Convenient Reparation of SiC-Coated C/C Composites by the Slurry Painting Method
Abstract
:1. Introduction
2. Experiment
2.1. Preparation and Pretreatment
2.2. Repair of Damaged Coating
2.2.1. Initial Repair by Heat Treatment Method
2.2.2. Slurry Brushing–Preoxidation Method for Further Repair
2.2.3. Process of Repairing Damaged Coating
2.3. Microstructure Characterization
2.4. Oxidation Test
3. Results and Discussion
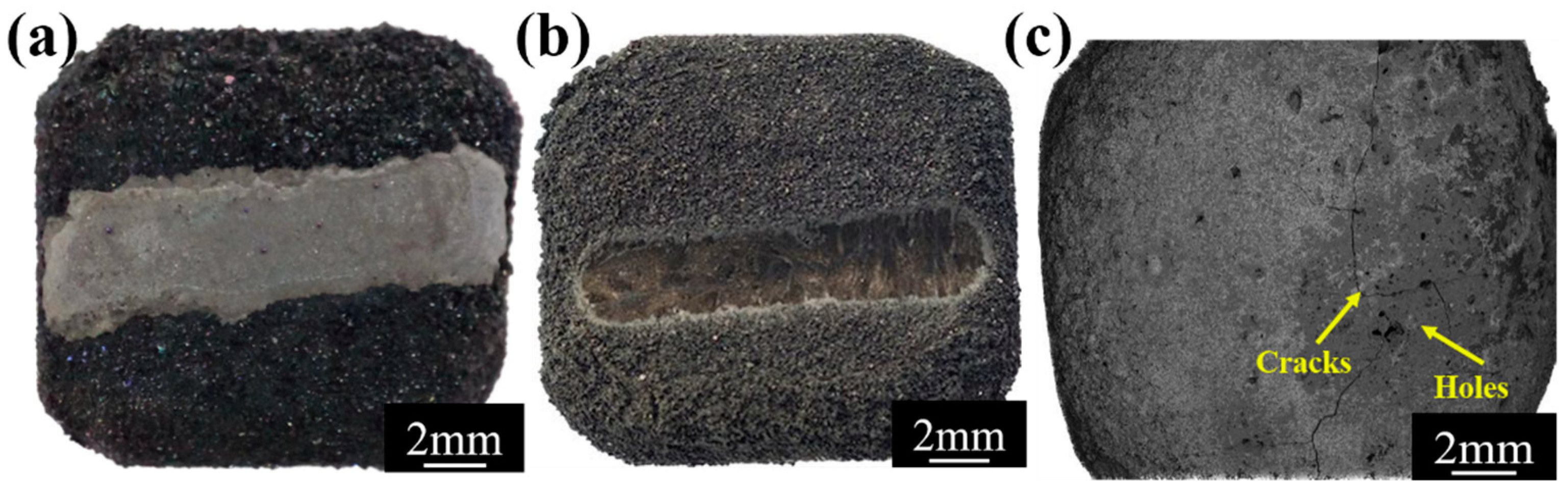
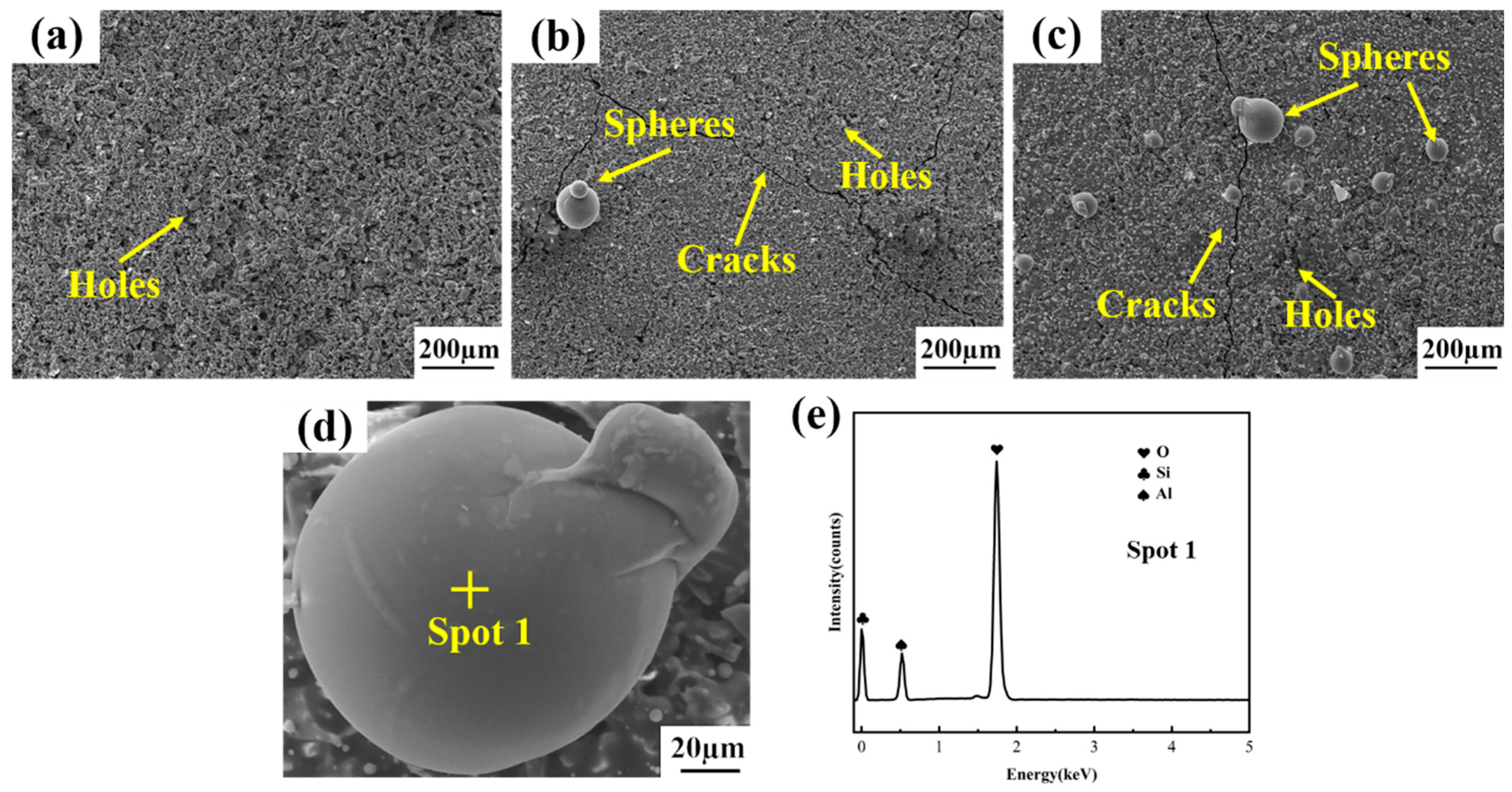
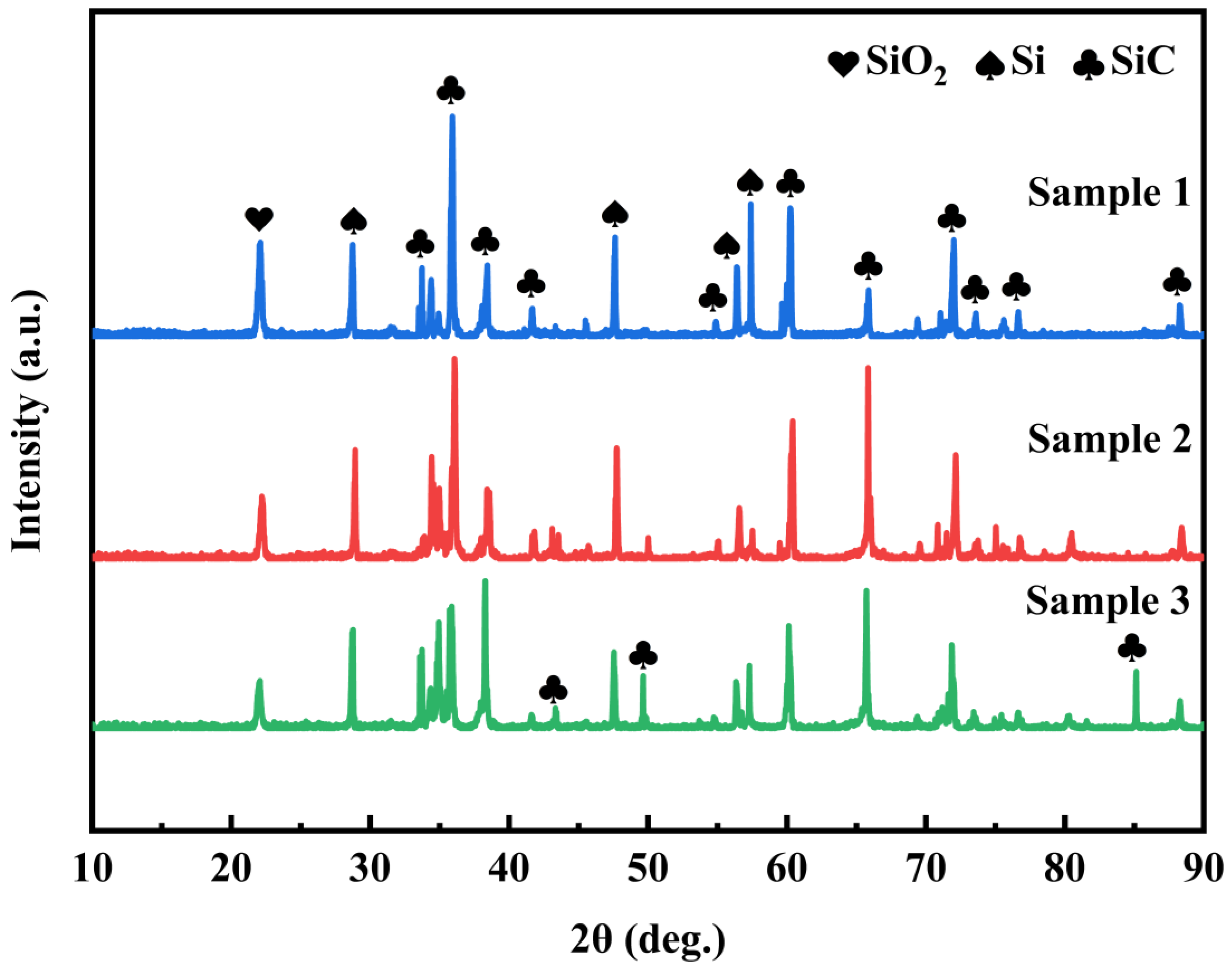
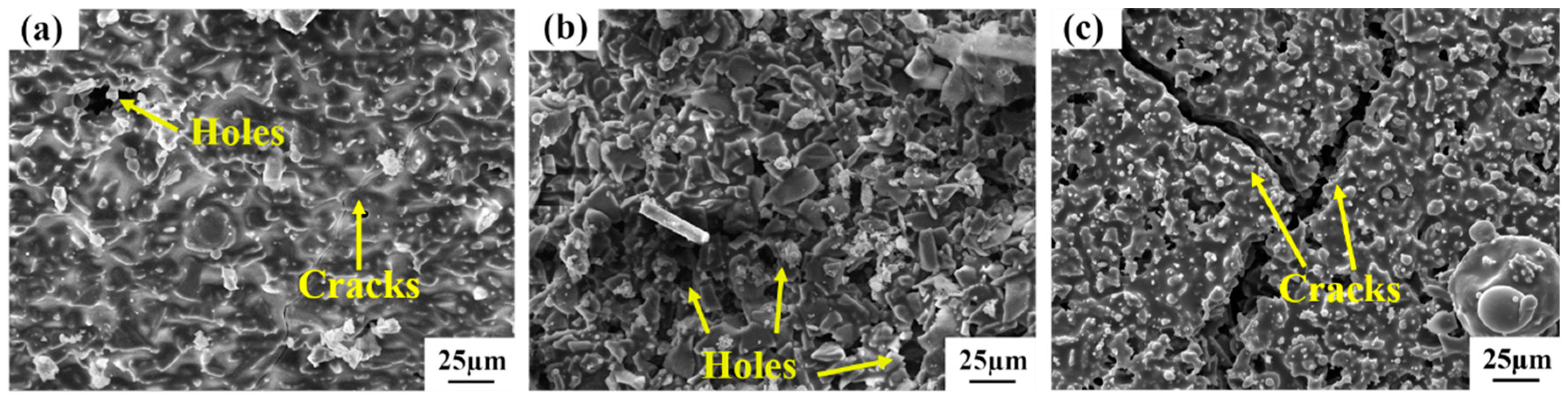
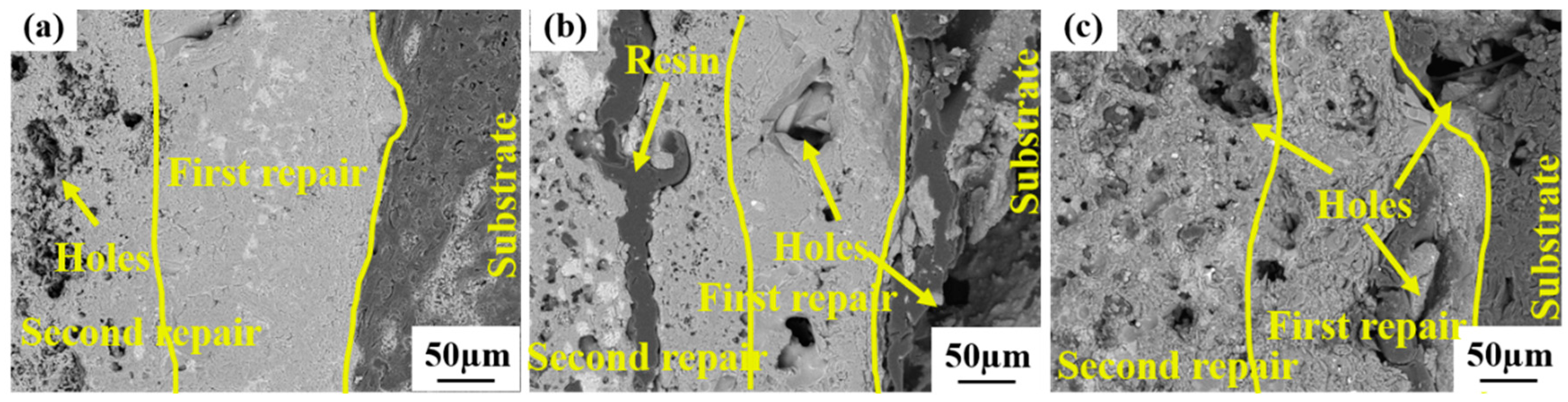
3.1. Characterization of Repair Coating Prepared by Different Process Parameters
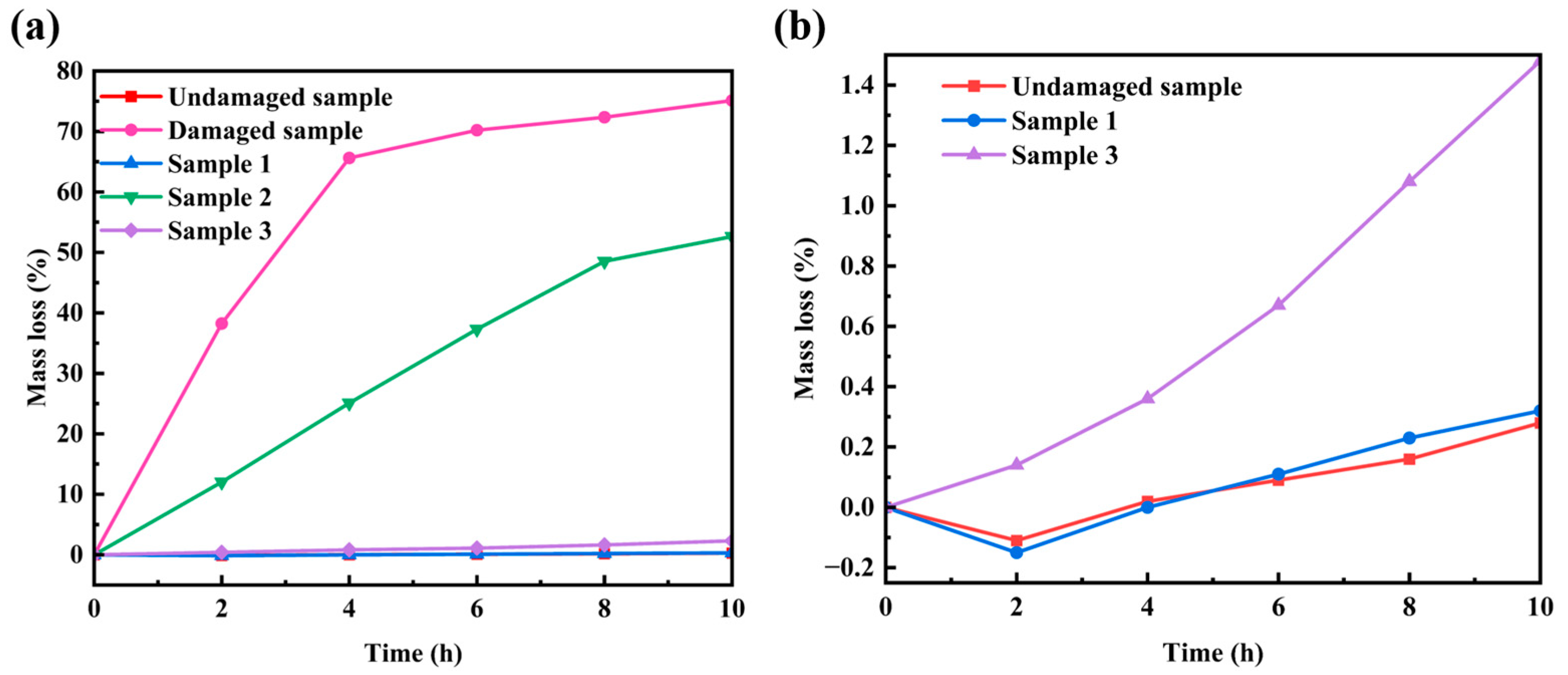
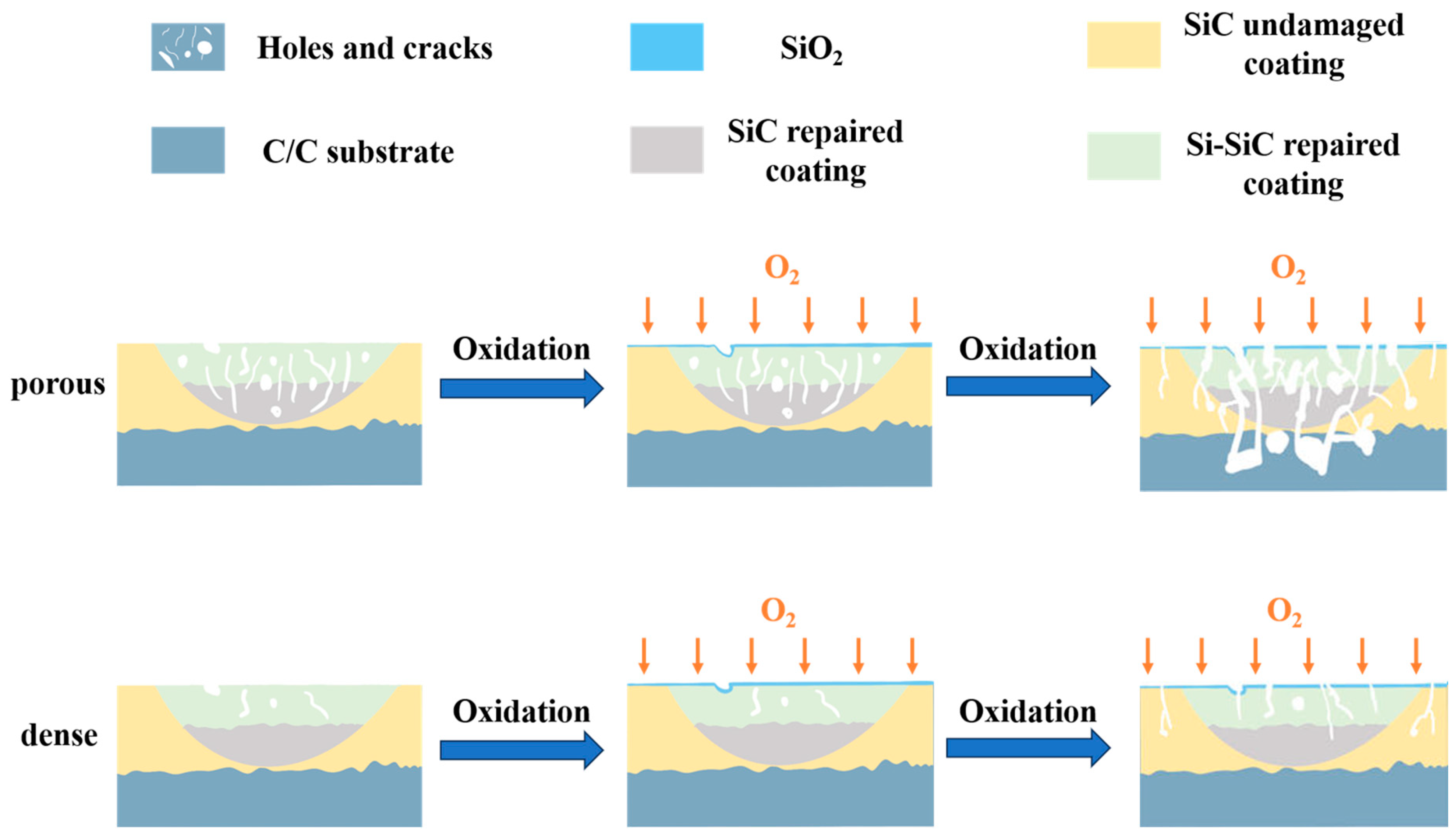
3.2. Oxidation Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manocha, L.M. High performance carbon-carbon composites. Sadhana 2003, 28, 349–358. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Zhang, J.; Chen, G.; Li, T. Radially one-dimensional hafnium carbide-carbon/carbon networks composites for ultra-high temperature ablation-resistance. Corros. Sci. 2021, 185, 109443. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, W.; Xiong, X.; Liu, F.; Luan, X. Ablation characteristics of mosaic structure ZrC-SiC coatings on low-density, porous C/C composites. J. Mater. Sci. Technol. 2019, 35, 2785–2798. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Shi, X.; Liu, X.; Kong, J.; Zhou, H. Repair of SiC coating on carbon/carbon composites by laser cladding technique. Ceram. Int. 2020, 46, 19537–19544. [Google Scholar] [CrossRef]
- Hu, C.; Pang, S.; Tang, S.; Yang, Z.; Wang, S.; Cheng, H.-M. Long-term oxidation behavior of carbon/carbon composites with a SiC/B4C–B2O3–SiO2–Al2O3 coating at low and medium temperatures. Corros. Sci. 2015, 94, 452–458. [Google Scholar] [CrossRef]
- Wang, H. Study on Laser Cladding Repair and Properties of MoSi2-SiC/SiC Coating on C/C Composites. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2023. [Google Scholar]
- Jia, Y. Investigation of Ablation Protection of Rare Earth Modified ZrC Coating System for C/C Composites. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2017. [Google Scholar]
- Feng, G.; Li, H.; Yao, X.; Zhou, H.; Yu, Y.; Lu, J. Ablation resistance of HfC-TaC/HfC-SiC alternate coating for SiC-coated carbon/carbon composites under cyclic ablation. J. Eur. Ceram. Soc. 2021, 41, 3207–3218. [Google Scholar] [CrossRef]
- Xie, W.; Fu, Q.; Cheng, C.; Wang, P.; Li, J.; Yan, N. Effect of Lu2O3 addition on the oxidation behavior of SiC-ZrB2 composite coating at 1500℃: Experimental and theoretical study. Corros. Sci. 2021, 192, 109803. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chu, Y.; Fu, Q.; Li, K. Preparation of oxidation protective ZrB2-SiC coating by in-situ reaction method on SiC-coated carbon/carbon composites. Surf. Coat. Technol. 2014, 247, 61–67. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Fu, Q.; Li, K. TaxHf1−xB2–SiC multiphase oxidation protective coating for SiC-coated carbon/carbon composites. Corros. Sci. 2014, 87, 479–488. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Fu, Q.; Li, K. Oxidation protective TaB2–SiC gradient coating to protect SiC–Si coated carbon/carbon composites against oxidation. Compos. Part B Eng. 2014, 66, 174–179. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, Z.; Tang, C.; Liu, X.; Li, Z.; Zhang, K. Study of SiC/SiO2 oxidation-resistant coatings on matrix graphite for HTR fuel element. Nucl. Eng. Des. 2014, 271, 217–220. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Li, Z.; Luo, W.; Zhou, W. Oxidation behavior of C/C composites with SiC/ZrSiO4–SiO2 coating. Trans. Nonferrous Met. Soc. China 2017, 27, 397–405. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, X.; Chu, H.; Lv, J.; Li, W.; Wang, W.; Yang, Q.; Feng, P. Oxidation inhibition behaviors of the HfB2-SiC-TaSi2 coating for carbon structural materials at 1700 °C. Corros. Sci. 2020, 177, 108982. [Google Scholar] [CrossRef]
- Chen, S.; Qiua, X.; Zhang, B.; Xua, J.; Zhong, F.; Zhu, B.; Zhang, Y.; Ou-Yang, J.; Yang, X. Advances in antioxidation coating materials for carbon/carbon composites. J. Alloys Compd. 2021, 886, 161143. [Google Scholar] [CrossRef]
- Jina, X.; Fana, X.; Lu, C.; Wang, T. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. J. Eur. Ceram. Soc. 2018, 38, 1–28. [Google Scholar] [CrossRef]
- Monteverde, F.; Savino, R.; De Stefano Fumo, M.; Di Maso, A. Plasma wind tunnel testing of ultra-high temperature ZrB2–SiC composites under hypersonic re-entry conditions. J. Eur. Ceram. Soc. 2010, 30, 2313–2321. [Google Scholar] [CrossRef]
- Li, H.; Xue, H.; Wang, Y.; Fu, Q.; Yao, D. A MoSi2–SiC–Si oxidation protective coating for carbon/carbon composites. Surf. Coat. Technol. 2007, 201, 9444–9447. [Google Scholar] [CrossRef]
- Williams, S.D.; Curry, D.M.; Chao, D.C.; Pham, V.T. Ablation analysis of the Shuttle Orbiter oxidation protected reinforced carbon-carbon. J. Thermophys. Heat Transf. 1995, 9, 478–485. [Google Scholar] [CrossRef]
- Wang, S.; Yin, J.; Xiefeng, M.; Tang, L.; Xiong, X.; Zhang, H.; Wen, Q.; Ma, D.; Zuo, J. Microstructure and ablation behaviour of C/C-SiC-ZrC-Cu composites prepared by reactive melt infiltration. Mater. Today Commun. 2024, 38, 108389. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Tong, Y.; Li, Y.; Xing, Y.; Lan, H.; Sun, Y.; Liang, X. Revealing thermal shock behaviors and damage mechanism of 3D needled C/C–SiC composites based on multi-scale analysis. J. Mater. Res. Technol. 2024, 29, 2016–2034. [Google Scholar] [CrossRef]
- Xue, L.; Li, K.; Jia, Y.; Zhang, S.; Ren, J.; You, Z. Effects of hypervelocity impact on ablation behavior of SiC coated C/C composites. Mater. Des. 2016, 108, 151–156. [Google Scholar] [CrossRef]
- Jiao, X.; He, Q.; Tan, Q.; Yin, X. Ablation behavior of mullite modified C/C-SiC-HfC composites under oxyacetylene torch for single and cyclic ablations with two heat fluxes. J. Eur. Ceram. Soc. 2023, 43, 5851–5862. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Q.; Zhao, F. A novel gradient SiC-ZrB2-MoSi2 coating for SiC coated C/C composites by supersonic plasma spraying. Surf. Coat. Technol. 2017, 313, 63–72. [Google Scholar]
- Lv, J.; Zhang, Y.; Li, W.; Zhu, X.; Li, J.; Zhang, J.; Li, T. Microstructure evolution of HfB2-SiC/SiC coating for C/C composites during long-term oxidation at 1700 °C. Corros. Sci. 2022, 206, 110524. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Q.; Hu, D.; Wei, Y.; Tong, M.; Zhang, J. Oxidation protective SiC-Si coating for carbon/carbon composites by gaseous silicon infiltration and pack cementation: A comparative investigation. J. Eur. Ceram. Soc. 2021, 41, 194–203. [Google Scholar] [CrossRef]
- Choi, W.J.; Lee, H.; Park, C.W.; Kim, Y.D.; Byun, J. High temperature oxidation behavior of molybdenum borides by silicon pack cementation process. Int. J. Refract. Met. Hard Mater. 2021, 100, 105609. [Google Scholar] [CrossRef]
- Zeng, J.; Hua, J.; Yang, X.; Xu, H.; Li, H.; Guo, N.; Dong, Q. Microstructure and formation mechanism of the Si-Cr dual-alloyed coating prepared by pack-cementation. Surf. Coat. Technol. 2020, 399, 126142. [Google Scholar] [CrossRef]
- Xianga, J.; Xiea, F.; Wua, X.; Yu, Y. Microstructure and tribological properties of Si-Y/Al two-step deposition coating prepared on Ti2AlNb based alloy by halide activated pack cementation technique. Tribol. Int. 2019, 136, 45–57. [Google Scholar] [CrossRef]



| Sample | Si wt/% | SiC wt/% | Al2O3 wt/% | Temperature/°C | Time/min |
|---|---|---|---|---|---|
| Sample-1 | 25 | 72 | 3 | 1450 | 30 |
| Sample-2 | 25 | 72 | 3 | 1350 | 30 |
| Sample-3 | 35 | 62 | 3 | 1450 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Shi, X.; Jiao, F.; Ti, X.; Du, L. Convenient Reparation of SiC-Coated C/C Composites by the Slurry Painting Method. Materials 2024, 17, 4515. https://doi.org/10.3390/ma17184515
Peng H, Shi X, Jiao F, Ti X, Du L. Convenient Reparation of SiC-Coated C/C Composites by the Slurry Painting Method. Materials. 2024; 17(18):4515. https://doi.org/10.3390/ma17184515
Chicago/Turabian StylePeng, Hui, Xiaohong Shi, Fan Jiao, Xutong Ti, and Linyi Du. 2024. "Convenient Reparation of SiC-Coated C/C Composites by the Slurry Painting Method" Materials 17, no. 18: 4515. https://doi.org/10.3390/ma17184515





