Investigation of Microstructure and Interfacial Reactions of Diffusion Bonding of Ni-Ti6Al4V Materials Joined by Using Ag Interlayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ni Samples
2.2. Diffusion Bonding of Ni-Ti6Al4V by Using the Ag Interlayer
2.3. Microstructure Analysis, Microhardness, and Lap-Shear Tests
3. Results and Discussion
3.1. Microstructure
3.2. Microhardness
3.3. EDS and X-ray Diffraction Analysis
3.4. The Lap-Shear Test
4. Conclusions
- Hardness changes in the regions are in parallel with the microstructure. It has been determined that low hardness values are associated with the silver-rich interlayer region.
- Ag diffusion into the main materials was observed at rates varying between 10 and 40 µm in the samples assembled at 900 and 950 °C. In EDS point analyses, it was generally observed that the diffusion of the Ag interlayer into the main material on the Ti6Al4V side was more intense, and the diffusion of Ni into the Ag interlayer was more intense on the Ni side, and the NiAg phase was also frequent.
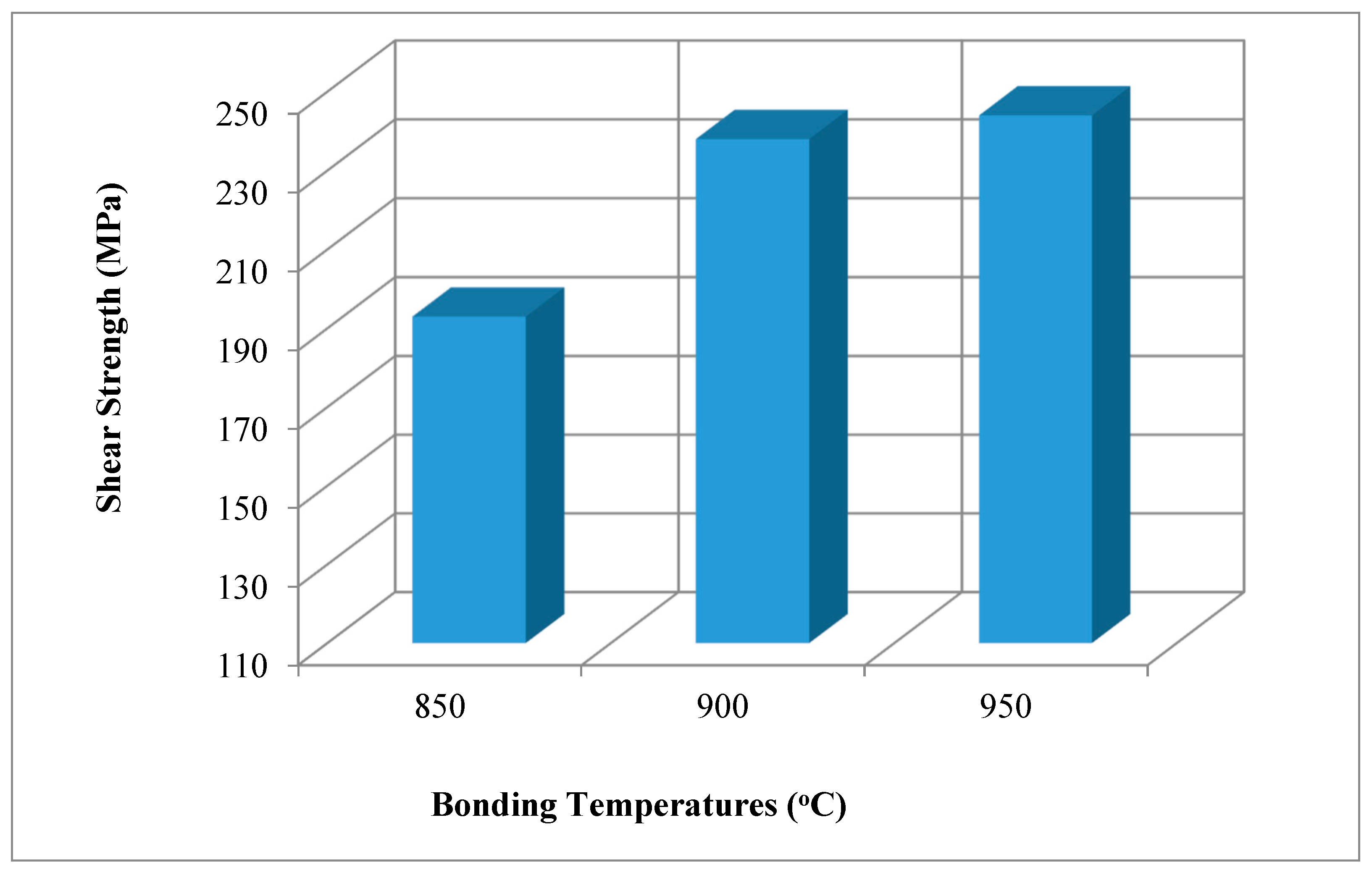
- The highest shear strength value was obtained from the sample joined at the 950 °C test condition. Higher test temperature increased the deformation at contact, and this also increased the amount of diffusion. By increasing the welding temperature, the lap-shear strength can be increased.
- For all the experiments, the SEM and EDS results show that reliable joints along the interface can be achieved at the process temperature of 950 °C for 60 min under 5 MPa pressure condition. This can be associated with the fact that the phases Ti92Ni33Al17 and Ag11Ni16 were more common at joints made at 950 °C.
- As the bonding time increased, due to continuous diffusion and the structural heterogeneity disappeared in the nickel materials obtained by the powder metallurgy method, the pores gradually decreased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.; Geng, Y.; Zeng, X.; Gao, Z.; Song, X. Uncovering the features of nickel flows in China. Resour. Conserv. Recycl. 2023, 188, 106702. [Google Scholar] [CrossRef]
- Astafurova, E.; Reunova, K.; Zagibalova, E.; Astapov, D.; Astafurov, S.; Kolubaev, E. Microstructure, Phase Composition, and Mechanical Properties of Intermetallic Ni-Al-Cr Material Produced by Dual-Wire Electron-Beam Additive Manufacturing. Metals 2024, 14, 75. [Google Scholar] [CrossRef]
- Duan, W.; Sun, Y.; Liu, C.; Liu, S.; Li, Y.; Ding, C.; Yu, L. Study on the formation mechanism of the glaze film formed on Ni/Ag composites. Tribol. Int. 2016, 95, 324–332. [Google Scholar] [CrossRef]
- Yang, D.; Sun, H.; Ji, G.; Xiang, Y.; Wang, J. Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy. Coatings 2024, 14, 615. [Google Scholar] [CrossRef]
- Güney, J.; Kejanlı, H. Combination of SX Steel with Copper Intermediate and GR5 (Ti6Al4V) Titanium Alloy by Diffusion Welding. DUJE 2023, 14, 315–323. [Google Scholar] [CrossRef]
- Guezmil, M.; Salem, A.; Bensalah, W.; Mezlini, S. Investigation of the Tribological Behavior of TiAl6V4 for Bio-application. Arab. J. Sci. Eng. 2023, 49, 10561–10571. [Google Scholar] [CrossRef]
- Gobbo, V.A.; Lallukka, M.; Gamna, F.; Prato, M.; Vitale, A.; Ferraris, S.; Spriano, S. Functionalization of a chemically treated Ti6Al4V-ELI alloy with nisin for antibacterial purposes. Appl. Surf. Sci. 2023, 620, 156820. [Google Scholar] [CrossRef]
- Gil, F.J.; Ginebra, M.P.; Manero, J.M.; Planell, J.A. Formation of a-Widmanstätten structure: Effects of grain size and cooling rate on the Widmanstätten morphologies and on the mechanical properties in Ti6Al4V alloy. J. Alloys Compd. 2001, 329, 142–152. [Google Scholar] [CrossRef]
- Kejanli, H. Diffusion welding of stainless steel 304L/Monel K-500 composite materials produced with different methods. Adv. Compos. Lett. 2020, 29, 2633366X20917983. [Google Scholar] [CrossRef]
- Berry, T.; Hoppin, G. Activated diffusion bonding (Combined vacuum furnace brazing with diffusion welding for joining high strength Ni base superalloys). Weld. J. Res. Suppl. 1970, 49. [Google Scholar]
- Akca, E.; Gürsel, A. The importance of interlayers in diffusion welding—A review. Period. Eng. Nat. Sci. 2015, 3, 12–16. [Google Scholar] [CrossRef]
- Lee, C.S.; Li, H.; Chandel, R.S. Vacuum-free diffusion bonding of aluminium metal matrix composite. J. Mater. Process Technol. 1999, 89–90, 326–330. [Google Scholar] [CrossRef]
- Cooke, K.O.; Atieh, A.M. Current trends in dissimilar diffusion bonding of titanium alloys to stainless steels, aluminium and magnesium. J. Manuf. Mater. Proc. 2020, 4, 39. [Google Scholar] [CrossRef]
- Peters, M.; Hemptenmacher, J.; Kumpfert, J.; Leyens, C. Structure and properties of titanium and titanium alloys. In Titanium and Titanium Alloys: Fundamentals and Applications; Leyens, C., Peters, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–36. ISBN 9783527602117. [Google Scholar] [CrossRef]
- Henriques, V.A.R.; Campos, P.P.D.; Cairo, C.A.A.; Bressiani, J.C. Production of titanium alloys for advanced aerospace systems by powder metallurgy. Mater. Res. 2005, 8, 443–446. [Google Scholar] [CrossRef]
- Choe, H.; Abkowitz, S.M.; Abkowitz, S.; Dunand, D.C. Effect of tungsten additions on the mechanical properties of Ti-6Al-4V. Mater. Sci. Eng. A 2005, 396, 99–106. [Google Scholar] [CrossRef]
- Yin, M.G.; Cai, Z.B.; Li, Z.Y.; Zhou, Z.R.; Wang, W.J.; He, W.F. Improving impact wear resistance of Ti-6Al-4V alloy treated by laser shock peening. Trans. Nonferrous Met. Soc. China 2019, 29, 1439–1448. [Google Scholar] [CrossRef]
- Jiang, P.; He, X.L.; Li, X.A.; Yu, L.G.; Wang, H.M. Wear resistance of a laser surface alloyed Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 24–28. [Google Scholar] [CrossRef]
- Dalili, N.; Edrisy, A.; Farokhzadeh, K.; Li, J.; Lo, J.; Riahi, A.R. Improving the wear resistance of Ti–6Al–4V/TiC composites through thermal oxidation (TO). Wear 2010, 269, 590–601. [Google Scholar] [CrossRef]
- Tsai, C.H.; Huang, W.C.; Kao, C.R. Development of Ag–In alloy pastes by mechanical alloying for die attachment of high-power semiconductor devices. Materials 2022, 15, 1397. [Google Scholar] [CrossRef]
- Coutu, R.A., Jr.; Kladitis, P.E.; Leedy, K.D.; Crane, R.L. Selecting metal alloy electric contact materials for MEMS switches. J. Micromech. Microeng. 2004, 14, 1157. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, S.; Li, J.G.; Chen, J.; Xie, M.; Li, X.; Sun, X. Morphology-controllable synthesis and thermal decomposition of Ag and Ni oxalate for Ag-Ni alloy electrical contact materials. Mater. Des. 2016, 108, 640–647. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, S.; Sun, X.; Xie, M.; Li, J.; Li, X.; Liu, M. The effects of citric acid on the synthesis and performance of silver–tin oxide electrical contact materials. J. Alloys Compd. 2014, 588, 30–35. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, Y.H. Adhesive strength and tensile fracture of Ni particle enhanced Sn–Ag composite solder joints. Mater. Sci. Eng. A 2006, 419, 172–180. [Google Scholar] [CrossRef]
- Wang, J.; Hu, D.; Zhu, Y.; Guo, P. Electrical properties of in situ synthesized Ag-graphene/Ni composites. Materials 2022, 15, 6423. [Google Scholar] [CrossRef]
- Ozoliņš, V.; Wolverton, C.; Zunger, A. Cu-Au, Ag-Au, Cu-Ag, and Ni-Au intermetallics: First-principles study of temperature-composition phase diagrams and structures. Phys. Rev. B 1998, 57, 6427. [Google Scholar] [CrossRef]
- Samavatian, M.; Zakipour, S.; Paidar, M. Effect of bonding pressure on microstructure and mechanical properties of Ti-6Al-4V diffusion-bonded joint. Weld. World 2017, 61, 69–74. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Wang, B.; Gao, C.; Sun, Z.; He, P. Dissimilar metal joining of Q235 mild steel to Ti6Al4V via resistance spot welding with Ni–Cu interlayer. J. Mater. Res. Technol. 2021, 15, 4086–4101. [Google Scholar] [CrossRef]
- Chen, T.; Liu, F.; Pang, L.; Hu, H.; Gao, P. Microstructure and performance study of Al/Cu Laser welding with Ag interlayer. Int. J. Precis. Eng. Manuf. 2024, 25, 79–89. [Google Scholar] [CrossRef]
- Pietrzak, K.; Frydman, K.; Wojcik-Grzybek, D.; Gładki, A.; Bańkowska, A.; Borkowski, P. Effect of carbon forms on properties of Ag-C composites contact materials. Mater. Sci. 2018, 24, 69–74. [Google Scholar] [CrossRef]
- Iltaf, A.; Barka, N.; Dehghan, S. Microstructural evolution and the nanomechanical response of fiber laser-welded dissimilar joints of AA7075–Ti–6Al–4 V alloys using Ag as an interlayer. J. Adv. Manuf. Technol. 2024, 132, 3939–3964. [Google Scholar] [CrossRef]
- Lis, A.; Leinenbach, C. Effect of process and service conditions on TLP-bonded components with (Ag, Ni–) Sn interlayer combinations. J. Electron. Mater. 2015, 44, 4576–4588. [Google Scholar] [CrossRef]
- Gayathri, S.; Kumar, N.; Krishnan, R.; Ravindran, T.R.; Dash, S.; Tyagi, A.K.; Sridharan, M. Tribological properties of pulsed laser deposited DLC/TM (TM = Cr, Ag, Ti and Ni) multilayers. Tribol. Int. 2012, 53, 87–97. [Google Scholar] [CrossRef]
- Barrena, M.I.; Matesanz, L.; de Salazar, J.M.G. Al2O3/Ti6Al4V diffusion bonding joints using Ag-Cu interlayer. Mater. Charact. 2009, 60, 1263–1267. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Hua, P.; Wang, M.; Zhou, W. Nickel interlayer on the microstructure and property of TC6 to copper alloy diffusion bonding. J. Adhes. Sci. Technol. 2018, 32, 1548–1559. [Google Scholar] [CrossRef]
- Taskin, M.; Kejanli, H.; Caligulu, U. The Effect on The Connecting Charasteristic of Welding Temperatures on The Joining with The Diffusion Bonding Using Ni And Cu Interlayer of Ni-Ti-Cu Composites Manufactured By Powder Metallurgy Method. E-J. New World Sci. Acad. 2008, 3, 556–568, ISSN 1306-3111. [Google Scholar]
- de Castilho, B.C.; Munagala, V.N.V.; Alidokht, S.A.; Sharifi, N.; Bessette, S.; Makowiec, M.E.; Chromik, R.R. Insights on silver migration mechanisms and their influence on the wear behavior of thermally sprayed self-lubricating coatings up to 350 °C. Tribol. Lett. 2022, 70, 120. [Google Scholar] [CrossRef]
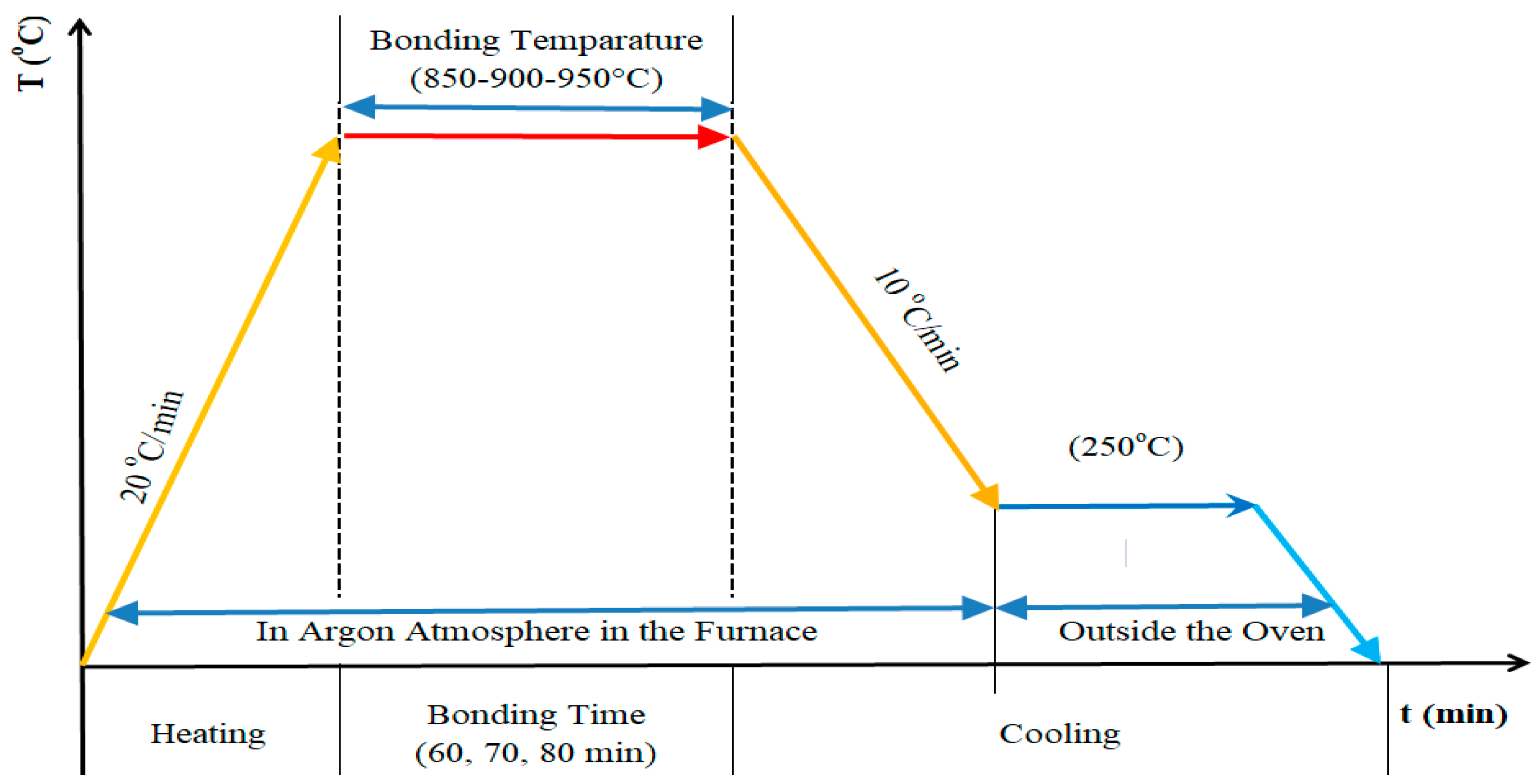
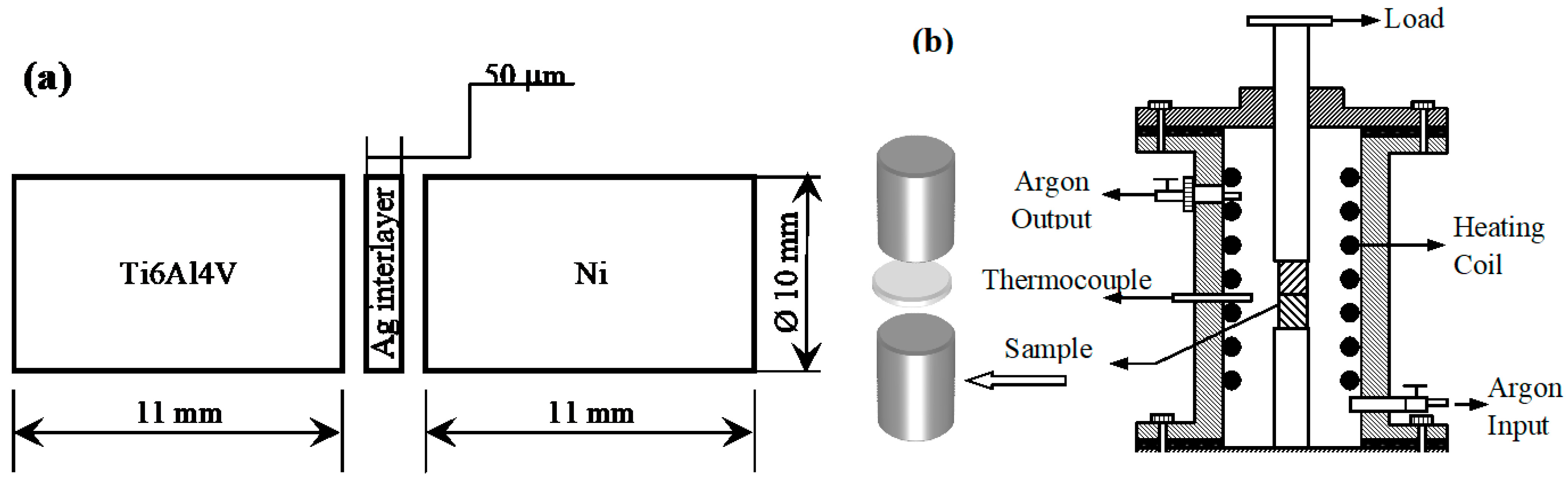

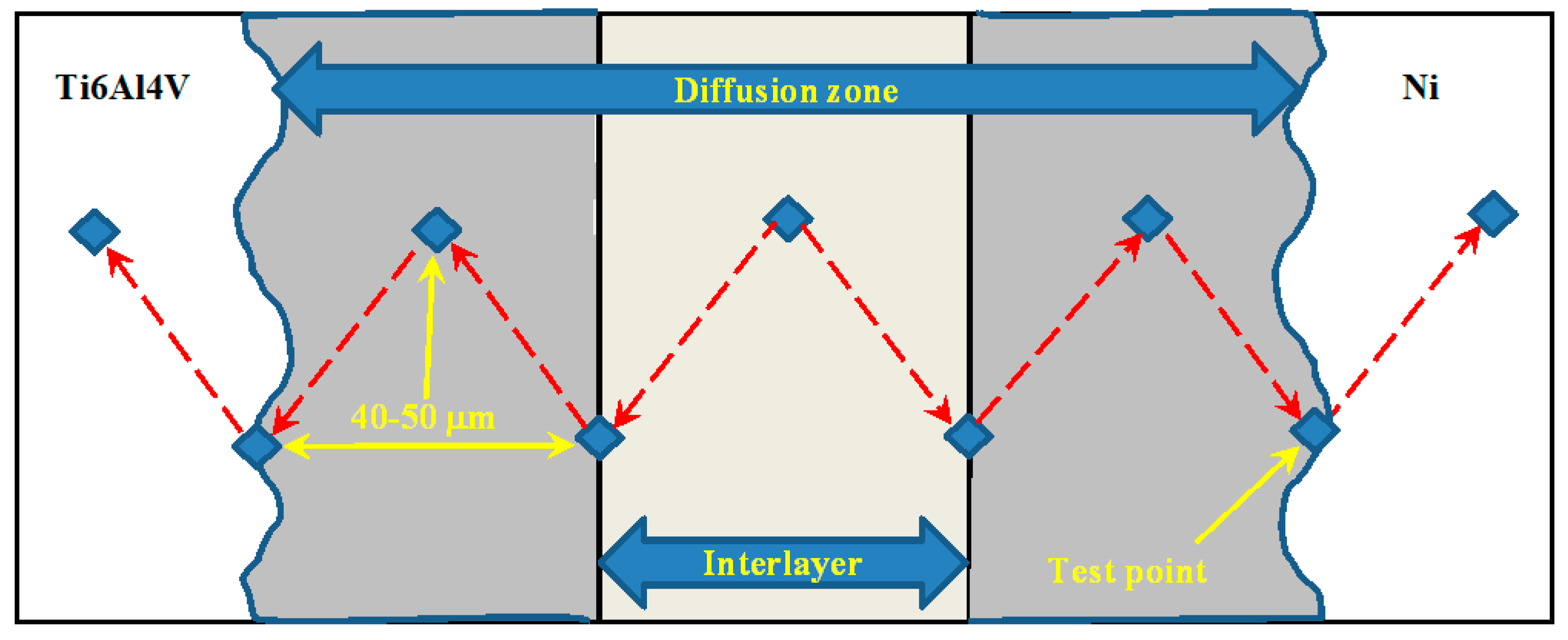
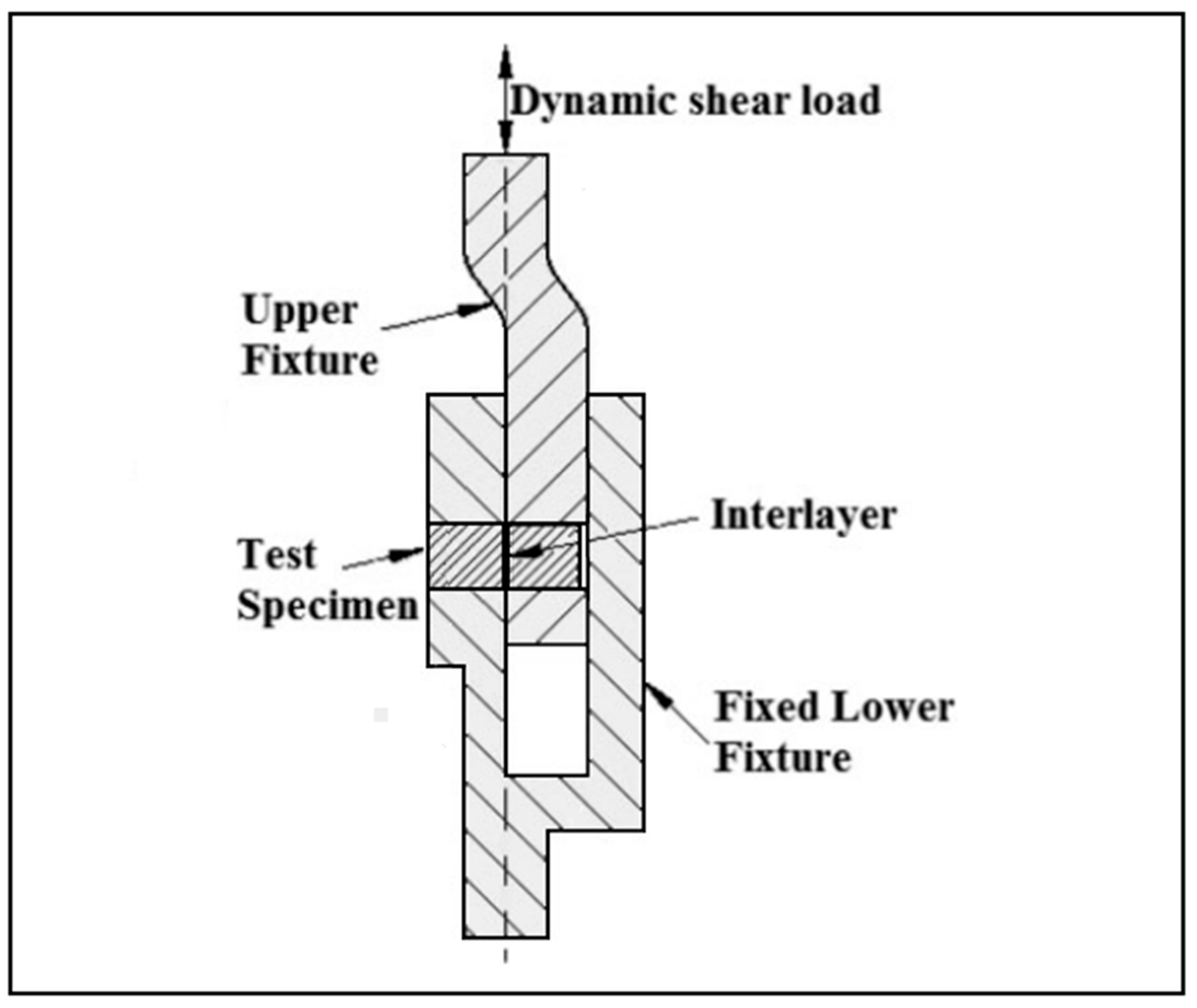


| Elements | Ti | Al | V | H (max.%) | Fe (max.) |
|---|---|---|---|---|---|
| (at.%) | 88.74–91 | 5.5–6.75 | 3.5–4.5 | 0.015 | 0.25 |
| Specimen No | N1 | N2 | N3 |
|---|---|---|---|
| Bonding pressure (MPa) | 5 | 5 | 5 |
| Bonding temperature (°C) | 850 | 900 | 950 |
| Holding time (min) | 60 | 60 | 60 |
| Test Point | Elemental Ratio (at.%) | Possible Phase | ||||
|---|---|---|---|---|---|---|
| Al | Ti | V | Ni | Ag | ||
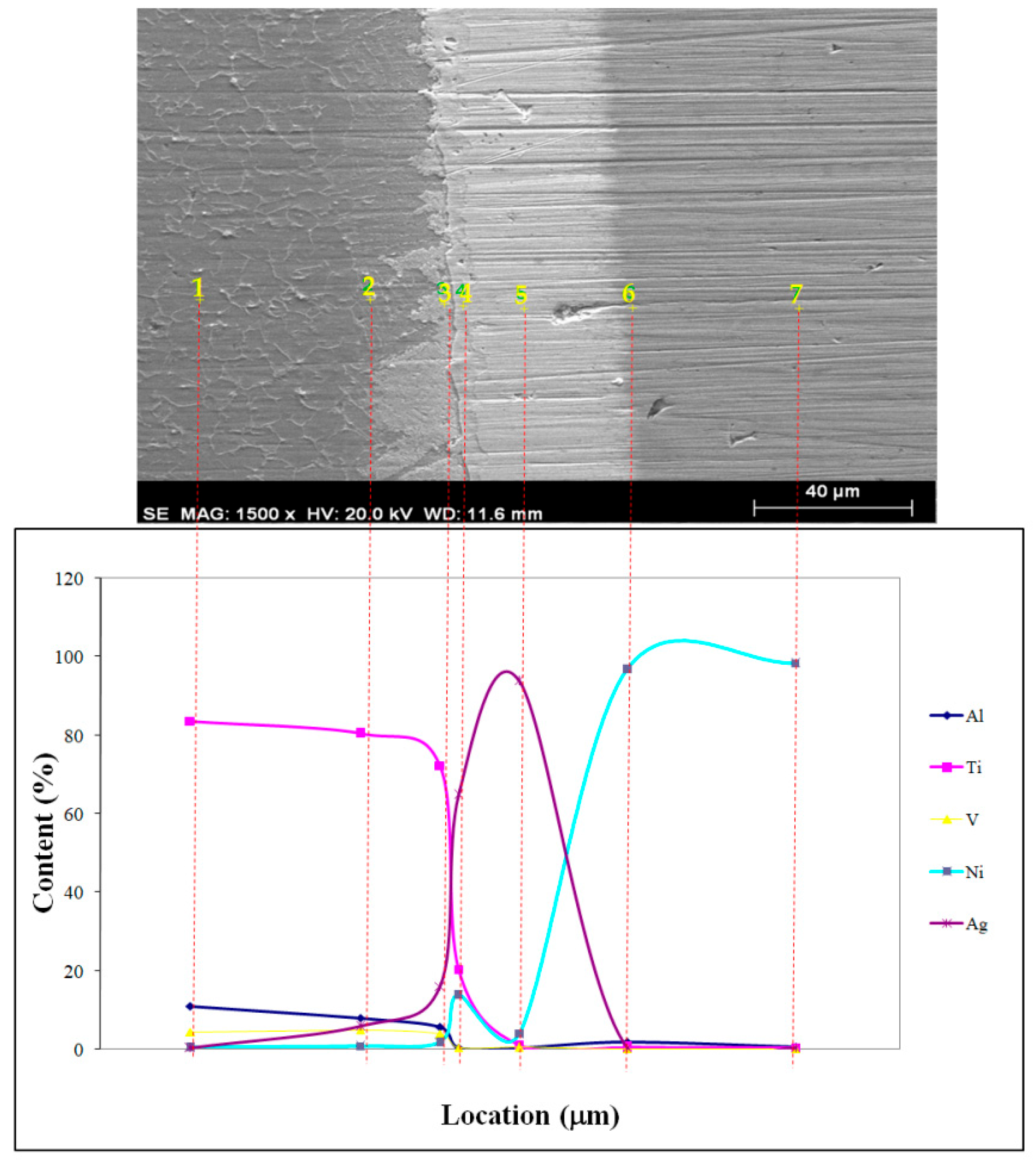
| 1 | 11.08 | 83.6 | 4.44 | 0.61 | 0.27 | Ti642Al151V32Ag3 |
| 2 | 7.91 | 80.44 | 4.96 | 0.82 | 5.87 | Ti156Al27V9Ag5 |
| 3 | 5.69 | 72.27 | 4 | 1.92 | 16.11 | - |
| 4 | 0.45 | 20.31 | 0.46 | 13.99 | 64.8 | Ag58Ti41Ni23 |
| 5 | 0.41 | 1.22 | 0.53 | 4.01 | 93.83 | Ag140Ni11 |
| 6 | 1.9 | 0.55 | 0.24 | 96.91 | 0.39 | Rich in Ni |
| 7 | 0.6 | 0.44 | 0.2 | 98.38 | 0.39 | Rich in Ni |
| Test Point | Elemental Ratio (at.%) | Possible Phase | ||||
|---|---|---|---|---|---|---|
| Al | Ti | V | Ni | Ag | ||
| 8 | 11.74 | 84.07 | 3.27 | 0.65 | 0.27 | Ti363Al90V17 |
| 9 | 9.56 | 76.71 | 5.67 | 1.37 | 6.69 | - |
| 10 | 1.49 | 3.98 | 0.57 | 8.69 | 85.27 | Ag224Ni41Ti23 |
| 11 | 0.59 | 0.8 | 0.52 | 8.87 | 89.22 | Ag11Ni2 |
| 12 | 1.93 | 2.9 | 0.52 | 7.11 | 87.55 | Ag80Ni12Ti11 |
| 13 | 0.22 | 0.5 | 0.26 | 98.56 | 0.45 | Rich in Ni |
| Test Point | Elemental Ratio (at.%) | Possible Phase | ||||
|---|---|---|---|---|---|---|
| Al | Ti | V | Ni | Ag | ||
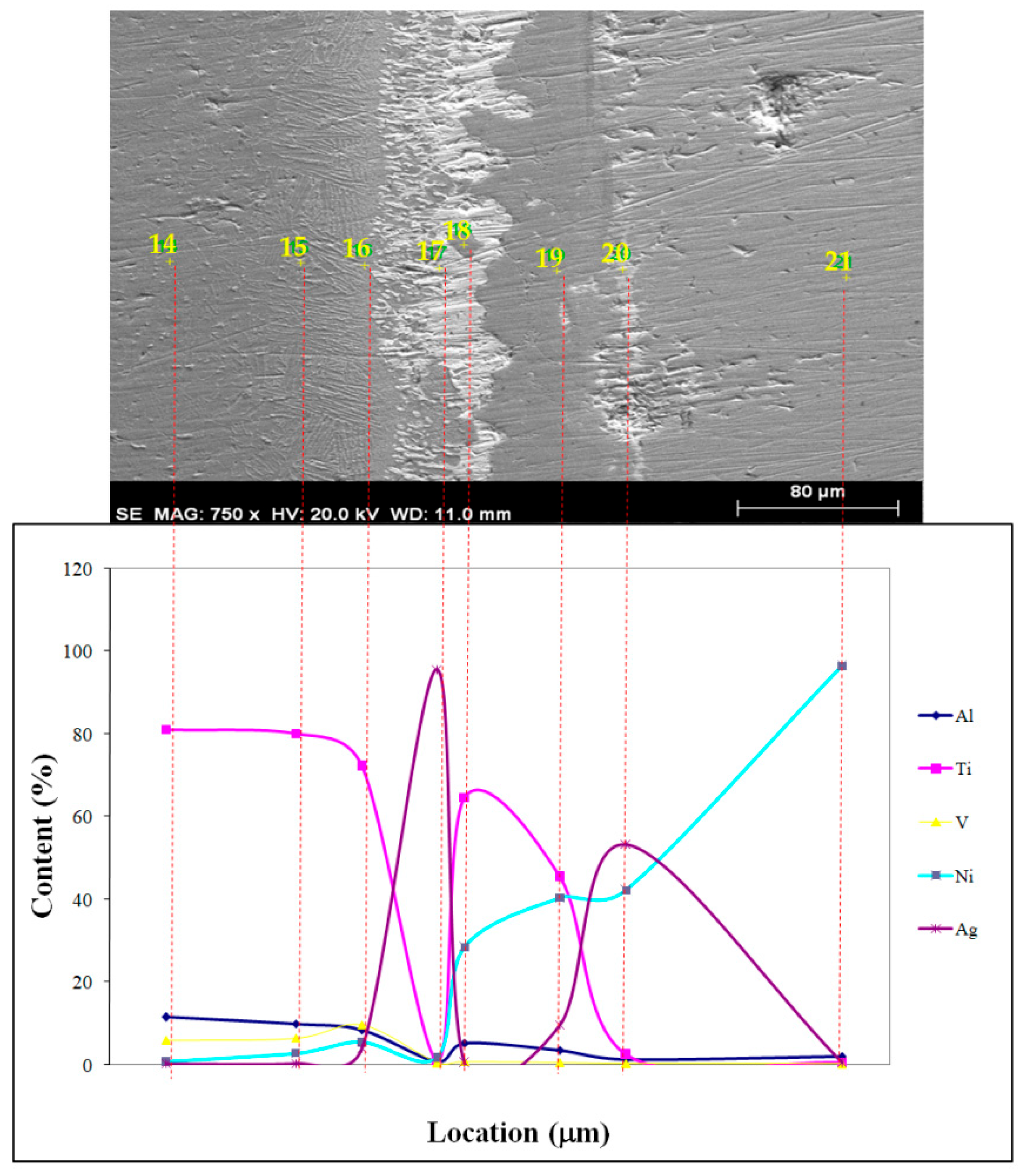
| 14 | 11.56 | 81.19 | 6.04 | 0.89 | 0.33 | Ti12Al3V |
| 15 | 9.95 | 80.17 | 6.49 | 2.94 | 0.46 | Ti725Al147V23 |
| 16 | 8.44 | 72.19 | 9.7 | 5.54 | 4.13 | |
| 17 | 0.76 | 1.19 | 0.56 | 1.87 | 95.62 | Rich in Ag |
| 18 | 5.3 | 64.76 | 0.9 | 28.49 | 0.55 | Ti92Ni33Al17 |
| 19 | 3.57 | 45.45 | 0.73 | 40.44 | 9.81 | Ni16Ag11 |
| 20 | 1.43 | 2.53 | 0.43 | 42.21 | 53.4 | |
| 21 | 2.11 | 0.59 | 0.28 | 96.51 | 0.51 | Rich in Ni |
| Specimens | Temperatures | Durations (min.) | Lap-Shear (MPa) |
|---|---|---|---|
| N1 | 850 °C | 60 | 193 |
| N2 | 900 °C | 60 | 238 |
| N3 | 950 °C | 60 | 244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çetinkaya, Ş.; Kejanli, H. Investigation of Microstructure and Interfacial Reactions of Diffusion Bonding of Ni-Ti6Al4V Materials Joined by Using Ag Interlayer. Materials 2024, 17, 4462. https://doi.org/10.3390/ma17184462
Çetinkaya Ş, Kejanli H. Investigation of Microstructure and Interfacial Reactions of Diffusion Bonding of Ni-Ti6Al4V Materials Joined by Using Ag Interlayer. Materials. 2024; 17(18):4462. https://doi.org/10.3390/ma17184462
Chicago/Turabian StyleÇetinkaya, Şükrü, and Haluk Kejanli. 2024. "Investigation of Microstructure and Interfacial Reactions of Diffusion Bonding of Ni-Ti6Al4V Materials Joined by Using Ag Interlayer" Materials 17, no. 18: 4462. https://doi.org/10.3390/ma17184462






