Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
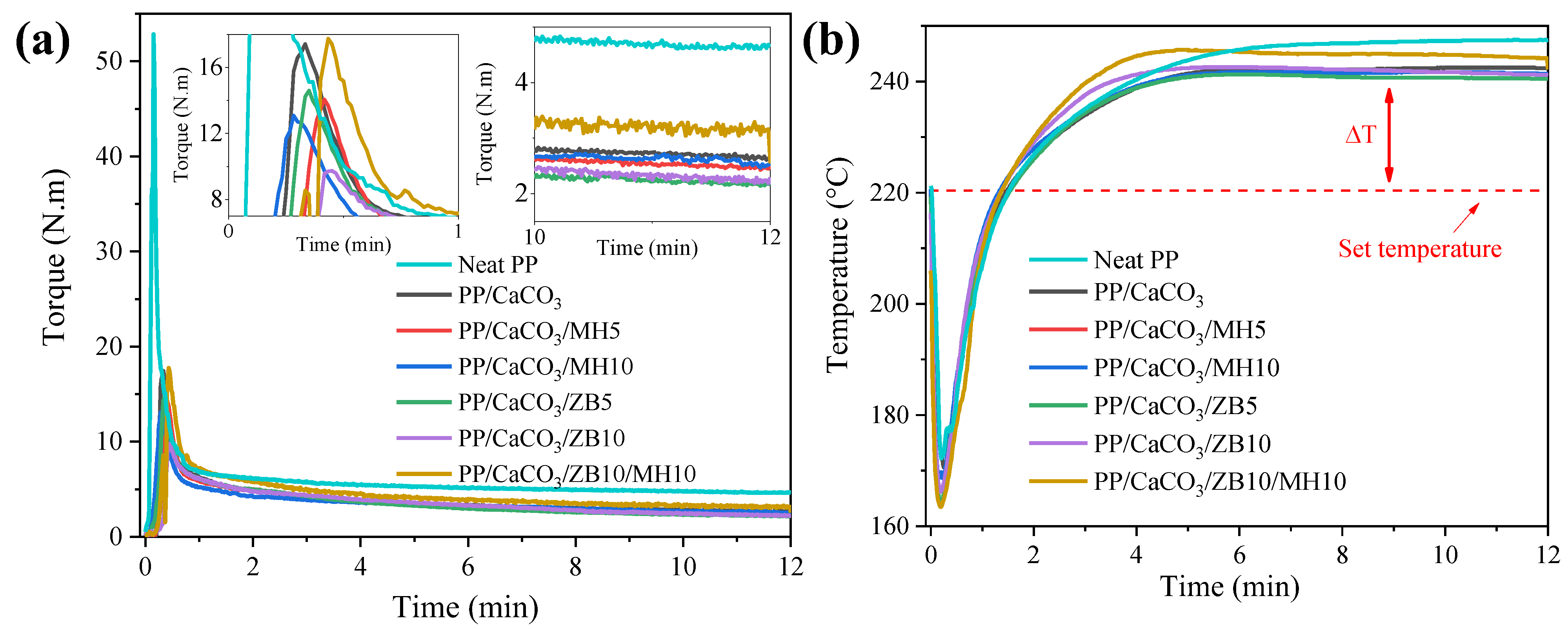
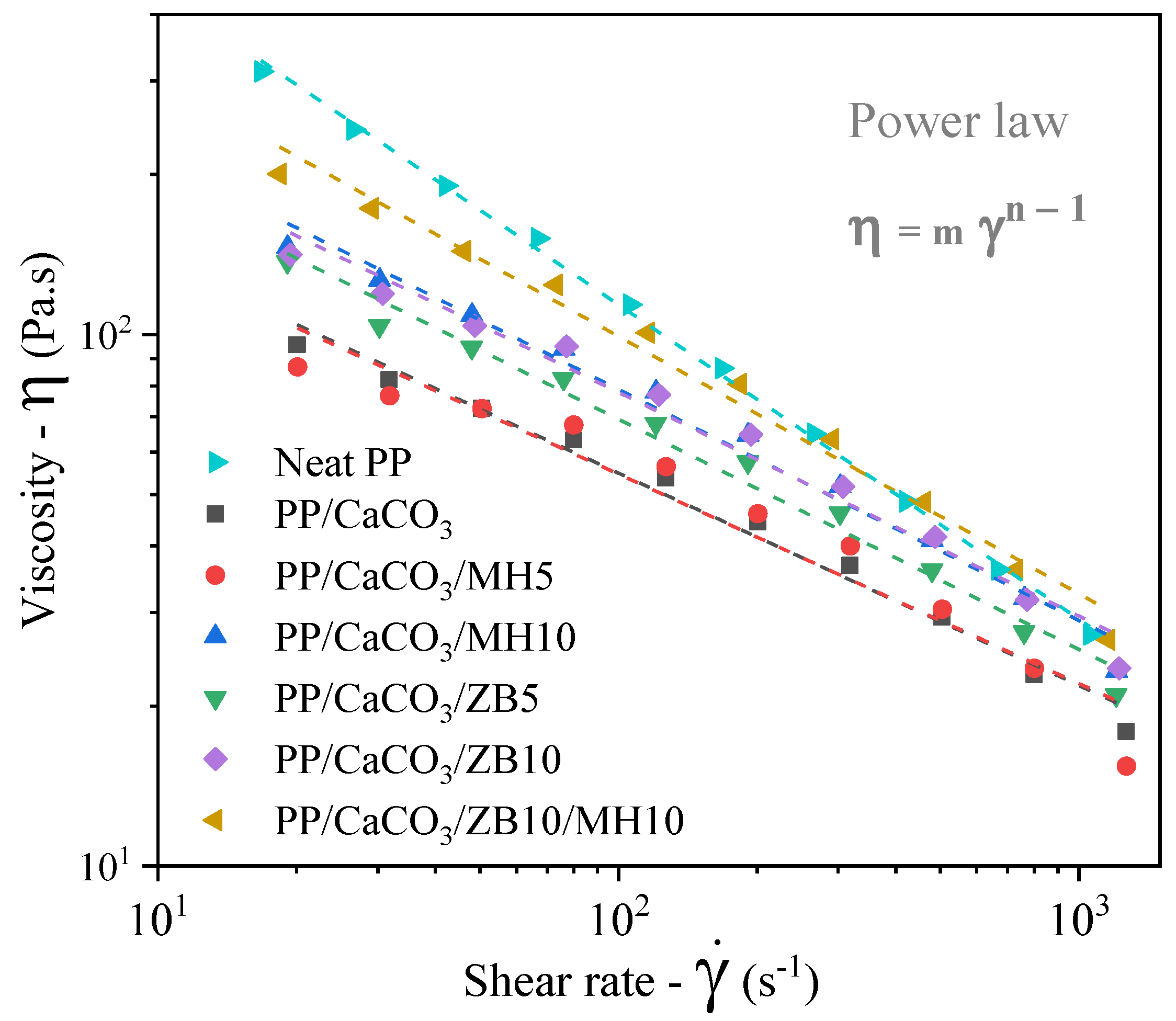
3.1. Processability and Rheological Properties
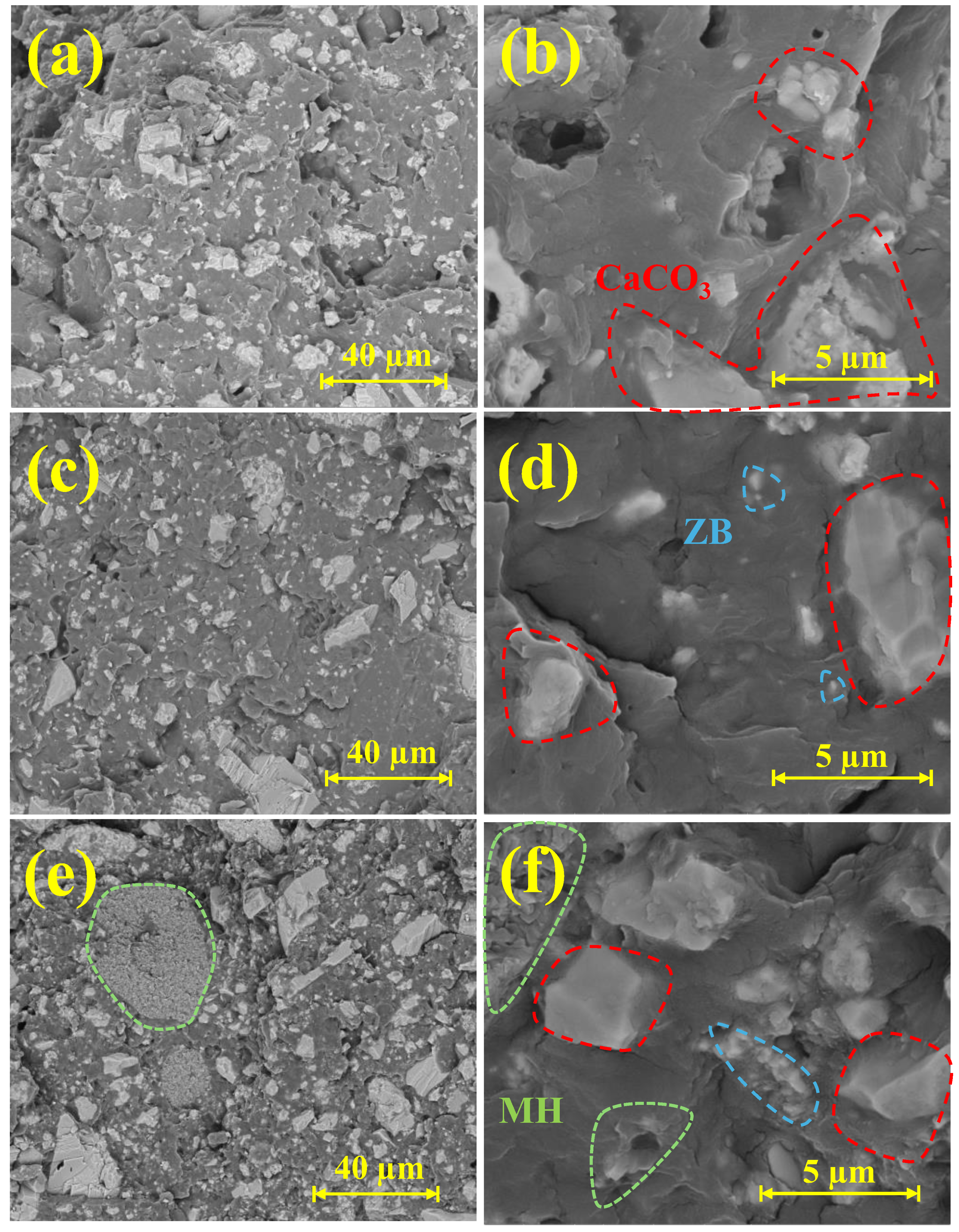
3.2. Morphological Evaluation (SEM with EDS Analysis)
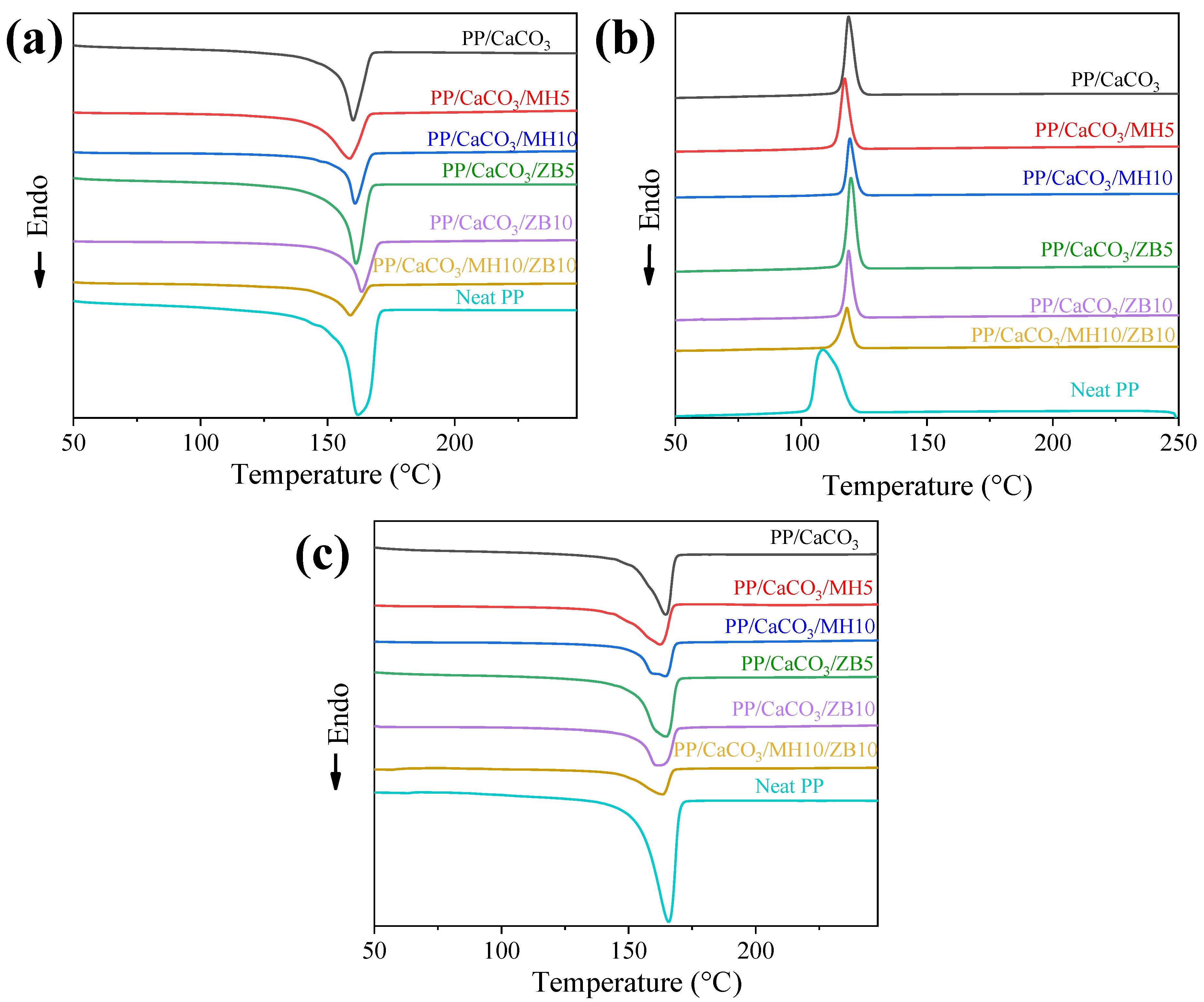
3.3. Differential Scanning Calorimetry (DSC)
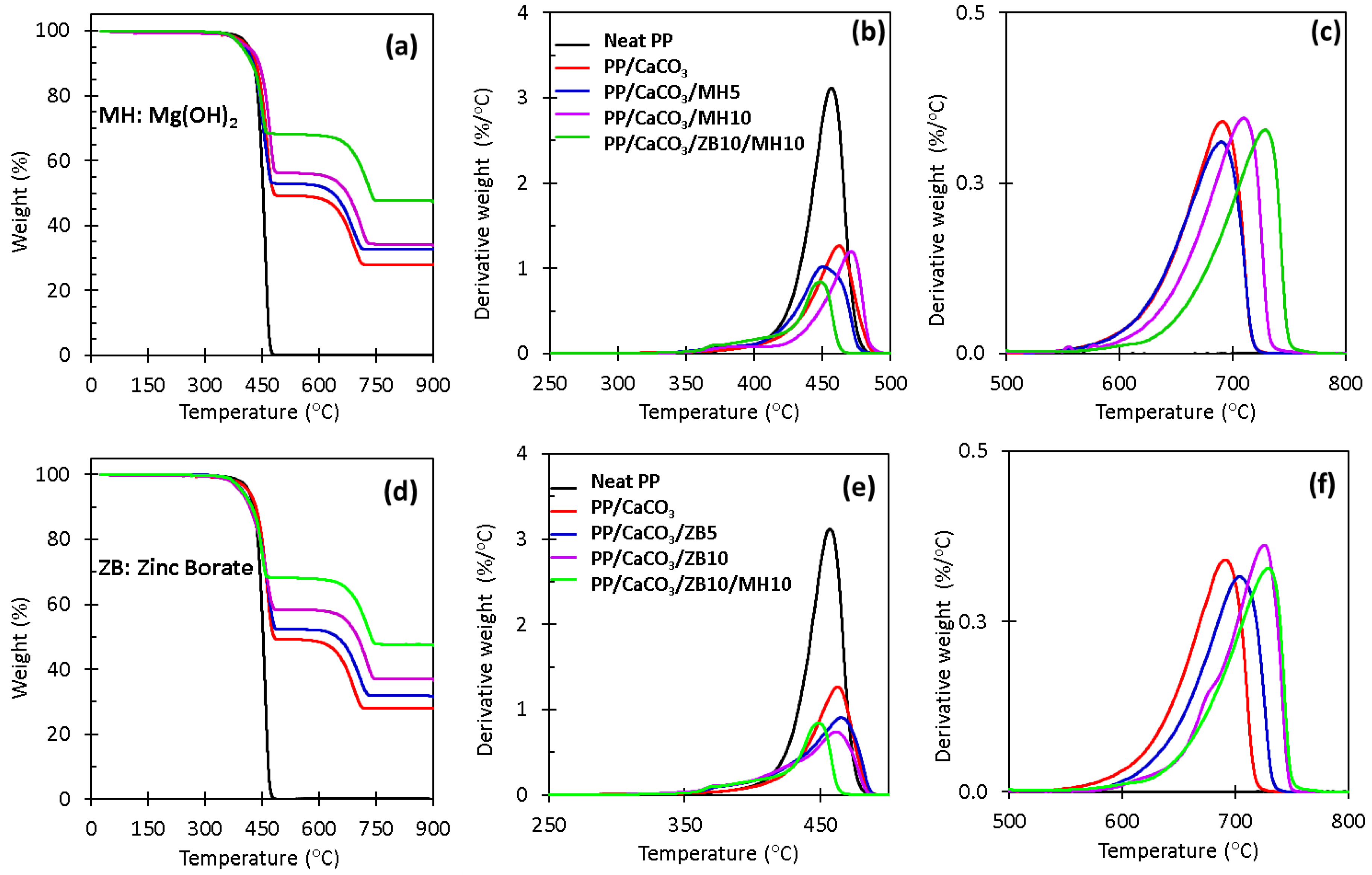
3.4. Thermogravimetric Analysis (TGA)
3.5. Flame Retardancy
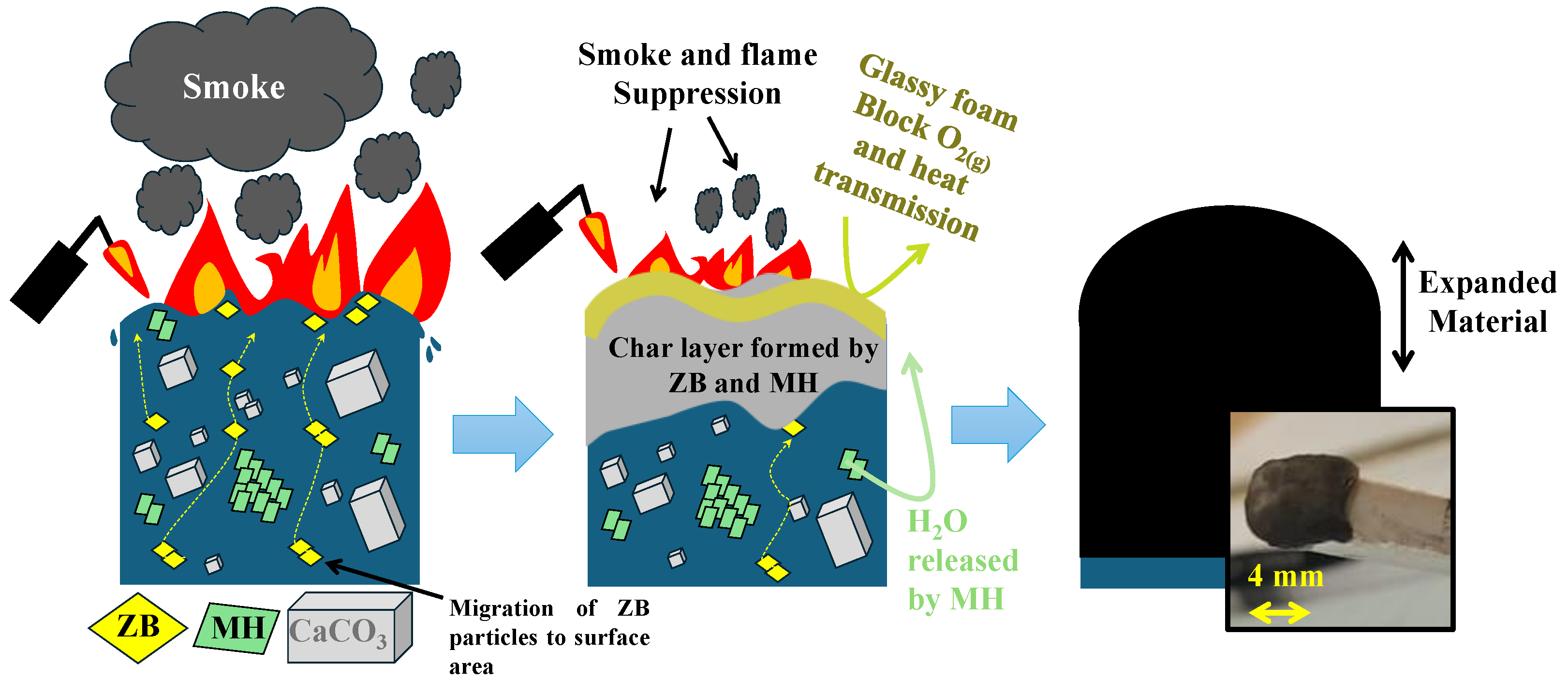
3.5.1. Mechanism for Flame Retardancy Action and Synergistic Effect of ZB and MH in the PP
3.5.2. Char Expansion Analysis of PP/CaCO3 Composites Reinforced with Flame Retardants
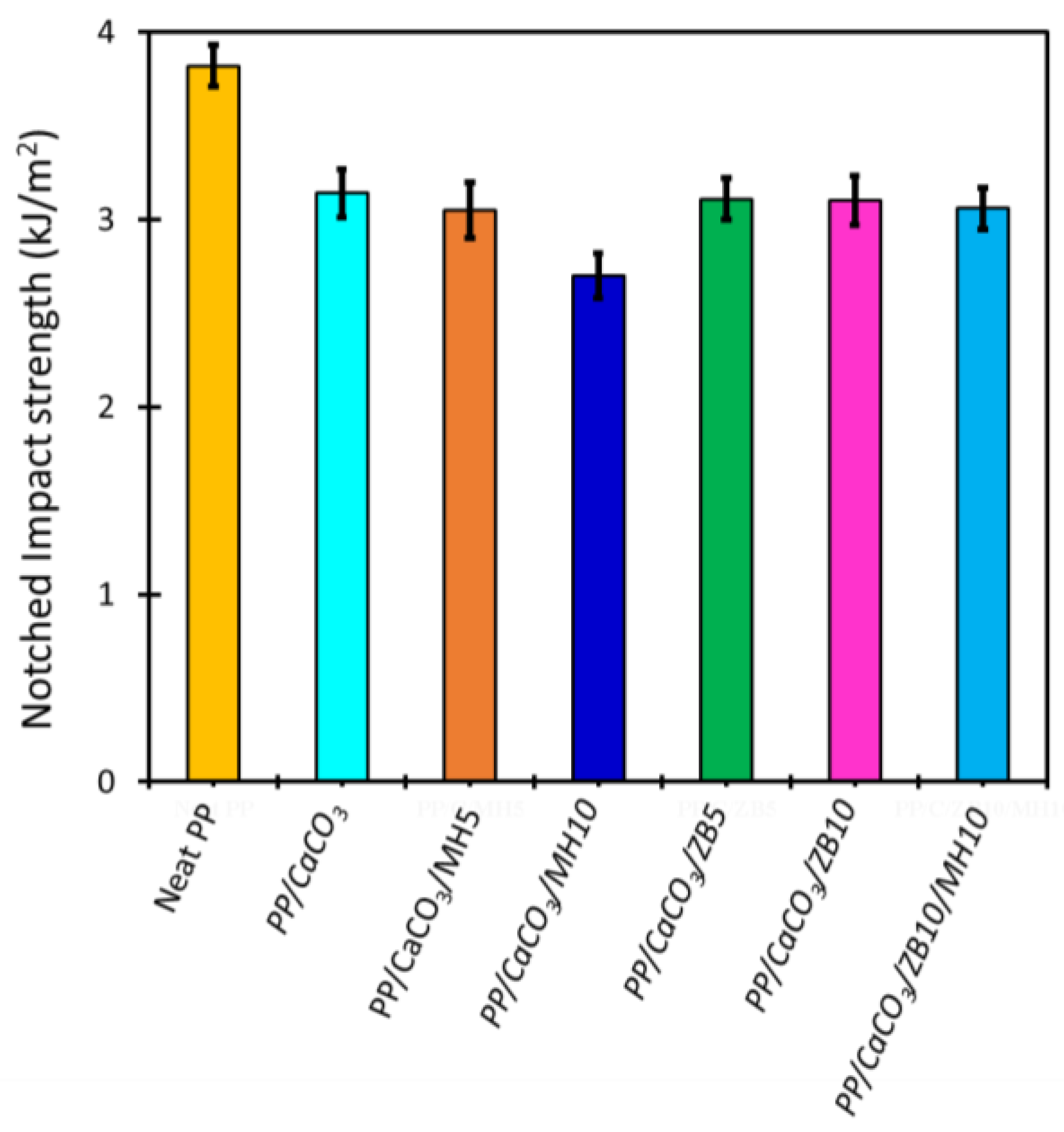
3.5.3. Evaluation of the Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mapossa, A.B.; Mehdipour, N.M.; Sundararaj, U. Thermal, Mechanical, Flammability Properties and Morphologies of Polypropylene/Calcium Carbonate-Based on Carbon Nanomaterials and Layered Silicates Composites: A Future Perspective of New Sheet Material for Flooring Application. J. Vinyl Addit. Technol. 2024. [Google Scholar]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent Advances in Carbon-Based Nanomaterials for Flame Retardant Polymers and Composites. Compos. B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Cheng, Y.; Zhang, A.; Liu, W. Enhanced Mechanical and Flame-Resistant Properties of Polypropylene Nanocomposites with Reduced Graphene Oxide-Functionalized Ammonium Polyphosphate and Pentaerythritol. J. Appl. Polym. Sci. 2019, 136, 48036. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame Retardancy through Carbon Nanomaterials: Carbon Black, Multiwall Nanotubes, Expanded Graphite, Multi-Layer Graphene and Graphene in Polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Gong, J.; Liu, J.; Tian, N.; Wang, Y.; Jiang, Z.; Qiu, J.; Tang, T. Thermal and Flammability Properties of Polypropylene/Carbon Black Nanocomposites. Polym. Degrad. Stab. 2012, 97, 793–801. [Google Scholar] [CrossRef]
- Genovese, A.; Shanks, R.A. Structural and Thermal Interpretation of the Synergy and Interactions between the Fire Retardants Magnesium Hydroxide and Zinc Borate. Polym. Degrad. Stab. 2007, 92, 2–13. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Zhou, C.; Li, J.; Shen, L.; Lin, H. A Biobased Flame Retardant towards Improvement of Flame Retardancy and Mechanical Property of Ethylene Vinyl Acetate. Chin. Chem. Lett. 2023, 34, 107202. [Google Scholar] [CrossRef]
- Worrell, E.; Reuter, M.A. Handbook of Recycling; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123964595. [Google Scholar]
- Stark, N.M.; White, R.H.; Mueller, S.A.; Osswald, T.A. Evaluation of Various Fire Retardants for Use in Wood Flour–Polyethylene Composites. Polym. Degrad. Stab. 2010, 95, 1903–1910. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Akbulut, T.; Dundar, T.; White, R.H.; Mengeloglu, F.; Buyuksari, U.; Candan, Z.; Avci, E. Effect of Boron and Phosphate Compounds on Physical, Mechanical, and Fire Properties of Wood–Polypropylene Composites. Constr. Build. Mater. 2012, 33, 63–69. [Google Scholar] [CrossRef]
- Kind, D.J.; Hull, T.R. A Review of Candidate Fire Retardants for Polyisoprene. Polym. Degrad. Stab. 2012, 97, 201–213. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Wang, L.; Liu, T. Evaluation of Intumescent Fire Retardants and Synergistic Agents for Use in Wood Flour/Recycled Polypropylene Composites. Constr. Build. Mater. 2015, 76, 273–278. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, R.; Mu, J.; Ding, Y.; Jiang, J. Synergistic Flame Retardancy of Linear Low-Density Polyethylene with Surface Modified Intumescent Flame Retardant and Zinc Borate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128400. [Google Scholar] [CrossRef]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2021, 14, 82. [Google Scholar] [CrossRef]
- Doğan, M.; Yılmaz, A.; Bayramlı, E. Synergistic Effect of Boron Containing Substances on Flame Retardancy and Thermal Stability of Intumescent Polypropylene Composites. Polym. Degrad. Stab. 2010, 95, 2584–2588. [Google Scholar] [CrossRef]
- Bi, Q.; Yao, D.; Yin, G.-Z.; You, J.; Liu, X.-Q.; Wang, N.; Wang, D.-Y. Surface Engineering of Magnesium Hydroxide via Bioinspired Iron-Loaded Polydopamine as Green and Efficient Strategy to Epoxy Composites with Improved Flame Retardancy and Reduced Smoke Release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Jiao, L.-L.; Zhao, P.-C.; Liu, Z.-Q.; Wu, Q.-S.; Yan, D.-Q.; Li, Y.-L.; Chen, Y.-N.; Li, J.-S. Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers 2022, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of Flame Retardant Magnesium Hydroxide on the Mechanical Properties of High Density Polyethylene Composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Ren, M.; Yang, M.; Li, S.; Chen, G.; Yuan, Q. High Throughput Preparation of Magnesium Hydroxide Flame Retardant via Microreaction Technology. RSC Adv. 2016, 6, 92670–92681. [Google Scholar] [CrossRef]
- Yao, D.; Yin, G.; Bi, Q.; Yin, X.; Wang, N.; Wang, D.-Y. Basalt Fiber Modified Ethylene Vinyl Acetate/Magnesium Hydroxide Composites with Balanced Flame Retardancy and Improved Mechanical Properties. Polymers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M. Thermoforming of Compostable PLA/PBS Blends Reinforced with Highly Hygroscopic Calcium Carbonate. J. Manuf. Process 2020, 56, 1185–1192. [Google Scholar] [CrossRef]
- Radebe, L.; Wesley-Smith, J.; Focke, W.W.; Ramjee, S. Formulating Calcium Carbonate Masterbatches. J. Polym. Eng. 2023, 43, 80–88. [Google Scholar] [CrossRef]
- Barczewski, M.; Lewandowski, K.; Schmidt, M.; Szostak, M. Melt Fracture and Rheology of Linear Low Density Polyethylene—Calcium Carbonate Composites. Polym. Eng. Sci. 2017, 57, 998–1004. [Google Scholar] [CrossRef]
- Viljoen, D.; Fischer, M.; Kühnert, I.; Labuschagné, J. The Tensile Behaviour of Highly Filled High-Density Polyethylene Quaternary Composites: Weld-Line Effects, DIC Curiosities and Shifted Deformation Mechanisms. Polymers 2021, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Dou, Q. Investigation of the Microstructures, Properties, and Toughening Mechanism of Polypropylene/Calcium Carbonate Toughening Masterbatch Composites. J. Appl. Polym. Sci. 2017, 134, 45515. [Google Scholar] [CrossRef]
- Donmez, S.; Tuzenli, Z.; Bayram, G.; Savaskan Yilmaz, S. Flame Retardancy and Mechanical Properties of Polypropylene Composites Containing Intumescent Flame Retardants, Preceramic Polymers, and Other Additives. SPE Polym. 2024, 5, 318–330. [Google Scholar] [CrossRef]
- Demirhan, Y.; Yurtseven, R.; Usta, N. The Effect of Boric Acid on Flame Retardancy of Intumescent Flame Retardant Polypropylene Composites Including Nanoclay. J. Thermoplast. Compos. Mater. 2023, 36, 1187–1214. [Google Scholar] [CrossRef]
- Doğan, M.; Bayramlı, E. Synergistic Effect of Boron Containing Substances on Flame Retardancy and Thermal Stability of Clay Containing Intumescent Polypropylene Nanoclay Composites. Polym. Adv. Technol. 2011, 22, 1628–1632. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Jiang, X.; Liu, T.; Zhao, L. Synergistic Effects of Intumescent Flame Retardant and Nano-CaCO3 on Foamability and Flame-Retardant Property of Polypropylene Composites Foams. J. Cell. Plast. 2018, 54, 615–631. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Lin, Y.; Chan, C.; Wu, J. Morphology and Mechanical Property of Binary and Ternary Polypropylene Nanocomposites with Nanoclay and CaCO3 Particles. J. Appl. Polym. Sci. 2007, 106, 3409–3416. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Characterization of the Performance of an Intumescent Fire Protective Coating. Surf. Coat. Technol. 2006, 201, 979–987. [Google Scholar] [CrossRef]
- Gillani, Q.F.; Ahmad, F.; Abdul Mutalib, M.I.; Megat-Yusoff, P.S.M.; Ullah, S.; Messet, P.J.; Zia-ul-Mustafa, M. Thermal Degradation and Pyrolysis Analysis of Zinc Borate Reinforced Intumescent Fire Retardant Coatings. Prog. Org. Coat. 2018, 123, 82–98. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Polymers 2020, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Ariff, Z.M.; Ariffin, A.; Jikan, S.S.; Rahim, N.A.A. Rheological Behaviour of Polypropylene Through Extrusion and Capillary Rheometry. In Polypropylene; Dogan, F., Ed.; InTech: London, UK, 2012; pp. 29–45. [Google Scholar]
- Chafidz, A.; Kaavessina, M.; Al-Zahrani, S.; Al-Otaibi, M.N. Rheological and Mechanical Properties of Polypropylene/Calcium Carbonate Nanocomposites Prepared from Masterbatch. J. Thermoplast. Compos. Mater. 2016, 29, 593–622. [Google Scholar] [CrossRef]
- Supaphol, P.; Harnsiri, W. Rheological and Isothermal Crystallization Characteristics of Neat and Calcium Carbonate-filled Syndiotactic Polypropylene. J. Appl. Polym. Sci. 2006, 100, 4515–4525. [Google Scholar] [CrossRef]
- Fuad, M.Y.A.; Hanim, H.; Zarina, R.; Ishak, Z.A.M. Azman Hassan Polypropylene/Calcium Carbonate Nanocomposites—Effects of Processing Techniques and Maleated Polypropylene Compatibiliser. Express Polym. Lett. 2010, 4, 611–620. [Google Scholar] [CrossRef]
- Sahebian, S.; Mosavian, M.H. Thermal Stability of CaCO3/Polyethylene (PE) Nanocomposites. Polym. Polym. Compos. 2019, 27, 371–382. [Google Scholar] [CrossRef]
- Moaref, R.; Shajari, S.; Sundararaj, U. From Waste to Value Added Products: Manufacturing High Electromagnetic Interference Shielding Composite from End-of-Life Vehicle (ELV) Waste. Polymers 2023, 16, 120. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, T.; Yoshida, H.; Takenaka, S.; Funabiki, T.; Yoshida, S.; Murata, T. A XANES Study on the Dehydration Process of Magnesium Hydroxide. Physica B Condens. Matter 1995, 208–209, 581–582. [Google Scholar] [CrossRef]
- Pondelak, A.; Škapin, A.S.; Knez, N.; Knez, F.; Pazlar, T. Improving the Flame Retardancy of Wood Using an Eco-Friendly Mineralisation Process. Green. Chem. 2021, 23, 1130–1135. [Google Scholar] [CrossRef]
- Schinazi, G.; Price, E.J.; Schiraldi, D.A. Fire Testing Methods of Bio-Based Flame-Retardant Polymeric Materials. In Bio-Based Flame-Retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–95. [Google Scholar]
- John, M.J. Flammability Performance of Biocomposites. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–58. [Google Scholar]
- Bourbigot, S.; Fontaine, G. Flame Retardancy of Polylactide: An Overview. Polym. Chem. 2010, 1, 1413. [Google Scholar] [CrossRef]
- Bieniawski, Z.T. Engineering Rock Mass Classifications: A Complete Manual for Engineers and Geologists in Mining, Civil, and Petroleum Engineering; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Available online: https://www.matweb.com/search/datasheet.aspx?MatGUID=2e64171d3dad4522bf11a6b0bc61903a (accessed on 8 September 2024).

| Samples | PP (wt.%) | CaCO3 (wt.%) | ZB (wt.%) | MH (wt.%) |
|---|---|---|---|---|
| Neat PP | 100 | 0 | 0 | 0 |
| PP/CaCO3 | 50 | 50 | 0 | 0 |
| PP/CaCO3/MH5 | 45 | 50 | 5 | 0 |
| PP/CaCO3/MH10 | 40 | 50 | 10 | 0 |
| PP/CaCO3/ZB5 | 45 | 50 | 0 | 5 |
| PP/CaCO3/ZB10 | 40 | 50 | 0 | 10 |
| PP/CaCO3/ZB10/MH10 | 30 | 50 | 10 | 10 |
| Samples | Intercept (Log m) | Slope (n − 1) | Adjusted-R2 | Consistence Index (m) 101 (Pa.sn) | Pseudoplasticity Index (n) |
|---|---|---|---|---|---|
| Neat PP | 3.24 ± 0.02 | −0.59 ± 0.01 | 0.998 | 174 ± 72 | 0.41 ± 0.01 |
| PP/CaCO3 | 2.54 ± 0.04 | −0.4 ± 0.02 | 0.984 | 35 ± 3 | 0.60 ± 0.02 |
| PP/CaCO3/MH5 | 2.53 ± 0.08 | −0.39 ± 0.04 | 0.931 | 33 ± 6 | 0.61 ± 0.04 |
| PP/CaCO3/MH10 | 2.77 ± 0.04 | −0.43 ± 0.02 | 0.982 | 58 ± 6 | 0.57 ± 0.02 |
| PP/CaCO3/ZB5 | 2.71 ± 0.04 | −0.43 ± 0.02 | 0.983 | 51 ± 5 | 0.57 ± 0.02 |
| PP/CaCO3/ZB10 | 2.73 ± 0.05 | −0.42 ± 0.02 | 0.977 | 54 ± 6 | 0.58 ± 0.02 |
| PP/CaCO3/ZB10/MH10 | 2.97 ± 0.05 | −0.48 ± 0.02 | 0.984 | 92 ± 10 | 0.52 ± 0.02 |
| Samples | Tm (°C) | ΔHm (J/g) | Xc (%) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|---|
| Neat PP | 166 | 87 | 42 | 109 | 99 | 162 | 91 | 44 |
| PP/CaCO3 | 165 | 86 | 42 | 119 | 52 | 160 | 91 | 44 |
| PP/CaCO3/MH5 | 162 | 83 | 40 | 117 | 46 | 159 | 89 | 43 |
| PP/CaCO3/MH10 | 165 | 80 | 39 | 119 | 38 | 161 | 85 | 41 |
| PP/CaCO3/ZB5 | 165 | 81 | 39 | 120 | 43 | 165 | 82 | 40 |
| PP/CaCO3/ZB10 | 162 | 84 | 40 | 119 | 39 | 161 | 87 | 42 |
| PP/CaCO3/ZB10/MH10 | 164 | 68 | 33 | 118 | 26 | 159 | 73 | 35 |
| Samples | First Stage of Temperature (°C) | Second Stage of Temperature (°C) | Third Stage of Temperature (°C) | Char Residue (%) | LOI (%) |
|---|---|---|---|---|---|
| Neat PP | N/A | 340–480 | N/A | 0.0 | 17.9 |
| PP/CaCO3 | N/A | 323–493 | 555–723 | 28.1 | 19.7 |
| PP/CaCO3/MH5 | 309–409 | 410–495 | 542–727 | 32.5 | 21.2 |
| PP/CaCO3/MH10 | 334–413 | 414–500 | 579–752 | 33.9 | 23.1 |
| PP/CaCO3/ZB5 | 300–400 | 412–497 | 579–743 | 31.3 | 22.2 |
| PP/CaCO3/ZB10 | 304–400 | 404–500 | 542–757 | 36.4 | 24.9 |
| PP/CaCO3/ZB10/MH10 | 294–409 | 410–472 | 583–771 | 47.2 | 29.4 |
| Samples | Elastic Modulus (MPa) | Ultimate Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|---|
| Neat PP | 1158 ± 18 | 31.4 ± 0.9 | 6.0 ± 1.0 |
| PP/CaCO3 | 2427 ± 156 | 23.4 ± 1.1 | 1.5 ± 0.2 |
| PP/CaCO3/MH5 | 2610 ± 260 | 21.2 ± 0.6 | 1.2 ± 0.2 |
| PP/CaCO3/MH10 | 3095 ± 102 | 22.3 ± 0.6 | 0.9 ± 0.1 |
| PP/CaCO3/ZB5 | 2446 ± 136 | 19.7 ± 0.3 | 1.0 ± 0.1 |
| PP/CaCO3/ZB10 | 2989 ± 241 | 20.0 ± 0.9 | 1.5 ± 0.2 |
| PP/CaCO3/ZB10/MH10 | 4804 ± 438 | 20.1 ± 6.0 | 0.6 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapossa, A.B.; dos Anjos, E.G.R.; Sundararaj, U. Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants. Materials 2024, 17, 4553. https://doi.org/10.3390/ma17184553
Mapossa AB, dos Anjos EGR, Sundararaj U. Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants. Materials. 2024; 17(18):4553. https://doi.org/10.3390/ma17184553
Chicago/Turabian StyleMapossa, Antonio Benjamim, Erick Gabriel Ribeiro dos Anjos, and Uttandaraman Sundararaj. 2024. "Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants" Materials 17, no. 18: 4553. https://doi.org/10.3390/ma17184553





