Abstract
High-temperature adhesives find extensive application in diverse domains, encompassing repairs, production processes, and material joining. However, achieving their curing at ambient temperatures remains a formidable challenge due to the inherent requirement of elevated temperatures, typically exceeding 500 °C, for the curing reaction. To overcome this limitation, in this study, we developed a distinctive inorganic phosphate-based composite adhesive by incorporating dual-functional calcium monoaluminate (CA) into a traditional adhesive blend comprising Al(H2PO4)3 and MgO. This distinctive approach significantly diminishes the curing temperature, enabling it to occur at room temperature. Firstly, CA’s facile hydration reaction effectively scavenges surrounding water molecules, thereby accelerating the dehydration curing process of Al(H2PO4)3. Secondly, as hydration is an exothermic process, it locally generates heat around the Al(H2PO4)3, fostering optimal conditions for its curing reaction. Moreover, the adhesive’s strength is substantially bolstered through the strategic inclusion of Nano-Al2O3 (enhancing the availability of reaction sites) and Nano-SiO2 (improving overall stability). As a demonstration, the adhesive formulation with added CA containing 2% Nano-Al2O3 and 2% Nano-SiO2 achieved a remarkable tensile strength of 32.48 MPa at room temperature, underscoring its potential as an efficient solution for various practical adhesive applications. The adhesive prepared in this study harnesses the hydration properties of CA to absorb moisture and release substantial heat, introducing a novel method for ambient temperature curing. This development promises to broaden its applications in refractory materials, coatings, and equipment repair.
1. Introduction
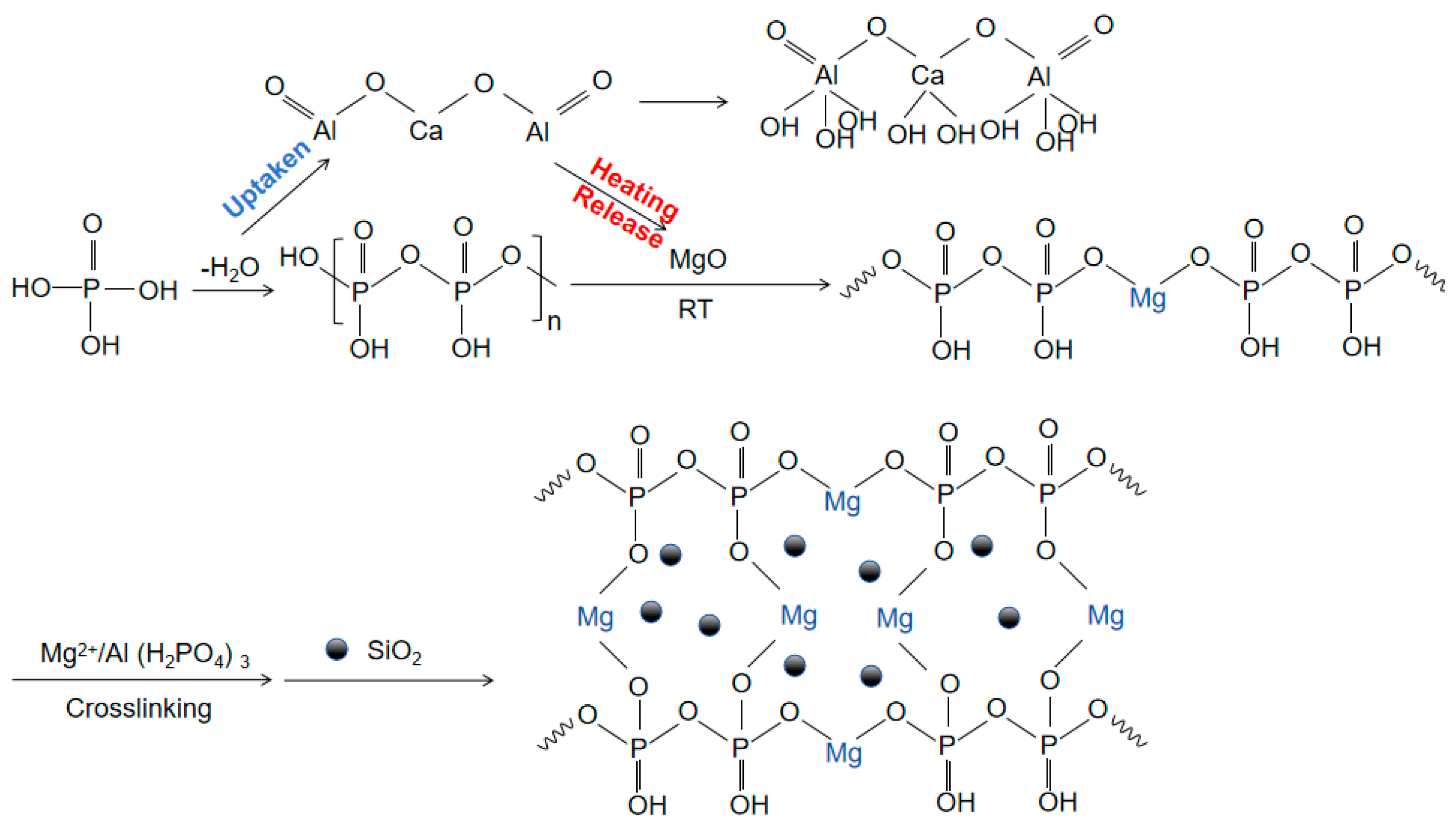
With the rapid development of industries such as aerospace and resource extraction, and the increasing use of special devices in extreme environments such as high temperature and high pressure [1,2,3,4,5,6], the demand for the performance of adhesive materials, especially ultra-high-temperature adhesive materials, continues to increase. Inorganic phosphate binders have broad application prospects and potential industrial value due to their simple structure, high bonding strength, and non-toxic and odorless properties [7,8,9]. However, the phosphate binder faces a challenge of curing at room temperature due to the typical high temperature of dehydration condensation reactions, which is approximately 500 °C, limiting the usability and practical application. Using curing agents to substitute other curing reactions that require lower reaction temperatures is now a common strategy [10,11,12]. MgO, through its ability to undergo a series of chemical reactions with the binder matrix, significantly lowers the curing temperature of the binder and is a commonly used curing agent. Taking Al(H2PO4)3 and MgO as an example, upon the addition of MgO, the binder system forms Mg(H2PO4)2·4H2O with superior bonding properties, significantly reducing the PO43− content in the solution and enhancing gel formation within the binder. This reaction is exothermic, with the generated heat facilitating the curing reaction. Subsequently, MgO encourages dehydration and condensation among phosphate molecules, leading to the formation of a comprehensive three-dimensional network structure. Mg2+ plays a pivotal role in this network structure, acting as a connecting bridge (Figure 1) [13,14,15,16]. J. A. Fernando [17] and Li Yizhen [18] lowered the curing temperature of the adhesive to 200 and 120 °C by controlling the P/Al ratio doped with MgO, but its curing temperature was still significantly higher than the ambient temperature.
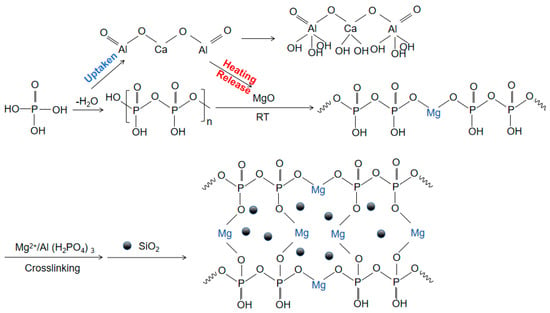
Figure 1.
Mechanism diagram of hydration reaction of adhesive.
As we know, calcium aluminate (CaO·Al2O3, CA) can easily react with water through a hydration process at room temperature, with the reaction equation being (T < 15 °C) [19,20]. Incorporating CA into the traditional adhesive blend of phosphate and MgO, on the one hand, can consume water molecules around the phosphate, promoting the dehydration process, but, on the other hand, the hydration of CA is an exothermic process, which generates heat around the Al(H2PO4)3, providing the necessary temperature conditions for the curing reaction without needing additional heating. Thus, this approach holds promise for achieving the curing reaction of phosphate high-temperature adhesive at room temperature [21,22,23,24,25].
Figure 1 shows a schematic diagram of the hydration reaction. Phosphoric acid is dehydrated by heating to form concentrated phosphoric acid. CA hydration absorbs water molecules generated by the dehydration condensation of Al(H2PO4)3, promoting the dehydration reaction. The horizontal and vertical binding between concentrated phosphoric acid and magnesium oxide forms a large molecular network structure through ‘-O-Mg-O-’. CA hydration releases a large amount of heat to promote the formation of the large molecular network structure. Nano-Al2O3 provides more nucleation sites [19,26,27,28]. Nano-SiO2, as an inert filler, does not participate in the reaction and plays a role in enhancing the thermal stability of the structure in the cross-linked network [29,30,31].
Herein, we developed a distinctive inorganic phosphate-based composite adhesive by incorporating the dual-functional CA into a traditional adhesive blend comprising Al(H2PO4)3 and MgO. The CA can simultaneously consume H2O and provide heat around the Al(H2PO4)3, thereby promoting the curing reaction. This distinctive approach significantly diminishes the curing temperature, enabling it to occur at room temperature. Moreover, the adhesive’s strength is substantially bolstered through the strategic inclusion of Nano-Al2O3 (enhancing the availability of reaction sites) and Nano-SiO2 (improving overall stability). As a demonstration, the adhesive formulation with CA containing 2% Nano-Al2O3 and 2% Nano-SiO2 achieved a remarkable tensile strength of 32.48 MPa at room temperature, underscoring its potential as an efficient solution for various practical adhesive applications.
2. Material and Methods
2.1. Materials and Manufacturing
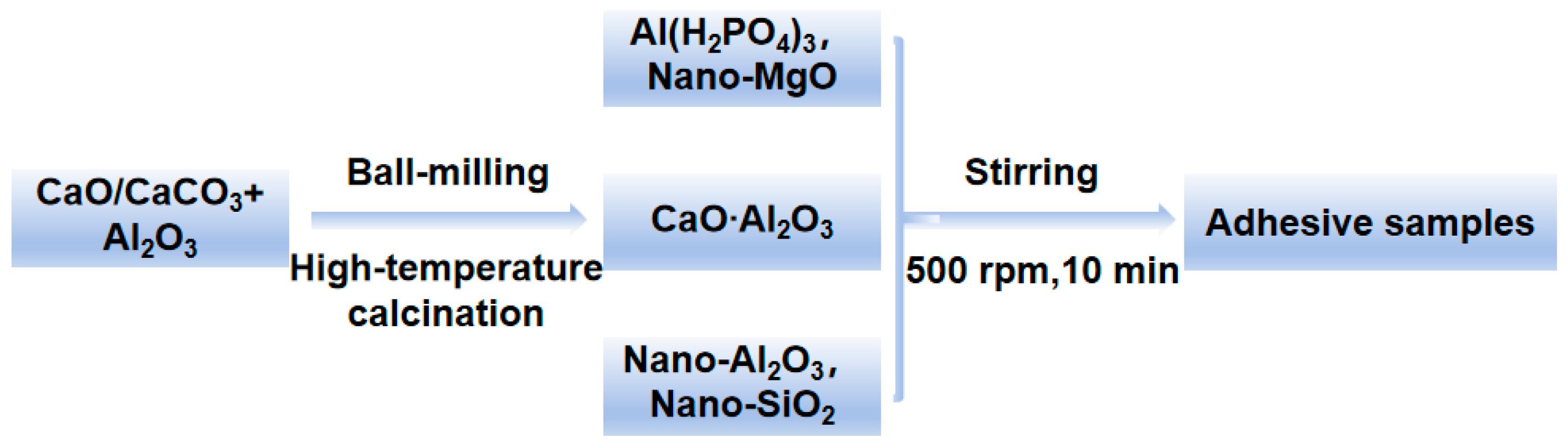
Phosphate binders are mainly composed of matrix, curing agent, filler, etc. The matrix used in the adhesive is aluminum dihydrogen phosphate (Al (H2PO4)3) and calcium aluminate. During this process, magnesium oxide is used as the curing agent, and the fillers are composed of nano silica and nano alumina. Add CA to a beaker containing Al (H2PO4)3 at room temperature and stir mechanically at 500 rpm for 10 min to dissolve and mix evenly. Use a 400 μm coating rod to apply the adhesive onto the surface of the ceramic sheet (40 mm*40 mm*1 mm) for bonding, and cure at room temperature for 3 days. The process and formula are shown in Figure 2 and Table 1. The raw materials used in the experiment are shown in Table 2.
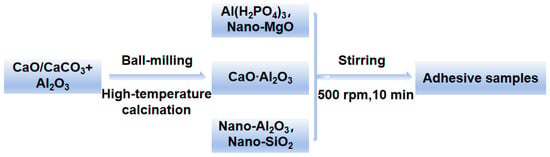
Figure 2.
Process for preparing the inorganic phosphate adhesive.

Table 1.
Curing promoter formulations (wt.%).

Table 2.
Experimental raw material.
2.2. Tensile Strength Test
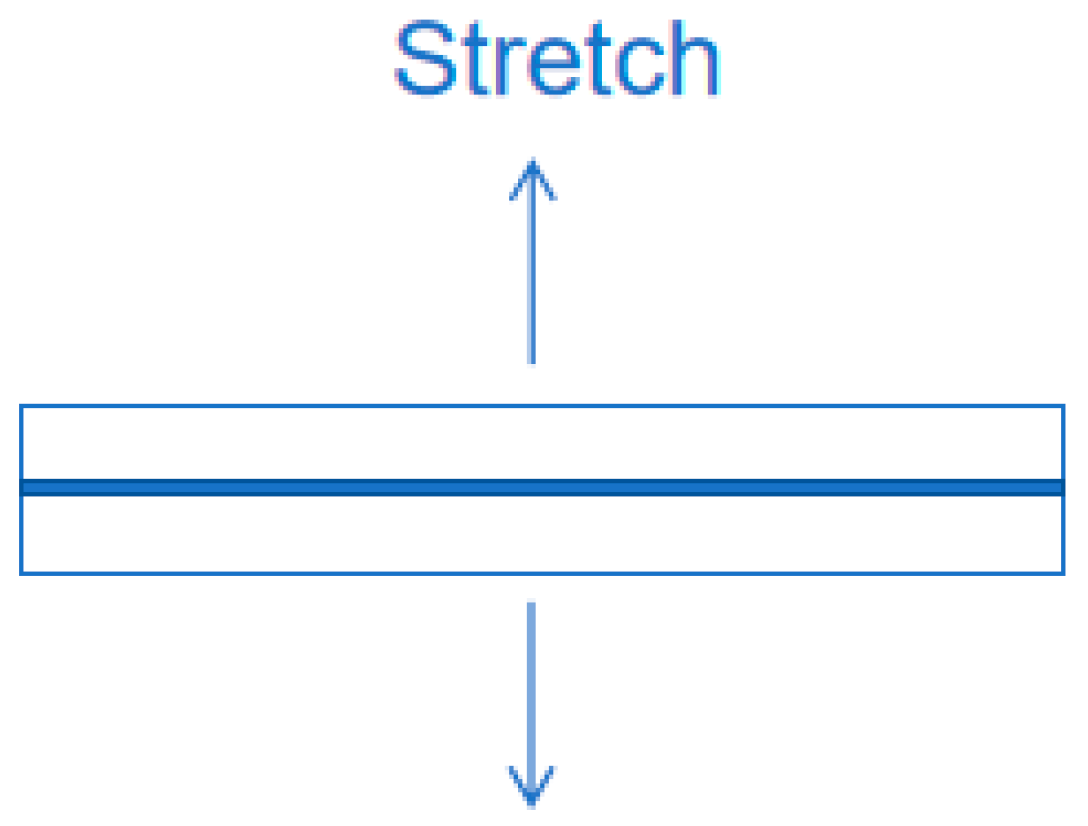
The bonding effect of phosphate binder is evaluated by testing the tensile strength of the binder bonded to ceramic tiles (30 mm*30 mm). After curing at room temperature and various environmental resistance tests, the binder is subjected to tensile testing using a universal testing machine to obtain the tensile strength (Figure 3). The test conditions are an inlet force of −1.25 N and a strain rate of 5 mm/min until the ceramic sample separates. Data analysis is based on the arithmetic mean of at least six measurements taken for each group of samples under the same conditions to ensure the accuracy of the experimental measurements. The calculation of tensile strength is shown in the following formula:
σ = Fm/So
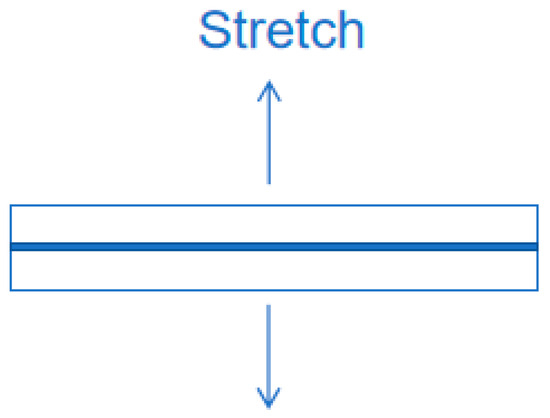
Figure 3.
Schematic diagram of tensile strength test.
σ—sample tensile strength (MPa); Fm—maximum force (N) experienced during sample separation; S0—original cross-sectional area of the sample (mm2).
2.3. Thermogravimetric Differential Scanning Calorimetry (TG-DSC)
This study uses the TA-SDT650 synchronous thermal analyzer from TA Instrument from the United States (New Castle, DE, USA) to conduct thermal analysis on the cured powder of phosphate binder, determining the complete curing temperature of phosphate binder and characterizing its thermal behavior. The test temperature was raised from room temperature to 1200 °C, with a heating rate of 10 °C min−1, and the atmospheric conditions were an air atmosphere.
2.4. X-ray Diffraction Analysis (XRD)
This study analyzes the phase changes of synthesized calcium aluminate and the solidified binder using an ARLX’TRA X-ray diffractometer from American thermal power company (Thermo Fisher Scientific, Waltham, MA, USA). The testing conditions are Cu target K α ray wavelength λ = 0.15046 nm, scanning rate of 10° min−1, and 2θ angle ranging from 10° to 80°. We collected XRD data of the products using Crystallographica Search-Match 3.1 and Jade 5.0 analysis software to analyze and process the measured data.
2.5. X-ray Photon–Electron Spectroscopy (XPS)
We examine the alterations in binding energy following the incorporation of nanoparticles, thereby analyzing the progression of the hydration reaction. Place the sample to be tested into the Kratos AXIS Ultra DLD electron spectrometer from Thermo Fisher Scientific (Waltham, MA, USA) to begin X-ray photoelectron spectroscopy testing. Irradiate with X-rays, adjust the electron detector, calibrate the beam intensity and energy position of the X-rays, and collect data. Conduct qualitative analysis on the surface elements of the test sample based on the obtained data, including the valence state of metal elements, the bonding structure of metal or non-metal elements, etc. The binding energy in the measurement results was corrected using the C1s (284.80 eV) binding energy.
2.6. Scanning Electron Microscopy Analysis (SEM)
This study characterizes the microstructure of the cured calcium aluminate powder and phosphate-based composite coating using a JSM-IT200 scanning electron microscope from Nippon Electric Corporation (Minato City, Tokyo). Before testing, the powder sample is dispersed on the silicon wafer and dried and coated with gold spray. We then analyze its constituent elements through EDS spectrometer.
3. Results and Discussion
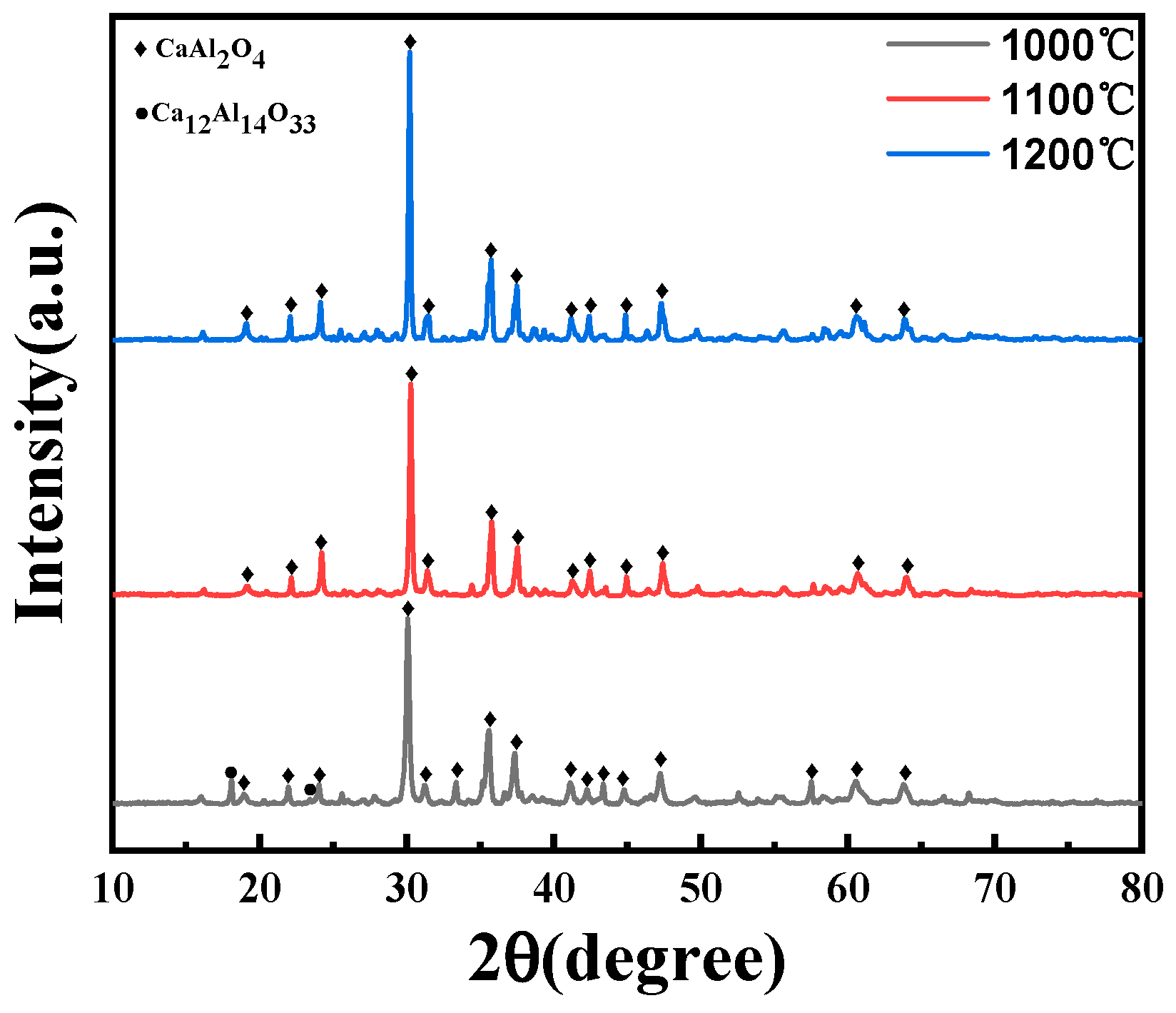
From Figure 4, when the calcination temperature is at 1100 and 1200 °C, the diffraction peak of the product is the pure CA phase, mainly because the Ca12Al14O33 phase begins to decompose at 900 and 1000 °C, and the following reaction occurs:
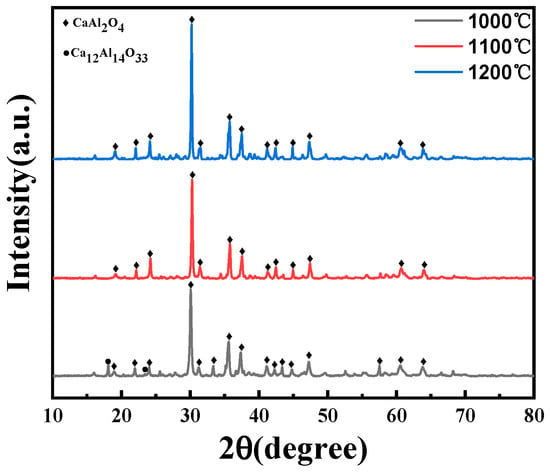
Figure 4.
XRD patterns of samples synthesized at different sintering temperatures.
The reaction produces the CaAl2O4 and Al2O3 phases. At a heat treatment temperature of 1000 °C, the decomposition reaction is incomplete, and distinct Ca12Al14O33 diffraction peaks are still observable within the XRD diffraction patterns. Nevertheless, at temperatures of 1100 and 1200 °C, the decomposition reaction is complete, and the phase composition is dominated by CA.
At a heat treatment temperature of 1000 °C, blurred grain boundaries are observable, with some grains undergoing phase transformation, suggesting they have not yet reached a state of complete growth (Figure 5d). At 1100 °C, the grains are well developed, with smaller size and more uniform distribution (Figure 5e). At 1200 °C, excessively high temperatures result in increased particle size and a non-uniform grain structure (Figure 5f). Consequently, 1100 °C is chosen as the heat treatment temperature for CA.
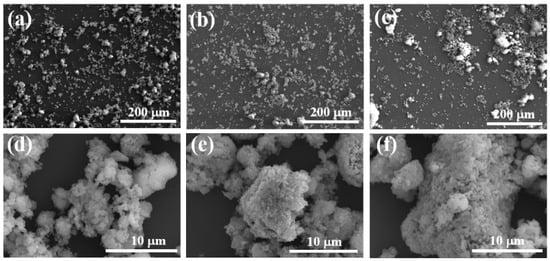
Figure 5.
SEM images of synthetic samples at different sintering temperatures: (a,d) 1000 °C; (b,e) 1100 °C; (c,f) 1200 °C.
When only CA and Al(H2PO4)3 are used, the cured sample’s adhesive surface appears powdery upon peeling, suggesting an unstable internal structure. As the CA content rises, the powdering of the adhesive worsens. This could be because the high CA content swiftly depletes the phosphate adhesive’s moisture, triggering hydration reactions that disrupt the phosphate’s three-dimensional network, leading to reduced adhesive efficacy, weakened ceramic bonding, and resultant ceramic detachment. Therefore, CA needs to be used in conjunction with other curing agents.
3.1. Thermal Properties
DSC curves of the adhesives with various curing promoters are shown in Figure 6.
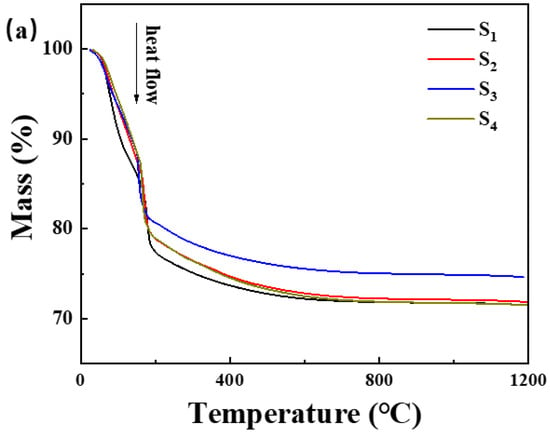
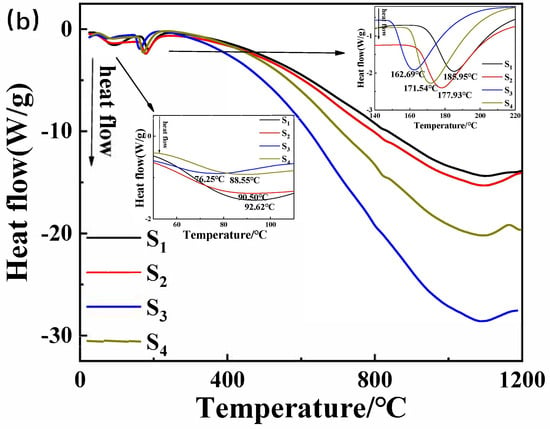
Figure 6.
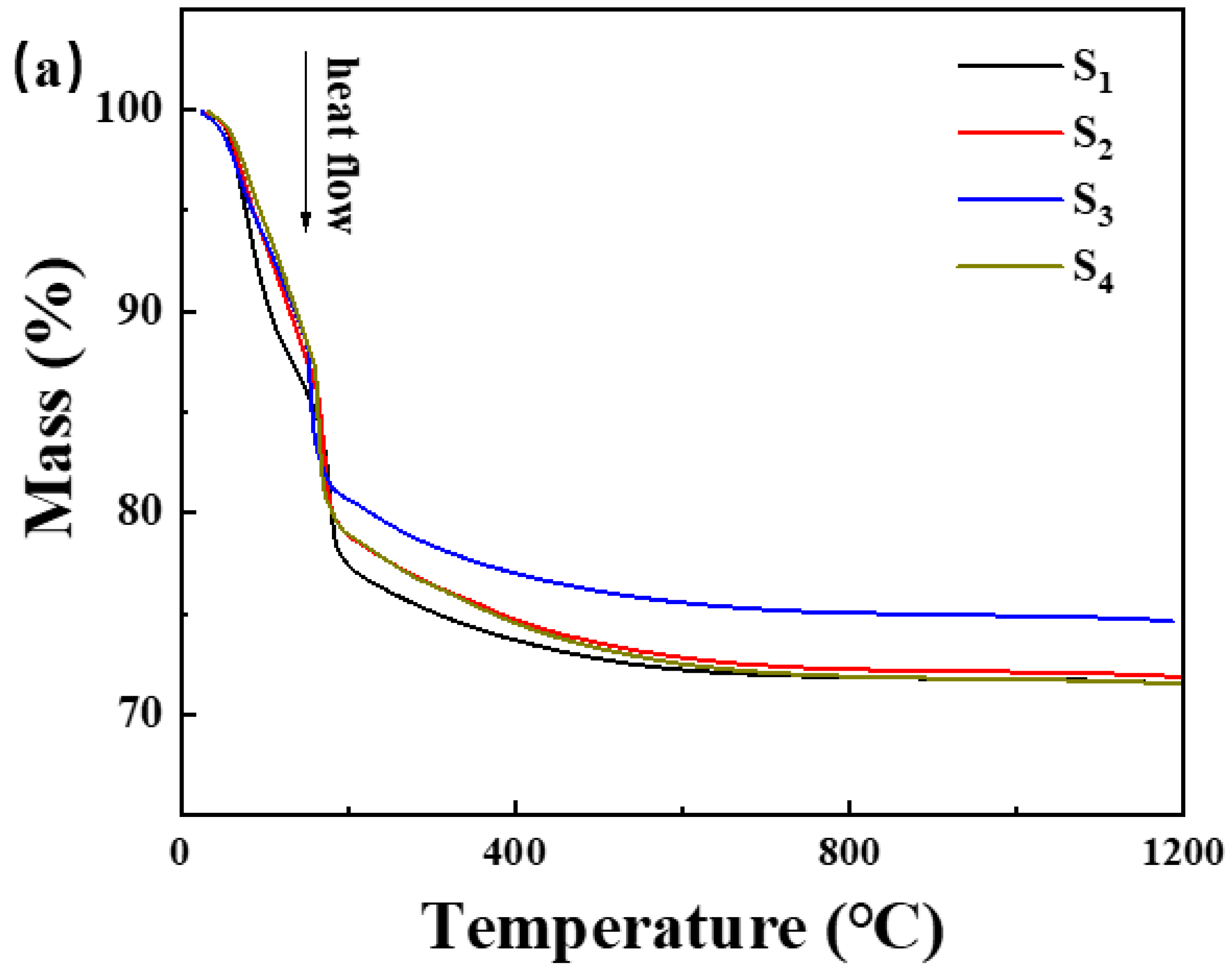
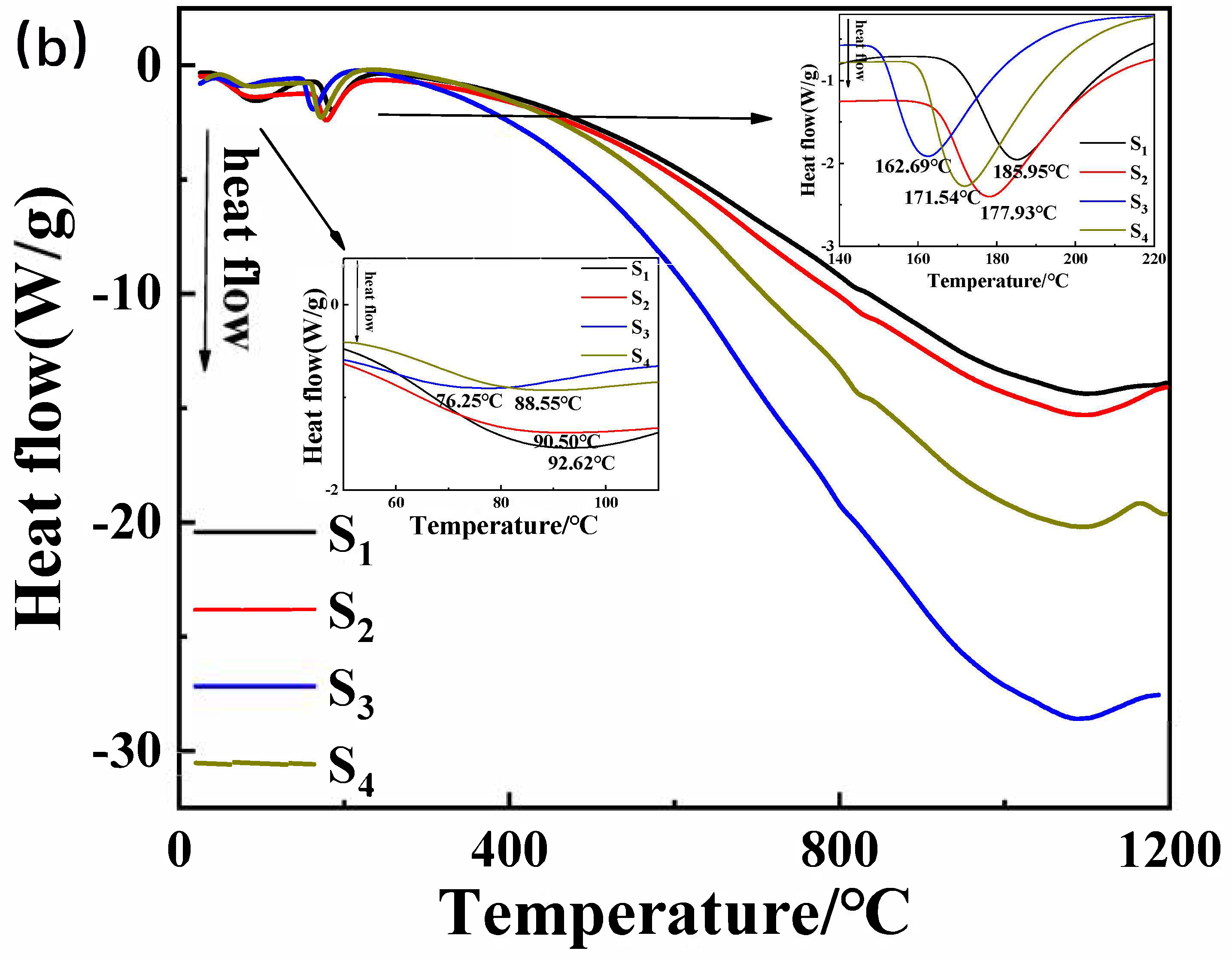
(a) Thermogravimetric curve and (b) differential scanning calorimetry curve of the samples in Table 1.
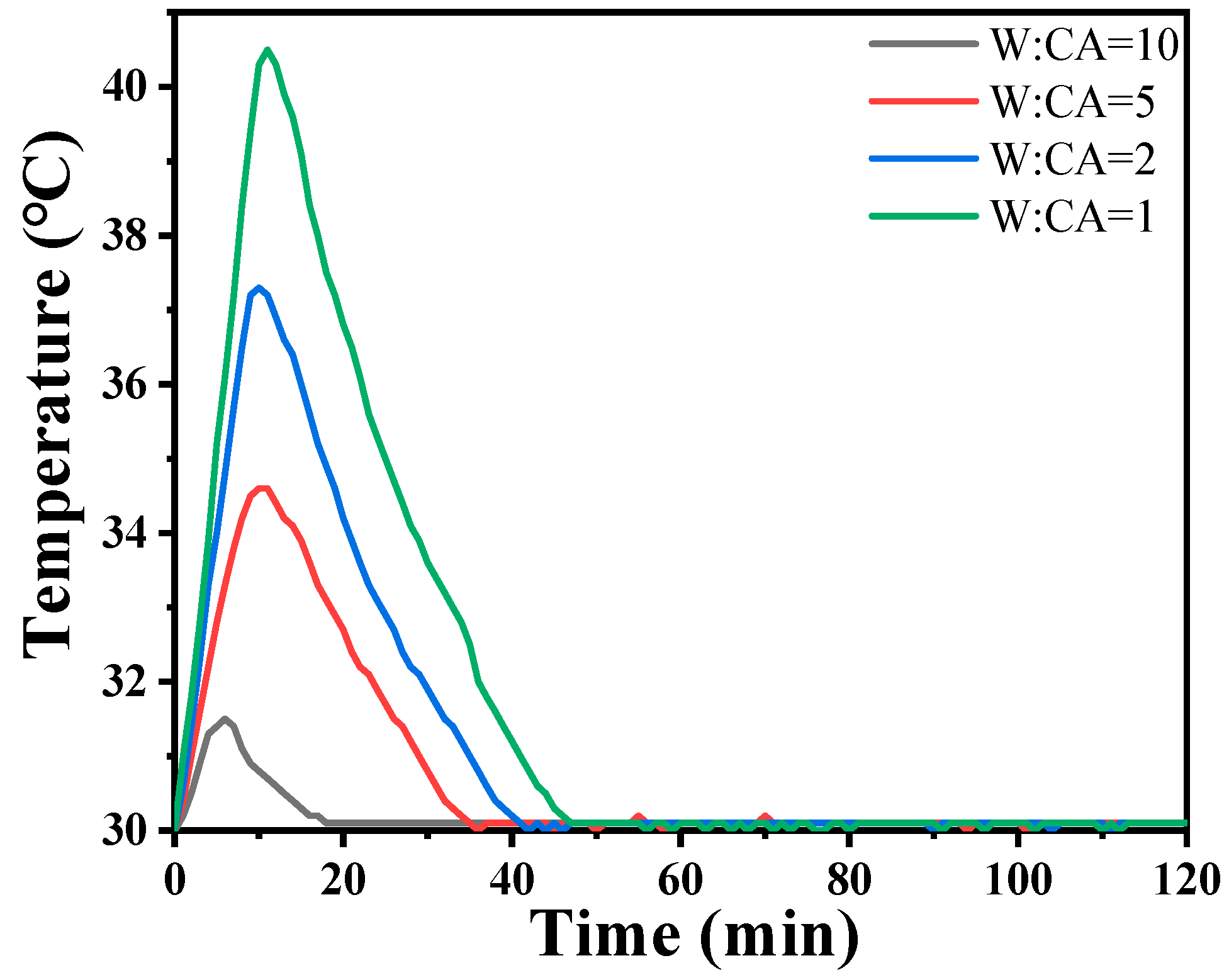
The incorporation of Nano-MgO alone does not allow the phosphate reaction mixture to set at room temperature. However, the inclusion of CA enables the absorption of moisture within the mixture, elevates its internal temperature, and facilitates the solidification of phosphate. In Figure 7, we prove that the hydration of CA promptly liberates a significant amount of heat. When the mass ratio of water to CA is 10:1, the temperature peaks at an increase of 1.5 degrees Celsius within 8 min. However, when the mass ratio of water to CA was 1:1, the temperature soared to a maximum of 10.5 degrees Celsius within 9 min. All other samples can be cured. The addition of nanoparticles increases the reaction sites and connection points. To determine the curing reaction process of phosphate-based composite binders, four sets of samples were ground into powder and subjected to thermogravimetric differential thermal analysis. The test results are shown in Figure 7. It can be seen from Figure 6 that there are two obvious endothermic peaks on the DSC curves of the four groups of binder samples with different inorganic fillers added, corresponding to the preliminary curing peak and the complete curing peak of the phosphate-based composite binder, respectively. The preliminary and complete curing peaks of binders without inorganic fillers (S1) were 92.62 and 188.95 °C; the preliminary and complete curing peaks of binders with 4 wt.% Nano-Al2O3 (S2) were 90.50 and 177.93 °C; the preliminary and complete curing peaks of binders with 4 wt.% Nano-SiO2 (S3) were 76.25 and 162.69 °C; and the preliminary and complete curing peaks of binders with 2 wt.% Nano-Al2O3 and Nano-SiO2 (S4) were 88.55 and 177.93 °C. It is worth noting that after adding inorganic fillers, the curing temperature significantly decreases, and the most significant decrease in curing temperature occurs when nano alumina is added. Due to the acidic nature of phosphates and the higher alkalinity of nano alumina compared to silica, alumina can serve as both an inorganic filler and a curing agent to promote the curing of binders. Therefore, adding nano alumina will generate strong acid–base reactions, promote the curing process, and further reduce the curing temperature.
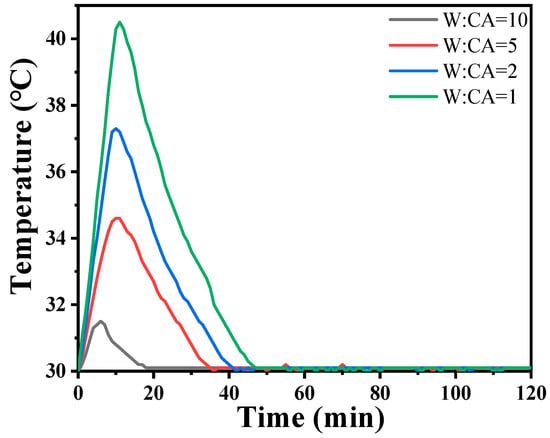
Figure 7.
Hydration heat curves of CA solution at 30 °C.
In summary, the TG-DSC curve indicated that the incorporation of CA absorbed moisture during the reaction, elevated the internal temperature, and facilitated the solidification of phosphate, thus lowering the curing temperature of the binder. Nano-Al2O3 and Nano-SiO2 underwent robust acid–base reactions, accelerating the curing process and further reducing the curing temperature.
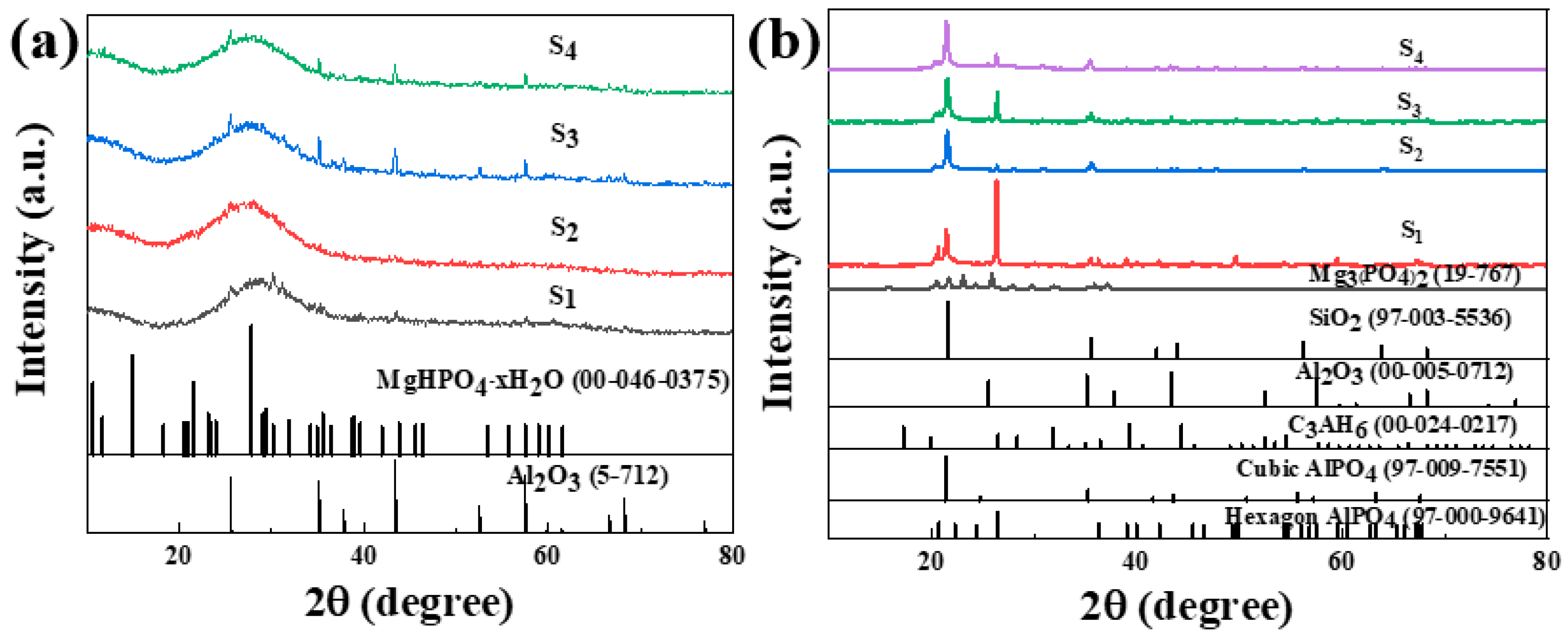
3.2. XRD Patterns of the Specimens
Through the TG-DSC analysis mentioned above, it was determined that the complete solidification peak of the sample is around 200 °C. The sample was heat-treated at 200 °C for 2 h in a tube furnace and cooled down with the furnace. The XRD test was conducted on the original sample and heat-treated sample. The test results are shown in Figure 8.
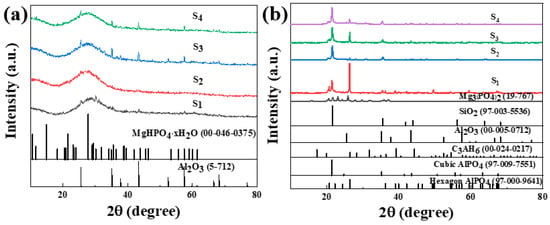
Figure 8.
XRD patterns of phosphate-based composite binder before (a) and after heat treatment (200 °C for 2 h) (b).
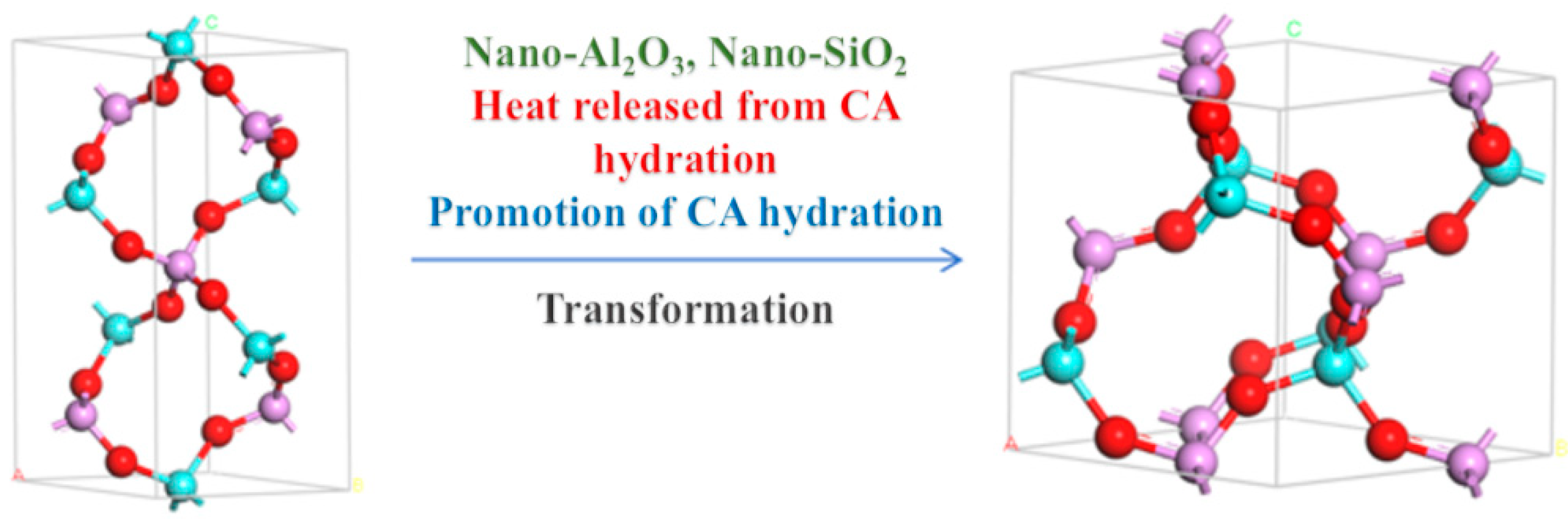
Figure 8a shows the XRD pattern of the phosphate-based composite adhesive after curing at room temperature for 5 days. After natural curing at room temperature for 5 days, a large hump appeared between 20 and 35°. The sample doped with a small amount of Nano-Al2O3 exhibited an incomplete reaction Al2O3 (ICDD-PDF 5-712) diffraction peak, and the incomplete reaction Al2O3 diffraction peak increased with an increase in the doping amount. The diffraction peak of MgHPO4∙xH2O (00-046-0375) appears in the sample due to the high activity of the reaction between MgO and the aluminum phosphate matrix. In addition, the system contains a small amount of CA hydration products, CAH10, C2AH8, and C3AH6 diffraction peaks, while other components exist in amorphous form. Figure 8b shows the XRD pattern of the phosphate-based composite adhesive after heat treatment. After heat treatment at 200 °C, the hump between 20 and 35° disappeared, and the samples were in a fully cured state. The hydration reaction of CA was complete in the four samples, and only the diffraction peak of the final product C3AH6 of CA hydration could be seen in XRD, indicating that the intermediate products CAH10 and C2AH8 were completely converted to C3AH6, with the strongest diffraction peak in S1. This is mainly because its CA content is the highest, resulting in the highest content of the hydration product C3AH6. The acceleration of the CA hydration process led to an increase in the reaction temperature in the system, accelerated the consumption of water in the system, and, thus, promoted the curing of the phosphate binder. Diffraction peaks of hexagonal AlPO4 and Mg3(PO4)2 of S1 were observed, indicating that Mg2+ entered the structure of the aluminum phosphate system and played a connecting role in the structure. With the addition of Nano-Al2O3, the diffraction peak of hexagonal AlPO4 decreases, while the diffraction peak of cubic AlPO4 relatively increases. Nano-Al2O3 promotes the transformation of hexagonal AlPO4 generated by the reaction into more stable cubic AlPO4 (Figure 9). Observing S2 and S4, in addition to the diffraction peaks of reaction-inert Nano-SiO2, Nano-SiO2 can also promote the transformation of hexagonal AlPO4 to cubic AlPO4 generated by the reaction. The addition of inorganic fillers can provide a large number of reaction sites, promoting the transition of the system to a more stable crystal phase. Therefore, both Nano-Al2O3 and Nano-SiO2 can serve as curing agents for phosphate binders to a certain extent, promoting the curing process of phosphate. Meanwhile, Nano-Al2O3 can accelerate the formation of CA hydration products, resulting in higher crystallinity of its hydration product C3AH6.
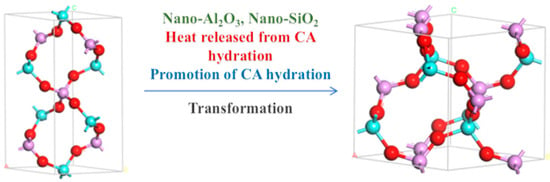
Figure 9.
Crystal transformation during heat treatment process.
To ascertain the hydration process of the binder reaction, the binder reaction was initially accelerated through heat treatment. XRD results indicated that when only CA and Nano-MgO were doped, the product was completely transformed into C3AH6, with diffraction peaks of hexagonal AlPO4 and Mg3(PO4)2 of S1 observed. It was concluded that the accelerated CA hydration process elevated the system’s reaction temperature, thereby accelerating water consumption and enhancing the curing of phosphate binders. As seen in S2 and S4, the addition of nano fillers further facilitates the transformation of the reaction-formed hexagonal AlPO4 to cubic AlPO4. This is attributed to the numerous reaction sites provided by the inorganic fillers, which encourage the system’s transition to a more stable crystal phase. Consequently, the synergistic effect of CA and nano fillers enhances the binder’s stability.
3.3. Micromorphology
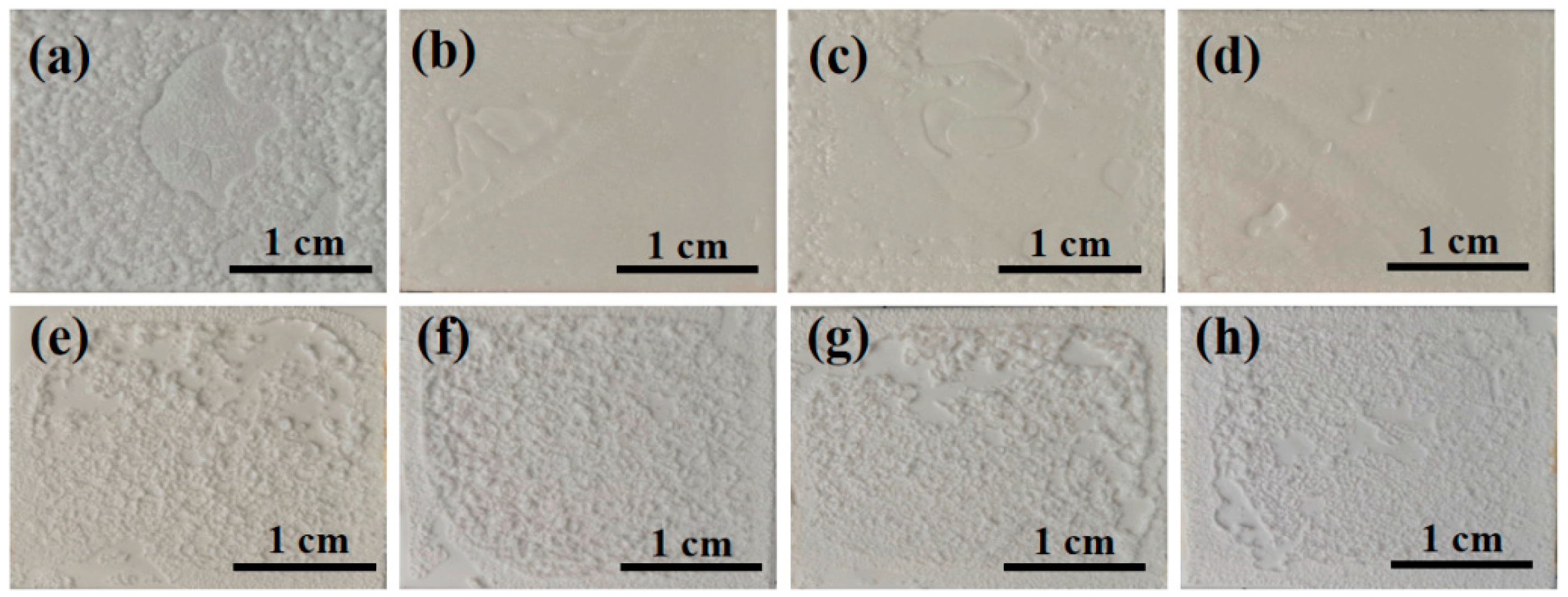
Figure 10 shows the apparent morphology of the sample after hydration and heat treatment. It can be seen from the figure that at room temperature, with the addition of nano fillers, the binder structure becomes denser, resulting in higher tensile strength. Heat treatment can observe significant volume expansion of the adhesive, greatly reducing its tensile strength.
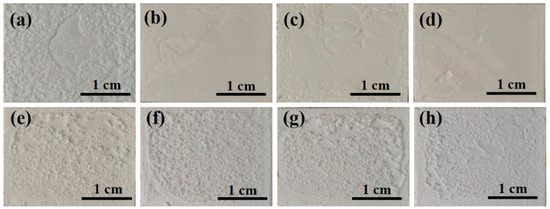
Figure 10.
Surface morphology diagram of samples (a–d) after hydration and (e–h) after heat treatment.
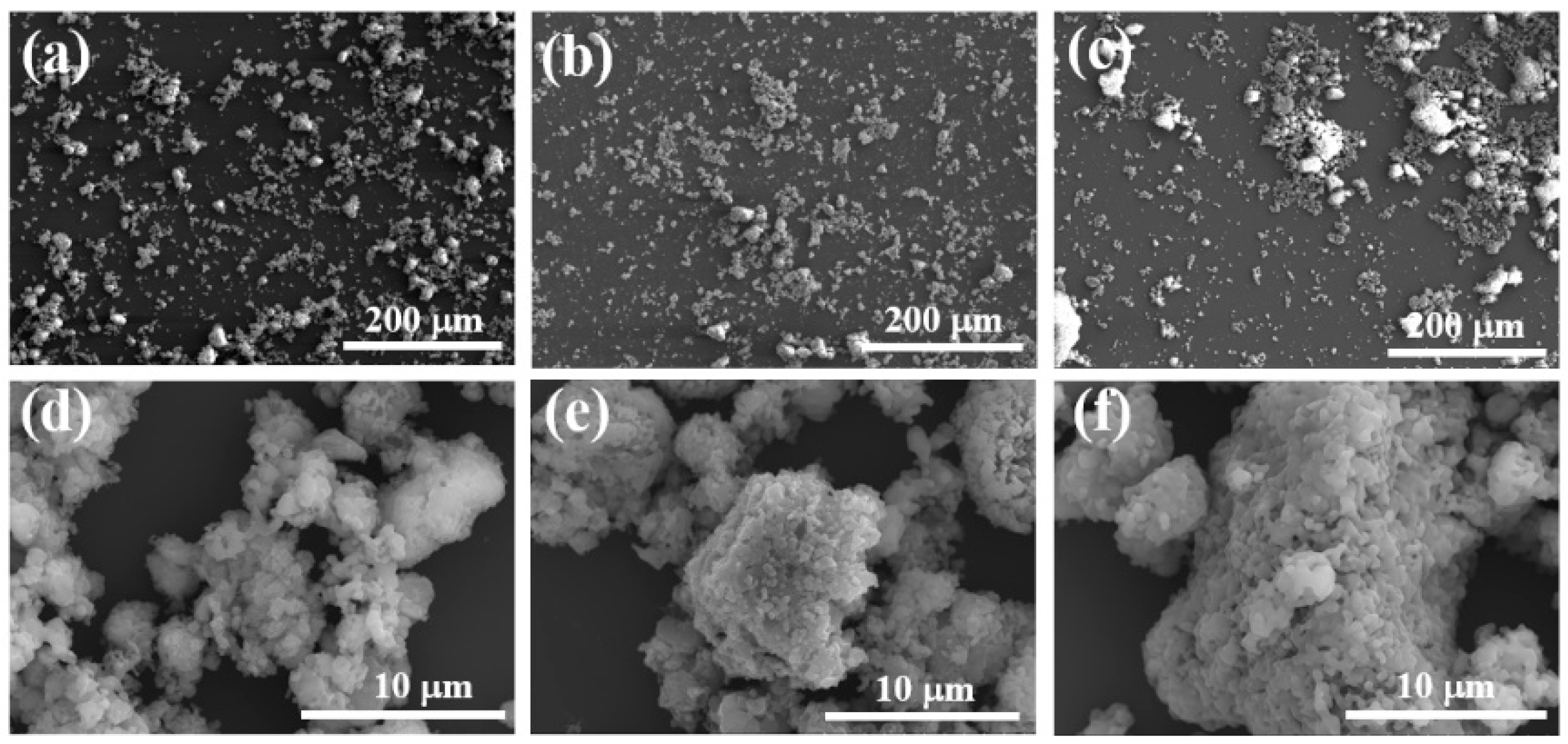
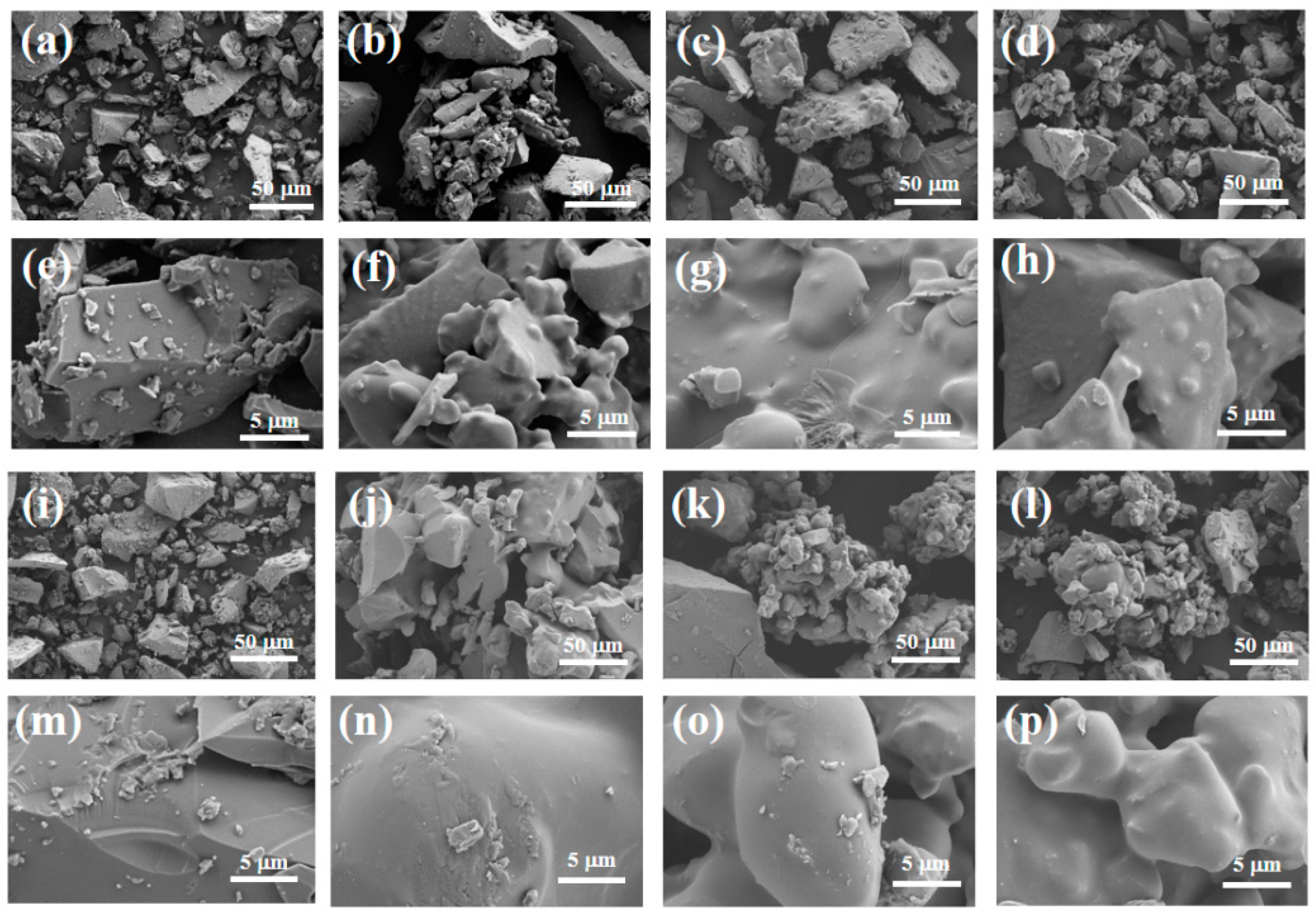
Figure 11 shows the surface microstructure of the adhesive at a magnification of 500 and 5000 times. It can be clearly observed from Figure 11e,m that there are many exposed particles on the surface of the adhesive, and there are cracks in S1. The surface quality of the adhesive is relatively rough. This is mainly because the CA content added by the binder is relatively high, resulting in more moisture in the coating, while the AP content is low, which is not enough to generate a large amount of the AlPO4 bonding phase to wrap the ceramic particles. When the coating is cured at high temperature, the liquid water evaporates due to heat and will be dispersed from between the exposed ceramic particles, leading to crack defects on the surface of the coating. However, the adhesive and surface in Figure 11f,h,n,p are flat and smooth, without obvious holes or cracks, and the density of the coating is significantly improved. This indicates that with an increase in the content of nano-SiO2, the particles can be better encapsulated, the adhesion between particles is improved, and the quality of the binder gradually improves. Therefore, it can be concluded that an increase in the content of nano-SiO2 helps to improve the surface quality of the binder.
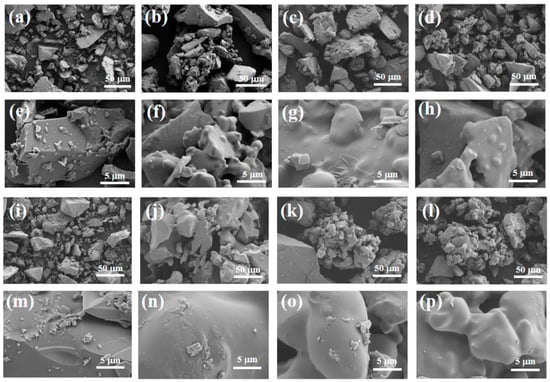
Figure 11.
Scanning electron microscopy of samples (a–h) after hydration and (i–p) after heat treatment.
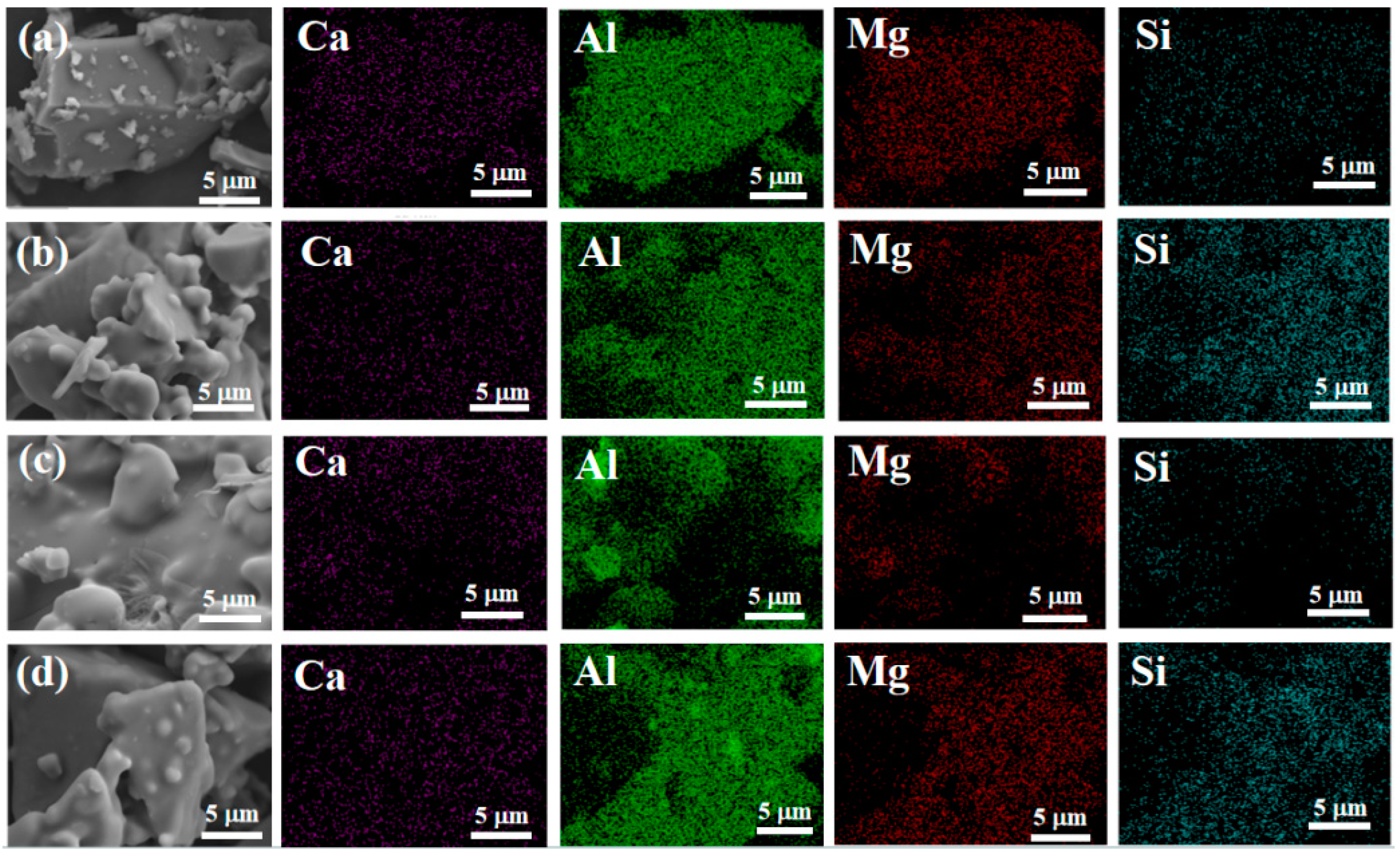
Figure 12 shows the distribution of silicon elements on the surface of the sample. It can be seen that silicon elements are uniformly distributed in samples S2 and S4, indicating that the nano fillers are uniformly mixed into the binder.
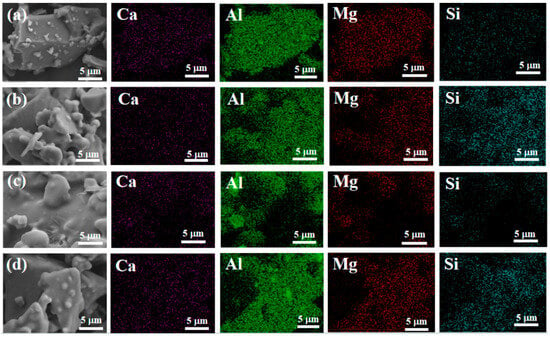
Figure 12.
Energy-dispersive spectrometry of samples after hydration. ((a)-S1, (b)-S2, (c)-S3, (d)-S4).
Overall, at room temperature, as nano fillers were added to the binder, the structure became denser, and the tensile strength increased. After heat treatment, the adhesive structure underwent significant volume expansion, greatly reducing its tensile strength. Under scanning electron microscopy, we found that when only doped with CA, due to insufficient AP content, the adhesive reaction was too rapid, and there were still many unreacted exposed particles on the surface, which affected its tensile strength. However, after adding nano fillers, the surface was smoother. With an increase in nano fillers, the particles were better embedded, the adhesion between particles was improved, and the quality of the adhesive gradually improved. Therefore, it can be concluded that the increase in nano fillers helps to improve the surface quality of adhesives. Scanning the energy spectrum also revealed that the silicon element was uniformly distributed in samples S2 and S4, indicating that the nano fillers were uniformly mixed into the binder.
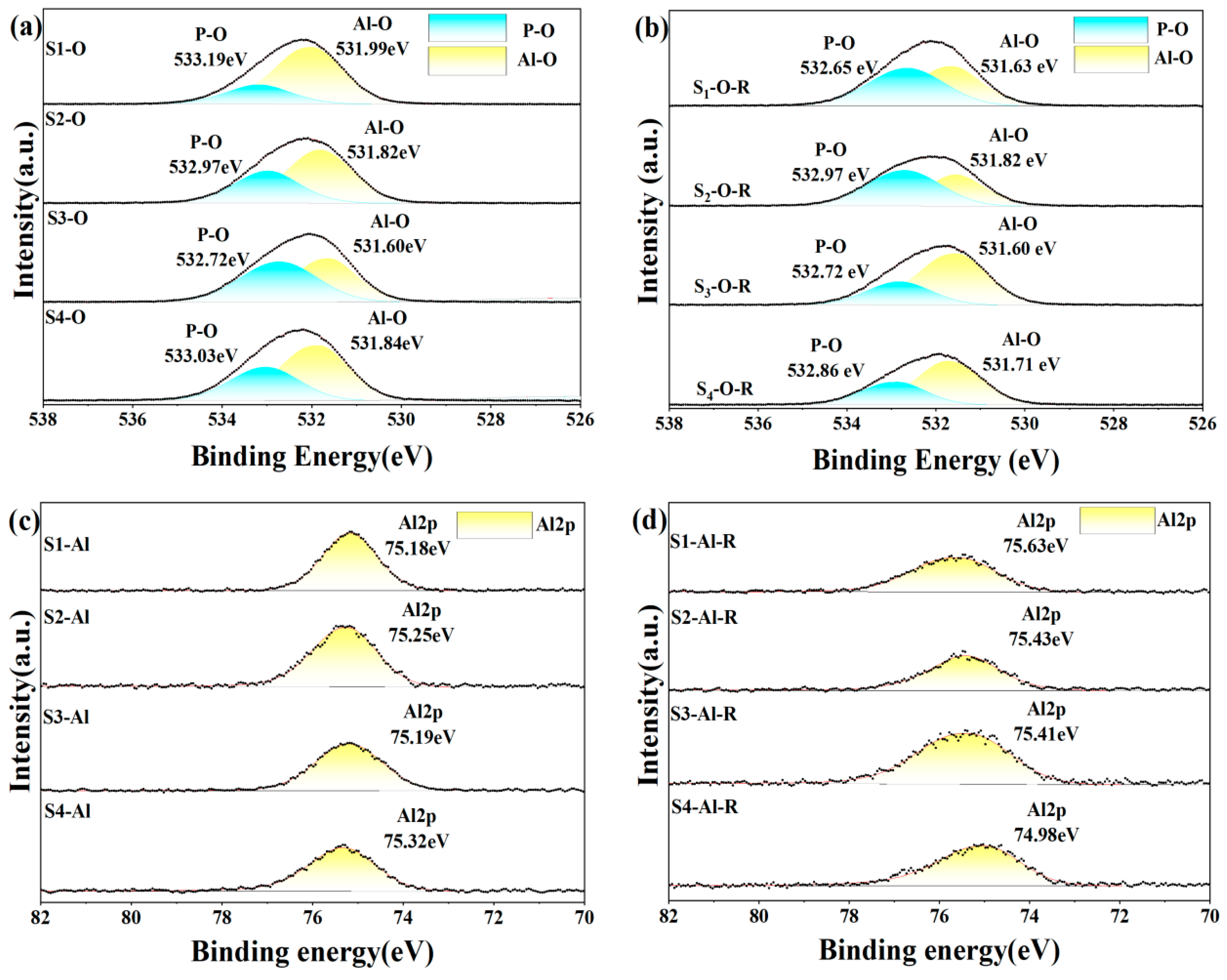
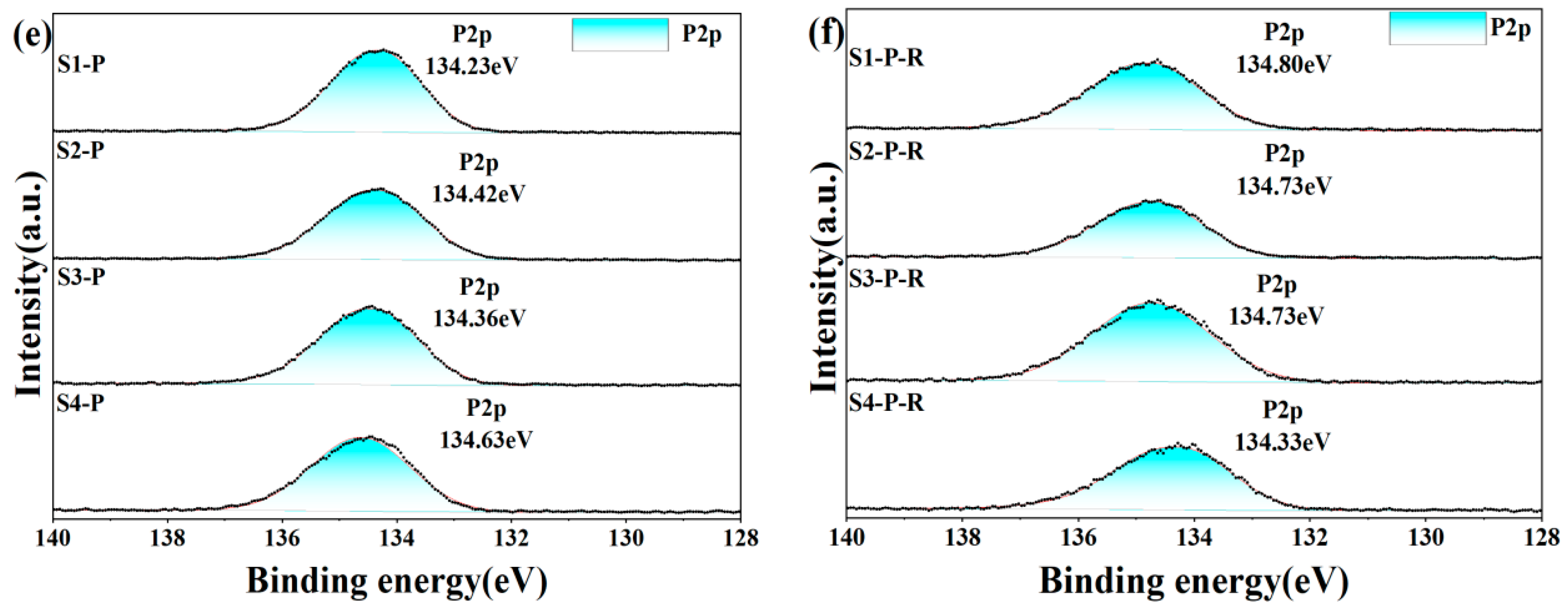
3.4. XPS
To further understand how different inorganic oxides and phosphates bind to each other, we analyzed XPS narrow-spectrum scans of different elements before and after heat treatment of the sample. In the four groups of samples after five days of hydration, the Al2p and P2p spectra (Figure 13) showed that the binding energies of the doped inorganic nano fillers Al2p and P2p increased, the doped binding energy decreased, and the density of the outer electron cloud on the surface increased; the incorporation of nanoparticles promoted the hydration reaction. O1s spectra can be divided into groups of independent component peaks that overlap with each other from different groups. Using different nano-inorganic particles as a comparison sample, the O1s binding energy (531.82 eV) in the Al-O bond of the S1 sample was higher than that of the S3 sample (531.60 eV) in the O1s spectrum. In the O1s spectra of S2 and S3, the O1s binding energy (533.03 eV) in the P-O bond of S4 samples was higher than that of S2 and S3 samples, and the results showed that under the same conditions, the combined action of silica and alumina had a greater effect on the oxygen atoms on the P-O bond, and the combined action of silica and alumina promoted the curing process of phosphate. Among the four groups of samples heat-treated, it was found that the binding energy of S1 of the undoped nano filler increased the most after heat treatment, indicating that the heat treatment could accelerate the transformation of hexagonal AlPO4 to a more stable cubic AlPO4. It was found that the binding energies of Al2p and P2p in S2 and S3 samples increased but lower than that of the undoped S1 samples, indicating that the steric hindrance effect of nanoparticles (the steric hindrance effect primarily denotes the spatial obstruction arising from the proximity of certain atoms or groups within a molecule, and, with the incorporation of Nano-SiO2 and Nano-Al2O3, there is an elevation in activity at elevated temperatures, which sterically obstructs the reaction sites of the CA hydration, thus delaying the hydration reaction’s progression) hindered the progress of the hydration reaction under high-temperature conditions, and it was found that the binding energy of Al2p and P2p in S4 decreased, maybe because Nano-Al2O3 had high activity as a filler and reacted with Nano-SiO2 at high temperature, coupled with the steric hindrance effect, which seriously hindered the progress of the hydration reaction. The binding energy of the O1s spectrum was lower than that of the samples before heat treatment, which proved that the hexagonal crystal system AlPO4 changed to cubic AlPO4 after heat treatment.
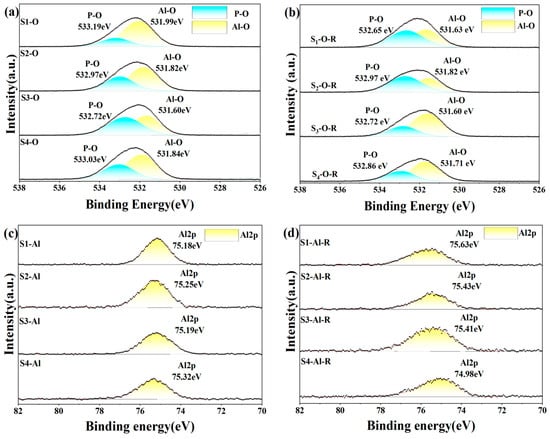
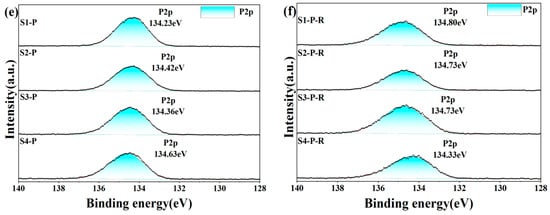
Figure 13.
XPS spectrum of hydration samples before (a,c,e) and after heat treatment (200 °C for 2 h) (b,d,f).
3.5. Tensile Strength Test
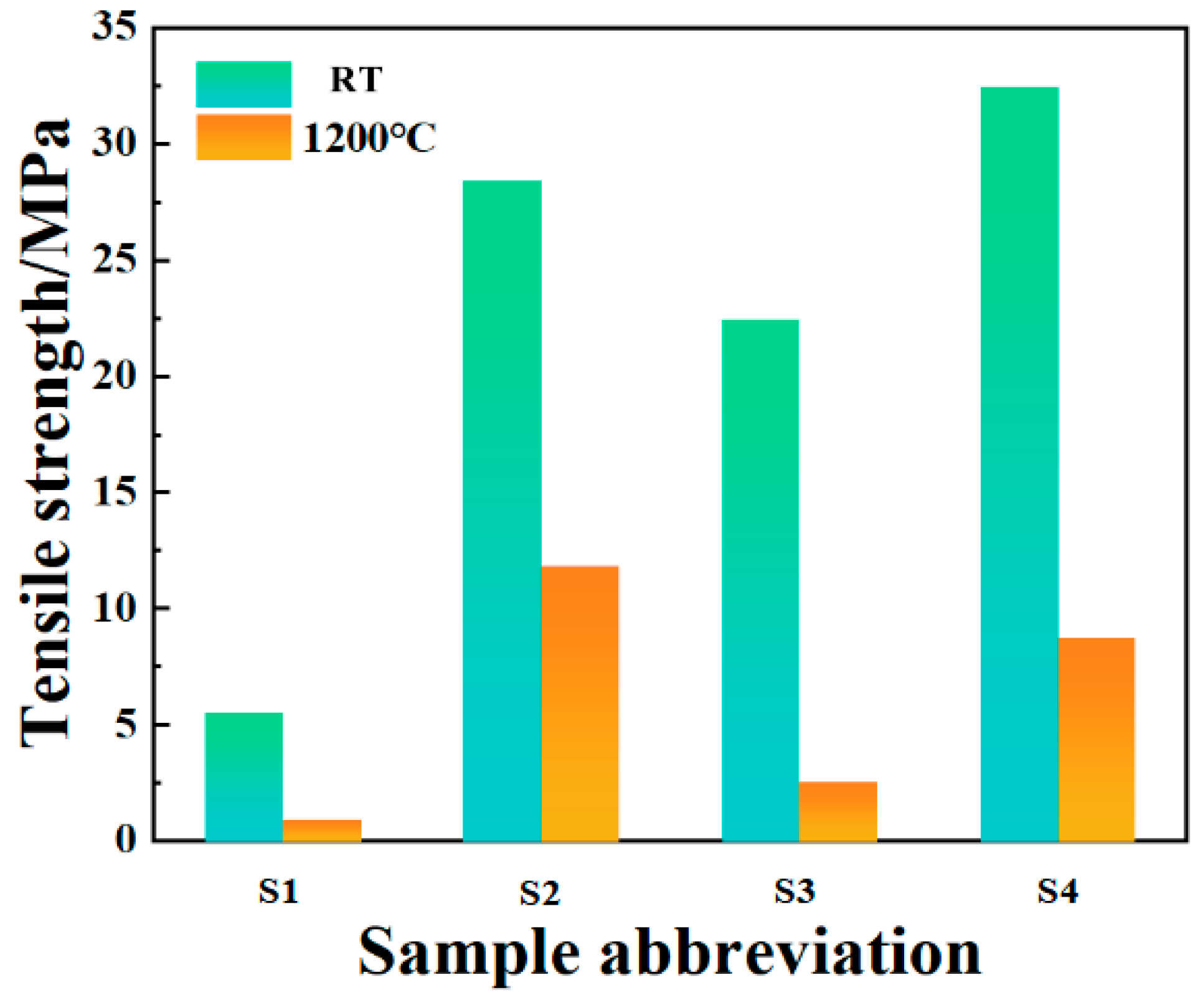
Figure 14 shows the tensile strength of the bonded sample after room-temperature curing and high-temperature resistance testing. It can be seen that the bonding strength of the sample after room temperature curing is lower when no filler is added. This may be due to the high content of CA, excessive water consumption during hydration, and the hydration products filling the interior of the phosphate bonding agent to damage the network structure, resulting in a decrease in its strength. The addition of nano inorganic fillers as curing agents significantly improves the density and viscosity of the binder system. On the one hand, the addition of inorganic fillers can increase the density and viscosity of the binder system, provide a large number of reactive sites, and fill the pores of the binder to reduce the porosity, which helps the diffusion rate of reactants in the system. Rapid diffusion enables reactants to contact and react more quickly, thereby accelerating the curing process. On the other hand, Nano-Al2O3 can serve as a curing agent for phosphate binders and accelerate the formation of CA hydration products, further promoting the curing reaction. Among them, when the doping levels of Nano-Al2O3 and Nano-SiO2 are both 2 wt.%, the tensile strength is the highest, reaching 32.48 MPa. Compared to the values reported in the literature (7.56 MPa) [32], the tensile strength has significantly increased.
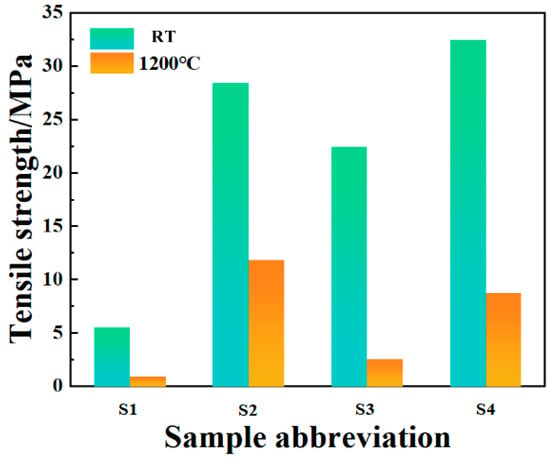
Figure 14.
Tensile strength of bonded samples after room-temperature curing and high-temperature test.
After curing at room temperature, the samples were subjected to high-temperature resistance tests at 1200 °C (Table 3). The tensile strength of the samples after removal is shown in the figure. It can be seen that the bonding ability of the samples without inorganic fillers is basically lost, indicating poor high-temperature resistance. However, after adding inorganic fillers, the tensile strength of the samples showed a certain degree of decrease. Among them, the samples in S3 and S4 experienced severe volume expansion, which may be due to the grain growth phenomenon of the particles under high-temperature conditions with the addition of Nano-Al2O3. Small grains migrate and rearrange at the interface to become larger grains. This grain growth causes the material to expand in volume, leading to the destruction of the network structure of the system by the fillers filled in the pores of the phosphate binder and further resulting in a significant loss of tensile strength in the system. Among them, when the inorganic filler is only Nano-SiO2 and the dosage is 4 wt.%, the decrease rate of the system’s tensile strength is the lowest, reaching 58.27%, and the tensile strength is 11.86 MPa at this time. Compared to the values reported in the literature (5.06 MPa) [32], the tensile strength has significantly increased.

Table 3.
Tensile strength of comparison sample and bonded samples after room-temperature curing and high-temperature test.
Based on the TG-DSC and XRD analysis, it can be concluded that the curing mechanism of the aluminum phosphate-based composite adhesive is as follows: during the curing process of the aluminum phosphate composite adhesive, CA continuously consumes water in the hydration process of the system to generate hydration products of CAH10, C2AH8, and C3AH6, and the intermediate products continue to transform towards the stable state of C3AH6. The addition of inorganic filler Nano-Al2O3 can promote a higher degree of crystallization of C3AH6, which is conducive to the improvement in the adhesive strength of the system. CA continuously releases heat during the hydration process, which increases the temperature of the system and provides temperature conditions for the curing of aluminum phosphate. The addition of curing agent Nano-MgO can react with the adhesive. Mg2+ enters the interior of the binder and connects the chain like phosphate through ionic bonds, promoting the formation of a three-dimensional network structure and ultimately promoting the curing of the binder into a film. The addition of nano inorganic fillers fills the pores of the system, increasing the density and viscosity of the binder system while providing a large number of active sites for the reaction, promoting the curing reaction, reducing the curing temperature, and further improving the adhesive strength of the binder.
4. Conclusions
This study addresses the performance of an ultra-high-temperature inorganic phosphate adhesive that is cured at room temperature. It has been demonstrated that the hydration reaction of CA involves the absorption of substantial amounts of water and generates a significant amount of heat, thereby enhancing the curing process of the adhesive. Two types of nano fillers are incorporated to improve the adhesive’s performance, with Nano-Al2O3 increasing its reaction sites and Nano-SiO2 boosting its thermal stability. Ultimately, it was found that the adhesive formulation added CA containing 2% Nano-Al2O3 and 2% Nano-SiO2 achieved a remarkable tensile strength of 32.48 MPa at room temperature. After undergoing heat treatment at 1200 °C, the adhesive formulation fortified with 4% Nano-SiO2 in CA exhibited the least pronounced decline in tensile strength, with a reduction of merely 58.27%, resulting in a residual tensile strength of 11.86 MPa, underscoring its potential as an efficient solution for various practical adhesive applications. This strategy provides an efficient and reliable solution for a diverse range of practical high-temperature adhesive applications.
Author Contributions
Conceptualization, Y.N.; Formal analysis, L.Z.; Investigation, Z.D.; Resources, Z.F.; Data curation, Z.D. and Z.F.; Writing—original draft, L.Z. and K.Y.; Writing—review & editing, K.Y. and Y.L.; Supervision, Y.N. and C.L.; Funding acquisition, Y.L. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, J.; Li, M.; Liu, J.; Liu, L.; Chen, Z.; Yang, F.; Shi, C. Corrosion Resistance of Cement-Based Materials with Sugarcane Bagasse Ash-Metakaolin-Portland Cement Binder under Carbonic Acid Water Environment. J. Build. 2024, 87, 109065. [Google Scholar] [CrossRef]
- Stepanov; Antonov, P.A.; Atroshchenko, V.V.; Nikulina, I.G.; Stroeva, O.V.; Borodai, O.A.; Ya, F.; Nepovinnykh, L.K. Radioparent Heat-Resistant Coating Based on Inorganic Binder. Refract. Ind. Ceram. 2017, 58, 239–241. [Google Scholar] [CrossRef]
- Wu, W.; Li, L.; Yu, J.; Xu, S.; Zhang, C.; Xue, L. Investigation on Thermo-Oxidative Aging Properties of Asphalt Binder with Hindered Phenolic Antioxidant. J. Test. Eval. 2018, 46, 624–630. [Google Scholar] [CrossRef]
- Barmouz, M.; Steinhäuser, F.; Azarhoushang, B.; Khosravi, J. Influence of Bond Thermal and Mechanical Properties on the Additively Manufactured Grinding Wheels Performance: Mechanical, Wear, Surface Integrity, and Topography Analysis. Wear 2024, 538–539, 205215. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Zhou, K.; Liu, X.; Wang, J.; Zhao, B. Enhanced Mechanical Stability and Corrosion Resistance of Superhydrophobic Coating Reinforced with Inorganic Binder. J. Cent. South Univ. 2024, 17, 3998. [Google Scholar] [CrossRef]
- Jarnerud, T.; Karasev, A.V.; Jnsson, P.G. Briquetting of Wastes from Pulp and Paper Industries by Using AOD Converter Slag as Binders for Application in Metallurgy. Materials 2019, 12, 2888. [Google Scholar] [CrossRef]
- Wang, F.; Cui, X.; Yang, Q. Fabrication and Posttreatment for Inorganic Binder Jetting Sand Molds for Casting. Addit. Manuf. 2023, 73, 103690. [Google Scholar] [CrossRef]
- Tchio, J.A.; Yerima, N.; Kaze, C.R. Design and Characterization of Iron-Calcium-Aluminum-Silicate-Hydrate as Low-Temperature Binder. Innov. Infrastruct. Solut. 2024, 9, 42. [Google Scholar] [CrossRef]
- Song, L.; Liu, W.; Xin, F.; Li, Y. Study of Adhesion Properties and Mechanism of Sodium Silicate Binder Reinforced with Silicate Fume. Int. J. Adhes. Adhes. 2021, 106, 102820. [Google Scholar] [CrossRef]
- Ma, C.; Chen, H.; Wang, C.; Zhang, J.; Qi, H. Effects of Nano-Aluminum Nitride on the Performance of an Ultrahigh-Temperature Inorganic Phosphate Adhesive Cured at Room Temperature. Materials 2017, 10, 1266. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, S.; Zhang, X. Preparation, Structures and Properties of Phosphate/S-PEEK Hybrid Composites Utilized Sintering Al(OH)3 Particles as Curing Agents. Ceram 2021, 47, 9711–9716. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Nkwaju, R.Y. Preparation of Acid Aluminum Phosphate Solutions for Metakaolin Phosphate Geopolymer Binder. RSC Adv. 2021, 11, 32258–32268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bian, D.; Zhao, Y. The Influence of Curing Agents on Thermal Property and Corrosion Resistance of Chemically Bonded Phosphate Ceramic Coatings. J. Sol-Gel Sci. Technol. 2019, 89, 403–415. [Google Scholar] [CrossRef]
- Banganayi, F.C.; Nyembwe, D.K.; Polzin, H. Optimisation of an Environmentally Friendly Foundry Inorganic Binder Core Making Process for the Replacement of an Organic Binder. MRS Adv. 2020, 5, 1323–1330. [Google Scholar] [CrossRef]
- Lu, W.; Chung, D.D.L. Oxidation Protection of Carbon Materials by Acid Phosphate Impregnation. Carbon 2002, 40, 1249–1254. [Google Scholar] [CrossRef]
- Zárybnická, L.; Machotová, J.; Mácová, P.; Machová, D.; Viani, A. Design of Polymeric Binders to Improve the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2021, 290, 123202. [Google Scholar] [CrossRef]
- Fernando, J.A.; Chung, D.D.L. Improving an Alumina Fiber Filter Membrane for Hot Gas Filtration Using an Acid Phosphate Binder. J. Mater. 2001, 36, 5079–5085. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhu, S. Preparation of an Aluminium Phosphate Binder and Its Influence on the Bonding Strength of Coating. Bull. Mater. Sci. 2019, 42, 200. [Google Scholar] [CrossRef]
- Xie, L.; Li, Y.; Hu, W.; Fang, S.; Chen, X.Q. A Novel Inorganic Phosphate-Based Adhesive for Bonding Archaeological Pottery: A Preliminary Exploration. Herit. Sci. 2024, 12, 164. [Google Scholar] [CrossRef]
- Shirani, S.; Cuesta, A.; Torre, A. CAC Conversion Analysed by Ptychographic Nanotomography. Cem. Concr. Res. 2020, 137, 106201. [Google Scholar] [CrossRef]
- Lapyote, P.; Issara, S. Dissolution, Nucleation and Crystal Growth Mechanism of CAC. J. Sustain. Cem.-Based Mater. 2019, 8, 180–197. [Google Scholar]
- Hertel, T.; Neubauer, J.; Goetz-Neunhoeffer, F. Study of Hydration Potential and Kinetics of the Ferrite Phase in Iron-Rich CAC. Cem. Concr. Res. 2016, 83, 79–85. [Google Scholar] [CrossRef]
- Klaus, S.R.; Neubauer, J.; Goetz-Neunhoeffer, F. Influence of the Specific Surface Area of Alumina Fillers on CAC Hydration Kinetics. Adv. Cem. Res. 2016, 28, 62–70. [Google Scholar] [CrossRef]
- Hueller, F.; Naber, C.; Neubauer, J. Impact of Initial CA Dissolution on the Hydration Mechanism of CAC. Cem. Concr. Res. 2018, 113, 41–54. [Google Scholar] [CrossRef]
- Alonso, M.M.; Palacios, M.; Puertas, F. Effect of Polycarboxylate-Ether Admixtures on CAC Pastes. Part 2: Hydration studies. Ind. Eng. Chem. Res. 2013, 52, 17330–17340. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Zhang, J.; Cao, H. Preparation and Properties of Phosphate Base Heat-resisting Composites. Chem. Adhes. 2005, 27, 67–70. [Google Scholar]
- Cao, X.; Zhao, K.; Wang, L. Robust, Thermally Insulating, and High-Temperature Resistant Phosphate-Enhanced Mullite Fiber Porous Ceramic Composites. Ceram 2024, 50, 23733–23743. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, W.; Zhan, Z. Novel High-Temperature-Resistant Phosphates: Thermal Ablation Behavior of La-Al System Phosphates at 2000 °C. ACS Omega 2022, 7, 3452–3461. [Google Scholar] [CrossRef]
- Nouri-Khezrabad, M.; Braulio, M.A.L.; Pandolfelli, V.C. Nano-Bonded Refractory Castables. Ceram 2013, 39, 3479–3497. [Google Scholar] [CrossRef]
- Burgos-Montes, O.; Álvarez, M.; Aza, A.D. The Main Role of Silica-Based Cement Free Binders on the Microstructural Evolution and Mechanical Behaviour of High Alumina Castables. J. Eur. Ceram. Soc. 2018, 38, 4137–4148. [Google Scholar] [CrossRef]
- Hossain, S.K.S.; Roy, P.K. Waste Rice Husk Ash Derived Sol: A Potential Binder in High Alumina Refractory Castables as a Replacement of Hydraulic Binder. J. Alloys Compd. 2020, 817, 152806. [Google Scholar] [CrossRef]
- Hao, R.; Liu, J.; Dong, X.; Guo, A.; Hou, Z.; Wang, M. High-Temperature Shear Strength and Bonding Mechanism of the Mullite Ceramic/Fiber Brick Component Joined by Phosphate Adhesive. Int. J. Appl. Ceram. Technol. 2022, 10, 978–985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).