Fabrication of Silicon Carbide Nanoparticles Using Pulsed Laser Ablation in Liquid and Viscosity Optimization via Solvent Tuning
Abstract
1. Introduction
2. Experimental
2.1. NP Synthesis
2.2. NP Characterization
3. Results and Discussion
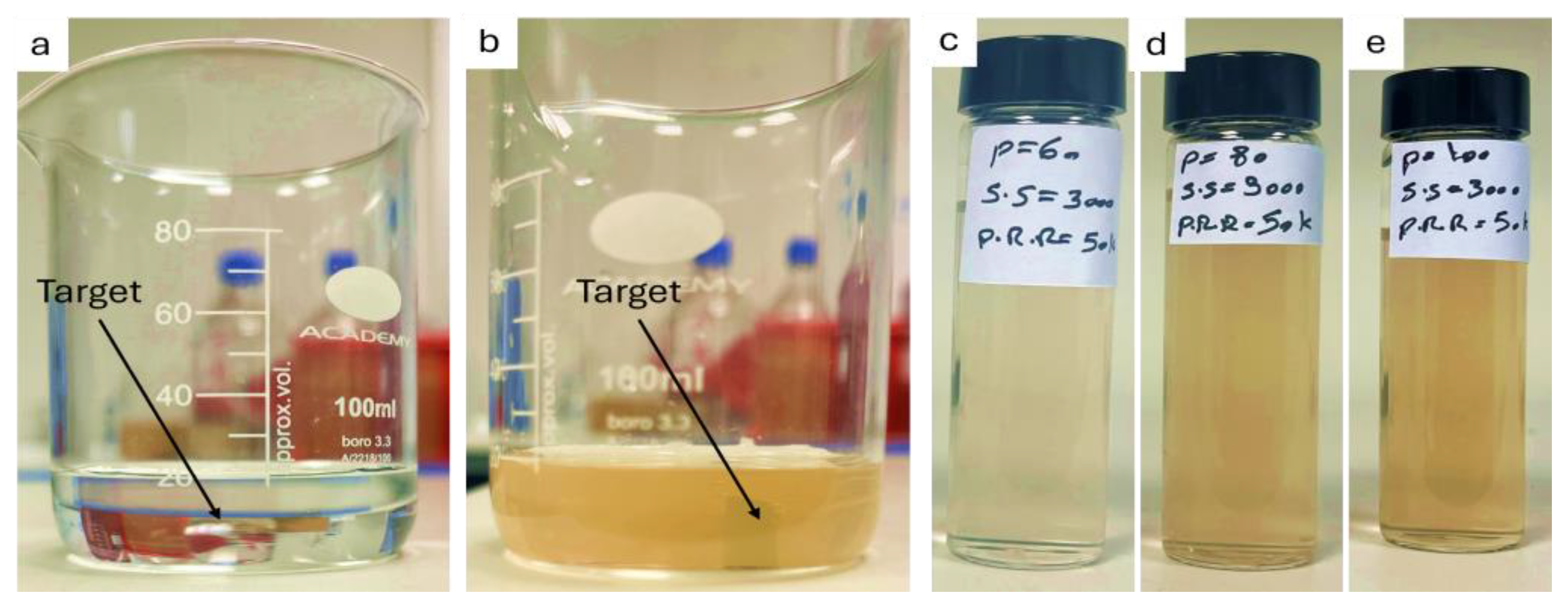
3.1. Visual Inspection and Ultraviolet–Visible Spectroscopy
3.2. Dynamic Light Scattering (DLS)
3.3. Field Emission Scanning Electron Microscopy (FESEM) and Scanning Transmission Electron Microscopy (STEM)
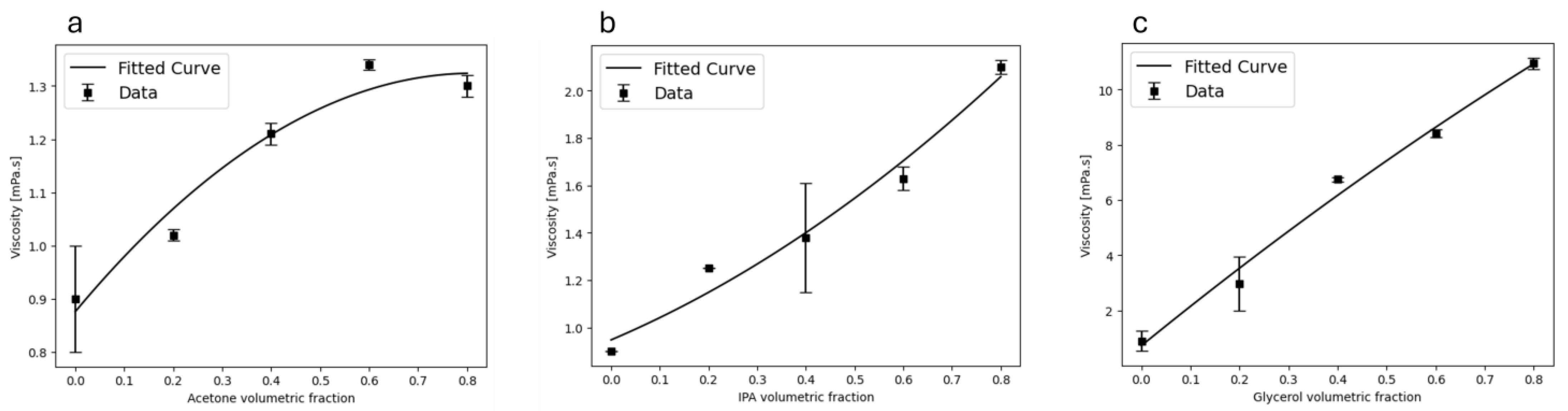
3.4. Viscosity Optimization and Effect of Liquid Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dell, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Adv. Synth. Funct. Nanoparticles Lasers Liq.—Fundam. Appl. Catal. Energy Sci. Biomed. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: A review. Appl. Phys. A 2021, 127, 810. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Adv. Colloid Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Magnesium Nanoparticle Synthesis from Powders via Pulsed Laser Ablation in Liquid for Nanocolloid Production. Appl. Sci. 2021, 11, 10974. [Google Scholar] [CrossRef]
- Pereira, H.; Moura, C.G.; Miranda, G.; Silva, F.S. Influence of liquid media and laser energy on the production of MgO nanoparticles by laser ablation. Opt. Laser Technol. 2021, 142, 107181. [Google Scholar] [CrossRef]
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.S.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D.; et al. Liquid Phase—Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Fitzpatrick, B.; Brabazon, D. Effect of liquid medium and laser processing parameters on the fabrication of carbon nanoparticles via pulsed laser ablation in liquid towards paper electronics. Colloids Surf. Physicochem. Eng. Asp. 2022, 636, 128151. [Google Scholar] [CrossRef]
- Urusov, A.E.; Petrakova, A.; Kuzmin, P.; Zherdev, A.; Sveshnikov, P.; Shafeev, G.; Dzantiev, B. Application of gold nanoparticles produced by laser ablation for immunochromatographic assay labeling. Anal. Biochem. 2015, 491, 65–71. [Google Scholar] [CrossRef]
- McCarthy, É.; Sreenilayam, S.P.; Ronan, O.; Ayub, H.; McCann, R.; McKeon, L.; Fleischer, K.; Nicolosi, V.; Brabazon, D. Silver nanocolloid generation using dynamic Laser Ablation Synthesis in Solution system and drop-casting. Nano-Struct. Nano-Objects 2022, 29, 100841. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Izaak, T.I.; Kharlamova, T.S.; Martynova, D.O.; Lapin, I.N.; Svetlichnyi, V.A. Ag/SiOx nanocomposite powders synthesized from colloids obtained by pulsed laser ablation. Colloids Surf. Physicochem. Eng. Asp. 2018, 553, 80–88. [Google Scholar] [CrossRef]
- Shuaib, E.P.; Yogesh, G.K.; Sastikumar, D. Amorphous and photoluminescent crystalline silicon carbide nanoparticles synthesized by laser ablation in liquids. Natl. Conf. Mater. Sci. 2022, 50, 2745–2750. [Google Scholar] [CrossRef]
- Lin, H.; Gerbec, J.A.; Sushchikh, M.; McFarland, E.W. Synthesis of amorphous silicon carbide nanoparticles in a low temperature low pressure plasma reactor. Nanotechnology 2008, 19, 325601. [Google Scholar] [CrossRef] [PubMed]
- Askari, S.; Haq, A.U.; Macias-Montero, M.; Levchenko, I.; Yu, F.; Zhou, W.; Ostrikov, K.; Maguire, P.; Svrcek, V.; Mariotti, D. Ultra-small photoluminescent silicon-carbide nanocrystals by atmospheric-pressure plasmas. Nanoscale 2016, 8, 17141–17149. [Google Scholar] [CrossRef]
- Khashan, K.S.; Hadi, A.; Mahdi, M.; Hamid, M.K. Nanosecond pulse laser preparation of InZnO (IZO) nanoparticles NPs for high-performance photodetector. Appl. Phys. A 2019, 125, 51. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Abdulameer, M.R.; Kadhim, M.M.; Mutlak, F.A.-H. Plasma parameters of Au nano-particles ablated on porous silicon produced via Nd-YAG laser at 355 nm for sensing NH3 gas. Optik 2022, 249, 168260. [Google Scholar] [CrossRef]
- Khashan, K.S.; Ismail, R.A.; Mahdi, R.O. Synthesis of SiC nanoparticles by SHG 532 nm Nd:YAG laser ablation of silicon in ethanol. Appl. Phys. A 2018, 124, 443. [Google Scholar] [CrossRef]
- Fromme, T.; Reichenberger, S.; Tibbetts, K.M.; Barcikowski, S. Laser synthesis of nanoparticles in organic solvents—Products, reactions, and perspectives. Beilstein J. Nanotechnol. 2024, 15, 638–663. [Google Scholar] [CrossRef]
- Harb, N.H.; Mutlak, F.A.-H. Production and characterization of porous silicon via laser-assisted etching: Effect of gamma irradiation. Optik 2021, 246, 167800. [Google Scholar] [CrossRef]
- Jwied, D.H.; Nayef, U.M.; Mutlak, F.A.-H. Synthesis of C:Se nanoparticles via laser ablated with magnetic field on porous silicon for gas sensor applications. Optik 2021, 242, 167207. [Google Scholar] [CrossRef]
- Wagener, P.; Schwenke, A.; Chichkov, B.N.; Barcikowski, S. Pulsed Laser Ablation of Zinc in Tetrahydrofuran: Bypassing the Cavitation Bubble. J. Phys. Chem. C 2010, 114, 7618–7625. [Google Scholar] [CrossRef]
- Dittrich, S.; Kohsakowski, S.; Wittek, B.; Hengst, C.; Gökce, B.; Barcikowski, S.; Reichenberger, S. Increasing the Size-Selectivity in Laser-Based g/h Liquid Flow Synthesis of Pt and PtPd Nanoparticles for CO and NO Oxidation in Industrial Automotive Exhaust Gas Treatment Benchmarking. Nanomaterials 2020, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, S.; Streubel, R.; McDonnell, C.; Huber, H.P.; Barcikowski, S.; Gökce, B. Comparison of the productivity and ablation efficiency of different laser classes for laser ablation of gold in water and air. Appl. Phys. A 2019, 125, 432. [Google Scholar] [CrossRef]
- Somiya, S.; Inomata, Y. Silicon Carbide Ceramics: Gas Phase Reactions, Fibers and Whisker, Joining; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991; Volume 2. [Google Scholar]
- Rajarao, R.; Ferreira, R.; Sadi, S.H.F.; Khanna, R.; Sahajwalla, V. Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Mater. Lett. 2014, 120, 65–68. [Google Scholar] [CrossRef]
- Castelletto, S.; Almutairi, A.F.M.; Thalassinos, G.; Lohrmann, A.; Buividas, R.; Lau, D.W.M.; Reineck, P.; Juodkazis, S.; Ohshima, T.; Gibson, B.C.; et al. Fluorescent color centers in laser ablated 4H-SiC nanoparticles. Opt. Lett. 2017, 42, 1297–1300. [Google Scholar] [CrossRef]
- Yamada, T.; Araki, F.; Ishihara, J.; Miyajima, K. Fabrication of silicon carbide nanoparticles using picosecond pulsed laser ablation in acetone with characterizations from TEM and XRD. AIP Adv. 2019, 9, 105011. [Google Scholar] [CrossRef]
- Guo, X.; Song, H.; Li, Y.; Wang, P.; Liu, S. Fabrication of 4H–SiC nanoparticles using femtosecond pulsed laser ablation in deionized water. Opt. Mater. 2022, 132, 112817. [Google Scholar] [CrossRef]
- Tarasenka, N.; Kornev, V.; Rzheutski, M.; Lutsenko, E.; Chakrabarti, S.; Velusamy, T.; Mariotti, D.; Tarasenko, N. Fabrication of luminescent silicon carbide nanoparticles by pulsed laser synthesis in liquid. Appl. Phys. A 2022, 128, 749. [Google Scholar] [CrossRef]
- Shi, W.; Zheng, Y.; Peng, H.; Wang, N.; Lee, C.S.; Lee, S.-T. Laser ablation synthesis and optical characterization of silicon carbide nanowires. J. Am. Ceram. Soc. 2000, 83, 3228–3230. [Google Scholar] [CrossRef]
- Molian, P.; Pecholt, B.; Gupta, S. Picosecond pulsed laser ablation and micromachining of 4H-SiC wafers. Appl. Surf. Sci. 2009, 255, 4515–4520. [Google Scholar] [CrossRef]
- Bagga, K.; McCann, R.; Wang, M.; Stalcup, A.; Vázquez, M.; Brabazon, D. Laser assisted synthesis of carbon nanoparticles with controlled viscosities for printing applications. J. Colloid Interface Sci. 2015, 447, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.H. Effects of Different Laser Pulse Regimes (Nanosecond, Picosecond and Femtosecond) on the Ablation of Materials for Production of Nanoparticles in Liquid Solution. In High Energy and Short Pulse Lasers; Viskup, R., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Kobayashi, M.; Liu, S.-M.; Sato, S.; Yao, H.; Kimura, K. Optical Evaluation of Silicon Nanoparticles Prepared by Arc Discharge Method in Liquid Nitrogen. Jpn. J. Appl. Phys. 2006, 45, 6146. [Google Scholar] [CrossRef]
- Bo, B.; Jian, Y.; Yi, C.; Zi-Qin, W. Transmission electron microscopy of annealed a-Si1−xCx:H/Al films with different Al grain sizes. Thin Solid. Films 1993, 230, 160–166. [Google Scholar] [CrossRef]
- Alekseev, S.; Shamatulskaya, E.; Volvach, M.; Gryn, S.; Korytko, D.; Bezverkhyy, I.; Iablokov, V.; Lysenko, V. Size and Surface Chemistry Tuning of Silicon Carbide Nanoparticles. Langmuir 2017, 33, 13561–13571. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, R.; Morjan, I.; Borsella, E.; Botti, S.; Fantoni, R.; Dikonimos-Makris, T.; Giorgi, R.; Enzo, S. Composite ceramic powders obtained by laser induced reactions of silane and amines. J. Mater. Res. 1991, 6, 2442–2451. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, J.; Lin, G. Effect of the scanning speed on the microgroove formation regime in nanosecond-pulsed laser scanning ablation of cermet. Int. J. Adv. Manuf. Technol. 2020, 107, 97–107. [Google Scholar] [CrossRef]
- Claverie, F.; Fernández, B.; Pécheyran, C.; Alexis, J.; Donard, O.F. Elemental fractionation effects in high repetition rate IR femtosecond laser ablation ICP-MS analysis of glasses. J. Anal. At. Spectrom. 2009, 24, 891–902. [Google Scholar] [CrossRef]
- Sugioka, K.; Meunier, M.; Piqué, A. Laser Precision Microfabrication; Springer: Berlin/Heidelberg, Germany, 2010; Available online: https://books.google.ie/books?id=Ho_QDAEACAAJ (accessed on 3 September 2010).
- Aiyyzhy, K.O.; Barmina, E.V.; Voronov, V.V.; Shafeev, G.A.; Novikov, G.G.; Uvarov, O.V. Laser ablation and fragmentation of Boron in liquids. Opt. Laser Technol. 2022, 155, 108393. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem. Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef]
- Heidarinassab, S.; Nyabadza, A.; Ahad, I.U.; Brabazon, D. Investigation of ablation efficiency and properties of silicon carbide nanoparticles synthesised using pulsed laser ablation in liquid. Opt. Lasers Eng. 2024, 180, 108341. [Google Scholar] [CrossRef]
- Nikolov, A.S.; Balchev, I.I.; Nedyalkov, N.N.; Kostadinov, I.K.; Karashanova, D.B.; Atanasova, G.B. Influence of the laser pulse repetition rate and scanning speed on the morphology of Ag nanostructures fabricated by pulsed laser ablation of solid target in water. Appl. Phys. A 2017, 123, 719. [Google Scholar] [CrossRef]
- Streubel, R.; Bendt, G.; Gökce, B. Pilot-scale synthesis of metal nanoparticles by high-speed pulsed laser ablation in liquids. Nanotechnology 2016, 27, 205602. [Google Scholar] [CrossRef] [PubMed]
- Wazeer, A.; Das, A.; Sinha, A.; Karmakar, A. Nanomaterials Synthesis via Laser Ablation in Liquid: A Review. J. Inst. Eng. India Ser. D 2023, 104, 413–426. [Google Scholar] [CrossRef]
- Bajaj, G.; Soni, R.K. Effect of liquid medium on size and shape of nanoparticles prepared by pulsed laser ablation of tin. Appl. Phys. A 2009, 97, 481–487. [Google Scholar] [CrossRef]
- Yoo, J.H.; Jeong, S.H.; Mao, X.L.; Greif, R.; Russo, R.E. Evidence for phase-explosion and generation of large particles during high power nanosecond laser ablation of silicon. Appl. Phys. Lett. 2000, 76, 783–785. [Google Scholar] [CrossRef]
- Lee, C.; Kim, N.R.; Koo, J.; Lee, Y.J.; Lee, H.M. Cu-Ag core–shell nanoparticles with enhanced oxidation stability for printed electronics. Nanotechnology 2015, 26, 455601. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; Grilo, J.P.d.F.; Nascimento, R.M.D.; Silva, F.S. Effects of laser fluence and liquid media on preparation of small Ag nanoparticles by laser ablation in liquid. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Vázquez, M.; Brabazon, D. Post-fabrication adjustment of metalloid Mg–C-graphene nanoparticles via Pulsed Laser Ablation for paper electronics and process optimisation. Mater. Des. 2024, 240, 112869. [Google Scholar] [CrossRef]
- Al-Antaki, A.H.M.; Luo, X.; Duan, X.; Lamb, R.N.; Hutchison, W.D.; Lawrance, W.; Raston, C.L. Continuous Flow Copper Laser Ablation Synthesis of Copper(I and II) Oxide Nanoparticles in Water. ACS Omega 2019, 4, 13577–13584. [Google Scholar] [CrossRef]
- Sreenilayam, S.P.; McCarthy, É.; McKeon, L.; Ronan, O.; McCann, R.; Fleischer, K.; Freeland, B.; Nicolosi, V.; Brabazon, D. Additive-free silver nanoparticle ink development using flow-based Laser Ablation Synthesis in Solution and Aerosol Jet printing. Chem. Eng. J. 2022, 449, 137817. [Google Scholar] [CrossRef]
- Kalus, M.-R.R.; Lanyumba, R.; Barcikowski, S.; Gökce, B. Discrimination of ablation, shielding, and interface layer effects on the steady-state formation of persistent bubbles under liquid flow conditions during laser synthesis of colloids. J. Flow. Chem. 2021. [Google Scholar] [CrossRef]
- Freeland, B.; McCann, R.; Alkan, G.; Friedrich, B.; Foley, G.; Brabazon, D. Stable nano-silver colloid production via Laser Ablation Synthesis in Solution (LASiS) under laminar recirculatory flow. Adv. Mater. Process. Technol. 2020, 6, 677–685. [Google Scholar] [CrossRef]
- Freeland, B.; McCann, R.; O’neill, P.; Sreenilayam, S.; Tiefenthaler, M.; Dabros, M.; Juillerat, M.; Foley, G.; Brabazon, D. Real-time monitoring and control for high-efficiency autonomous laser fabrication of silicon nanoparticle colloids. Int. J. Adv. Manuf. Technol. 2021, 114, 291–304. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771. [Google Scholar] [CrossRef]


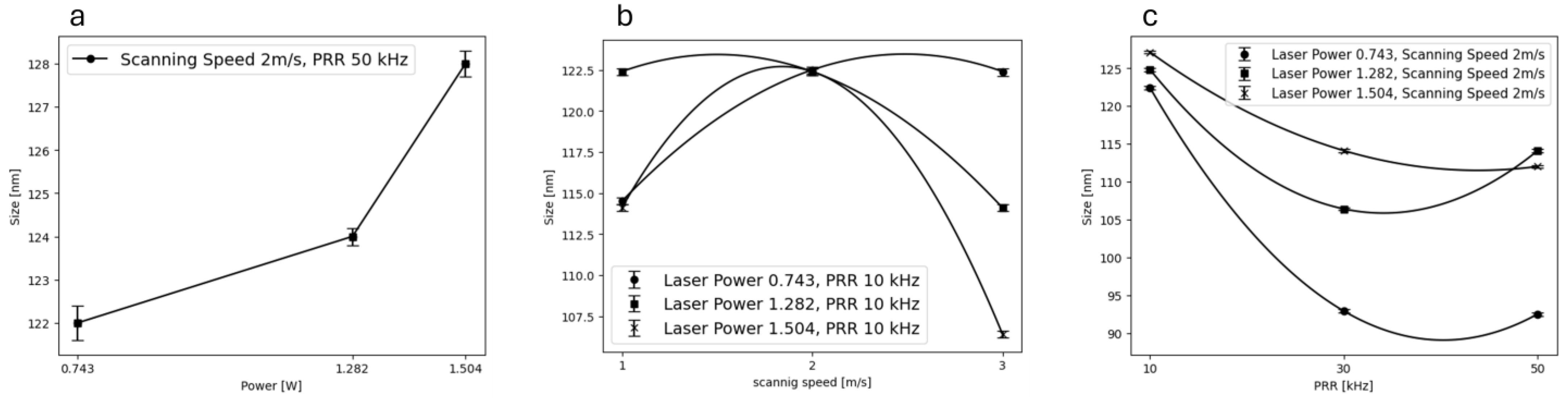

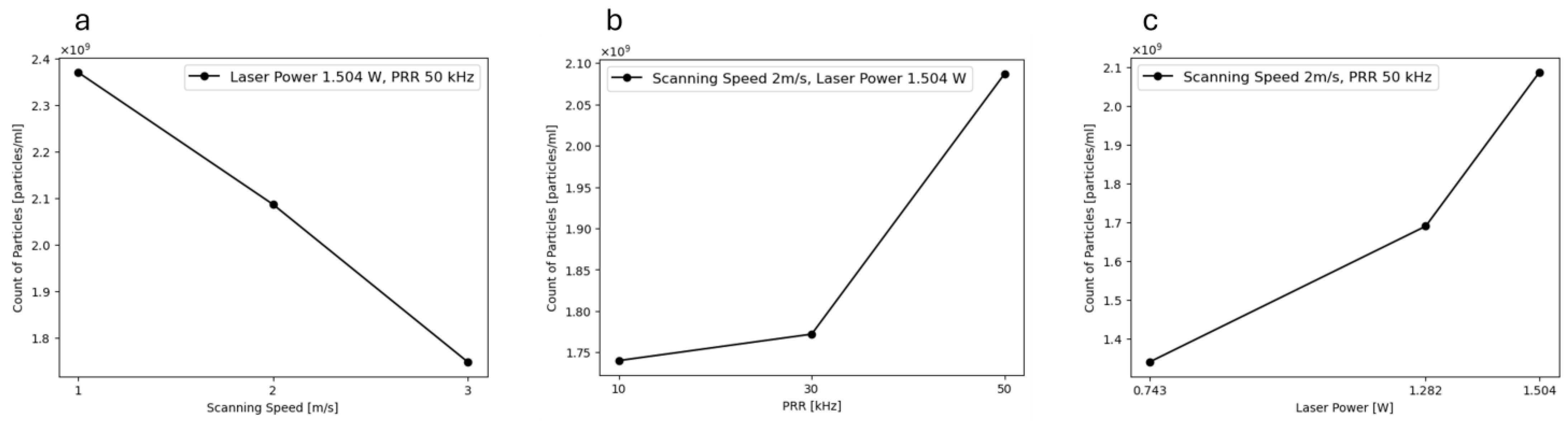

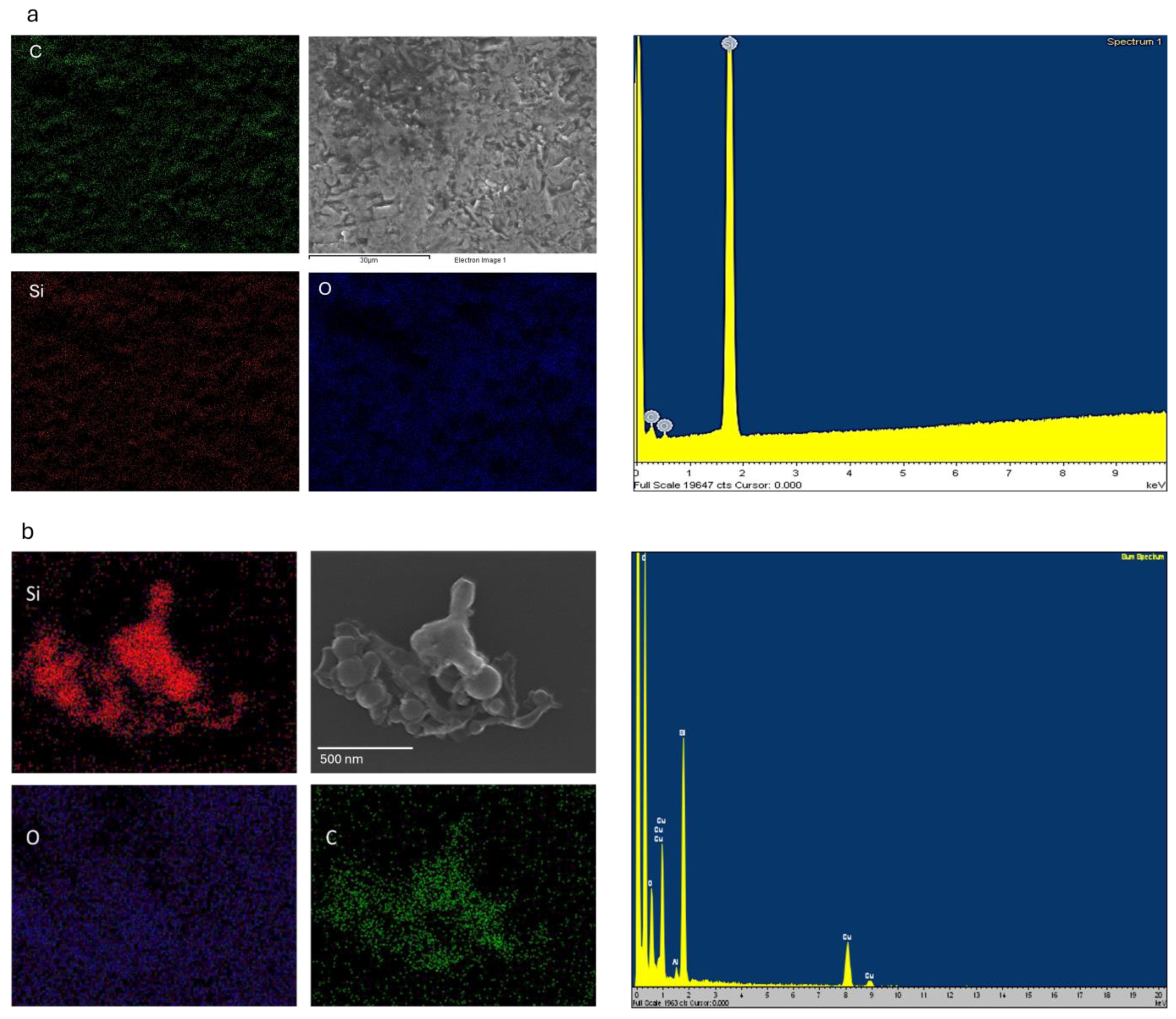

| Level | −1 | 0 | 1 |
|---|---|---|---|
| Laser power (W) | 0.743 | 1.282 | 1.504 |
| Scanning speed (m/s) | 1 | 2 | 3 |
| PRR (kHz) | 10 | 30 | 50 |
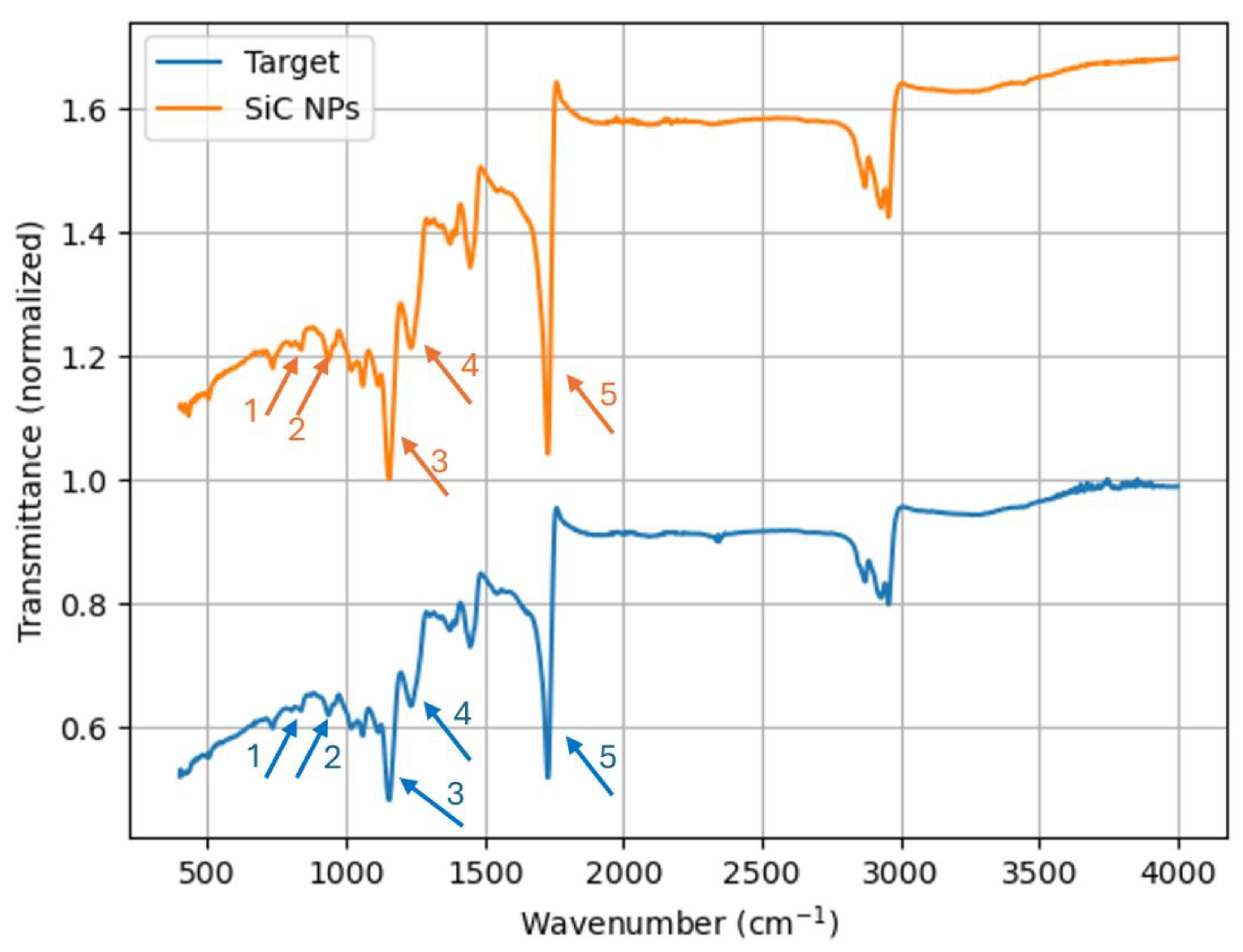
| Peak Number | Bonding | Associated Material |
|---|---|---|
| 1 | Si-C | Silicon Carbide |
| 2 | Si-O-Si | Silicon Oxide/TO and LO Scratching of SiC Lattice |
| 3 | Si-O-Si | Silicon Oxide |
| 4 | Si-O-Si | Silicon Oxide |
| 5 | C=O | Carbonyl Compound or Carbide |
| Element | Weight Percentage (Target) | Weight Percentage (NPs) |
|---|---|---|
| Carbon | 32.06 | 25.4 |
| Silicon | 61.95 | 41.46 |
| Oxygen | 4.89 | 18.71 |
| Copper | 0.27 | 14.03 |
| Aluminium | 0.83 | 0.4 |
| Solvent | Viscosity (mPa·s) |
|---|---|
| Acetone | 0.95 |
| IPA | 2.93 |
| Glycerol | 10.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarinassab, S.; Nyabadza, A.; Ahad, I.U.; Brabazon, D. Fabrication of Silicon Carbide Nanoparticles Using Pulsed Laser Ablation in Liquid and Viscosity Optimization via Solvent Tuning. Materials 2024, 17, 4527. https://doi.org/10.3390/ma17184527
Heidarinassab S, Nyabadza A, Ahad IU, Brabazon D. Fabrication of Silicon Carbide Nanoparticles Using Pulsed Laser Ablation in Liquid and Viscosity Optimization via Solvent Tuning. Materials. 2024; 17(18):4527. https://doi.org/10.3390/ma17184527
Chicago/Turabian StyleHeidarinassab, Saeid, Anesu Nyabadza, Inam Ul Ahad, and Dermot Brabazon. 2024. "Fabrication of Silicon Carbide Nanoparticles Using Pulsed Laser Ablation in Liquid and Viscosity Optimization via Solvent Tuning" Materials 17, no. 18: 4527. https://doi.org/10.3390/ma17184527
APA StyleHeidarinassab, S., Nyabadza, A., Ahad, I. U., & Brabazon, D. (2024). Fabrication of Silicon Carbide Nanoparticles Using Pulsed Laser Ablation in Liquid and Viscosity Optimization via Solvent Tuning. Materials, 17(18), 4527. https://doi.org/10.3390/ma17184527







