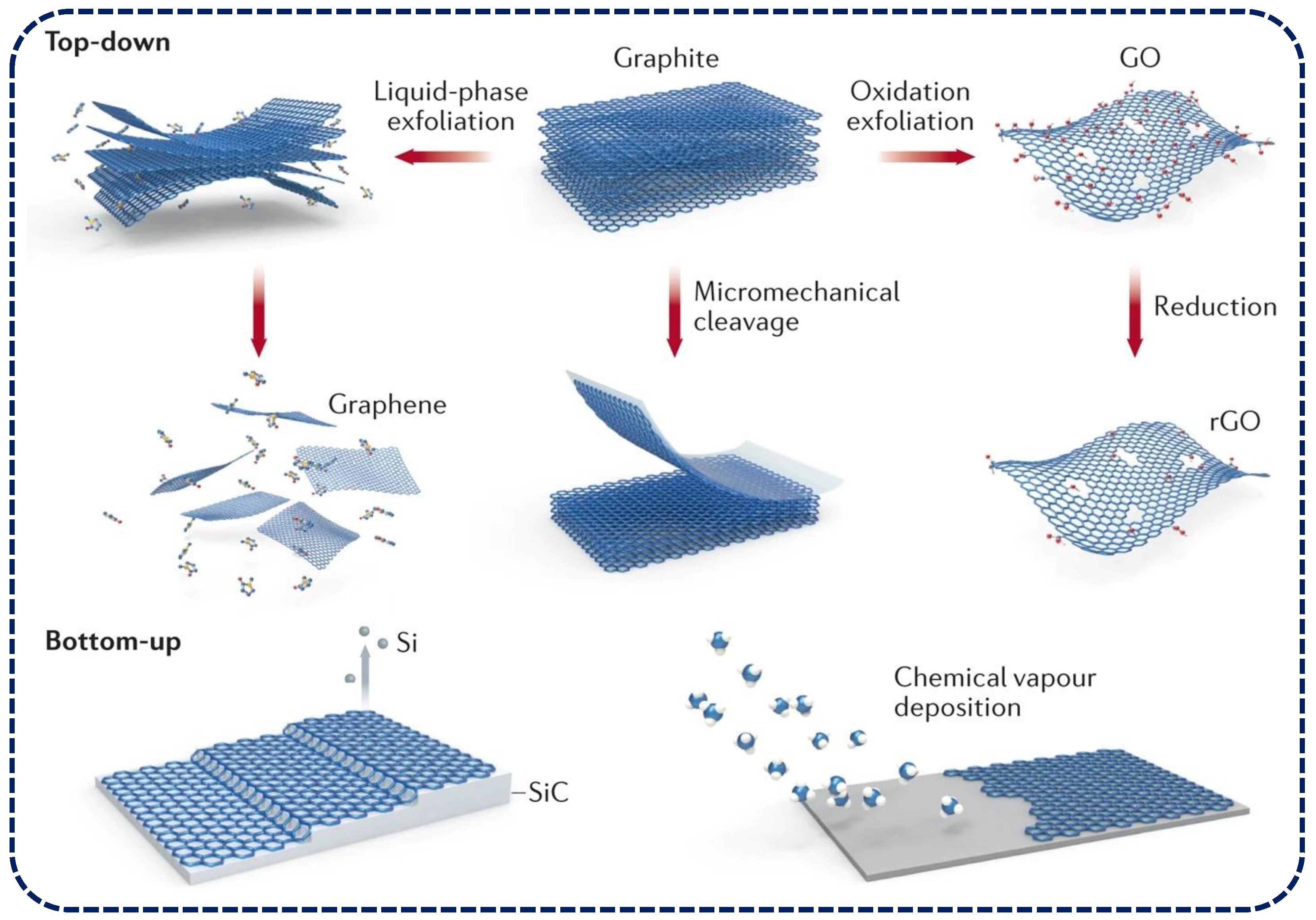
1. Introduction
2. Graphene–Liquid Crystal Composites in Sensor Applications
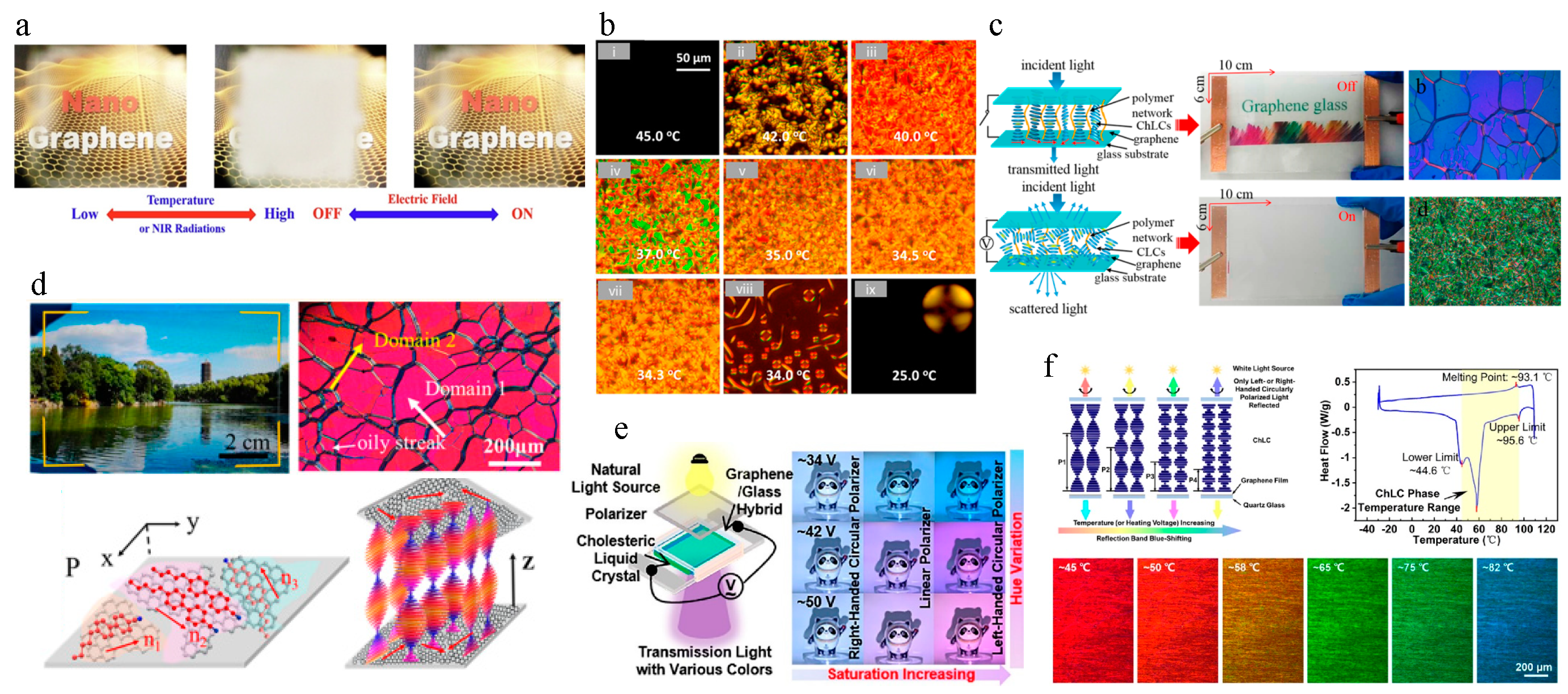
2.1. Thermal and Infrared Sensing
2.2. Flexible and Wearable Actuators
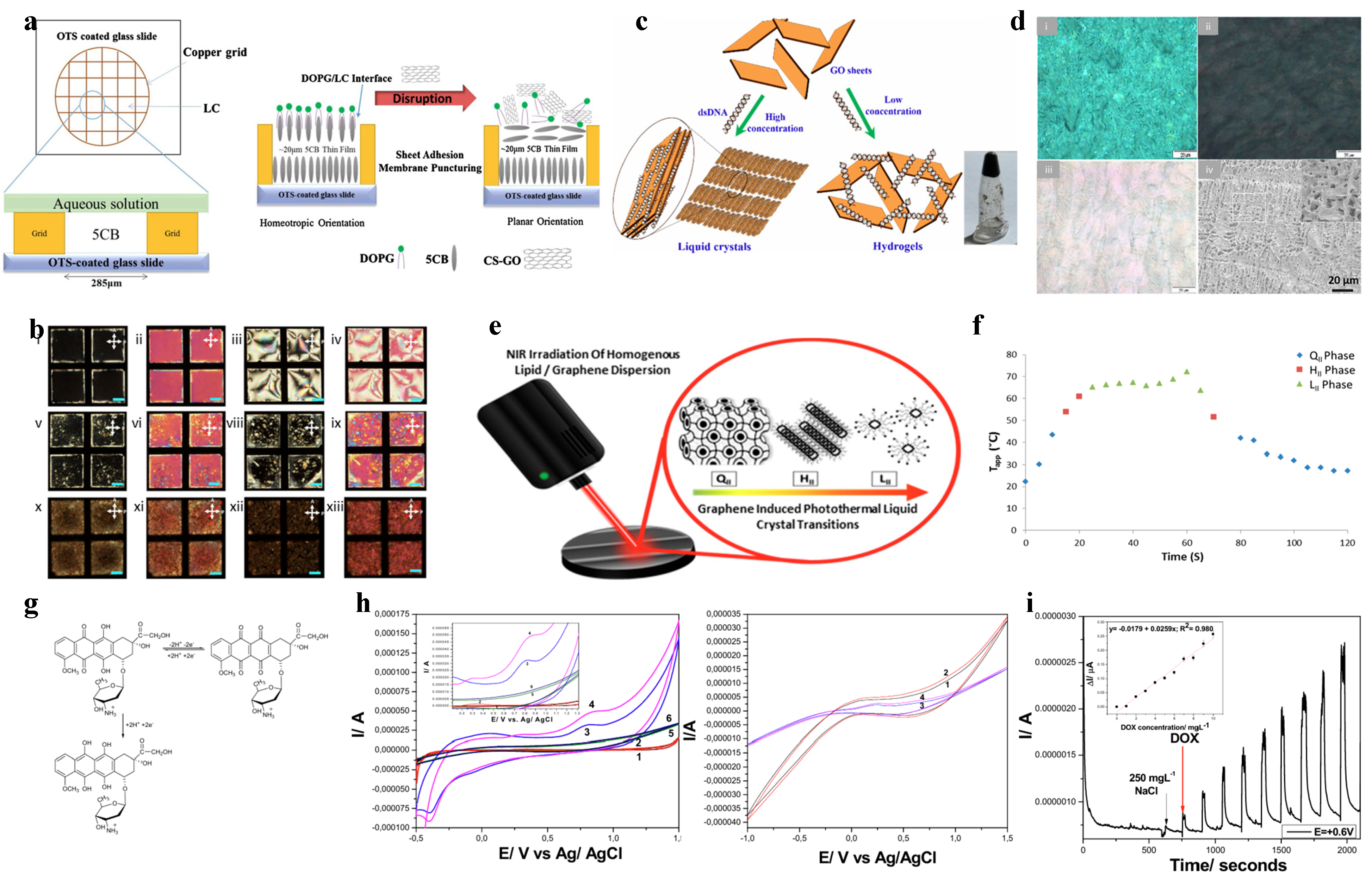
2.3. Chemical and Biological Detection
2.4. Environmental Monitoring Systems
3. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashok Kumar, S.S.; Bashir, S.; Ramesh, K.; Ramesh, S. A Review on Graphene and Its Derivatives as the Forerunner of the Two-Dimensional Material Family for the Future. J. Mater. Sci. 2022, 57, 12236–12278. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, B.; Gao, C.; Guo, J.; Zhao, C.; Jia, C.; Guo, X. Graphene: Preparation, Tailoring, and Modification. Exploration 2023, 3, 20210233. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of Thermally and Chemically Reduced Graphene Oxides by Thermal Analysis and Raman Spectroscopy. J. Therm. Anal. Calorim. 2020, 142, 331–337. [Google Scholar] [CrossRef]
- Bagani, K.; Ray, M.K.; Satpati, B.; Ray, N.R.; Sardar, M.; Banerjee, S. Contrasting Magnetic Properties of Thermally and Chemically Reduced Graphene Oxide. J. Phys. Chem. C 2014, 118, 13254–13259. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Li, X.; You, H.; Li, X.; Pei, C.; Huang, X.; Li, H. Direct CVD Growth of MoS2 on Chemically and Thermally Reduced Graphene Oxide Nanosheets for Improved Photoresponse. APL Mater. 2021, 9, 051105. [Google Scholar] [CrossRef]
- Priestly, E. Introduction to Liquid Crystals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 1-4684-2175-1. [Google Scholar]
- Collings, P.J.; Goodby, J.W. Introduction to Liquid Crystals: Chemistry and Physics; Crc Press: Boca Raton, FL, USA, 2019; ISBN 1-315-09834-2. [Google Scholar]
- Stewart, I.W. The Static and Dynamic Continuum Theory of Liquid Crystals: A Mathematical Introduction; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1-315-27258-X. [Google Scholar]
- Kumar, M.B.; Kang, D.; Jung, J.; Park, H.; Hahn, J.; Choi, M.; Bae, J.-H.; Kim, H.; Park, J. Compact Vari-Focal Augmented Reality Display Based on Ultrathin, Polarization-Insensitive, and Adaptive Liquid Crystal Lens. Opt. Lasers Eng. 2020, 128, 106006. [Google Scholar] [CrossRef]
- Bharath Kumar, M.; Awwal Adeshina, M.; Kang, D.; Jee, Y.; Kim, T.; Choi, M.; Park, J. Enhancement of Birefringence in Reduced Graphene Oxide Doped Liquid Crystal. Nanomaterials 2020, 10, 842. [Google Scholar] [CrossRef]
- Dierking, I.; San, S.E. Magnetically Steered Liquid Crystal-Nanotube Switch. Appl. Phys. Lett. 2005, 87, 233507. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Draude, A.P.; Dierking, I. Thermotropic Liquid Crystals with Low-Dimensional Carbon Allotropes. Nano Express 2021, 2, 012002. [Google Scholar] [CrossRef]
- Therrien, B. Thermotropic Liquid-Crystalline Materials Based on Supramolecular Coordination Complexes. Inorganics 2019, 8, 2. [Google Scholar] [CrossRef]
- Ula, S.W.; Traugutt, N.A.; Volpe, R.H.; Patel, R.R.; Yu, K.; Yakacki, C.M. Liquid Crystal Elastomers: An Introduction and Review of Emerging Technologies. Liq. Cryst. Rev. 2018, 6, 78–107. [Google Scholar] [CrossRef]
- Herbert, K.M.; Fowler, H.E.; McCracken, J.M.; Schlafmann, K.R.; Koch, J.A.; White, T.J. Synthesis and Alignment of Liquid Crystalline Elastomers. Nat. Rev. Mater. 2021, 7, 23–38. [Google Scholar] [CrossRef]
- Kim, H.; Gibson, J.; Maeng, J.; Saed, M.O.; Pimentel, K.; Rihani, R.T.; Pancrazio, J.J.; Georgakopoulos, S.V.; Ware, T.H. Responsive, 3D Electronics Enabled by Liquid Crystal Elastomer Substrates. ACS Appl. Mater. Interfaces 2019, 11, 19506–19513. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3-527-30725-7. [Google Scholar]
- Kumar, M.; Gowda, A.; Kumar, S. Discotic Liquid Crystals with Graphene: Supramolecular Self-assembly to Applications. Part. Part. Syst. Charact. 2017, 34, 1700003. [Google Scholar] [CrossRef]
- Adeshina, M.A.; Kumar, M.B.; Kang, D.; Choi, B.; Park, J. Impact of Electric Field on Propagation Velocity of Phase Boundary Between Nematic and Isotropic Phases of 5CB Liquid Crystal. J. Sens. Sci. Technol. 2019, 28, 341–344. [Google Scholar] [CrossRef]
- Kim, W.; Im, J.; Kim, H.; Choi, J.; Choi, Y.; Kim, Y. Liquid Crystalline Systems from Nature and Interaction of Living Organisms with Liquid Crystals. Adv. Mater. 2023, 35, 2204275. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on Graphene and Its Derivatives: Synthesis Methods and Potential Industrial Implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Wang, X. Applications of Graphene-Based Materials in Sensors: A Review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision Synthesis versus Bulk-Scale Fabrication of Graphenes. Nat. Rev. Chem. 2017, 2, 0100. [Google Scholar] [CrossRef]
- Adeshina, M.A.; Lee, H.; Mareddi, B.; Kang, D.; Ogunleye, A.M.; Kim, H.; Kim, T.; Choi, M.; Park, H.; Park, J. Liquid Phase IR Detector Based on the Photothermal Effect of Reduced Graphene Oxide-Doped Liquid Crystals. Nanoscale 2023, 15, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, Y.; Zeng, X.; Xiao, R.; Lü, P.; Wang, L.; Lu, Y. Visual Measurement of the Microscopic Temperature of Porous Graphene Based on Cholesteric Liquid Crystal Microcapsules. Chin. Opt. Lett. 2020, 18, 031201. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Sayed, F.A.; Aly, A.H. Graphene Deposited Liquid Crystal and Thermal Sensitivity Using Photonic Crystals. Phys. Scr. 2021, 96, 035503. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Shu, J.; Cao, W.; Li, C.; Cao, M. Graphene Implanted Shape Memory Polymers with Dielectric Gene Dominated Highly Efficient Microwave Drive. Adv. Funct. Mater. 2023, 33, 2303560. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, W.; Peng, R.; He, C.; Pang, X.; Shi, D.; Jiang, T.; Lin, Z. Graphene-Enabled Superior and Tunable Photomechanical Actuation in Liquid Crystalline Elastomer Nanocomposites. Adv. Mater. 2015, 27, 6376–6381. [Google Scholar] [CrossRef]
- De Jesús-Téllez, M.A.; Felix-Serrano, I.; Rodríguez-González, R.J.; Navarro-Rodríguez, D.; Larios-López, L. Effect of Gold and Graphene Oxide Nanoparticles on the Thermo- and Photo-Actuation of Monodomain Liquid Crystal Elastomers. Polymer 2020, 205, 122837. [Google Scholar] [CrossRef]
- Wei, R.; Wang, Z.; Zhang, H.; Liu, X. Photo-Responsive Liquid Crystalline Elastomer with Reduced Chemically Modified Graphene Oxide. Liq. Cryst. 2016, 43, 1009–1016. [Google Scholar] [CrossRef]
- Marotta, A.; Lama, G.C.; Ambrogi, V.; Cerruti, P.; Giamberini, M.; Gentile, G. Shape Memory Behavior of Liquid-Crystalline Elastomer/Graphene Oxide Nanocomposites. Compos. Sci. Technol. 2018, 159, 251–258. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Liu, Y.; Xu, Y.; Zhang, A. NIR–UV Responsive Actuator with Graphene Oxide/Microchannel-Induced Liquid Crystal Bilayer Structure for Biomimetic Devices. ACS Appl. Mater. Interfaces 2020, 12, 6727–6735. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, C.-H. Liquid Crystal as Sensing Platforms for Determining the Effect of Graphene Oxide-Based Materials on Phospholipid Membranes and Monitoring Antibacterial Activity. Sens. Actuators B Chem. 2018, 254, 72–80. [Google Scholar] [CrossRef]
- Kurapati, R.; Reddy, U.V.; Raichur, A.M.; Suryaprakash, N. Facile Synthesis of Graphene Oxide/Double-Stranded DNA Composite Liquid Crystals and Hydrogels. J. Chem. Sci. 2016, 128, 325–330. [Google Scholar] [CrossRef]
- Quinn, M.D.J.; Du, J.; Boyd, B.J.; Hawley, A.; Notley, S.M. Lipid Liquid-Crystal Phase Change Induced through near-Infrared Irradiation of Entrained Graphene Particles. Langmuir 2015, 31, 6605–6609. [Google Scholar] [CrossRef] [PubMed]
- Motoc Ilies, S.; Schinteie, B.; Pop, A.; Negrea, S.; Cretu, C.; Szerb, E.I.; Manea, F. Graphene Quantum Dots and Cu(I) Liquid Crystal for Advanced Electrochemical Detection of Doxorubicine in Aqueous Solutions. Nanomaterials 2021, 11, 2788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bisoyi, H.K.; Zheng, Z.; Gutierrez-Cuevas, K.G.; Singh, G.; Kumar, S.; Bunning, T.J.; Li, Q. Stimuli-Directed Self-Organized Chiral Superstructures for Adaptive Windows Enabled by Mesogen-Functionalized Graphene. Mater. Today 2017, 20, 230–237. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Wang, L.; Chen, X.; Chen, Z.; Qi, Y.; Cui, G.; Xie, H.; Zhang, Y.; Liu, Z. Graphene Glass Inducing Multidomain Orientations in Cholesteric Liquid Crystal Devices toward Wide Viewing Angles. ACS Nano 2018, 12, 6443–6451. [Google Scholar] [CrossRef]
- Zhou, F.; Lan, R.; Li, Z.; Liu, B.; Xie, Q.; Bao, J.; Liu, J.; Gao, P.; Yang, H.; Zhang, Y.; et al. Graphene/Cholesteric Liquid-Crystal-Based Electro-Driven Thermochromic Light Modulators toward Wide-Gamut Dynamic Light Color-Tuning-Related Applications. Nano Lett. 2023, 23, 4617–4626. [Google Scholar] [CrossRef]
- Politano, G.G.; Filice, F.; Versace, C. Alignment of Nematic Liquid Crystal 5CB Using Graphene Oxide. Crystals 2023, 13, 1500. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeshina, M.A.; Ogunleye, A.M.; Lee, H.; Mareddi, B.; Kim, H.; Park, J. Graphene–Liquid Crystal Synergy: Advancing Sensor Technologies across Multiple Domains. Materials 2024, 17, 4431. https://doi.org/10.3390/ma17174431
Adeshina MA, Ogunleye AM, Lee H, Mareddi B, Kim H, Park J. Graphene–Liquid Crystal Synergy: Advancing Sensor Technologies across Multiple Domains. Materials. 2024; 17(17):4431. https://doi.org/10.3390/ma17174431
Chicago/Turabian StyleAdeshina, Mohammad A., Abdulazeez M. Ogunleye, Hakseon Lee, Bharathkumar Mareddi, Hyunmin Kim, and Jonghoo Park. 2024. "Graphene–Liquid Crystal Synergy: Advancing Sensor Technologies across Multiple Domains" Materials 17, no. 17: 4431. https://doi.org/10.3390/ma17174431
APA StyleAdeshina, M. A., Ogunleye, A. M., Lee, H., Mareddi, B., Kim, H., & Park, J. (2024). Graphene–Liquid Crystal Synergy: Advancing Sensor Technologies across Multiple Domains. Materials, 17(17), 4431. https://doi.org/10.3390/ma17174431






