Thin Conducting Films: Preparation Methods, Optical and Electrical Properties, and Emerging Trends, Challenges, and Opportunities
Abstract
:1. Introduction
2. Preparation/Methodology
2.1. Vaccum-Based Depositions
2.1.1. Physical Vapor Deposition (PVD)
Evaporation
Thermal Evaporation
Electron Beam Evaporation (EBE)
Molecular Beam Epitaxy (MBE)
Laser Evaporation (or Laser Ablation)
Resistance Heating Evaporation
Ion Plating
Sputtering
Radio-Frequency (RF) Sputtering
Reactive Sputtering
Ion Beam Sputtering (IBS)
DC Diode and Triode Sputtering
Magnetron Sputtering
2.1.2. Low-Pressure Chemical Vapor Deposition (LPCVD)
2.1.3. Plasma-Enhanced Chemical Vapor Deposition (PECVD)
2.1.4. Hot-Wall and Cold-Wall CVD
- Industry of semiconductors: The semiconductor industry uses both hot-wall and cold-wall CVD to create thin coatings on silicon wafers. Hot-wall CVD is frequently utilized due to its ease of use and adaptability. Cold-wall CVD is used when exact control over film characteristics and little contamination from undesired reactions on chamber walls are essential [130].
- Solar energy photovoltaics: Solar cells and gadgets cannot be produced without thin sheets. Layers can be deposited using hot- and cold-wall CVD techniques to fabricate solar cell structures [131].
- Optical layers: Lenses, mirrors, and other optical components can have optical coatings applied via hot-wall and cold-wall CVD. These coatings can improve optical elements’ transmissive or reflective qualities [132].
- Protective coatings: Electrochemical vapor deposition is used in both hot-wall and cold-wall applications to provide barrier coatings that shield electronic equipment from moisture and oxygen. Hot-wall and cold-wall CVD are crucial to creating thin films for energy, optical, and electronic-related uses. The demand for temperature control, the quality of the film, and specific material requirements all influence the choice between various designs [132].
2.1.5. Atomic Layer Deposition (ALD)
2.1.6. Pulsed Laser Deposition (PLD)
2.2. Solution-Processed (Non-Vacuum) Depositions
2.2.1. Atmospheric Pressure Chemical Vapor Deposition (APCVD)
2.2.2. Chemical Bath Deposition (CBD)
2.2.3. Spin-Coating
2.2.4. Electrodeposition
2.2.5. Sol–Gel Deposition
2.2.6. Spray Pyrolysis
2.2.7. Anodization Methods
2.3. Mixed Category
Metal–Organic Chemical Vapor Deposition (MOCVD)
3. Requirements for Thin Films to Be Conductive
- Selecting proper material: Either intrinsic or extrinsic conductivity is required for the thin-film material. Extrinsic conductivity results from adding dopants to a material to change its conductivity, whereas intrinsic conductivity results from the substance’s intrinsic electronic structure. Metals (like Au, Ag, and Cu), metal oxides (like ITO), and conducting polymers are frequently utilized as conductive materials in thin films.
- Carrier density: For electrical conduction to occur, the thin film must have an adequate concentration of charge carriers, such as electrons or holes [260]. Doping or intrinsic carrier production techniques like photoexcitation or thermal activation can be used to accomplish this.
- Charge carriers’ mobility: When an electric field is applied, the charge carriers in the thin film must be allowed to flow freely through the substance [261]. Efficient electrical conduction requires high carrier mobility, which is determined by various factors, including defects, interactions with lattice vibrations, and the crystal structure [262].
- Film thickness: The conductivity of the thin layer may vary depending on its thickness. Thinner films can occasionally have higher conductivity because of better carrier transport qualities and less charge carrier scattering [263]. However, quantum confinement phenomena may cause overly thin films to have higher resistance.
- Uniformity of a film and purity: The thin film’s surface should have a consistent thickness and composition to guarantee constant electrical properties. Furthermore, impurities or flaws in the film can decrease its conductivity and impede charge carrier mobility [264].
- High electrical conductivity and low resistivity: High electrical conductivity refers to the thin layer’s ability to permit the passage of electrical current with little resistance. This is accomplished by minimizing imperfections that impede charge carrier mobility, decreasing the scattering mechanisms, and optimizing the material’s electronic band structure [265]. The value of this resistivity should be in the range of 10−6 to 10−2 Ohm cm or lower [266]. The resistivity is inversely correlated with conductivity [267].
- Optical bandgap: Since photon energy is inversely related to wavelength, a material with a wider bandgap tends to absorb shorter wavelengths (like visible or ultraviolet light) and be transparent to longer wavelengths (like infrared). As a result, the material selection and doping level can influence the thin film’s optical characteristics and transparency at various wavelengths [268]. Table 6 overviews various conductive thin films and their required properties.
4. Optical Properties of Different TCFs
4.1. Absorption Coefficient
4.1.1. Organic Thin Films
Polymer Thin Films
OLED Film
4.1.2. Inorganic Thin Films
AZO Thin Film
ITO Thin Film
CuI Thin Film
Cadmium Oxide (CdO) Thin Film
| TCFs | Factors | Effects on the Absorption Coefficients | Authors |
|---|---|---|---|
| AZO | Doping |
| Alam et al. [214], Mamat et al. [213], Muiva et al. [216], Mia et al. [211], Silva et al. [212], Manjakkal et al. [322], Majumder et al. [323] |
| Mamat et al. [213] | ||
| The absorption spectrum shifts monotonically to a higher energy | Muiva et al. [216] | ||
| AZO films are expected to be useful as UV detector | Mia et al. [211] | ||
| Al doping produces donor levels below the conducting band, bending the band edge and reducing the bandgap. | Kaur et al. [230] | ||
| Caglar et al. [215] | ||
| Tuna et al. [324] | ||
| Free Carrier Concertation | An increase in the absorption with increasing carrier | Alam et al. [214], Babu et al. [217] | |
| Bandgap widening is proportional to N2/3 | Mosbah et al. [221] | ||
| Temperature | Annealing temperature did not result in any appreciable difference. | Majumder et al. [323] | |
| Bandgap increases with increasing substrate temperature. | |||
| decreases with increasing temperature | Barhoumi et al. [219] | ||
| Thickness | The bandgap decreases with the increasing film thickness | Kaur et al. [230] | |
| ITO | Doping |
| Tuna et al. [324] |
| Kim et al. [273] | ||
| Free carrier concentration | Since the thinnest film has the lowest carrier concentration, fewer occupied states are in the valence band, resulting in a tiny bandgap value | Eshaghi and Graeli [278] | |
| The carrier density decreases with Sn doping over the critical Sn doping | Kim et al. [273] | ||
| Senthilkumar et al. [202] | ||
| Thickness | The bandgap decreased, and the absorption coefficient rose with thickness. | Eshaghi and Graeli [278] | |
| Ahmed et al. [341] | ||
| Wet Etching | Intense light absorption by the substrate can achieve entire material removal and the formation of residue-free etch lines without over-etching the substrate. | Yavas and Takai [279] | |
| Temperature | With an increase in substrate temperature, the absorption coefficient increases, and the bandgap rises as well. | Tuna et al. [324] | |
| The absorbance coefficient decreases as the annealing temperature increases. | Mohamed [270] | ||
| CuI | Doping | Optical absorption increased in CuI-thin-film with doping silver concentration. | Wang et al. [329] |
| La-doped CuI films show strong optical absorption in UV spectra, and their bandgap value drops for up to 6 wt.% of doping. | Naveena et al. [330] | ||
| The produced CuI thin films exhibit remarkable transparency in the visible and near-infrared (NIR) spectra. | Yang et al. [188] | ||
| Thickness |
| Amalina et al. [331] | |
| Greater grain sizes cause more empty intergranular volume, which lowers absorption per unit thickness. | Abbas et al. [332] | ||
| Temperature |
| Villegas et al. [327] | |
| John and Wang [328] | ||
| As the temperature increased, the absorption spectra decreased. | Zhu and Zhao [175] | ||
| CdO | Doping | Absorbance drops as the concentration of Al is doped in CdO thin films | Kumaravel et al. [335] |
| Moss et al. [336], Burstein et al. [337] | ||
| Usharani et al. [338] | ||
| Abdolahzadeh Ziabari et al. [339] | ||
| Velusamy et al. [340] | ||
| Temperature | When annealing was conducted at higher temperatures, the absorption edge decreased. | Ullah et al. [333] | |
| The absorption edge changes to higher wavelengths for the film annealed at higher temperatures. | Santos-Cruz et al. [334] | ||
| Polymer thin film | Doping |
| Yoon et al. [294], Geethalakshmi et al. [301] |
| An increase in the absorption coefficient with the increasing dopant on PANI-HCl | Banerjee et al. [295], Ayad et al. [296] | ||
| Geethalakshmi et al. [301] | ||
| When the doping concentration increases, the optical features of PMMA thin films alter, and the resulting composite materials may show some degree of electrical conductivity. | Chan and Kammer [297], Ou et al. [298] | ||
| Molecular structure | Polymers like polyacetylene or polyaniline have conjugated π-electron systems that delocalize π-electrons along the polymer backbone, leading to higher in the visible and ultraviolet areas. | Kar et al. [302] | |
| Polymers with aliphatic or saturated structures could have lower absorption coefficients in the visible and ultraviolet spectra because they lack conjugated π-electron systems. | Kiebooms et al. [303] | ||
| While polyanilines have different bands and molecular structures from other conducting polymers, their absorption spectra are comparable to those of other conjugated polymers. | Stubb et al. [300] | ||
| Temperature |
| Stejskal et al. [305] | |
| Geethalakshmi et al. [301], Banerjee et al. [295], Ayad et al. [296]. | ||
| INO | Temperature |
| Beena et al. [342] |
| Due to the Burstein–Moss shift, the bandgap increases with temperature increasing. | Prathap et al. [343], Gupta et al. [344], Senthilkumar and Vickraman [345] | ||
| Particle size |
| Beena et al. [342] |
4.2. Transmittance
4.2.1. AZO Thin Film
Effect of Film Thickness
Effect of Temperature
Effect of Doping Concentrations
Effect of Surface Morphology
Effect of Coating
4.2.2. ITO Thin Film
Effect of Film Thickness
Effect on Changing Compositions
Effect of Grain Size
Effect of Surface Morphology
Effect of Deposition Power and Time
4.2.3. CuI Thin Film
Effect of Deposition Technique
Effect of Temperature
Effect of Doping Concentration
4.2.4. Polymer Thin Films
Effect of Wavelength
Effect of Doping Concentrations
Effect of Polymer Type and Molecular Structure
Effect of Film Thickness
4.2.5. INO Thin Film
Effect of Temperature
Effect of Doping Concentration
4.2.6. CdO Thin Film
Effect of Oxygen Pressure
Effect of Temperature
Effect of Doping Concentration
4.3. Reflectance
4.3.1. AZO Thin Film
Effect of Temperature
Effect of Carrier Concentration
Effect of Bandgap
Effect of Film Thickness
4.3.2. ITO Thin Film
Effect of Film Thickness
Effect of Oxygen Pressure
Effect of Tin-Dopant Concentration
Effect of Temperature
4.3.3. CdO Thin Film
Effect of Doping
Effect of Surface Morphology
4.3.4. INO Thin Film
Effect of Doping
Effect of Temperature
| Thin Films | Factors | Effects on Reflectance | Authors |
|---|---|---|---|
| AZO | Temperature |
| Miao et al. [222] |
| Mosbah and Aida [221] | ||
| Fu et al. [386] | ||
| Carrier concentration | IR reflectance increases as product N increases. | Chaabouni et al. [387] | |
| Bandgap | The bandgap value decreases with an Al concentration of 6 and 10 at. %, while it increases with an increase in Al concentration from 0 to 4 at. %. | Takci et al. [349] | |
| Film thickness | Since the aspect ratio of crystallites varies with thickness, the change in optical reflectance may result from morphological changes in films. | Kaur et al. [230] | |
| ITO | Oxygen pressure | Reflection decreases with increasing oxygen pressure, indicating that the film’s grain size increases. | Tynell et al. [231] |
| Tin dopant |
| Tynell et al. [231] | |
| Temperature | Transmittance increases due to a drop in specular reflectance caused by increases in substrate temperature and negative bias voltage. | Miao et al. [222] | |
| CdO | Doping | The reflectance is reasonably consistent, with a magnitude difference of less than 10%. In contrast to the 5 at. % Al-doped film, the 1 at. % Al-doped film has a little greater reflectivity. | Ziabari et al. [339] |
| Surface morphology |
| Abdolahzadeh Ziabari et al. [339], Bennett and Porteus [388], Ghodsi et al. [389] | |
| INO | Doping |
| Alqahtani et al. [374] |
| Temperature |
| Beena et al. [342] |
4.4. Refractive Index (RI)
4.4.1. AZO Thin Film
Effect of Wavelength
Effect of Film Thickness
Effect of Substrate Tilt Angle and Substrate Rotating
Effect of Doping
Effect of Annealing Temperature
4.4.2. ITO Thin Film
Effect of Doping Concentration
Effect of Packing Density
Effect of Porosity
Effect of Power and Deposition Time
Effect of ZnO Ratio
Effect of Temperature
4.4.3. CdO Thin Film
Effect of Doping Concentration
Effect of Roughness
4.4.4. CuI Thin Film
Effect of Temperature
Effect of Changing the Media of Methodology
Effect of Film Thickness
| Thin Films | Factors | Effects on Refractive Index | Authors |
|---|---|---|---|
| AZO | Wavelength | The refractive index initially decreases with increasing wavelength and then becomes constant at higher wavelengths. | Alam and Cameron [214], Caglar et al. [215] |
| Film thickness |
| Reddy et al. [223] | |
| Doping |
| Caglar et al. [215] | |
| Temperature | The refractive index rose as annealing temperatures rose. | Gareso et al. [210] | |
| ITO | Doping |
| Senthilkumar et al. [202] |
| The refractive index progressively drops with SnO2 content up to 5% of the total weight before significantly increasing to 15%. | Mohamed [270], Chen and Robinson [404], Ohhata et al. [405] | ||
| Packing density decreased with Sn doping concentration, which affects the refractive index. | Harris et al. [400] | ||
| As the Sn doping concentration increases, the ITO films’ porosity rises, impacting the RI. | Senthilkumar et al. [202] | ||
| Power and deposition time | The refractive index rises with increasing power and longer deposition times in the visible and infrared areas. | Tchenka et al. [325], Kim et al. [397], Kumar et al. [398] | |
| ZnO ratio | As the ZnO ratio increases up to x = 0.15, the refractive index increases, but, as the ratio increases further, the values decrease slightly. | Mohamed [270] | |
| Temperature | The refractive index decreases as the ZnO content and annealing temperature rise. The increase in carrier mobility, which raises the relaxation time and mean free path, could cause the refractive index to decrease. | Mohamed [270] | |
| The refractive index falls when the substrate’s deposition temperature rises. A rise in the films’ carrier concentration can also account for this drop in refractive index. | Kim et al. [273] | ||
| CdO | Doping concentration | Al dopant in the films often causes structural alterations, resulting in decreased refractive index. | Ziabari et al. [339] |
| With the increasing amount of CdO doping, the refractive index gradually increased. | Vinodkumar et al. [402] | ||
| Roughness | A high refractive index was associated with higher film roughness, higher Al doping, and smaller grain sizes. | Ziabari et al. [339] | |
| CuI | Temperature | The refractive index of CuI films formed at room temperature falls with increasing substrate temperature and does not significantly vary with laser energy. | Zhu and Zhao [190] |
| Changing the media of methodology | The film generated in the chloroform bath, where the particle size was somewhat smaller than the others, has a lower refractive index. | Kariper [403] | |
| Film thickness | As the film thickness increases, the RI also increases | Kariper [403] |
5. Electrical Properties of Different Thin Films
5.1. Resistivity
5.1.1. AZO Thin Film
Effect of Doping Concentration
Effect of Temperature
Effect of Deposition Techniques
Effect of Film Thickness
5.1.2. ITO Thin Film
Effect of Film Thickness
Effect of Power and Deposition Time
Effect of Temperature
Effect of Grain Size
Effect on Bandgap
Effect of Sputtering Voltage
Effect of Carrier Concentration
5.1.3. CuI Thin Film
Effect of Temperature
Effect of I/Cu Ratio
Effect of Doping Concentration
5.1.4. Polymer Thin Films
Insulating Behavior
Conducting Behavior
Semiconducting Behavior
5.1.5. INO Thin Film
Effect of Oxygen Pressure
Effect of Temperature
5.2. Electrical Conductivity
5.2.1. Ti-Doped Al2O3 Thin Film
5.2.2. CdO Thin Film
Effect of Doping
Effect of Deposition Technique
6. Recent Trends of Conductive Thin Films
6.1. Graphene
- Nour et al. [453] investigated using an in-pot approach; polyaniline nanofibers (PANI-NFs) were directly produced on a glass substrate with GR sheets exfoliated from graphite in an aqueous solution. Titanium dioxide nanoparticles (TiO2NPs) were added for decoration, resulting in a smart 2D nanocomposite film called PANI-NF-GR-TiO2 NP. The intriguing 2D structure network thin-film device for various applications is then adorned with a thin layer of polypyrrole (PPY) using vapor phase polymerization. The study also showed that the novel 2D thin film has several active sites and an intriguing device structure for various applications. It comprises four layers of one-dimensional (1D) polyaniline nanofibers, 2D graphene adorned with zero-dimensional (0D) nanoparticles, and a thin polypyrrole layer.
- With its distinct structural, physical, and electrical characteristics, graphene is predicted to favor the advancement of thin-film solar cells. The study [454] determined if graphene may produce solar cells as an active interfacial layer and front and back connections. The study found that graphene has been demonstrated to be a good substitute for the TCO layer in the CdTe and CIGS systems due to its exceptionally high carrier mobility, transparency, and appropriate work function. While the back contacts of graphene should be sufficiently thick to avoid additional current leakage, the front contacts of the material could have a monolayer structure. The experiment revealed that the graphene layer requires more carrier transportation, complete surface coverage, and higher sheet resistance in a lateral orientation. Even though graphene outperformed the traditional hole transport layer in the long-term stability test, the device parameter loss must be corrected before commercialization.
- According to the role of graphene, which acts as an electrode material and a channel layer in FET, the recent development of flexible thin-film transistors based on graphene and graphene/semiconductor heterostructures was demonstrated in the study conducted by Zhongcheng et al. [455]. The study also stated that, although graphene still has several technological challenges to be solved, it appears to hold great promise for flexible electronic applications that are challenging to implement with the traditional materials. The large-scale integration of two-dimensional materials, particularly graphene, with materials of different dimensionalities is anticipated to significantly influence semiconductor technology in the future. Moreover, it is crucial to have single-crystalline films with consistent thickness and few voids, wrinkles, ripples, and other impurities. As a result, the direct synthesis of superior 2D materials on insulating substrates ought to be paid top attention. Overall, the enormous mixed-dimensional integration opportunities point to significant future growth potential for this sector in applied technology and basic research.
- In a study [456], two distinct graphene strategies were presented for engineering strain in graphene. These strategies used the shrinkage feature of thin films deposited on graphene in a planar shape, eliminating the requirement to bend the substrate. In a controlled manner, graphene was found to exhibit both biaxially strained states and isotropic compressive strained states. The techniques presented here promise to advance graphene strain engineering and investigate novel physics in strained graphene. Furthermore, this work broadly applies to other two-dimensional crystals, including atomically thin metal dichalcogenides.
- According to the investigation of Shuaishuai et al. [457], graphene films with a regulated number of layers or superior monolayer graphene films have been found to grow when thin-metal films are used as the substrate. Cu (111) and CuNi (111), in particular, are single-crystal metal films and alloys that have also been reported and studied as substrates for the reported formation of single-crystal graphene. Single-crystal films with a regulated number of layers might be achievable by expanding these methods to produce other single-crystal metal or alloy films. Further investigation into binary alloys and their extension to ternary (and other) alloy films appears to have ample potential as substrates for forming n-layer graphene films, enabling the exact control of n-layers.
- Owing primarily to its physical and chemical characteristics, graphene oxide (GO) is a versatile material that has been making waves in the biomedical industry in recent years. As mentioned throughout this study [458], many applications can benefit from incorporating GO. These applications include those that use it as a carrier given the large amount of drug it can encapsulate and transport; as a structural component that keeps the drug delivery system (DDS) intact until it reaches its target, preventing the leakage of bioactive compounds; or as a capping layer or barrier that enables the DDS to be fine-tuned and encourages the release of biomolecules in a timely and sequential manner. Furthermore, new and interesting uses of GO are being researched daily, even though its potential for medicinal applications still requires further evaluation.
6.1.1. Single-Layer Graphene
- Flexible Electronics: Graphene’s distinct mechanical and electrical characteristics, coupled with its high optical transmittance, have made it a potential material for flexible transparent conducting electrodes. Additionally, because of its adaptability, graphene is perfect for wearable and flexible electronics, like displays and sensors. A study by Han et al. [470] presented flexible electronic devices on plastic substrates as a promising technology for solar cells and displays in the future and called for graphene to take the place of the expensive and fragile ITO electrode. Because of its special qualities, graphene is a great option for transparent conducting electrodes in flexible electronics, but, before it can be used in real applications, some issues need to be resolved. The review examined the use of graphene in solar cells, FETs, and LEDs. It presented solutions to address its drawbacks and create flexible, extremely stable electronic devices. It also outlined prospective avenues for future study in this area.
- Energy Storage: As single-layer graphene has high surface area and electrical conductivity, it has the potential to be used in batteries, supercapacitors, and other energy storage devices. Pomerantseva et al. [471] emphasized in their research how single-layer graphene, in particular, has the potential to revolutionize energy storage technologies. Electrodes based on nanomaterials have enhanced electronic conductivity and ionic transport, resulting in rapid ion diffusion and high specific capacities. Because these electrodes can tolerate high currents, they present a viable option for high-power and high-energy storage. Despite the current obstacles, a wide range of nanomaterials, from oxides to carbon-based structures, are available. These materials can be used to create novel energy storage solutions, such as wearable and structural energy storage technologies, which were previously impractical to achieve with the conventional materials.
- Sensors: Graphene’s sensitivity to environmental changes allows it to be used in a variety of sensing applications, such as gas detection, biological sensing, and environmental monitoring. Tung et al. [472] emphasized the necessity for sensors with qualities like low cost, high selectivity, and quick response times, pointing out the growing interest in next-generation sensor devices across a range of industries in the study. Because of their remarkable qualities, graphene and its derivatives are positioned as the perfect materials to satisfy these needs. The research investigated the latest developments in the synthesis of graphene-based superstructures in a range of forms and dimensions, such as fibers, thin films, foams, and aerogels. These materials’ excellent sensing capabilities and adaptability are demonstrated by the variety of applications they are used in, including piezoresistive sensors, gas sensors, and biological sensors.
- Composite Materials: Adding graphene to composite materials can improve their mechanical, electrical, and thermal qualities. This can result in the creation of high-performance, lightweight materials for the building, automobile, and aerospace industries. The state of composite materials containing graphene and carbon nanotubes, long viewed as promising developments in nanotechnology applications, has been critically examined by different authors [471,472]. There are still unanswered practical questions about the efficacy of these composites after almost 20 years of research. Their broad use is hampered by problems such as poor load transfer, challenges with interfacial engineering, and processing difficulties. Furthermore, rules for choosing graphene or nanotubes based on particular application needs are lacking. The study by Kinloch et al. [473] provides insights into the future trends regarding this subject by outlining different strategies to tackle these problems and highlighting the prospects and challenges in the development of high-strength, low-density, and high-conductivity materials incorporating nanotubes or graphene.
- Biomedical Applications: Drug transport, tissue engineering, and biosensing are just a few of the biomedical applications that graphene’s special qualities and biocompatibility make possible. Because of its special physicochemical qualities, graphene is gaining attention for a variety of uses in the physical, chemical, and biological sciences according to [474]. The study by Yang et al. [475] examines the new developments in graphene-based materials for biomedical applications, with a particular emphasis on drug delivery, bioimaging, biosensors for biomolecule detection, and photothermal therapy. The review offers insights into the future trends of graphene in biological applications and also addresses the opportunities and potential obstacles in this quickly developing field.
6.1.2. Double-Layer Graphene
6.2. Tungsten Disulfide (WS2)
6.2.1. Conductivity
6.2.2. Transmittance
6.3. Molybdenum Disulfide (MoS2)
6.3.1. Conductivity
6.3.2. Transmittance
6.4. Silver-Nanowire-Based Electrodes for Flexible TCFs
Tips for Optimizing AgNW-Based TCFs
- Length of Nanowire:
- Long AgNWs: These improve conductivity and lessen electrical resistance by reducing the number of junctions in the network. They are perfect for situations where strong conductivity is essential [539].
- Short AgNWs: They are more straightforward to handle but often have more connections, which could lead to increased resistance. To lessen this, make sure the density is higher or maximize the uniformity of the deposition [540].
- Arrangement of Nanowires:
- Uniform Arrangement: The electrical conductivity and optical transparency increase when AgNWs are distributed uniformly throughout the substrate. Methods such as controlled spin-coating can achieve this homogeneity [541].
- Random Arrangement: When nanowires are positioned randomly, hotspots of high resistance and inconsistent performance could exist across the film. This can be resolved by applying post-treatment techniques to enhance the connection or streamline the deposition process [542].
- Density of Deposition: Careful control of the AgNW content in the solution and the deposition conditions is necessary to balance transparency and conductivity. A network that is too dense could be less transparent, whereas one that is too sparse might not have enough conductivity [543].
- Surface Coatings: Applying coatings like graphene (AgNW-G) or zinc oxide after deposition strengthens the electrical connectivity at the nanowire junctions and increases mechanical stability, both of which are advantageous in flexible or stretchable applications [544].
7. Challenges and Opportunities
7.1. Challenges
7.2. Opportunities
- Flexible electronics: Flexible electronics represent one of the most promising applications of conductive thin films. Their natural flexibility opens the door to a new era of wearable and flexible electronics by creating roll-up solar cells, bendable screens, and wearable sensors [551].
- Energy technologies: Conductive thin films are essential for improving the efficiency of batteries, supercapacitors, and solar cells [552]. These films help to improve energy conversion and storage efficiency by enhancing electron mobility and charge transport.
- Biomedical applications: Due to their electrical properties and biocompatibility, conductive thin films have unique possibilities for various applications in biomedical devices. These films have enormous potential to transform healthcare technology, from drug delivery systems to biosensors and neurological probes [553,554].
- Manufacturing advancements: The printability of conductive thin films makes large-scale manufacturing processes like inkjet and screen printing easier [555]. This paves the way for the affordable and scalable manufacturing of electronic components, opening up new opportunities.
- Photonic devices: Photonic devices, such as OLEDs, LEDs, and photodetectors, use conductive thin films. Their capacity to regulate the flow of photons makes improvements in lighting, displays, and optical communications possible, thus also improving these devices’ efficiency and performance [556,557].
- Flexible sensors: Stretchable and flexible sensors are being developed for various uses, and conductive thin films play a vital role in this process [558]. Utilizing the characteristics of thin films, these sensors identify and transfer essential data in wearable health monitors, fitness trackers, environmental sensors, and human–machine interfaces.
- Aerospace and defense: Conductive thin films are essential for temperature management, radar-absorbing materials, and electromagnetic interference (EMI) shielding in these sectors [559]. Because of their conductive, lightweight, and firm characteristics, they are perfect for improving the dependability and performance of military hardware, satellites, and airplanes [560].
- Automotive electronics: Conductive thin films are helpful in the automotive industry for touch-sensitive controls, transparent conductive coatings for windows and displays, and parts for electric vehicles [561]. These films fuel innovation in the automobile sector, improving safety systems, energy economy, and vehicle connectivity.
- Environmental monitoring: Conductive thin films are an essential component of environmental monitoring systems. They make it easier to identify and analyze pollutants, gases, and contaminants. Thin-film sensors support environmental conservation and protection initiatives by helping with environmental surveillance, water quality evaluation, and air quality monitoring [562].
- Smart textiles: Incorporating conductive thin films into textiles makes wearable electronics and smart clothing possible. With their seamless integration of electronics into regular clothing, these textiles transform sports, fashion, and healthcare. They can track physical activity, monitor vital signs, and provide interactive functions [563].
- Transparent conductors: Transparent conductive films provide the conductivity and transparency needed for responsive touch interfaces. They are utilized in touchscreens found in smartphones, tablets, and other devices [555]. They also play a crucial role as transparent electrodes in optoelectronic devices, including OPVs and LEDs.
- Radio-frequency identification (RFID) and antennas: When the conventional rigid antennas are impractical, conductive thin films are employed to create flexible antennas for wearable technology, drones, and Internet of Things (IoT) applications [564]. These films can also create RFID tags, which are necessary for contactless payments, supply chain tracking, and inventory management.
- Imaging sensors: Infrared and UV light sensors are essential for applications including night vision, environmental monitoring, and medical imaging. These sensors are composed of conductive thin films [565]. In terms of high-resolution images, conductive thin films can be utilized to improve the sensitivity and resolution of the detectors in image sensors, especially for industrial or medical inspection, enabling more precise and detailed imaging.
- Anti-counterfeiting and security and safe packaging: To provide tamper-evident seals or integrate RFID technology for tracking and identification, conductive thin films can be used in packing materials. This helps to ensure product integrity and prevent counterfeiting. These films can be utilized to create smart labels, providing additional security for expensive or delicate goods by changing the color or displaying information in response to specific stimuli [566].
8. Conclusions
- The review delved into the preparation of thin films through various methods. The preparation methods included the most commonly used techniques: PVD, CVD, ALD, CBD, spin-coating, electrodeposition, sol–gel deposition, spray pyrolysis, and PLD. Each method was explored thoroughly, highlighting the respective advantages and drawbacks. A comprehensive understanding of the techniques used in conductive thin-film preparation was outlined.
- The optical properties, including the absorption coefficient, transmittance, reflectance, and refractive index, were discussed for the different conductive thin films in this article. The variations in the aforementioned properties with the changes in the atomic concentrations, doping concentrations, temperature (annealing temperature, substrate temperature, etc.), surface morphology, bandgap, packing density, porosity, wavelength, molarity, deposition techniques, film thickness, power and deposition time, etc., were discussed briefly. Numerous research studies were investigated and discussed to understand the underlying factors responsible for the alterations observed in the optical characteristics of the different thin films.
- The electronic resistivity for the different conductive thin films was discussed thoroughly. The variations in the electronic resistivity due to various influencing parameters, including temperature, deposition techniques, film thickness, power and deposition time, grain size, sputtering voltage, carrier concentration, etc., were discussed. Additionally, electronic resistivity’s inverse relationship with electronic conductivity was also highlighted in this article.
- The review article also identified the potential future trends regarding conductive thin films, focusing on 2D TCF materials such as graphene, WS2, MoS2, and AgNW. Some challenges related to conductive thin films were discussed, along with some potential solutions by other authors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.; Kuanr, S.K.; Babu, K.S. Thin Film: Deposition, Growth Aspects, and Characterization. In Thin Film Structures in Energy Applications; Moorthy, S.B.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–49. [Google Scholar] [CrossRef]
- Sanchez, C.; Boissière, C.; Grosso, D.; Laberty, C.; Nicole, L. Design, Synthesis, and Properties of Inorganic and Hybrid Thin Films Having Periodically Organized Nanoporosity. Chem. Mater. 2008, 20, 682–737. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Eslamian, M. Inorganic and Organic Solution-Processed Thin Film Devices. Nano-Micro Lett. 2016, 9, 3. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, Y.; Wang, Q.; Shao, Y.; Bai, Y.; Deng, Y.; Dong, Q.; Hu, M.; Bi, C.; Huang, J. Thin-film semiconductor perspective of organometal trihalide perovskite materials for high-efficiency solar cells. Mater. Sci. Eng. R Rep. 2016, 101, 1–38. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Sharshir, S.W.; Ahmed Ali, M.K.; Shaibo, J.; Edreis, E.M.A.; Abdelhamid, T.; Du, C.; Haiou, Z. Thin film technology for solar steam generation: A new dawn. Sol. Energy 2019, 177, 561–575. [Google Scholar] [CrossRef]
- Schlom, D.G.; Chen, L.-Q.; Pan, X.; Schmehl, A.; Zurbuchen, M.A. A Thin Film Approach to Engineering Functionality into Oxides. J. Am. Ceram. Soc. 2008, 91, 2429–2454. [Google Scholar] [CrossRef]
- Myny, K. The development of flexible integrated circuits based on thin-film transistors. Nat. Electron. 2018, 1, 30–39. [Google Scholar] [CrossRef]
- Franklin, A.D. Nanomaterials in transistors: From high-performance to thin-film applications. Science 2015, 349, aab2750. [Google Scholar] [CrossRef]
- Shanmugam, N.; Pugazhendhi, R.; Elavarasan, R.M.; Kasiviswanathan, P.; Das, N. Anti-Reflective Coating Materials: A Holistic Review from PV Perspective. Energies 2020, 13, 2631. [Google Scholar] [CrossRef]
- Piegari, A.; Flory, F. Optical Thin Films and Coatings: From Materials to Applications; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Arya, R.K.; Verros, G.D.; Davim, J.P. Functional Coatings: Innovations and Challenges; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; Pinyou, P.; Zampardi, G. Research Frontiers in Energy-Related Materials and Applications for 2020–2030. Adv. Sustain. Syst. 2020, 4, 1900145. [Google Scholar] [CrossRef]
- Nistor, P.A.; May, P.W. Diamond thin films: Giving biomedical applications a new shine. J. R. Soc. Interface 2017, 14, 20170382. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302. [Google Scholar] [CrossRef]
- Hegedus, S. Thin film solar modules: The low cost, high throughput and versatile alternative to Si wafers. Prog. Photovolt. Res. Appl. 2006, 14, 393–411. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, Z.; Yang, Z.; Chen, X.; Arnold, M.S.; Zhang, Y.; Xu, J.; Chi, Z.; Aldred, M.P. Recent developments of truly stretchable thin film electronic and optoelectronic devices. Nanoscale 2018, 10, 5764–5792. [Google Scholar] [CrossRef]
- Delbos, S. Kësterite thin films for photovoltaics: A review. EPJ Photovolt. 2012, 3, 35004. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Mascaro, D.J. Organic thin-film transistors: A review of recent advances. IBM J. Res. Dev. 2001, 45, 11–27. [Google Scholar] [CrossRef]
- Kumar, B.; Kaushik, B.K.; Negi, Y.S. Organic Thin Film Transistors: Structures, Models, Materials, Fabrication, and Applications: A Review. Polym. Rev. 2014, 54, 33–111. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. AO 2002, 41, 3053–3060. [Google Scholar] [CrossRef]
- Vook, R.W. Structure and growth of thin films. Int. Met. Rev. 1982, 27, 209–245. [Google Scholar] [CrossRef]
- Fu, Y.; Du, H.; Huang, W.; Zhang, S.; Hu, M. TiNi-based thin films in MEMS applications: A review. Sens. Actuators A Phys. Vol. 2004, 112, 395–408. [Google Scholar] [CrossRef]
- Lee, T.D.; Ebong, A.U. A review of thin film solar cell technologies and challenges. Renew. Sustain. Energy Rev. 2017, 70, 1286–1297. [Google Scholar] [CrossRef]
- Chopra, K.L.; Paulson, P.D.; Dutta, V. Thin-film solar cells: An overview. Prog. Photovolt. Res. Appl. 2004, 12, 69–92. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Sheng, J.; Jeong, H.-J.; Han, K.-L.; Hong, T.; Park, J.-S. Review of recent advances in flexible oxide semiconductor thin-film transistors. J. Inf. Disp. 2017, 18, 159–172. [Google Scholar] [CrossRef]
- Khan, A.; Abas, Z.; Kim, H.S.; Oh, I.-K. Piezoelectric thin films: An integrated review of transducers and energy harvesting. Smart Mater. Struct. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Craciun, F.; Verardi, P.; Dinescu, M. Chapter 4—Piezoelectric thin films: Processing and properties. In Handbook of Thin Films; Nalwa, H.S., Ed.; Academic Press: Burlington, NJ, USA, 2002; pp. 231–308. [Google Scholar] [CrossRef]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible CIGS, CdTe and a-Si:H based thin film solar cells: A review. Prog. Mater. Sci. 2020, 110, 100619. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.; Kim, H.J. Review of solution-processed oxide thin-film transistors. Jpn. J. Appl. Phys. 2014, 53, 02BA02. [Google Scholar] [CrossRef]
- O’Hara, J.F.; Withayachumnankul, W.; Al-Naib, I. A Review on Thin-film Sensing with Terahertz Waves. J. Infrared. Milli Terahz. Waves 2012, 33, 245–291. [Google Scholar] [CrossRef]
- Setter, N.; Damjanovic, D.; Eng, L.; Fox, G.; Gevorgian, S.; Hong, S.; Kingon, A.; Kohlstedt, H.; Park, N.Y.; Stephenson, G.B.; et al. Ferroelectric thin films: Review of materials, properties, and applications. J. Appl. Phys. 2006, 100, 051606. [Google Scholar] [CrossRef]
- Adedokun, O. Review on Transparent Conductive Oxides Thin Films deposited by Sol-gel spin coating technique. IJESA 2018, 2, 88–97. Available online: https://dergipark.org.tr/en/pub/ijesa/issue/39397/427973 (accessed on 1 April 2024).
- Afre, R.A.; Sharma, N.; Sharon, M.; Sharon, M. Transparent Conducting Oxide Films for Various Applications: A Review. Rev. Adv. Mater. Sci. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Elanjeitsenni, V.P.; Vadivu, K.S.; Prasanth, B.M. A review on thin films, conducting polymers as sensor devices. Mater. Res. Express 2022, 9, 022001. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Silva, R.; Hechenleitner, A.A.W.; Radovanovic, E.; Melo, M.A.C.; Pineda, E.A.G. Synthesis and characterization of ZnO, CuO and a mixed Zn and Cu oxide. Mater. Chem. Phys. 2009, 115, 110–115. [Google Scholar] [CrossRef]
- Kanani, N. Electroplating: Basic Principles, Processes and Practice; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wang, H.; Lin, N.; Nouri, M.; Liu, Z.; Yu, Y.; Zeng, Q.; Ma, G.; Fan, J.; Li, D.; Wu, Y. Improvement in surface performance of stainless steel by nitride and carbon-based coatings prepared via physical vapor deposition for marine application. J. Mater. Res. Technol. 2023, 27, 6021–6046. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Rosenblatt, G.M. Evaporation from Solids. In Treatise on Solid State Chemistry: Volume 6A Surfaces I; Hannay, N.B., Ed.; Springer: Boston, MA, USA, 1976; pp. 165–240. [Google Scholar] [CrossRef]
- Shah, S.I.; Jaffari, G.H.; Yassitepe, E.; Ali, B. Chapter 4—Evaporation: Processes, Bulk Microstructures, and Mechanical Properties. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 135–252. [Google Scholar] [CrossRef]
- Patil, N.D.; Bange, P.G.; Bhardwaj, R.; Sharma, A. Effects of Substrate Heating and Wettability on Evaporation Dynamics and Deposition Patterns for a Sessile Water Droplet Containing Colloidal Particles. Langmuir 2016, 32, 11958–11972. [Google Scholar] [CrossRef] [PubMed]
- Paul, B. Compilation of Evaporation Coefficients. ARS J. 1962, 32, 1321–1328. [Google Scholar] [CrossRef]
- Safarian, J.; Engh, T.A. Vacuum Evaporation of Pure Metals. Metall. Mater. Trans. A 2013, 44, 747–753. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Ghasemi, H. Evaporation in nano/molecular materials. Adv. Colloid Interface Sci. 2021, 290, 102385. [Google Scholar] [CrossRef]
- Rockett, A. Physical Vapor Deposition. In The Materials Science of Semiconductors; Springer: Boston, MA, USA, 2008; pp. 505–572. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Wang, N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 2002, 81, 757–759. [Google Scholar] [CrossRef]
- Moditswe, C.; Muiva, C.M.; Luhanga, P.; Juma, A. Effect of annealing temperature on structural and optoelectronic properties of γ-CuI thin films prepared by the thermal evaporation method. Ceram. Int. 2017, 43, 5121–5126. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Zhang, J.; Cao, Y. A novel application of the CuI thin film for preparing thin copper nanowires. Phys. B Condens. Matter 2005, 362, 231–235. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Selvaraj, M.; Ramu, S.; Subrahmanyam, A. Thermal evaporated Copper Iodide (CuI) thin films: A note on the disorder evaluated through the temperature dependent electrical properties. Sol. Energy Mater. Sol. Cells 2017, 165, 52–58. [Google Scholar] [CrossRef]
- Merkel, J.J.; Sontheimer, T.; Rech, B.; Becker, C. Directional growth and crystallization of silicon thin films prepared by electron-beam evaporation on oblique and textured surfaces. J. Cryst. Growth 2013, 367, 126–130. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Harlin, A.; Karppinen, M. Comparison of some coating techniques to fabricate barrier layers on packaging materials. Thin Solid Film. 2010, 518, 5463–5466. [Google Scholar] [CrossRef]
- Pham, A.T.T.; Ngo, N.M.; Le, O.K.T.; Hoang, D.V.; Nguyen, T.H.; Phan, T.B.; Tran, V.C. High-mobility sputtered F-doped ZnO films as good-performance transparent-electrode layers. J. Sci. Adv. Mater. Devices 2021, 6, 446–452. [Google Scholar] [CrossRef]
- Tahar, R.B.H.; Ban, T.; Ohya, Y.; Takahashi, Y. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Luo, X.; Ding, Y. Enhancing antibacterial property of porous titanium surfaces with silver nanoparticles coatings via electron-beam evaporation. J. Mater. Sci. Mater. Med. 2022, 33, 57. [Google Scholar] [CrossRef]
- Guo, C.; Kong, M. Fabrication of Ultralow Stress TiO2/SiO2 Optical Coatings by Plasma Ion-Assisted Deposition. Coatings 2020, 10, 720. [Google Scholar] [CrossRef]
- Kosiorek, A.; Kandulski, W.; Chudzinski, P.; Kempa, K.; Giersig, M. Shadow Nanosphere Lithography: Simulation and Experiment. Nano Lett. 2004, 4, 1359–1363. [Google Scholar] [CrossRef]
- Logothetidis, S.; Laskarakis, A.; Gika, A.; Patsalas, P. In situ and real-time ellipsometry diagnostic techniques towards the monitoring of the bonding structure and growth kinetics: Silicon oxide coatings. Surf. Coat. Technol. 2002, 151–152, 204–208. [Google Scholar] [CrossRef]
- Henini, M. Molecular Beam Epitaxy: From Research to Mass Production; Newnes: Oxford, UK, 2012. [Google Scholar]
- Pershin, S.M.; Lednev, V.N.; Bunkin, A.F. Laser ablation of alloys: Selective evaporation model. Phys. Wave Phen. 2011, 19, 261–274. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef]
- Liu, Y.; Du, G. Preparation and Flux-Pinning Properties of Multilayered Yttrium Barium Copper Oxide Thin Films Containing Alternating Barium Zirconate and Yttria Nanostructures. J. Electron. Mater. 2011, 40, 1512–1516. [Google Scholar] [CrossRef]
- Stamford, L.; Azapagic, A. Environmental impacts of copper-indium-gallium-selenide (CIGS) photovoltaics and the elimination of cadmium through atomic layer deposition. Sci. Total Environ. 2019, 688, 1092–1101. [Google Scholar] [CrossRef]
- Savu, R.; Joanni, E. Effect of Processing Conditions on the Microstructure and Electrical Resistance of Nanocrystalline ITO Thin Films Made by Laser Ablation. Mater. Sci. Forum 2006, 514–516, 1161–1165. [Google Scholar] [CrossRef]
- Ngaffo, F.F.; Caricato, A.P.; Fernandez, M.; Martino, M.; Romano, F. Structural properties of single and multilayer ITO and TiO2 films deposited by reactive pulsed laser ablation deposition technique. Appl. Surf. Sci. 2007, 253, 6508–6511. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Kafle, B.P. Chapter 6—Introduction to nanomaterials and application of UV–Visible spectroscopy for their characterization. In Chemical Analysis and Material Characterization by Spectrophotometry; Kafle, B.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 147–198. [Google Scholar] [CrossRef]
- Kern, W. Chemical Vapor Deposition. In Microelectronic Materials and Processes; Levy, R.A., Ed.; Springer: Dordrecht, The Netherlands, 1989; pp. 203–246. [Google Scholar] [CrossRef]
- Scorzoni, A.; Caputo, D.; Petrucci, G.; Placidi, P.; Zampolli, S.; de Cesare, G.; Tavernelli, M.; Nascetti, A. Design and experimental characterization of thin film heaters on glass substrate for Lab-on-Chip applications. Sens. Actuators A Phys. 2015, 229, 203–210. [Google Scholar] [CrossRef]
- Ma, B.; Guye, K.; Dogruoz, B.; Agonafer, D. Molecular dynamics simulations of thin-film evaporation: The influence of interfacial thermal resistance on a graphene-coated heated silicon substrate. Appl. Therm. Eng. 2021, 195, 117142. [Google Scholar] [CrossRef]
- Taheri, M.; Hajiesmaeilbaigi, F.; Motamedi, A. Optical and structural characteristics of silicon nanoparticles thin film prepared by laser ablation. Thin Solid Film. 2011, 519, 7785–7788. [Google Scholar] [CrossRef]
- Ashfold, M.N.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef]
- Krishnaswamy, J.; Rengan, A.; Narayan, J.; Vedam, K.; McHargue, C.J. Thin-film deposition by a new laser ablation and plasma hybrid technique. Appl. Phys. Lett. 1989, 54, 2455–2457. [Google Scholar] [CrossRef]
- Matthias, E.; Siegel, J.; Petzoldt, S.; Reichling, M.; Skurk, H.; Käding, O.; Neske, E. In-situ investigation of laser ablation of thin films. Thin Solid Film. 1995, 254, 139–146. [Google Scholar] [CrossRef]
- Okamoto, A.; Ohata, K. Selective epitaxial growth of gallium arsenide by molecular beam epitaxy. Appl. Phys. Lett. 1987, 51, 1512–1514. [Google Scholar] [CrossRef]
- Fontcuberta i Morral, A.; Colombo, C.; Abstreiter, G.; Arbiol, J.; Morante, J.R. Nucleation mechanism of gallium-assisted molecular beam epitaxy growth of gallium arsenide nanowires. Appl. Phys. Lett. 2008, 92, 063112. [Google Scholar] [CrossRef]
- Fallah, H.R.; Ghasemi, M.; Hassanzadeh, A. Influence of heat treatment on structural, electrical, impedance and optical properties of nanocrystalline ITO films grown on glass at room temperature prepared by electron beam evaporation. Phys. E Low-Dimens. Syst. Nanostructures 2007, 39, 69–74. [Google Scholar] [CrossRef]
- Dey, M.; Das, N.K.; Sen Gupta, A.K.; Dey, M.; Hossain, M.S.; Matin, M.A.; Amin, N. Deposition of CdS Thin Film by Thermal Evaporation. In Proceedings of the 2019 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’s Bazar, Bangladesh, 7–9 February 2019; pp. 1–5. [Google Scholar]
- Lee, S.-M.; Cahill, D.G. Heat transport in thin dielectric films. J. Appl. Phys. 1997, 81, 2590–2595. [Google Scholar] [CrossRef]
- Shinar, J.; Shinar, R. 1.04—An Overview of Organic Light-Emitting Diodes and their Applications. In Comprehensive Nanoscience and Technology; Andrews, D.L., Scholes, G.D., Wiederrecht, G.P., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 73–107. [Google Scholar] [CrossRef]
- The Molecular-Beam Epitaxy (MBE) Process. Available online: https://resources.pcb.cadence.com/blog/2024-the-molecular-beam-epitaxy-mbe-process (accessed on 28 March 2024).
- Saunders, S.R.J.; Nicholls, J.R. Chapter 14—Oxidation, hot corrosion and Protection of metallic materials. In Physical Metallurgy, 4th ed.; Cahn, R.W., Haasen†, P., Eds.; North-Holland: Oxford, UK, 1996; pp. 1291–1361. [Google Scholar] [CrossRef]
- Fancey, K.S.; Matthews, A. Evaporative ion plating: Process mechanisms and optimization. IEEE Trans. Plasma Sci. 1990, 18, 869–877. [Google Scholar] [CrossRef]
- Barshilia, H.C. Surface Modification Technologies for Aerospace and Engineering Applications: Current Trends, Challenges and Future Prospects. Trans Indian Natl. Acad. Eng. 2021, 6, 173–188. [Google Scholar] [CrossRef]
- Mattox, D.M. Ion plating—Past, present and future. Surf. Coat. Technol. 2000, 133–134, 517–521. [Google Scholar] [CrossRef]
- Hauert, R.; Patscheider, J. From Alloying to Nanocomposites—Improved Performance of Hard Coatings. Adv. Eng. Mater. 2000, 2, 247–259. [Google Scholar] [CrossRef]
- Wang, L.; Wan, S.; Wang, S.C.; Wood, R.J.K.; Xue, Q.J. Gradient DLC-Based Nanocomposite Coatings as a Solution to Improve Tribological Performance of Aluminum Alloy. Tribol. Lett. 2010, 38, 155–160. [Google Scholar] [CrossRef]
- Dimitrijević, S.; Rajčić-Vujasinović, M.; Alagić, S.; Grekulović, V.; Trujić, V. Formulation and characterization of electrolyte for decorative gold plating based on mercaptotriazole. Electrochim. Acta 2013, 104, 330–336. [Google Scholar] [CrossRef]
- Constantin, R.; Miremad, B. Performance of hard coatings, made by balanced and unbalanced magnetron sputtering, for decorative applications. Surf. Coat. Technol. 1999, 120–121, 728–733. [Google Scholar] [CrossRef]
- Williams, P. The sputtering process and sputtered ion emission. Surf. Sci. 1979, 90, 588–634. [Google Scholar] [CrossRef]
- Kelly, R. The mechanisms of sputtering part I.: Prompt and slow collisional sputtering. Radiat. Eff. 1984, 80, 273–317. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Thin film deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A 2003, 21, S74–S87. [Google Scholar] [CrossRef]
- Sigmund, P. Sputtering by ion bombardment theoretical concepts. In Sputtering by Particle Bombardment I: Physical Sputtering of Single-Element Solids; Behrisch, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 9–71. [Google Scholar] [CrossRef]
- Rossnagel, S.M.; Schatz, K.; Whitehair, S.J.; Guarnieri, R.C.; Ruzic, D.N.; Cuomo, J.J. The effects of substrate potentials on electron cyclotron resonance plasmas. J. Vac. Sci. Technol. A 1991, 9, 702–706. [Google Scholar] [CrossRef]
- Ibano, K.; Surla, V.; Ruzic, D.N. Sputtering and Thermal Evaporation Studies of Lithiated ATJ Graphite. IEEE Trans. Plasma Sci. 2010, 38, 341–345. [Google Scholar] [CrossRef]
- Mahan, J.E.; Vantomme, A. A simplified collisional model of sputtering in the linear cascade regime. J. Vac. Sci. Technol. A 1997, 15, 1976–1989. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Tracton, A.A. Coatings Technology: Fundamentals, Testing, and Processing Techniques; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chaudhari, M.; Ahirrao, R.; Bagul, S. Thin film Deposition Methods: A Critical Review. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 5215–5232. [Google Scholar]
- Este, G.O.; Westwood, W.D. AC and RF Reactive Sputtering. In Handbook of Thin Film Process Technology; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Chen, C.; Cheng, Y.; Dai, Q.; Song, H. Radio Frequency Magnetron Sputtering Deposition of TiO2 Thin Films and Their Perovskite Solar Cell Applications. Sci. Rep. 2015, 5, 17684. [Google Scholar] [CrossRef]
- Piazza, G.; Felmetsger, V.; Muralt, P.; Iii, R.H.O.; Ruby, R. Piezoelectric aluminum nitride thin films for microelectromechanical systems. MRS Bull. 2012, 37, 1051–1061. [Google Scholar] [CrossRef]
- Chauhan, R.N.; Tiwari, N. Preparation of optically transparent and conducting radio-frequency sputtered indium tin oxide ultrathin films. Thin Solid Film. 2021, 717, 138471. [Google Scholar] [CrossRef]
- Danışman, Ş.; Odabaş, D.; Teber, M. The Effect of TiN, TiAlN, TiCN Thin Films Obtained by Reactive Magnetron Sputtering Method on the Wear Behavior of Ti6Al4V Alloy: A Comparative Study. Coatings 2022, 12, 1238. [Google Scholar] [CrossRef]
- Iqbal, A.; Mohd-Yasin, F. Reactive sputtering of aluminum nitride (002) thin films for piezoelectric applications: A review. Sensors 2018, 18, 1797. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.K.; Mayer, J.W.; Nicolet, M.-A.; Buck, T.M.; Amsel, G.; Eisen, F. Principles and applications of ion beam techniques for the analysis of solids and thin films. Thin Solid Film. 1973, 17, 1–41. [Google Scholar] [CrossRef]
- Schmidt, B.; Wetzig, K. Ion Beams in Materials Processing and Analysis; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Mateev, M.; Lautenschläger, T.; Spemann, D.; Finzel, A.; Gerlach, J.W.; Frost, F.; Bundesmann, C. Systematic investigation of the reactive ion beam sputter deposition process of SiO2. Eur. Phys. J. B 2018, 91, 1–8. [Google Scholar] [CrossRef]
- Park, Y.-S.; Park, H.-K.; Cho, S.-W.; Jeong, J.-A.; Choi, K.-H.; Kim, H.-K.; Lee, J.-Y.; Lee, J.-H.; Bae, H.-D.; Tak, Y.-H.; et al. Transparent Conducting AZO Cosputtered ITO Anode Films Grown by a Dual Target DC Magnetron Sputtering for OLEDs. Electrochem. Solid-State Lett. 2008, 11, J85. [Google Scholar] [CrossRef]
- Golan, G.; Axelevitch, A.; Gorenstein, B.; Peled, A. Novel type of indium oxide thin films sputtering for opto-electronic applications. Appl. Surf. Sci. 2007, 253, 6608–6611. [Google Scholar] [CrossRef]
- Bhatt, V.; Chandra, S. Silicon dioxide films by RF sputtering for microelectronic and MEMS applications. J. Micromech. Microeng. 2007, 17, 1066. [Google Scholar] [CrossRef]
- Bradley, J.W. The plasma properties adjacent to the target in a magnetron sputtering source. Plasma Sources Sci. Technol. 1996, 5, 622. [Google Scholar] [CrossRef]
- Yusupov, M.; Bultinck, E.; Depla, D.; Bogaerts, A. Behavior of electrons in a dual-magnetron sputter deposition system: A Monte Carlo model. New J. Phys. 2011, 13, 033018. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films composed of metal nanoparticles (Au, Ag, Cu) dispersed in AlN: The influence of composition and thermal annealing on the structure and plasmonic response. Thin Solid Film. 2019, 676, 12–25. [Google Scholar] [CrossRef]
- Jain, I.P.; Agarwal, G. Ion beam induced surface and interface engineering. Surf. Sci. Rep. 2011, 66, 77–172. [Google Scholar] [CrossRef]
- Luo, P.; Zhou, S.; Xia, W.; Cheng, J.; Xu, C.; Lu, Y. Chemical Vapor Deposition of Perovskites for Photovoltaic Application. Adv. Mater. Interfaces 2017, 4, 1600970. [Google Scholar] [CrossRef]
- Acosta, E. Thin Films/Properties and Applications. In Thin Films; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Martinu, L.; Zabeida, O.; Klemberg-Sapieha, J.E. Chapter 9—Plasma-Enhanced Chemical Vapor Deposition of Functional Coatings. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 392–465. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Stoffel, A.; Kovács, A.; Kronast, W.; Müller, B. LPCVD against PECVD for micromechanical applications. J. Micromech. Microeng. 1996, 6, 1. [Google Scholar] [CrossRef]
- Crowell, J.E. Chemical methods of thin film deposition: Chemical vapor deposition, atomic layer deposition, and related technologies. J. Vac. Sci. Technol. A 2003, 21, S88–S95. [Google Scholar] [CrossRef]
- Carlsson, J.-O.; Martin, P.M. Chapter 7—Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 314–363. [Google Scholar] [CrossRef]
- Morosanu, C.E. Thin Films by Chemical Vapour Deposition; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Alarifi, I.M. Advanced selection materials in solar cell efficiency and their properties—A comprehensive review. Mater. Today Proc. 2023, 81, 403–414. [Google Scholar] [CrossRef]
- Herman, I.P. Optical Diagnostics for Thin Film Processing; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Zhang, J.; Li, Y.; Cao, K.; Chen, R. Advances in Atomic Layer Deposition. Nanomanuf. Metrol. 2022, 5, 191–208. [Google Scholar] [CrossRef]
- Detavernier, C.; Dendooven, J.; Sree, S.P.; Ludwig, K.F.; Martens, J.A. Tailoring nanoporous materials by atomic layer deposition. Chem. Soc. Rev. 2011, 40, 5242–5253. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.-B.-R.; Maeng, W.-J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Film. 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- Oke, J.A.; Jen, T.-C. Atomic layer deposition and other thin film deposition techniques: From principles to film properties. J. Mater. Res. Technol. 2022, 21, 2481–2514. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Li, Y.; Goei, R.; Jiamin Ong, A.; Zou, Y.; Shpatz Dayan, A.; Rahmany, S.; Etgar, L.; Iing Yoong Tok, A. Atomic layer deposition of piezoelectric materials: A timely review. Mater. Today Energy 2024, 39, 101457. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Liu, J.; Adair, K.; Zhao, F.; Sun, Y.; Wu, T.; Bi, X.; Amine, K.; Lu, J.; et al. Atomic/molecular layer deposition for energy storage and conversion. Chem. Soc. Rev. 2021, 50, 3889–3956. [Google Scholar] [CrossRef]
- Doyle, S. New Materials via Combinatorial Atomic Layer Deposition. 2022. Available online: https://hdl.handle.net/10468/13206 (accessed on 17 March 2024).
- Rao, M.C. Pulsed laser deposition—Ablation mechanism and applications. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 355–360. [Google Scholar] [CrossRef]
- Krebs, H.-U.; Weisheit, M.; Faupel, J.; Süske, E.; Scharf, T.; Fuhse, C.; Störmer, M.; Sturm, K.; Seibt, M.; Kijewski, H.; et al. Pulsed Laser Deposition (PLD)—A Versatile Thin Film Technique. In Advances in Solid State Physics; Kramer, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 505–518. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Sanjeeviraja, C.; Jayachandran, M.; Sankaranarayanan, K.; Misra, P.; Kukreja, L.M. Development of a novel high optical quality ZnO thin films by PLD for III–V opto-electronic devices. Curr. Appl. Phys. 2006, 6, 103–108. [Google Scholar] [CrossRef]
- Chen, R.; Lan, L. Solution-processed metal-oxide thin-film transistors: A review of recent developments. Nanotechnology 2019, 30, 312001. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Kashyap, A.; Singh, N.K.; Soni, M.; Soni, A. Chapter 3—Deposition of thin films by chemical solution-assisted techniques. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Das, S., Dhara, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–117. [Google Scholar] [CrossRef]
- Levy, D.; Castellón, E. Transparent Conductive Materials: Materials, Synthesis, Characterization, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Pawar, S.M.; Pawar, B.S.; Kim, J.H.; Joo, O.-S.; Lokhande, C.D. Recent status of chemical bath deposited metal chalcogenide and metal oxide thin films. Curr. Appl. Phys. 2011, 11, 117–161. [Google Scholar] [CrossRef]
- Aida, M.S.; Hariech, S. Cadmium Sulfide Thin Films by Chemical Bath Deposition Technique. In Advances in Energy Materials; Ikhmayies, S.J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–75. [Google Scholar] [CrossRef]
- Della Gaspera, E.; Kennedy, D.F.; van Embden, J.; Chesman, A.S.R.; Gengenbach, T.R.; Weber, K.; Jasieniak, J.J. Flash-Assisted Processing of Highly Conductive Zinc Oxide Electrodes from Water. Adv. Funct. Mater. 2015, 25, 7263–7271. [Google Scholar] [CrossRef]
- Patel, S.R.; Chaki, S.H.; Bhatt, S.V.; Deshpande, M.P.; Soni, S.S.; Bariya, S.N. Thorough investigation of the optical, electrical and thermal properties of Cu3Se2 thin film deposited by chemical bath deposition. Thin Solid Film. 2024, 791, 140242. [Google Scholar] [CrossRef]
- Tyona, M.D. A comprehensive study of spin coating as a thin film deposition technique and spin coating equipment. Adv. Mater. Res. 2013, 2, 181–193. Available online: http://techno-press.org/content/?page=article&journal=amr&volume=2&num=4&ordernum=1 (accessed on 17 March 2024).
- Tyona, M.D. A theoritical study on spin coating technique. Adv. Mater. Res. 2013, 2, 195–208. Available online: http://techno-press.org/content/?page=article&journal=amr&volume=2&num=4&ordernum=2 (accessed on 17 March 2024).
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Padmanaban, M.; Cho, J.; Kudo, T.; Rahman, D.; Yao, H.; McKenzie, D.; Dioses, A.; Mullen, S.; Wolfer, E.; Yamamoto, K.; et al. Progress in Spin-on Hard Mask Materials for Advanced Lithography. J. Photopolym. Sci. Technol. 2014, 27, 503–509. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Y.; Yang, X.; Chen, J.; Yang, N.; Zhu, Y. Stability of silver nanowire transparent conductive film and strategies for improvement. Crit. Rev. Solid State Mater. Sci. 2024, 1–53. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Yu, H.; Wang, L.; Zain-ul-Abdin; Amer, W.A.; Akram, M.; Khalid, H.; Chen, Y.; Saleem, M.; Sun, R.; et al. Preparation of silver nanowires and their application in conducting polymer nanocomposites. Mater. Chem. Phys. 2015, 166, 1–15. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef]
- Thanh, T.D.; Chuong, N.D.; Hien, H.V.; Kshetri, T.; Tuan, L.H.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Meng, X. An overview of molecular layer deposition for organic and organic–inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Prog. Mater. Sci. 2016, 75, 1–37. [Google Scholar] [CrossRef]
- Wu, T.; Kim, J.; Lim, J.-H.; Kim, M.-S.; Myung, N.V. Comprehensive Review on Thermoelectric Electrodeposits: Enhancing Thermoelectric Performance Through Nanoengineering. Front. Chem. 2021, 9, 762896. [Google Scholar] [CrossRef]
- Schubert, U. Chemistry and Fundamentals of the Sol–Gel Process. In The Sol-Gel Handbook; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–28. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sahu, B.K.; Dash, D.K. A brief review on basic fundamentals of nanoparticle (NPs). Nano Med. Mater. 2023, 3, 31. [Google Scholar] [CrossRef]
- Harun, K.; Hussain, F.; Purwanto, A.; Sahraoui, B.; Zawadzka, A.; Mohamad, A.A. Sol–gel synthesized ZnO for optoelectronics applications: A characterization review. Mater. Res. Express 2017, 4, 122001. [Google Scholar] [CrossRef]
- Guleryuz, H.; Kaus, I.; Filiàtre, C.; Grande, T.; Einarsrud, M.-A. Deposition of silica thin films formed by sol–gel method. J. Sol-Gel. Sci. Technol. 2010, 54, 249–257. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Chen, W.-H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Patil, P.S. Versatility of chemical spray pyrolysis technique. Mater. Chem. Phys. 1999, 59, 185–198. [Google Scholar] [CrossRef]
- Jung, D.S.; Park, S.B.; Kang, Y.C. Design of particles by spray pyrolysis and recent progress in its application. Korean J. Chem. Eng. 2010, 27, 1621–1645. [Google Scholar] [CrossRef]
- Dounia, R.; Migalska-Zalas, A.; Addou, M.; Bernede, J.C.; Outzourhit, A.; Benbrahim, M. Preparation and characterization of highly transparent and conductive indium-zinc oxide thin films deposited by pyrolysis spray technique. Opt. Quant Electron. 2016, 48, 339. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Schwank, J.; DiBattista, M.; Vasiliev, A. Peculiarities of SnO2 thin film deposition by spray pyrolysis for gas sensor application. Sens. Actuators B Chem. 2001, 77, 244–252. [Google Scholar] [CrossRef]
- Zhang, L.; Song, T.; Shi, L.; Wen, N.; Wu, Z.; Sun, C.; Jiang, D.; Guo, Z. Recent progress for silver nanowires conducting film for flexible electronics. J. Nanostruct. Chem. 2021, 11, 323–341. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Zheng, H.; Latham, K.; Wlodarski, W.; Kalantar-zadeh, K. Anodization of Ti Thin Film Deposited on ITO. Langmuir 2009, 25, 509–514. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Transparent Highly Ordered TiO2 Nanotube Arrays via Anodization of Titanium Thin Films. Adv. Funct. Mater. 2005, 15, 1291–1296. [Google Scholar] [CrossRef]
- Kant, K.; Low, S.P.; Marshal, A.; Shapter, J.G.; Losic, D. Nanopore Gradients on Porous Aluminum Oxide Generated by Nonuniform Anodization of Aluminum. ACS Appl. Mater. Interfaces 2010, 2, 3447–3454. [Google Scholar] [CrossRef]
- Hubarevich, A.; Marus, M.; Stsiapanau, A.; Smirnov, A.; Zhao, J.; Fan, W.; Wang, H.; Sun, X. Transparent conductive nanoporous aluminium mesh prepared by electrochemical anodizing. Phys. Status Solidi A 2015, 212, 2174–2178. [Google Scholar] [CrossRef]
- Roy, R.; Mondal, I.; Singh, A.K. Fabrication of an anodized nanoporous aluminium (AAO/Al) transparent electrode as an ITO alternative for PDLC smart windows. Mater. Adv. 2023, 4, 923–931. [Google Scholar] [CrossRef]
- Abermann, S. Non-vacuum processed next generation thin film photovoltaics: Towards marketable efficiency and production of CZTS based solar cells. Sol. Energy 2013, 94, 37–70. [Google Scholar] [CrossRef]
- Dick, K.A. A review of nanowire growth promoted by alloys and non-alloying elements with emphasis on Au-assisted III–V nanowires. Prog. Cryst. Growth Charact. Mater. 2008, 54, 138–173. [Google Scholar] [CrossRef]
- Grodzinski, P.; DenBaars, S.P.; Lee, H.C. From research to manufacture—The evolution of MOCVD. JOM 1995, 47, 25–32. [Google Scholar] [CrossRef]
- Thompson, A.G. MOCVD technology for semiconductors. Mater. Lett. 1997, 30, 255–263. [Google Scholar] [CrossRef]
- Advantage and Disadvantages of MOCVD. Available online: https://www.universitywafer.com/advantages-disadvantages-mocvd.html (accessed on 17 March 2024).
- Ledebo, L. MOCVD technology. In Proceedings of the New Developments in Semiconductor Physics; Ferenczi, G., Beleznay, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 268–280. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabata, K.; Hirose, M. Transparent, conductive CuI films prepared by rf-dc coupled magnetron sputtering. Thin Solid Film. 1996, 281–282, 179–181. [Google Scholar] [CrossRef]
- Yang, C.; Kneiβ, M.; Lorenz, M.; Grundmann, M. Room-temperature synthesized copper iodide thin film as degenerate p-type transparent conductor with a boosted figure of merit. Proc. Natl. Acad. Sci. USA 2016, 113, 12929–12933. [Google Scholar] [CrossRef]
- Sirimanne, P.M.; Rusop, M.; Shirata, T.; Soga, T.; Jimbo, T. Characterization of transparent conducting CuI thin films prepared by pulse laser deposition technique. Chem. Phys. Lett. 2002, 366, 485–489. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zhao, X.Z. Transparent conductive CuI thin films prepared by pulsed laser deposition. Phys. Status Solidi A 2011, 208, 91–96. [Google Scholar] [CrossRef]
- Amalina, M.N.; Azilawati, Y.; Rasheid, N.A.; Rusop, M. The Properties of Copper (I) Iodide (CuI) Thin Films Prepared by Mister Atomizer at Different Doping Concentration. Procedia Eng. 2013, 56, 731–736. [Google Scholar] [CrossRef]
- Sankapal, B.R.; Goncalves, E.; Ennaoui, A.; Lux-Steiner, M.C. Wide band gap p-type windows by CBD and SILAR methods. Thin Solid Film. 2004, 451–452, 128–132. [Google Scholar] [CrossRef]
- Zhao, K.; Ngongang Ndjawa, G.O.; Jagadamma, L.K.; Labban, A.E.; Hu, H.; Wang, Q.; Li, R.; Abdelsamie, M.; Beaujuge, P.M.; Amassian, A. Highly efficient organic solar cells based on a robust room-temperature solution-processed copper iodide hole transporter. Nano Energy 2015, 16, 458–469. [Google Scholar] [CrossRef]
- Inudo, S.; Miyake, M.; Hirato, T. Electrical properties of CuI films prepared by spin coating. Phys. Status Solidi A 2013, 210, 2395–2398. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.; Liu, C.; Ni, J.; Hou, G.; Zhao, Y.; Zhang, X. Highly transparent and conductive indium tin oxide thin films for solar cells grown by reactive thermal evaporation at low temperature. Appl. Phys. A 2014, 117, 815–822. [Google Scholar] [CrossRef]
- Chu, J.B.; Huang, S.M.; Zhu, H.B.; Xu, X.B.; Sun, Z.; Chen, Y.W.; Huang, F.Q. Preparation of indium tin oxide thin films without external heating for application in solar cells. J. Non-Cryst. Solids 2008, 354, 5480–5484. [Google Scholar] [CrossRef]
- Ren, B.; Liu, X.; Wang, M.; Xu, Y. Preparation and characteristics of indium tin oxide (ITO) thin films at low temperature by r.f. magnetron sputtering. Rare Met. 2006, 25, 137–140. [Google Scholar] [CrossRef]
- Parida, B.; Gil, Y.; Kim, H. Highly Transparent Conducting Indium Tin Oxide Thin Films Prepared by Radio Frequency Magnetron Sputtering and Thermal Annealing. J. Nanosci. Nanotechnol. 2019, 19, 1455–1462. [Google Scholar] [CrossRef]
- Liu, W.-S.; Cheng, H.-M.; Hu, H.-C.; Li, Y.-T.; Huang, S.-D.; Yu, H.-W.; Pu, N.-W.; Liang, S.-C. Indium tin oxide with titanium doping for transparent conductive film application on CIGS solar cells. Appl. Surf. Sci. 2015, 354, 31–35. [Google Scholar] [CrossRef]
- Gwamuri, J.; Marikkannan, M.; Mayandi, J.; Bowen, P.K.; Pearce, J.M. Influence of Oxygen Concentration on the Performance of Ultra-Thin RF Magnetron Sputter Deposited Indium Tin Oxide Films as a Top Electrode for Photovoltaic Devices. Materials 2016, 9, 63. [Google Scholar] [CrossRef]
- Fallah, H.R.; Ghasemi, M.; Hassanzadeh, A.; Steki, H. The effect of annealing on structural, electrical and optical properties of nanostructured ITO films prepared by e-beam evaporation. Mater. Res. Bull. 2007, 42, 487–496. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Vickraman, P.; Jayachandran, M.; Sanjeeviraja, C. Structural and optical properties of indium tin oxide (ITO) thin films with different compositions prepared by electron beam evaporation. Vacuum 2010, 84, 864–869. [Google Scholar] [CrossRef]
- Rozati, S.M.; Ganj, T. Transparent conductive Sn-doped indium oxide thin films deposited by spray pyrolysis technique. Renew. Energy 2004, 29, 1671–1676. [Google Scholar] [CrossRef]
- Parthiban, S.; Ramamurthi, K.; Elangovan, E.; Martins, R.; Fortunato, E. Spray deposited molybdenum doped indium oxide thin films with high near infrared transparency and carrier mobility. Appl. Phys. Lett. 2009, 94, 212101. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.-Y.; Zhao, J.; Ma, H.-L. Preparation and properties of indium tin oxide films deposited on polyester substrates by reactive evaporation. Thin Solid Film. 1997, 307, 200–202. [Google Scholar] [CrossRef]
- Adurodija, F.O.; Izumi, H.; Ishihara, T.; Yoshioka, H.; Yamada, K.; Matsui, H.; Motoyama, M. Highly conducting indium tin oxide (ITO) thin films deposited by pulsed laser ablation. Thin Solid Film. 1999, 350, 79–84. [Google Scholar] [CrossRef]
- Coutal, C.; Azema, A.; Roustan, J.C. Fabrication and characterization of ITO thin films deposited by excimer laser evaporation. Thin Solid Film. 1996, 288, 248–253. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, K.A.; Kim, G.H.; Lee, S.Y. Electrical, structural, and optical properties of ITO thin films prepared at room temperature by pulsed laser deposition. Appl. Surf. Sci. 2006, 252, 4834–4837. [Google Scholar] [CrossRef]
- Machet, J.; Guille, J.; Saulnier, P.; Robert, S. Deposition of conducting and transparent thin films of indium tin oxide by reactive ion plating. Thin Solid Film. 1981, 80, 149–155. [Google Scholar] [CrossRef]
- Gareso, P.L.; Heryanto, H.; Juarlin, E.; Taba, P. Effect of Annealing on the Structural and Optical Properties of ZnO/ITO and AZO/ITO Thin Films Prepared by Sol-Gel Spin Coating. Trends Sci. 2023, 20, 6521. [Google Scholar] [CrossRef]
- Mia, M.N.H.; Habiba, U.; Pervez, M.F.; Kabir, H.; Nur, S.; Hossen, M.F.; Sen, S.K.; Hossain, M.K.; Iftekhar, M.A.; Rahman, M.M. Investigation of aluminum doping on structural and optical characteristics of sol–gel assisted spin-coated nano-structured zinc oxide thin films. Appl. Phys. A 2020, 126, 162. [Google Scholar] [CrossRef]
- Silva, R.F.; Darbello Zaniquelli, M.E. Aluminium doped zinc oxide films: Formation process and optical properties. J. Non-Cryst. Solids 1999, 247, 248–253. [Google Scholar] [CrossRef]
- Mamat, M.H.; Sahdan, M.Z.; Amizam, S.; Rafaie, H.A.; Khusaimi, Z.; Rusop, M. Optical and electrical properties of aluminum doped zinc oxide thin films at various doping concentrations. J. Ceram. Soc. Jpn. 2009, 117, 1263–1267. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and properties of transparent conductive aluminum-doped zinc oxide thin films by sol–gel process. J. Vac. Sci. Technol. A 2001, 19, 1642–1646. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. The effects of Al doping on the optical constants of ZnO thin films prepared by spray pyrolysis method. J. Mater. Sci. Mater. Electron. 2008, 19, 704–708. [Google Scholar] [CrossRef]
- Muiva, C.M.; Sathiaraj, T.S.; Maabong, K. Effect of doping concentration on the properties of aluminium doped zinc oxide thin films prepared by spray pyrolysis for transparent electrode applications. Ceram. Int. 2011, 37, 555–560. [Google Scholar] [CrossRef]
- Babu, B.J.; Maldonado, A.; Velumani, S.; Asomoza, R. Electrical and optical properties of ultrasonically sprayed Al-doped zinc oxide thin films. Mater. Sci. Eng. B 2010, 174, 31–37. [Google Scholar] [CrossRef]
- Lee, K.E.; Wang, M.; Kim, E.J.; Hahn, S.H. Structural, electrical and optical properties of sol–gel AZO thin films. Curr. Appl. Phys. 2009, 9, 683–687. [Google Scholar] [CrossRef]
- Barhoumi, A.; Leroy, G.; Duponchel, B.; Gest, J.; Yang, L.; Waldhoff, N.; Guermazi, S. Aluminum doped ZnO thin films deposited by direct current sputtering: Structural and optical properties. Superlattices Microstruct. 2015, 82, 483–498. [Google Scholar] [CrossRef]
- Leem, J.W.; Yu, J.S. Structural, optical, and electrical properties of AZO films by tilted angle sputtering method. Thin Solid Film. 2010, 518, 6285–6288. [Google Scholar] [CrossRef]
- Mosbah, A.; Aida, M.S. Influence of deposition temperature on structural, optical and electrical properties of sputtered Al doped ZnO thin films. J. Alloys Compd. 2012, 515, 149–153. [Google Scholar] [CrossRef]
- Miao, D.; Jiang, S.; Shang, S.; Chen, Z. Effect of heat treatment on infrared reflection property of Al-doped ZnO films. Sol. Energy Mater. Sol. Cells 2014, 127, 163–168. [Google Scholar] [CrossRef]
- Subba Reddy, R.; Sreedhar, A.; Sivasankar Reddy, A.; Uthanna, S. Effect Of Film Thickness On The Structural Morphological And Optical Properties Of Nanocrystalline ZnO Films Formed By RF Magnetron Sputtering. Adv. Mater. Lett. 2012, 3, 239–245. [Google Scholar] [CrossRef]
- Minami, T.; Sato, H.; Ohashi, K.; Tomofuji, T.; Takata, S. Conduction mechanism of highly conductive and transparent zinc oxide thin films prepared by magnetron sputtering. J. Cryst. Growth 1992, 117, 370–374. [Google Scholar] [CrossRef]
- Wang, F.; Wu, M.Z.; Wang, Y.Y.; Yu, Y.M.; Wu, X.M.; Zhuge, L.J. Influence of thickness and annealing temperature on the electrical, optical and structural properties of AZO thin films. Vacuum 2013, 89, 127–131. [Google Scholar] [CrossRef]
- Suzuki, S.; Miyata, T.; Ishii, M.; Minami, T. Transparent conducting V-co-doped AZO thin films prepared by magnetron sputtering. Thin Solid Film. 2003, 434, 14–19. [Google Scholar] [CrossRef]
- Ghorannevis, Z.; Akbarnejad, E.; Salar Elahi, A.; Ghoranneviss, M. RETRACTED ARTICLE: Magnetron Sputtered AZO Thin Film Preparation for the Solar Cells Applications. J. Inorg. Organomet. Polym. 2015, 25, 1486–1489. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, H.; Lu, L.; Jiang, F.; Yang, C. Preparation and properties of AZO thin films on different substrates. Prog. Nat. Sci. Mater. Int. 2010, 20, 44–48. [Google Scholar] [CrossRef]
- Fang, G.J.; Li, D.J.; Yao, B.-L. Effect of Vacuum Annealing on the Properties of Transparent Conductive AZO Thin Films Prepared by DC Magnetron Sputtering. Phys. Status Solidi A 2002, 193, 139–152. [Google Scholar] [CrossRef]
- Kaur, G.; Mitra, A.; Yadav, K.L. Pulsed laser deposited Al-doped ZnO thin films for optical applications. Prog. Nat. Sci. Mater. Int. 2015, 25, 12–21. [Google Scholar] [CrossRef]
- Tynell, T.; Yamauchi, H.; Karppinen, M.; Okazaki, R.; Terasaki, I. Atomic layer deposition of Al-doped ZnO thin films. J. Vac. Sci. Technol. A 2012, 31, 01A109. [Google Scholar] [CrossRef]
- Tok, E.S.; Neave, J.H.; Ashwin, M.J.; Joyce, B.A.; Jones, T.S. Growth of Si-doped GaAs(110) thin films by molecular beam epitaxy; Si site occupation and the role of arsenic. J. Appl. Phys. 1998, 83, 4160–4167. [Google Scholar] [CrossRef]
- Ploog, K.; Fischer, A.; Künzel, H. The Use of Si and Be Impurities for Novel Periodic Doping Structures in GaAs Grown by Molecular Beam Epitaxy. J. Electrochem. Soc. 1981, 128, 400. [Google Scholar] [CrossRef]
- Georgakilas, A.; Panayotatos, P.; Stoemenos, J.; Mourrain, J.L.; Christou, A. Achievements and limitations in optimized GaAs films grown on Si by molecular-beam epitaxy. J. Appl. Phys. 1992, 71, 2679–2701. [Google Scholar] [CrossRef]
- Galiev, G.B.; Klimov, E.A.; Pushkarev, S.S.; Zaytsev, A.A.; Klochkov, A.N. Silicon-Doped Epitaxial Films Grown on GaAs(110) Substrates: The Surface Morphology, Electrical Characteristics, and Photoluminescence Spectra. Semiconductors 2020, 54, 1417–1423. [Google Scholar] [CrossRef]
- Vazquez-Cortas, D.; Shimomura, S.; Lopez-Lopez, M.; Cruz-Hernandez, E.; Gallardo-Hernandez, S.; Kudriavtsev, Y.; Mendez-Garcia, V.H. Electrical and optical properties of Si doped GaAs (631) layers studied as a function of the growth temperature. J. Cryst. Growth 2012, 347, 77–81. [Google Scholar] [CrossRef]
- Briones, F.; Collins, D.M. Low temperature photoluminescence of lightly Si-doped and undoped MBE GaAs. J. Electron. Mater. 1982, 11, 847–866. [Google Scholar] [CrossRef]
- Skromme, B.J.; Bose, S.S.; Lee, B.; Low, T.S.; Lepkowski, T.R.; DeJule, R.Y.; Stillman, G.E.; Hwang, J.C.M. Characterization of high-purity Si-doped molecular beam epitaxial GaAs. J. Appl. Phys. 1985, 58, 4685–4702. [Google Scholar] [CrossRef]
- Li, W.Q.; Bhattacharya, P.K.; Kwok, S.H.; Merlin, R. Molecular-beam epitaxial growth and characterization of silicon-doped AlGaAs and GaAs on (311)A GaAs substrates and their device applications. J. Appl. Phys. 1992, 72, 3129–3135. [Google Scholar] [CrossRef]
- Ruhstorfer, D.; Mejia, S.; Ramsteiner, M.; Döblinger, M.; Riedl, H.; Finley, J.J.; Koblmüller, G. Demonstration of n-type behavior in catalyst-free Si-doped GaAs nanowires grown by molecular beam epitaxy. Appl. Phys. Lett. 2020, 116, 052101. [Google Scholar] [CrossRef]
- Shimanoe, T.; Murotani, T.; Nakatani, M.; Otsubo, M.; Mitsui, S. High quality Si-doped GaAs layers grown by molecular beam epitaxy. Surf. Sci. 1979, 86, 126–136. [Google Scholar] [CrossRef]
- Müller, S.; von Wenckstern, H.; Splith, D.; Schmidt, F.; Grundmann, M. Control of the conductivity of Si-doped β-Ga2O3 thin films via growth temperature and pressure. Phys. Status Solidi A 2014, 211, 34–39. [Google Scholar] [CrossRef]
- Şenay, V.; Özen, S.; Pat, S.; Korkmaz, Ş. Some physical properties of a Si-doped nano-crystalline GaAs thin film grown by thermionic vacuum arc. Vacuum 2015, 119, 228–232. [Google Scholar] [CrossRef]
- Solis-Pomar, F.; Nápoles, O.; Vázquez Robaina, O.; Gutierrez-Lazos, C.; Fundora, A.; Colin, A.; Pérez-Tijerina, E.; Melendrez, M.F. Preparation and characterization of nanostructured titanium nitride thin films at room temperature. Ceram. Int. 2016, 42, 7571–7575. [Google Scholar] [CrossRef]
- Kavitha, A.; Kannan, R.; Sreedhara Reddy, P.; Rajashabala, S. The effect of annealing on the structural, optical and electrical properties of Titanium Nitride (TiN) thin films prepared by DC magnetron sputtering with supported discharge. J. Mater. Sci. Mater. Electron. 2016, 27, 10427–10434. [Google Scholar] [CrossRef]
- Solovan, M.N.; Brus, V.V.; Maistruk, E.V.; Maryanchuk, P.D. Electrical and optical properties of TiN thin films. Inorg Mater 2014, 50, 40–45. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Chen, Y.-H.; Liao, B.-H.; Chen, K.-P. Room temperature fabrication of titanium nitride thin films as plasmonic materials by high-power impulse magnetron sputtering. Opt. Mater. Express OME 2016, 6, 540–551. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Narayandass, S.K.; Mangalaraj, D.; Areva, S.; Mielczarski, J.A. Properties of titanium nitride films prepared by direct current magnetron sputtering. Mater. Sci. Eng. A 2007, 445–446, 223–236. [Google Scholar] [CrossRef]
- Yu, J.; Phang, P.; Samundsett, C.; Basnet, R.; Neupan, G.P.; Yang, X.; Macdonald, D.H.; Wan, Y.; Yan, D.; Ye, J. Titanium Nitride Electron-Conductive Contact for Silicon Solar Cells By Radio Frequency Sputtering from a TiN Target. ACS Appl. Mater. Interfaces 2020, 12, 26177–26183. [Google Scholar] [CrossRef]
- Kharitonov, A.V.; Yanilkin, I.V.; Gumarov, A.I.; Vakhitov, I.R.; Yusupov, R.V.; Tagirov, L.R.; Kharintsev, S.S.; Salakhov, M.K. Synthesis and characterization of titanium nitride thin films for enhancement and localization of optical fields. Thin Solid Film. 2018, 653, 200–203. [Google Scholar] [CrossRef]
- Mascaretti, L.; Barman, T.; Bricchi, B.R.; Münz, F.; Li Bassi, A.; Kment, Š.; Naldoni, A. Controlling the plasmonic properties of titanium nitride thin films by radiofrequency substrate biasing in magnetron sputtering. Appl. Surf. Sci. 2021, 554, 149543. [Google Scholar] [CrossRef]
- Ponon, N.K.; Appleby, D.J.R.; Arac, E.; King, P.J.; Ganti, S.; Kwa, K.S.K.; O’Neill, A. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Film. 2015, 578, 31–37. [Google Scholar] [CrossRef]
- Elstner, F.; Ehrlich, A.; Giegengack, H.; Kupfer, H.; Richter, F. Structure and properties of titanium nitride thin films deposited at low temperatures using direct current magnetron sputtering. J. Vac. Sci. Technol. A 1994, 12, 476–483. [Google Scholar] [CrossRef]
- Su, P.-J.; Liu, B.H. Ion-beam-sputter deposited titanium nitride thin films for conductive atomic force microscope probes. Thin Solid Film. 2013, 529, 317–321. [Google Scholar] [CrossRef]
- Saoula, N.; Henda, K.; Kesri, R. Influence of Nitrogen Content on the Structural and Mechanical Properties of TiN Thin Films. 2009. Available online: https://www.osti.gov/etdeweb/biblio/21510784 (accessed on 29 March 2024).
- Chou, W.-J.; Yu, G.-P.; Huang, J.-H. Deposition of TiN thin films on Si(100) by HCD ion plating. Surf. Coat. Technol. 2001, 140, 206–214. [Google Scholar] [CrossRef]
- Snyder, M.Q.; Trebukhova, S.A.; Ravdel, B.; Wheeler, M.C.; DiCarlo, J.; Tripp, C.P.; DeSisto, W.J. Synthesis and characterization of atomic layer deposited titanium nitride thin films on lithium titanate spinel powder as a lithium-ion battery anode. J. Power Sources 2007, 165, 379–385. [Google Scholar] [CrossRef]
- Yamada, T.; Miyake, A.; Makino, H.; Yamamoto, N.; Yamamoto, T. Effect of thermal annealing on electrical properties of transparent conductive Ga-doped ZnO films prepared by ion-plating using direct-current arc discharge. Thin Solid Film. 2009, 517, 3134–3137. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakemi, T.; Awai, K.; Shirakata, S. Dependence of carrier concentrations on oxygen pressure for Ga-doped ZnO prepared by ion plating method. Thin Solid Film. 2004, 451–452, 439–442. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Boix, P.P.; Wong, L.H.; Sun, L.; Lien, S.-Y. Towards high efficiency thin film solar cells. Prog. Mater. Sci. 2017, 87, 246–291. [Google Scholar] [CrossRef]
- Poncé, S.; Li, W.; Reichardt, S.; Giustino, F. First-principles calculations of charge carrier mobility and conductivity in bulk semiconductors and two-dimensional materials. Rep. Prog. Phys. 2020, 83, 036501. [Google Scholar] [CrossRef]
- Li, S.-L.; Tsukagoshi, K.; Orgiu, E.; Samorì, P. Charge transport and mobility engineering in two-dimensional transition metal chalcogenide semiconductors. Chem. Soc. Rev. 2016, 45, 118–151. [Google Scholar] [CrossRef]
- Han, H.; Theodore, N.D.; Alford, T.L. Improved conductivity and mechanism of carrier transport in zinc oxide with embedded silver layer. J. Appl. Phys. 2008, 103, 013708. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ma, H.L. Scattering mechanisms of charge carriers in transparent conducting oxide films. Appl. Phys. A 1996, 62, 487–492. [Google Scholar] [CrossRef]
- Gayner, C.; Amouyal, Y. Energy Filtering of Charge Carriers: Current Trends, Challenges, and Prospects for Thermoelectric Materials. Adv. Funct. Mater. 2020, 30, 1901789. [Google Scholar] [CrossRef]
- Appleyard, E.T.S.; Bristow, J.R.; Tyndall, A.M. The electrical conductivity of thin films of mercury. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1997, 172, 530–539. [Google Scholar] [CrossRef]
- Light, T.S.; Licht, S.; Bevilacqua, A.C.; Morash, K.R. The Fundamental Conductivity and Resistivity of Water. Electrochem. Solid-State Lett. 2004, 8, E16. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Beyond the Visible: Bioinspired Infrared Adaptive Materials. Adv. Mater. 2021, 33, 2004754. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, D.; Yu, Z.; Xiao, L.; Li, J. Preparation of aluminum doped zinc oxide films and the study of their microstructure, electrical and optical properties. Thin Solid Film. 2007, 515, 6909–6914. [Google Scholar] [CrossRef]
- Mohamed, H.A. The effect of annealing and ZnO dopant on the optoelectronic properties of ITO thin films. J. Phys. D Appl. Phys. 2007, 40, 4234. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Yang, Y.; Fan, W.-H.; Wang, C.; Wu, W.-Y.; Tseng, M.-C.; Wuu, D.-S.; Gao, P.; Kuo, H.-C.; Lien, S.-Y.; et al. Growth and characterization of Si-doped Ga2O3 thin films by remote plasma atomic layer deposition: Toward UVC-LED application. Surf. Coat. Technol. 2022, 435, 128252. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, F.; Ji, X. Optical and electrical properties of Al-doped ZnO thin films by atomic layer deposition. J. Mater. Sci. Mater. Electron. 2020, 31, 17365–17374. [Google Scholar] [CrossRef]
- Kim, H.; Piqué, A.; Horwitz, J.S.; Mattoussi, H.; Murata, H.; Kafafi, Z.H.; Chrisey, D.B. Indium tin oxide thin films for organic light-emitting devices. Appl. Phys. Lett. 1999, 74, 3444–3446. [Google Scholar] [CrossRef]
- Tsai, W.; Delfino, M.; Fair, J.A.; Hodul, D. Temperature dependence of the electrical resistivity of reactively sputtered TiN films. J. Appl. Phys. 1993, 73, 4462–4467. [Google Scholar] [CrossRef]
- Atique, N.; Harmon, E.S.; Chang, J.C.P.; Woodall, J.M.; Melloch, M.R.; Otsuka, N. Electrical and structural properties of Be- and Si-doped low-temperature-grown GaAs. J. Appl. Phys. 1995, 77, 1471–1476. [Google Scholar] [CrossRef]
- Mo, G.; Liu, J.; Lin, G.; Zou, Z.; Wei, Z.; Liu, Y.; He, H.; Fu, Y.; Shen, X. Characterization of low resistivity Ga-doped ZnO thin films on Si substrates prepared by pulsed laser deposition. Mater. Res. Express 2019, 6, 106421. [Google Scholar] [CrossRef]
- Antony, A.; Nisha, M.; Manoj, R.; Jayaraj, M.K. Influence of target to substrate spacing on the properties of ITO thin films. Appl. Surf. Sci. 2004, 225, 294–301. [Google Scholar] [CrossRef]
- Eshaghi, A.; Graeli, A. Optical and electrical properties of indium tin oxide (ITO) nanostructured thin films deposited on polycarbonate substrates “thickness effect”. Optik 2014, 125, 1478–1481. [Google Scholar] [CrossRef]
- Yavas, O.; Takai, M. Effect of substrate absorption on the efficiency of laser patterning of indium tin oxide thin films. J. Appl. Phys. 1999, 85, 4207–4212. [Google Scholar] [CrossRef]
- Sethi, R.; Ahmad, S.; Aziz, A.; Siddiqui, A.M. Structural, optical and electrical properties of tin oxide thin films for application as a wide band gap semiconductor. AIP Conf. Proc. 2015, 1675, 030039. [Google Scholar] [CrossRef]
- Mohan, S.; Major, S.S. Si doped GaN films grown by reactive co-sputtering of GaAs and Si. Mater. Res. Express 2018, 5, 096411. [Google Scholar] [CrossRef]
- Yang, C.; Kneiß, M.; Schein, F.-L.; Lorenz, M.; Grundmann, M. Room-temperature Domain-epitaxy of Copper Iodide Thin Films for Transparent CuI/ZnO Heterojunctions with High Rectification Ratios Larger than 109. Sci. Rep. 2016, 6, 21937. [Google Scholar] [CrossRef]
- Gullu, H.H.; Isik, M.; Gasanly, N.M.; Parlak, M. Temperature-dependent optical characteristics of sputtered Ga-doped ZnO thin films. Mater. Sci. Eng. B 2021, 263, 114834. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, F.; Li, H. Front Transparent Passivation of CIGS-Based Solar Cells via AZO. Molecules 2022, 27, 6285. [Google Scholar] [CrossRef]
- Kim, M.-J. A Study on Optimal Indium Tin Oxide Thickness as Transparent Conductive Electrodes for Near-Ultraviolet Light-Emitting Diodes. Materials 2023, 16, 4718. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.H.; Chang, C.-W.; Chen, Y.-L.; Chang, W.-Y.; Kuo, T.-J.; Lu, Y.-J.; Gwo, S.; Ahn, H. Ultrathin TiN Epitaxial Films as Transparent Conductive Electrodes. ACS Appl. Mater. Interfaces 2022, 14, 16839–16845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Willis, J.; Yang, Z.; Lian, X.; Chen, W.; Wang, L.-S.; Xu, X.; Lee, T.-L.; Chen, L.; Scanlon, D.O.; et al. Deep UV transparent conductive oxide thin films realized through degenerately doped wide-bandgap gallium oxide. Cell Rep. Phys. Sci. 2022, 3, 100801. [Google Scholar] [CrossRef]
- Coroa, J.; Faustino, B.M.M.; Marques, A.; Bianchi, C.; Koskinen, T.; Juntunen, T.; Tittonen, I.; Ferreira, I. Highly transparent copper iodide thin film thermoelectric generator on a flexible substrate. RSC Adv. 2019, 9, 35384–35391. [Google Scholar] [CrossRef]
- Ponja, S.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Highly conductive and transparent gallium doped zinc oxide thin films via chemical vapor deposition. Sci. Rep. 2020, 10, 638. [Google Scholar] [CrossRef]
- Hoffmann, M.; Soos, Z.G. Optical absorption spectra of the Holstein molecular crystal for weak and intermediate electronic coupling. Phys. Rev. B 2002, 66, 024305. [Google Scholar] [CrossRef]
- Oshina, I.; Spigulis, J. Beer–Lambert law for optical tissue diagnostics: Current state of the art and the main limitations. JBO 2021, 26, 100901. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, T. Based on the refractive angle method of complex refractive index measurement. Optik 2018, 174, 692–697. [Google Scholar] [CrossRef]
- Srivastava, S.; Haridas, M.; Basu, J.K. Optical properties of polymer nanocomposites. Bull. Mater. Sci. 2008, 31, 213–217. [Google Scholar] [CrossRef]
- Yoon, S.E.; Kang, Y.; Jeon, G.G.; Jeon, D.; Lee, S.Y.; Ko, S.-J.; Kim, T.; Seo, H.; Kim, B.-G.; Kim, J.H. Exploring Wholly Doped Conjugated Polymer Films Based on Hybrid Doping: Strategic Approach for Optimizing Electrical Conductivity and Related Thermoelectric Properties. Adv. Funct. Mater. 2020, 30, 2004598. [Google Scholar] [CrossRef]
- Banerjee, S.; Sarmah, S.; Kumar, A. Photoluminescence studies in HCl-doped polyaniline nanofibers. J. Opt. 2009, 38, 124–130. [Google Scholar] [CrossRef]
- Ayad, M.M.; Salahuddin, N.; Shenashin, M.A. The optimum HCl concentration for the in situ polyaniline film formation. Synth. Met. 2004, 142, 101–106. [Google Scholar] [CrossRef]
- Chan, C.H.; Kammer, H.-W. Low Frequency Dielectric Relaxation and Conductance of Solid Polymer Electrolytes with PEO and Blends of PEO and PMMA. Polymers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Ou, R.; Gupta, S.; Parker, C.A.; Gerhardt, R.A. Fabrication and Electrical Conductivity of Poly(methyl methacrylate) (PMMA)/Carbon Black (CB) Composites: Comparison between an Ordered Carbon Black Nanowire-Like Segregated Structure and a Randomly Dispersed Carbon Black Nanostructure. J. Phys. Chem. B 2006, 110, 22365–22373. [Google Scholar] [CrossRef]
- Glenis, S.; Benz, M.; LeGoff, E.; Schindler, J.L.; Kannewurf, C.R.; Kanatzidis, M.G. Polyfuran: A new synthetic approach and electronic properties. J. Am. Chem. Soc. 1993, 115, 12519–12525. [Google Scholar] [CrossRef]
- Stubb, H.; Punkka, E.; Paloheimo, J. Electronic and optical properties of conducting polymer thin films. Mater. Sci. Rep. 1993, 10, 85–140. [Google Scholar] [CrossRef]
- Geethalakshmi, D.; Muthukumarasamy, N.; Balasundaraprabhu, R. Effect of dopant concentration on the properties of HCl-doped PANI thin films prepared at different temperatures. Optik 2014, 125, 1307–1310. [Google Scholar] [CrossRef]
- Kar, P. Doping in Conjugated Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kiebooms, R.; Menon, R.; Lee, K. Chapter 1—Synthesis, electrical, and optical properties of conjugated polymers. In Handbook of Advanced Electronic and Photonic Materials and Devices; Singh Nalwa, H., Ed.; Academic Press: Burlington, NJ, USA, 2001; pp. 1–102. [Google Scholar] [CrossRef]
- McCall, R.P.; Ginder, J.M.; Leng, J.M.; Ye, H.J.; Manohar, S.K.; Masters, J.G.; Asturias, G.E.; MacDiarmid, A.G.; Epstein, A.J. Spectroscopy and defect states in polyaniline. Phys. Rev. B. 1990, 41, 5202–5213. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Prokeš, J.; Zemek, J. In-situ polymerized polyaniline films. Synth. Met. 1999, 105, 195–202. [Google Scholar] [CrossRef]
- Rault, J. Aging of oriented polymer glasses. J. Non-Cryst. Solids 2006, 352, 4946–4955. [Google Scholar] [CrossRef]
- Danielsen, P.L. Interchain effects and the anisotropic photogeneration of defects in trans-polyacetylene. Synth. Met. 1987, 20, 125–139. [Google Scholar] [CrossRef]
- Danno, T.; Kürti, J.; Kuzmany, H. Optical anisotropy and Raman scattering from highly oriented poly(octylthiophene) films. Phys. Rev. B 1991, 43, 4809–4819. [Google Scholar] [CrossRef]
- Abargues, R.; Abderrafi, K.; Pedrueza, E.; Gradess, R.; Marqués-Hueso, J.; Valdés, J.L.; Martínez-Pastor, J. Optical properties of different polymer thin films containing in situ synthesized Ag and Au nanoparticles. New J. Chem. 2009, 33, 1720–1725. [Google Scholar] [CrossRef]
- Gioti, M.; Kokkinos, D.; Chaidou, C.I.; Laskarakis, A.; Andreopoulou, A.K.; Kallitsis, J.K.; Logothetidis, S. A comprehensive study of the optical properties of emitting polymers for efficient flexible OLED devices. Phys. Status Solidi A 2016, 213, 2947–2953. [Google Scholar] [CrossRef]
- Tomita, Y. Alternative Transparent Electrodes for Organic Light Emitting Diodes. 2008. Available online: https://www.osti.gov/etdeweb/biblio/21223806 (accessed on 30 March 2024).
- Adetayo, A.E.; Ahmed, T.N.; Zakhidov, A.; Beall, G.W. Improvements of Organic Light-Emitting Diodes Using Graphene as an Emerging and Efficient Transparent Conducting Electrode Material. Adv. Opt. Mater. 2021, 9, 2002102. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Forrest, S.R.; Leo, K.; Thompson, M.E. Electrophosphorescent p–i–n Organic Light-Emitting Devices for Very-High-Efficiency Flat-Panel Displays. Adv. Mater. 2002, 14, 1633–1636. [Google Scholar] [CrossRef]
- Meerheim, R.; Lussem, B.; Leo, K. Efficiency and Stability of p-i-n Type Organic Light Emitting Diodes for Display and Lighting Applications. Proc. IEEE 2009, 97, 1606–1626. [Google Scholar] [CrossRef]
- Cao, X.A.; Li, X.M.; Li, S.; Liu, L.Y. Conductivity Enhancement in Organic Electronics by Delta Doping. IEEE Electron Device Lett. 2016, 37, 1628–1631. [Google Scholar] [CrossRef]
- Thejo Kalyani, N.; Dhoble, S.J. Novel materials for fabrication and encapsulation of OLEDs. Renew. Sustain. Energy Rev. 2015, 44, 319–347. [Google Scholar] [CrossRef]
- Kaur, P.; Karar, V.; Marriwala, N. Study of effect of environmental factors on organic light emitting diode (OLED) displays: A review. IOSR J. Electron. Commun. Eng. 2016, 1, 84–89. Available online: https://www.researchgate.net/profile/Palwinder-Kaur/publication/305622577_Study_of_Effect_of_Environmental_Factors_on_Organic_Light_Emitting_Diode_OLED_Displays_A_Review/links/59095f22a6fdcc4961680036/Study-of-Effect-of-Environmental-Factors-on-Organic-Light-Emitting-Diode-OLED-Displays-A-Review.pdf (accessed on 8 April 2024).
- Mahmood, N.; Tang, T.; Hou, Y. Nanostructured Anode Materials for Lithium Ion Batteries: Progress, Challenge and Perspective. Adv. Energy Mater. 2016, 6, 1600374. [Google Scholar] [CrossRef]
- Wei, M.-K.; Lin, C.-W.; Yang, C.-C.; Kiang, Y.-W.; Lee, J.-H.; Lin, H.-Y. Emission Characteristics of Organic Light-Emitting Diodes and Organic Thin-Films with Planar and Corrugated Structures. Int. J. Mol. Sci. 2010, 11, 1527–1545. [Google Scholar] [CrossRef]
- Vyas, S. A Short Review on Properties and Applications of Zinc Oxide Based Thin Films and Devices: ZnO as a promising material for applications in electronics, optoelectronics, biomedical and sensors. Johns. Matthey Technol. Rev. 2020, 64, 202–218. [Google Scholar] [CrossRef]
- Aktaruzzaman, A.F.; Sharma, G.L.; Malhotra, L.K. Electrical, optical and annealing characteristics of ZnO:Al films prepared by spray pyrolysis. Thin Solid Film. 1991, 198, 67–74. [Google Scholar] [CrossRef]
- Manjakkal, L.; Packia Selvam, I.; Potty, S.N.; Shinde, R.S. Electrical and optical properties of aluminium doped zinc oxide transparent conducting oxide films prepared by dip coating technique. Microelectron. Int. 2017, 34, 1–8. [Google Scholar] [CrossRef]
- Majumder, S.B.; Jain, M.; Dobal, P.S.; Katiyar, R.S. Investigations on solution derived aluminium doped zinc oxide thin films. Mater. Sci. Eng. B 2003, 103, 16–25. [Google Scholar] [CrossRef]
- Tuna, O.; Selamet, Y.; Aygun, G.; Ozyuzer, L. High quality ITO thin films grown by dc and RF sputtering without oxygen. J. Phys. D Appl. Phys. 2010, 43, 055402. [Google Scholar] [CrossRef]
- Tchenka, A.; Agdad, A.; Samba Vall, M.C.; Hnawi, S.K.; Narjis, A.; Nkhaili, L.; Ibnouelghazi, E.; Ech-Chamikh, E. Effect of RF Sputtering Power and Deposition Time on Optical and Electrical Properties of Indium Tin Oxide Thin Film. Adv. Mater. Sci. Eng. 2021, 2021, e5556305. [Google Scholar] [CrossRef]
- Xu, B.; Ren, X.-G.; Gu, G.-R.; Lan, L.-L.; Wu, B.-J. Structural and optical properties of Zn-doped SnO2 films prepared by DC and RF magnetron co-sputtering. Superlattices Microstruct. 2016, 89, 34–42. [Google Scholar] [CrossRef]
- Villegas, C.E.P.; Rocha, A.R.; Marini, A. Anomalous Temperature Dependence of the Band Gap in Black Phosphorus. Nano Lett. 2016, 16, 5095–5101. [Google Scholar] [CrossRef]
- John, S.; Wang, J. Quantum optics of localized light in a photonic band gap. Phys. Rev. B 1991, 43, 12772–12789. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.-Y. The Influence of Doping Ag on Photovoltaic Characteristic of CuI Film; Atlantis Press: Amsterdam, The Netherlands, 2014; pp. 63–67. [Google Scholar]
- Naveena, D.; Dhanabal, R.; Chandra Bose, A. Investigating the effect of La doped CuO thin film as absorber material for solar cell application. Opt. Mater. 2022, 127, 112266. [Google Scholar] [CrossRef]
- Amalina, M.N.; Zainun, A.R.; Rasheid, N.A.; Rusop, M. The electrical conductivity of copper (I) iodide (CuI) thin films prepared by mister atomizer. In Proceedings of the 2012 10th IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 19–21 September 2012; pp. 128–131. [Google Scholar]
- Abbas, M.M.; Shehab, A.A.-M.; Al-Samuraee, A.-K.; Hassan, N.-A. Effect of Deposition Time on the Optical Characteristics of Chemically Deposited Nanostructure PbS Thin Films. Energy Procedia 2011, 6, 241–250. [Google Scholar] [CrossRef]
- Ullah, H.; Rahaman, R.; Mahmud, S. Optical Properties of Cadmium Oxide (CdO) Thin Films. Indones. J. Electr. Eng. Comput. Sci. 2017, 5, 81–84. [Google Scholar] [CrossRef]
- Santos-Cruz, J.; Torres-Delgado, G.; Castanedo-Perez, R.; Jiménez-Sandoval, S.; Jiménez-Sandoval, O.; Zúñiga-Romero, C.I.; Márquez Marín, J.; Zelaya-Angel, O. Dependence of electrical and optical properties of sol–gel prepared undoped cadmium oxide thin films on annealing temperature. Thin Solid Film. 2005, 493, 83–87. [Google Scholar] [CrossRef]
- Kumaravel, R.; Menaka, S.; Snega, S.R.M.; Ramamurthi, K.; Jeganathan, K. Electrical, optical and structural properties of aluminum doped cadmium oxide thin films prepared by spray pyrolysis technique. Mater. Chem. Phys. 2010, 122, 444–448. [Google Scholar] [CrossRef]
- Moss, T.S. The Interpretation of the Properties of Indium Antimonide. Proc. Phys. Soc. B 1954, 67, 775. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous Optical Absorption Limit in InSb. Phys. Rev. 1954, 93, 632–633. [Google Scholar] [CrossRef]
- Usharani, K.; Balu, A.R.; Nagarethinam, V.S.; Suganya, M. Characteristic analysis on the physical properties of nanostructured Mg-doped CdO thin films—Doping concentration effect. Prog. Nat. Sci. Mater. Int. 2015, 25, 251–257. [Google Scholar] [CrossRef]
- Abdolahzadeh Ziabari, A.; Ghodsi, F.E.; Kiriakidis, G. Correlation between morphology and electro-optical properties of nanostructured CdO thin films: Influence of Al doping. Surf. Coat. Technol. 2012, 213, 15–20. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Viegas, J.; Elangovan, E. Structural, microstructural, optical and electrical properties of spray deposited rare-earth metal (Sm) ions doped CdO thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 4152–4164. [Google Scholar] [CrossRef]
- Ahmed, M.; Bakry, A.; Shaaban, E.R.; Dalir, H. Structural, electrical, and optical properties of ITO thin films and their influence on performance of CdS/CdTe thin-film solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 11107–11118. [Google Scholar] [CrossRef]
- Beena, D.; Lethy, K.J.; Vinodkumar, R.; Mahadevan Pillai, V.P.; Ganesan, V.; Phase, D.M.; Sudheer, S.K. Effect of substrate temperature on structural, optical and electrical properties of pulsed laser ablated nanostructured indium oxide films. Appl. Surf. Sci. 2009, 255, 8334–8342. [Google Scholar] [CrossRef]
- Prathap, P.; Devi, G.G.; Subbaiah, Y.P.V.; Ramakrishna Reddy, K.T.; Ganesan, V. Growth and characterization of indium oxide films. Curr. Appl. Phys. 2008, 8, 120–127. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ghosh, K.; Patel, R.; Kahol, P.K. Effect of substrate temperature on opto-electrical properties of Nb-doped In2O3 thin films. J. Cryst. Growth 2008, 310, 4336–4339. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Vickraman, P. Annealing temperature dependent on structural, optical and electrical properties of indium oxide thin films deposited by electron beam evaporation method. Curr. Appl. Phys. 2010, 10, 880–885. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, B.L.; Yen, W.T.; Ha, C.T.; Peng, C. Effect of process conditions on the optoelectronic characteristics of ZnO:Mo thin films prepared by pulsed direct current magnetron sputtering. Thin Solid Film. 2010, 518, 4928–4934. [Google Scholar] [CrossRef]
- Wang, H.-C.; Liao, C.-H.; Chueh, Y.-L.; Lai, C.-C.; Chou, P.-C.; Ting, S.-Y. Crystallinity improvement of ZnO thin film by hierarchical thermal annealing. Opt. Mater. Express OME 2013, 3, 295–306. [Google Scholar] [CrossRef]
- Gao, F.; Yu, K.M.; Mendelsberg, R.J.; Anders, A.; Walukiewicz, W. Preparation of high transmittance ZnO:Al film by pulsed filtered cathodic arc technology and rapid thermal annealing. Appl. Surf. Sci. 2011, 257, 7019–7022. [Google Scholar] [CrossRef]
- Takci, D.K.; Senadim Tuzemen, E.; Kara, K.; Yilmaz, S.; Esen, R.; Baglayan, O. Influence of Al concentration on structural and optical properties of Al-doped ZnO thin films. J. Mater. Sci. Mater. Electron. 2014, 25, 2078–2085. [Google Scholar] [CrossRef]
- Lee, H.W.; Lau, S.P.; Wang, Y.G.; Tse, K.Y.; Hng, H.H.; Tay, B.K. Structural, electrical and optical properties of Al-doped ZnO thin films prepared by filtered cathodic vacuum arc technique. J. Cryst. Growth 2004, 268, 596–601. [Google Scholar] [CrossRef]
- Maniscalco, B.; Kaminski, P.M.; Walls, J.M. Thin film thickness measurements using Scanning White Light Interferometry. Thin Solid Film. 2014, 550, 10–16. [Google Scholar] [CrossRef]
- Sujatha Devi, P.; Chatterjee, M.; Ganguli, D. Indium tin oxide nano-particles through an emulsion technique. Mater. Lett. 2002, 55, 205–210. [Google Scholar] [CrossRef]
- Ogihara, S.; Kinugawa, K. Characteristics of transparent conductive In2O3 films prepared by thermal decomposition of indium nitrile acetylacetonate. J. Ceram. Soc. Jpn. 1982, 90, 157–163. [Google Scholar]
- Katsube, Y. Transparent conducting films of In2O3 prepared by vacuum-evaporation. Appl. Phys. 1980, 49, 2. Available online: https://cir.nii.ac.jp/crid/1370016974112189964 (accessed on 19 March 2024).
- Kim, K.Y.; Park, S.B. Preparation and property control of nano-sized indium tin oxide particle. Mater. Chem. Phys. 2004, 86, 210–221. [Google Scholar] [CrossRef]
- Potts, J.E.; Hanson, R.C.; Walker, C.T.; Schwab, C. Raman scattering from CuBr and CuI. Solid State Commun. 1973, 13, 389–392. [Google Scholar] [CrossRef]
- Burns, G.; Dacol, F.H.; Shafer, M.W.; Alben, R. The Raman spectra of the superionic conductor CuI in its three phases. Solid State Commun. 1977, 24, 753–757. [Google Scholar] [CrossRef]
- Schein, F.-L.; von Wenckstern, H.; Grundmann, M. Transparent p-CuI/n-ZnO heterojunction diodes. Appl. Phys. Lett. 2013, 102, 092109. [Google Scholar] [CrossRef]
- Zi, M.; Li, J.; Zhang, Z.; Wang, X.; Han, J.; Yang, X.; Qiu, Z.; Gong, H.; Ji, Z.; Cao, B. Effect of deposition temperature on transparent conductive properties of γ-CuI film prepared by vacuum thermal evaporation. Phys. Status Solidi A 2015, 212, 1466–1470. [Google Scholar] [CrossRef]
- Moditswe, C.; Muiva, C.M.; Juma, A. Highly conductive and transparent Ga-doped ZnO thin films deposited by chemical spray pyrolysis. Optik 2016, 127, 8317–8325. [Google Scholar] [CrossRef]
- Carboni, M.; Carravetta, M.; Zhang, X.L.; Stulz, E. Efficient NIR light blockage with matrix embedded silver nanoprism thin films for energy saving window coating. J. Mater. Chem. C 2016, 4, 1584–1588. [Google Scholar] [CrossRef]
- Indolia, A.P.; Gaur, M.S. Optical properties of solution grown PVDF-ZnO nanocomposite thin films. J. Polym. Res. 2012, 20, 43. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Amb, C.M.; Reynolds, J.R. Spectral Engineering in π-Conjugated Polymers with Intramolecular Donor-Acceptor Interactions. Acc. Chem. Res. 2010, 43, 1396–1407. [Google Scholar] [CrossRef]
- Lahlouh, B. Optical Properties of Iodine Doped-Poly (Ethylene Oxide). Ph.D. Thesis, University of Jordan, Amman, Jordan, 2009. Available online: https://platform.almanhal.com/Files/4/32798 (accessed on 11 April 2024).
- Zhou, R.-J.; Burkhart, T. Optical properties of particle-filled polycarbonate, polystyrene, and poly(methyl methacrylate) composites. J. Appl. Polym. Sci. 2010, 115, 1866–1872. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 32, 1907296. [Google Scholar] [CrossRef]
- Klinger, M.; Tolbod, L.P.; Gothelf, K.V.; Ogilby, P.R. Effect of Polymer Cross-Links on Oxygen Diffusion in Glassy PMMA Films. ACS Appl. Mater. Interfaces 2009, 1, 661–667. [Google Scholar] [CrossRef]
- Good, P.; Cooper, T.; Querci, M.; Wiik, N.; Ambrosetti, G.; Steinfeld, A. Spectral reflectance, transmittance, and angular scattering of materials for solar concentrators. Sol. Energy Mater. Sol. Cells 2016, 144, 509–522. [Google Scholar] [CrossRef]
- Tsilingiris, P.T. Comparative evaluation of the infrared transmission of polymer films. Energy Convers. Manag. 2003, 44, 2839–2856. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J. Protection materials: Ultraviolet shielding, high energy visible light filtering and visible light transparency PETG composite films. Plast. Rubber Compos. 2015, 44, 368–375. [Google Scholar] [CrossRef]
- Szczyrbowski, J.; Bräuer, G.; Ruske, M.; Schilling, H.; Zmelty, A. New low emissivity coating based on TwinMag® sputtered TiO2 and Si3N4 layers. Thin Solid Film. 1999, 351, 254–259. [Google Scholar] [CrossRef]
- Solieman, A. Effect of sintering temperature on the structural, optical and electrical properties of sol–gel derived indium oxide thin films. J. Sol-Gel Sci. Technol. 2011, 60, 48–57. [Google Scholar] [CrossRef]
- Han, H.; Mayer, J.W.; Alford, T.L. Band gap shift in the indium-tin-oxide films on polyethylene napthalate after thermal annealing in air. J. Appl. Phys. 2006, 100, 083715. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Hadia, N.M.A.; Mohamed, S.H. Effects of V doping on magnetic and optical properties of oxygen-deficient In2O3 thin films. Optik 2017, 145, 377–386. [Google Scholar] [CrossRef]
- Beena, D.; Lethy, K.J.; Vinodkumar, R.; MahadevanPillai, V.P. Influence of substrate temperature on the properties of laser ablated indium tin oxide films. Sol. Energy Mater. Sol. Cells 2007, 91, 1438–1443. [Google Scholar] [CrossRef]
- Ortega, M.; Santana, G.; Acevedo, A.M. Optoelectronic properties of CdO-Si heterojunctions. Superf. Y Vacio 1999, 9, 294–295. Available online: https://www.redalyc.org/pdf/942/94200977.pdf (accessed on 31 March 2024).
- Saha, B.; Das, S.; Chattopadhyay, K.K. Electrical and optical properties of Al doped cadmium oxide thin films deposited by radio frequency magnetron sputtering. Sol. Energy Mater. Sol. Cells 2007, 91, 1692–1697. [Google Scholar] [CrossRef]
- Manjula, N.; Pugalenthi, M.; Nagarethinam, V.S.; Usharani, K.; Balu, A.R. Effect of doping concentration on the structural, morphological, optical and electrical properties of Mn-doped CdO thin films. Mater. Sci. Pol. 2015, 33, 774–781. [Google Scholar] [CrossRef]
- de Biasi, R.S.; Grillo, M.L.N. Measurement of small concentrations of manganese in cadmium oxide (CdO) using electron magnetic resonance. Ceram. Int. 2013, 39, 2171–2173. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Elangovan, E.; Viegas, J. Effect of La doping on the structural, optical and electrical properties of spray pyrolytically deposited CdO thin films. J. Alloys Compd. 2017, 708, 804–812. [Google Scholar] [CrossRef]
- Wu, X.; Coutts, T.J.; Mulligan, W.P. Properties of transparent conducting oxides formed from CdO and ZnO alloyed with SnO2 and In2O3. J. Vac. Sci. Technol. A 1997, 15, 1057–1062. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Dhakal, T.P.; Garner, S.M.; Cimo, P.; Lu, S.; Westgate, C.R. Strained Growth of Aluminum-Doped Zinc Oxide on Flexible Glass Substrate and Degradation Studies Under Cyclic Bending Conditions. IEEE Trans. Device Mater. Reliab. 2014, 14, 121–126. [Google Scholar] [CrossRef]
- Evans, G.C. An Area Survey Method of Investigating the Distribution of Light Intensity in Woodlands, with Particular Reference to Sunflecks. J. Ecol. 1956, 44, 391–428. [Google Scholar] [CrossRef]
- Anderson, M.C. Light Relations of Terrestrial Plant Communities and Their Measurement. Biol. Rev. 1964, 39, 425–481. [Google Scholar] [CrossRef]
- Ion, M.; Berbecaru, C.; Iftimie, S.; Filipescu, M.; Dinescu, M.; Antohe, S. Pld deposited al2o3 thin films for transparent electronics. Dig. J. Nanomater. Bios. 2012, 7, 1609–1614. [Google Scholar]
- Fu, E.-G.; Zhuang, D.-M.; Zhang, G.; Yang, W.-F.; Zhao, M. Substrate temperature dependence of the properties of ZAO thin films deposited by magnetron sputtering. Appl. Surf. Sci. 2003, 217, 88–94. [Google Scholar] [CrossRef]
- Chaabouni, F.; Abaab, M.; Rezig, B. Characterization of n-ZnO/p-Si films grown by magnetron sputtering. Superlattices Microstruct. 2006, 39, 171–178. [Google Scholar] [CrossRef]
- Bennett, H.E.; Porteus, J.O. Relation Between Surface Roughness and Specular Reflectance at Normal Incidence. J. Opt. Soc. Am. JOSA 1961, 51, 123–129. [Google Scholar] [CrossRef]
- Ghodsi, F.E.; Tepehan, F.Z.; Tepehan, G.G. Electrochromic properties of heat-treated thin films of CeO2–TiO2–ZrO2 prepared by sol–gel route. Sol. Energy Mater. Sol. Cells 2008, 92, 234–239. [Google Scholar] [CrossRef]
- Moore, E.A.; Smart, L.E. Optical Properties of Solids. In Solid State Chemistry; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Liu, Q.; Luo, D.; Zhang, X.; Li, S.; Tian, Z. Refractive index and absorption coefficient of blue phase liquid crystal in terahertz band. Liq. Cryst. 2017, 44, 348–354. [Google Scholar] [CrossRef]
- Sharme, R.K.; Islam, M.R.; Sarker, M.A.; Solayman, M.; Al Momin, M.; Islam, M.R. First-principles study on electronic, mechanical, and optical properties of pressure-induced vanadium-based perovskite KVO3. Phys. B Condens. Matter 2024, 681, 415785. [Google Scholar] [CrossRef]
- Solayman, M.; Sarker, M.A.; Muntasir, M.; Sharme, R.K.; Islam, M.R. Pressure-induced investigation of structural, electronic, optical, and mechanical properties of BaCeO3. Opt. Mater. 2024, 148, 114699. [Google Scholar] [CrossRef]
- Heavens, O.S. Optical properties of thin films. Rep. Prog. Phys. 1960, 23, 1. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Paul, G.K.; Sen, S.K. Study of optical properties of some sol–gel derived films of ZnO. Sol. Energy Mater. Sol. Cells 2002, 71, 103–113. [Google Scholar] [CrossRef]
- Xue, S.W.; Zu, X.T.; Zheng, W.G.; Deng, H.X.; Xiang, X. Effects of Al doping concentration on optical parameters of ZnO:Al thin films by sol–gel technique. Phys. B Condens. Matter 2006, 381, 209–213. [Google Scholar] [CrossRef]
- Kim, J.-H.; Seong, T.-Y.; Ahn, K.-J.; Chung, K.-B.; Seok, H.-J.; Seo, H.-J.; Kim, H.-K. The effects of film thickness on the electrical, optical, and structural properties of cylindrical, rotating, magnetron-sputtered ITO films. Appl. Surf. Sci. 2018, 440, 1211–1218. [Google Scholar] [CrossRef]
- Kumar, K.J.; Raju, N.R.C.; Subrahmanyam, A. Thickness dependent physical and photocatalytic properties of ITO thin films prepared by reactive DC magnetron sputtering. Appl. Surf. Sci. 2011, 257, 3075–3080. [Google Scholar] [CrossRef]
- Bragg, W.L.; Pippard, A.B. The form birefringence of macromolecules. Acta Crystallogr. 1953, 6, 865–867. Available online: https://journals.iucr.org/q/issues/1953/11-12/00/a01037/a01037.pdf (accessed on 19 March 2024).
- Ali, H.M.; Abd El-Raheem, M.M.; Megahed, N.M.; Mohamed, H.A. Optimization of the optical and electrical properties of electron beam evaporated aluminum-doped zinc oxide films for opto-electronic applications. J. Phys. Chem. Solids 2006, 67, 1823–1829. [Google Scholar] [CrossRef]
- Ellmer, K. Transparent Conductive Zinc Oxide and Its Derivatives. In Handbook of Transparent Conductors; Ginley, D.S., Ed.; Springer: Boston, MA, USA, 2011; pp. 193–263. [Google Scholar] [CrossRef]
- Vinodkumar, R.; Lethy, K.J.; Arunkumar, P.R.; Krishnan, R.R.; Venugopalan Pillai, N.; Mahadevan Pillai, V.P.; Philip, R. Effect of cadmium oxide incorporation on the microstructural and optical properties of pulsed laser deposited nanostructured zinc oxide thin films. Mater. Chem. Phys. 2010, 121, 406–413. [Google Scholar] [CrossRef]
- Kariper, I.A. CuI Film Produced by Chemical Extraction Method in Different Media. Mat. Res. 2016, 19, 991–998. [Google Scholar] [CrossRef]
- Chen, R.T.; Robinson, D. Electro-optic and all-optical phase modulator on an indium tin oxide single-mode waveguide. Appl. Phys. Lett. 1992, 60, 1541–1543. [Google Scholar] [CrossRef]
- Ohhata, Y.; Shinoki, F.; Yoshida, S. Optical properties of r.f. reactive sputtered tin-doped In2O3 films. Thin Solid Film. 1979, 59, 255–261. [Google Scholar] [CrossRef]
- Goyal, D.; Solanki, P.; Marathe, B.; Takwale, M.T.M.; Bhide, V.B.V. Deposition of Aluminum-Doped Zinc Oxide Thin Films by Spray Pyrolysis. Jpn. J. Appl. Phys. 1992, 31, 361. [Google Scholar] [CrossRef]
- Cossement, D.; Streydio, J.-M. Fabrication of ZnO polycrystalline layers by chemical spray. J. Cryst. Growth 1985, 72, 57–60. [Google Scholar] [CrossRef]
- Gómez, H.; Maldonado, A.; Castanedo-Pérez, R.; Torres-Delgado, G.; Olvera, M.D.L.L. Properties of Al-doped ZnO thin films deposited by a chemical spray process. Mater. Charact. 2007, 58, 708–714. [Google Scholar] [CrossRef]
- Hohenberger, G.; Tomandl, G. Sol-gel processing of varistor powders. J. Mater. Res. 1992, 7, 546–548. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; Maldonado, A.; Olvera, M.d.l.L. Effect of the [Al/Zn] ratio in the starting solution and deposition temperature on the physical properties of sprayed ZnO:Al thin films. Mater. Lett. 2007, 61, 1460–1464. [Google Scholar] [CrossRef]
- Ning, Z.Y.; Cheng, S.H.; Ge, S.B.; Chao, Y.; Gang, Z.Q.; Zhang, Y.X.; Liu, Z.G. Preparation and characterization of ZnO:Al films by pulsed laser deposition. Thin Solid Film. 1997, 307, 50–53. [Google Scholar] [CrossRef]
- Yanfeng, S.; Liu, W.; Zhidan, H.; Shaolin, L.; Zhao Yi, Z.; Du, G. Novel properties of AZO film sputtered in Ar+H2 ambient at high temperature. Vacuum 2006, 80, 981–985. [Google Scholar] [CrossRef]
- Achour, Z.B.; Ktari, T.; Ouertani, B.; Touayar, O.; Bessais, B.; Brahim, J.B. Effect of doping level and spray time on zinc oxide thin films produced by spray pyrolysis for transparent electrodes applications. Sens. Actuators A Phys. 2007, 134, 447–451. [Google Scholar] [CrossRef]
- Yu, X.; Ma, J.; Ji, F.; Wang, Y.; Cheng, C.; Ma, H. Thickness dependence of properties of ZnO:Ga films deposited by rf magnetron sputtering. Appl. Surf. Sci. 2005, 245, 310–315. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Garskaite, E.; Otter, B.; Fossheim, H.E.; Sæterli, R.; Holmestad, R.; Einarsrud, M.-A.; Grande, T. Transparent and conducting ITO thin films by spin coating of an aqueous precursor solution. J. Mater. Chem. 2012, 22, 15740–15749. [Google Scholar] [CrossRef]
- Bélanger, D.; Dodelet, J.P.; Lombos, B.A.; Dickson, J.I. Thickness Dependence of Transport Properties of Doped Polycrystalline Tin Oxide Films. J. Electrochem. Soc. 1985, 132, 1398. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Lyu, X.; Zhang, W. Preparation and investigation of nano-thick FTO/Ag/FTO multilayer transparent electrodes with high figure of merit. Sci. Rep. 2016, 6, 20399. [Google Scholar] [CrossRef]
- Lee, H.-C.; Ok Park, O. The evolution of the structural, electrical and optical properties in indium-tin-oxide thin film on glass substrate by DC reactive magnetron sputtering. Vacuum 2006, 80, 880–887. [Google Scholar] [CrossRef]
- Nishimoto, N.; Yamada, Y.; Ohnishi, Y.; Imawaka, N.; Yoshino, K. Effect of temperature on the electrical properties of ITO in a TiO2/ITO film. Phys. Status Solidi A 2013, 210, 589–593. [Google Scholar] [CrossRef]
- Geng, P.; Li, W.; Zhang, X.; Zhang, X.; Deng, Y.; Kou, H. A novel theoretical model for the temperature dependence of band gap energy in semiconductors. J. Phys. D Appl. Phys. 2017, 50, 40LT02. [Google Scholar]
- Ishibashi, S.; Higuchi, Y.; Ota, Y.; Nakamura, K. Low resistivity indium–tin oxide transparent conductive films. II. Effect of sputtering voltage on electrical property of films. J. Vac. Sci. Technol. A 1990, 8, 1403–1406. [Google Scholar] [CrossRef]
- Ali, A.H.; Hassan, Z.; Shuhaimi, A. Enhancement of optical transmittance and electrical resistivity of post-annealed ITO thin films RF sputtered on Si. Appl. Surf. Sci. 2018, 443, 544–547. [Google Scholar] [CrossRef]
- Boltz, J. Sputtered Tin Oxide and Titanium Oxide Thin Films as Alternative Transparent Conductive Oxides. 2011. Available online: https://www.osti.gov/etdeweb/biblio/21595030 (accessed on 20 March 2024).
- Kokubun, Y.; Watanabe, H.; Wada, M. Electrical Properties of CuI Thin Films. Jpn. J. Appl. Phys. 1971, 10, 864. [Google Scholar] [CrossRef]
- Rusop, M.; Soga, T.; Jimbo, T.; Umeno, M. Copper iodide thin films as a p-type electrical conductivity in dye-sensitized p-Cui|Dye|n-TiO2 heterojunction solid state solar cells. Surf. Rev. Lett. 2004, 11, 577–583. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kottegoda, I.R.M.; Perera, V.P.S.; Aponsu, G.M.L.P.; Wijayantha, K.G.U. Deposition of thin conducting films of CuI on glass. Sol. Energy Mater. Sol. Cells 1998, 55, 283–289. [Google Scholar] [CrossRef]
- Herrick, C.S.; Tevebaugh, A.D. Oxygen-Controlled Conduction in Thin Films of Cuprous Iodide: A Mixed Valency Anion Semiconductor. J. Electrochem. Soc. 1963, 110, 119. [Google Scholar] [CrossRef]
- Perera, V.P.S.; Tennakone, K. Recombination processes in dye-sensitized solid-state solar cells with CuI as the hole collector. Sol. Energy Mater. Sol. Cells 2003, 79, 249–255. [Google Scholar] [CrossRef]
- Devi, L.V.; Selvalakshmi, T.; Sellaiyan, S.; Uedono, A.; Sivaji, K.; Sankar, S. Effect of La doping on the lattice defects and photoluminescence properties of CuO. J. Alloys Compd. 2017, 709, 496–504. [Google Scholar] [CrossRef]
- Kardarian, K.; Nunes, D.; Maria Sberna, P.; Ginsburg, A.; Keller, D.A.; Vaz Pinto, J.; Deuermeier, J.; Anderson, A.Y.; Zaban, A.; Martins, R.; et al. Effect of Mg doping on Cu2O thin films and their behavior on the TiO2/Cu2O heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 27–36. [Google Scholar] [CrossRef]
- Uddin, J.; Sharmin, M.; Hasan, M.N.; Podder, J. Influence of Ni doping on the morphological, structural, optical and electrical properties of CuO thin films deposited via a spray pyrolysis. Opt. Mater. 2021, 119, 111388. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Saeid, M.H.; Gangi, F. Molecular structure and physical properties of E-beam crosslinked low-density polyethylene for wire and cable insulation applications. J. Appl. Polym. Sci. 2002, 86, 1959–1969. [Google Scholar] [CrossRef]
- Muntasir, M.; Sharme, R.K.; Uddin, M.B.; Haque, M.S.; Khan, F. Modeling Microstructural and Mechanical Properties of Solidified Al-Sn-Cu System. Malays. J. Compos. Sci. Manuf. 2023, 12, 84–101. [Google Scholar] [CrossRef]
- De Oliveira, M.C.C.; Diniz Cardoso, A.S.A.; Viana, M.M.; Lins, V.d.F.C. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, N.; Makino, T.; Yoshida, T. Stoichiometric indium oxide thin films prepared by pulsed laser deposition in pure inert background gas. J. Vac. Sci. Technol. A 2000, 18, 83–86. [Google Scholar] [CrossRef]
- Gupta, R.; Mamidi, N.; Ghosh, K.; Mishra, S.; Kahol, P. Growth and characterization of In2O3 thin films prepared by pulsed laser deposition. J. Optoelectron. Adv. Mater. 2007, 9, 2211–2216. Available online: https://bearworks.missouristate.edu/articles-cnas/1705 (accessed on 31 March 2024).
- Meredith, P.; Bettinger, C.J.; Irimia-Vladu, M.; Mostert, A.B.; Schwenn, P.E. Electronic and optoelectronic materials and devices inspired by nature. Rep. Prog. Phys. 2013, 76, 034501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ji, C.; Park, Y.-B.; Guo, L.J. Thin-Metal-Film-Based Transparent Conductors: Material Preparation, Optical Design, and Device Applications. Adv. Opt. Mater. 2021, 9, 2001298. [Google Scholar] [CrossRef]
- Yang, C.; Feng, B.; Wei, J.; Tochigi, E.; Ishihara, S.; Shibata, N.; Ikuhara, Y. Atomic and electronic band structures of Ti-doped Al2O3 grain boundaries. Acta Mater. 2020, 201, 488–493. [Google Scholar] [CrossRef]
- Unno, H.; Sato, Y.; Toh, S.; Yoshinaga, N.; Matsumura, S. Microstructures and electrical properties of TiO2-doped Al2O3 ceramics. J. Electron Microsc. 2010, 59, S107–S115. [Google Scholar] [CrossRef]
- Nakamura, A.; Mizoguchi, T.; Matsunaga, K.; Yamamoto, T.; Shibata, N.; Ikuhara, Y. Periodic Nanowire Array at the Crystal Interface. ACS Nano 2013, 7, 6297–6302. [Google Scholar] [CrossRef]
- Nakamura, A.; Matsunaga, K.; Tohma, J.; Yamamoto, T.; Ikuhara, Y. Conducting nanowires in insulating ceramics. Nat. Mater 2003, 2, 453–456. [Google Scholar] [CrossRef]
- Dou, Y.; Fishlock, T.; Egdell, R.G.; Law, D.S.L.; Beamson, G. Band-gap shrinkage in n-type-doped CdO probed by photoemission spectroscopy. Phys. Rev. B 1997, 55, R13381–R13384. [Google Scholar] [CrossRef]
- Maity, R.; Chattopadhyay, K.K. Synthesis and characterization of aluminum-doped CdO thin films by sol–gel process. Sol. Energy Mater. Sol. Cells 2006, 90, 597–606. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, B.O. Characteristics of Al-doped ZnO thin films obtained by ultrasonic spray pyrolysis: Effects of Al doping and an annealing treatment. Mater. Sci. Eng. B 2004, 106, 242–245. [Google Scholar] [CrossRef]
- Nunes, P.; Fortunato, E.; Tonello, P.; Fernandes, F.B.; Vilarinho, P.; Martins, R. Effect of different dopant elements on the properties of ZnO thin films. Vacuum 2002, 64, 281–285. [Google Scholar] [CrossRef]
- Geng, D.; Yang, H.Y. Recent Advances in Growth of Novel 2D Materials: Beyond Graphene and Transition Metal Dichalcogenides. Adv. Mater. 2018, 30, 1800865. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 2020, 8, 387–440. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.; Luo, J.; Zhang, J.; Wang, X.; Li, D.; Yao, Y.; Zhang, Z. Direct growth of nanocrystalline graphene/graphite all carbon transparent electrode for graphene glass and photodetectors. Carbon 2017, 111, 1–7. [Google Scholar] [CrossRef]
- Chandrashekhar, M.; Lu, J.; Spencer, M.G.; Qazi, M.; Koley, G. Large Area Nanocrystalline Graphite Films on SiC for Gas Sensing Applications. In Proceedings of the 2007 IEEE SENSORS, Atlanta, GA, USA, 28–31 October 2007; pp. 558–561. [Google Scholar] [CrossRef]
- Liu, D.; Yang, W.; Zhang, L.; Zhang, J.; Meng, J.; Yang, R.; Zhang, G.; Shi, D. Two-step growth of graphene with separate controlling nucleation and edge growth directly on SiO2 substrates. Carbon 2014, 72, 387–392. [Google Scholar] [CrossRef]
- Attia, N.F.; Hady, K.A.; Elashery, S.E.A.; Hashem, H.M.; Oh, H.; Refaat, A.M.; Hady, A.A. Greener synthesis route and characterization of smart hybrid graphene based thin films. Surf. Interfaces 2020, 21, 100681. [Google Scholar] [CrossRef]
- Shi, Z.; Jayatissa, A.H. The impact of graphene on the fabrication of thin film solar cells: Current status and future prospects. Materials 2017, 11, 36. Available online: https://www.mdpi.com/1996-1944/11/1/36 (accessed on 20 March 2024). [CrossRef]
- Zhu, Z.; Murtaza, I.; Meng, H.; Huang, W. Thin film transistors based on two dimensional graphene and graphene/semiconductor heterojunctions. RSC Adv. 2017, 7, 17387–17397. [Google Scholar] [CrossRef]
- Shioya, H.; Craciun, M.F.; Russo, S.; Yamamoto, M.; Tarucha, S. Straining Graphene Using Thin Film Shrinkage Methods. Nano Lett. 2014, 14, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, L.; Wang, B.; Ruoff, R.S. Chemical vapor deposition of graphene on thin-metal films. Cell Rep. Phys. Sci. 2021, 2, 100372. Available online: https://www.cell.com/cell-reports-physical-science/pdf/S2666-3864(21)00062-X.pdf (accessed on 20 March 2024).
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C 2019, 5, 67. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J. Graphene. In MoS2 Nanohybrid for Preprint not Peer Reviewed Preprint not Peer Reviewed; CRC Press: Boca Raton, FL, USA, 2012; Available online: https://api.taylorfrancis.com/content/books/mono/download?identifierName=doi&identifierValue=10.1201/b11259&type=googlepdf (accessed on 22 April 2024).
- Whitener, K.E., Jr.; Sheehan, P.E. Graphene synthesis. Diam. Relat. Mater. 2014, 46, 25–34. Available online: https://www.sciencedirect.com/science/article/pii/S0925963514000983 (accessed on 22 April 2024).
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. Available online: https://www.nature.com/articles/nature09579 (accessed on 22 April 2024).
- Cheun Lee, H.; Liu, W.-W.; Chai, S.-P.; Rahman Mohamed, A.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.S.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Gan, X.; Zhou, H.; Zhu, B.; Yu, X.; Jia, Y.; Sun, B.; Zhang, M.; Huang, X.; Liu, J.; Luo, T. A simple method to synthesize graphene at 633 K by dechlorination of hexachlorobenzene on Cu foils. Carbon 2012, 50, 306–310. [Google Scholar] [CrossRef]
- Orofeo, C.M.; Ago, H.; Hu, B.; Tsuji, M. Synthesis of large area, homogeneous, single layer graphene films by annealing amorphous carbon on Co and Ni. Nano Res. 2011, 4, 531–540. [Google Scholar] [CrossRef]
- Tanaka, H.; Arima, R.; Fukumori, M.; Tanaka, D.; Negishi, R.; Kobayashi, Y.; Kasai, S.; Yamada, T.K.; Ogawa, T. Method for controlling electrical properties of single-layer graphene nanoribbons via adsorbed planar molecular nanoparticles. Sci. Rep. 2015, 5, 12341. Available online: https://www.nature.com/articles/srep12341 (accessed on 22 April 2024).
- Alanyalıoğlu, M.; Segura, J.J.; Oro-Sole, J.; Casañ-Pastor, N. The synthesis of graphene sheets with controlled thickness and order using surfactant-assisted electrochemical processes. Carbon 2012, 50, 142–152. Available online: https://www.sciencedirect.com/science/article/pii/S0008622311006609 (accessed on 22 April 2024).
- Han, T.-H.; Kim, H.; Kwon, S.-J.; Lee, T.-W. Graphene-based flexible electronic devices. Mater. Sci. Eng. R Rep. 2017, 118, 1–43. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Rodrigo, D.; Tittl, A.; Limaj, O.; de Abajo, F.J.G.; Pruneri, V.; Altug, H. Double-layer graphene for enhanced tunable infrared plasmonics. Light Sci. Appl. 2017, 6, e16277. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, E.; Goto, H.; Eguchi, R.; Fujiwara, A.; Kubozono, Y. Electric double-layer capacitance between an ionic liquid and few-layer graphene. Sci. Rep. 2013, 3, 1595. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of double-layer graphene tubules. J. Appl. Phys. 1993, 73, 494–500. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Zhang, Q.; Huang, J.-Q.; Tian, G.-L.; Nie, J.-Q.; Peng, H.-J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium–sulphur batteries. Nat. Commun. 2014, 5, 3410. [Google Scholar] [CrossRef]
- Mink, M.P.; Stoof, H.T.C.; Duine, R.A.; MacDonald, A.H. Influence of remote bands on exciton condensation in double-layer graphene. Phys. Rev. B 2011, 84, 155409. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, M. Two-dimensional WS2/MoS2 heterostructures: Properties and applications. Nanoscale 2021, 13, 5594–5619. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. Available online: https://www.nature.com/articles/nnano.2012.193 (accessed on 22 April 2024).
- Cong, C.; Shang, J.; Wang, Y.; Yu, T. Optical Properties of 2D Semiconductor WS2. Adv. Opt. Mater. 2018, 6, 1700767. [Google Scholar] [CrossRef]
- Hussain, W.; Algarni, S.; Rasool, G.; Shahzad, H.; Abbas, M.; Alqahtani, T.; Irshad, K. Advances in Nanoparticle-Enhanced Thermoelectric Materials from Synthesis to Energy Harvesting: A Review. ACS Omega 2024, 9, 11081–11109. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Okla, M.K. Exploring the electrochemical and photocatalytic potential of metal chalcogenide thin film Cu2S:Ni3S4. Ionics 2024, 30, 1587–1602. [Google Scholar] [CrossRef]
- Thiehmed, Z.; Shakoor, A.; Altahtamouni, T. Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts 2021, 11, 1283. [Google Scholar] [CrossRef]
- Ye, M.; Winslow, D.; Zhang, D.; Pandey, R.; Yap, Y.K. Recent Advancement on the Optical Properties of Two-Dimensional Molybdenum Disulfide (MoS2) Thin Films. Photonics 2015, 2, 288–307. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.F.; Jothiramalingam, R.; Noora Ibrahim, S.; Al-Lohedan, H.A. A screen-printed electrode modified with tungsten disulfide nanosheets for nanomolar detection of the arsenic drug roxarsone. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Li, J.; Cao, L.; Hao, W.; Xu, Z. Improved Na Storage Performance with the Involvement of Nitrogen-Doped Conductive Carbon into WS2 Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 23899–23908. [Google Scholar] [CrossRef]
- Han, P.; Zha, J.-W.; Zheng, M.-S.; Wen, Y.-Q.; Dang, Z.-M. Nonlinear electric conductivity and thermal conductivity of WS2/EPDM field grading materials. J. Appl. Phys. 2017, 122, 195106. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Q.; Fan, A.; Yu, L.; Wang, H.; Ma, W.; Lv, R.; Zhang, X. Reduction in thermal conductivity of monolayer WS2 caused by substrate effect. Nano Res. 2022, 15, 9578–9587. [Google Scholar] [CrossRef]
- Yakovleva Galina, E.; Romanenko Anatoly, I.; Berdinsky Alexander, S.; Kuznetsov Vitalii, A.; Ledneva Alexandra, Y.; Fedorov Vladimir, E. The research of temperature dependences of electrical conductivity and thermopower of WS2 and WSe2 with partial replacement of w on Nb. J. Sib. Fed. Univ. Math. Phys. 2018, 11, 459–464. Available online: https://cyberleninka.ru/article/n/the-research-of-temperature-dependences-of-electrical-conductivity-and-thermopower-of-ws2-and-wse2-with-partial-replacement-of-w-on-nb (accessed on 23 April 2024).
- Magnozzi, M.; Ferrera, M.; Piccinini, G.; Pace, S.; Forti, S.; Fabbri, F.; Coletti, C.; Bisio, F.; Canepa, M. Optical dielectric function of two-dimensional WS2 on epitaxial graphene. 2D Mater. 2020, 7, 025024. [Google Scholar] [CrossRef]
- Bin Rafiq, M.K.S.; Amin, N.; Alharbi, H.F.; Luqman, M.; Ayob, A.; Alharthi, Y.S.; Alharthi, N.H.; Bais, B.; Akhtaruzzaman, M. WS2: A New Window Layer Material for Solar Cell Application. Sci. Rep. 2020, 10, 771. [Google Scholar] [CrossRef]
- Zeng, L.; Tao, L.; Tang, C.; Zhou, B.; Long, H.; Chai, Y.; Lau, S.P.; Tsang, Y.H. High-responsivity UV-Vis Photodetector Based on Transferable WS2 Film Deposited by Magnetron Sputtering. Sci. Rep. 2016, 6, 20343. [Google Scholar] [CrossRef]
- Vincent, T.; Liang, J.; Singh, S.; Castanon, E.G.; Zhang, X.; McCreary, A.; Jariwala, D.; Kazakova, O.; Al Balushi, Z.Y. Opportunities in electrically tunable 2D materials beyond graphene: Recent progress and future outlook. Appl. Phys. Rev. 2021, 8, 041320. [Google Scholar] [CrossRef]
- Zhu, C. Light-Tunable Two-Dimensional Electronic Devices; Nanyang Technological University: Singapore, 2019. [Google Scholar] [CrossRef]
- Ye, W.; Du, P.; Xiao, S.; Li, M. Effect of annealing temperature on properties of WS2 thin films. Surf. Eng. 2022, 38, 411–416. [Google Scholar] [CrossRef]
- Hotovy, I.; Spiess, L.; Mikolasek, M.; Kostic, I.; Sojkova, M.; Romanus, H.; Hulman, M.; Buc, D.; Rehacek, V. Layered WS2 thin films prepared by sulfurization of sputtered W films. Appl. Surf. Sci. 2021, 544, 148719. [Google Scholar] [CrossRef]
- Hotovy, I.; Spiess, L.; Sojkova, M.; Kostic, I.; Mikolasek, M.; Predanocy, M.; Romanus, H.; Hulman, M.; Rehacek, V. Structural and optical properties of WS2 prepared using sulfurization of different thick sputtered tungsten films. Appl. Surf. Sci. 2018, 461, 133–138. [Google Scholar] [CrossRef]
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Atomically Thin-Layered Molybdenum Disulfide (MoS2) for Bulk-Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 3223–3245. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayan, A.S.; Shebeeb, C.M.; Akshay, K.S.; Mathew, K.P.J.; Sajith, V. A review on tailoring the corrosion and oxidation properties of MoS2-based coatings. J. Mater. Chem. A 2023, 11, 3172–3209. [Google Scholar] [CrossRef]
- Strachan, J.; Masters, A.F.; Maschmeyer, T. 3R-MoS2 in Review: History, Status, and Outlook. ACS Appl. Energy Mater. 2021, 4, 7405–7418. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Guo, W.; Zhao, M.; Fan, X.; Dong, Y.; Xu, C.; Deng, J.; Fu, Y. Synthesis Methods of Two-Dimensional MoS2: A Brief Review. Crystals 2017, 7, 198. [Google Scholar] [CrossRef]
- Yang, J.; Xing, Y.; Wu, Z.; Huang, P.; Liu, L. Ultrathin molybdenum disulfide (MoS2) film obtained in atomic layer deposition: A mini-review. Sci. China Technol. Sci. 2021, 64, 2347–2359. [Google Scholar] [CrossRef]
- Xu, H.; Akbari, M.K.; Kumar, S.; Verpoort, F.; Zhuiykov, S. Atomic layer deposition—State-of-the-art approach to nanoscale hetero-interfacial engineering of chemical sensors electrodes: A review. Sens. Actuators B Chem. 2021, 331, 129403. [Google Scholar] [CrossRef]
- Schwartzberg, A.M.; Olynick, D. Complex Materials by Atomic Layer Deposition. Adv. Mater. 2015, 27, 5778–5784. [Google Scholar] [CrossRef] [PubMed]
- Jurca, T.; Moody, M.J.; Henning, A.; Emery, J.D.; Wang, B.; Tan, J.M.; Lohr, T.L.; Lauhon, L.J.; Marks, T.J. Low-Temperature Atomic Layer Deposition of MoS2 Films. Angew. Chem. Int. Ed. 2017, 56, 4991–4995. [Google Scholar] [CrossRef]
- Letourneau, S.; Young, M.J.; Bedford, N.M.; Ren, Y.; Yanguas-Gil, A.; Mane, A.U.; Elam, J.W.; Graugnard, E. Structural Evolution of Molybdenum Disulfide Prepared by Atomic Layer Deposition for Realization of Large Scale Films in Microelectronic Applications. ACS Appl. Nano Mater. 2018, 1, 4028–4037. [Google Scholar] [CrossRef]
- Jeon, W.; Cho, Y.; Jo, S.; Ahn, J.H.; Jeong, S.J. Wafer-Scale Synthesis of Reliable High-Mobility Molybdenum Disulfide Thin Films via Inhibitor-Utilizing Atomic Layer Deposition. Adv. Mater. 2017, 29, 1703031. [Google Scholar] [CrossRef]
- Shi, M.L.; Chen, L.; Zhang, T.B.; Xu, J.; Zhu, H.; Sun, Q.Q.; Zhang, D.W. Top-Down Integration of Molybdenum Disulfide Transistors with Wafer-Scale Uniformity and Layer Controllability. Small 2017, 13, 1603157. [Google Scholar] [CrossRef]
- Singh Nalwa, H. A review of molybdenum disulfide (MoS2) based photodetectors: From ultra-broadband, self-powered to flexible devices. RSC Adv. 2020, 10, 30529–30602. [Google Scholar] [CrossRef]
- Mouloua, D.; Kotbi, A.; Deokar, G.; Kaja, K.; El Marssi, M.; EL Khakani, M.A.; Jouiad, M. Recent Progress in the Synthesis of MoS2 Thin Films for Sensing, Photovoltaic and Plasmonic Applications: A Review. Materials 2021, 14, 3283. [Google Scholar] [CrossRef]
- Yang, X.; Li, B. Monolayer MoS2 for nanoscale photonics. Nanophotonics 2020, 9, 1557–1577. [Google Scholar] [CrossRef]
- Xu, B.-H.; Lin, B.-Z.; Sun, D.-Y.; Ding, C. Preparation and electrical conductivity of polyethers/WS2 layered nanocomposites. Electrochim. Acta 2007, 52, 3028–3034. [Google Scholar] [CrossRef]
- Yang, J.; Bussolotti, F.; Kawai, H.; Goh, K.E.J. Tuning the Conductivity Type in Monolayer WS2 and MoS2 by Sulfur Vacancies. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2020, 14, 2000248. [Google Scholar] [CrossRef]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.; Shi, L.; Ye, J. Engineering the Edges of MoS2 (WS2) Crystals for Direct Exfoliation into Monolayers in Polar Micromolecular Solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wu, Z.; Ma, P.; Wang, G.; Zhang, B. TiS2,MoS2,WS2/Sb2Te3 mixed nanosheets saturable absorber for dual-wavelength passively Q-switched Nd:GYSGG Laser. Infrared Phys. Technol. 2018, 92, 1–5. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, L.; Zhou, F.; Zhang, Y. Wafer-Scale Uniform Synthesis of 2D Transition Metal Dichalcogenides Single Crystals via Chemical Vapor Deposition. Acc. Mater. Res. 2022, 3, 161–174. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, J.; Ding, F. Strategies, Status, and Challenges in Wafer Scale Single Crystalline Two-Dimensional Materials Synthesis. Chem. Rev. 2021, 121, 6321–6372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Sun, K.; He, J.; Zheng, Y.; Xu, C.; Zhang, Y.; Chen, Y.; Li, M. Improving ionic/electronic conductivity of MoS2 Li-ion anode via manganese doping and structural optimization. Chem. Eng. J. 2019, 372, 665–672. [Google Scholar] [CrossRef]
- Souder, A.; Brodie, D.E. Electrical Contacts and Conductivity of MoS2 Layer Structures. Can. J. Phys. 1971, 49, 2565–2571. [Google Scholar] [CrossRef]
- Mao, Y.; Fang, Y.; Yuan, K.; Huang, F. Effect of vanadium doping on the thermoelectric properties of MoS2. J. Alloys Compd. 2022, 903, 163921. [Google Scholar] [CrossRef]
- Ko, T.-S.; Huang, C.-C.; Lin, D.-Y. Optical and Transport Properties of Ni-MoS2. Appl. Sci. 2016, 6, 227. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, X.; Zhang, T.; Chen, H.; Liu, M. Experimental study on thermal conductivity and rectification of monolayer and multilayer MoS2. Int. J. Heat Mass Transf. 2021, 170, 121013. [Google Scholar] [CrossRef]
- Yuan, S.; Roldán, R.; Katsnelson, M.I.; Guinea, F. Effect of point defects on the optical and transport properties of MoS2 and WS2. Phys. Rev. B 2014, 90, 041402. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, Z.; Meng, Y. Effect of annealing temperature on the microstructure and optical properties of MoS2 films. IOP Conf. Ser. Mater. Sci. Eng. 2020, 892, 012007. [Google Scholar] [CrossRef]
- Chen, G.; Lu, B.; Cui, X.; Xiao, J. Effects of Deposition and Annealing Temperature on the Structure and Optical Band Gap of MoS2 Films. Materials 2020, 13, 5515. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Tan, F.; Zhang, R. Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering. Crystals 2021, 11, 1183. [Google Scholar] [CrossRef]
- Park, J.; Choudhary, N.; Smith, J.; Lee, G.; Kim, M.; Choi, W. Thickness modulated MoS2 grown by chemical vapor deposition for transparent and flexible electronic devices. Appl. Phys. Lett. 2015, 106, 012104. [Google Scholar] [CrossRef]
- Sachidanand, P.S.; Sreelal, M.M.; Reshmi, S.; Viswan, G.; Mohan, M.; Gautam, S.K.; Singh, R.K.; Bhattacharjee, K. MoS2 nanostructures as transparent material: Optical transmittance measurements. Mater. Today Proc. 2020, 26, 104–107. [Google Scholar] [CrossRef]
- Yoon, J.; Park, W.; Bae, G.-Y.; Kim, Y.; Jang, H.S.; Hyun, Y.; Lim, S.K.; Kahng, Y.H.; Hong, W.-K.; Lee, B.H.; et al. Highly Flexible and Transparent Multilayer MoS2 Transistors with Graphene Electrodes. Small 2013, 9, 3295–3300. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Fang, P.; Zhang, Z.; Zhang, J.Z. Synthesis, properties, and optoelectronic applications of two-dimensional MoS2 and MoS2-based heterostructures. Chem. Soc. Rev. 2018, 47, 6101–6127. [Google Scholar] [CrossRef]
- Dutta, R.; Bala, A.; Sen, A.; Spinazze, M.R.; Park, H.; Choi, W.; Yoon, Y.; Kim, S. Optical Enhancement of Indirect Bandgap 2D Transition Metal Dichalcogenides for Multi-Functional Optoelectronic Sensors. Adv. Mater. 2023, 35, 2303272. [Google Scholar] [CrossRef]
- Bai, S.; Guo, X.; Chen, T.; Zhang, Y.; Yang, H. Solution Process Fabrication of Silver Nanowire Composite Transparent Conductive Films with Tunable Work Function. Thin Solid Film. 2020, 709, 138096. [Google Scholar] [CrossRef]
- Morales-Masis, M.; De Wolf, S.; Woods-Robinson, R.; Ager, J.W.; Ballif, C. Transparent Electrodes for Efficient Optoelectronics. Adv. Electron. Mater. 2017, 3, 1600529. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Z.; Yang, J.; Kim, J.-K. Graphene oxide-based transparent conductive films. Prog. Mater. Sci. 2014, 64, 200–247. [Google Scholar] [CrossRef]
- Hu, H.; Wang, S.; Wang, S.; Liu, G.; Cao, T.; Long, Y. Aligned Silver Nanowires Enabled Highly Stretchable and Transparent Electrodes with Unusual Conductive Property. Adv. Funct. Mater. 2019, 29, 1902922. [Google Scholar] [CrossRef]
- You, W.; Liao, B.; Wan, S.; Guo, X. Research progress on the stability of transparent conductive films for silver nanowires. Microelectron. Reliab. 2024, 156, 115394. [Google Scholar] [CrossRef]
- Shi, Y.; He, L.; Deng, Q.; Liu, Q.; Li, L.; Wang, W.; Xin, Z.; Liu, R. Synthesis and Applications of Silver Nanowires for Transparent Conductive Films. Micromachines 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.-P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: A Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, Y.; Liu, X.; Liu, G.; Shan, F. Welded silver nanowire networks as high-performance transparent conductive electrodes: Welding techniques and device applications. Appl. Mater. Today 2020, 20, 100634. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Goyat, M.S.; Avasthi, D.K. A review of the latest developments in the production and applications of Ag-nanowires as transparent electrodes. Mater. Today Commun. 2022, 33, 104433. [Google Scholar] [CrossRef]
- Litzelman, S.J.; Hertz, J.L.; Jung, W.; Tuller, H.L. Opportunities and Challenges in Materials Development for Thin Film Solid Oxide Fuel Cells. Fuel Cells 2008, 8, 294–302. [Google Scholar] [CrossRef]
- Zhang, J.; Ricote, S.; Hendriksen, P.V.; Chen, Y. Advanced Materials for Thin-Film Solid Oxide Fuel Cells: Recent Progress and Challenges in Boosting the Device Performance at Low Temperatures. Adv. Funct. Mater. 2022, 32, 2111205. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E.; Ermakova, L.; Bogdanovich, N. Challenges of Formation of Thin-Film Solid Electrolyte Layers on Non-Conductive Substrates by Electrophoretic Deposition. Coatings 2021, 11, 805. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Liu, J.; Wöll, C. Surface-supported metal–organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, M. Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges. Coatings 2014, 4, 60–84. [Google Scholar] [CrossRef]
- Baran, D.; Corzo, D.; Blazquez, G.T. Flexible Electronics: Status, Challenges and Opportunities. Front. Electron. 2020, 1, 594003. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, N.; Lin, X.; Nie, S. Emerging challenges in the thermal management of cellulose nanofibril-based supercapacitors, lithium-ion batteries and solar cells: A review. Carbohydr. Polym. 2020, 234, 115888. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef]
- Li, D.; Lai, W.-Y.; Zhang, Y.-Z.; Huang, W. Printable Transparent Conductive Films for Flexible Electronics. Adv. Mater. 2018, 30, 1704738. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L. Light Management with Patterned Micro- and Nanostructure Arrays for Photocatalysis, Photovoltaics, and Optoelectronic and Optical Devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Chen, H.; Xue, J. Transparent electrodes for organic optoelectronic devices: A review. JPE 2014, 4, 040990. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.; Lai, W.-Y.; Huang, W. Stretchable Thin-Film Electrodes for Flexible Electronics with High Deformability and Stretchability. Adv. Mater. 2015, 27, 3349–3376. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y. Thin Films and/or Coating for Electromagnetic Interference and Stealth. In Inorganic and Organic Thin Films; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 587–614. [Google Scholar] [CrossRef]
- Kumar, P. Ultrathin 2D Nanomaterials for Electromagnetic Interference Shielding. Adv. Mater. Interfaces 2019, 6, 1901454. [Google Scholar] [CrossRef]
- Gordon, R.G. Criteria for Choosing Transparent Conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Zhang, L.; Yang, B.; Shu, L.; Yang, Y.; Ren, M.; Wang, Y.; Chen, J.; Chen, W.; et al. Smart Textile-Integrated Microelectronic Systems for Wearable Applications. Adv. Mater. 2020, 32, 1901958. [Google Scholar] [CrossRef]
- Alobaidi, O.R.; Chelvanathan, P.; Tiong, S.K.; Bais, B.; Uzzaman, M.A.; Amin, N. Transparent Antenna for Green Communication Feature: A Systematic Review on Taxonomy Analysis, Open Challenges, Motivations, Future Directions and Recommendations. IEEE Access 2022, 10, 12286–12321. [Google Scholar] [CrossRef]
- Gazotti, W.A.; Nogueira, A.F.; Girotto, E.M.; Micaroni, L.; Martini, M.; das Neves, S.; De Paoli, M.-A. Chapter 2—Optical devices based on conductive polymers. In Handbook of Advanced Electronic and Photonic Materials and Devices; Singh Nalwa, H., Ed.; Academic Press: Burlington, NJ, USA, 2001; pp. 53–98. [Google Scholar] [CrossRef]
- Solin, K.; Borghei, M.; Sel, O.; Orelma, H.; Johansson, L.-S.; Perrot, H.; Rojas, O.J. Electrically Conductive Thin Films Based on Nanofibrillated Cellulose: Interactions with Water and Applications in Humidity Sensing. ACS Appl. Mater. Interfaces 2020, 12, 36437–36448. [Google Scholar] [CrossRef]
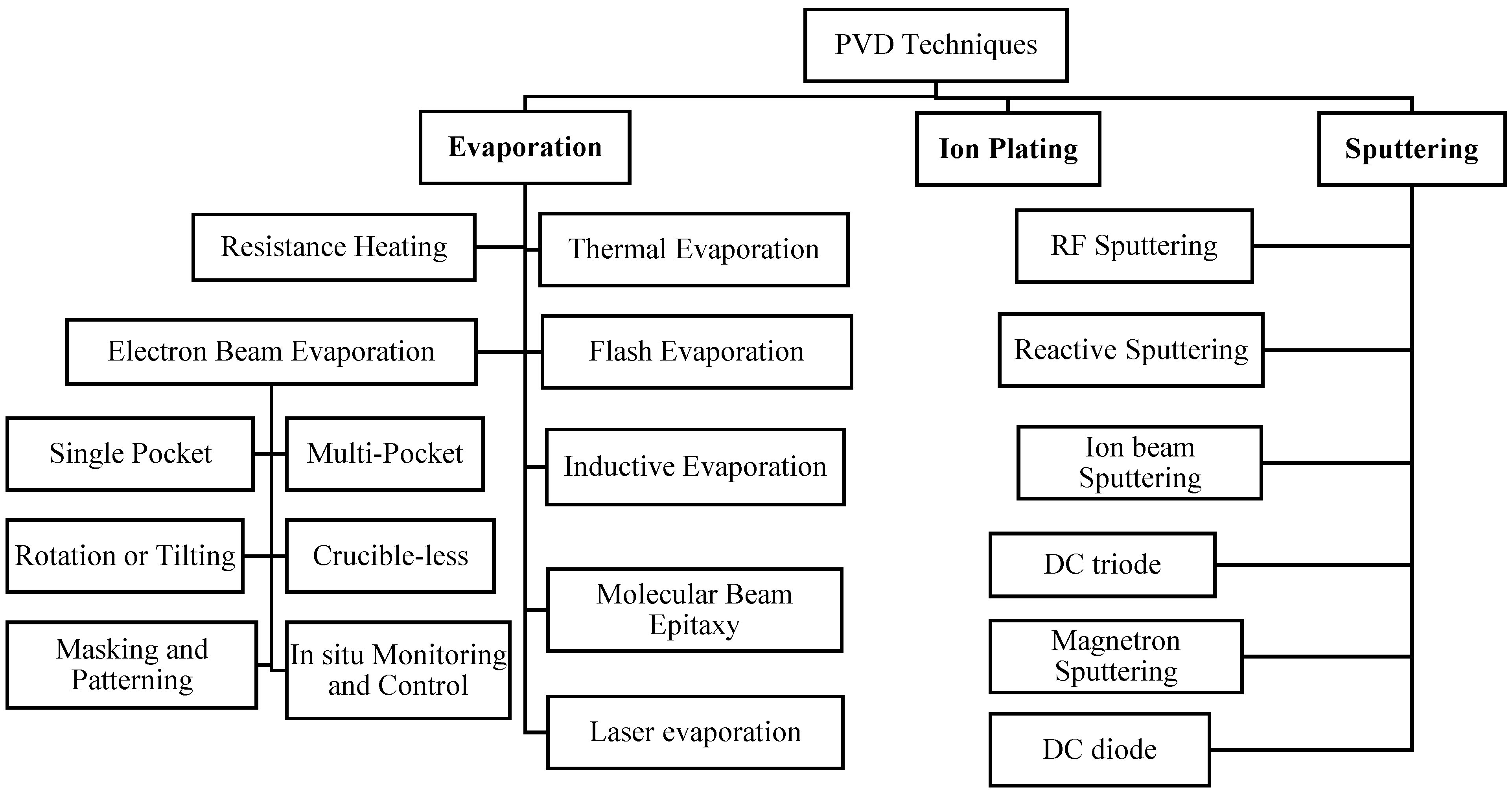


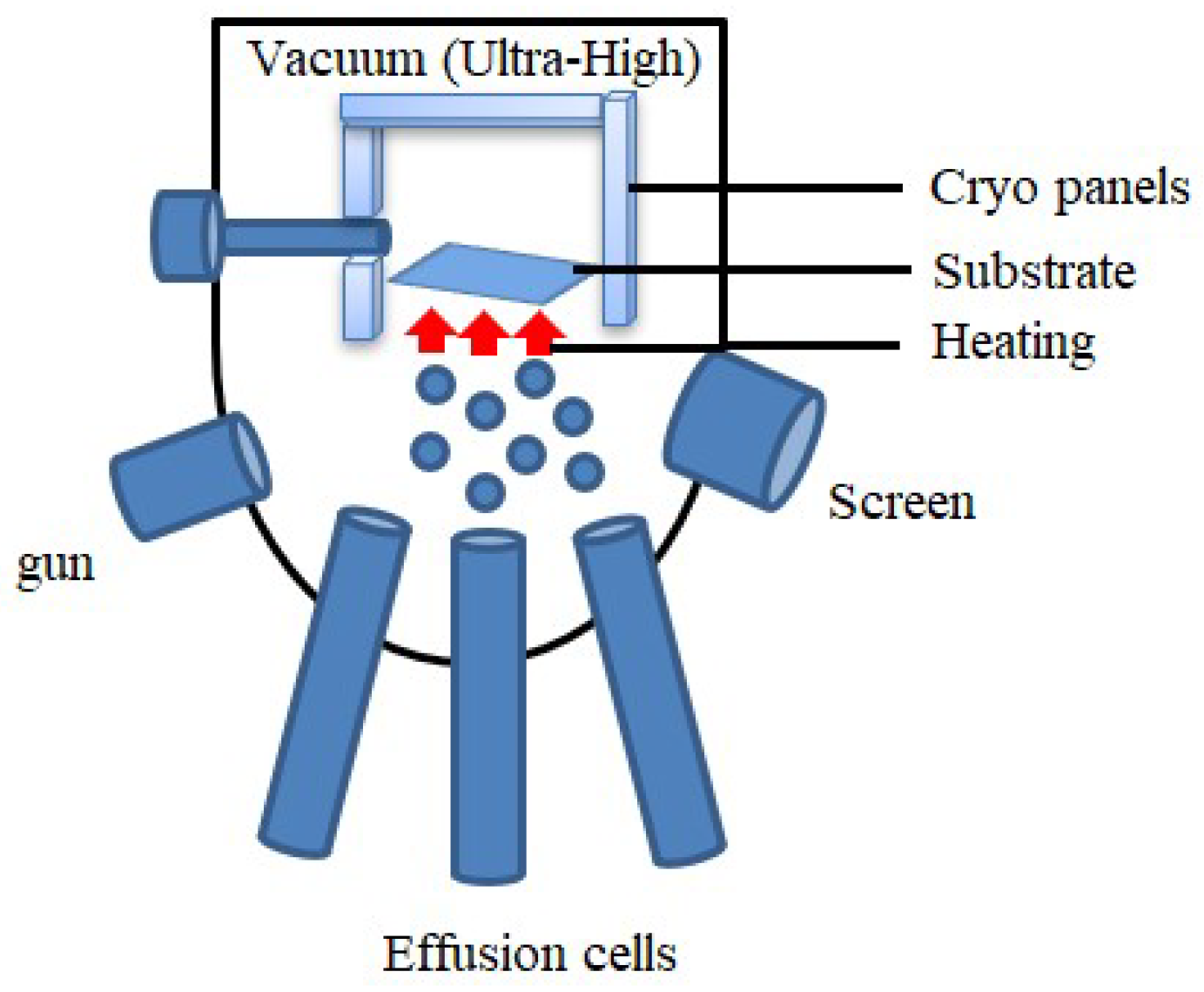
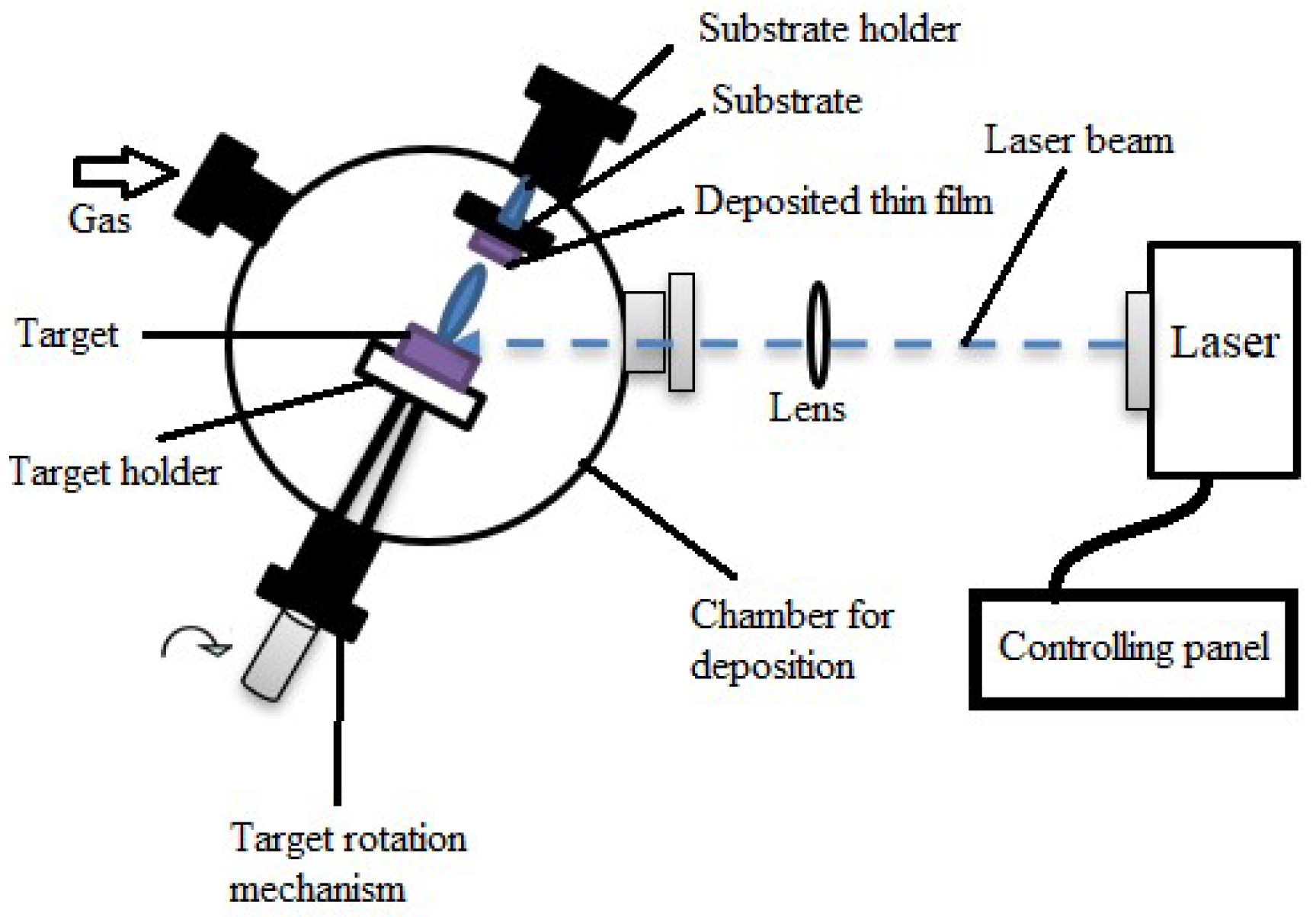
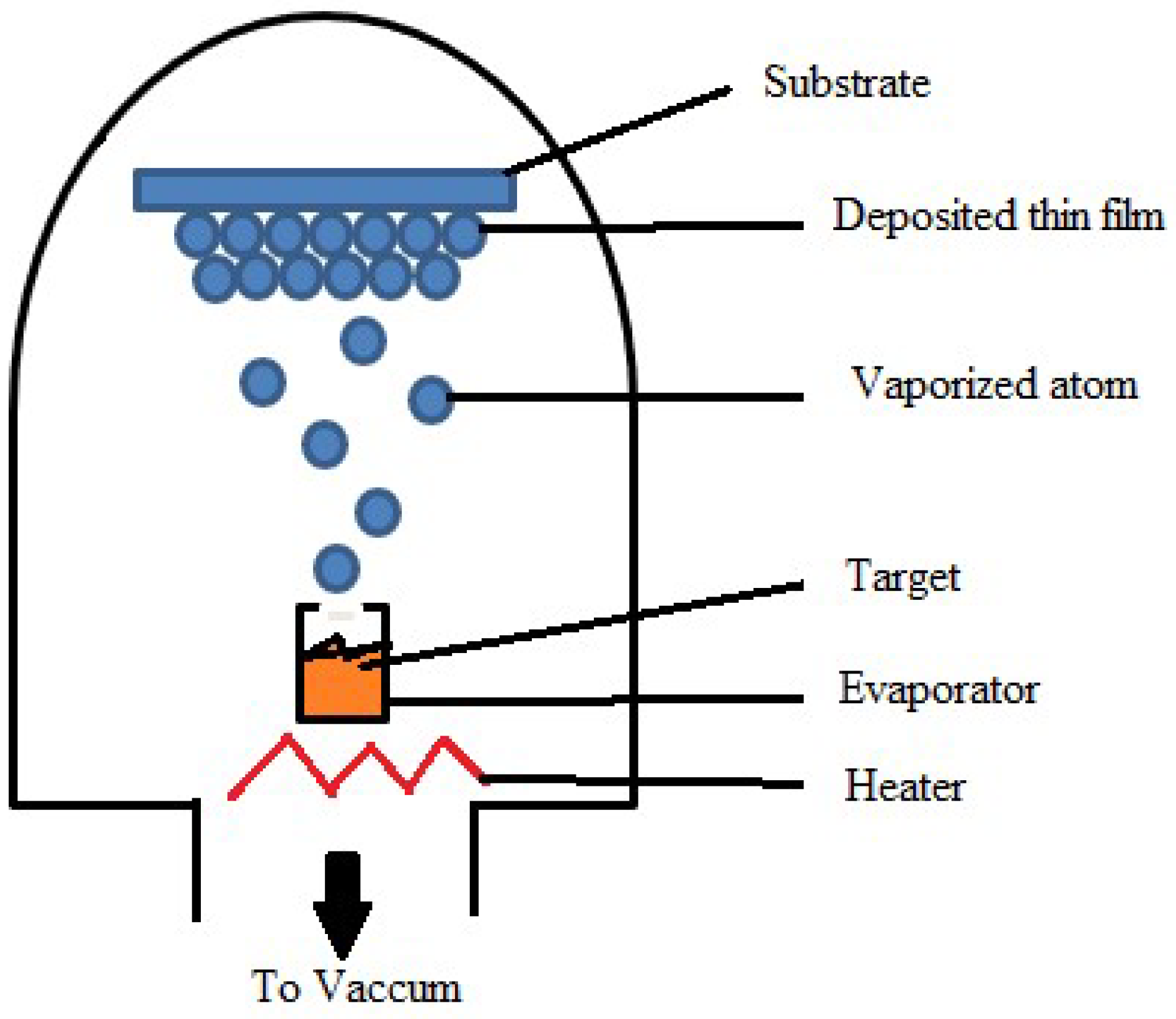
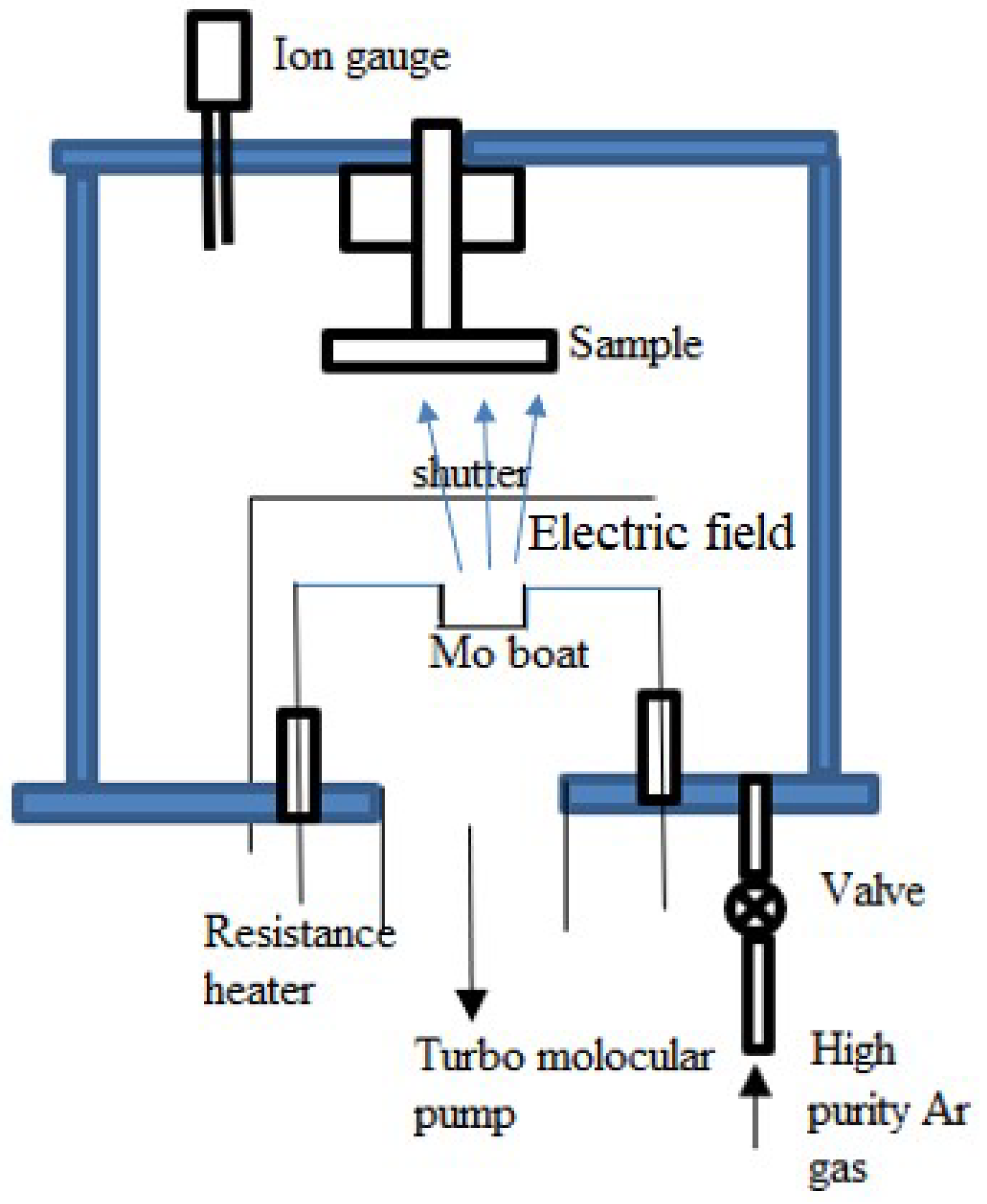
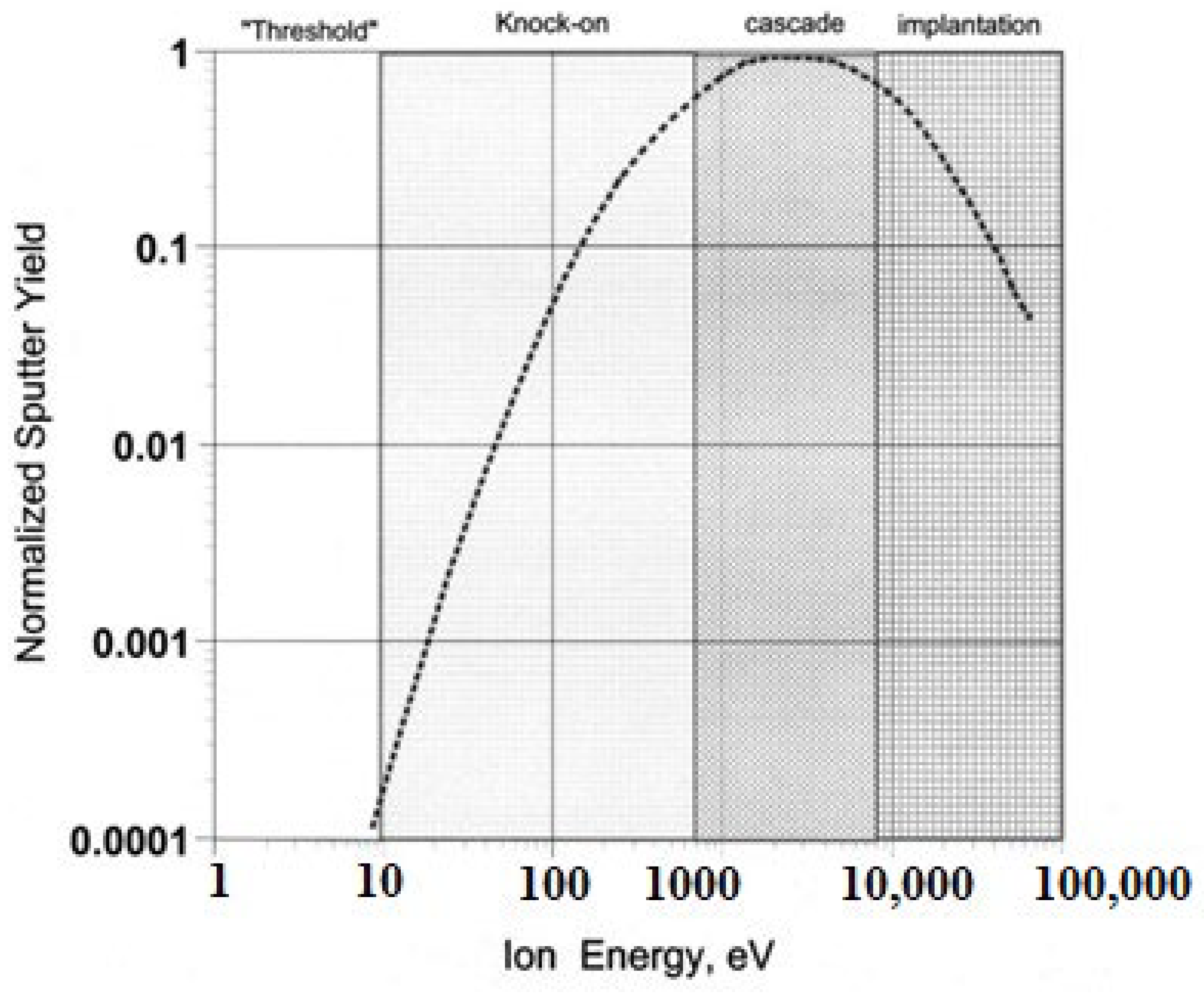
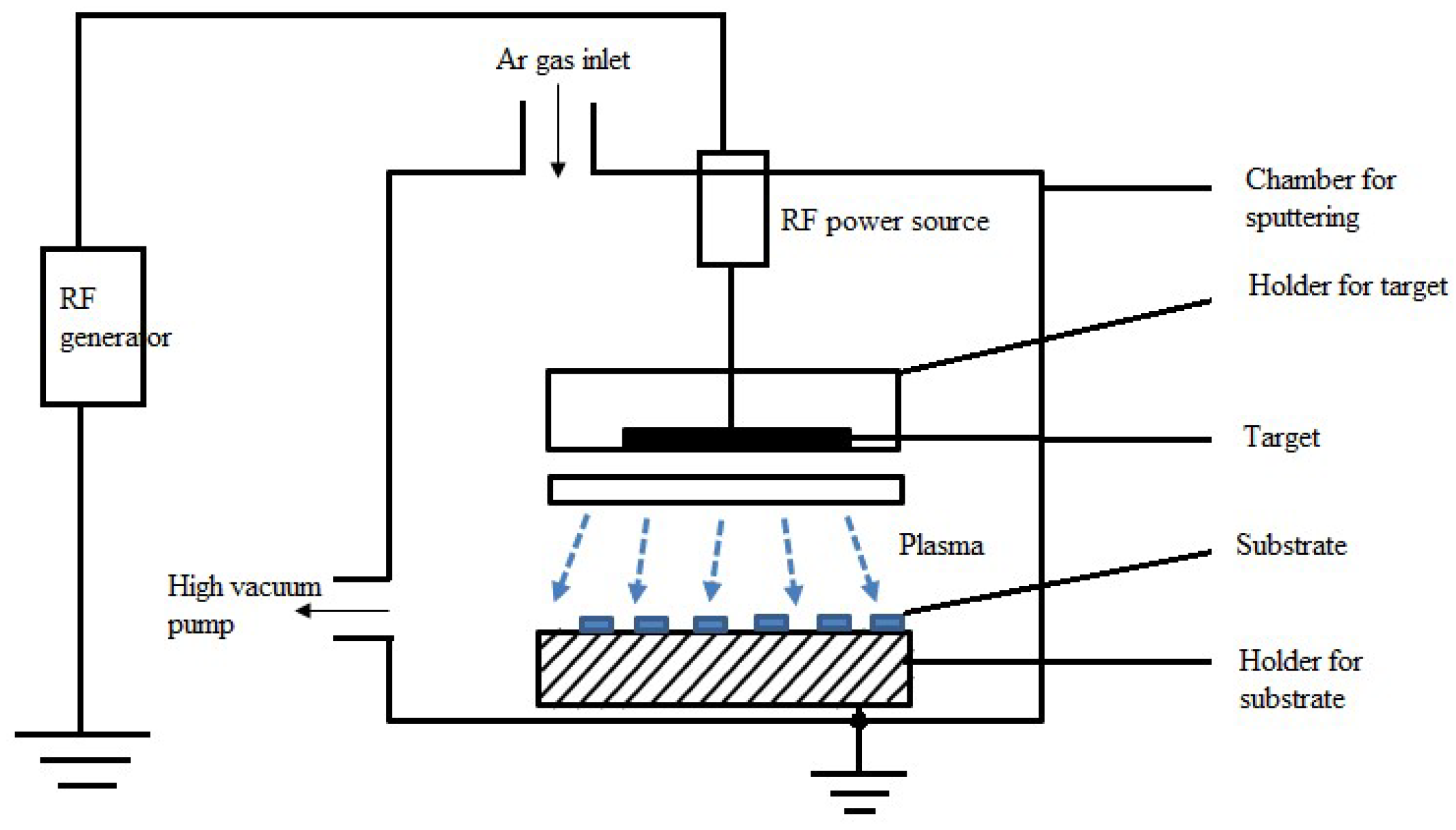
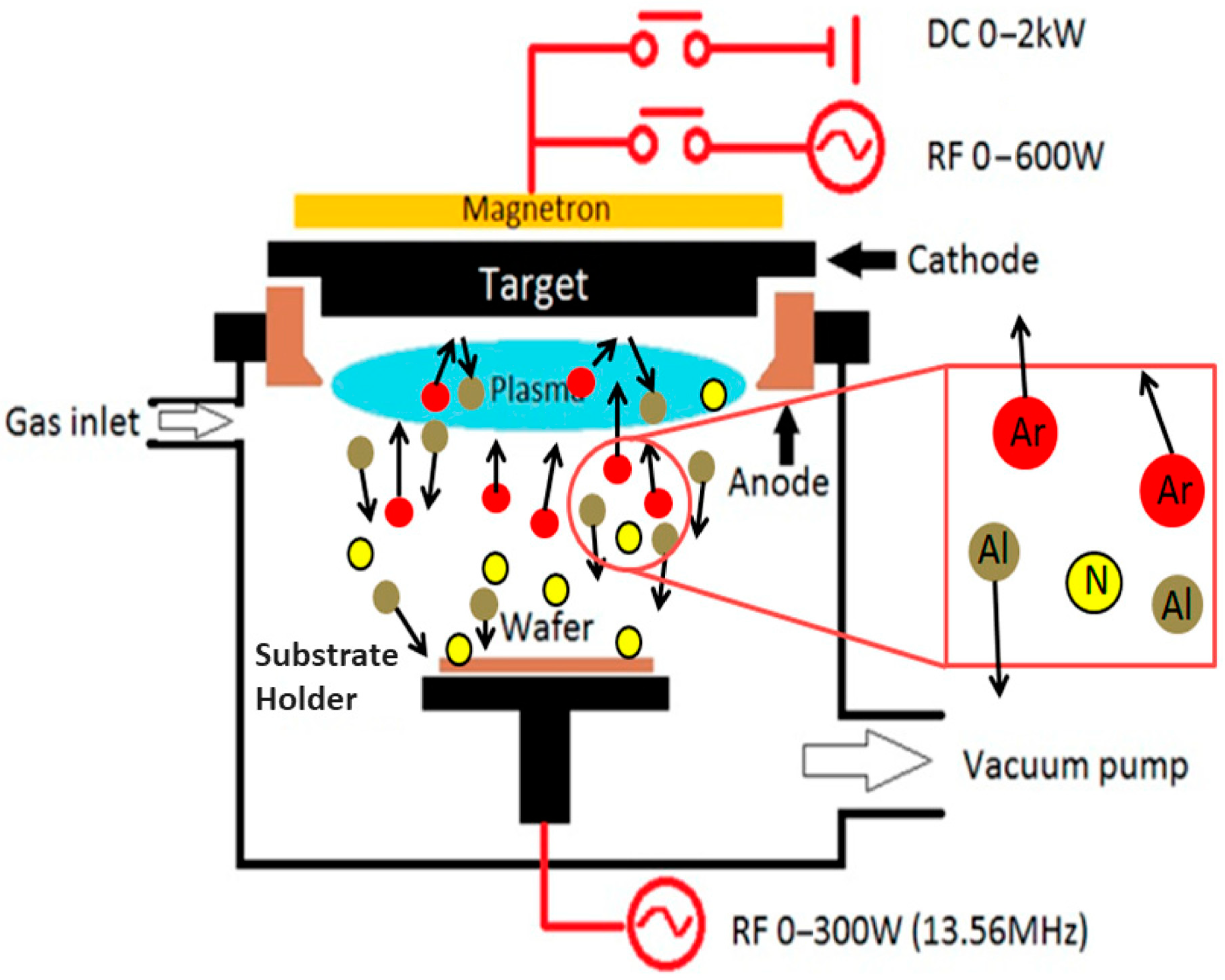
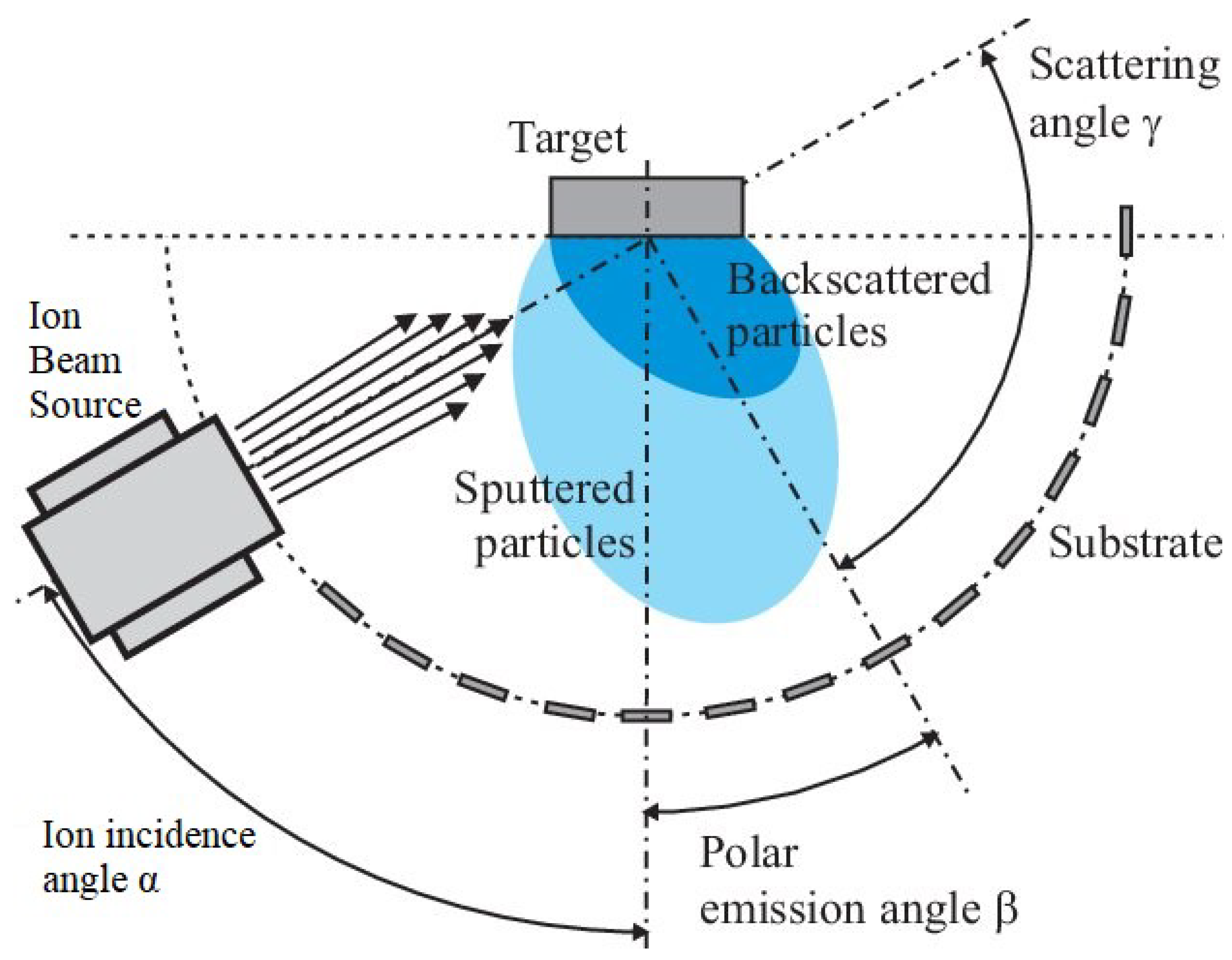

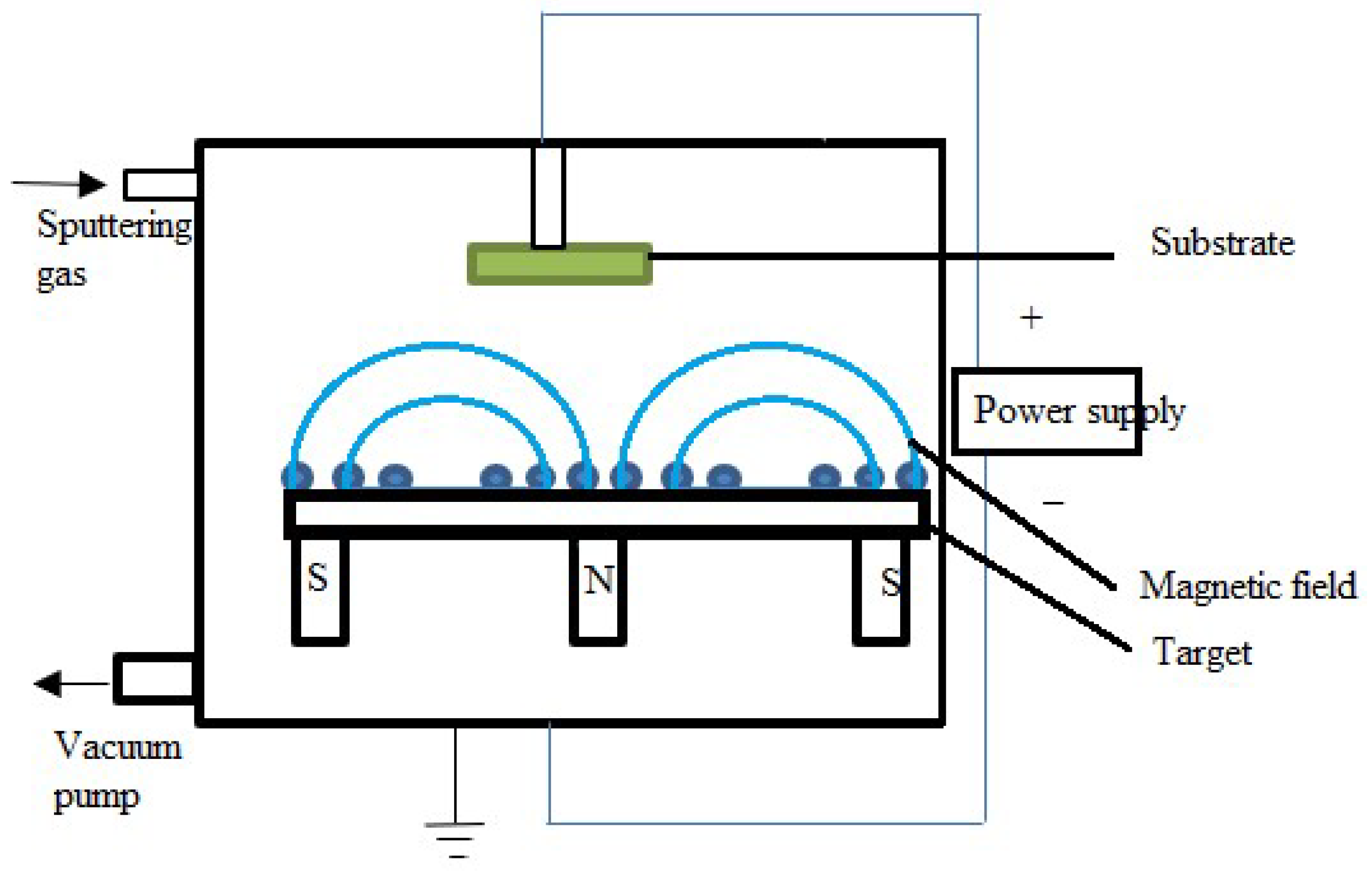
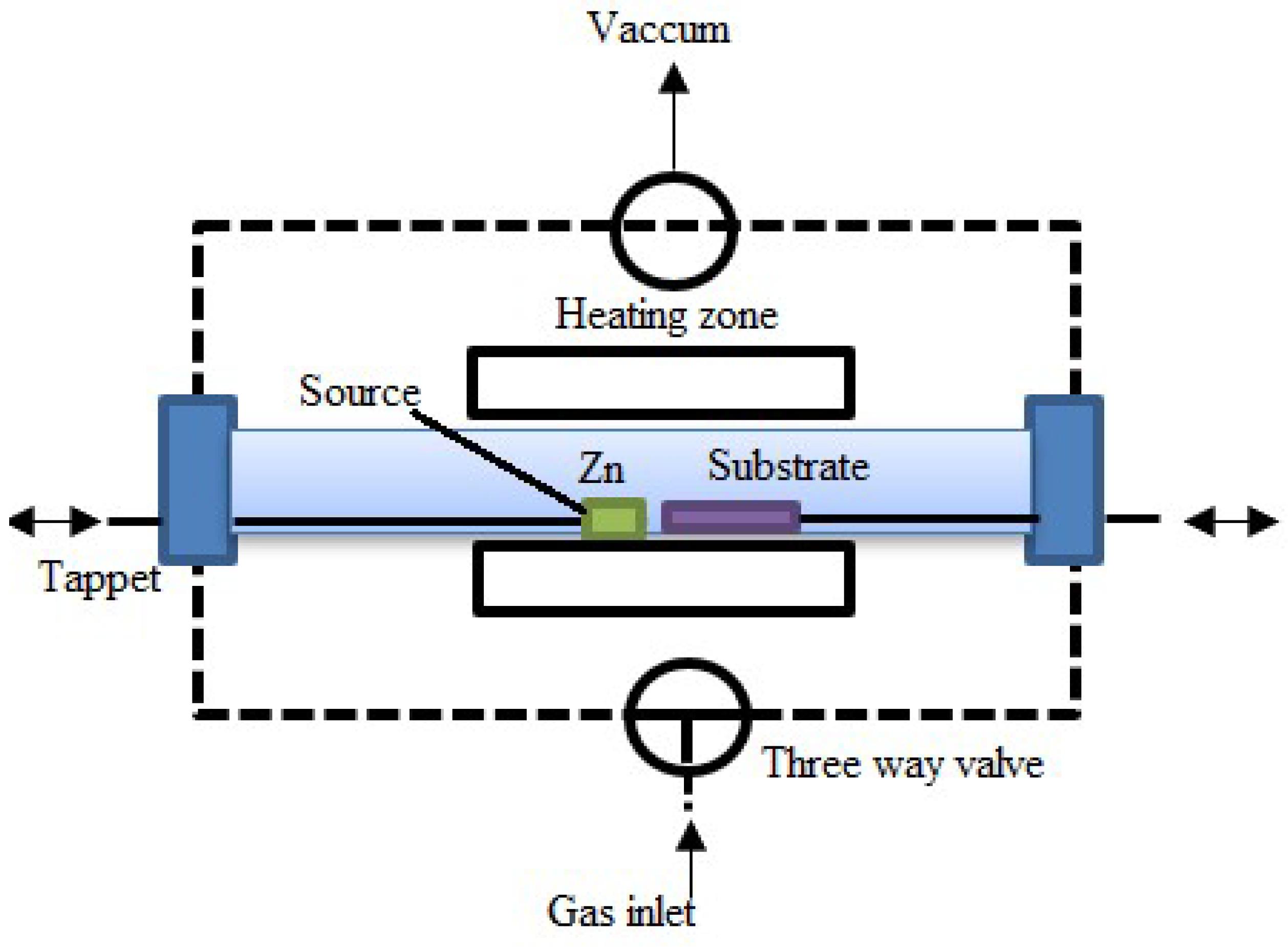

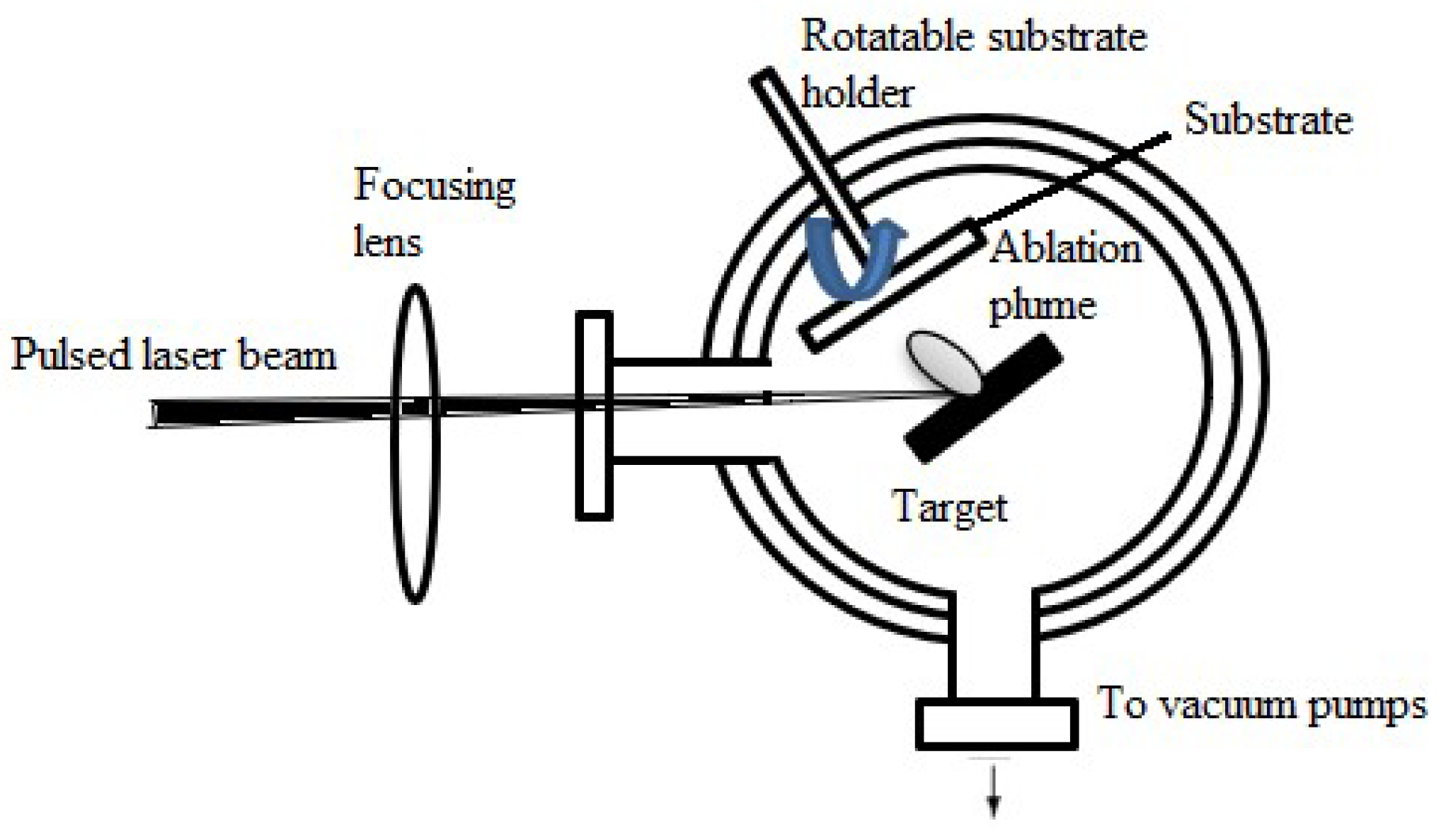
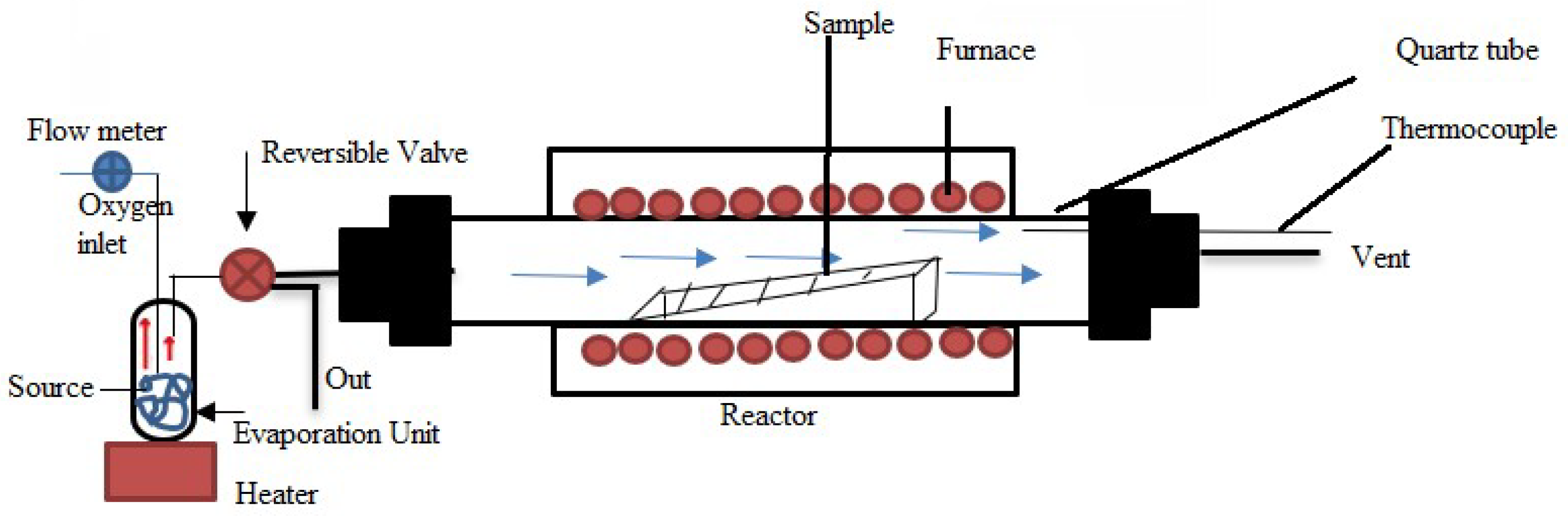

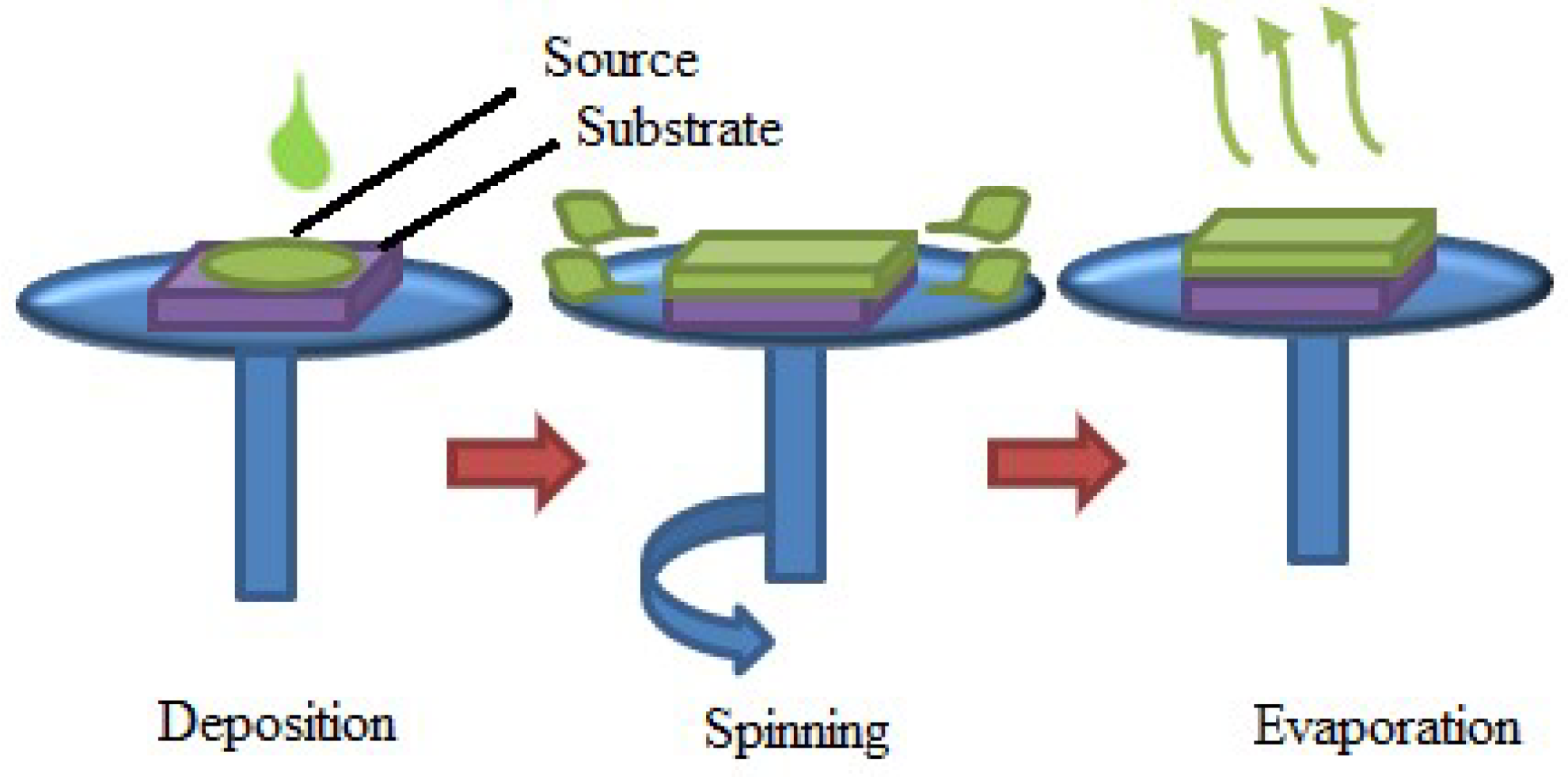
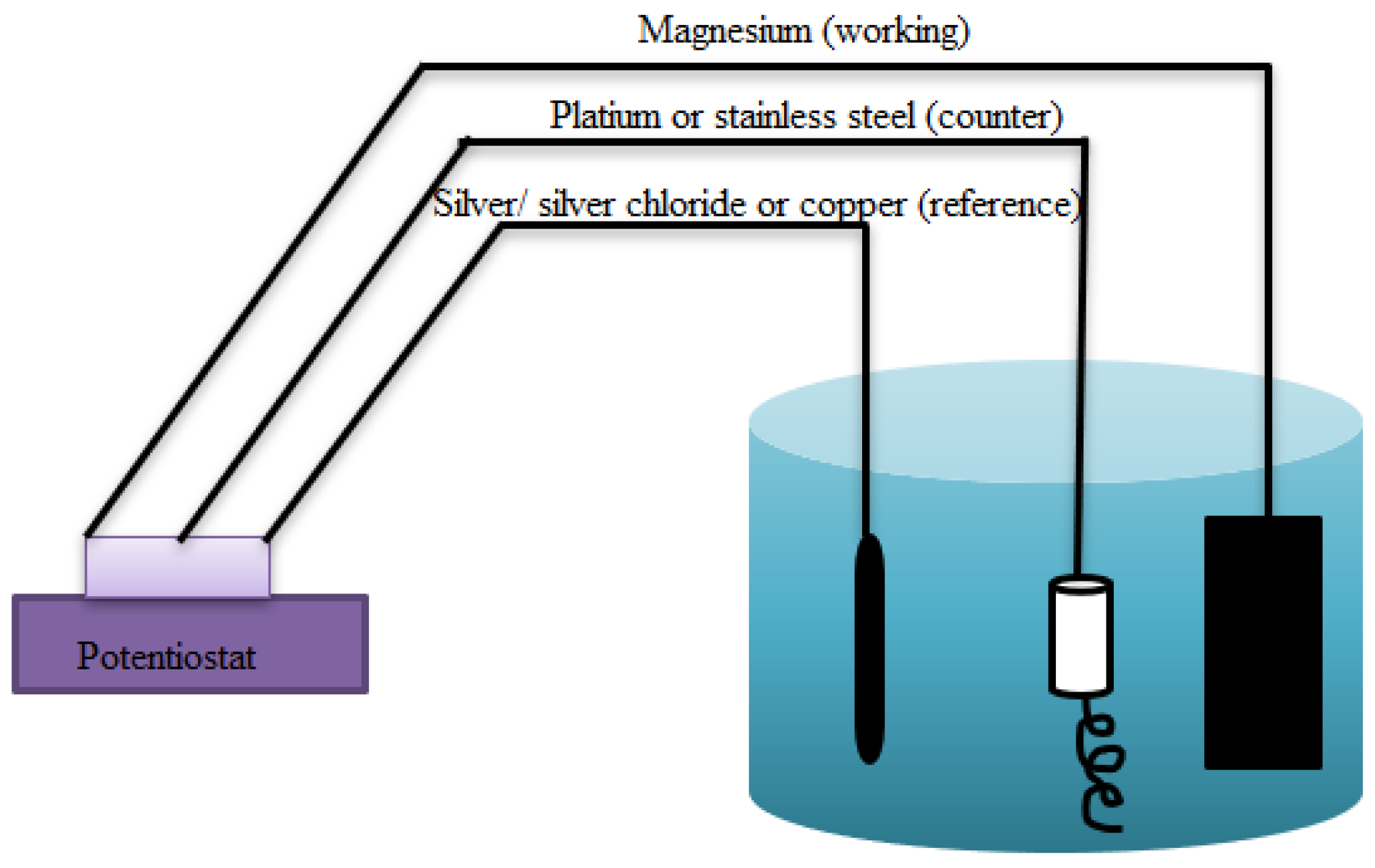

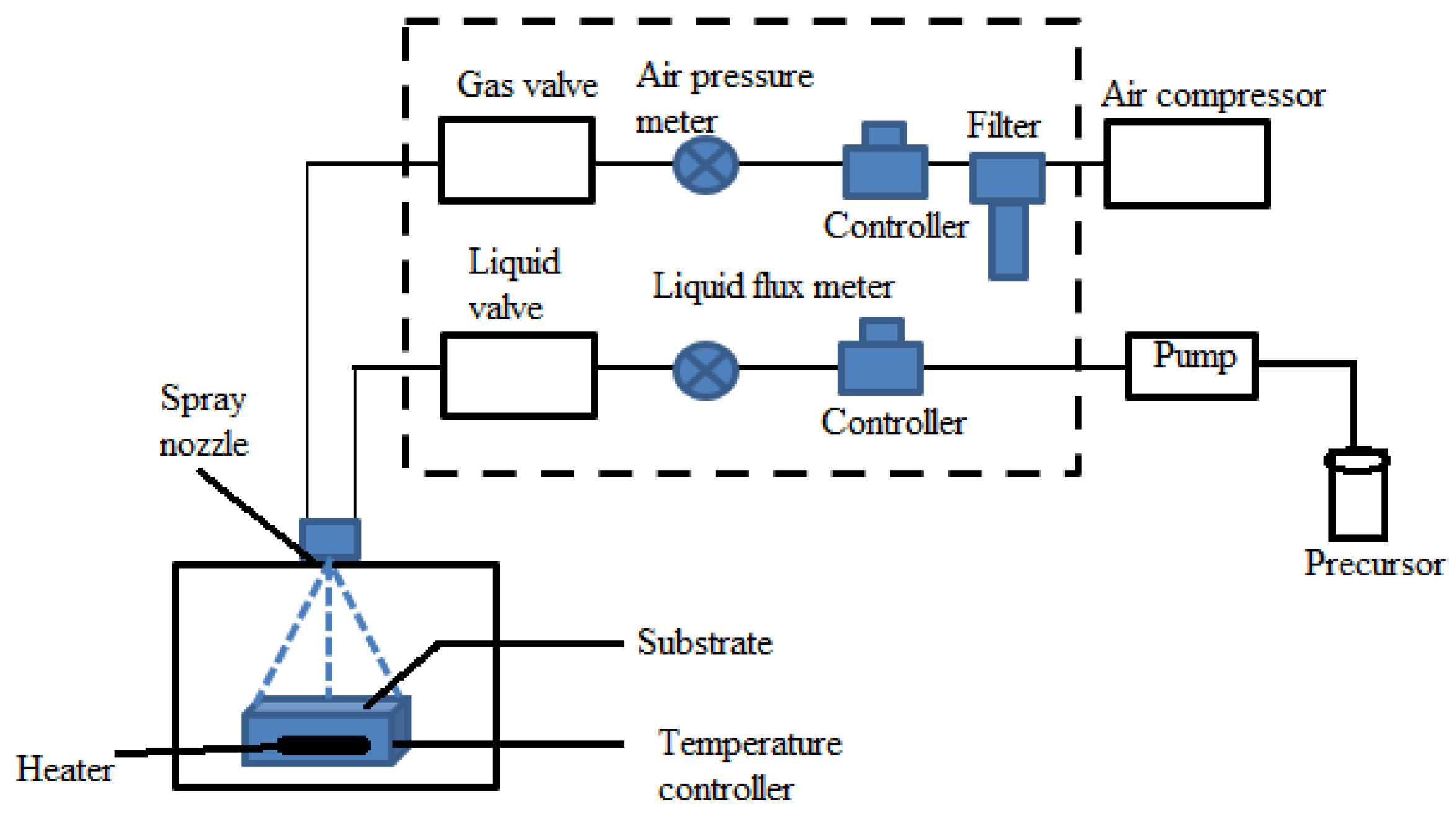
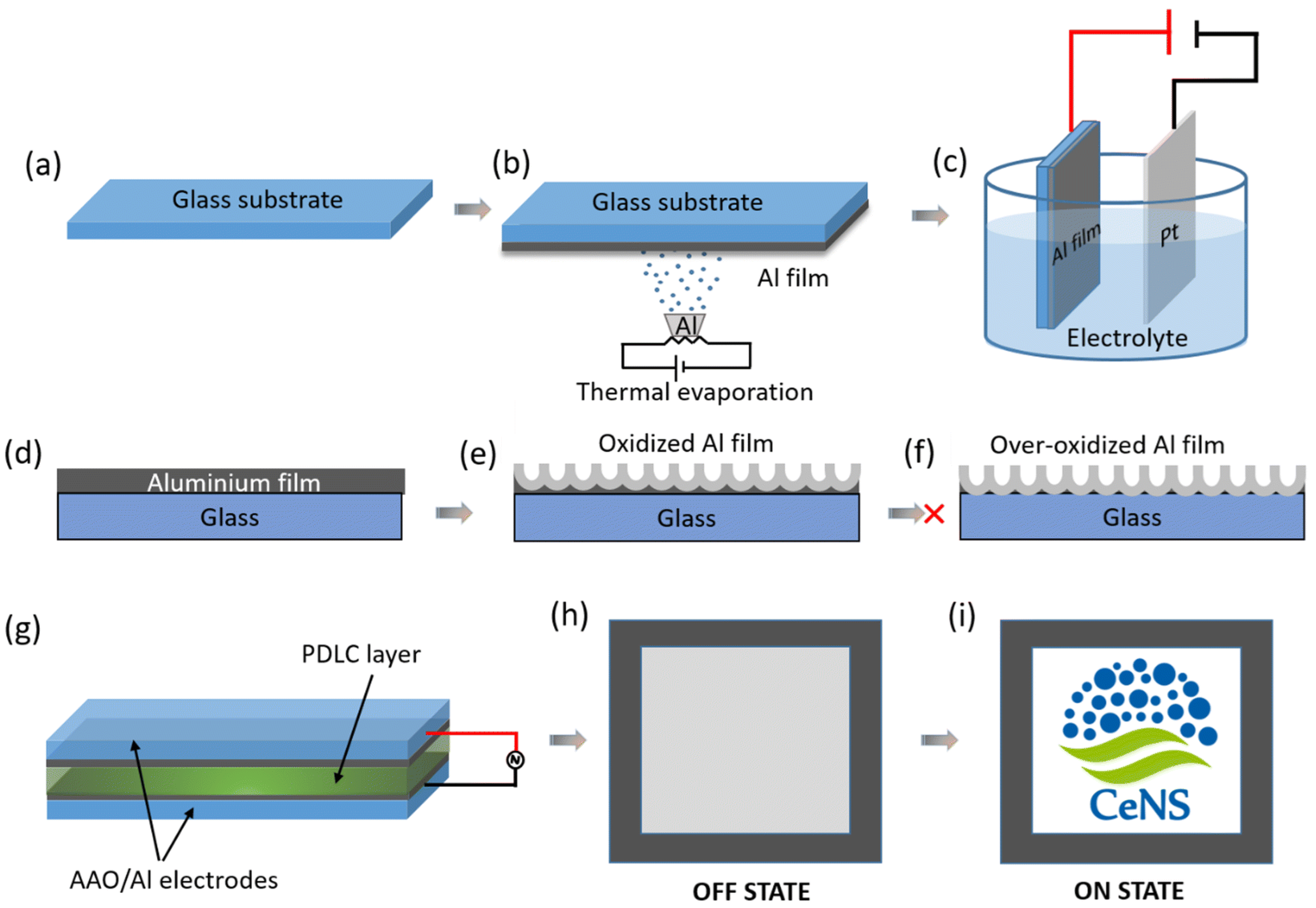
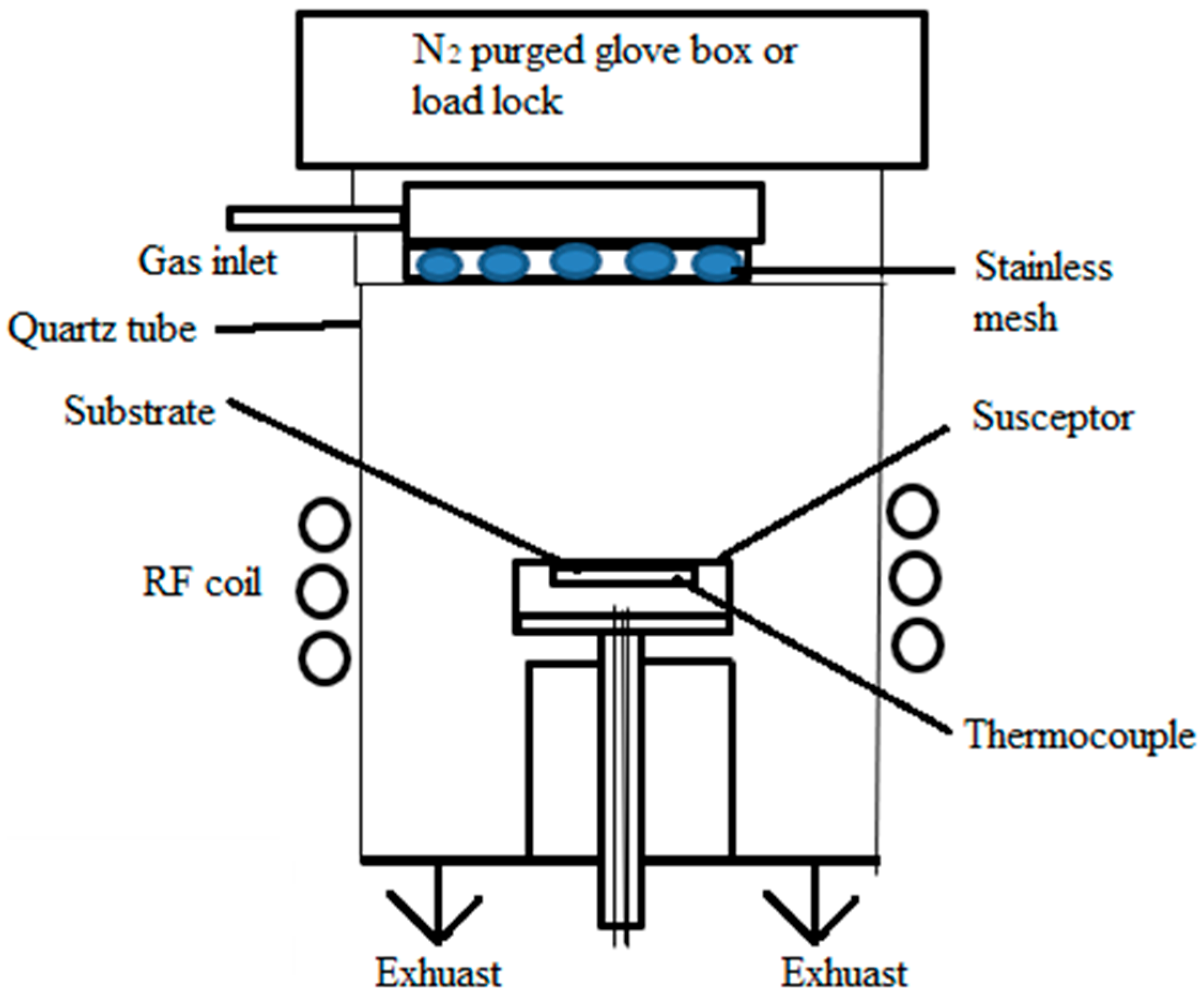
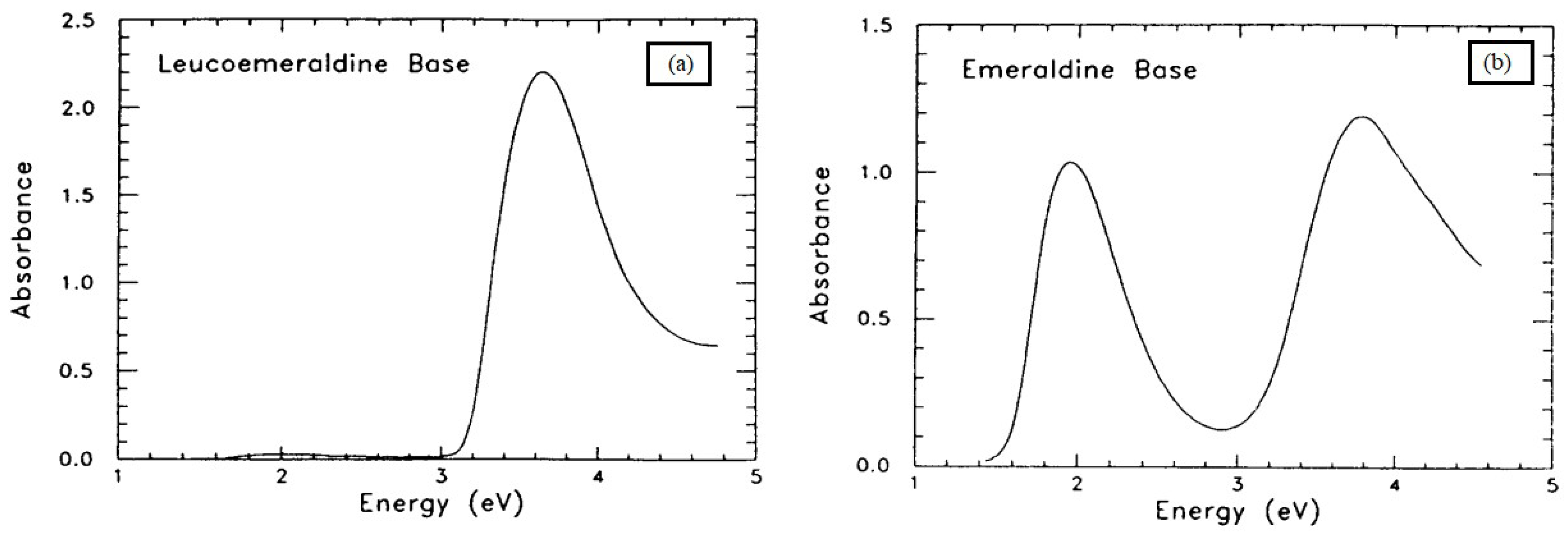
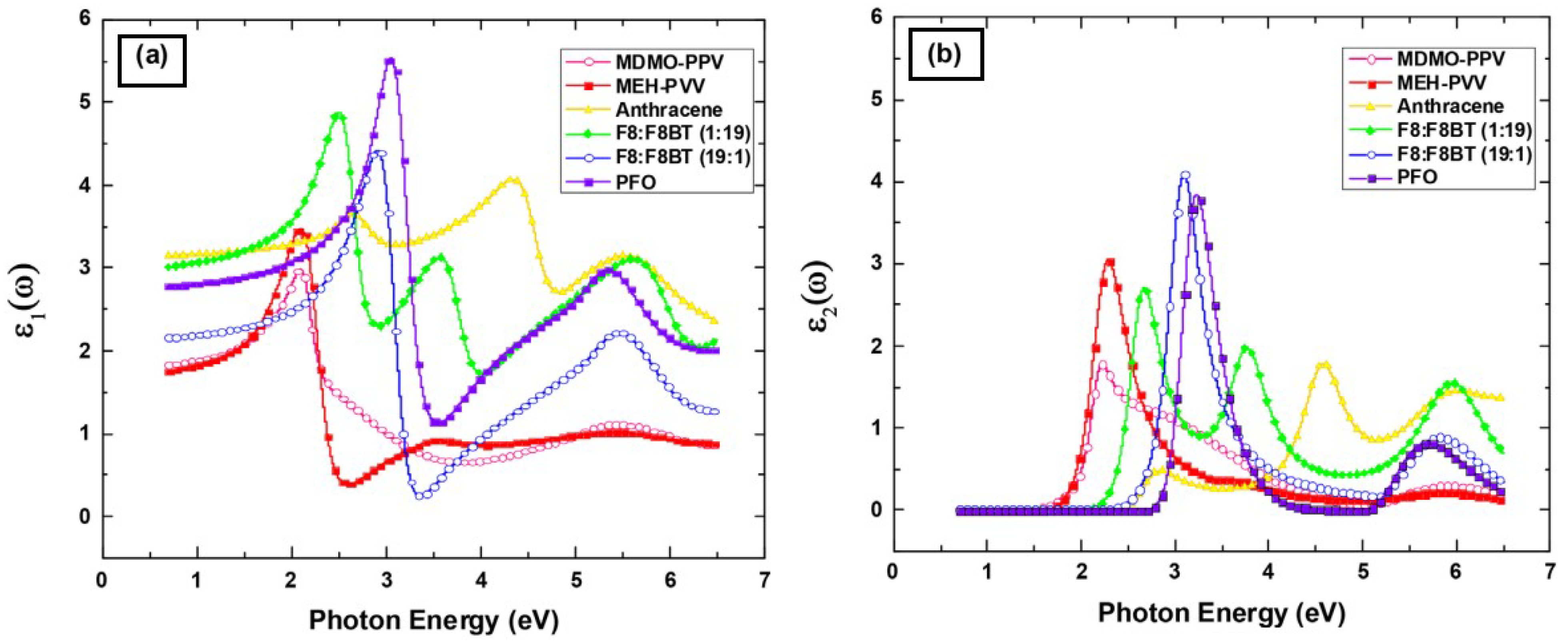

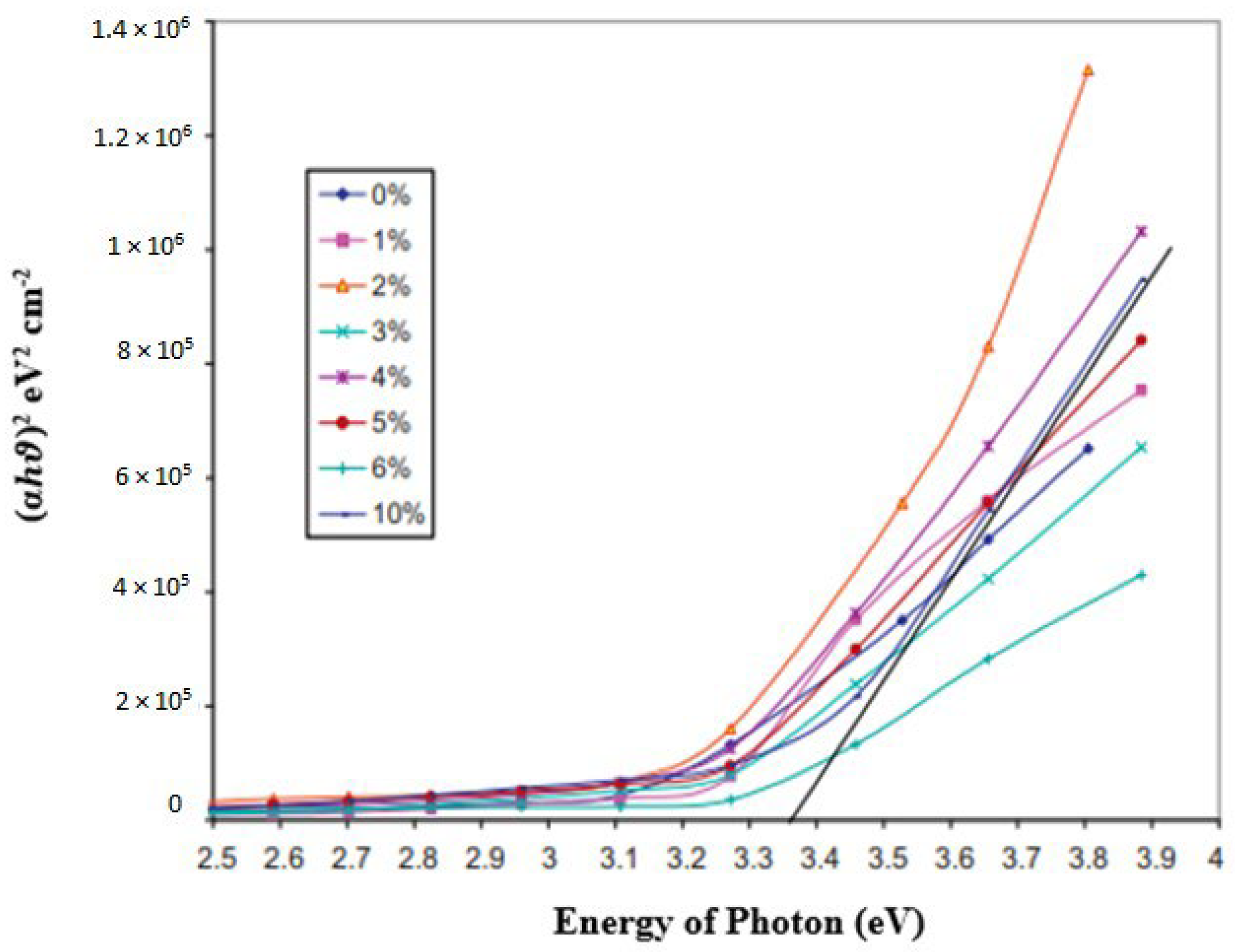
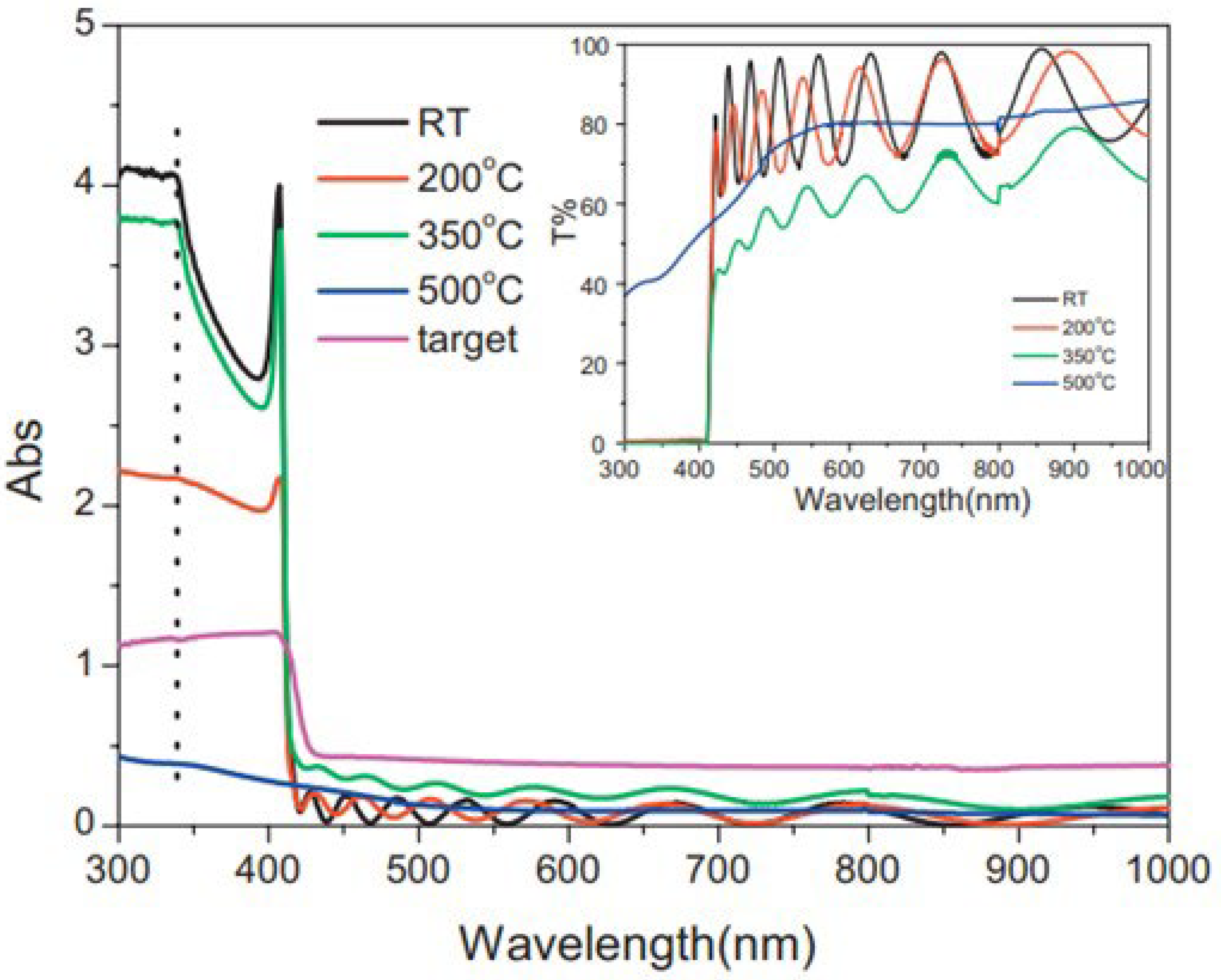
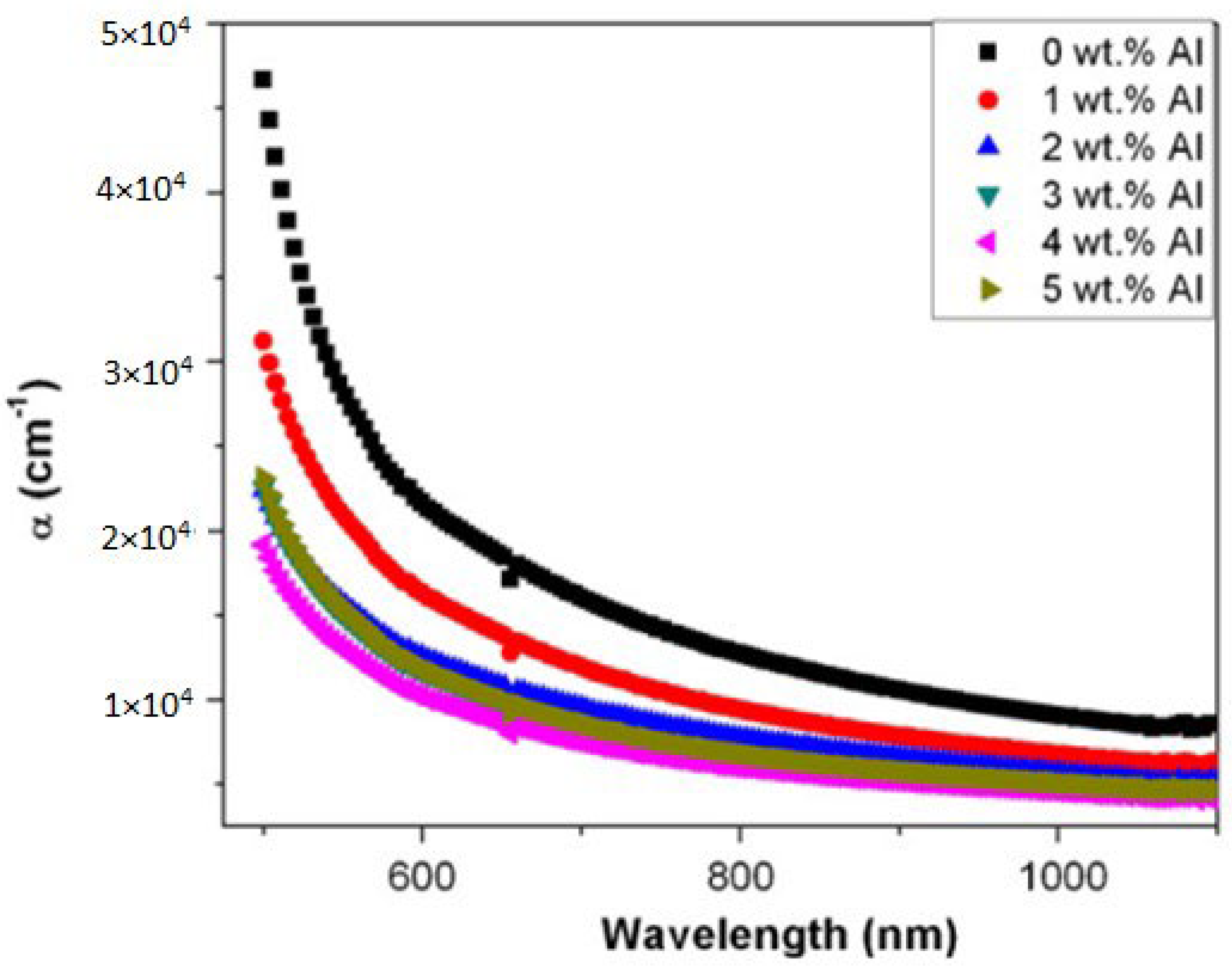

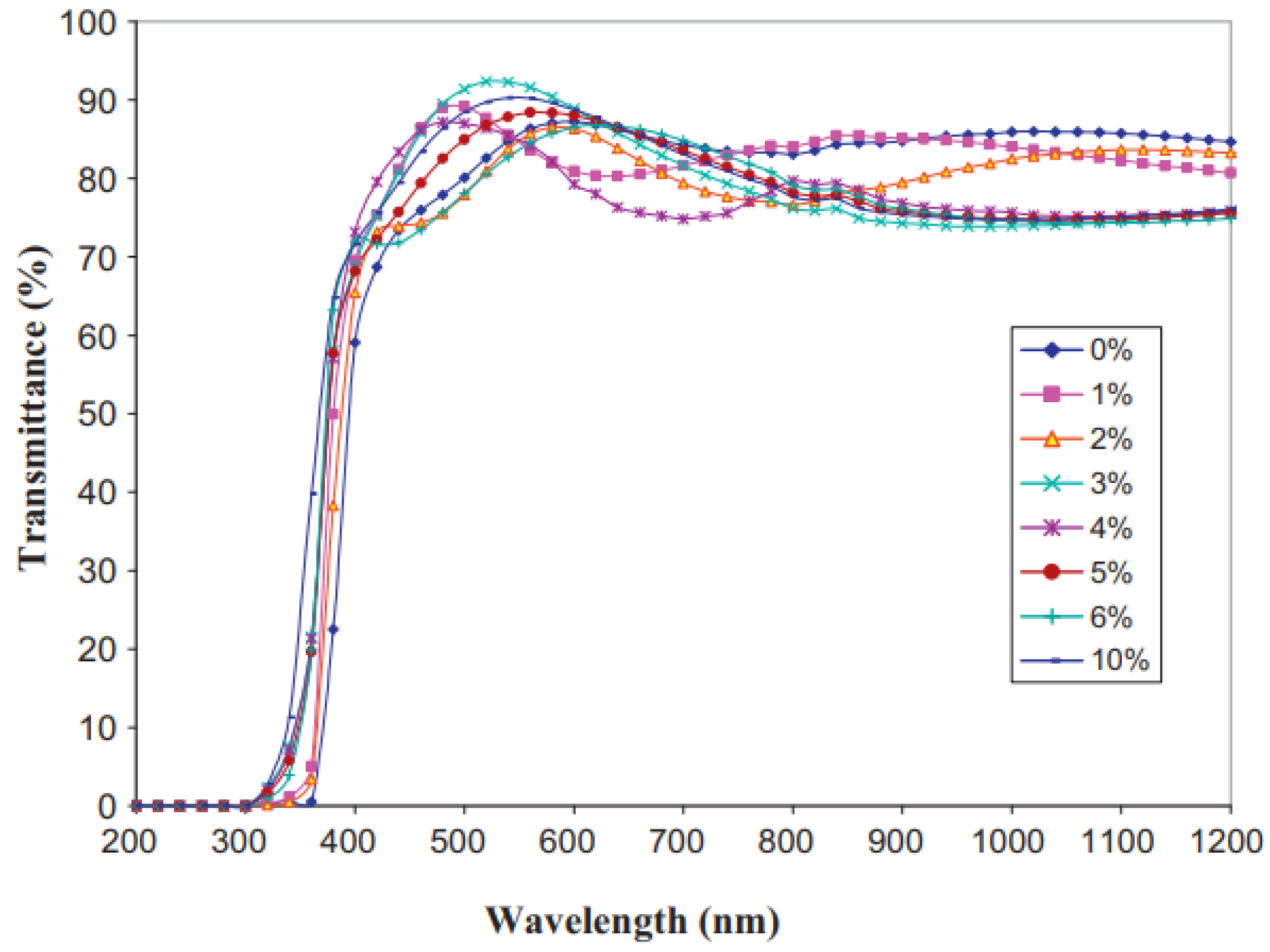
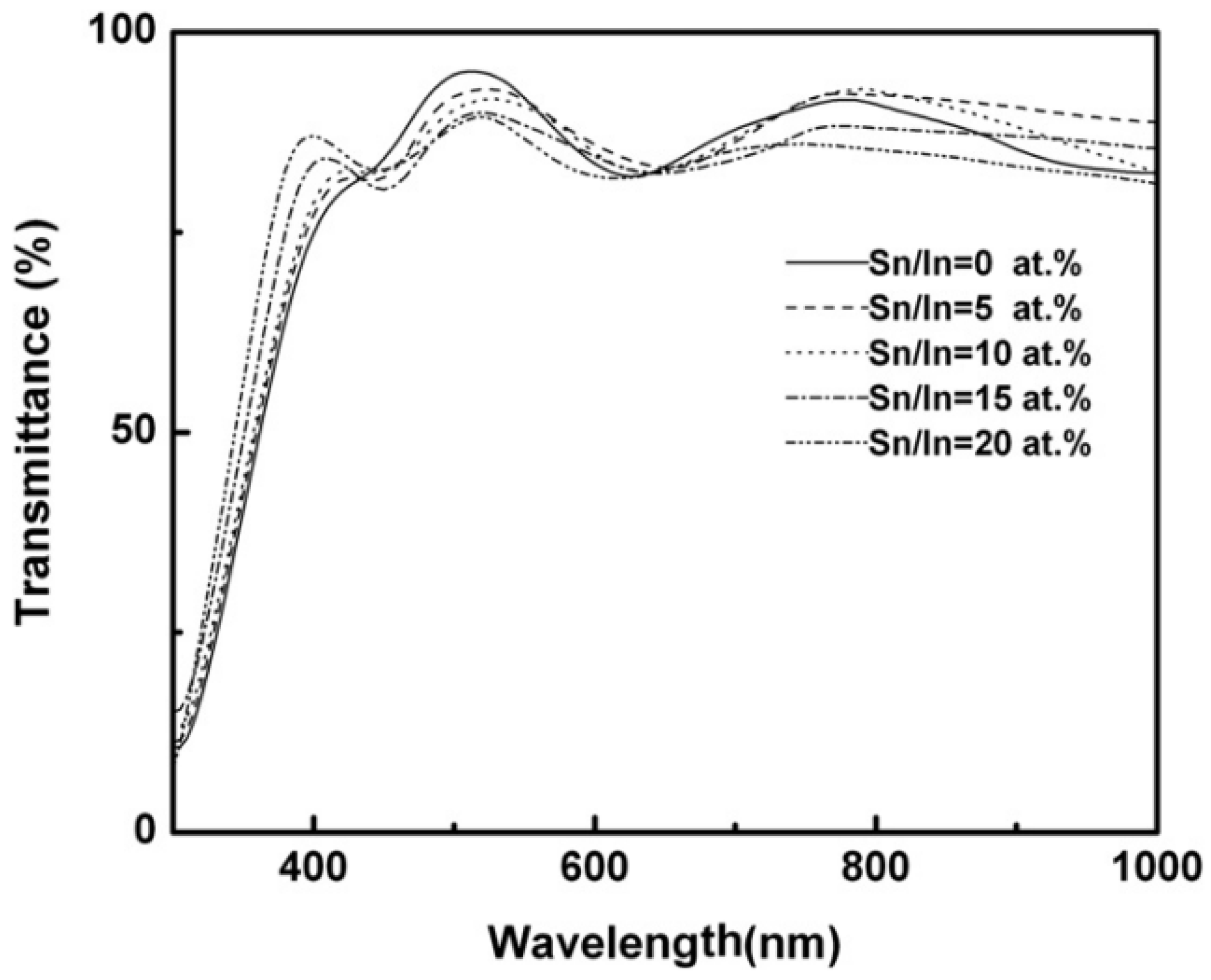
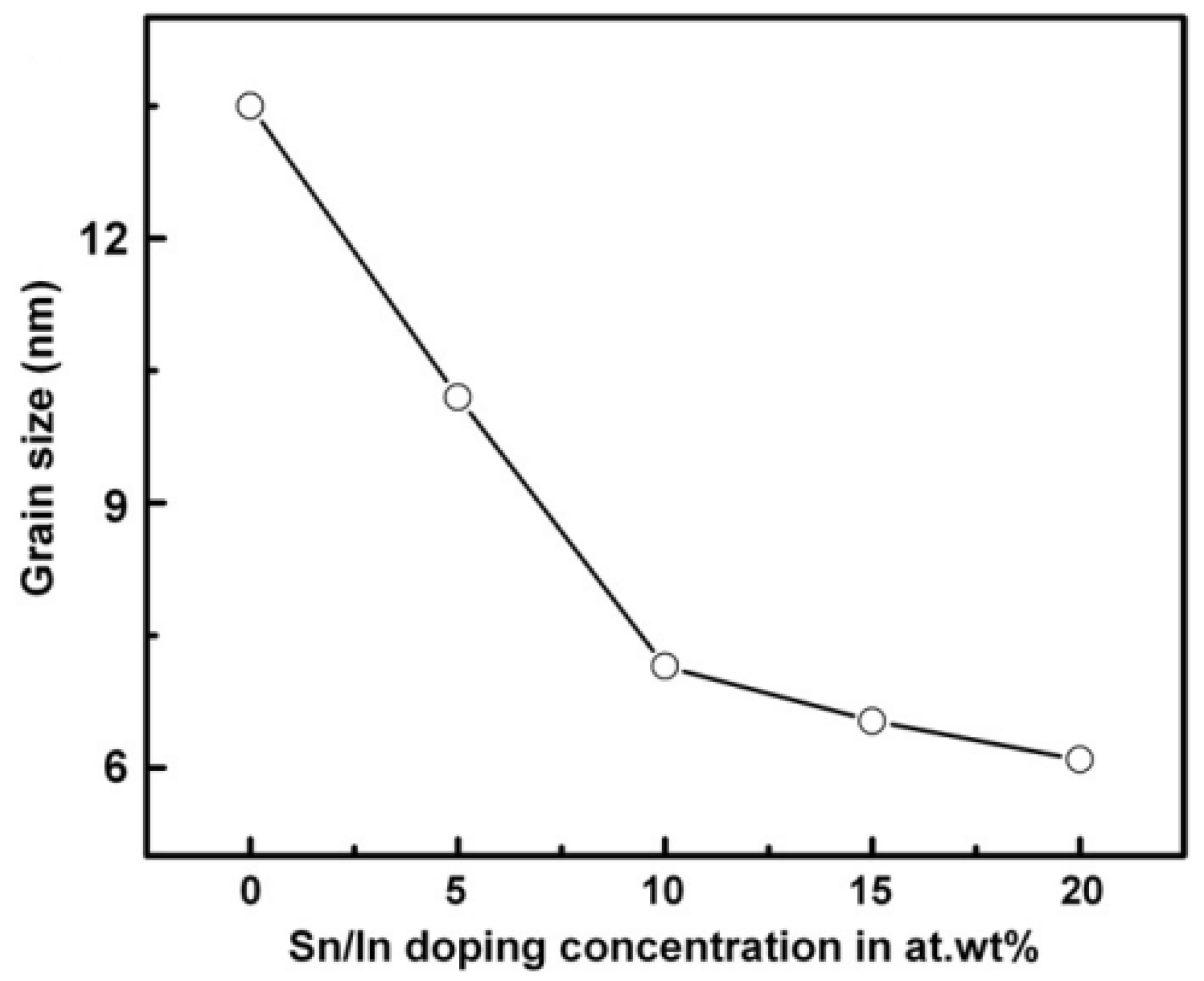
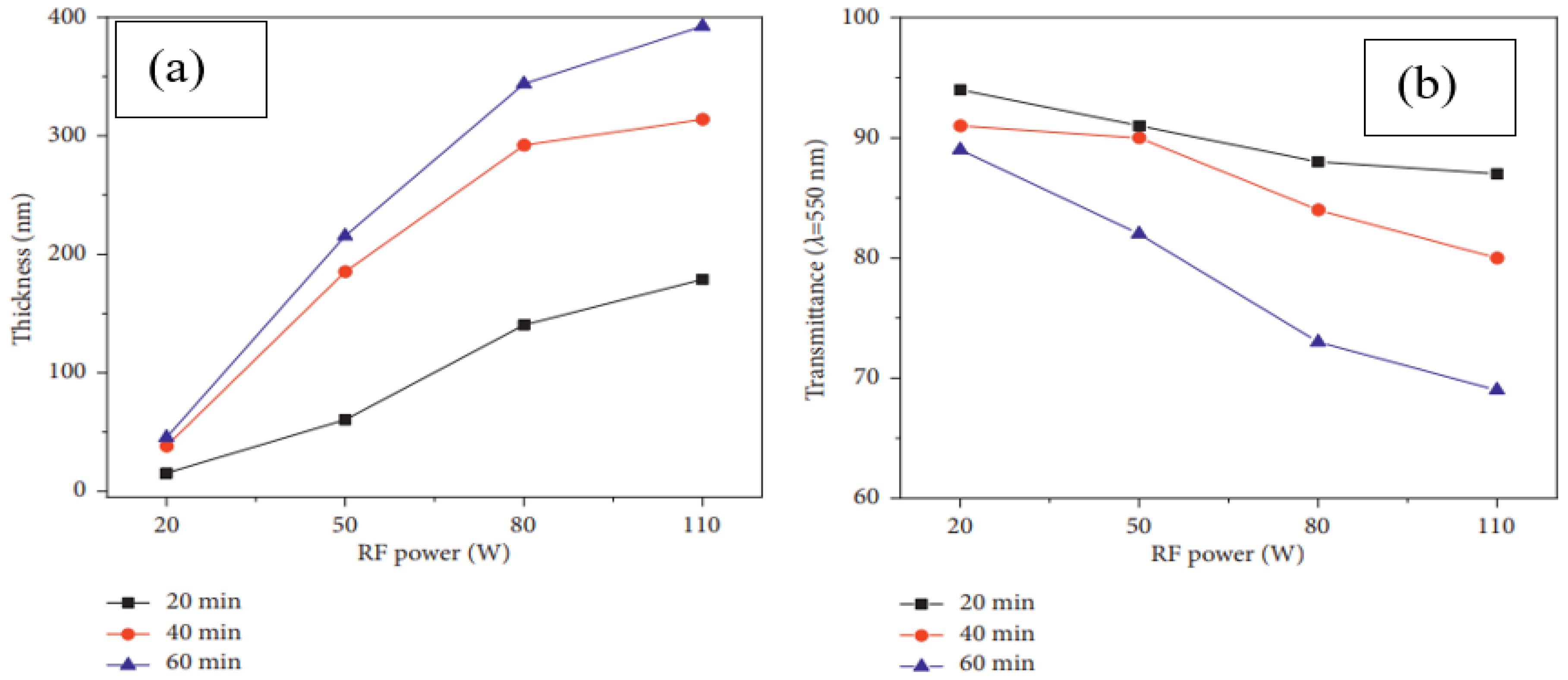
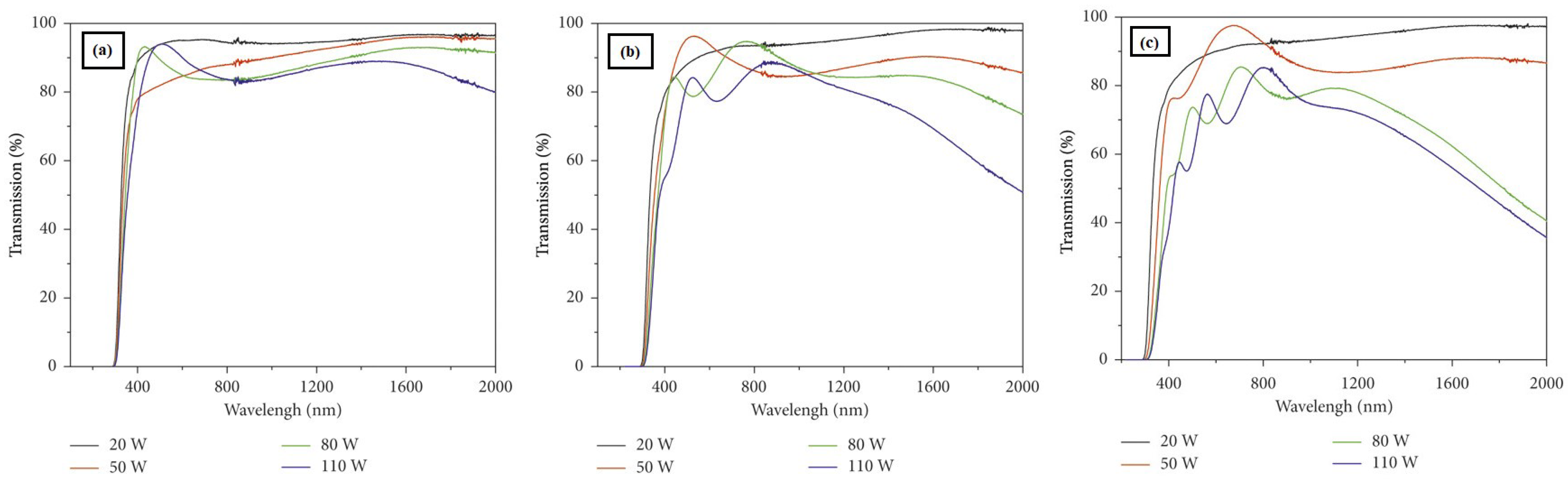
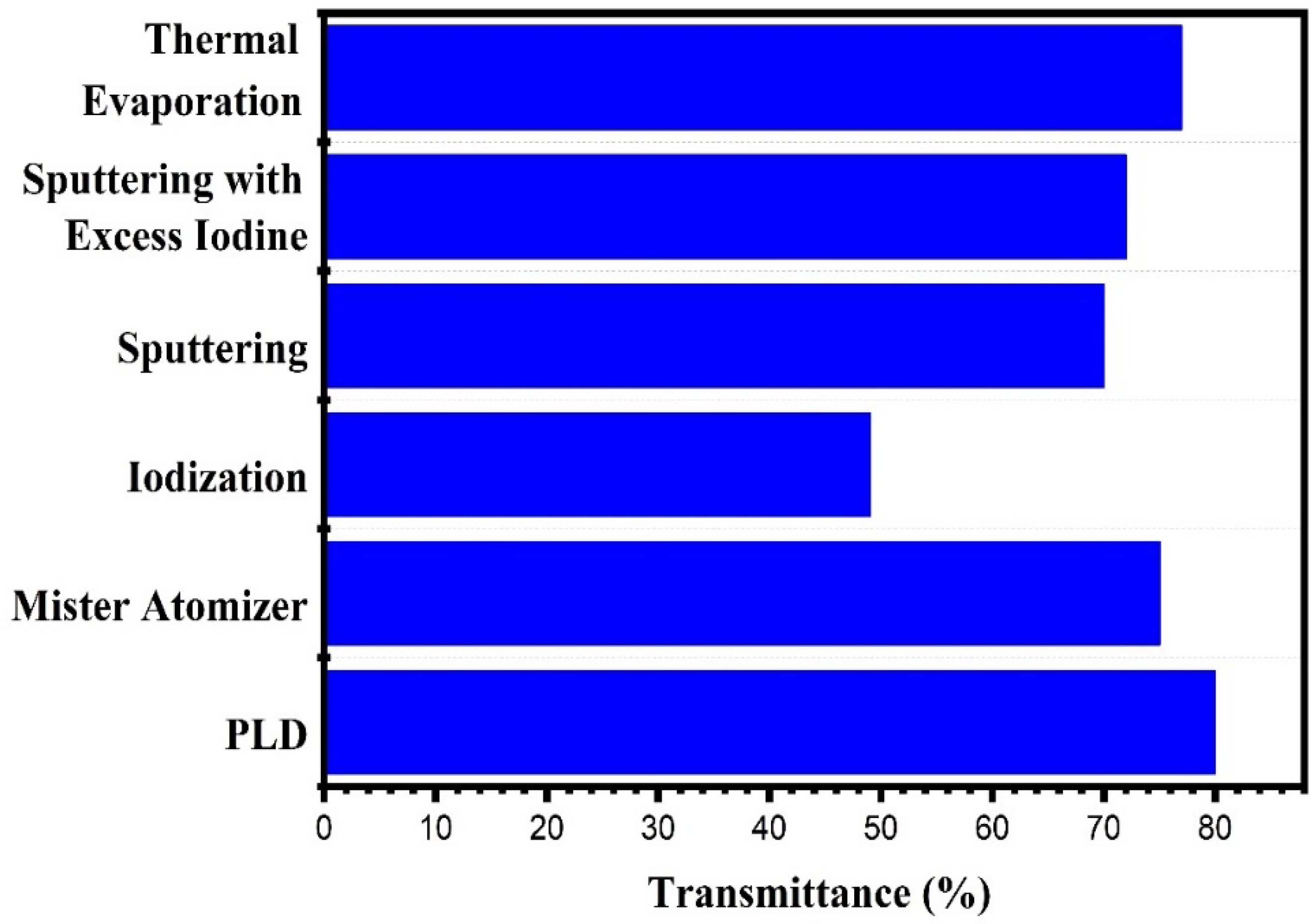

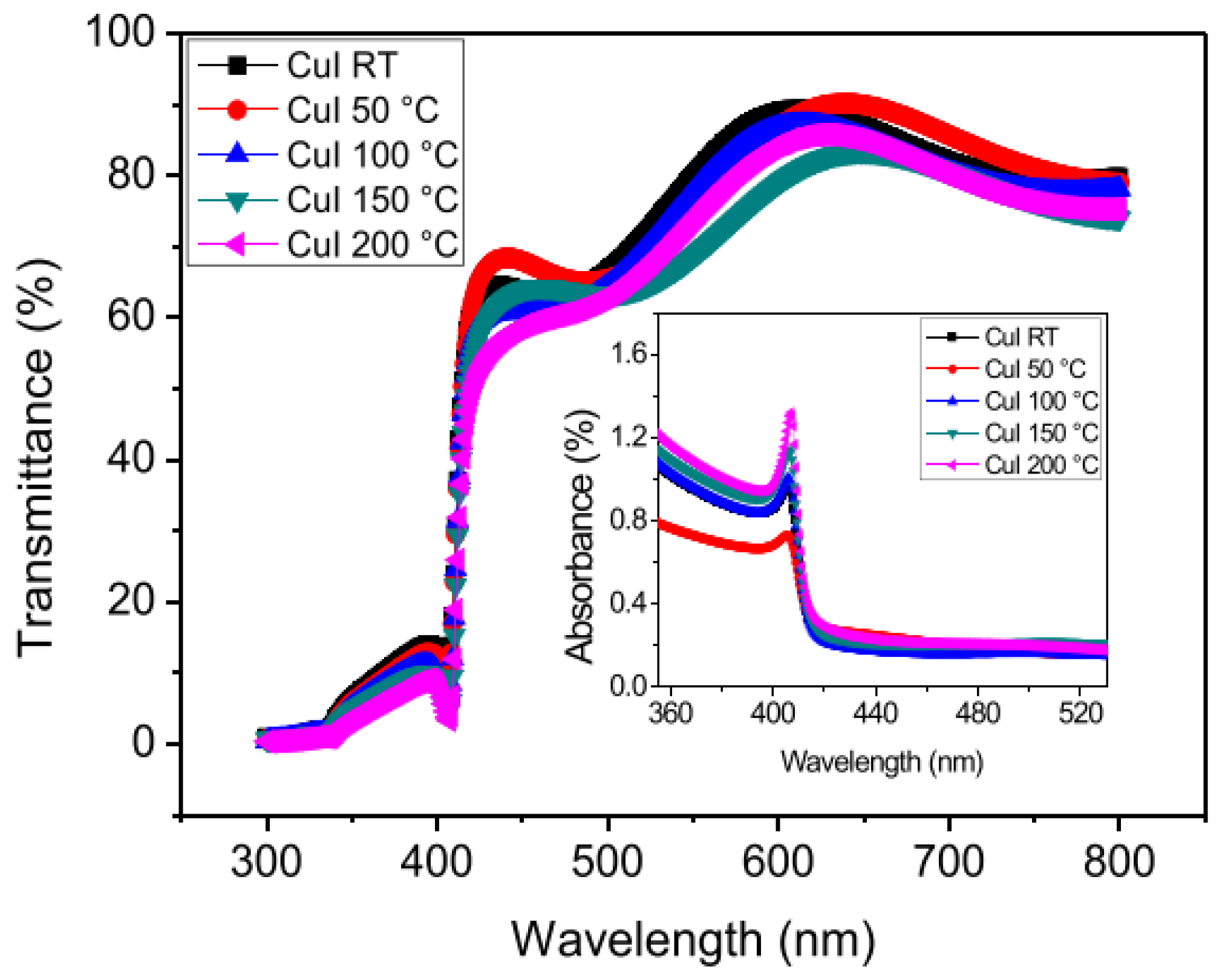

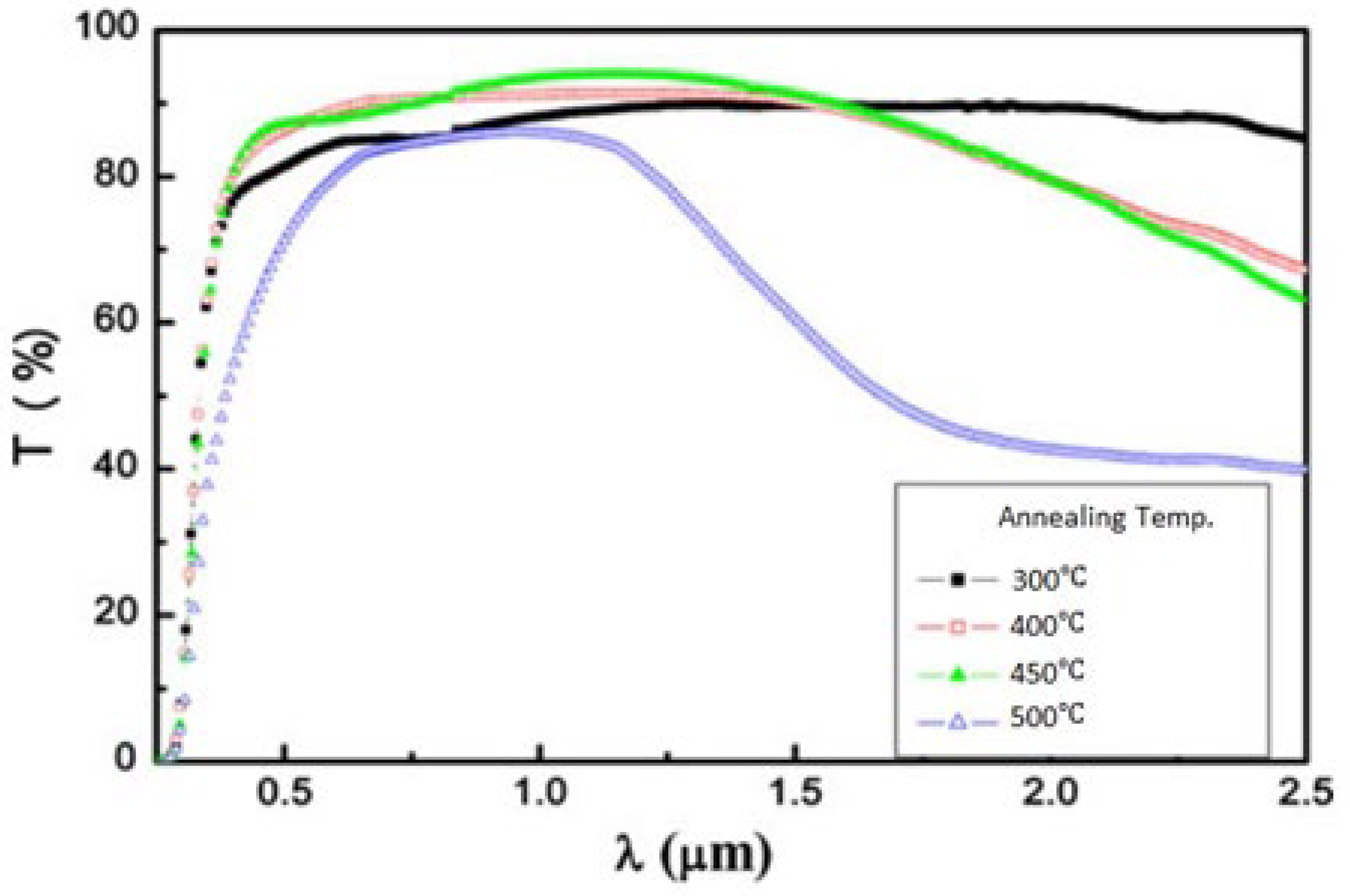

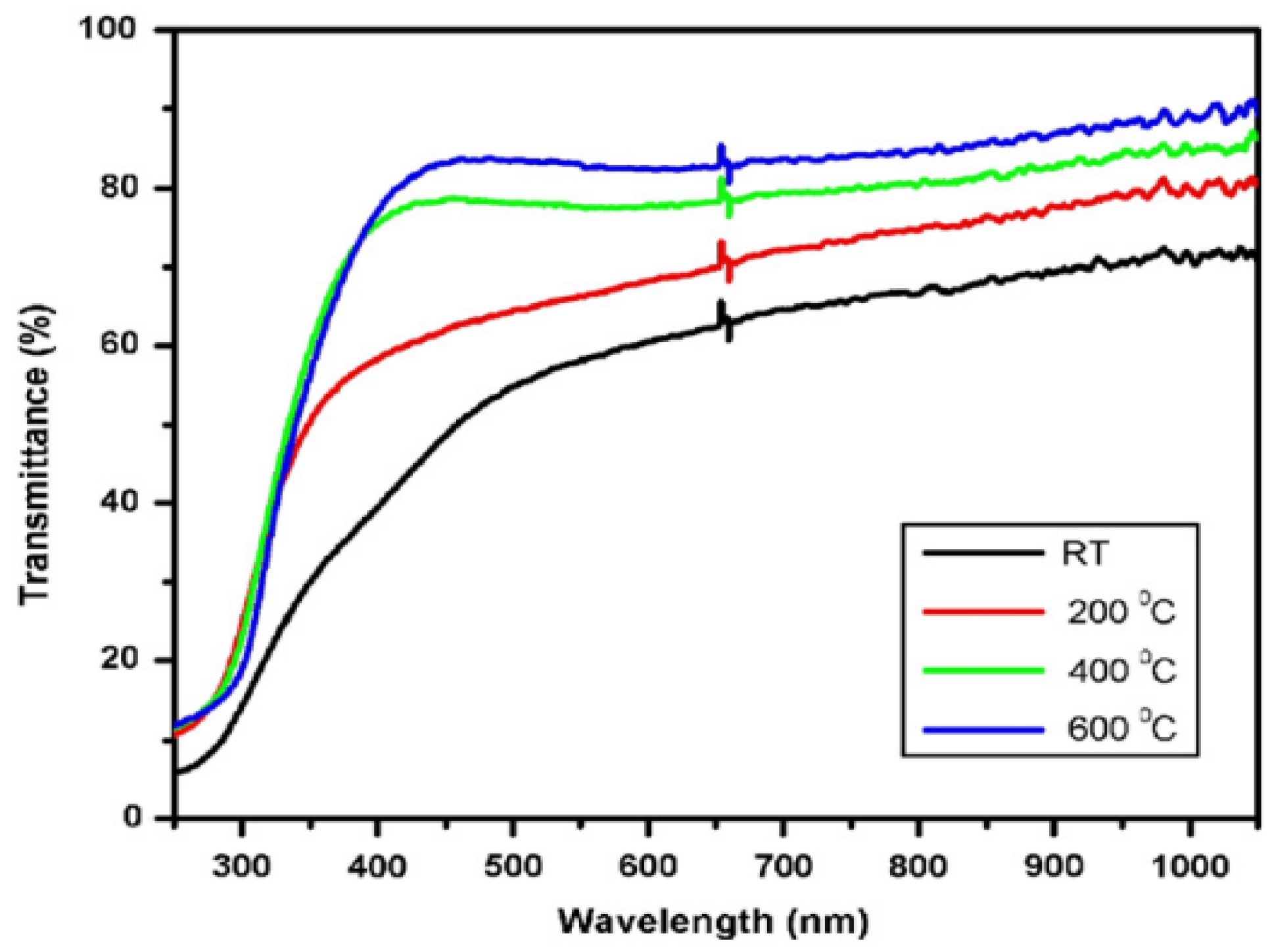
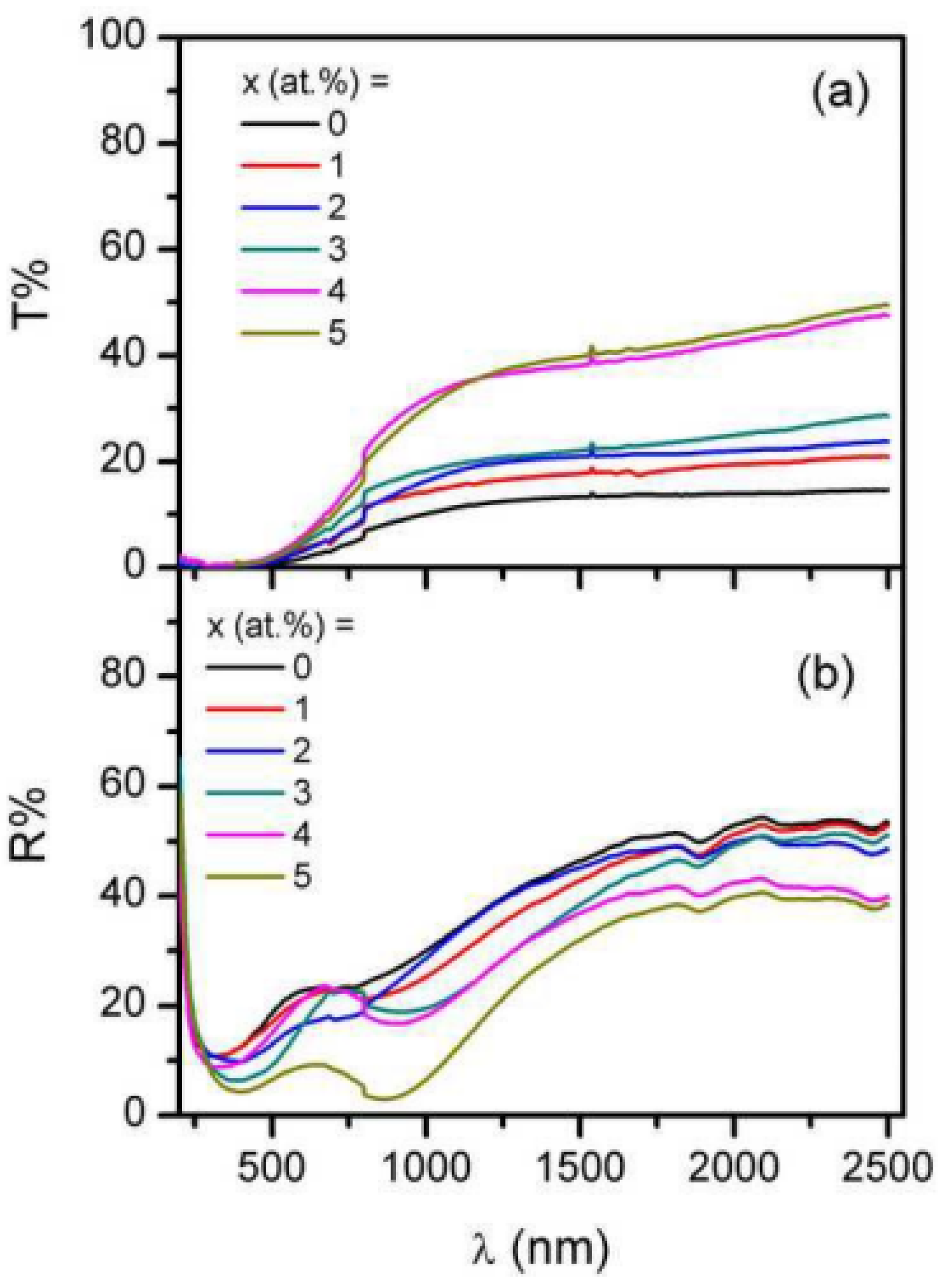


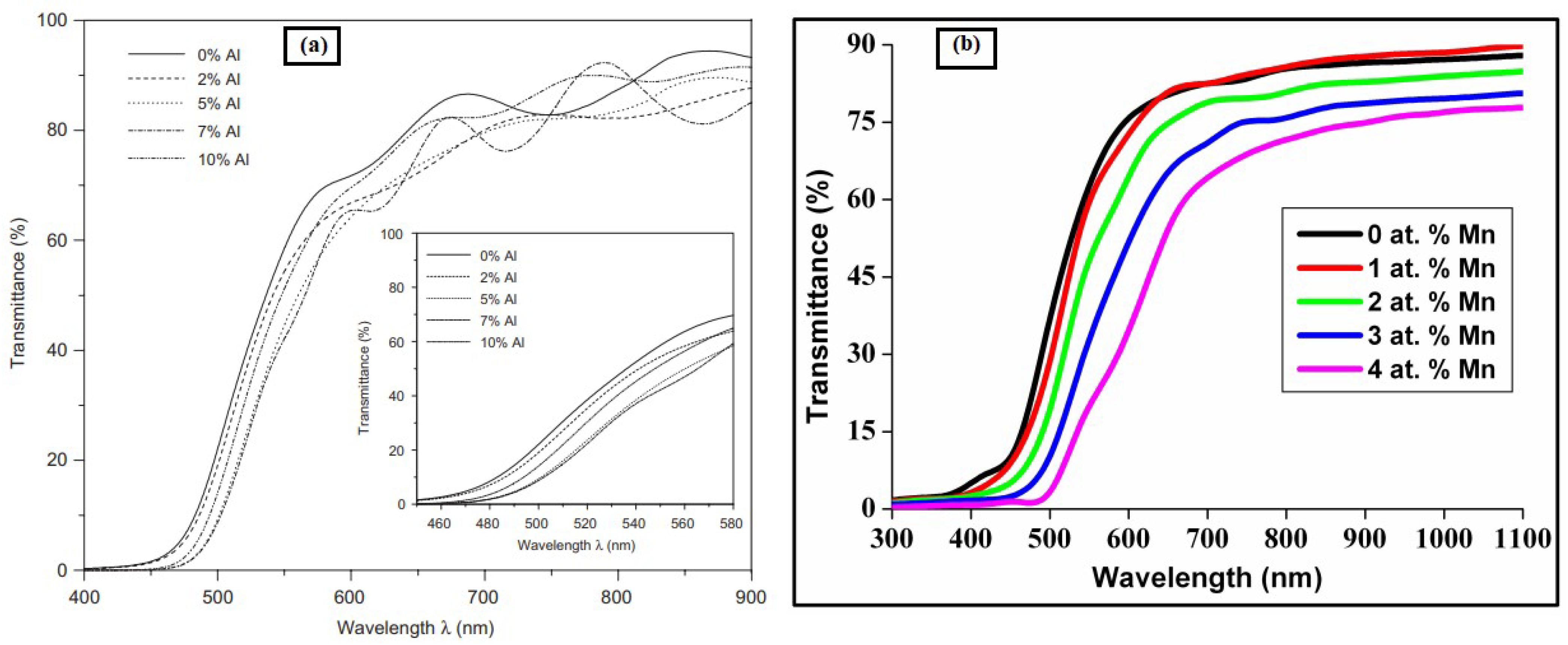
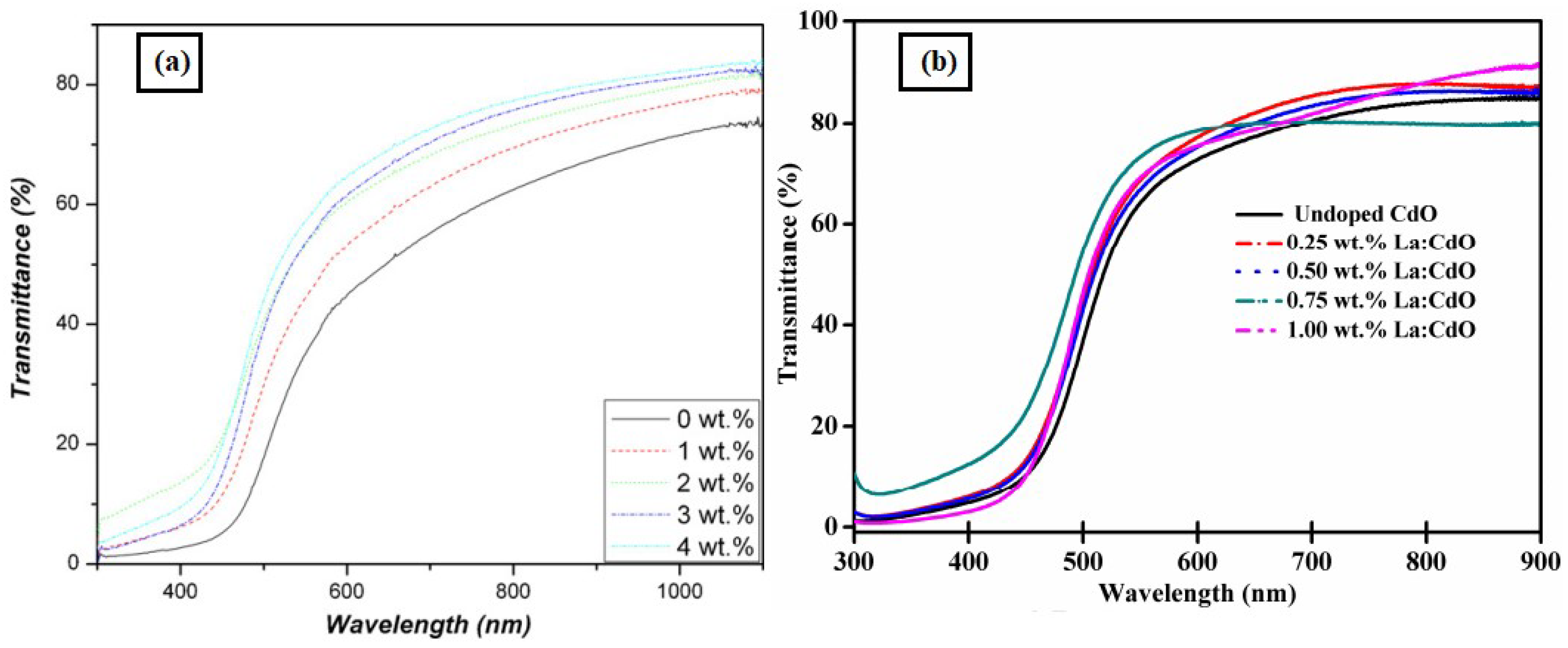
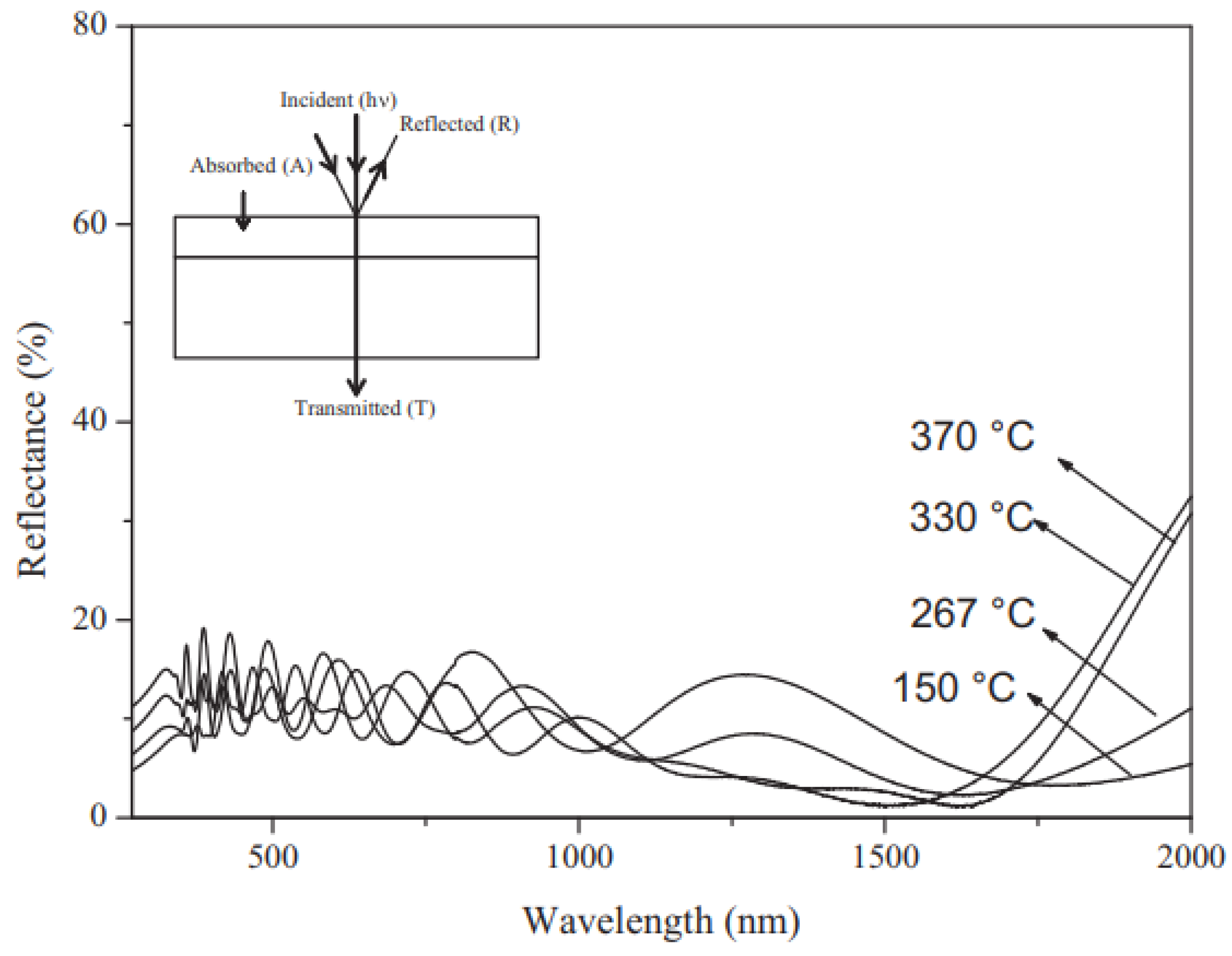
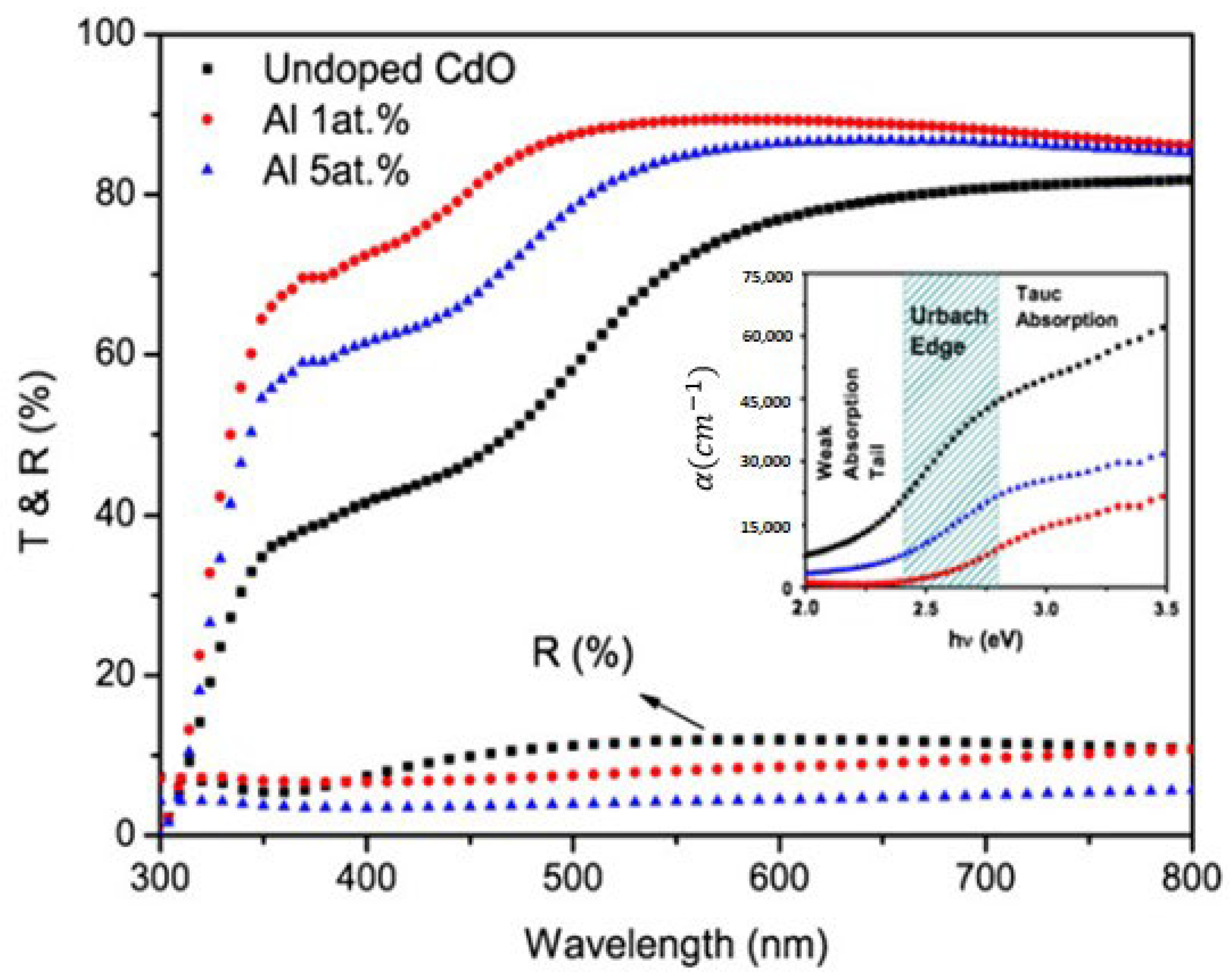

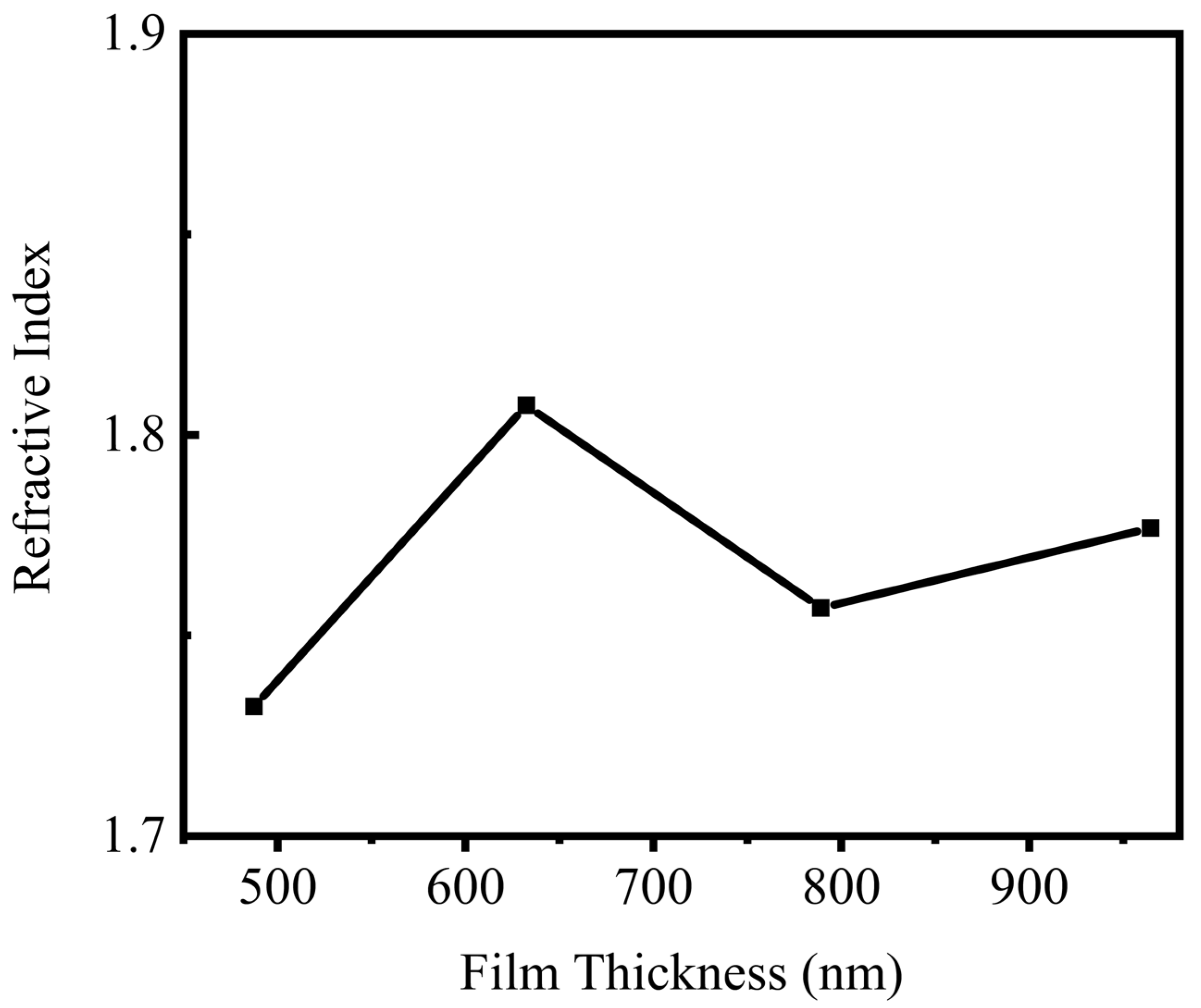
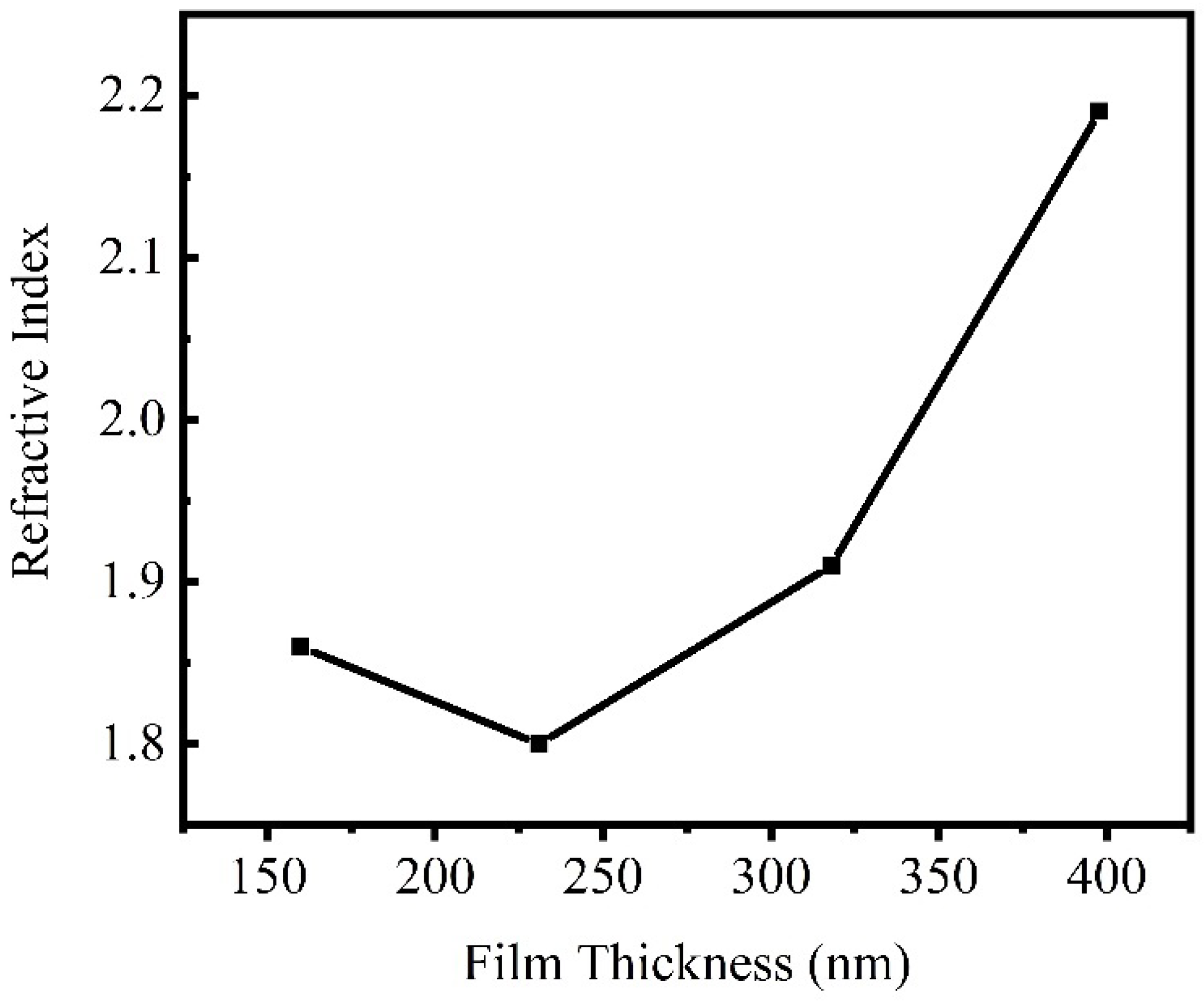
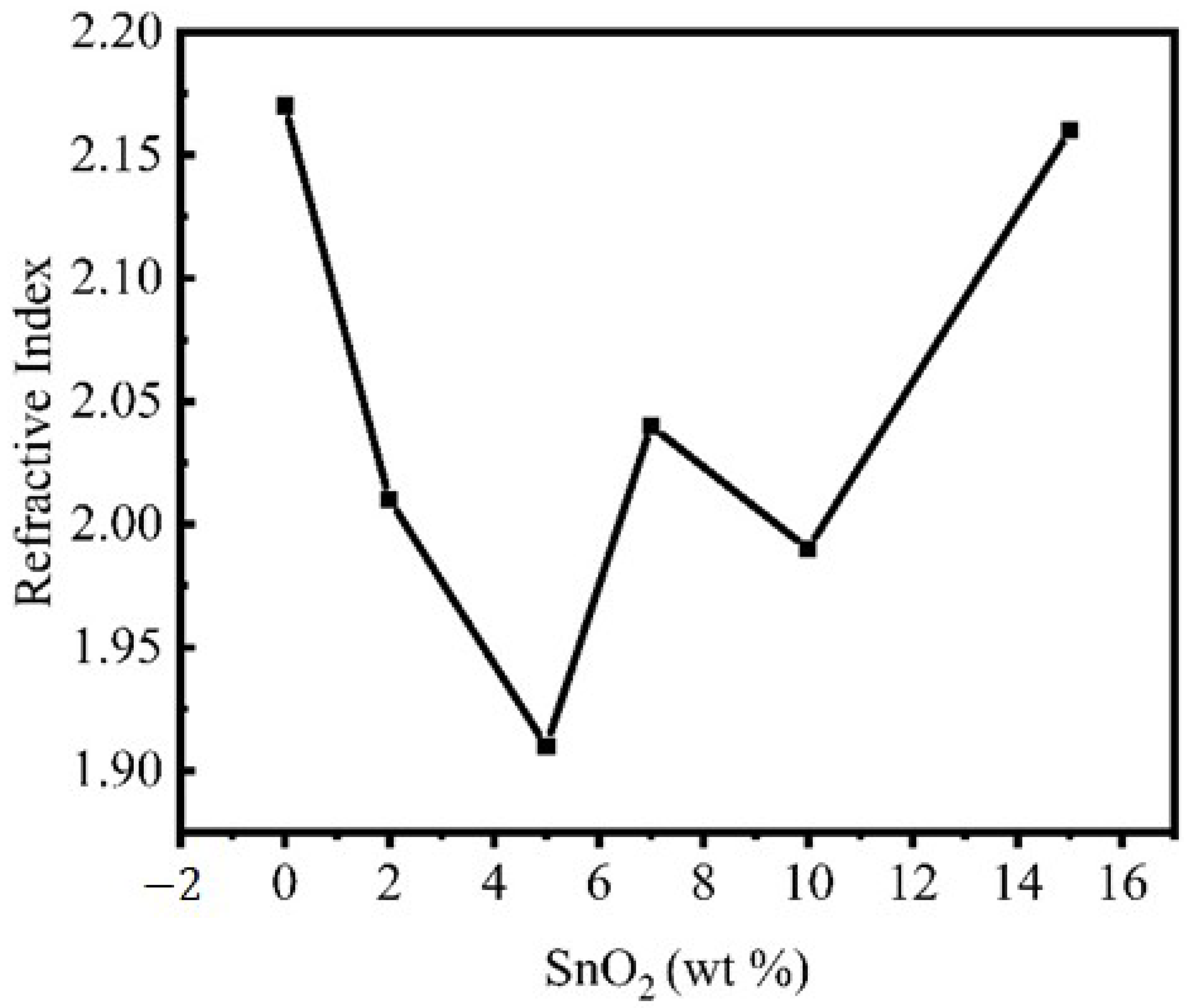
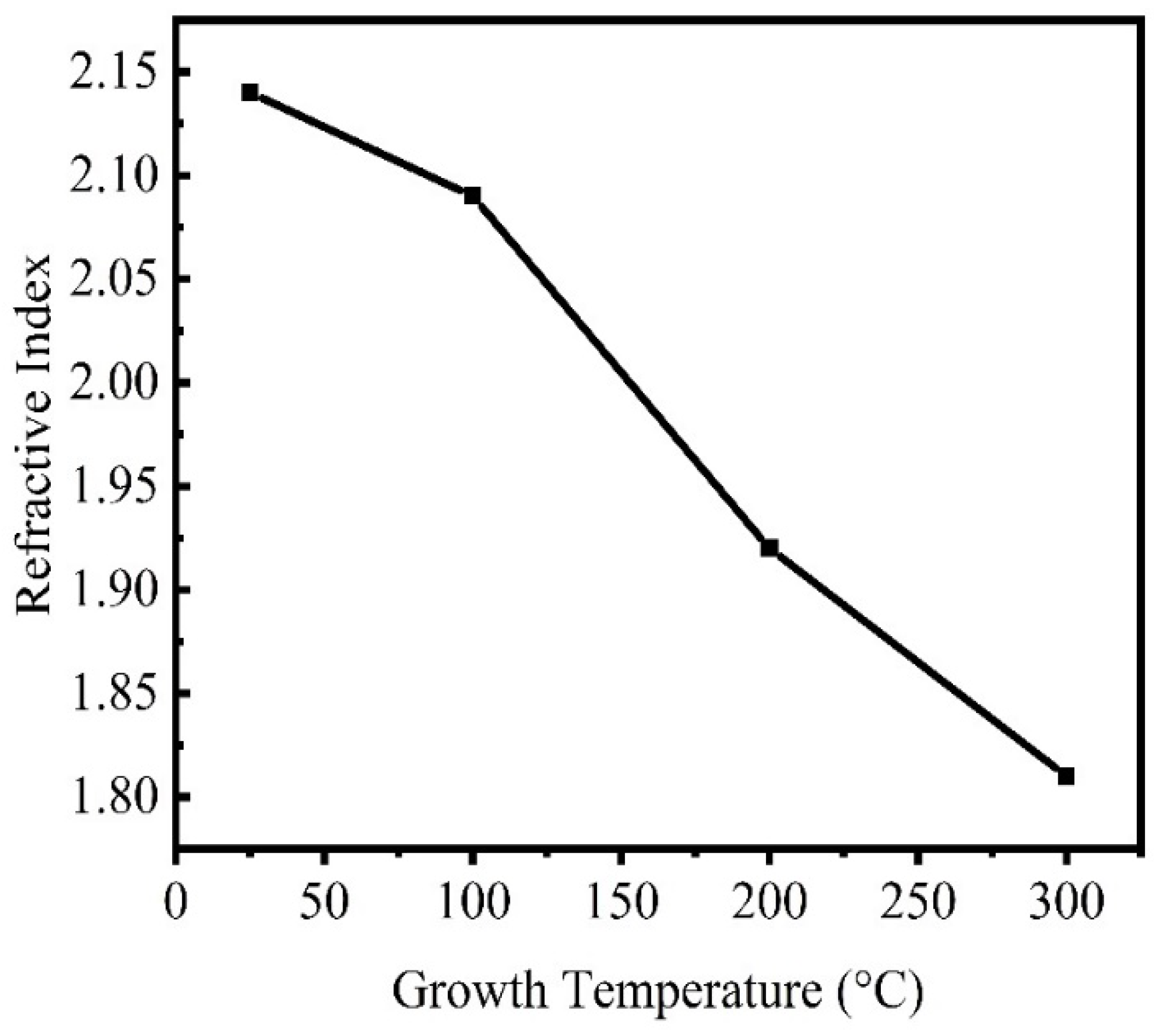
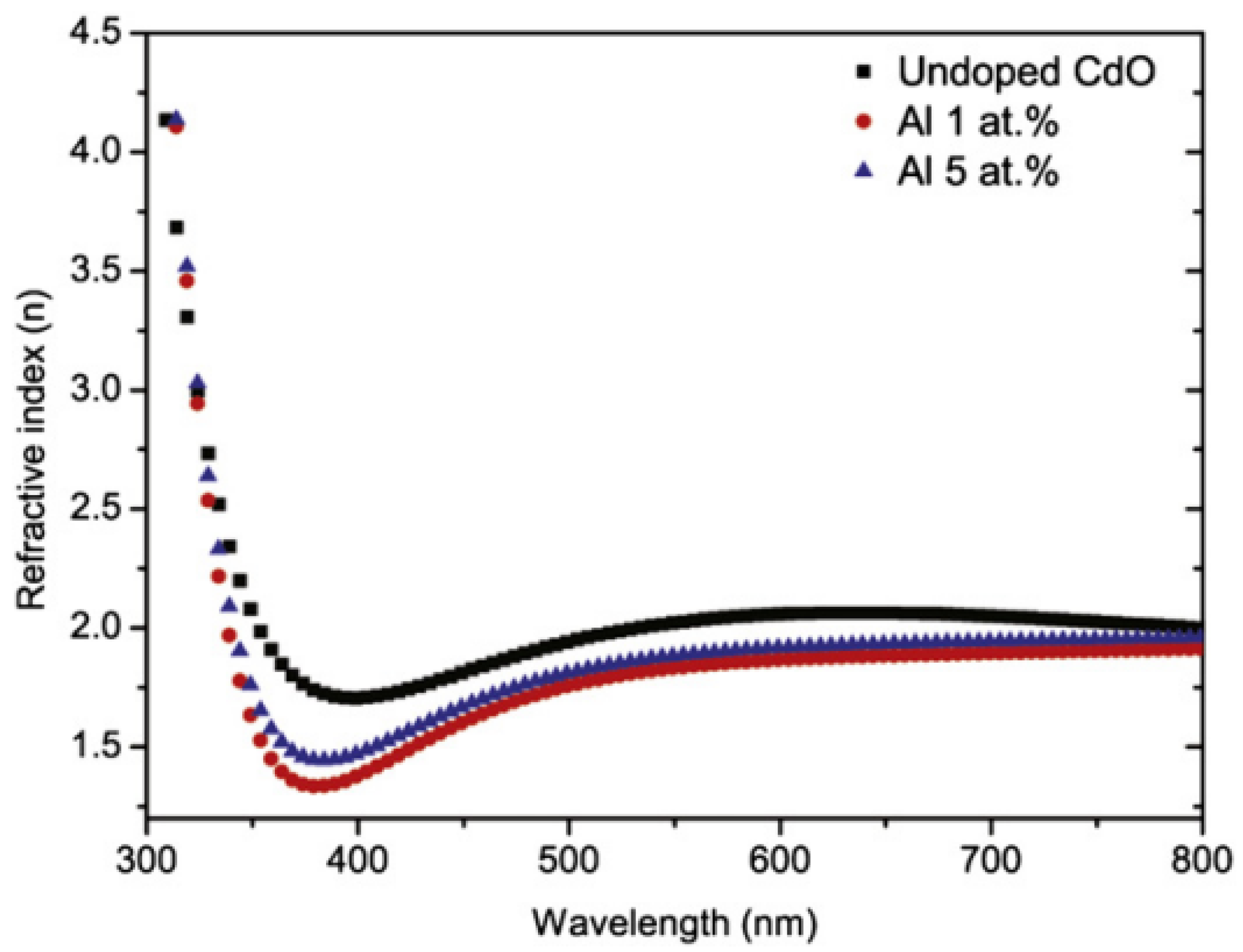
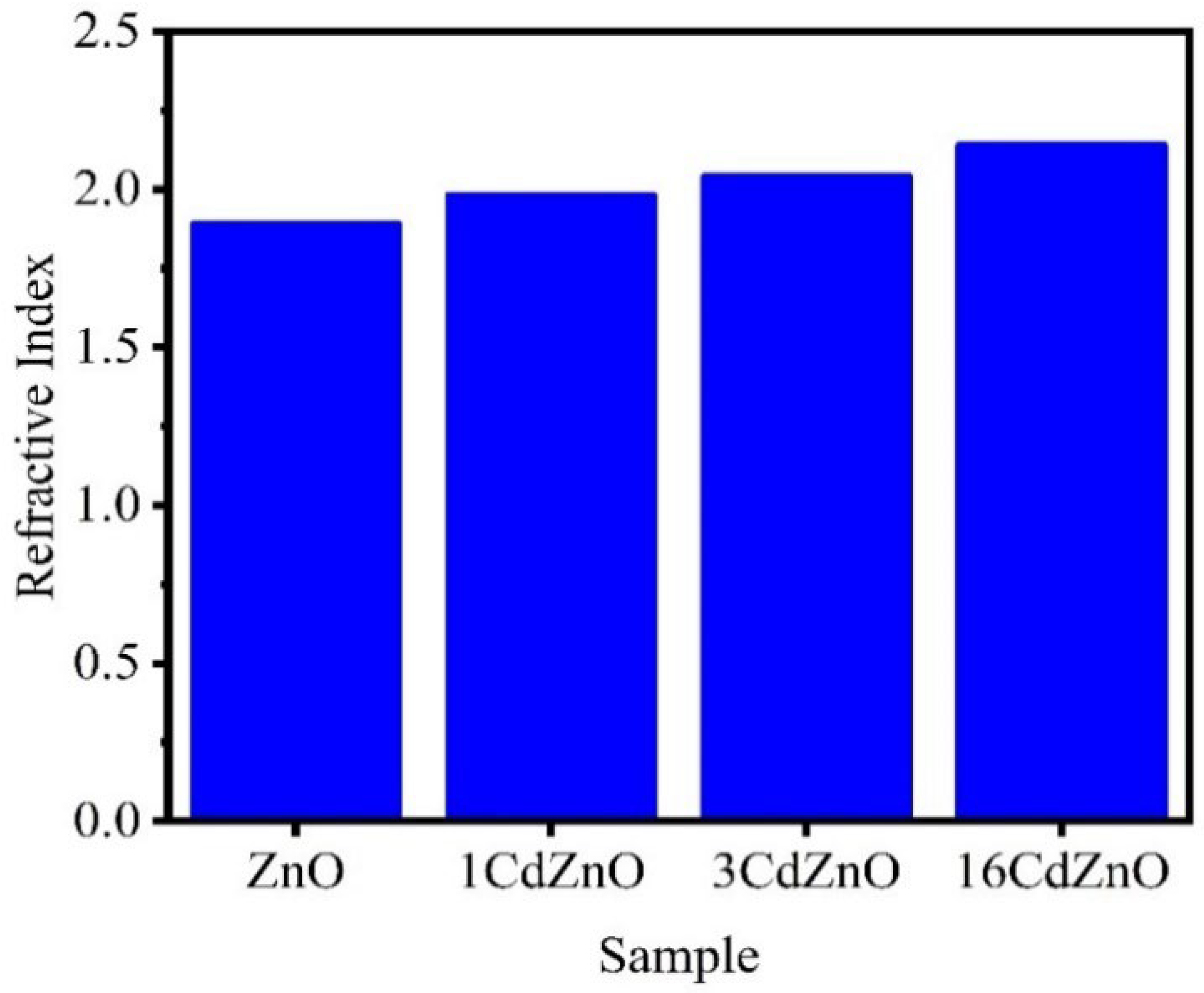
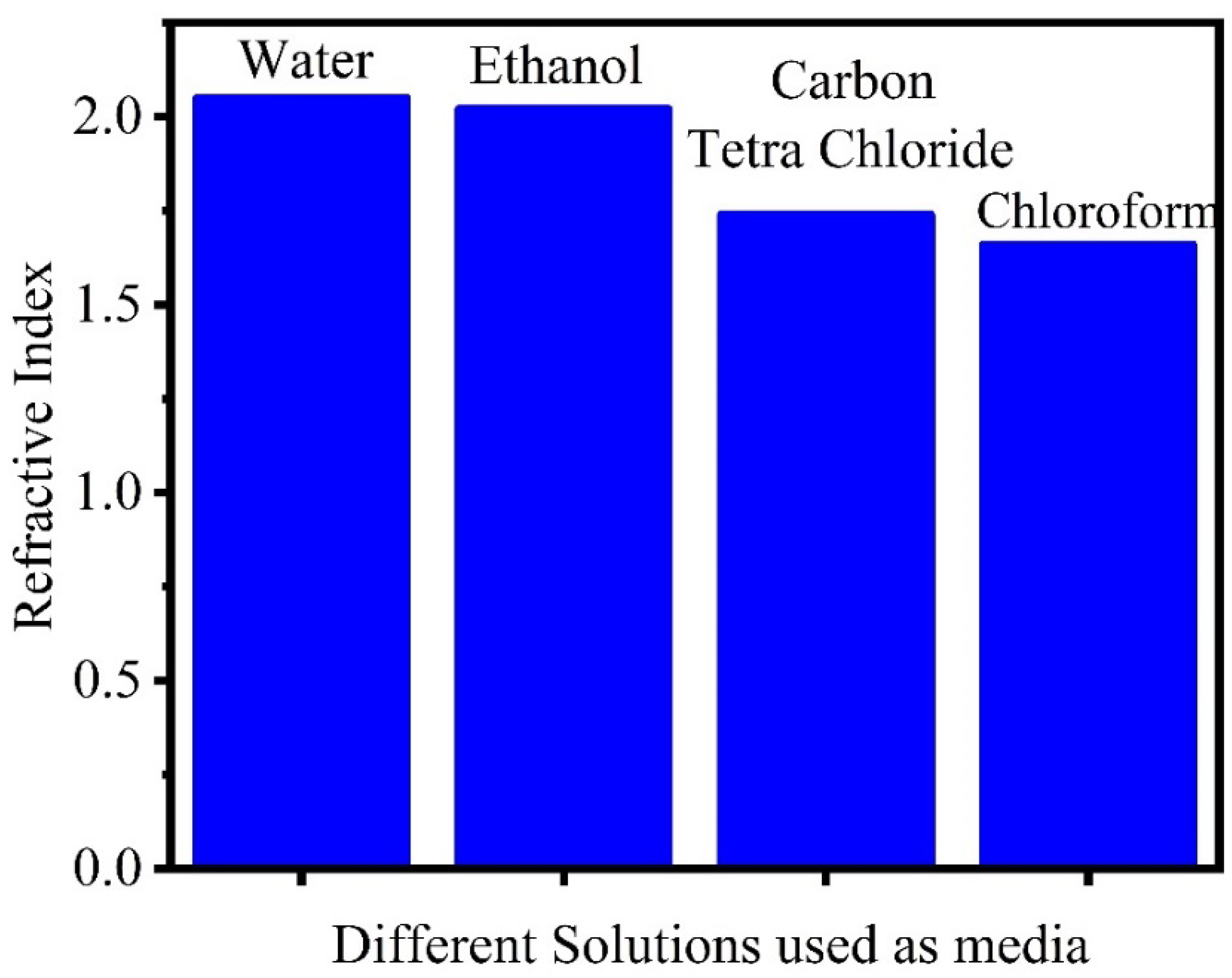

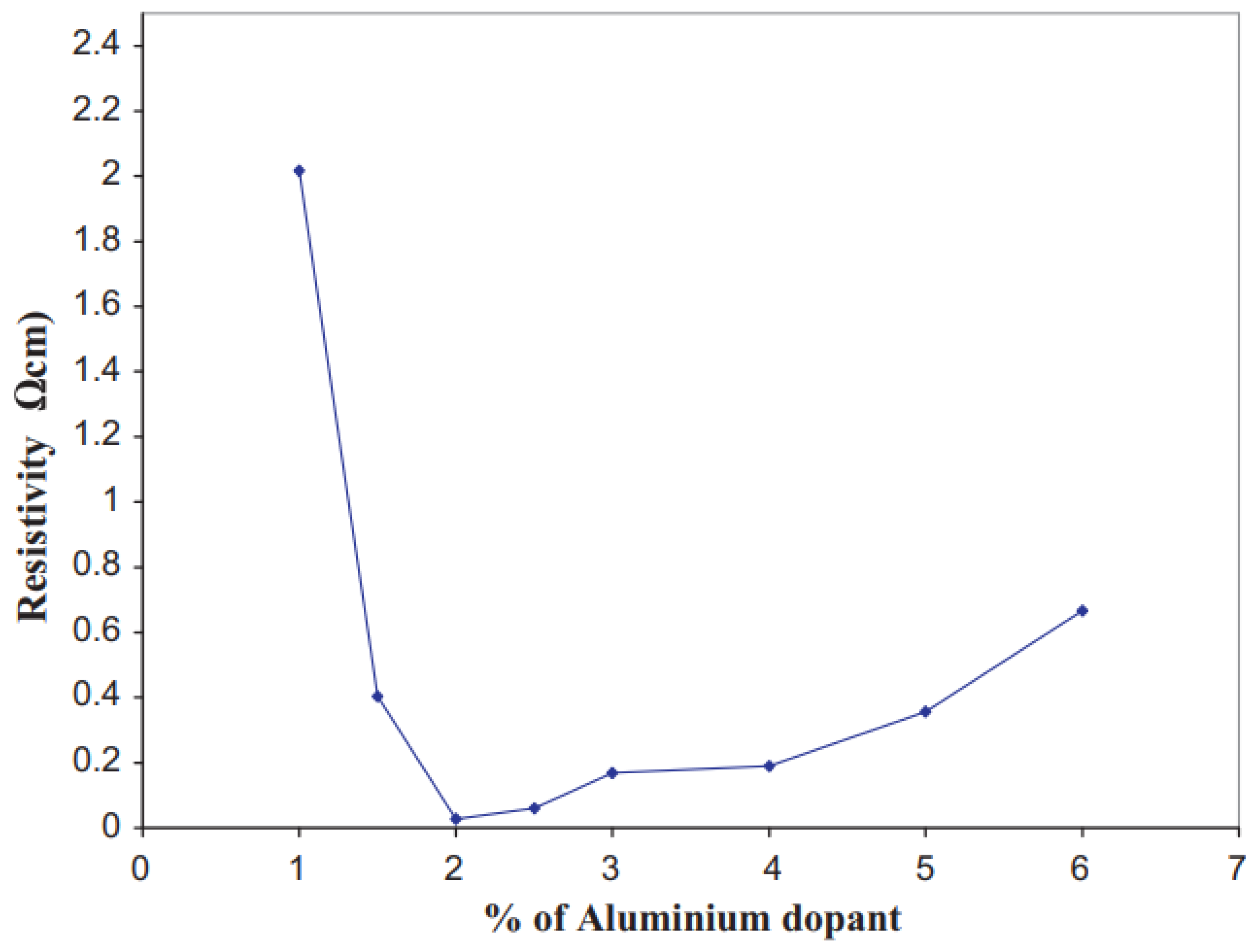
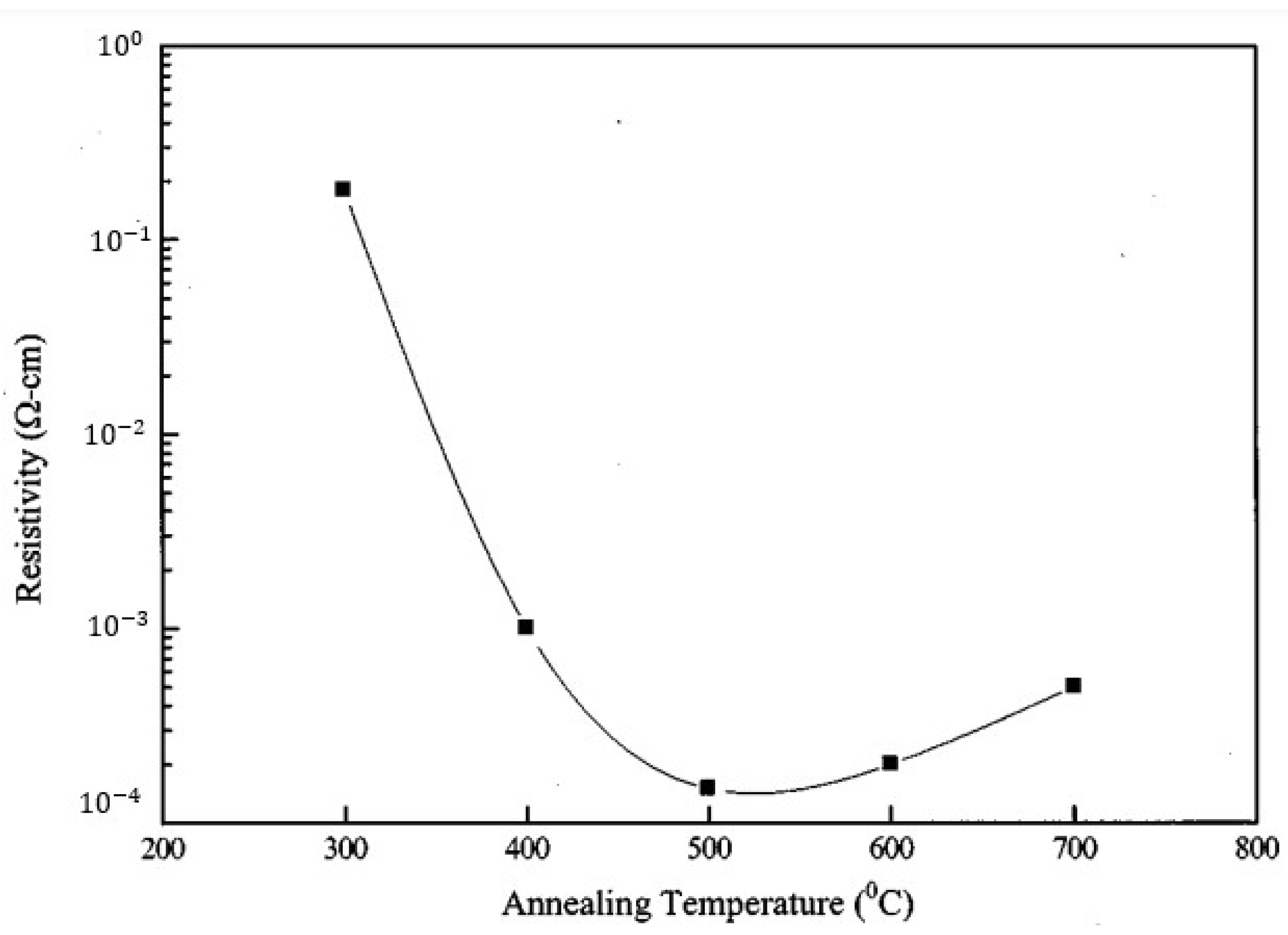
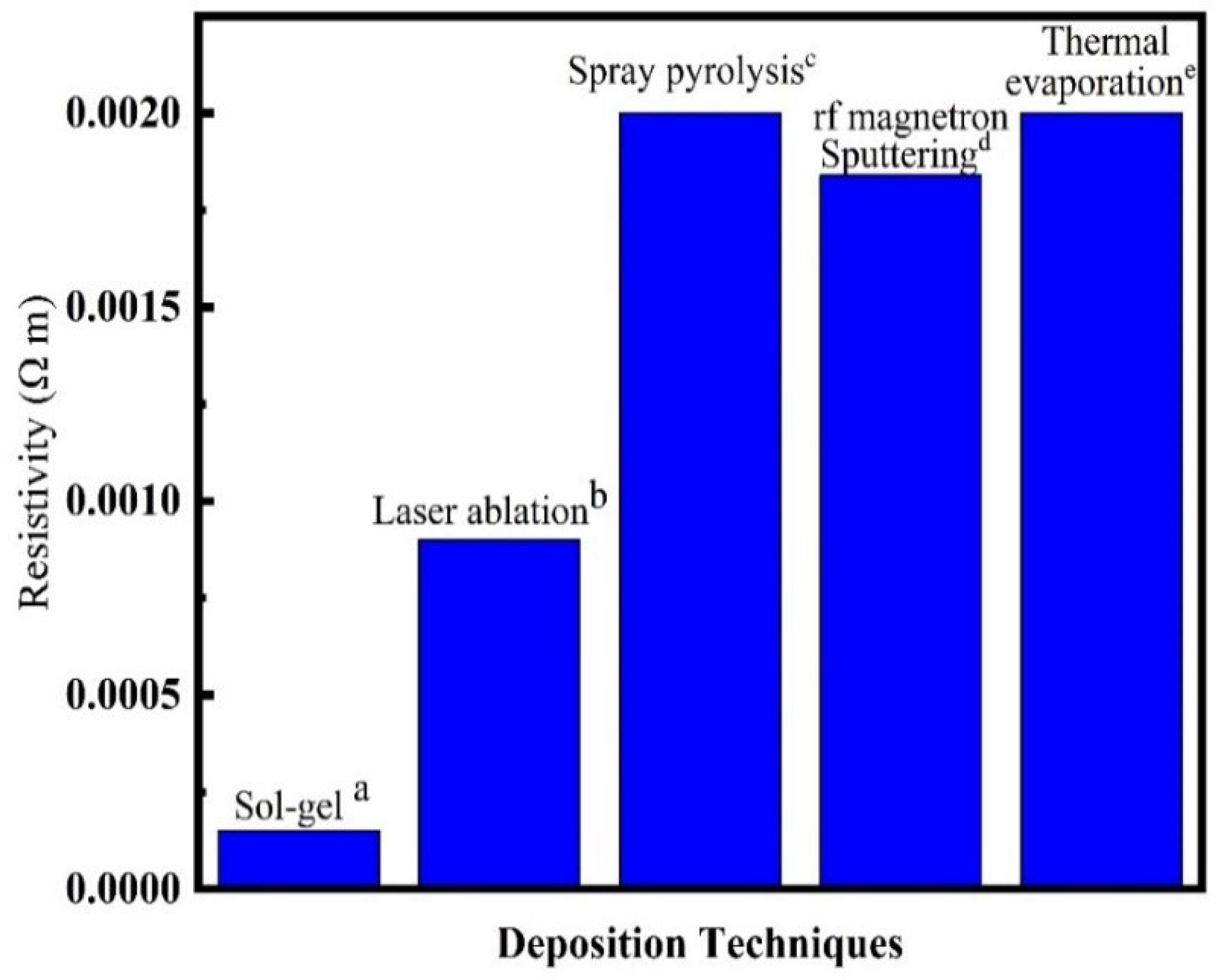
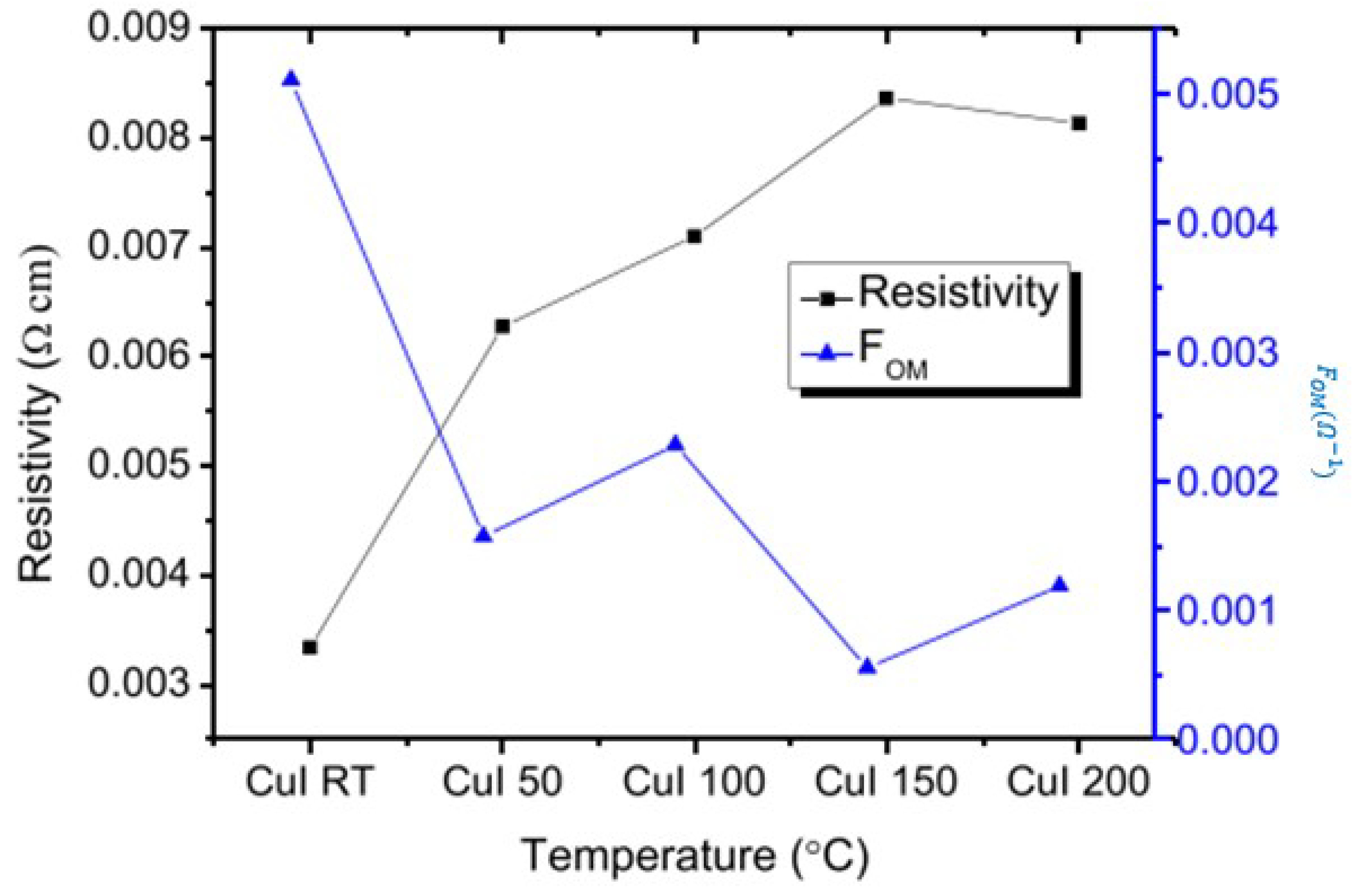
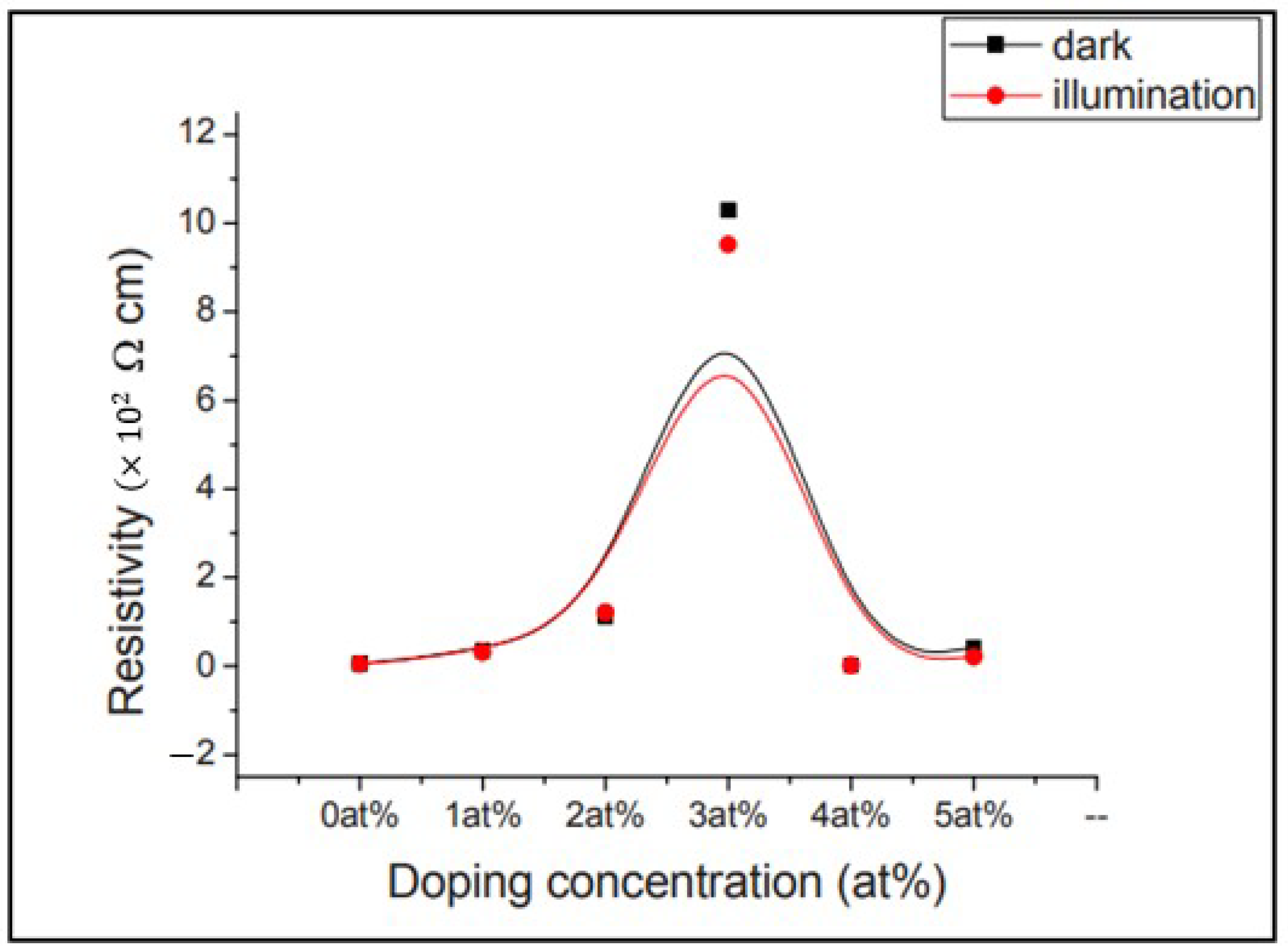
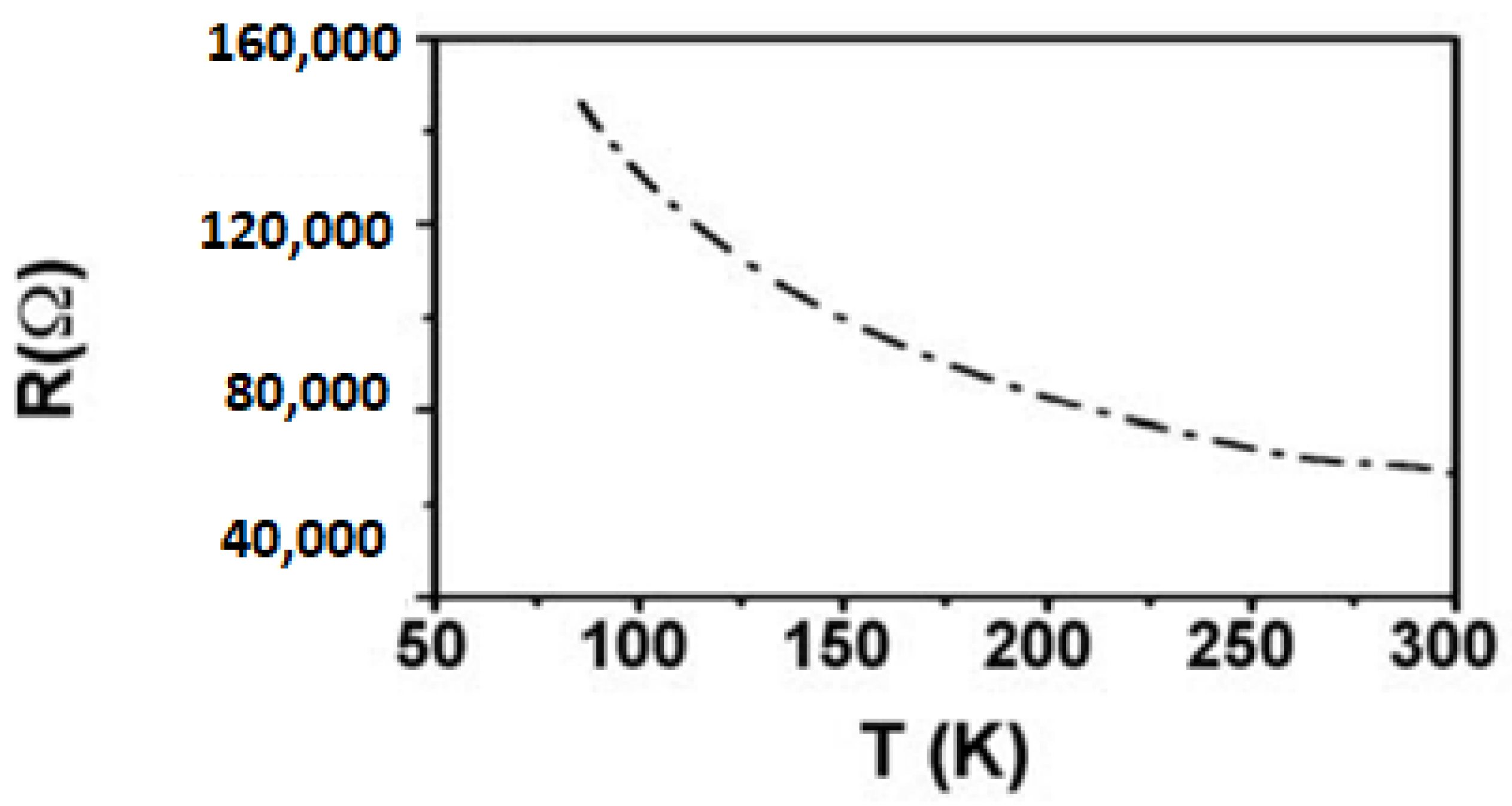
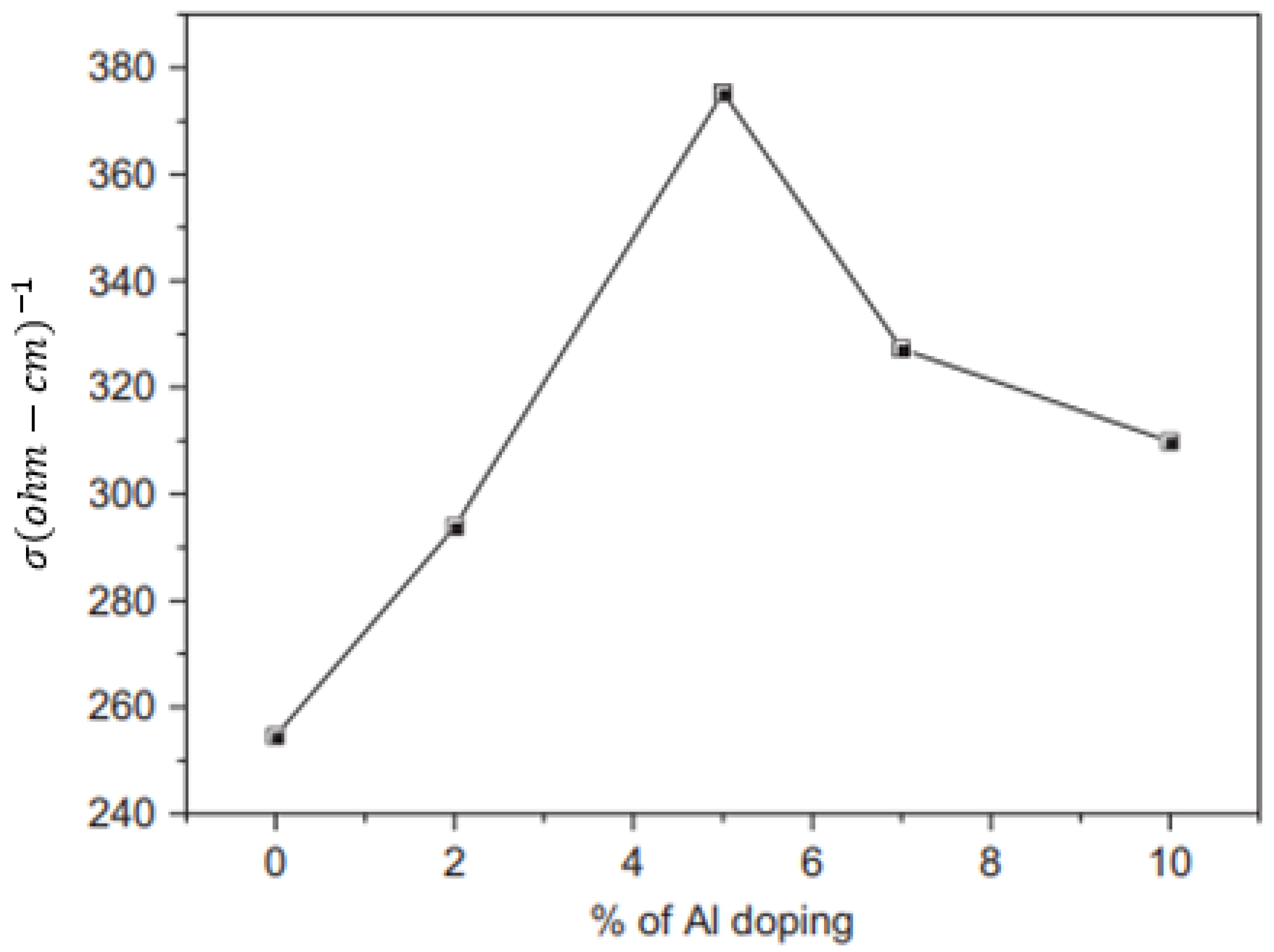
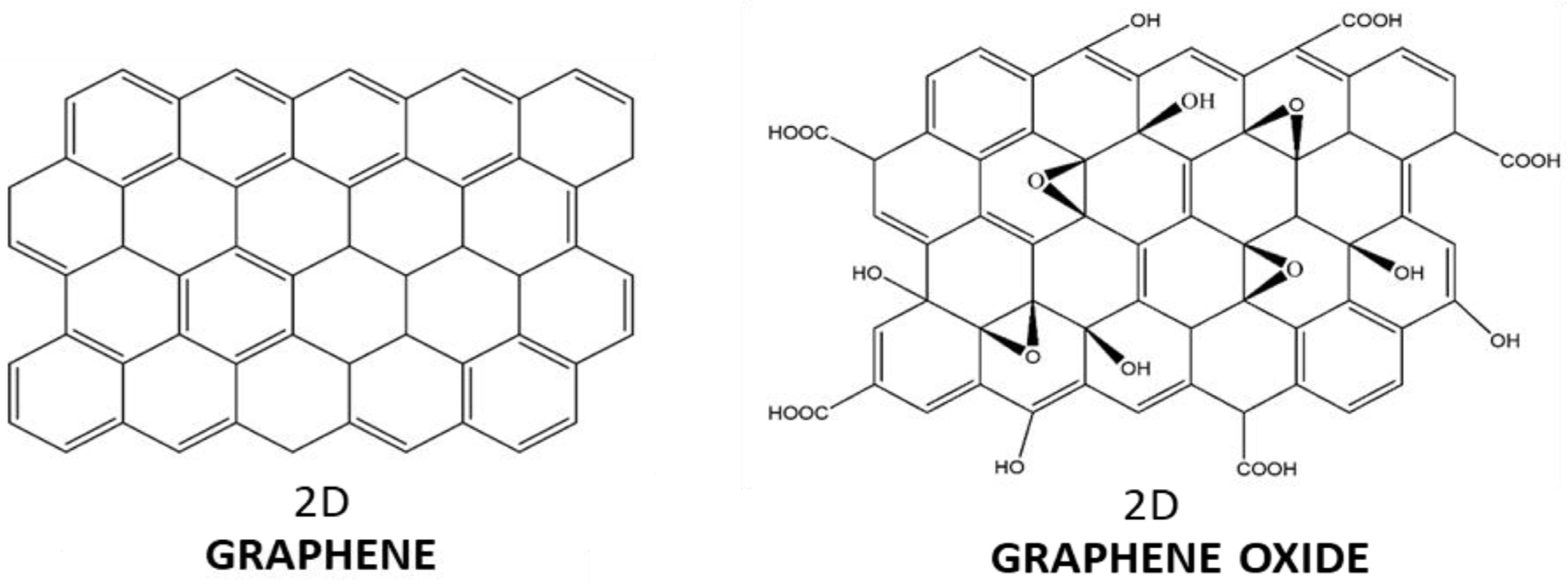
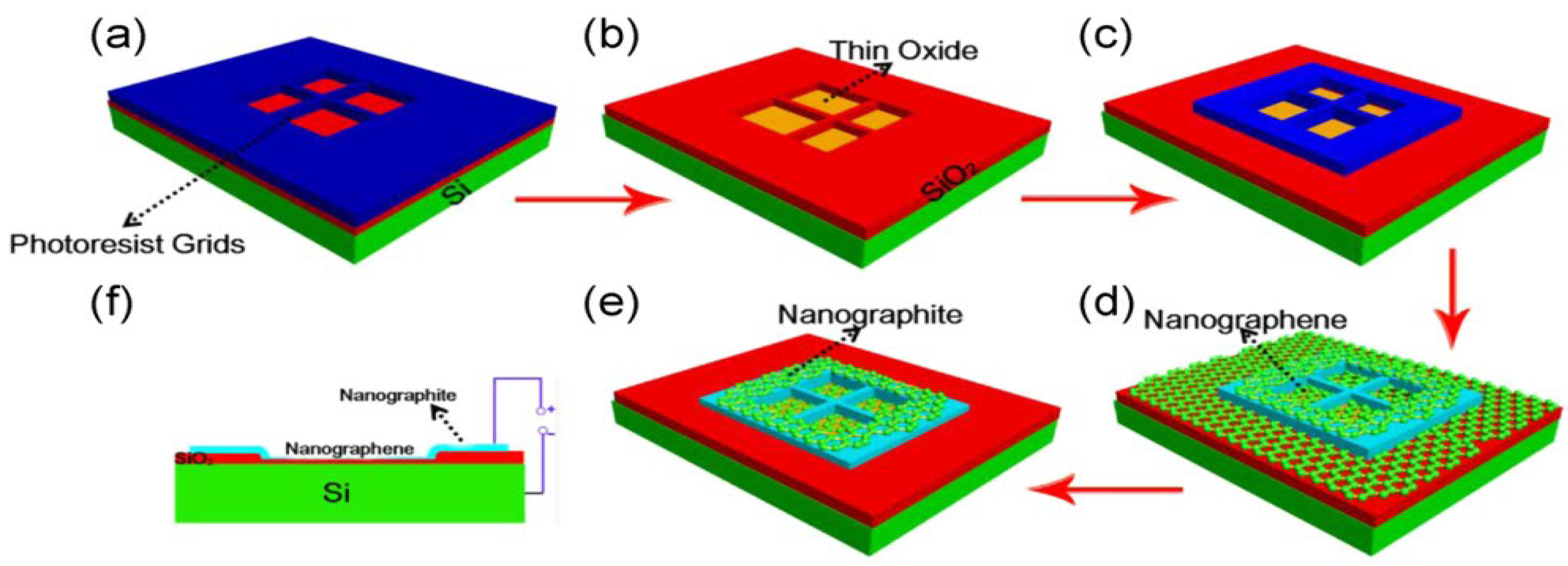
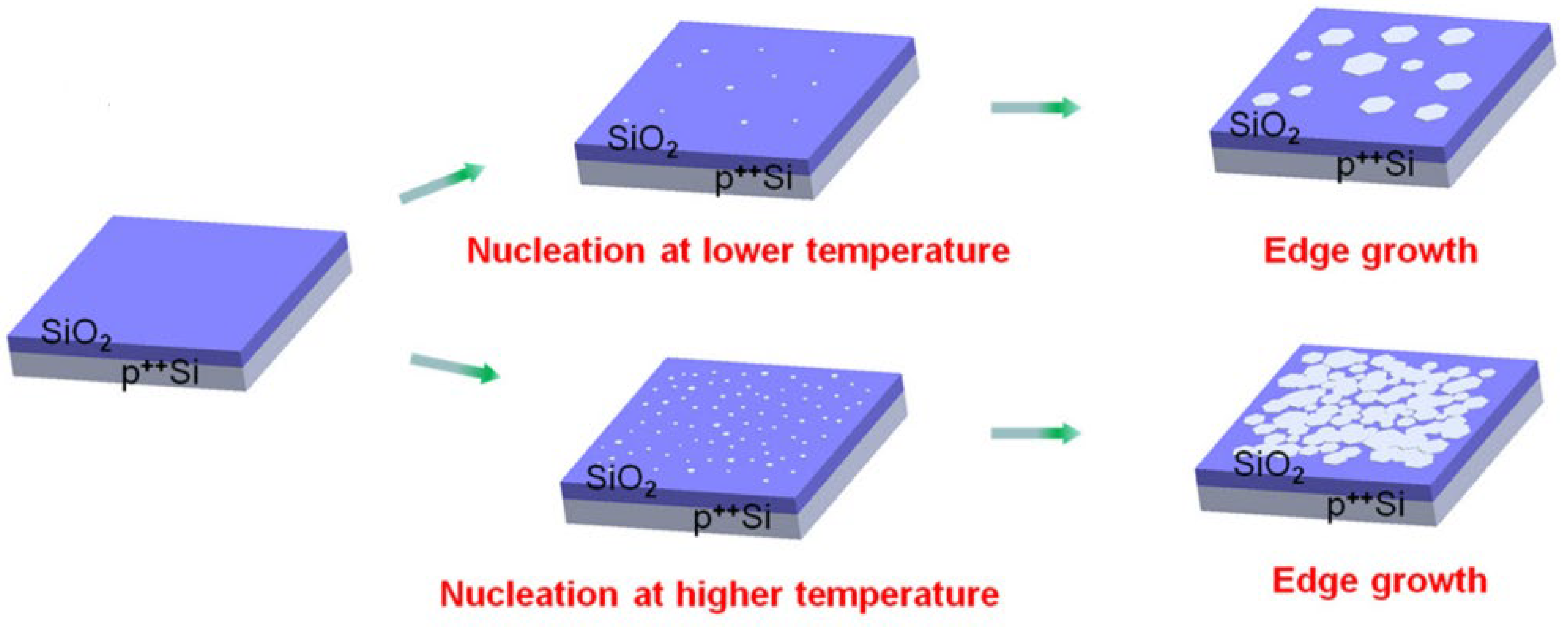
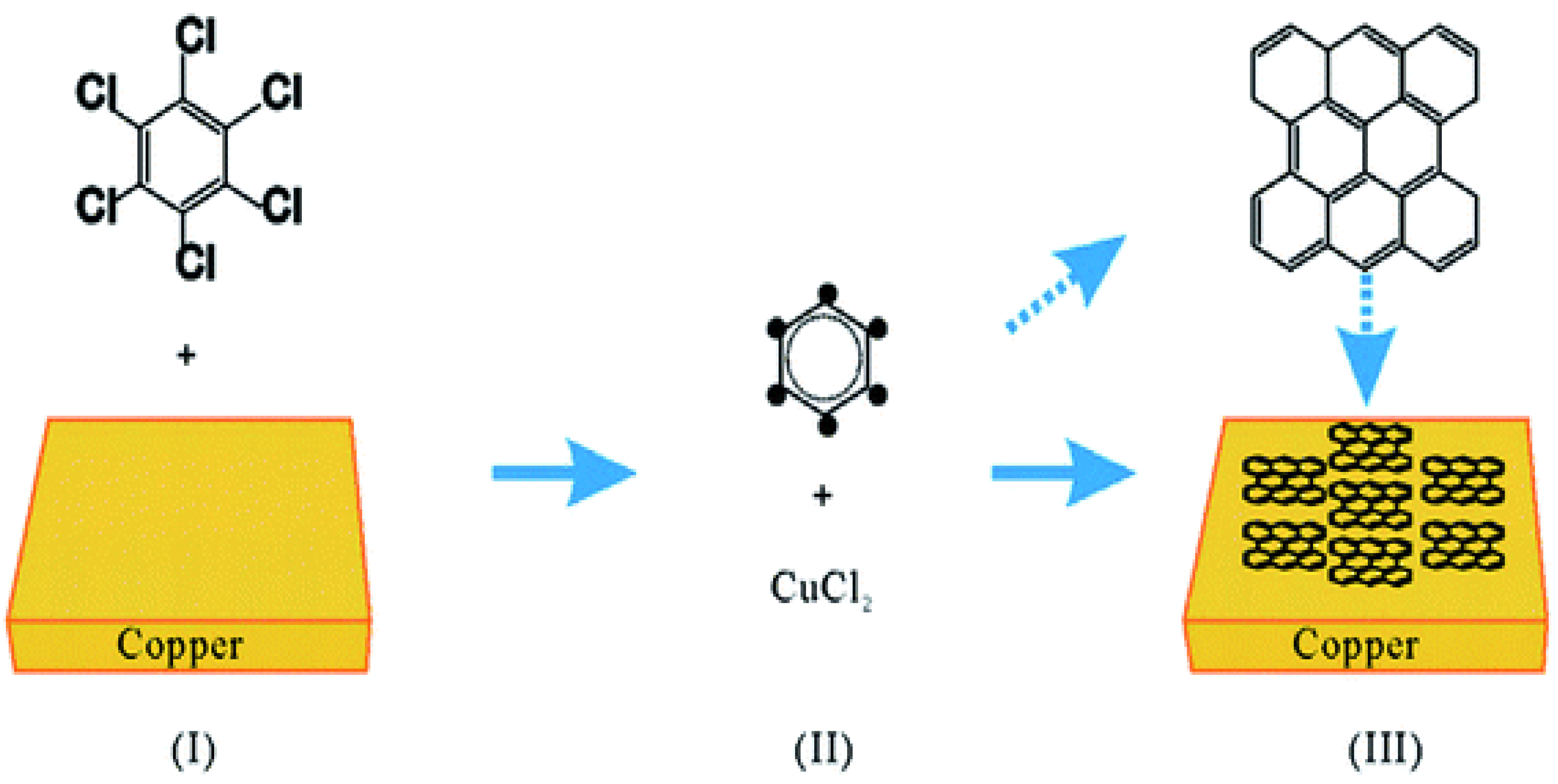
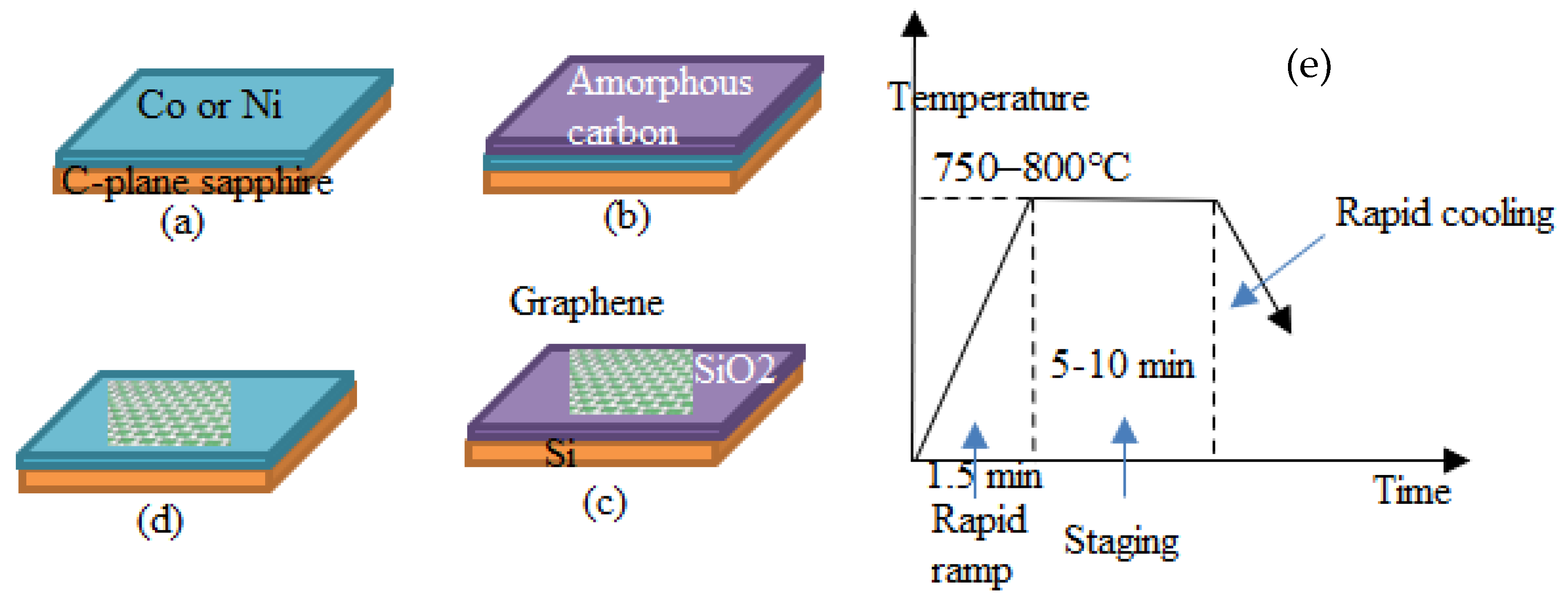

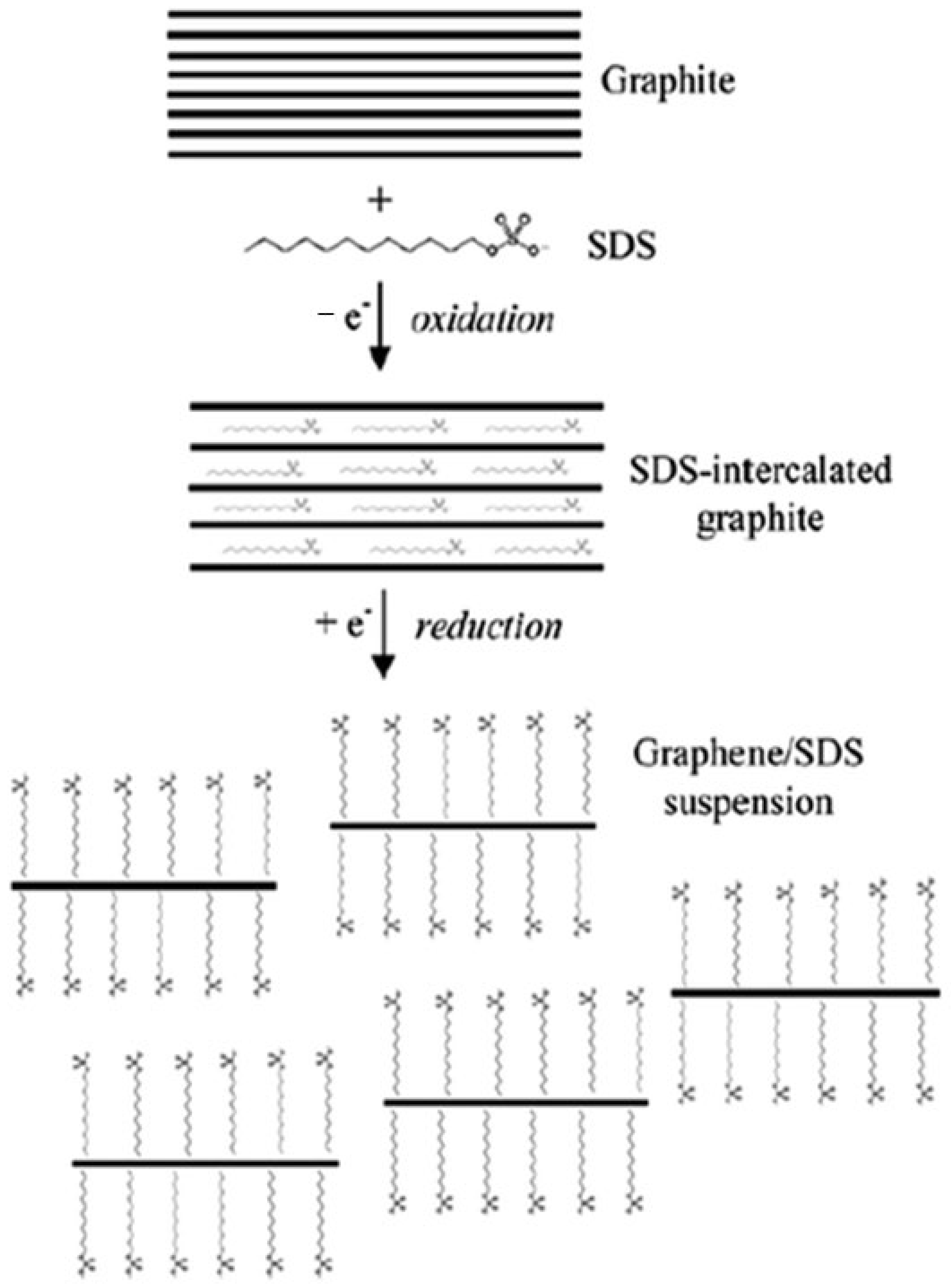
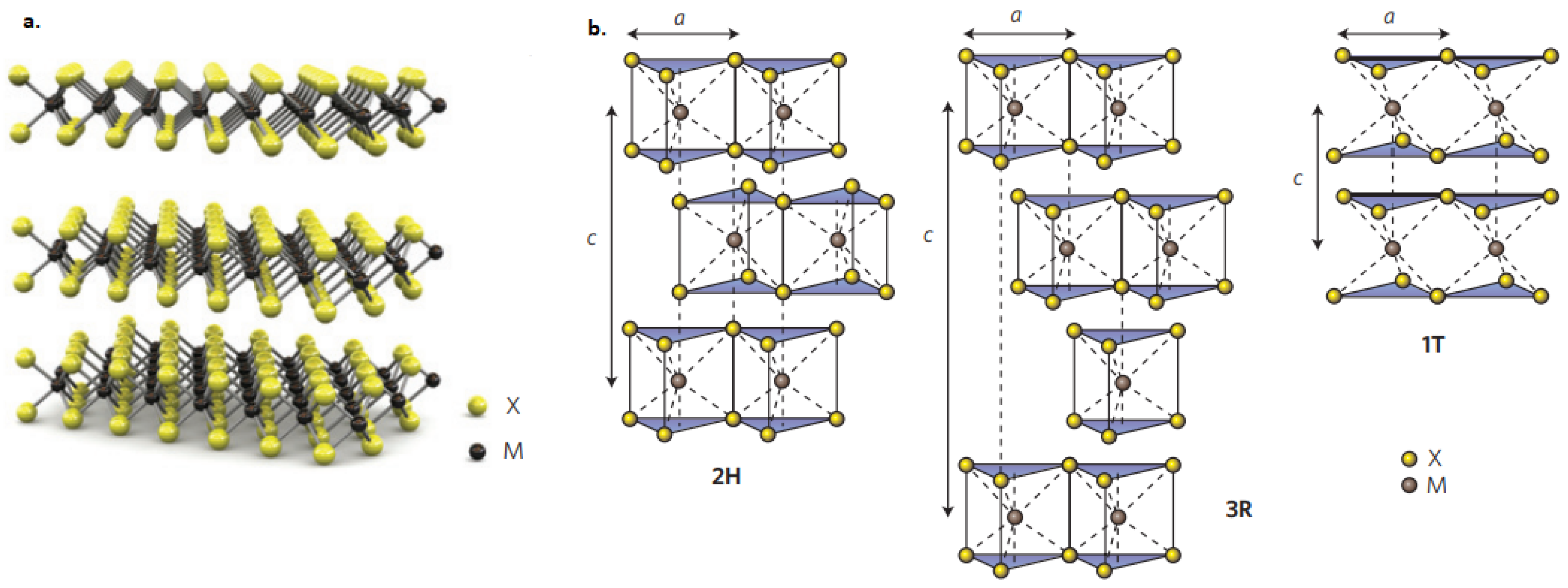



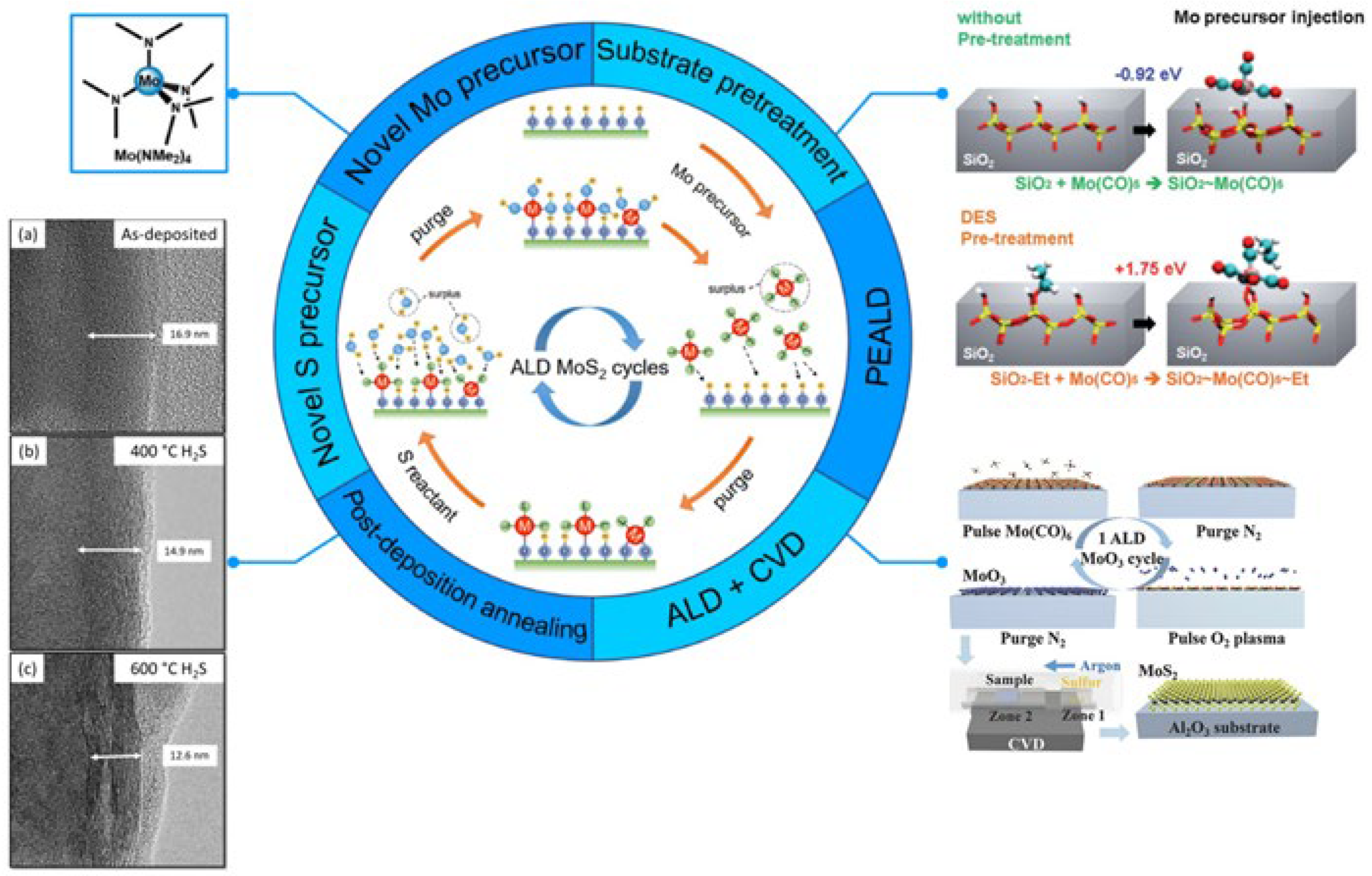
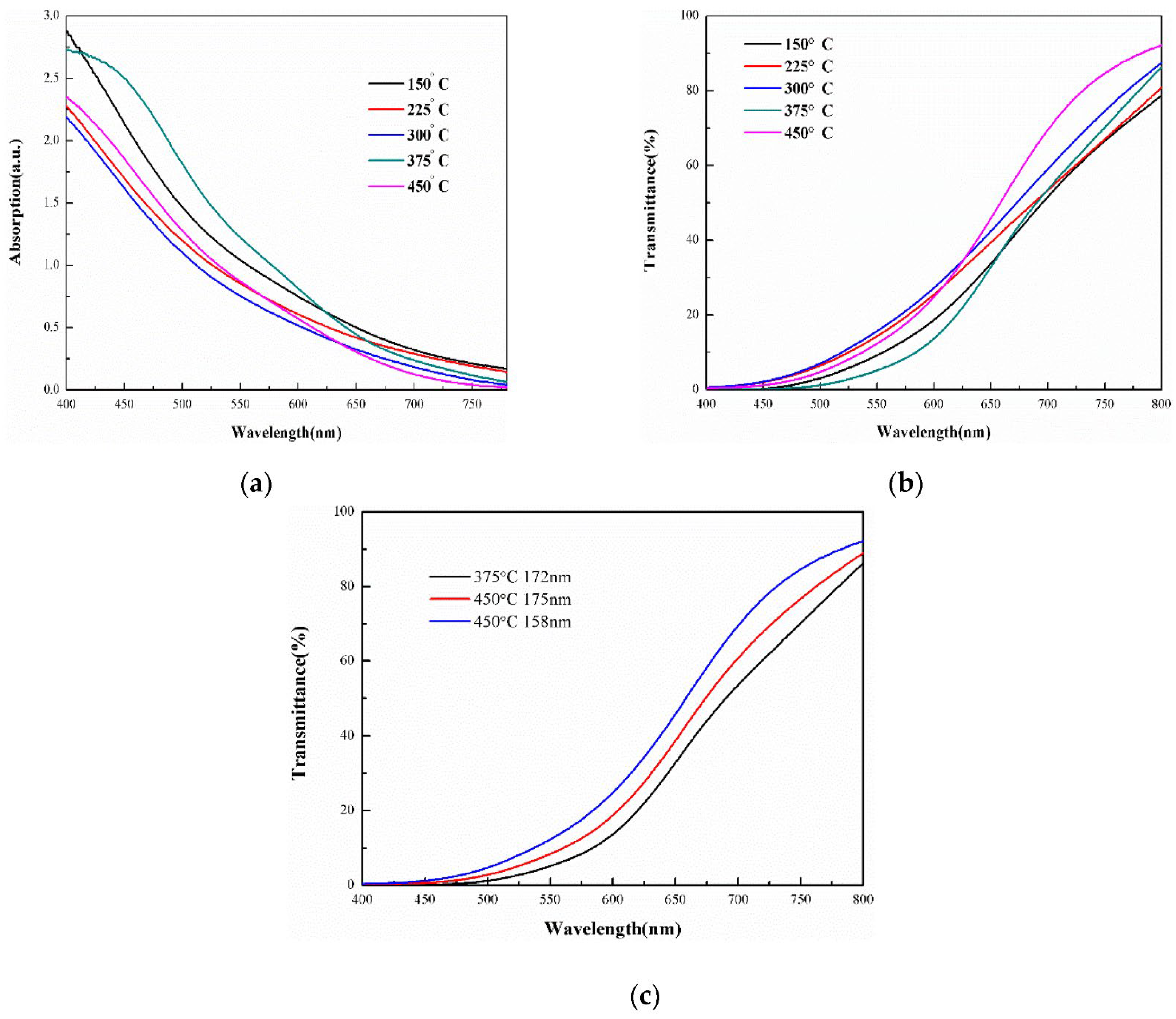
| Vacuum-based | Physical Vapor Deposition |
| Low-Pressure Chemical Vapor Deposition | |
| Plasma-Enhanced Chemical Vapor | |
| Hot-wall and cold-wall CVD | |
| Atomic Layer Deposition | |
| Solution-Processed (non-vacuum) | Atmospheric Pressure Chemical Vapor Deposition |
| Chemical Bath Deposition | |
| Spin-coating | |
| Electrodeposition | |
| Sol–gel Process | |
| Spray Pyrolysis | |
| Anodization Process | |
| Mixed Category | Metal–Organic Chemical Vapor Deposition |
| Deposition process | Resistance heating evaporation | Laser evaporation/laser ablation | MBE | EBE | Thermal evaporation |
| Source of energy | Electrical resistance heating | Laser | E-beam or thermal heating | Electron beam bombardment | Thermal Heating |
| Substrate heating | Generally, not required | Heating can be required | Generally, not required | Generally, not required | Heating can be required |
| Commonly used substrate | Quartz [74], Glass [75], silicon [76] | Glass [77,78], Silicon [79,80] | GaAs [81], Silicon Wafer [82] | Glass [83] | Glass [84], Silicon [85] |
| Rate of deposition | Moderate to high | High | Moderate to high | High | Moderate to high |
| Uniformity and deposition quality | Good | Good | Excellent | Good | Good |
| Control of film thickness | Good | Good | Excellent | Excellent | Good |
| Vacuum requirements | High (~10−6 Torr) [86] | High | High vacuum to Ultra-high (10−8 to 10−12 Torr) [87] | High (7.5 × 10−5 Torr) [88] | High (~10−6 Torr) [86] |
| Complexity in the process | Mostly low to moderate | Moderate | Generally high | Moderate | Mostly Low to moderate |
| Sputtering Method | Thin-Film Quality | Key Characteristics |
|---|---|---|
| RF Sputtering | Strong adhesion and high homogeneity; similar to Magnetron but more versatile for substrates that are heat sensitive. | Versatile, suitable for heat-sensitive substrates [118,120]. |
| Reactive Sputtering | Controlled stoichiometry is better than DC but less effective than Magnetron; it is perfect for compound synthesis. | Precise control over the composition of the film, particularly for nitrides and oxides [106]. |
| Ion Beam Sputtering | Finer control than RF and Reactive; exceptional precision, very uniform, and defect-free. | Ideal for films without defects, with the best control over film characteristics [123,124]. |
| DC Diode and Triode Sputtering | Good homogeneity and adherence, optimal for conductive materials, limited control over complex formulations. | Effective for conductive films, easy to set up [117]. |
| Magnetron Sputtering | Better than DC in complicated formulations, it combines scalability and quality with high uniformity, great adhesion, and efficient deposition. | Scalable, effective, and featuring improved control over ionization [128,129]. |
| Classification Type | Different Anodization Methods |
|---|---|
| Electrolyte type | Chromic Acid Anodizing |
| Sulfuric Acid Anodizing | |
| Hard Anodizing or Hardcoat Anodizing | |
| Phosphoric Acid Anodizing | |
| Oxalic Acid Anodizing | |
| Anodization Conditions | Low-Voltage Anodization |
| High-Voltage Anodization | |
| Temperature of the Anodization Process | Room-Temperature Anodization |
| Low-Temperature Anodization | |
| High-Temperature Anodization |
| TCFs | Authors | Deposition Methods | Major Applications |
|---|---|---|---|
| CuI | Shi et al. [55], Moditswe et al. [54], Kaushik et al. [56] | Thermal evaporation | Nanowires (Nanodevices and Sensors), Nanoscale electric circuits, Solar cells |
| Tanaka et al. [187], Yang et al. [188] | Sputtering | ||
| Sirimanne et al. [189], Zhu and Zhao [190] | PLD | ||
| Amalina et al. [191] | Spray pyrolysis | ||
| Sankapal et al. [192], Kui Zhao [193] | CBD | ||
| Inudo et al. [194] | Spin-coating | ||
| ITO | Du et al. [195] | Thermal evaporation | CIGS solar cells, Batteries, Flat panel displays, Smart windows, LEDs, Optoelectronic devices |
| Chu et al. [196], REN et al. [197], Parida et al. [198], Liu et al. [199], Gwamuri et al. [200] | Sputtering | ||
| Fallah et al. [201], Senthilkumar et al. [202] | EBE | ||
| Rozati et al. [203], Parthiban et al. [204] | Spray pyrolysis | ||
| Ma et al. [205] | Reactive evaporation | ||
| Adurodija [206] | PLD | ||
| Savu et al. [70], Coutal et al. [207], Kim et al. [208], Ngaffo et al. [71] | Laser ablation | ||
| Machet et al. [209] | Ion Plating Technique | ||
| AZO | Gareso et al. [210], Mia et al. [211], Silva et al. [212], MAMAT et al. [213], Alam et al. [214] | Sol–gel | Solar Cells, OLED displays, General illumination, Transparent heaters |
| Caglar et al. [215], Muiva et al. [216], Babu et al. [217], Lee et al. [218] | Spray pyrolysis | ||
| Barhoumi et al. [219], Leem et al. [220], Mosbah et al. [221], Miao et al. [222], Reddy et al. [223], Minami et al. [224], Wang et al. [225], Suzuki et al. [226], Ghorannevis et al. [227], SHEN et al. [228], Fang et al. [229] | Sputtering | ||
| Kaur et al. [230] | PLD | ||
| Tynell et al. [231] | ALD | ||
| Si-doped GaAs | Tok et al. [232], Ploog et al. [233], Georgakilas et al. [234], Galiev et al. [235], Cortas et al. [236], Briones et al. [237], Skromme et al. [238], Li et al. [239], Ruhstorfer et al. [240], Shimanoe et al. [241] | MBE | High-speed electronic devices, Telecommunications, Optical fiber communication, Photovoltaic applications, Monolithic Microwave Integrated Circuits (MMICs), Integrated circuits |
| Müller et al. [242] | PLD | ||
| Şenay et al. [243] | Thermionic Vacuum Arc | ||
| TiN | Pomar et al. [244], Kavitha et al. [245], Solovan et al. [246], Yang et al. [247], Jeyachandran et al. [248], Yu et al. [249], Kharitonov et al. [250], Mascaretti et al. [251], Ponon et al. [252], Elstner et al. [253], Su et al. [254], Saoula et al. [255] | Sputtering | Decorative coatings, Tool coatings, Hard coatings, Dental tools, Surgical instruments, Infrared filters |
| Chou et al. [256] | Ion plating | ||
| Snyder et al. [257] | ALD | ||
| Ga doped ZnO | Yamada et al. [258], Yamamoto et al. [259] | Ion plating | LEDs, solar cells, Gas sensors, wearable electronics, Photovoltaic devices, Flexible displays and electronics |
| Carrier concentration | High, around 9.41 × 1019 cm3 [269] | High, in the range of ∼1020 to ∼1019 cm−3 [270] | High | High, carrier density is found in the range of 1015 to 1019 cm−3 [271] | High, mobilities around 0.5–2 cm2 V−1 s−1 [194] | High, around 1.07 × 1021 cm−3 [272] |
| Electrical resistivity | Zhou et al. found that the lowest electrical resistance was 3.2 × 102 Ω cm with 1% Al [269] | Low, 2−4 × 10−4 Ω cm [273] | Single crystal TiN shows resistivity of 23 μΩ, whereas polycrystalline shows 1000 μΩ [274] | As low as −2.6 × 10−3 Ω cm is obtained [275] | The resistivity is of the order of 10−2 Ω cm [56,196] | Low around 1.84 × 10–4 Ω cm [276] |
| Intrinsic/Extrinsic | Extrinsic | Extrinsic | Intrinsic | Extrinsic | Intrinsic | Extrinsic |
| Bandgap | 3.3–3.4 eV, direct bandgap [221,224] | , direct bandgap [277,278,279,280,281] | 3.35–3.45 eV, direct bandgap [250] | Depending on the doping concentration, it shows the Burstein—Moss shift. Typically have narrow band direct bandgap of 1.25 eV [247] | , direct bandgap [282] | [283] |
| Transparency region | High optical transmittance in the near-infrared. Region approximately 300–1500 nm [284] | Transparent in the visible spectrum. Region approximately 400–700 nm [285] | Opaque, low transmittance in the visible spectrum. Region approximately 350–800 nm [286] | High transparency over a wide spectrum. Region approximately 800 nm to 1.7 μm [287] | Extremely high transmittance (>70%) in the visible region, approximately 400–700 nm [288] | Highly transparent, shows >80% visible light (400–800 nm) transmittance [289] |
| Material | Zinc oxide with aluminum dopant | INO with tin dopant | Titanium and nitrogen | Gallium arsenide with silicon Si dopant | Copper and iodine | Zinc oxide with gallium dopant |
| Thin film | AZO | ITO | TiN | Si-doped GaAs | CuI | GZO |
| Thin Films | Factors | Effects on Transmittance | Authors |
|---|---|---|---|
| AZO | Doping | With increasing doping concentrations, the transmittance usually decreases. | Wang et al. [347], Alam and Cameron [214], Babu et al. [217] |
| Lee et al. [350] | ||
| Peng et al. [382], Caglar et al. [215] | ||
| Babu et al. [217] | ||
| Temperature | ZnO: Al films exhibit enhanced optical transmission in the visible spectrum upon annealing at elevated temperatures. | Alam and Cameron [214] | |
| Babu et al. [217] | ||
| Barhoumi et al. [219] | ||
| Gao et al. [348], Takci et al. [349] | ||
| Thickness | Transmittance may decrease due to increased light absorption in the film caused by thickness. | Subba Reddy et al. [223], Lin et al. [346] | |
| The transmittance dropped to 50–70% from 70–90% when the deposited film thickness increased. | Hoon et al. [346] | ||
| The films’ optical transmittance rose as the film thickness increased to 231 nm and dropped to a higher thickness of 398 nm. | Subba Reddy et al. [223] | ||
| Surface morphology |
| Babu et al. [217] | |
| Coating |
| Silva and Darbello Zaniquelli [212] | |
| Tilted angle | Despite the distorted structure, an average transmittance of >90% was achieved, demonstrating the generally high transparency of AZO film on a glass substrate. | Leem and Yu [220] | |
| ITO | Film thickness |
| Eshaghi and Graeli [278] |
| Maniscalco et al. [351] | ||
| Changing compositions |
| Senthilkumar et al. [202] | |
| Grain size |
| Senthilkumar et al. [202], Ogihara et al. [353], Katsube et al. [354] | |
| Deposition power and time |
| Tchenka et al. [325] | |
| CuI | Deposition technique |
| Kaushik et al. [56], Potts et al. [356], Burns et al. [357] |
| Temperature |
| Zi et al. [359], Zhu and Zhao [190] | |
| Moditswe et al. [54] | ||
| Doping concentration |
| Amalina et al. [191], Muiva et al. [216] | |
| Polymer | Wavelength | 20% of the composites are the ideal concentration, resulting in films with good visual transparency in polymeric PMMA that are 0.1 mm thick. | Carboni et al. [361] |
| Doping concentration |
| Indolia and Gaur [362] | |
| Polymer type and molecular structure | Polyethylene has a linear molecular structure comprising repeated ethylene units (-CH2-CH2-), so light can travel through it without absorption or scattering, resulting in outstanding optical transmittance. | Lahlouh [364] | |
| Zhou and Burkhart [365], Zhao et al. [366] | ||
| PMMA thin films have a homogeneous molecular structure and little chain branching or crosslinking; as a result, they offer optical clarity and high transmittance throughout the visible spectrum | Klinger et al. [367] | ||
| Film thickness |
| Good et al. [368], Tsilingiris [369] | |
| As the PET film thickness rises, the polymer material absorbs more incident light, lowering optical clarity and transmittance. | Zhang and Zhang [370] | ||
| INO | Temperature |
| Solieman [372] |
| Beena et al. [342] | ||
| As substrate temperature rises, the films’ transmittance also increases. | Gupta et al. [344] | ||
| Senthilkumar et al. [345] | ||
| Crystallization improves the electrical and transmittance qualities of thin films. | Han et al. [373] | ||
| Doping concertation |
| Alqahtani et al. [374] | |
| CdO | Oxygen pressure |
| Beena et al. [342] |
| Temperature |
| Ullah et al. [333] | |
| When a film is annealed at a higher temperature, the absorption edge changes to higher wavelengths. | Santos-Cruz et al. [334] | ||
| Doping concentration | With 10 wt. % Al doping, the transparency falls between 70 and 80 percent in the visible spectrum. | Saha et al. [377] | |
| Manjula et al. [378], de Biasi and Grillo [379] | ||
| Adding Al dopant to CdO thin films improves transmittance. The transmittance of the undoped films is 62%, which increased to 77% with 4 wt. % Al doping. | Kumaravel et al. [335] | ||
| Ziabari et al. [339] | ||
| Velusamy et al. [340] | ||
| Usharani et al. [338] |
| Thin Films | Factors | Effects on Electrical Resistivity and Conductivity | Authors |
|---|---|---|---|
| AZO | Doping |
| [214] |
| Goyal et al. [406] | ||
| Babu et al. [217] | ||
| Muiva et al. [216], Lee and Park [445], Nunes et al. [446], Gómez-Pozos et al. [410] | ||
| Surface morphology | Surface leakage reduces the accuracy, impacts, and other characteristics of a high-resistive film’s resistivity values. | Majumder et al. [323] | |
| Temperature | The resistivity decreased with increasing temperature up to 500 °C; after that, the resistivity began to increase. | Alam et al. [214] | |
| Ning et al. [213] | ||
| Babu et al. [217] | ||
| Deposition technique | According to the study analysis, the sol–gel films perform significantly better. | Alam et al. [214] | |
| Film thickness |
| Achour et al. [413] | |
| ITO | Film thickness | Conductivity increases as grain size and grain boundary decrease, reducing resistivity. | Eshaghi and Graeli [278], Ohhata et al. [405] |
| Thinner films might be more vulnerable to substrate effects, which could alter the film’s resistivity. | Bélanger et al. [416] | ||
| Power and deposition time | As a function of power, the rise in carrier concentration causes the resistivity to grow. | Yu et al. [417] | |
| Similar electrical experiments showed that the resistivity falls when the RF power and the deposition time increase. | Tchenka et al. [325] | ||
| Temperature |
| Tuna et al. [324] | |
| Grain size |
| Tina et al. [324], Nishimoto et al. [419] | |
| Bandgap | As substrate temperature rises, the bandgap increases, and resistivity falls. | Geng et al. [420]. | |
| Sputtering voltage |
| Ishibashi et al. [421], Sunde et al. [415] | |
| Carrier concentration |
| Tchenka et al. [325] | |
| CuI | Temperature |
| Kaushik et al. [56], Inudo et al. [194], Zhu and Zhao [190] |
| Zi et al. [359] | ||
| The resistivity rises to the order of 1 Ω cm as the substrate temperature rises to 350 °C. | Zhu and Zhao [190] | ||
| The CuI thin-film resistivity increased with increased annealing temperature. | Moditswe et al. [54] | ||
| Cu/I ration |
| Rusop et al. [425], Tanaka et al. [187], Tennakone et al. [426] | |
| Addition of oxygen |
| Herrick and Tevebaugh [427] | |
| Doping concentration |
| Amalina et al. [191] | |
| Perera et al. [428], Amalina et al. [191] | ||
| High optical conductivity and lower electrical resistance were reported for CuO thin films doped with Zn. | Nesa et al. [429] | ||
| Ti dopant improves the conductivity of the films while maintaining the samples’ optical qualities and crystallinity. | Saied et al. [430] | ||
| As the Ni concentration of the CuO thin film increases, both the optical bandgap and electrical resistance increase. | Uddin et al. [431] | ||
| INO | Oxygen pressure | The INO films created in an oxygen-free atmosphere show a low value of electrical resistivity. | Beena et al. [342], Yamada et al. obtained [435] |
| A lower DC electrical resistivity value (1.7 × 10−6) Ω m for INO films created at an oxygen pressure of 10−3 mbar has been found. | Gupta et al. [436] | ||
| Solieman [372] | ||
| Temperature | With the increasing temperature, the resistivity decreases. | Beena et al. [342] | |
| Ti-doped Al2O3 | Doping |
| Yang et al. [439], Unno et al. [440] |
| CdO | Doping |
| Saha et al. [377], Maity and Chattopadhyay [444] |
| Deposition technique |
| Saha et al. [377], Maity and Chattopadhyay [444] |
| Authors | Challenges Addressed | Possible Solutions | |
|---|---|---|---|
| Issues | Abbreviation | ||
| Naghdi et al. [30] | Sintering process and substrate compatibility | The selection of substrates is limited by the sintering process’s temperature requirements, which are necessary to enhance the conductivity of thin coatings. Although raising the sintering temperature can improve conductivity, it also increases the risk of pore formation and reduced functionality. To increase the variety of substrates, the sintering temperature must be lowered. | Reducing the size of nanomaterials can increase film conductivity, increase substrate possibilities, and decrease the sintering temperature. |
| Problem associated with film thickness | A thicker film has better electrical conductivity but less optical transparency. The ideal thickness must be determined to balance these opposing qualities. | Determining the ideal thin-film thickness that balances optical transparency and electrical conductivity should be maintained. | |
| Aspect ratio | Increasing the aspect ratio lowers the percolation threshold, which is essential for cutting production costs without sacrificing mechanical qualities. | Conductive metal nanoparticles can be incorporated into polymers or mixed with other nanomaterials to generate hybrid materials with improved mechanical properties, conductivity, and customized functionality. | |
| Adhesion problem | Poor adhesion between the substrate and the thin film during deposition can cause problems when the film is stretched or bent, jeopardizing its integrity and performance. | By employing polydopamine, the substrate’s wettability can be changed. The hydrophilic condition of the substrate surface facilitates the deposition of Ag NWs, which improves the adhesion between the thin film and the elastomeric substrate. This method may create a transparent Ag NW thin film with improved adhesion, excellent optical transparency, minimal sheet resistance, and stability, even after the sample has been stretched. | |
| Litzelman et al. [545] | Variations in the properties | The characteristics of nanostructured electrode and electrolyte components produced using thin-film fabrication techniques can differ greatly from those of bulk materials. | Minimizing differences in characteristics from bulk materials by improving thin-film fabrication methods. |
| Variations in ionic conductivity | The performance of SOFCs can be affected by changes in ionic conductivity caused by internal interfaces within the nanostructured components. | Designing interfaces to improve ionic conductivity or reduce adverse effects, maybe by changing the materials’ surface or chemistry. | |
| Long-term stability | Increased cation diffusion along grain boundaries may affect the devices’ long-term stability. Enhancing membranes’ mechanical stability and thermomechanical dependability, especially when exposed to greater temperatures and for extended periods of time, is crucial. | Finding the sources of lingering tensions and the progression of stresses in the films is crucial to achieving this. Furthermore, using stress-tolerant designs is a possible remedy to alleviate these problems. | |
| Zhang et al. [546] | Achieving high performance and stability at a low cost | The technical challenge is to minimize costs while maintaining high performance and stability. This requires creating large cells with low grain boundary resistance and thin, gas-tight electrolytes. | Using theoretical insights from density functional theory investigations, and machine learning can be used in material design to expedite the process and find useful characteristics for innovative materials. |
| Kalinina et al. [547] | Creating conductivity on non-conductive substrates | Conductivity must be established on the surface of non-conductive substrates in order to apply electrophoretic deposition (EPD). | Developing methods for the successful deposition of the Ce0.8Sm0.2O1.9 (SDC) electrolyte film, such as the formation of conductive sublayers such as PPy and Pt on the front surface of the non-conductive substrate. |
| Mechanical stresses in the formation of bilayer films | The EPD of CuO-modified BaCe0.5Zr0.3Y0.1Yb0.1O3−δ (BCZYYbO-CuO/SDC) and SDC films on reduced Ni-SDC substrates results in significant mechanical strains that cause cracks in the bilayer film and disintegration of the single SDC film during the oxidative co-sintering process that follows. | Changing the anode substrate’s composition or altering the deposition process’s characteristics | |
| Patil et al. [548] | Achieving high energy density and power density | A difficulty in developing lithium-based thin-film rechargeable batteries is achieving higher energy and power densities without sacrificing appropriate power densities. | By increasing the energy density of thin-film batteries, concentrating on technology development and improvements in the electrode process, within the next five to six years, it is possible to obtain energy densities exceeding 500 Wh/L and 200 Wh/kg by investigating novel active materials and electrode technologies. |
| Liu and Wöll [549] | Thin-film quality | For MOF thin films to find widespread use, their surfaces must be smooth, crystalline, aligned, compact, uniform, and free of pinholes, yet this is still a difficult task. | The film can be improved and oriented by employing techniques such as ALD and Liquid Phase Epitaxy (LPE). |
| Defect control | Flaws must be controlled for MOF thin films to function as well as possible, yet characterizing and managing defects is difficult. | Advanced characterization methods, such as electron microscopy, spectroscopy, and synchrotron X-ray diffraction, can be utilized to study and manage flaws in MOF thin films. Techniques like “defective linkers” or controlled heating introduce defects in a controlled manner. | |
| Enhancing electrical conductivity | The intrinsic features of most <span id=“zotero-drag”/> MOFs make them poor electrical conductors, which restricts their use in energy storage systems. | Techniques include loading organic guest molecules into MOF voids, creating conductive polymers inside MOF frameworks, or creating MOFs with intrinsic conductive qualities by utilizing highly conjugated linkers, which can all be used to improve electrical conductivity. | |
| Eslamian [550] | Development of Continuous and Integrated Thin Films | Spray coating presents a difficulty in producing thin layers that are consistent, high-quality, and integrated. The efficiency of current approaches is frequently lower than that of spin-coating, indicating a need for improved spray coating techniques. | Applying spray-coating-specific optimal processing conditions can improve the homogeneity and quality of thin films. Fine-tuning variables can enhance film morphology and device performance, including substrate temperature, nozzle distance, and spray rate. |
| Effective Charge Separation, Transfer, and Collection | One of the largest challenges in spray-on layers is to ensure effective charge separation, transfer, and collection. | Using numerous spray passes increases the device’s overall efficiency by improving the coating’s thickness and coverage. Iterative deposition methods enable improved control over the film’s shape and characteristics. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharme, R.K.; Quijada, M.; Terrones, M.; Rana, M.M. Thin Conducting Films: Preparation Methods, Optical and Electrical Properties, and Emerging Trends, Challenges, and Opportunities. Materials 2024, 17, 4559. https://doi.org/10.3390/ma17184559
Sharme RK, Quijada M, Terrones M, Rana MM. Thin Conducting Films: Preparation Methods, Optical and Electrical Properties, and Emerging Trends, Challenges, and Opportunities. Materials. 2024; 17(18):4559. https://doi.org/10.3390/ma17184559
Chicago/Turabian StyleSharme, Razia Khan, Manuel Quijada, Mauricio Terrones, and Mukti M. Rana. 2024. "Thin Conducting Films: Preparation Methods, Optical and Electrical Properties, and Emerging Trends, Challenges, and Opportunities" Materials 17, no. 18: 4559. https://doi.org/10.3390/ma17184559
APA StyleSharme, R. K., Quijada, M., Terrones, M., & Rana, M. M. (2024). Thin Conducting Films: Preparation Methods, Optical and Electrical Properties, and Emerging Trends, Challenges, and Opportunities. Materials, 17(18), 4559. https://doi.org/10.3390/ma17184559








