Investigating the Effects of Transition Metals and Activated Carbon on Hydrogenation Characteristics of Severely Deformed ZK60 Processed by High-Energy Ball Milling
Abstract
1. Introduction
2. Experimental Procedures and Methods
3. Results and Discussion
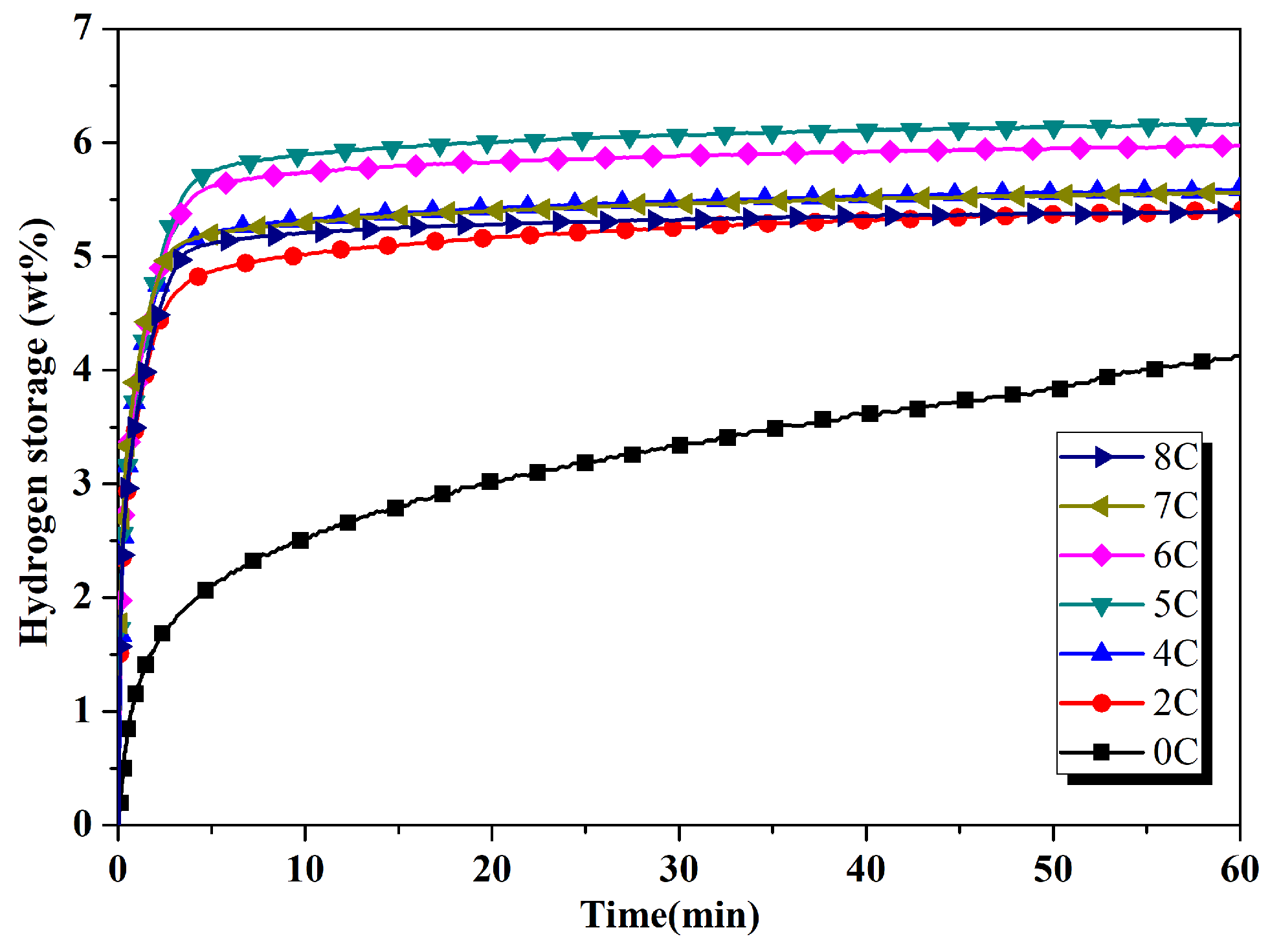
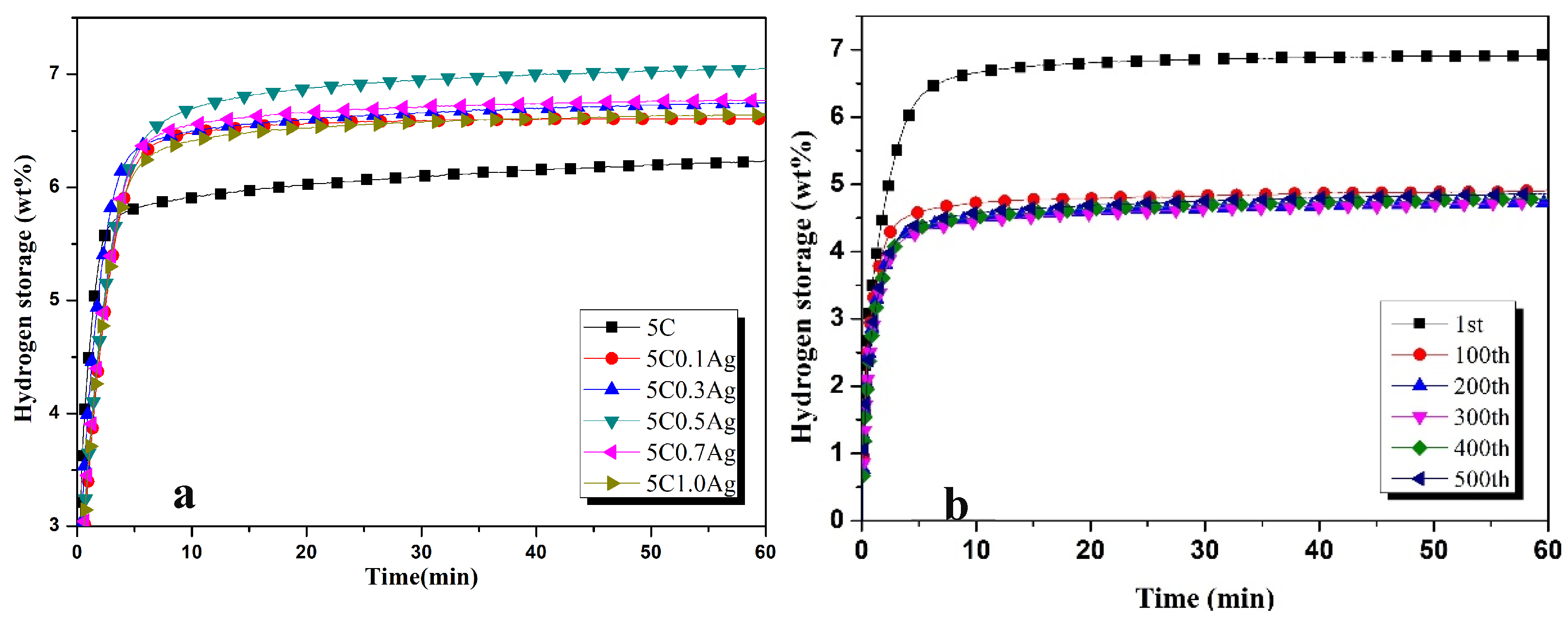
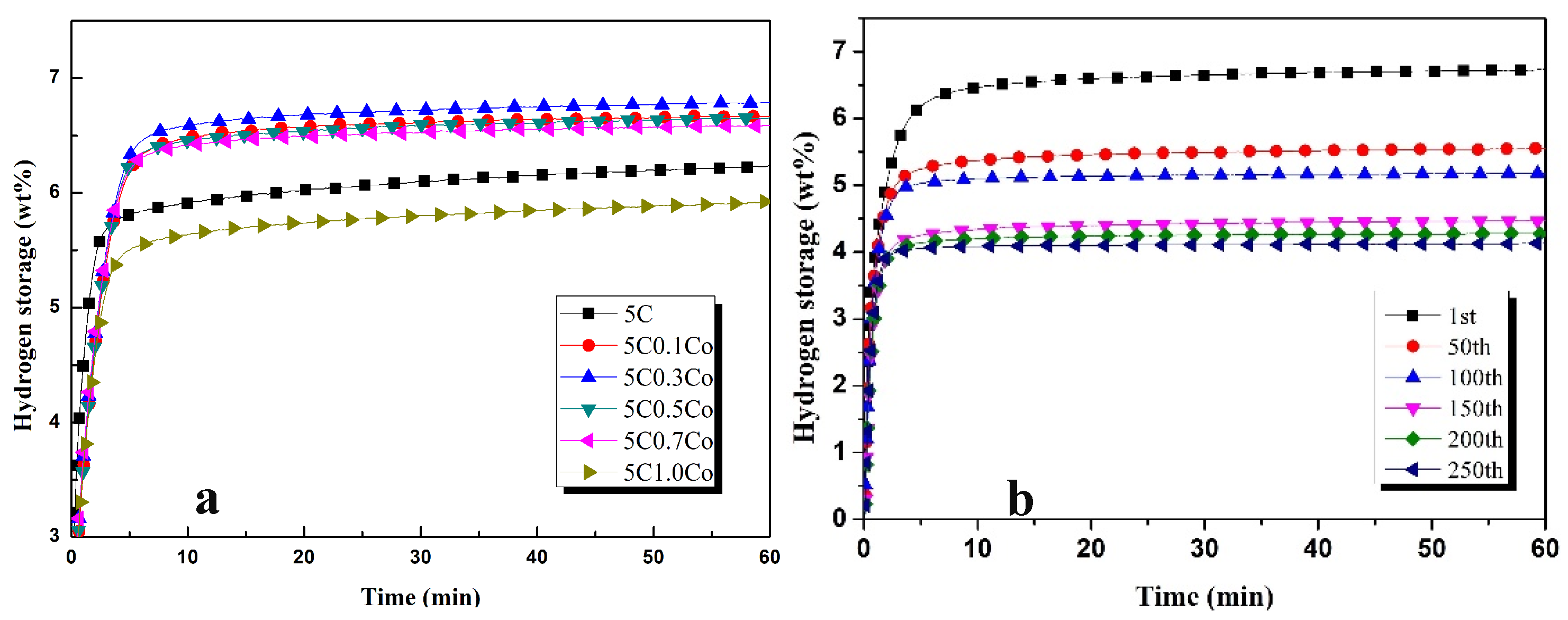
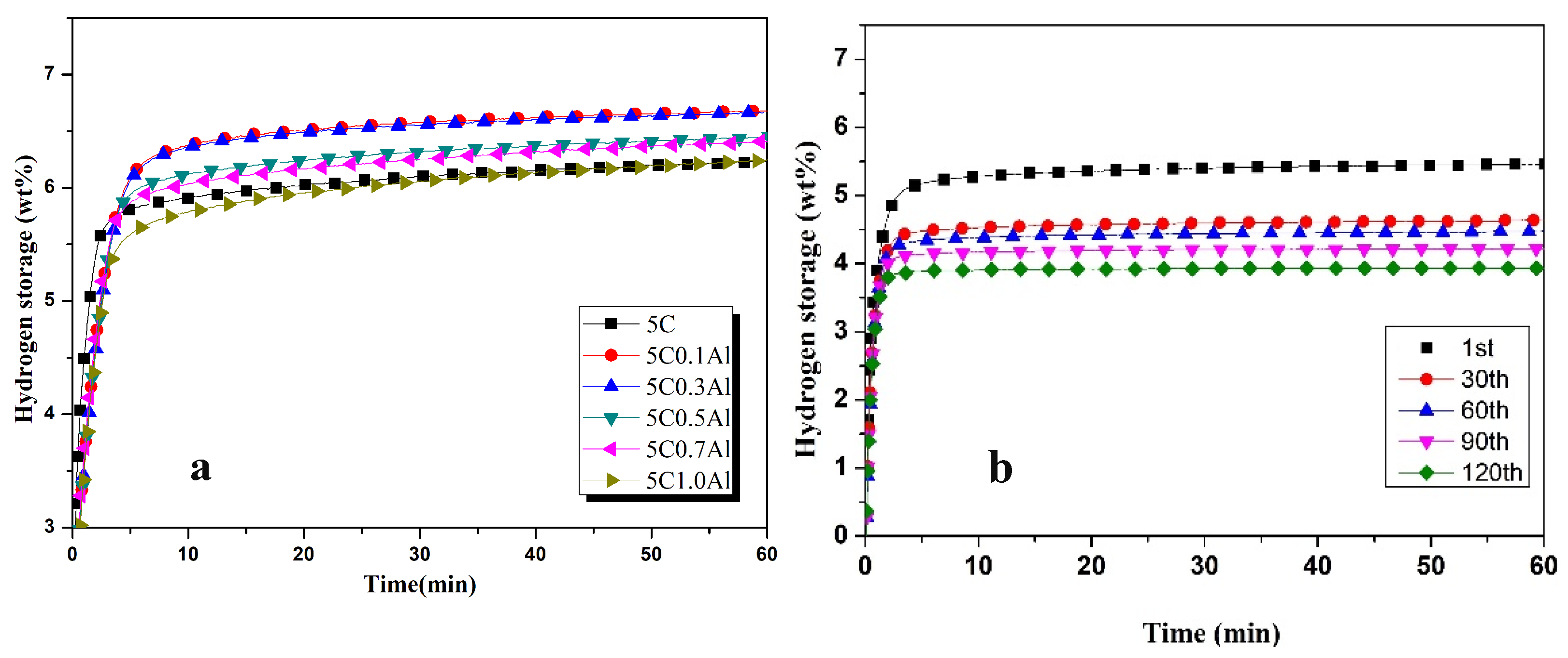
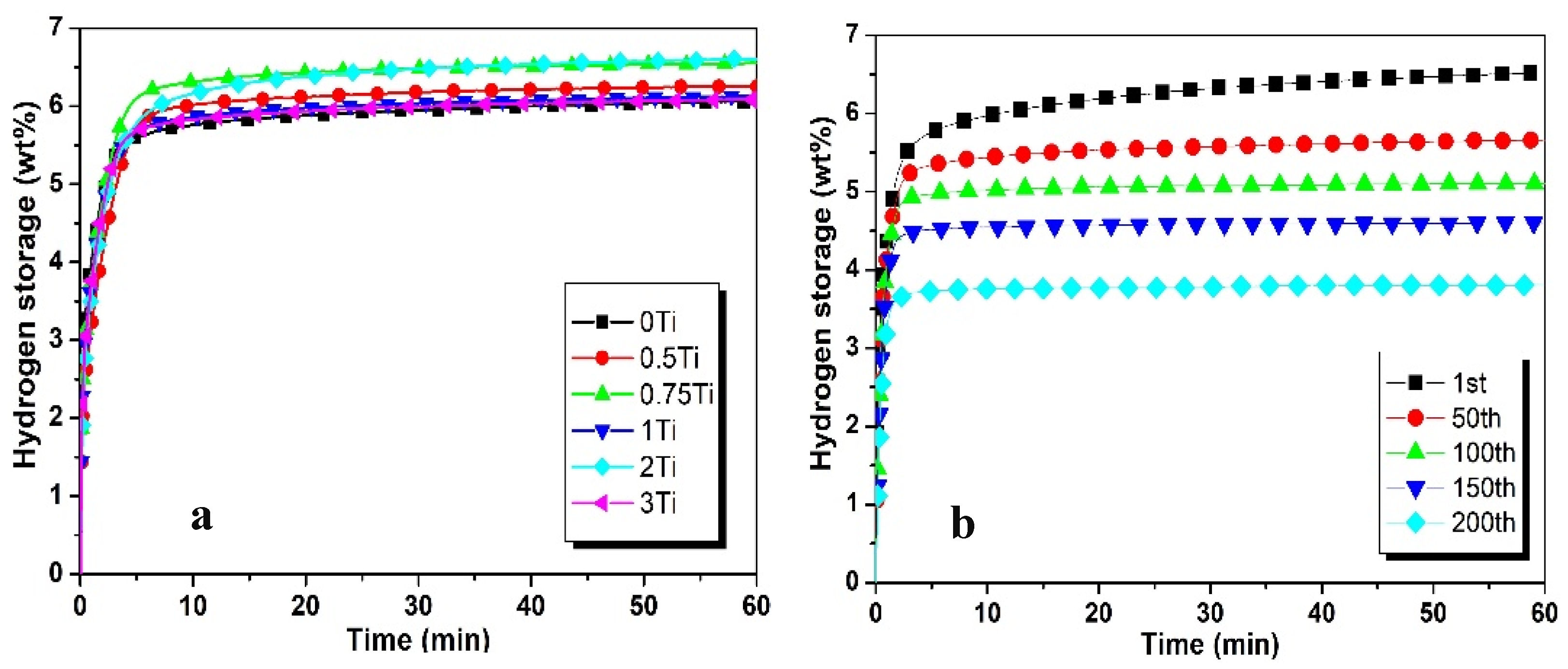
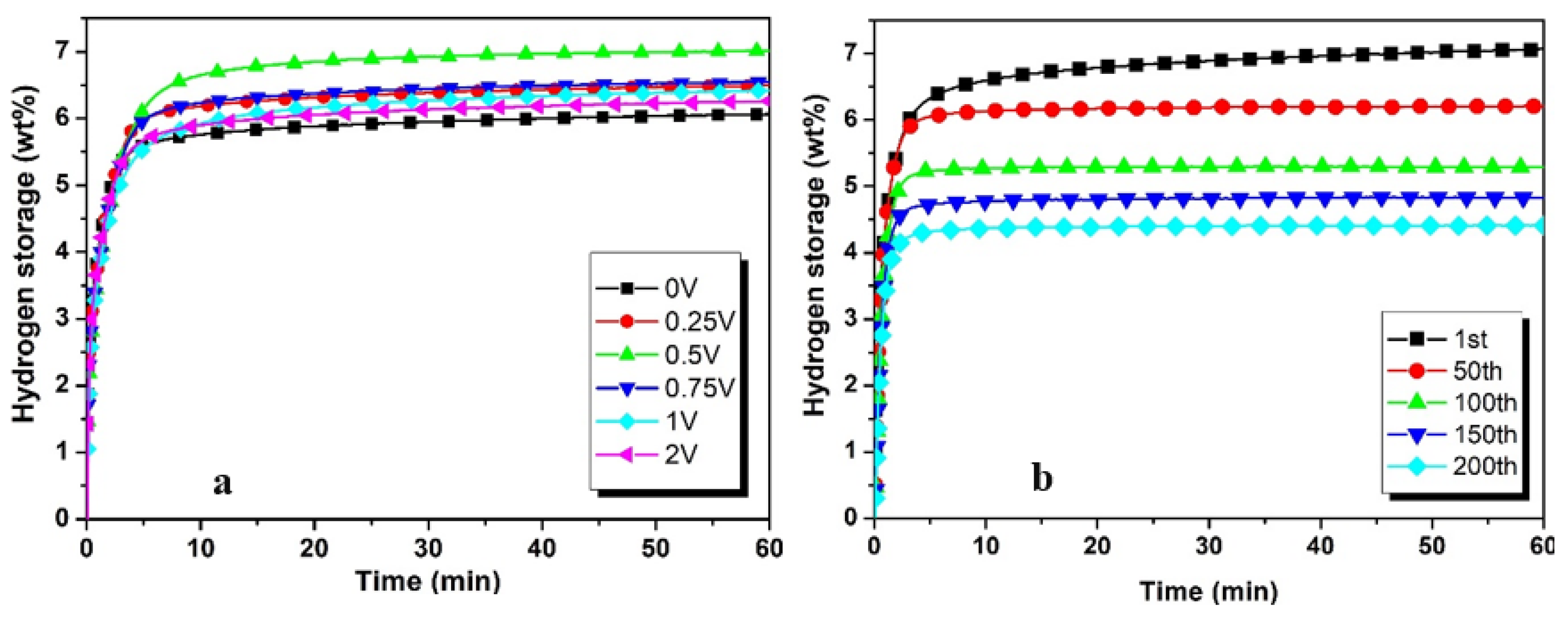
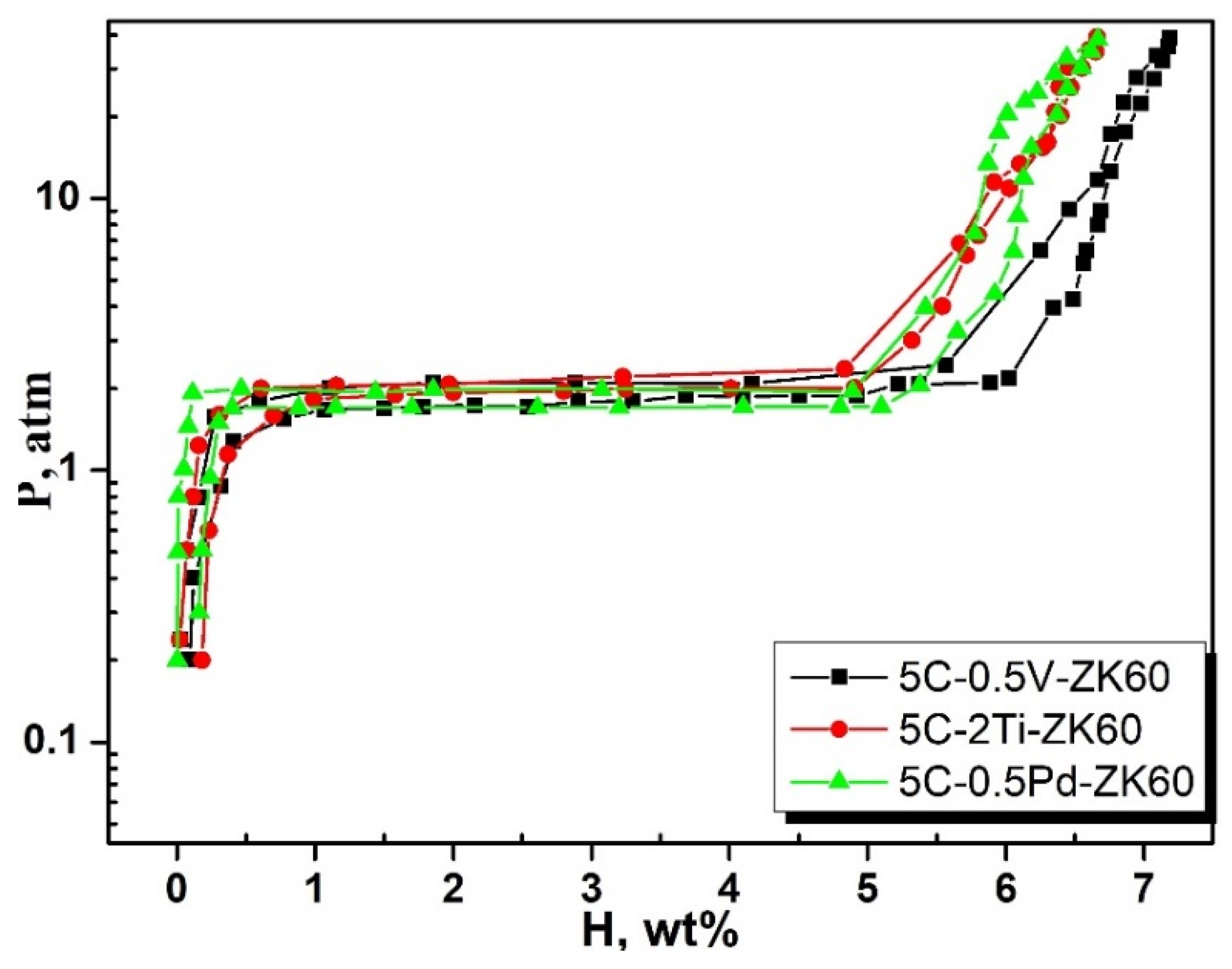
3.1. Hydrogenation Characteristics
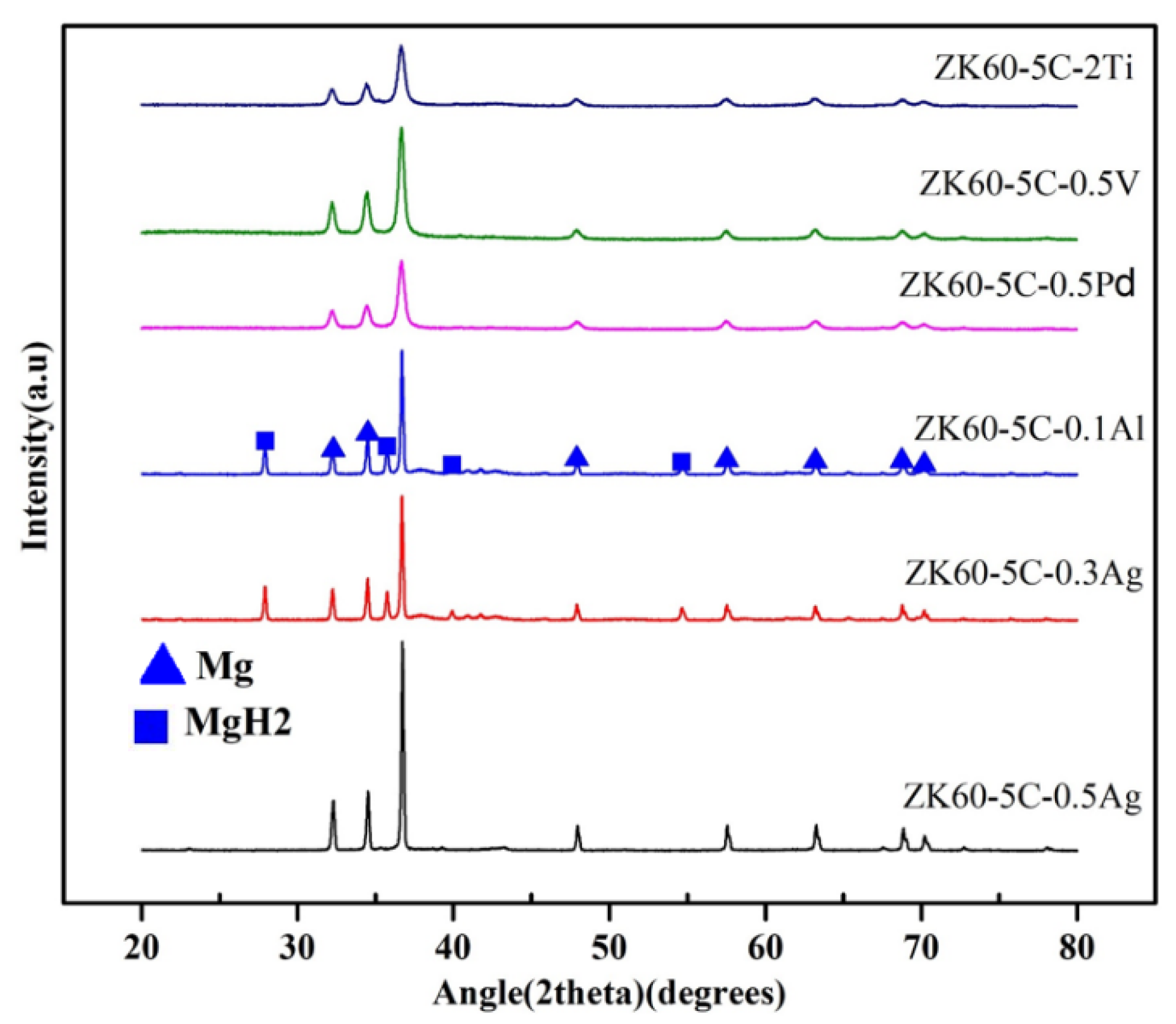
3.2. Microstructural Characterization
4. Conclusions
- The addition of 5 wt% activated carbon increased hydrogen absorption capacity from 4.06 wt% to the maximum level of 6.2 wt%.
- The addition of different contents of transition metals increased the hydrogen absorption capacity of ZK60 + 5C, and maximum hydrogen capacities in the first cycle after 1 h were observed as 7.1 wt%, 6.8 wt%, 6.7 wt%, 6.64 wt%, 6.65 wt%, and 7.06 wt% for 0.5Ag, 0.3Co, 0.1Al, 0.5Pd, 2Ti, and 0.5V, respectively.
- Hydrogen absorption capacities decreased by 35.21%, 26.47%, 41.79%, 21.68%, 26.31%, and 26.34% for 0.5Ag, 0.3Co, 0.1Al, 0.5Pd, 2Ti, and 0.5V, respectively, after 100 cycles.
- Hydrogen absorption in ZK60 with 5C0.5Ag and 5C0.5Pd remained the same in the cycles higher than 100 but decreased in all other samples.
- The hydrogen absorption percentage in the first five minutes was higher than 90% in all samples and effectively improved the hydrogen absorption characteristics.
- There was no obvious difference in microstructure between samples with different additives, and hydrogen was completely desorbed in all samples except 5C0.1Al and 5C0.3Ag at higher cycles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhang, X.; Zhang, L.; Zhang, W.; Liu, H.; Huang, Z.; Yang, L.; Gu, C.; Sun, W.; Gao, M.; et al. Recent advances in catalyst-modified Mg-based hydrogen storage materials. J. Mater. Sci. Technol. 2023, 163, 182–211. [Google Scholar] [CrossRef]
- Damizia, M.; Lloreda-Jurado, P.J.; De Filippis, P.; de Caprariis, B.; Chicardi, E.; Sepúlveda, R. Green hydrogen production using doped Fe2O3 foams. Int. J. Hydrog. Energy 2024, 51, 834–845. [Google Scholar] [CrossRef]
- Belkhiria, S.; Briki, C.; Dhaou, M.H.; Jemni, A. Experimental study of a metal –hydrogen reactor’s behavior under the action of an external magnetostatic field during absorption and desorption. Int. J. Hydrog. Energy 2020, 45, 4673–4684. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sakaki, K.; Kim, H.; Asano, K.; Watanuki, T.; Machida, A. Reaction paths via a new transient phase in non-equilibrium hydrogen absorption of LaNi2Co3. Int. J. Hydrog. Energy 2020, 45, 21655–21665. [Google Scholar] [CrossRef]
- Xie, L.; Xu, M.; Zhang, C.; Wu, T. Composition dependent hydrogen storage performance and desorption factors of Mg–Ce based alloys. Int. J. Hydrog. Energy 2020, 45, 9865–9876. [Google Scholar] [CrossRef]
- Cengeri, P.; Kimoto, Y.; Janoska, M.; Abbasi, Z.; Morisada, Y.; Fujii, H.; Enzinger, N.; Sommitsch, C.; Boczkal, G.; Krexner, G.; et al. Long term hydrogen storage properties of ZK60 Mg-alloy as processed by different methods of, S.P.D. J. Mater. Sci. 2024, 59, 5906–5922. [Google Scholar] [CrossRef]
- Kichigin, V.I.; Shein, A.B. The kinetics of hydrogen evolution reaction accompanied by hydrogen absorption reaction with consideration of subsurface hydrogen as an adsorbed species: Polarization curve. J. Electroanal. Chem. 2020, 873, 114427. [Google Scholar] [CrossRef]
- Chen, Y.; Yong, H.; Wang, S.; Xie, C.; Zhao, B.; Liu, B.; Hu, J.; Li, Y.; Zhang, Y. Investigation of microstructure characteristics, kinetics, and thermodynamics of Mg–Ni-RE (RE = Y and RE = Ce) hydrogen storage alloys. Int. J. Hydrog. Energy 2024, 69, 1329–1340. [Google Scholar] [CrossRef]
- Song, W.; Dong, H.; Zhang, G.; Liu, J.; Yang, G.; Liu, Y.; Li, Y.; Li, J.; Shen, J.; Chen, Y.; et al. Enhanced hydrogen absorption kinetics by introducing fine eutectic and long-period stacking ordered structure in ternary eutectic Mg–Ni–Y alloy. J. Alloys Compd. 2020, 820, 153187. [Google Scholar] [CrossRef]
- Huang, S.J.; Muneeb, A.; Abbas, A.; Sankar, R. The effect of Mg content and milling time on the solid solubility and microstructure of Ti–Mg alloys processed by mechanical milling. J. Mater. Res. Technol. 2021, 11, 1424–1433. [Google Scholar] [CrossRef]
- He, T.; Wang, X.; Liu, H.; Gao, S.; Wang, Y.; Li, S.; Yan, M. Enhanced hydrogen desorption/absorption properties of magnesium hydride with CeF3@Gn. Int. J. Hydrog. Energy 2020, 45, 4754–4764. [Google Scholar] [CrossRef]
- Yong, H.; Yu, S.; Wang, X.; Zhao, Y.; Wang, S.; Wang, Y.; Hu, J.; Liu, B.; Zhang, Y. Study on catalytic mechanism of Yb2O3 on RE-Mg based hydrogen storage alloys. J. Alloys Compd. 2024, 971, 172777. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, H.; Guo, S.; Zhao, D.; Li, J.; Zhang, Y. Hydrogen storage properties of Mg-based alloys modified with metal-organic frameworks and carbon-based porous materials: A review and summary. Int. J. Hydrog. Energy 2024, 57, 1373–1388. [Google Scholar] [CrossRef]
- Comanescu, C. Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals 2023, 13, 878. [Google Scholar] [CrossRef]
- Abdi, S.; Mansournia, M. A new approach to synthesize ammonium uranate decorated reduced graphene oxide nanosheets and their performance in electrochemical hydrogen storage. Fuel 2023, 342, 127704. [Google Scholar] [CrossRef]
- Zhang, Z.; He, D.; Xing, X.; Liu, Y.; Liu, T. Al and Zr addition to improve the hydrogen storage kinetics of Mg-based nanocomposites: Synergistic effects of multiphase nanocatalysts. J. Alloys Compd. 2023, 942, 169098. [Google Scholar] [CrossRef]
- Abbas, A.; Lin, H.-P.; Lin, K.-M.; Lin, H.-C. Effects of differential speed rolling and H2S poison on hydrogen storage performance of ZK60 alloys ball-milled with C, Pd, Ag, and Zr. Renew. Energy 2024, 226, 120379. [Google Scholar] [CrossRef]
- Huang, S.-J.; Rajagopal, V.; Chen, Y.L.; Chiu, Y.-H. Improving the hydrogenation properties of AZ31-Mg alloys with different carbonaceous additives by high energy ball milling (HEBM) and equal channel angular pressing (ECAP). Int. J. Hydrog. Energy 2020, 45, 22291–22301. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, S.-J. ECAP effects on microstructure and mechanical behavior of annealed WS2/AZ91 metal matrix composite. J. Alloys Compd. 2020, 835, 155466. [Google Scholar] [CrossRef]
- Yang, S.; Luo, Z.; Yang, G.; Lv, L.; Xu, L.; Leng, H.; Han, X.; Zhu, J.; Liu, W.; Zhu, P.; et al. Influence of rare earth doping on the hydrogen absorption properties of Zr7V5Fe alloy. J. Rare Earths 2024. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, D.; Feng, D.; Ren, H.; Zhang, Y. Effect of Y content on the hydrogen storage properties of ball-milled Mg2.4-Y Ni (x = 0.05, 0.1, 0.15, 0.2) alloys. J. Phys. Chem. Solids 2023, 178, 111320. [Google Scholar] [CrossRef]
- Hara, M.; Morozumi, S.; Watanabe, K. Effect of a magnesium depletion on the Mg–Ni–Y alloy hydrogen absorption properties. J. Alloys Compd. 2006, 414, 207–214. [Google Scholar] [CrossRef]
- Yong, H.; Guo, S.; Yuan, Z.; Qi, Y.; Zhao, D.; Zhang, Y. Catalytic effect of in situ formed Mg2Ni and REHx (RE: Ce and Y) on thermodynamics and kinetics of Mg-RE-Ni hydrogen storage alloy. Renew. Energy 2020, 157, 828–839. [Google Scholar] [CrossRef]
- Yong, H.; Wei, X.; Wang, Y.; Guo, S.; Yuan, Z.; Qi, Y.; Zhao, D.; Zhang, Y. Phase evolution, thermodynamics and kinetics property of transition metal (TM = Zr, Ti, V) catalyzed Mg–Ce–Y–Ni hydrogen storage alloys. J. Phys. Chem. Solids 2020, 144, 109516. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, W.; Yang, X.-S.; Wang, H.; Ouyang, L.; Zhu, M. Enhancing de/hydrogenation kinetics properties of the Mg/MgH2 system by adding ANi5 (A = Ce, Nd, Pr, Sm, and Y) alloys via ball milling. J. Rare Earths 2020, 39, 1010–1016. [Google Scholar] [CrossRef]
- Uyor, U.O.; Popoola, P.A.; Popoola, O.M.; Aigbodion, V.S. A review of recent advances on the properties of polypropylene—Carbon nanotubes composites. J. Thermoplast. Compos. Mater. 2023, 36, 3737–3770. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, S.; Zhou, Z.; Zhou, W.; Guo, J.; Lan, Z. Effect of transition metal on the hydrogen storage properties of Mg–Al alloy. J. Mater. Sci. 2017, 52, 2392–2399. [Google Scholar] [CrossRef]
- Chen, D.; Chen, L.; Liu, S.; Ma, C.X.; Chen, D.M.; Wang, L.B. Microstructure and hydrogen storage property of Mg/MWNTs composites. J. Alloys Compd. 2004, 372, 231–237. [Google Scholar] [CrossRef]
- Mose, M.P.; Huang, S.-J. Enhanced hydrogen storage capacity and kinetics in AZ61 alloy with nickel and cobalt additives: Insights into reaction mechanisms and activation energy. J. Alloys Compd. 2024, 984, 173934. [Google Scholar] [CrossRef]
- Huang, S.-J.; Rajagopal, V.; Skripnyuk, V.; Rabkin, E.; Fang, C. A comparative study of hydrogen storage properties of AZ31 and AZ91 magnesium alloys processed by different methods. J. Alloys Compd. 2023, 935, 167854. [Google Scholar] [CrossRef]
- Guo, S.; Yu, Z.; Li, Y.; Fu, Y.; Zhang, Z.; Han, S. Preparation of Mg-Mg2Ni/C composite and its excellent hydrogen storage properties. J. Alloys Compd. 2024, 976, 173035. [Google Scholar] [CrossRef]
- Anik, M.; Akay, I.; Özdemir, G.; Baksan, B. Electrochemical hydrogen storage performance of Mg–Ti–Zr–Ni alloys. Int. J. Hydrog. Energy 2009, 34, 9765–9772. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Fu, J.; Guo, Q.; Pan, F.; Deng, S.; Li, J.; Zhao, G. The synthesis and hydrogen storage properties of Mg 2 Ni substituted with Cu, Co. J. Mater. Res. 2009, 24, 1311–1316. [Google Scholar] [CrossRef]
- Andreasen, A. Hydrogenation properties of Mg–Al alloys. Int. J. Hydrog. Energy 2008, 33, 7489–7497. [Google Scholar] [CrossRef]
- Abbas, A.; Lin, Z.-B.; Ma, R.-L.; Lin, K.-M.; Lin, H.-C. Effects of CNTs, graphene, and organic additives on hydrogen storage performance of severely deformed ZK60 alloy. Int. J. Hydrog. Energy 2024, 52, 1175–1184. [Google Scholar] [CrossRef]
- Abbas, A.; Hu, K.-C.; Lin, H.-C.; Lin, K.-M. Influence of Severe Plastic Deformation and some Additives on Hydrogenation of ZK60 Alloy. J. Phys. Chem. Solids 2020, 151, 109927. [Google Scholar] [CrossRef]
- Abbas, A.; Hu, K.-C.; Lin, H.-C.; Lin, K.-M. Effects of ball milling and additives (activated carbon and copper) on hydrogen absorption characteristics of ZK60 alloy. Mater. Chem. Phys. 2021, 271, 124950. [Google Scholar] [CrossRef]
- Nateq, B.; Haddad-Sabzevar, M.; Sajjadi, S.A.; Pellizzari, M. Interfacial structure-property relationship in a carbon nanotube-reinforced aluminum alloy matrix composite fabricated by an advanced method. Mater. Charact. 2023, 203, 113159. [Google Scholar] [CrossRef]
- Pęska, M.; Smektalska, K.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Dębski, A.; Polański, M. Hydrogen sorption behavior of mechanically synthesized Mg–Ag alloys. Int. J. Hydrog. Energy 2021, 46, 33152–33163. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Sypien, A.; Gąsior, W.; Pęska, M.; Polański, M. Hydrogen Sorption Behavior of Cast Ag-Mg Alloys. Materials 2021, 15, 270. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wei, X.; Gao, J.-L.; Hu, F.; Qi, Y.; Zhao, D.-L. Electrochemical hydrogen storage behaviors of as-milled Mg–Ti–Ni–Co–Al-based alloys applied to Ni-MH battery. Electrochim. Acta 2020, 342, 136123. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Alumina based doped templated carbons: A comparative study with zeolite and silica gel templates. Microporous Mesoporous Mater. 2018, 257, 241–252. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Feng, H.; Yanan, D.; Ting, X.; Yongzhi, L.; Xin, Z.; Jianyi, X.; Guofang, Z.; Ying, C.; Yanghuan, Z. Influence of adding graphene on the hydrogen storage thermodynamics and kinetics of as-milled CeMg12–Ni alloy. Int. J. Hydrog. Energy 2023, 48, 13213–13226. [Google Scholar] [CrossRef]
- Qi, J.; Ma, L.; Gong, P.; Rainforth, W.M. Investigation of the wear transition in CoCrMo alloys after heat treatment to produce an HCP structure. Wear 2023, 518–519, 204649. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; You, L.; Cao, X.; Lu, Z.; Song, X. Investigation on the activation mechanism of hydrogen absorption in TiZrNbTa high entropy alloy. J. Alloys Compd. 2019, 781, 613–620. [Google Scholar] [CrossRef]
- Darvishnejad, M.H.; Afshari, M.; Cheshme Khavar, A.H. Transition metal atoms (TM = Sc, Ti, V, Cr, and Mn) decorated PBCF-graphene as a possible reversible hydrogen storage material: A DFT-D2 investigation. Int. J. Hydrog. Energy 2024, 78, 40–51. [Google Scholar] [CrossRef]
- Yong, H.; Guo, S.; Yuan, Z.; Qi, Y.; Zhao, D.; Zhang, Y. Improved hydrogen storage kinetics and thermodynamics of RE-Mg-based alloy by co-doping Ce–Y. Int. J. Hydrog. Energy 2019, 44, 16765–16776. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, X.; Yuan, Z.; Hou, Z.; Qi, Y.; Guo, S. Hydrogen storage thermodynamics and dynamics of Mg–Y–Ni–Cu based alloys synthesized by melt spinning. J. Phys. Chem. Solids 2020, 138, 109252. [Google Scholar] [CrossRef]


| Alloy | Absorption Temperature (°C) | Hydrogen Absorption Capacity (wt%) | References |
|---|---|---|---|
| Mg-Ni | 300 | 3.6 | [31] |
| Mg-5 wt%Ti | 320 | 6.0 | [32] |
| Mg-10 wt%V | 250 | 6.5 | [33] |
| Mg-Al-Ti | 360 | 4.28 | [34] |
| AZ61 | 375 | 3.9 | [27] |
| AZ61-Ni + Co | 375 | 5.13 | [29] |
| ZK60-Graphene + (1 mL) Toluene | 320 | 6.36 | [35] |
| ZK60-Graphene +(2 mL) Cyclohexane | 320 | 6.77 | [35] |
| ZK60-5C + 2V | 350 | 6.7 | [36] |
| ZK60-5 wt% activated carbon | 300 | 6.6 | [37] |
| Sample | 60 min (wt%) | 2nd Min | 5th Min | ||
|---|---|---|---|---|---|
| wt% | % | wt% | % | ||
| ZK60 + C | 6.2 | 4.7 | 75.8 | 5.7 | 91.93 |
| 0.1 Ag | 6.6 | 4.6 | 69.69 | 6.2 | 93.93 |
| 0.3 Ag | 6.7 | 5.2 | 77.61 | 6.4 | 95.52 |
| 0.5 Ag | 7.1 | 4.8 | 67.6 | 6.4 | 90.14 |
| 0.7 Ag | 6.8 | 4.7 | 67.64 | 5.3 | 67.64 |
| 1.0 Ag | 6.7 | 4.6 | 68.65 | 6.2 | 92.53 |
| 0.1 Co | 6.7 | 4.7 | 70.14 | 6.3 | 94.02 |
| 0.3 Co | 6.8 | 4.8 | 70.58 | 6.4 | 94.11 |
| 0.5 Co | 6.7 | 4.7 | 70.14 | 6.3 | 94.02 |
| 0.7 Co | 6.6 | 4.8 | 72.72 | 6.2 | 93.93 |
| 1.0 Co | 5.9 | 4.6 | 77.96 | 5.6 | 94.91 |
| 0.1 Al | 6.7 | 4.7 | 70.14 | 6.2 | 92.53 |
| 0.3 Al | 6.7 | 4.6 | 68.65 | 6.2 | 92.53 |
| 0.5 Al | 6.5 | 4.7 | 72.3 | 6.0 | 92.3 |
| 0.7 Al | 6.4 | 4.7 | 73.43 | 6.3 | 98.43 |
| 1Al | 6.3 | 4.5 | 71.42 | 5.6 | 88.88 |
| 0.25Pd | 6.44 | 5.09 | 79.03 | 6.01 | 93.32 |
| 0.5Pd | 6.64 | 4.97 | 74.84 | 6.16 | 92.77 |
| 0.75Pd | 6.56 | 5.5 | 83.84 | 6.13 | 93.44 |
| 1Pd | 6.36 | 4.79 | 75.31 | 5.91 | 92.92 |
| 2Pd | 5.67 | 4.93 | 86.94 | 5.39 | 95.06 |
| 0.5Ti | 6.25 | 4.82 | 77.12 | 5.7 | 91.2 |
| 0.75Ti | 6.55 | 4.82 | 73.85 | 6.11 | 93.28 |
| 1Ti | 6.57 | 4.9 | 74.58 | 6.42 | 97.71 |
| 2Ti | 6.65 | 4.57 | 68.72 | 5.79 | 87.06 |
| 3Ti | 6.09 | 4.71 | 77.33 | 5.68 | 93.26 |
| 0.25V | 6.50 | 4.81 | 74 | 5.99 | 92.15 |
| 0.5V | 7.06 | 4.71 | 66.71 | 6.18 | 88.15 |
| 0.75V | 6.72 | 4.62 | 68.75 | 6.0 | 89.28 |
| 1V | 6.47 | 4.74 | 73.26 | 6.02 | 93.04 |
| 2V | 6.31 | 4.8 | 76.06 | 5.83 | 92.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.; Hsu, T.-C.; Lin, J.-Y.; Ho, H.-C.; Lin, K.-M.; Lin, H.-C. Investigating the Effects of Transition Metals and Activated Carbon on Hydrogenation Characteristics of Severely Deformed ZK60 Processed by High-Energy Ball Milling. Materials 2024, 17, 4562. https://doi.org/10.3390/ma17184562
Abbas A, Hsu T-C, Lin J-Y, Ho H-C, Lin K-M, Lin H-C. Investigating the Effects of Transition Metals and Activated Carbon on Hydrogenation Characteristics of Severely Deformed ZK60 Processed by High-Energy Ball Milling. Materials. 2024; 17(18):4562. https://doi.org/10.3390/ma17184562
Chicago/Turabian StyleAbbas, Aqeel, Tzu-Chieh Hsu, Jhe-Yi Lin, Hung-Cheng Ho, Kun-Ming Lin, and Hsin-Chih Lin. 2024. "Investigating the Effects of Transition Metals and Activated Carbon on Hydrogenation Characteristics of Severely Deformed ZK60 Processed by High-Energy Ball Milling" Materials 17, no. 18: 4562. https://doi.org/10.3390/ma17184562
APA StyleAbbas, A., Hsu, T.-C., Lin, J.-Y., Ho, H.-C., Lin, K.-M., & Lin, H.-C. (2024). Investigating the Effects of Transition Metals and Activated Carbon on Hydrogenation Characteristics of Severely Deformed ZK60 Processed by High-Energy Ball Milling. Materials, 17(18), 4562. https://doi.org/10.3390/ma17184562






