Ionic Liquid Crystals as Chromogenic Materials
Abstract
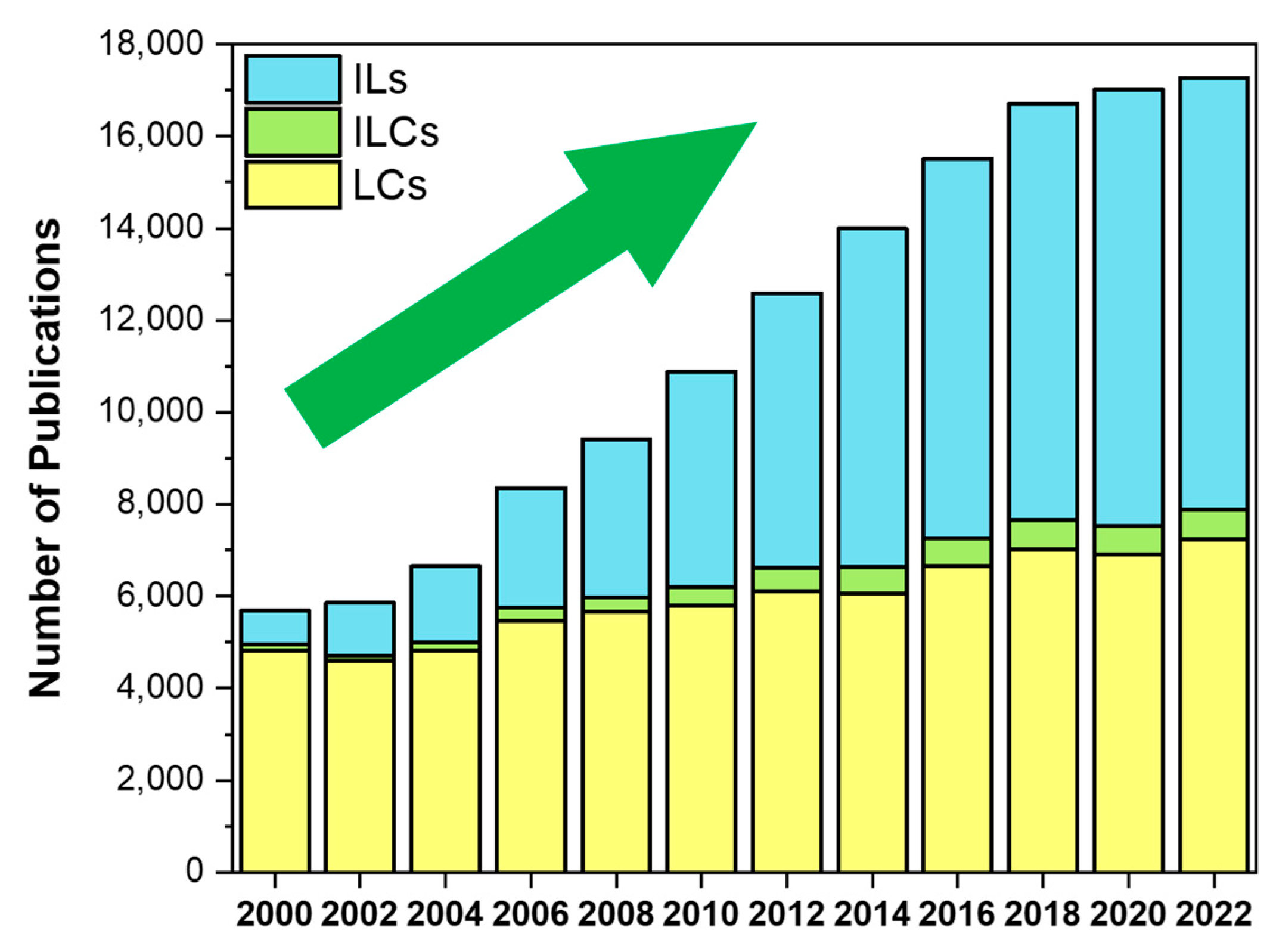
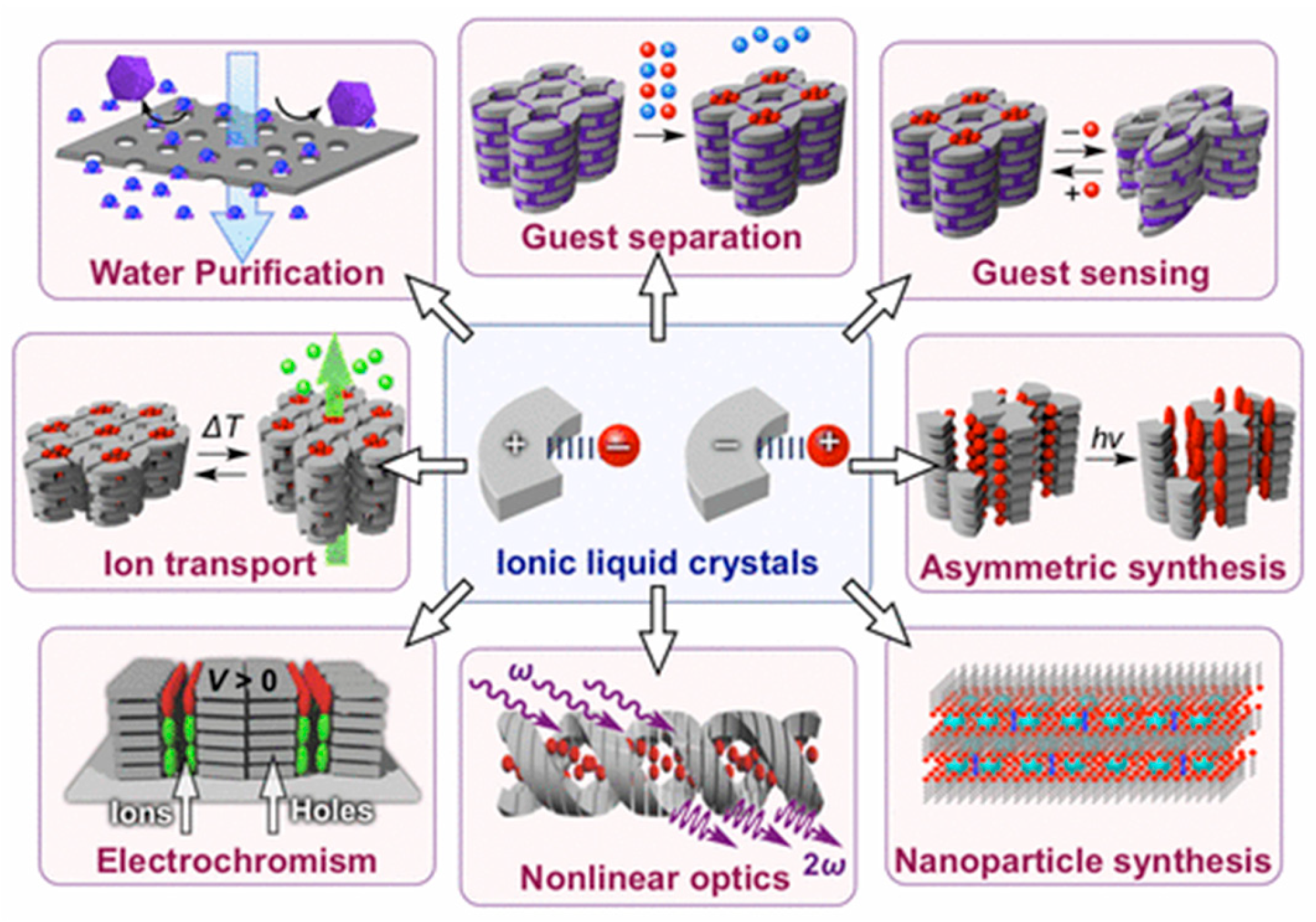
1. Introduction
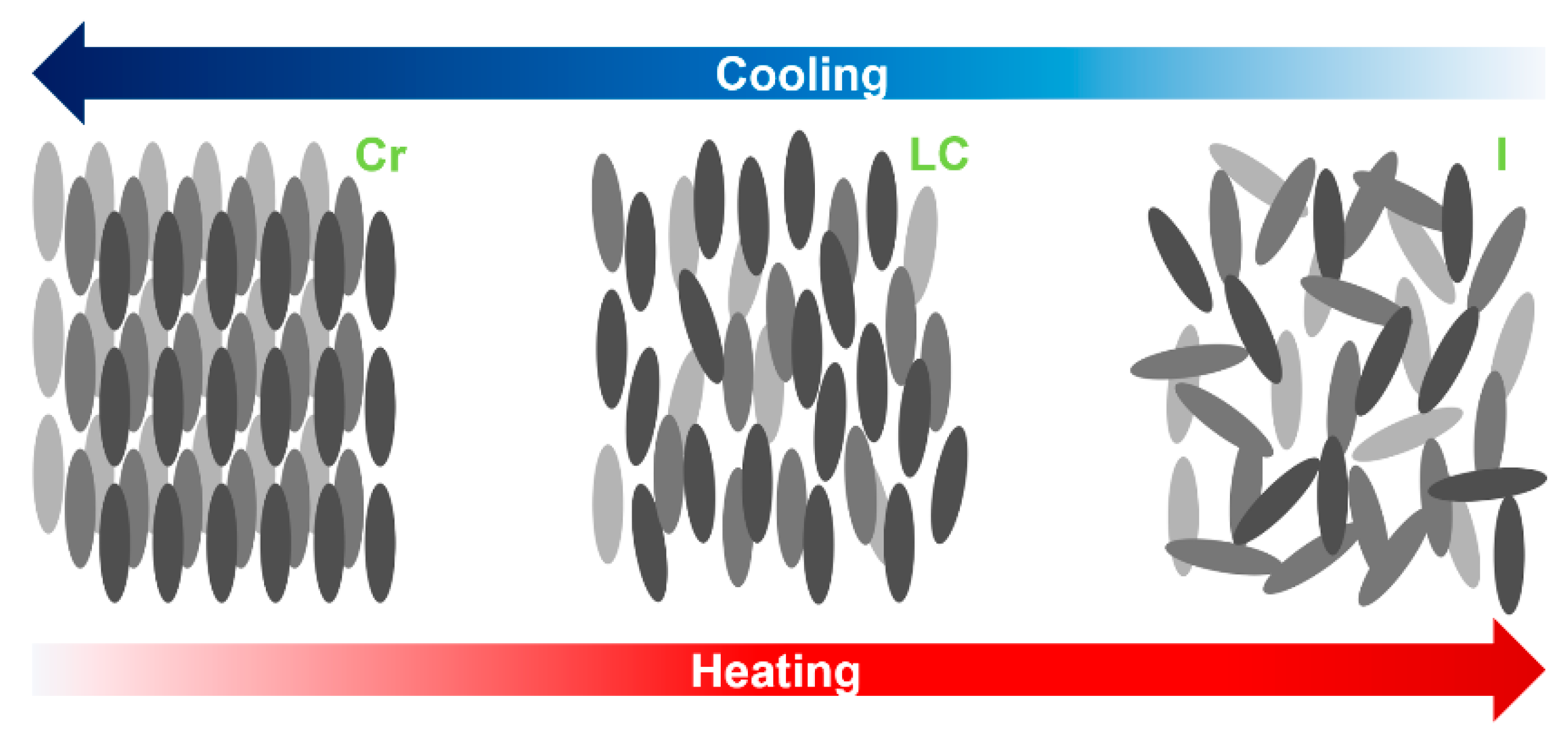
2. Liquid Crystals

3. Classification of Liquid Crystals

- Nematic:
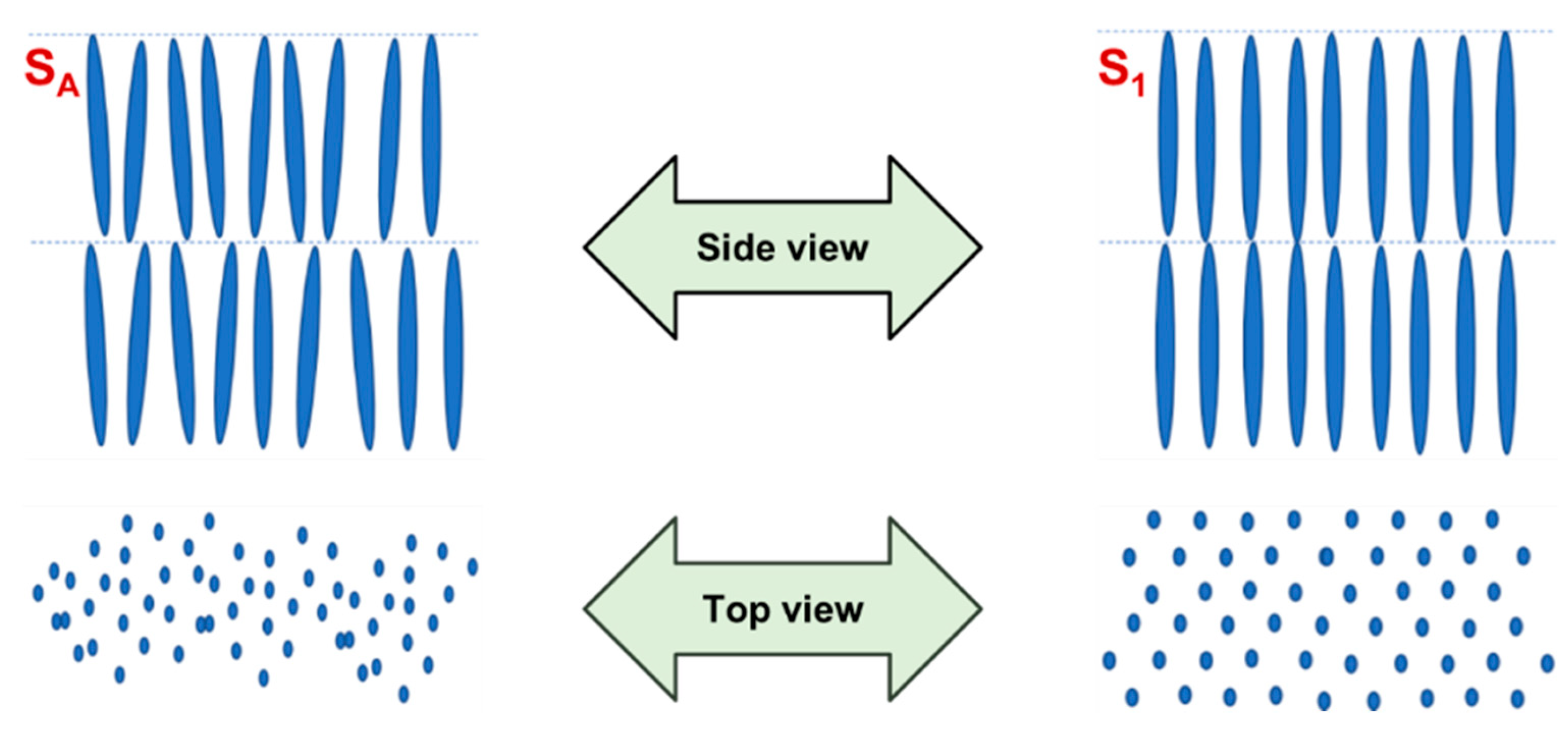
- Smectic:
- Columnar:
- Cubic:
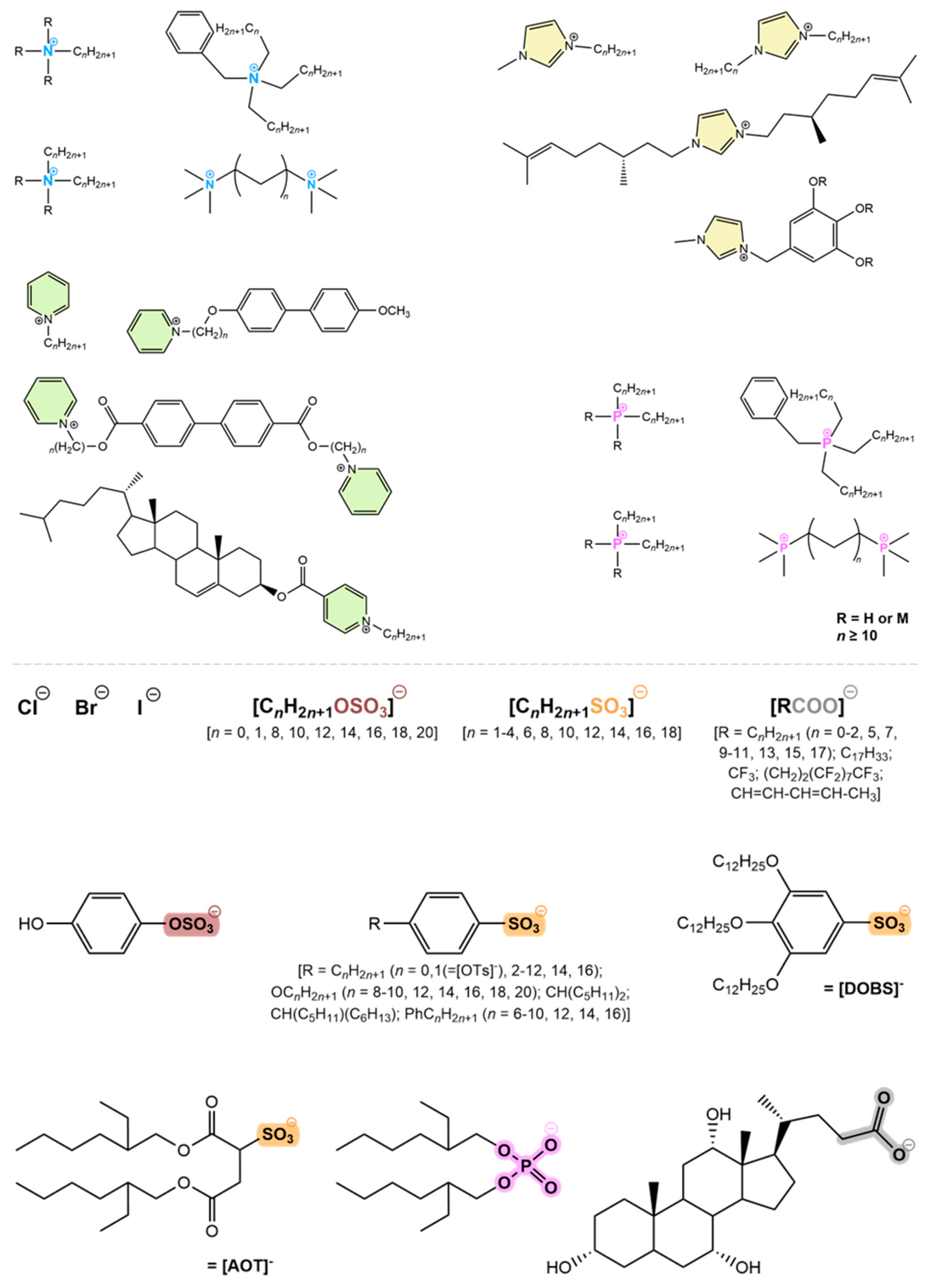
4. Ionic Liquid Crystals
- Ionic liquids:
- Ionic liquid crystals:
- Thermotropic and lyotropic ionic liquid crystals:
- Confinement of ionic liquid crystals:
5. Characterisation of Ionic Liquid Crystals
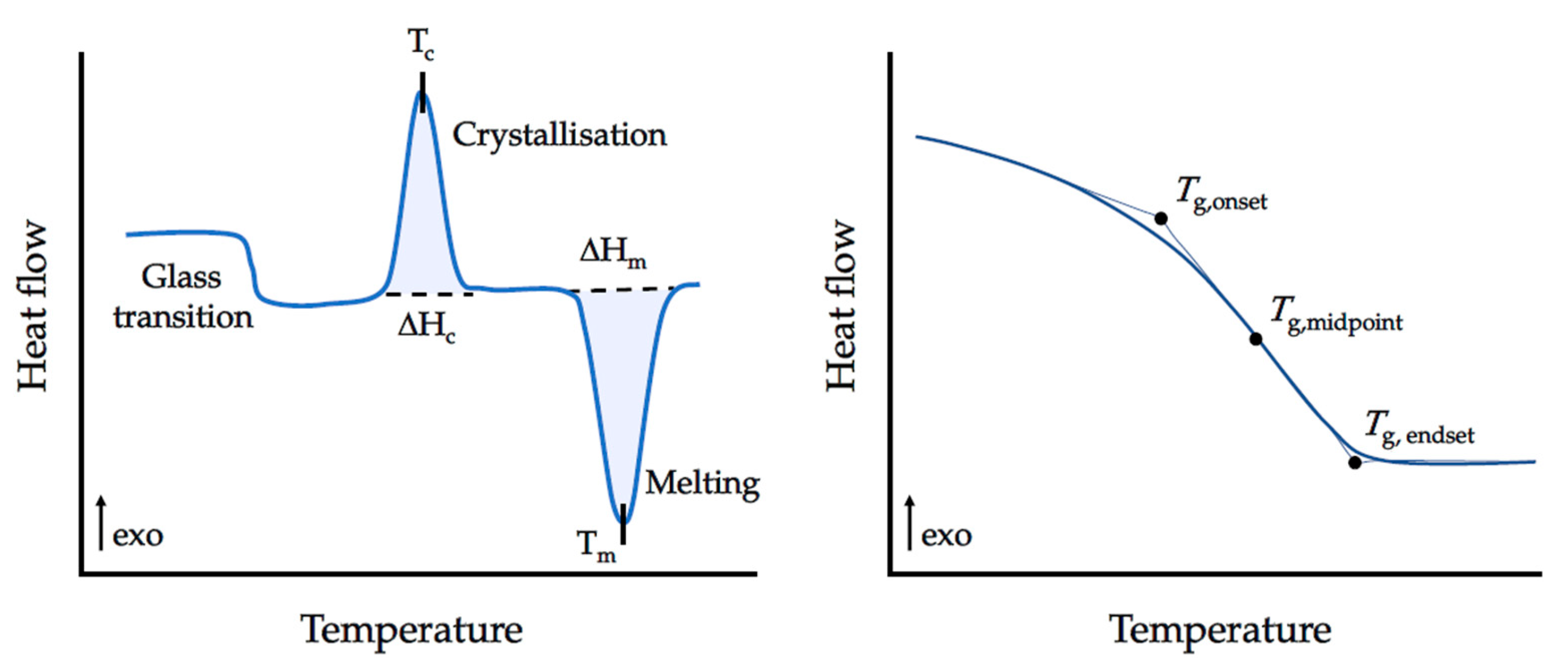
- Differential Scanning Calorimetry:
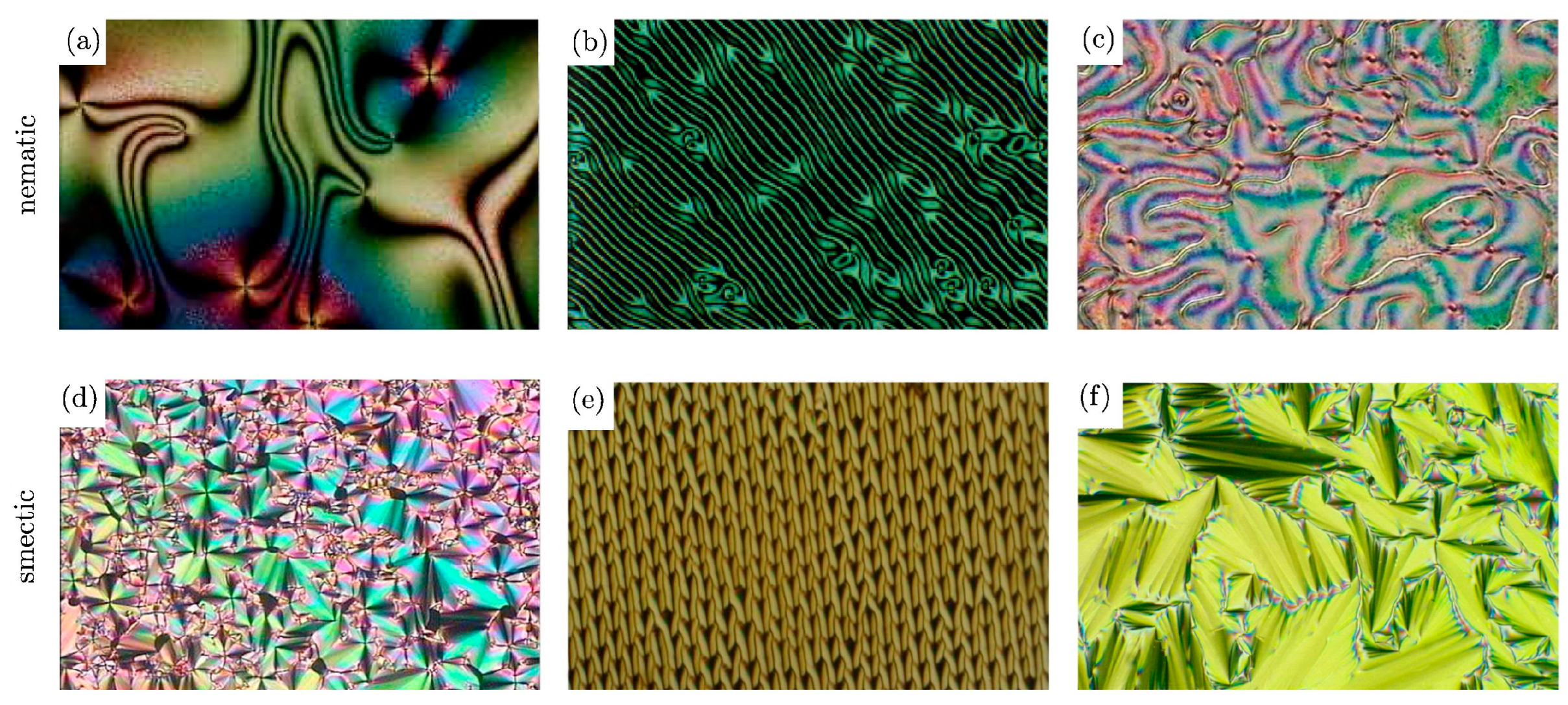
- Polarised Optical Microscopy:
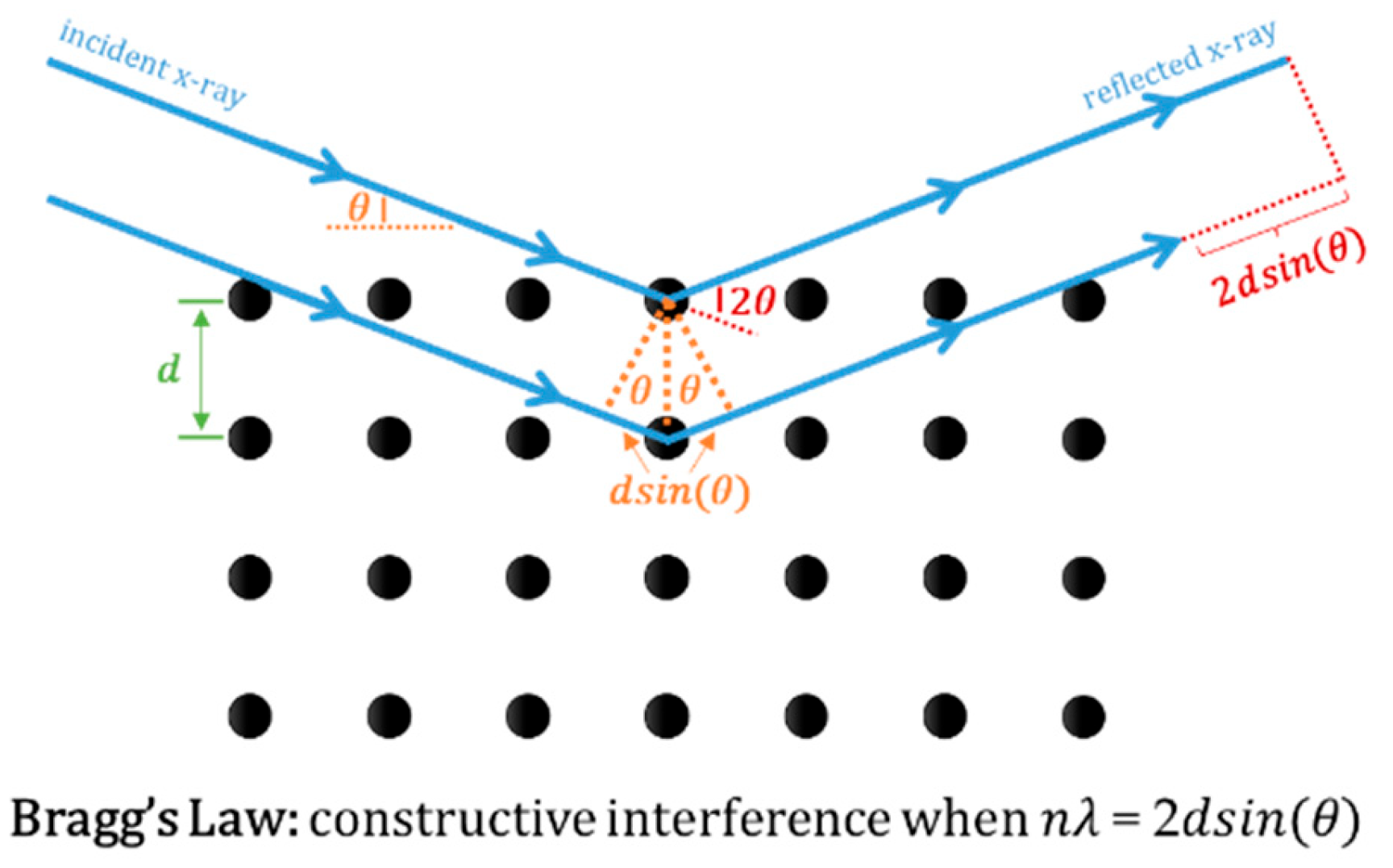
- X-Ray Powder Diffraction:
6. Applications of Ionic Liquid Crystals as Chromogenic Materials
| Compound | Liquid Crystalline Phase | Colour Change | Reference | |
|---|---|---|---|---|
| Photochromism | [(C12ImC1)2Azo][Br]2 | Smectic A phase | n.d. a | [185] |
| [(C14ImC1)2Azo][Br]2 | Smectic A phase | n.d. a | [185] | |
| [(C10ImC2O)C1Azo][Br] | Smectic A phase | n.d. a | [185] | |
| [(C12ImC2O)C1Azo][Br] | Smectic A phase | n.d. a | [185] | |
| [(C16ImC6O)C1Azo][Br] | Smectic A phase | n.d. a | [185] | |
| Electrochromism | [(C1)2BPyr][DOBS]2 | Hexagonal columnar phase | Yellow → Blue | [186] |
| [(C8Ph)2BPyr][NTf2]2 | Smectic A phase | Colourless → Green | [187] | |
| [(C10Ph)2BPyr][NTf2]2 | Smectic A phase | Colourless → Green | [187] | |
| [(C14Ph)2BPyr][NTf2]2 | Smectic A phase | Colourless → Green | [187] | |
| [(C14)(C2CF3)BPyr][NTf2]2 | Smectic X phase b | Colourless → Violet | [188] | |
| Thermochromism | [C10-4-(SC12-ODZ)-Pyr][Br] | Smectic A phase | Yellow → Red | [189] |
| [C12-4-(SC12-ODZ)-Pyr][Br] | Smectic A phase | Yellow → Red | [189,190] | |
| [C14-4-(SC12-ODZ)-Pyr][Br] | Smectic A phase | Yellow → Red | [189] | |
| [C16-4-(SC12-ODZ)-Pyr][Br] | Smectic A phase | Yellow → Red | [189] | |
| [C1-3-(C7F15-ODZ)-Pyr][I] | Smectic A phase | Yellow → Red | [191] | |
| [C4VIM]m[MnClxBry] | Hexagonal columnar phase | Green → Red d | [192] | |
| [C8VIM]m[MnClxBry] | n.d. c | Red → Green d | [192] | |
| [C12VIM]m[MnClxBry] | Smectic A phase | Yellow → Red d | [192] | |
| [(C1)2BPyr][DOBS]2 | Hexagonal columnar phase | Yellow → Blue | [186] |
- Photochromism:
- Electrochromism:
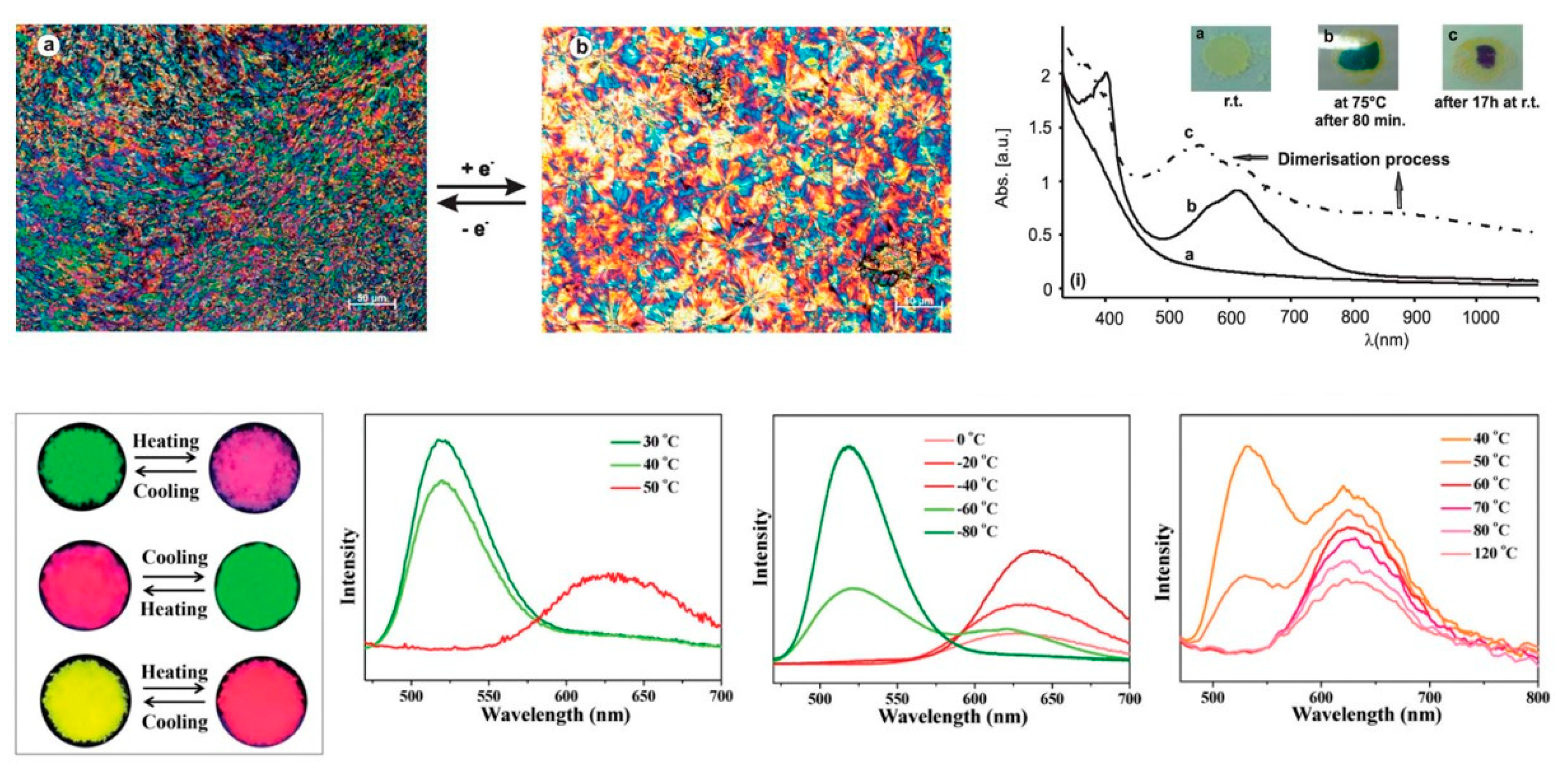
- Thermochromism:
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.C.; Mun, S.; Ko, H.-U.; Zhai, L.; Kafy, A.; Kim, J. Renewable Smart Materials. Smart Mater. Struct. 2016, 25, 073001. [Google Scholar] [CrossRef]
- Rogers, C.A. Intelligent Material Systems—The Dawn of a New Materials Age. J. Intell. Mater. Syst. Struct. 1993, 4, 4–12. [Google Scholar]
- Spillman, W.B., Jr.; Sirkis, J.S.; Gardiner, P.T. Smart Materials and Structures: What Are They? Smart Mater. Struct. 1996, 5, 247–254. [Google Scholar] [CrossRef]
- Lampert, C.M. Chromogenic Smart Materials. Mater. Today 2004, 7, 28–35. [Google Scholar]
- Cox, L.M.; Killgore, J.P.; Li, Z.; Zhang, Z.; Hurley, D.C.; Xiao, J.; Ding, Y. Morphing Metal–Polymer Janus Particles. Adv. Mater. 2014, 26, 899–904. [Google Scholar] [CrossRef]
- Ge, Q.; Dunn, C.K.; Qi, H.J.; Dunn, M.L. Active Origami by 4D Printing. Smart Mater. Struct. 2014, 23, 094007. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. Chromogenic Applications. In Metal Oxide Nanostructures: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–147. ISBN 9780128115121. [Google Scholar]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Yuan, D.; Dong, H.; Liu, J.; Yuan, X.; Fang, W.; Zhao, G.; Zhang, H. Ionic Liquid Crystal Electrolytes: Fundamental, Applications and Prospects. Nano Energy 2023, 106, 108087. [Google Scholar] [CrossRef]
- Jordão, N.; Ferreira, P.; Cruz, H.; Parola, A.J.; Branco, L.C. Photochromic Room Temperature Ionic Liquids Based on Anionic Diarylethene Derivatives. ChemPhotoChem 2019, 3, 525–528. [Google Scholar]
- Cui, J.; Li, Y.; Chen, D.; Zhan, T.-G.; Zhang, K.-D. Ionic Liquid-Based Stimuli-Responsive Functional Materials. Adv. Funct. Mater. 2020, 30, 2005522. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chu, Y.-H. Expeditious Discovery of Small-Molecule Thermoresponsive Ionic Liquid Materials: A Review. Molecules 2023, 28, 6817. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Yang, J.; Xu, C.; Zhang, Q.; Peng, Z. Ionic Gels and Their Applications in Stretchable Electronics. Macromol. Rapid Commun. 2018, 39, 1800246. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic Liquids for Electrochemical Energy Storage Devices Applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Choudhary, G.; Dhariwal, J.; Saha, M.; Trivedi, S.; Banjare, M.K.; Kanaoujiya, R.; Behera, K. Ionic Liquids: Environmentally Sustainable Materials for Energy Conversion and Storage Applications. Environ. Sci. Pollut. Res. 2023, 31, 10296–10316. [Google Scholar] [CrossRef]
- Akamatsu, N.; Hisano, K.; Tatsumi, R.; Aizawa, M.; Barrett, C.J.; Shishido, A. Thermo-, Photo-, and Mechano-Responsive Liquid Crystal Networks Enable Tunable Photonic Crystals. Soft Matter 2017, 13, 7486–7491. [Google Scholar] [CrossRef]
- Hill, M.; Moaddel, T. 2—Soap Structure and Phase Behavior. In Soap Manufacturing Technology: Second Edition; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 35–54. ISBN 9781630670665. [Google Scholar]
- Oliveira, L.B.A.; de Oliveira, R.P.; Oliveira, C.; Raposo, N.R.B.; Brandão, M.A.F.; Ferreira, A.d.O.; Polonini, H. Cosmetic Potential of a Liotropic Liquid Crystal Emulsion Containing Resveratrol. Cosmetics 2017, 4, 54. [Google Scholar] [CrossRef]
- Caritá, A.C.; de Azevedo, J.R.; Buri, M.V.; Bolzinger, M.-A.; Chevalier, Y.; Riske, K.A.; Leonardi, G.R. Stabilization of Vitamin C in Emulsions of Liquid Crystalline Structures. Int. J. Pharm. 2021, 592, 120092. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, B.; Ruan, J.; Zhao, P.; Chen, L.; Chen, D.; Ye, F. 3D-Printed Biomimetic Systems with Synergetic Color and Shape Responses Based on Oblate Cholesteric Liquid Crystal Droplets. Adv. Mater. 2021, 33, 2006361. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Funahashi, M. Chiral Liquid Crystalline Electronic Systems. Symmetry 2021, 13, 672. [Google Scholar] [CrossRef]
- Chigrinov, V.G.; Kudreyko, A.A.; Podgornov, F.V. Optically Rewritable Liquid Crystal Displays: Characteristics and Performance. Crystals 2021, 11, 1053. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Kato, T.; Ohno, H. Dimension Control of Ionic Liquids. Chem. Commun. 2019, 55, 8205–8214. [Google Scholar] [CrossRef]
- Kapernaum, N.; Lange, A.; Ebert, M.; Grunwald, M.A.; Haege, C.; Marino, S.; Zens, A.; Taubert, A.; Giesselmann, F.; Laschat, S. Current Topics in Ionic Liquid Crystals. ChemPlusChem 2022, 87, e202100397. [Google Scholar] [CrossRef]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced Functional Liquid Crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef]
- Salikolimi, K.; Sudhakar, A.A.; Ishida, Y. Functional Ionic Liquid Crystals. Langmuir 2020, 36, 11702–11731. [Google Scholar] [CrossRef]
- de Gennes, P.-G.; Prost, J. The Physics of Liquid Crystals. In International Series of Monographs on Physics; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Oswald, P.; Pieranski, P. Nematic and Cholesteric Liquid Crystals: Concepts and Physical Properties Illustrated by Experiments; Taylor & Francis: New York, NY, USA, 2005; ISBN 9780429215742. [Google Scholar]
- Collings, P.J.; Goodby, J.W. Introduction to Liquid Crystals: Chemistry and Physics, 2nd ed.; Taylor & Francis: New York, NY, USA, 2019; ISBN 9781315098340. [Google Scholar]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, UK, 2020; ISBN 9780199655441. [Google Scholar]
- Reinitzer, F. Beiträge Zur Kenntniss Des Cholesterins. Monatshefte für Chemie und Verwandte Teile Anderer Wissenschaften; Springer: Berlin/Heidelberg, Germany, 1888; Volume 9, pp. 421–441. [Google Scholar] [CrossRef]
- Lehmann, O. Über Fliessende Krystalle. Zeitschrift für Phys. Chemie 1889, 4U, 462–472. [Google Scholar] [CrossRef]
- DiLisi, G.A. History. In An Introduction to Liquid Crystals; Morgan & Claypool Publishers: San Rafael, CA, USA, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Mohanty, S. Liquid Crystals—The “Fourth” Phase of Matter. Resonance 2003, 8, 52–70. [Google Scholar]
- Dunmur, D.; Luckhurst, G. 38. Liquid Crystals. In Springer Handbook of Electronic and Photonic Materials; Springer: Berlin/Heidelberg, Germany, 2007; pp. 917–952. [Google Scholar]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics. In Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1997; ISBN 9781351989244. [Google Scholar]
- Andrienko, D. Introduction to Liquid Crystals. J. Mol. Liq. 2018, 267, 520–541. [Google Scholar] [CrossRef]
- Inoue, M. Review of Various Measurement Methodologies of Migration Ion Influence on LCD Image Quality and New Measurement Proposal beyond LCD Materials. J. Soc. Inf. Disp. 2020, 28, 92–110. [Google Scholar] [CrossRef]
- Kato, T.; Gupta, M.; Yamaguchi, D.; Gan, K.P.; Nakayama, M. Supramolecular Association and Nanostructure Formation of Liquid Crystals and Polymers for New Functional Materials. Bull. Chem. Soc. Jpn. 2021, 94, 357–376. [Google Scholar] [CrossRef]
- Zhang, P.; de Haan, L.T.; Debije, M.G.; Schenning, A.P.H.J. Liquid Crystal-Based Structural Color Actuators. Light Sci. Appl. 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Mitov, M. Cholesteric Liquid Crystals in Living Matter. Soft Matter 2017, 13, 4176–4209. [Google Scholar] [CrossRef]
- Robinson, C. Liquid-Crystalline Structures in Polypeptide Solutions. Tetrahedron 1961, 13, 219–234. [Google Scholar]
- Livolant, F.; Leforestier, A. Condensed Phases of DNA: Structures and Phase Transitions. Prog. Polym. Sci. 1996, 21, 1115–1164. [Google Scholar]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef]
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid Crystalline Substances from Virus-Infected Plants. Nature 1936, 138, 1051–1052. [Google Scholar] [CrossRef]
- Lapointe, J.; Marvin, D.A. Filamentous Bacterial Viruses VIII. Liquid Crystals of Fd. Mol. Cryst. Liq. Cryst. 1973, 19, 269–278. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Smectic Phase in a Colloidal Suspension of Semiflexible Virus Particles. Phys. Rev. Lett. 1997, 78, 2417–2420. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Cholesteric Phase in Virus Suspensions. Langmuir 2000, 16, 7820–7824. [Google Scholar] [CrossRef]
- Liu, S.; Zan, T.; Chen, S.; Pei, X.; Li, H.; Zhang, Z. Thermoresponsive Chiral to Nonchiral Ordering Transformation in the Nematic Liquid-Crystal Phase of Rodlike Viruses: Turning the Survival Strategy of a Virus into Valuable Material Properties. Langmuir 2015, 31, 6995–7005. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-Based Biomimetics and Their Applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef]
- da Rosa, R.R.; Fernandes, S.N.; Mitov, M.; Godinho, M.H. Cellulose and Chitin Twisted Structures: From Nature to Applications. Adv. Funct. Mater. 2023, 34, 2304286. [Google Scholar] [CrossRef]
- Seago, A.E.; Brady, P.; Vigneron, J.-P.; Schultz, T.D. Gold Bugs and beyond: A Review of Iridescence and Structural Colour Mechanisms in Beetles (Coleoptera). J. R. Soc. Interface 2009, 6, S165–S184. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Whitney, H.M.; Glover, B.J.; Steiner, U.; Vignolini, S. Natural Helicoidal Structures: Morphology, Self-Assembly and Optical Properties. Mater. Today Proc. 2014, 1, 177–185. [Google Scholar] [CrossRef]
- Gebeshuber, I.C.; Lee, D.W. Nanostructures for Coloration (Organisms Other Than Animals). In Encyclopedia of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–19. ISBN 9789400761780. [Google Scholar]
- Kerkam, K.; Viney, C.; Kaplan, D.; Lombardi, S. Liquid Crystallinity of Natural Silk Secretions. Nature 1991, 349, 596–598. [Google Scholar] [CrossRef]
- Willcox, P.J.; Gido, S.P.; Muller, W.; Kaplan, D.L. Evidence of a Cholesteric Liquid Crystalline Phase in Natural Silk Spinning Processes. Macromolecules 1996, 29, 5106–5110. [Google Scholar] [CrossRef]
- Giraud-Guille, M.-M.; Besseau, L.; Martin, R. Liquid Crystalline Assemblies of Collagen in Bone and in Vitro Systems. J. Biomech. 2003, 36, 1571–1579. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M.; Mosser, G.; Belamie, E. Liquid Crystallinity in Collagen Systems in Vitro and in Vivo. Curr. Opin. Colloid Interface Sci. 2008, 13, 303–313. [Google Scholar] [CrossRef]
- Mouquinho, A.; Saavedra, M.; Maiau, A.; Petrova, K.; Barros, M.T.; Figueirinhas, J.L.; Sotomayor, J. Films Based on New Methacrylate Monomers: Synthesis, Characterisation and Electro-Optical Properties. Mol. Cryst. Liq. Cryst. 2011, 542, 132/[654]–140/[662]. [Google Scholar] [CrossRef]
- Selevou, A.; Papamokos, G.; Yildirim, T.; Duran, H.; Steinhart, M.; Floudas, G. Eutectic Liquid Crystal Mixture E7 in Nanoporous Alumina. Effects of Confinement on the Thermal and Concentration Fluctuations. RSC Adv. 2019, 9, 37846–37857. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Tiwari, G.; Tiwari, S.N. Electronic and Electro-Optical Properties of 5CB and 5CT Liquid Crystal Molecules: A Comparative DFT Study. Pramana J. Phys. 2021, 95, 71. [Google Scholar] [CrossRef]
- Sanyal, N.K.; Tiwari, S.N.; Roychoudhury, M. Liquid Crystalline Behaviour of Para-Azoxyanisole—A Theoretical Study of the Role of Intermolecular Interactions. Mol. Cryst. Liq. Cryst. 1986, 140, 179–193. [Google Scholar] [CrossRef]
- Pardey, R.; Zhang, A.; Gabori, P.A.; Harris, F.W.; Cheng, S.Z.D.; Adduci, J.; Facinelli, J.V.; Lenz, R.W. Monotropic Liquid Crystal Behavior in Two Poly(Ester Imides) with Even and Odd Flexible Spacers. Macromolecules 1992, 25, 5060–5068. [Google Scholar] [CrossRef]
- Shete, A.; Nadaf, S.; Doijad, R.; Killedar, S. Liquid Crystals: Characteristics, Types of Phases and Applications in Drug Delivery. Pharm. Chem. J. 2021, 55, 106–118. [Google Scholar] [CrossRef]
- Hiltrop, K. Lyotropic Liquid Crystals. In Liquid Crystals; Steinkopff: Heidelberg, Germany, 1994; pp. 143–171. [Google Scholar]
- Lydon, J. Chromonic Liquid Crystal Phases. Curr. Opin. Colloid Interface Sci. 1998, 3, 458–466. [Google Scholar] [CrossRef]
- Dierking, I.; Neto, A.M.F. Novel Trends in Lyotropic Liquid Crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Priestley, E.B. Liquid Crystal Mesophases. In Introduction to Liquid Crystals; Elsevier: Amsterdam, The Netherlands, 1975; pp. 1–13. [Google Scholar]
- Tschierske, C. Amphotropic Liquid Crystals. Curr. Opin. Colloid Interface Sci. 2002, 7, 355–370. [Google Scholar] [CrossRef]
- Sharma, V.S.; Vishwakarma, V.K.; Shrivastav, P.S.; Ammathnadu Sudhakar, A.; Sharma, A.S.; Shah, P.A. Calixarene Functionalized Supramolecular Liquid Crystals and Their Diverse Applications. ACS Omega 2022, 7, 45752–45796. [Google Scholar] [CrossRef]
- Hird, M. Banana-Shaped and Other Bent-Core Liquid Crystals. Liq. Cryst. Today 2005, 14, 9–21. [Google Scholar] [CrossRef]
- Goodby, J.W. Materials and Phase Structures of Calamitic and Discotic Liquid Crystals. In Handbook of Visual Display Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1243–1287. [Google Scholar]
- Singh, S. Phase Transitions in Liquid Crystals. Phys. Rep. 2000, 324, 107–269. [Google Scholar] [CrossRef]
- Demus, D. Phase Types, Structures and Chemistry of Liquid Crystals. In Liquid Crystals; Steinkopff: Heidelberg, Germany, 1994; pp. 1–50. [Google Scholar]
- Friedel, G. Les États Mésomorphes de La Matière. Ann. Phys. 1922, 9, 273–474. [Google Scholar] [CrossRef]
- Singh, S. Liquid Crystals: Fundamentals; World Scientific: Singapore, 2002; ISBN 978-981-02-4250-3. [Google Scholar]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Walden, P. Ueber Die Molekulargrösse Und Elektrische Leitfähigkeit Einiger Geschmolzenen Salze. Bull. l’Académie Impériale des Sci. St.-Pétersbg. 1914, 8, 405–422. [Google Scholar]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Fayer, M.D. Dynamics and Structure of Room Temperature Ionic Liquids. Chem. Phys. Lett. 2014, 616–617, 259–274. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Encyclopedia of Analytical Science; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 218–225. ISBN 9780081019832. [Google Scholar]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The Third Evolution of Ionic Liquids: Active Pharmaceutical Ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [PubMed]
- Mohammad, A.; Inamuddin, D. Green Solvents II: Properties and Applications of Ionic Liquids; Mohammad, A., Inamuddin, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-2890-5. [Google Scholar]
- Zalewska, K.; Branco, L.C. Organocatalysis with Chiral Ionic Liquids. Mini. Rev. Org. Chem. 2014, 11, 141–153. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Hejazifar, M.; Lanaridi, O.; Bica-Schröder, K. Ionic Liquid Based Microemulsions: A Review. J. Mol. Liq. 2020, 303, 112264. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic Liquid Gel Materials: Applications in Green and Sustainable Chemistry. Green Chem. 2015, 18, 105–128. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Tang, S.-F.; Mudring, A.-V. Highly Luminescent Ionic Liquids Based on Complex Lanthanide Saccharinates. Inorg. Chem. 2019, 58, 11569–11578. [Google Scholar] [CrossRef]
- Donato, M.T.; Colaço, R.; Branco, L.C.; Saramago, B. A Review on Alternative Lubricants: Ionic Liquids as Additives and Deep Eutectic Solvents. J. Mol. Liq. 2021, 333, 116004. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic Liquids for Advanced Materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Liu, C.; Chen, B.; Shi, W.; Huang, W.; Qian, H. Ionic Liquids for Enhanced Drug Delivery: Recent Progress and Prevailing Challenges. Mol. Pharm. 2022, 19, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.P.; Gindri, I.M.; Tier, A.Z.; Buriol, L.; Moreira, D.N.; Martins, M.A.P. Pharmaceutical Salts: Solids to Liquids by Using Ionic Liquid Design. In Ionic Liquids—New Aspects for the Future; IntechOpen: London, UK, 2013; pp. 557–579. [Google Scholar]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Rogers, R.D. Ionic Liquids: New Forms of Active Pharmaceutical Ingredients with Unique, Tunable Properties. Chem. Rev. 2023, 123, 11894–11953. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse Applications of Ionic Liquids: A Comprehensive Review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Knight, G.A.; Shaw, B.D. Long-Chain Alkylpyridines and Their Derivatives. New Examples of Liquid Crystals. J. Chem. Soc. 1938, 682–683. [Google Scholar] [CrossRef]
- Alvarez Fernandez, A.; Kouwer, P.H.J. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2016, 17, 731. [Google Scholar] [CrossRef]
- Arkas, M.; Paleos, C.M.; Skoulios, A. Crystal and Liquid Crystal Behaviour of N-Cyanoalkyl-N-Alkyl-N,N-Dimethylammonium Bromides: Role of the Dipole Interactions of the Cyano Groups. Liq. Cryst. 1997, 22, 735–742. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, R.; Wang, Y.; Saielli, G. Effect of the Chain Length on the Structure of Ionic Liquids: From Spatial Heterogeneity to Ionic Liquid Crystals. J. Phys. Chem. B 2013, 117, 1104–1109. [Google Scholar] [CrossRef]
- Sharma, V.S.; Vekariya, R.H.; Sharma, A.S.; Patel, R.B. Mesomorphic Properties of Liquid Crystalline Compounds with Chalconyl Central Linkage in Two Phenyl Rings. Liq. Cryst. Today 2017, 26, 46–54. [Google Scholar] [CrossRef]
- Sasi, R.; Sarojam, S.; Devaki, S.J. High Performing Biobased Ionic Liquid Crystal Electrolytes for Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 3535–3543. [Google Scholar] [CrossRef]
- Devaki, S.J.; Sasi, R. Ionic Liquids/Ionic Liquid Crystals for Safe and Sustainable Energy Storage Systems. In Progress and Developments in Ionic Liquids; IntechOpen: London, UK, 2017; pp. 313–336. ISBN 0000957720. [Google Scholar]
- Quevillon, M.J.; Whitmer, J.K. Charge Transport and Phase Behavior of Imidazolium-Based Ionic Liquid Crystals from Fully Atomistic Simulations. Materials 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; He, Y.; Tao, X.; Xu, X.-Q.; Wu, X.; Wang, Y. Crystal-Confined Freestanding Ionic Liquids for Reconfigurable and Repairable Electronics. Nat. Commun. 2019, 10, 547. [Google Scholar] [CrossRef]
- Yuan, F.; Chi, S.; Dong, S.; Zou, X.; Lv, S.; Bao, L.; Wang, J. Ionic Liquid Crystal with Fast Ion-Conductive Tunnels for Potential Application in Solvent-Free Li-Ion Batteries. Electrochim. Acta 2019, 294, 249–259. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, J. Ionic Liquids in Surface Electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, Q. A New Class of Ion-Ion Interaction: Z-Bond. Sci. China Chem. 2015, 58, 495–500. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, J. Understanding the Hydrogen Bonds in Ionic Liquids and Their Roles in Properties and Reactions. Chem. Commun. 2016, 52, 6744–6764. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Wang, C.; Lu, Y.; Dong, K.; Huo, F.; Zhang, S. Insights into Ionic Liquids: From Z-Bonds to Quasi-Liquids. JACS Au 2022, 2, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Z.; Fu, Y.-C.; Wei, Y.-M.; Yan, J.-W.; Mao, B.-W. The Electrode/Ionic Liquid Interface: Electric Double Layer and Metal Electrodeposition. ChemPhysChem 2010, 11, 2764–2778. [Google Scholar] [CrossRef]
- Wang, X.; Salari, M.; Jiang, D.; Chapman Varela, J.; Anasori, B.; Wesolowski, D.J.; Dai, S.; Grinstaff, M.W.; Gogotsi, Y. Electrode Material–Ionic Liquid Coupling for Electrochemical Energy Storage. Nat. Rev. Mater. 2020, 5, 787–808. [Google Scholar] [CrossRef]
- Abe, H.; Nemoto, F.; Hiroi, K.; Takata, S. Nanoconfined Water in Ionic Liquid and Lyotropic Ionic Liquid Crystals by Small- and Wide-Angle X-ray and Neutron Scattering: 1-Decyl-3-Methylimidazolium Nitrate. J. Mol. Liq. 2023, 386, 122551. [Google Scholar] [CrossRef]
- Song, Z.; Xin, X.; Shen, J.; Jiao, J.; Xia, C.; Wang, S.; Yang, Y. Manipulation of Lyotropic Liquid Crystal Behavior of Ionic Liquid-Type Imidazolium Surfactant by Amino Acids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 7–14. [Google Scholar] [CrossRef]
- Goujon, N.; Dumée, L.F.; Byrne, N.; Bryant, G.; Forsyth, M. Impact of Comonomer Chemistry on Phase Behavior of Polymerizable Lyotropic Ionic Liquid Crystals: A Pre- and Post-Polymerization Study. Macromol. Chem. Phys. 2018, 219, 1800097. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Koval’chuk, A.; Grydyakina, A.; Bugaychuk, S.; Mirnaya, T.; Klimusheva, G. Electrical Conductivity of Lyotropic and Thermotropic Ionic Liquid Crystals Consisting of Metal Alkanoates. Liq. Cryst. 2007, 34, 599–603. [Google Scholar] [CrossRef]
- Bordyuh, A.; Garbovskiy, Y.; Bugaychuk, S.; Klimusheva, G.; Reshetnyak, V. Fast Nolinear Optical Mechanisms in Bi-Layered Cells Composed by Lyotropic Ionic Liquid Crystals with Dye and Viologen Films. Mol. Cryst. Liq. Cryst. 2009, 508, 296/[658]–308/[670]. [Google Scholar] [CrossRef]
- Bugaychuk, S. Fast Nonlinear Optical Mechanism of Photoconversion in Systems of Lyotropic Ionic Liquid Crystals-Viologen Impurities. Mol. Phys. 2011, 109, 1567–1574. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Nedeltchev, A.K.; Han, H. Synthesis, Thermal and Lyotropic Liquid Crystalline Properties of Protic Ionic Salts. Liq. Cryst. 2008, 35, 757–764. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Bao, Z.; Su, B.; Zhang, Z.; Ren, Q.; Yang, Y.; Xing, H. Nonaqueous Lyotropic Ionic Liquid Crystals: Preparation, Characterization, and Application in Extraction. Chem. A Eur. J. 2015, 21, 9150–9156. [Google Scholar] [CrossRef]
- Misono, T.; Sekihara, R.; Endo, T.; Sakai, K.; Abe, M.; Sakai, H. Ternary Phase Behavior of Phytosterol Ethoxylate, Water, and Imidazolium-Based Ionic Liquid Systems—Lyotropic Liquid Crystal Formation over a Wide Range of Compositions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 117–123. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Stillinger, F.H. Supercooled Liquids and the Glass Transition. Nature 2001, 410, 259–267. [Google Scholar] [CrossRef]
- Kremer, F.; Huwe, A.; Schönhals, A.; Różański, S.A. Molecular Dynamics in Confining Space. In Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 171–224. [Google Scholar]
- Kremer, F. Dynamics in Geometrical Confinement; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-319-06099-6. [Google Scholar]
- Ryu, S.H.; Gim, M.-J.; Lee, W.; Choi, S.-W.; Yoon, D.K. Switchable Photonic Crystals Using One-Dimensional Confined Liquid Crystals for Photonic Device Application. ACS Appl. Mater. Interfaces 2017, 9, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Spengler, M.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J.; Giese, M. Hydrogen-Bonded Liquid Crystals in Confined Spaces—Toward Photonic Hybrid Materials. Adv. Funct. Mater. 2018, 28, 1800207. [Google Scholar] [CrossRef]
- d’Alessandro, A.; Asquini, R. Light Propagation in Confined Nematic Liquid Crystals and Device Applications. Appl. Sci. 2021, 11, 8713. [Google Scholar] [CrossRef]
- Galluzzi, M.; Bovio, S.; Milani, P.; Podestà, A. Surface Confinement Induces the Formation of Solid-Like Insulating Ionic Liquid Nanostructures. J. Phys. Chem. C 2018, 122, 7934–7944. [Google Scholar] [CrossRef]
- Grigoriadis, C.; Duran, H.; Steinhart, M.; Kappl, M.; Butt, H.-J.; Floudas, G. Suppression of Phase Transitions in a Confined Rodlike Liquid Crystal. ACS Nano 2011, 5, 9208–9215. [Google Scholar] [CrossRef]
- Kohler, F.T.U.; Morain, B.; Weiß, A.; Laurin, M.; Libuda, J.; Wagner, V.; Melcher, B.U.; Wang, X.; Meyer, K.; Wasserscheid, P. Surface-Functionalized Ionic Liquid Crystal–Supported Ionic Liquid Phase Materials: Ionic Liquid Crystals in Mesopores. ChemPhysChem 2011, 12, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Kolmangadi, M.A.; Zhuoqing, L.; Smales, G.J.; Pauw, B.R.; Wuckert, E.; Raab, A.; Laschat, S.; Huber, P.; Schönhals, A. Confinement-Suppressed Phase Transition and Dynamic Self-Assembly of Ionic Superdiscs in Ordered Nanochannels: Implications for Nanoscale Applications. ACS Appl. Nano Mater. 2023, 6, 15673–15684. [Google Scholar] [CrossRef]
- Li, Z.; Raab, A.; Kolmangadi, M.A.; Busch, M.; Grunwald, M.; Demel, F.; Bertram, F.; Kityk, A.V.; Schönhals, A.; Laschat, S.; et al. Self-Assembly of Ionic Superdiscs in Nanopores. ACS Nano 2024, 18, 14414–14426. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.M.; Viciosa, M.T.; Matos, I.; Sotomayor, J.C.; Figueirinhas, J.L.; Godinho, M.H.; Branco, L.C.; Dias, C.J.; Dionísio, M. Impact of Nanoconfinement on the Physical State and Conductivity Mechanisms of a 2-Picolinium Ionic Liquid Crystal. J. Mol. Liq. 2024, 403, 124830. [Google Scholar] [CrossRef]
- Nobori, H.; Fujimoto, D.; Yoshioka, J.; Fukao, K.; Konishi, T.; Taguchi, K. Phase Transitions and Dynamics in Ionic Liquid Crystals Confined in Nanopores. J. Chem. Phys. 2024, 160, 044902. [Google Scholar] [CrossRef]
- Krause, C.; Schönhals, A. Phase Transitions and Molecular Mobility of a Discotic Liquid Crystal. J. Phys. Chem. C 2013, 117, 19712–19720. [Google Scholar]
- Kobayashi, T.; Ichikawa, T.; Kato, T.; Ohno, H. Development of Glassy Bicontinuous Cubic Liquid Crystals for Solid Proton-Conductive Materials. Adv. Mater. 2017, 29, 1604429. [Google Scholar] [CrossRef]
- Busch, M.; Kityk, A.V.; Piecek, W.; Hofmann, T.; Wallacher, D.; Całus, S.; Kula, P.; Steinhart, M.; Eich, M.; Huber, P. A Ferroelectric Liquid Crystal Confined in Cylindrical Nanopores: Reversible Smectic Layer Buckling, Enhanced Light Rotation and Extremely Fast Electro-Optically Active Goldstone Excitations. Nanoscale 2017, 9, 19086–19099. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A Tool to Study the Thermal Behavior of Lipid Bilayers and Liposomal Stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Lukas, K.; LeMaire, P.K. Differential Scanning Calorimetry: Fundamental Overview. Resonance 2009, 14, 807–817. [Google Scholar] [CrossRef]
- Sarge, S.M.; Höhne, G.W.H.; Hemminger, W. Calorimetry: Fundamentals, Instrumentation and Applications; Wiley: Hoboken, NJ, USA, 2014; ISBN 9783527327614. [Google Scholar]
- Bhugra, C.; Pikal, M.J. Role of Thermodynamic, Molecular, and Kinetic Factors in Crystallization from the Amorphous State. J. Pharm. Sci. 2008, 97, 1329–1349. [Google Scholar] [CrossRef]
- Varghese, N.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. A Calorimetric Investigation of the Assembly of Gold Nanorods to Form Necklaces. Chem. Phys. Lett. 2008, 450, 340–344. [Google Scholar] [CrossRef]
- Brown, M.E. Handbook of Thermal Analysis and Calorimetry: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Clas, S.-D.; Dalton, C.R.; Hancock, B.C. Differential Scanning Calorimetry: Applications in Drug Development. Pharm. Sci. Technol. Today 1999, 2, 311–320. [Google Scholar] [CrossRef]
- Robertson, L.A. Antoni van Leeuwenhoek 1723–2023: A Review to Commemorate Van Leeuwenhoek’s Death, 300 Years Ago. Antonie Van Leeuwenhoek 2023, 116, 919–935. [Google Scholar] [CrossRef]
- Olympus Basics of Polarizing Microscopy. 2013. Available online: https://www.yumpu.com/en/document/view/17552622/basics-of-polarizing-microscopy-olympus (accessed on 29 July 2024).
- Oldenbourg, R. Polarized Light Microscopy: Principles and Practice. Cold Spring Harb. Protoc. 2013, 11, 1023–1036. [Google Scholar] [CrossRef]
- Goodby, J.W. Introduction to Defect Textures in Liquid Crystals. In Handbook of Visual Display Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1897–1924. ISBN 9783319143460. [Google Scholar]
- Dierking, I. Textures of Liquid Crystals; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527307258. [Google Scholar]
- Sackmann, H.; Demus, D. The Polymorphism of Liquid Crystals. Mol. Cryst. 1966, 2, 81–102. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Introduction to Diffraction. In Principles and Applications of Powder Diffraction; Wiley: Hoboken, NJ, USA, 2009; pp. 73–122. [Google Scholar]
- Bragg, W.H.; Bragg, W.L. The Reflection of X-rays by Crystals. Proc. R. Soc. A 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Liu, L.; Boldon, L.; Urquhart, M.; Wang, X. Small and Wide Angle X-Ray Scattering Studies of Biological Macromolecules in Solution. J. Vis. Exp. 2013, 71, e4160. [Google Scholar] [CrossRef]
- Seddon, J.M. Structural Studies of Liquid Crystals by X-ray Diffraction. In Handbook of Liquid Crystals; Wiley-VCH Verlag: Hoboken, NJ, USA, 1998; pp. 635–679. [Google Scholar]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer After Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Goossens, K.; Rakers, L.; Heinrich, B.; Ahumada, G.; Ichikawa, T.; Donnio, B.; Shin, T.J.; Bielawski, C.W.; Glorius, F. Anisotropic, Organic Ionic Plastic Crystal Mesophases from Persubstituted Imidazolium Pentacyanocyclopentadienide Salts. Chem. Mater. 2019, 31, 9593–9603. [Google Scholar] [CrossRef]
- Timmermans, J. Plastic Crystals: A Historical Review. J. Phys. Chem. Solids 1961, 18, 1–8. [Google Scholar] [CrossRef]
- Simonov, A.; Goodwin, A.L. Designing Disorder into Crystalline Materials. Nat. Rev. Chem. 2020, 4, 657–673. [Google Scholar] [CrossRef]
- Mudring, A.-V. Solidification of Ionic Liquids: Theory and Techniques. Aust. J. Chem. 2010, 63, 544–564. [Google Scholar] [CrossRef]
- Kimata, H.; Mochida, T. Effects of Molecular Structure on Phase Transitions of Ionic Plastic Crystals Containing Cationic Sandwich Complexes. Cryst. Growth Des. 2018, 18, 7562–7569. [Google Scholar] [CrossRef]
- d’Agostino, S.; Fornasari, L.; Braga, D. Binary and Ternary Solid Solutions of Ionic Plastic Crystals, and Modulation of Plastic Phase Transitions. Cryst. Growth Des. 2019, 19, 6266–6273. [Google Scholar] [CrossRef]
- Park, C.B.; Sung, B.J. Heterogeneous Rotational Dynamics of Imidazolium-Based Organic Ionic Plastic Crystals. J. Phys. Chem. B 2020, 124, 6894–6904. [Google Scholar] [CrossRef]
- Sirigiri, N.; Chen, F.; Forsyth, C.M.; Yunis, R.; O’Dell, L.; Pringle, J.M.; Forsyth, M. Factors Controlling the Physical Properties of an Organic Ionic Plastic Crystal. Mater. Today Phys. 2022, 22, 100603. [Google Scholar] [CrossRef]
- Santos, A.F.M.; Figueirinhas, J.L.; Dias, C.J.; Godinho, M.H.; Branco, L.C.; Dionísio, M. Study of the Mesomorphic Properties and Conductivity of N-Alkyl-2-Picolinium Ionic Liquid Crystals. J. Mol. Liq. 2023, 377, 121456. [Google Scholar] [CrossRef]
- Chazhengina, S.Y.; Kotelnikova, E.N.; Filippova, I.V.; Filatov, S.K. Phase Transitions of N-Alkanes as Rotator Crystals. J. Mol. Struct. 2003, 647, 243–257. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Rotator Phases in Alkane Systems: In Bulk, Surface Layers and Micro/Nano-Confinements. Adv. Colloid Interface Sci. 2019, 269, 7–42. [Google Scholar] [CrossRef]
- Cholakova, D.; Tsvetkova, K.; Tcholakova, S.; Denkov, N. Rheological Properties of Rotator and Crystalline Phases of Alkanes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127926. [Google Scholar] [CrossRef]
- Burrows, S.A.; Lin, E.E.; Cholakova, D.; Richardson, S.; Smoukov, S.K. Structure of the Hexadecane Rotator Phase: Combination of X-ray Spectra and Molecular Dynamics Simulation. J. Phys. Chem. B 2023, 127, 7772–7784. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; He, Z.; Su, F.; Zhang, J.; Wang, Y.; Xin, Y.; Wang, H.; Yao, D.; Li, M.; et al. Dual Stimuli-Responsive Porous Ionic Liquids with the Reversible Phase Transition Behavior Based on Ionic Liquid Crystals for CO2 and C2H4 Adsorption. J. Mater. Chem. A 2022, 10, 13333–13344. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ogawa, T.; Nada, H.; Nakatsuji, K.; Mitani, M.; Soberats, B.; Kawata, K.; Yoshio, M.; Tomioka, H.; Sasaki, T.; et al. Development of Nanostructured Water Treatment Membranes Based on Thermotropic Liquid Crystals: Molecular Design of Sub-Nanoporous Materials. Adv. Sci. 2018, 5, 1700405. [Google Scholar] [CrossRef]
- Avilés, M.D.; Sánchez, C.; Pamies, R.; Sanes, J.; Bermúdez, M.D. Ionic Liquid Crystals in Tribology. Lubricants 2019, 7, 72. [Google Scholar] [CrossRef]
- Xia, X.; Peng, J.; Wan, Q.; Wang, X.; Fan, Z.; Zhao, J.; Li, F. Functionalized Ionic Liquid-Crystal Additive for Perovskite Solar Cells with High Efficiency and Excellent Moisture Stability. ACS Appl. Mater. Interfaces 2021, 13, 17677–17689. [Google Scholar] [CrossRef]
- Liu, C.; Yoshio, M. Ionic Liquid Crystal−Polymer Composite Electromechanical Actuators: Design of Two-Dimensional Molecular Assemblies for Efficient Ion Transport and Effect of Electrodes on Actuator Performance. ACS Appl. Mater. Interfaces 2024, 16, 27750–27760. [Google Scholar] [CrossRef]
- Stappert, K.; Muthmann, J.; Spielberg, E.T.; Mudring, A.-V. Azobenzene-Based Organic Salts with Ionic Liquid and Liquid Crystalline Properties. Cryst. Growth Des. 2015, 15, 4701–4712. [Google Scholar] [CrossRef]
- Asaftei, S.; Ciobanu, M.; Lepadatu, A.M.; Song, E.; Beginn, U. Thermotropic Ionic Liquid Crystals by Molecular Assembly and Ion Pairing of 4,4′-Bipyridinium Derivatives and Tris(Dodecyloxy)Benzenesulfonates in a Non-Polar Solvent. J. Mater. Chem. 2012, 22, 14426–14437. [Google Scholar] [CrossRef]
- Veltri, L.; Cavallo, G.; Beneduci, A.; Metrangolo, P.; Corrente, G.A.; Ursini, M.; Romeo, R.; Terraneo, G.; Gabriele, B. Synthesis and Thermotropic Properties of New Green Electrochromic Ionic Liquid Crystals. New J. Chem. 2019, 43, 18285–18293. [Google Scholar] [CrossRef]
- Pibiri, I.; Beneduci, A.; Carraro, M.; Causin, V.; Casella, G.; Corrente, G.A.; Chidichimo, G.; Pace, A.; Riccobono, A.; Saielli, G. Mesomorphic and Electrooptical Properties of Viologens Based on Non-Symmetric Alkyl/Polyfluoroalkyl Functionalization and on an Oxadiazolyl-Extended Bent Core. J. Mater. Chem. C 2019, 7, 7974–7983. [Google Scholar] [CrossRef]
- Haristoy, D.; Tsiourvas, D. Novel Ionic Liquid-Crystalline Compounds Bearing Oxadiazole and Pyridinium Moieties as Prospective Materials for Optoelectronic Applications. Chem. Mater. 2003, 15, 2079–2083. [Google Scholar] [CrossRef]
- Haristoy, D.; Tsiourvas, D. Effect of Counterions on the Thermotropic and Thermochromic Properties of Ionic Liquid Crystals. Liq. Cryst. 2004, 31, 697–703. [Google Scholar] [CrossRef]
- Lo Celso, F.; Pibiri, I.; Triolo, A.; Triolo, R.; Pace, A.; Buscemi, S.; Vivona, N. Study on the Thermotropic Properties of Highly Fluorinated 1,2,4-Oxadiazolylpyridinium Salts and Their Perspective Applications as Ionic Liquid Crystals. J. Mater. Chem. 2007, 17, 1201–1208. [Google Scholar] [CrossRef]
- Huang, Z.; Yi, M.; Xu, Y.; Qi, P.; Liu, Y.; Song, A.; Hao, J. Fluorescent Magnetic Ionic Liquids with Multiple Responses to Temperature, Humidity and Organic Vapors. J. Mater. Chem. C 2021, 9, 13276–13285. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q. Photochromism into Nanosystems: Towards Lighting up the Future Nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, J.; Liu, H.; Chen, X. A Luminescent Lyotropic Liquid Crystal with UV Irradiation Induced Photochromism. Soft Matter 2020, 16, 1170–1178. [Google Scholar] [CrossRef]
- Jordão, N.; Cabrita, L.; Pina, F.; Branco, L.C. Novel Bipyridinium Ionic Liquids as Liquid Electrochromic Devices. Chem. A Eur. J. 2014, 20, 3982–3988. [Google Scholar] [CrossRef]
- Jordão, N.; Cruz, H.; Branco, A.; Pina, F.; Branco, L.C. Electrochromic Devices Based on Disubstituted Oxo-Bipyridinium Ionic Liquids. ChemPlusChem 2015, 80, 202–208. [Google Scholar] [CrossRef]
- Jordão, N.; Cruz, H.; Branco, A.; Pinheiro, C.; Pina, F.; Branco, L.C. Switchable Electrochromic Devices Based on Disubstituted Bipyridinium Derivatives. RSC Adv. 2015, 5, 27867–27873. [Google Scholar] [CrossRef]
- Jordão, N.; Cruz, H.; Branco, A.; Pina, F.; Branco, L.C. Bis(Bipyridinium) Salts as Multicolored Electrochromic Devices. ChemPlusChem 2017, 82, 1211–1217. [Google Scholar] [CrossRef]
- Jordão, N.; Cruz, H.; Pina, F.; Branco, L.C. Studies of Bipyridinium Ionic Liquids and Deep Eutectic Solvents as Electrolytes for Electrochromic Devices. Electrochim. Acta 2018, 283, 718–726. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, L.; Guan, F.; Li, X.; Wang, R.; Xu, J.; Qin, Y.; Chen, G. Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens. Materials 2021, 14, 1702. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-H.; Eo, H.; Choi, T.-H.; Kim, W.-S.; Oh, S.-W. A Simulation of Diffractive Liquid Crystal Smart Window for Privacy Application. Sci. Rep. 2022, 12, 11384. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Han, J.; Yang, L.; Li, J.; Song, Z.; Wang, T.; Zhu, J. Advanced Liquid Crystal-Based Switchable Optical Devices for Light Protection Applications: Principles and Strategies. Light Sci. Appl. 2023, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Mirnaya, T.A.; Volkov, S.V. Ionic Liquid Crystals as Universal Matrices (Solvents). In Green Industrial Applications of Ionic Liquids; Springer: Dordrecht, The Netherlands, 2002; pp. 439–456. [Google Scholar]
- Ichikawa, T.; Kuwana, M.; Suda, K. Chromonic Ionic Liquid Crystals Forming Nematic and Hexagonal Columnar Phases. Crystals 2022, 12, 1548. [Google Scholar] [CrossRef]
- Kondo, M.; Yanai, S.; Shirata, S.; Kakibe, T.; Nishida, J.; Kawatsuki, N. Multichromic Behavior of Liquid Crystalline Composite Polymeric Films. Crystals 2023, 13, 786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.F.M.; Figueirinhas, J.L.; Dionísio, M.; Godinho, M.H.; Branco, L.C. Ionic Liquid Crystals as Chromogenic Materials. Materials 2024, 17, 4563. https://doi.org/10.3390/ma17184563
Santos AFM, Figueirinhas JL, Dionísio M, Godinho MH, Branco LC. Ionic Liquid Crystals as Chromogenic Materials. Materials. 2024; 17(18):4563. https://doi.org/10.3390/ma17184563
Chicago/Turabian StyleSantos, Andreia F. M., João L. Figueirinhas, Madalena Dionísio, Maria H. Godinho, and Luis C. Branco. 2024. "Ionic Liquid Crystals as Chromogenic Materials" Materials 17, no. 18: 4563. https://doi.org/10.3390/ma17184563
APA StyleSantos, A. F. M., Figueirinhas, J. L., Dionísio, M., Godinho, M. H., & Branco, L. C. (2024). Ionic Liquid Crystals as Chromogenic Materials. Materials, 17(18), 4563. https://doi.org/10.3390/ma17184563








