Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
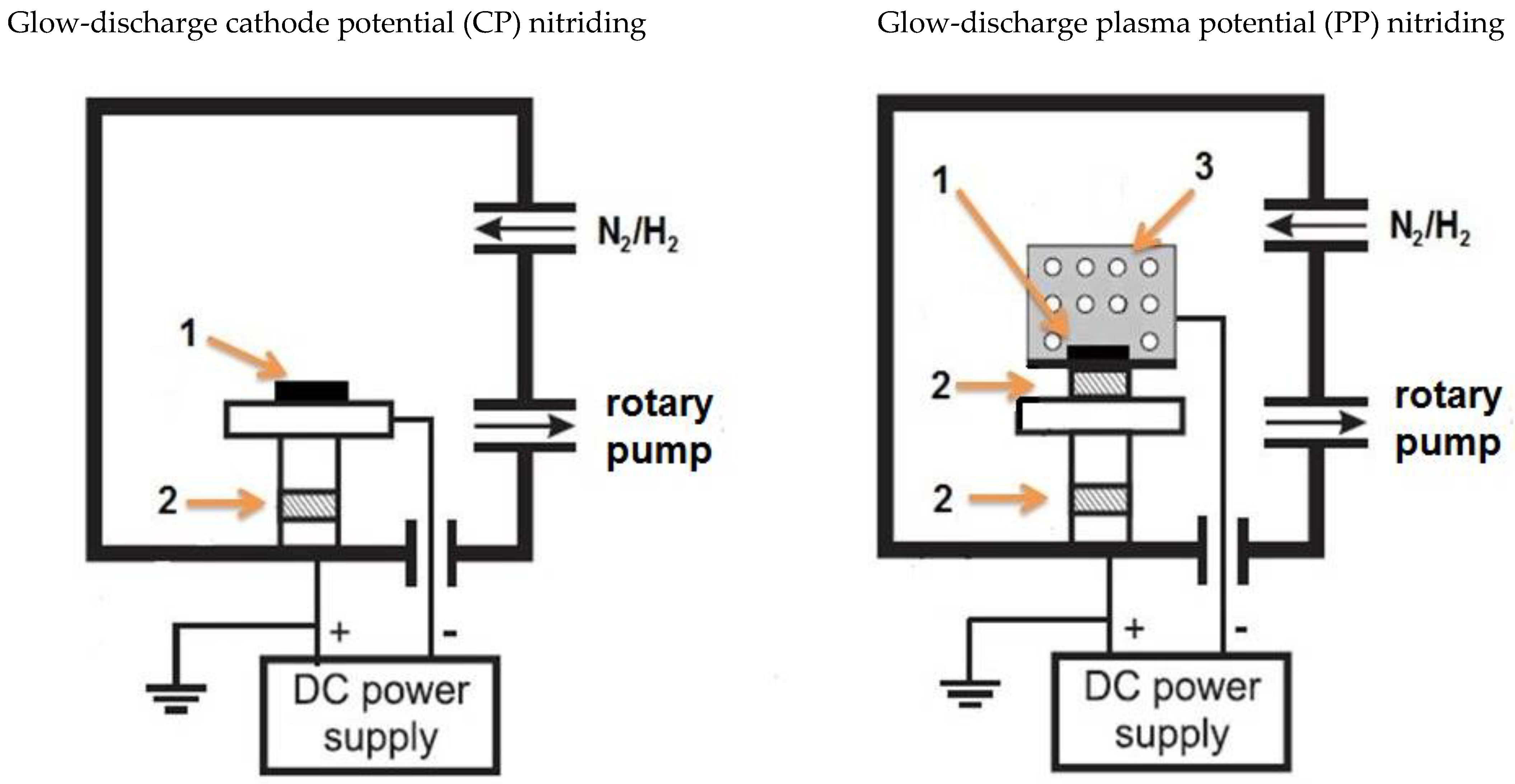
2.2. Glow Nitriding Process
2.3. Nanohardness of the Nitrided Specimen
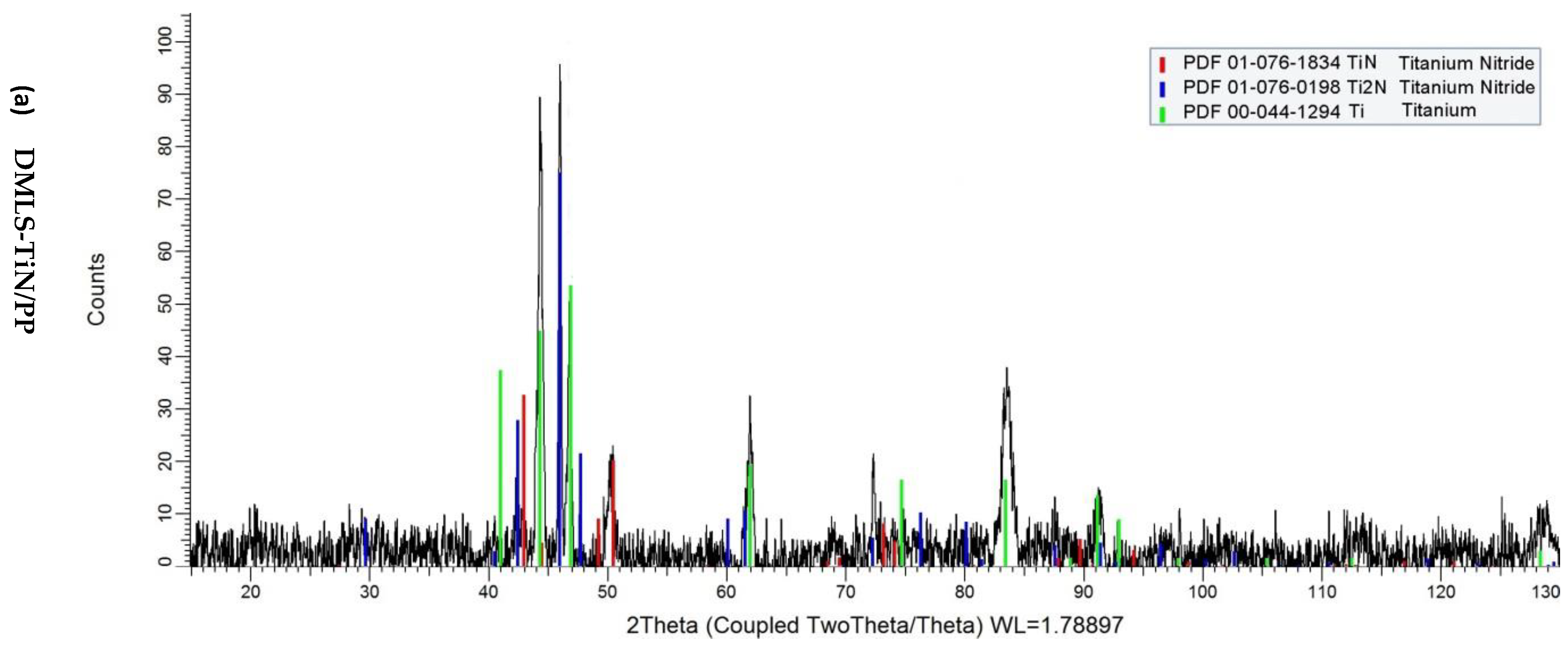
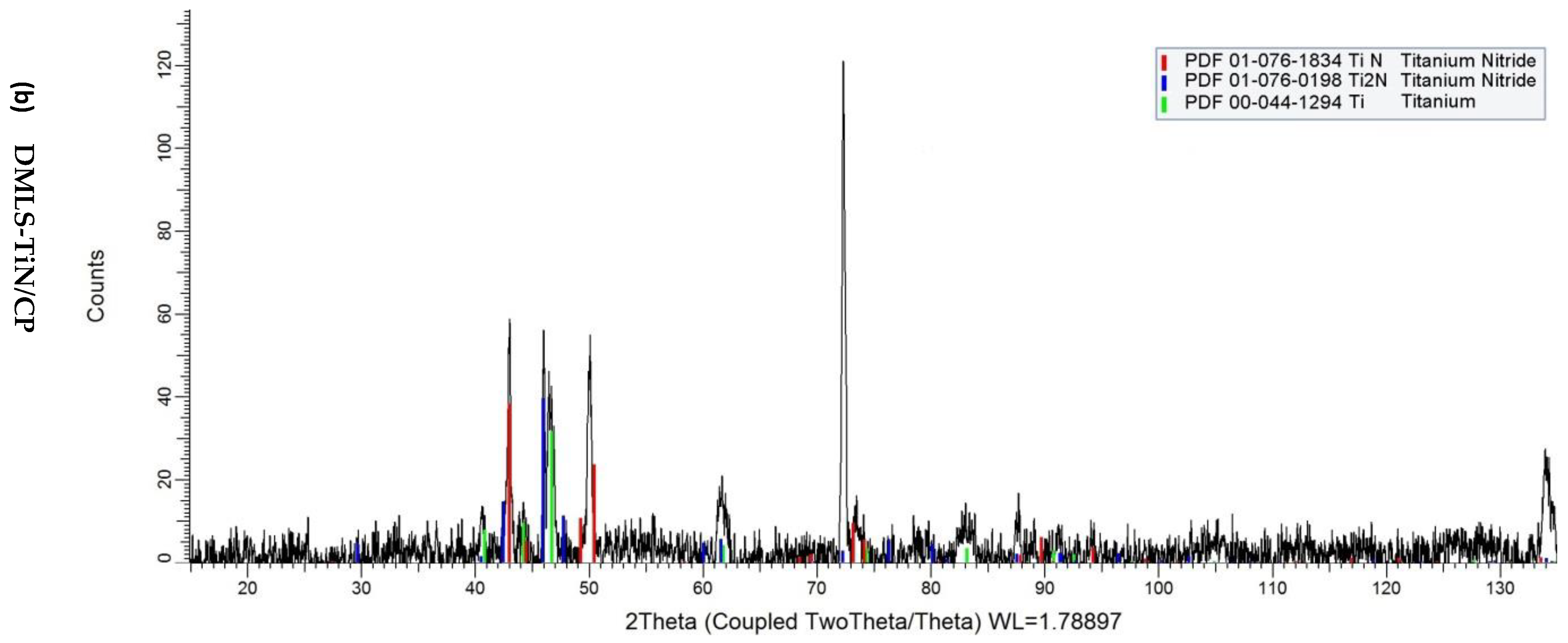
2.4. XRD Diffraction Test
2.5. Corrosion Resistance Test
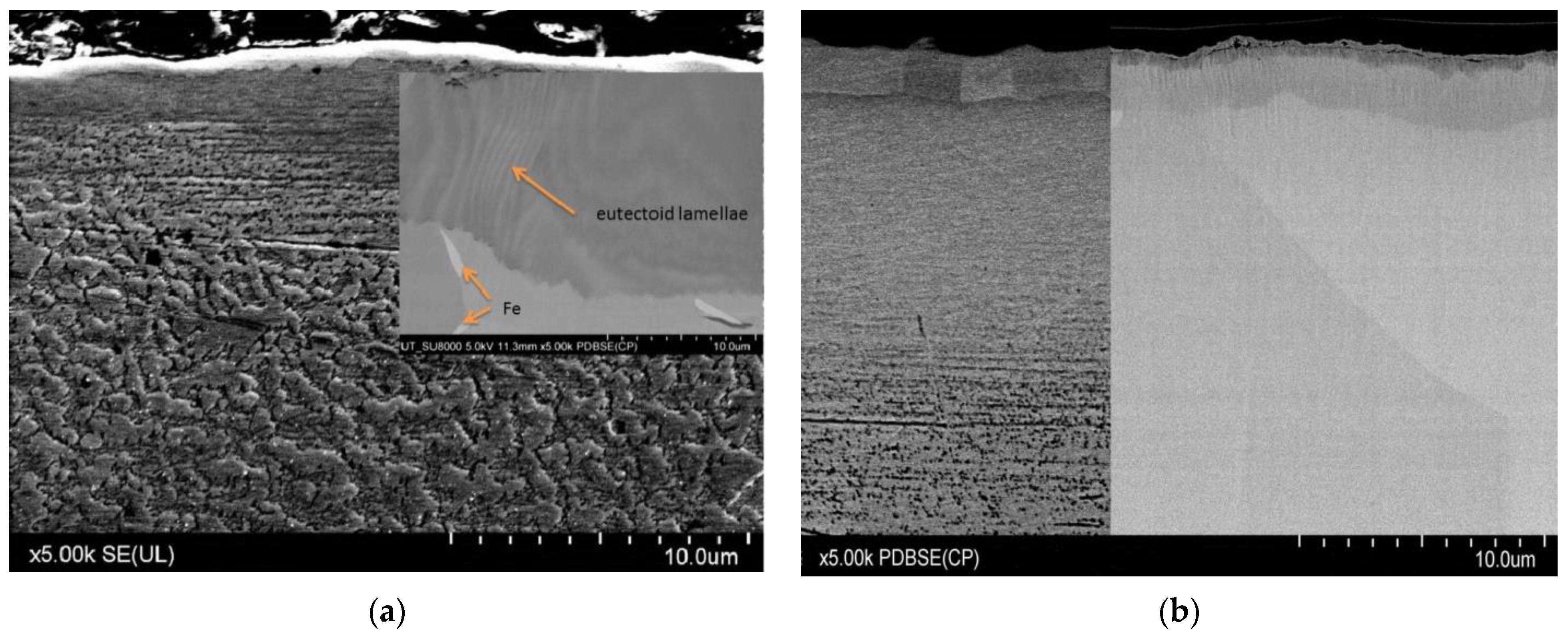
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys; a brief review. Rev. Adv. Mater. Sci. 2012, 32, 14–34. [Google Scholar]
- Singh, P.; Pungotra, H.; Kalsi, N.S. On the characteristics of titanium alloys for the aircraft applications. Mater. Today Proc. 2017, 4, 8971–8982. [Google Scholar] [CrossRef]
- She, D.; Yue, W.; Fu, Z.; Wang, C.; Yang, X.; Liu, J. Effects of nitriding temperature on microstructures and vacuum tribological properties of plasma-nitrided titanium. Surf. Coat. Technol. 2015, 264, 32–40. [Google Scholar] [CrossRef]
- Osip, H.; Wojcik, J. The Use of Titanium Alloys in Shipbuilding. Weld. Int. 1995, 9, 597–600. [Google Scholar] [CrossRef]
- Gilbert, J.R.B. The uses of titanium. Mater. Sci. Technol. 1985, 1, 257–262. [Google Scholar] [CrossRef]
- Gooch, W.A. The Design and Application of Titanium Alloys to U.S. Army Platforms. In Proceedings of the International Titanium Association Titanium 2010, Kissimee, FL, USA, 3–6 October 2010. [Google Scholar]
- Meda, U.S.; Vora, K.; Athreya, Y.; Mandi, U.A. Titanium dioxide based heterogeneous and heterojunction photocatalysts for pollution control applications in the construction industry. Process Saf. Environ. Prot. 2022, 161, 771–787. [Google Scholar] [CrossRef]
- Adamus, J. Applications of titanium sheets in modern building construction. Adv. Mater. Res. 2014, 1020, 9–14. [Google Scholar] [CrossRef]
- Brunette, D.M.; Teengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine, Materials Science, Surface Science, Engineering, Biological Responses and Medical Application; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2001; p. 1005. [Google Scholar]
- Jackson, M.J.; Kopac, J.; Balazic, M.; Bombac, D.; Brojan, M.; Kosel, F. Titanium and Titanium Alloy Applications in Medicine; Surgical Tools and Medical Devices; Ahmed, W., Jackson, M.J., Eds.; Springer: Cham, Switzerland, 2016; p. 691. [Google Scholar] [CrossRef]
- Dobrzański, L.A. Descriptive Metal Science of Non-Ferrous Metal Alloys; Publishing House of Silesian University of Technology: Gliwice, Poland, 2008; p. 406. [Google Scholar]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New Titanium Alloys, Promising Materials for Medical Devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef]
- Panda, K.B.; Ravi Chandran, K.S. Titanium-titanium boride (Ti-TiB) functionally graded materials through reaction sintering: Synthesis, microstructure, and properties. Met. Mater. Trans. A 2003, 34, 1993–2003. [Google Scholar] [CrossRef]
- Eger, M.; Sterer, N.; Liron, T.; Kohavi, D.; Gabet, Y. Scaling of titanium implants entrains inflammation-induced osteolysis. Sci. Rep. 2017, 7, 39612. [Google Scholar] [CrossRef]
- Dwivedi, D.K. Surface Engineering Enhancing Life of Tribological Components; Springer (India) Pvt. Ltd.: New Delhi, India, 2018; p. 220. [Google Scholar]
- Wierzchon, T.; Czarnowska, E.; Grzonka, J.; Sowinska, A.; Tarnowski, M.; Kaminski, J.; Kulikowski, K.; Borowski, T.; Kurzydłowski, K.J. Glow discharge assisted oxynitriding process of titanium for medical application. App. Surf. Sci. 2015, 334, 74–79. [Google Scholar] [CrossRef]
- Tarnowski, M.; Borowski, T.; Skrzypek, S.; Kulikowski, K.; Wierzchon, T. Shaping the structure and properties of titanium and Ti6Al7Nb titanium alloy in low-temperature plasma nitriding processes. J. Alloys Comp. 2021, 864, 158896. [Google Scholar] [CrossRef]
- Roliński, E. Isothermal and Cyclic Plasma Nitriding of Titanium Alloys. Sur. Eng. 1986, 2, 35–42. [Google Scholar] [CrossRef]
- Menthe, E.; Rie, K.T. Further investigation of the structure and properties of austenitic stainless steel after plasma nitriding. Surf. Coat. Technol. 1999, 116–119, 199–204. [Google Scholar] [CrossRef]
- Sitek, R.; Kaminski, J.; Mizera, J. Corrosion Resistance of the Inconel 740H Nickel Alloy after Pulse Plasma Nitriding at a Frequency of 10 kHz. Acta Phys. Pol. A 2016, 129, 584–587. [Google Scholar] [CrossRef]
- Solouk, A.; Cousins, B.G.; Mirzadeh, H.; Seifalian, A.M. Application of plasma face modification techniques to improve hemocompatibility of vascular grafts: A review. Biotechnol. Appl. Biochem. 2011, 58, 311–327. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Affonço, L.J.; Lisboa-Filho, P.N.; da Silva, J.H.D.; Rocha, L.A.; Pinto, A.M.P.; Toptan, F. Corrosion and tribocorrosion behaviour of titanium nitride thin films grown on titanium under different deposition times. Surf. Coat. Technol. 2019, 374, 878–888. [Google Scholar] [CrossRef]
- Roliński, E. Nitriding of Titanium Alloys. In ASM Handbook, Heat Treating of Nonferrous Alloys; Totten, G.E., Ed.; ASM International: West Conshohocken, PA, USA, 2016; Volume 4E, pp. 604–621. [Google Scholar]
- Morgiel, J.; Maj, Ł.; Szymkiewicz, K.; Pomorska, M.; Ozga, P.; Toboła, D.; Tarnowski, M.; Wierzchoń, T. Surface roughening of Ti-6Al-7Nb alloy plasma nitrided at cathode potential. Appl. Surf. Sci. 2022, 574, 151639. [Google Scholar] [CrossRef]
- Sowinska, A.; Czarnowska, E.; Tarnowski, M.; Witkowska, J.; Wierzchon, T. Structure and hemocompatibility of nanocrystalline titanium nitride produced under glow-discharge conditions. Appl. Surf. Sci. 2018, 436, 382–390. [Google Scholar] [CrossRef]
- Majchrowicz, K.; Pakiela, Z.; Kaminski, J.; Plocinska, M.; Kurzynowski, T.; Chlebus, E. The Effect of Rhenium Addition on Microstructure and Corrosion Resistance of Inconel 718 Processed by Selective Laser Melting. Metal. Mater. Trans. A 2018, 49A, 6479–6489. [Google Scholar] [CrossRef]
- Sitek, R.; Kaminski, J.; Adamczyk-Cieslak, B.; Molak, R.; Spychalski, M.; Cowell, B.; McCann, J.; Rolinski, E. Effect of Plasma Nitriding on Structure and Properties of Titanium Grade2 Produced by Direct Metal Laser Sintering. Metal. Microstr. Anal. 2022, 11, 852–863. [Google Scholar] [CrossRef]
- Kaminski, J.; Witkowska, J.; Wierzchon, T. Corrosion resistance of NiTi shape memory alloy afer nitriding and oxynitriding processes under glow discharge condition for medical application. Key Eng. Mater. 2016, 687, 92–97. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, A.; Warchomicka, F.; Pichon, L. Depth profiling characterization of the nitride layers on gas nitrided commercially pure titanium. Mater. Charact. 2021, 181, 111453. [Google Scholar] [CrossRef]
- Szymkiewicz, K.; Morgiel, J.; Maj, L.; Pomorska, M.; Tarnowski, M.; Tkachuk, O.; Pohrelyuk, I.; Wierzchon, T. Effect of nitriding conditions of Ti6Al7Nb on microstructure of TiN surface layer. J. Alloys Comp. 2020, 845, 156320. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008; p. 525. [Google Scholar]
- Alhammad, M.; Esmaeili, S.; Toyserkani, E. Surface modifcation of Ti–6Al–4V alloy using laser-assisted deposition of a Ti–Si compound. Surf. Coat. Technol. 2008, 203, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.Q.; Lou, D.J.; Qin, C.X.; Jiang, J.; Fang, X.Y.; Guo, Y.B. Microstructure anisotropy and its implication in mechanical properties of biomedical titanium alloy processed by electron beam melting. Mat. Sci. Eng. A 2019, 743, 123–137. [Google Scholar] [CrossRef]
- Omura, R.; Yagi, J.; Mukai, K.; Oyaidzu, M.; Ochiai, K.; Kasugai, A.; Konishi, S. Analysis of nitrogen distribution in iron-titanium alloys after nitrogen trapping in liquid lithium by using soft X-ray emission spectroscopy. Fusion Eng. Des. 2021, 170, 11254. [Google Scholar] [CrossRef]
- Yu, S.; Zeng, Q.; Oganov, A.R.; Frappere, G.; Zhanga, L. Phase stability, chemical bonding and mechanical properties of titanium nitrides: A first-principles study. Phys. Chem. Chem. Phys. 2015, 17, 11763. [Google Scholar] [CrossRef]
- Rost, M.J.; Quist, D.A.; Frenken, J.W.M. Grains, Growth, and Grooving. Phys. Rev. Lett. 2003, 91, 026101. [Google Scholar] [CrossRef]
- Glaser, A.; Surnev, S.; Netzer, F.P.; Fateh, N.; Fontalvo, G.A.; Mitterer, C. Oxidation of vanadium nitride and titanium nitride coatings. Surf. Sci. 2007, 601, 1153–1159. [Google Scholar] [CrossRef]
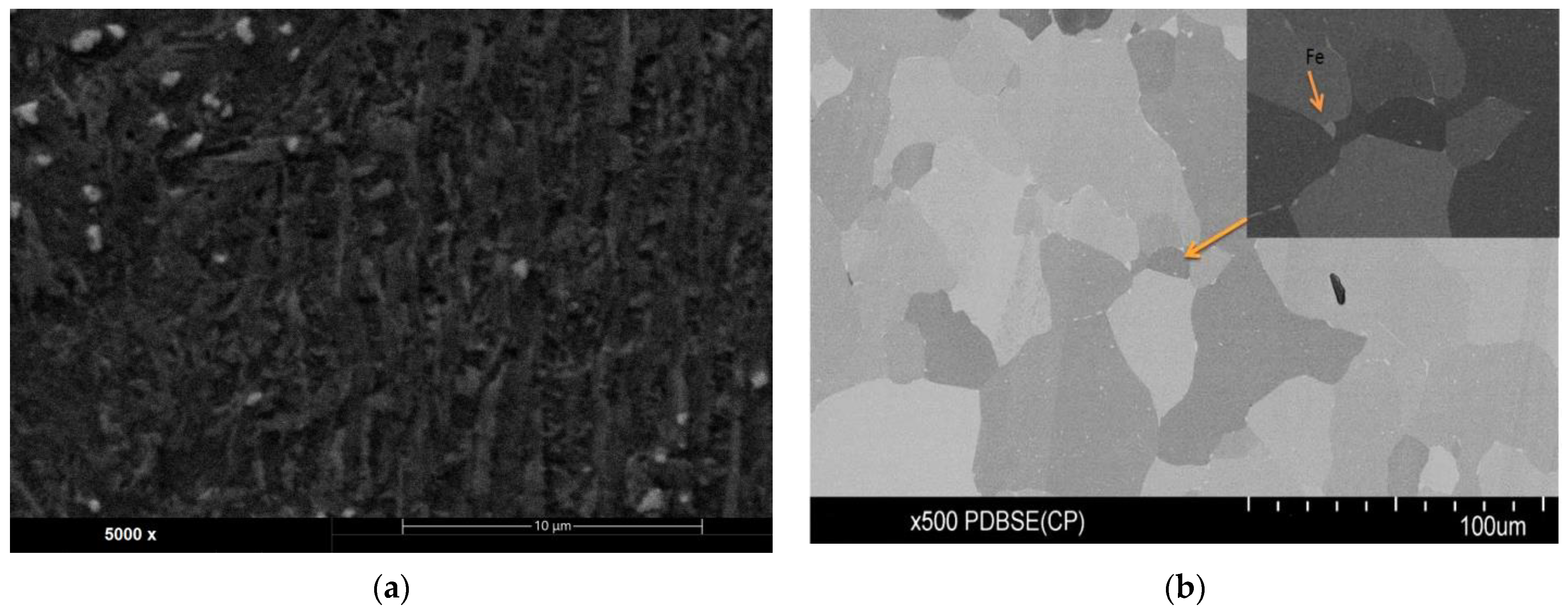
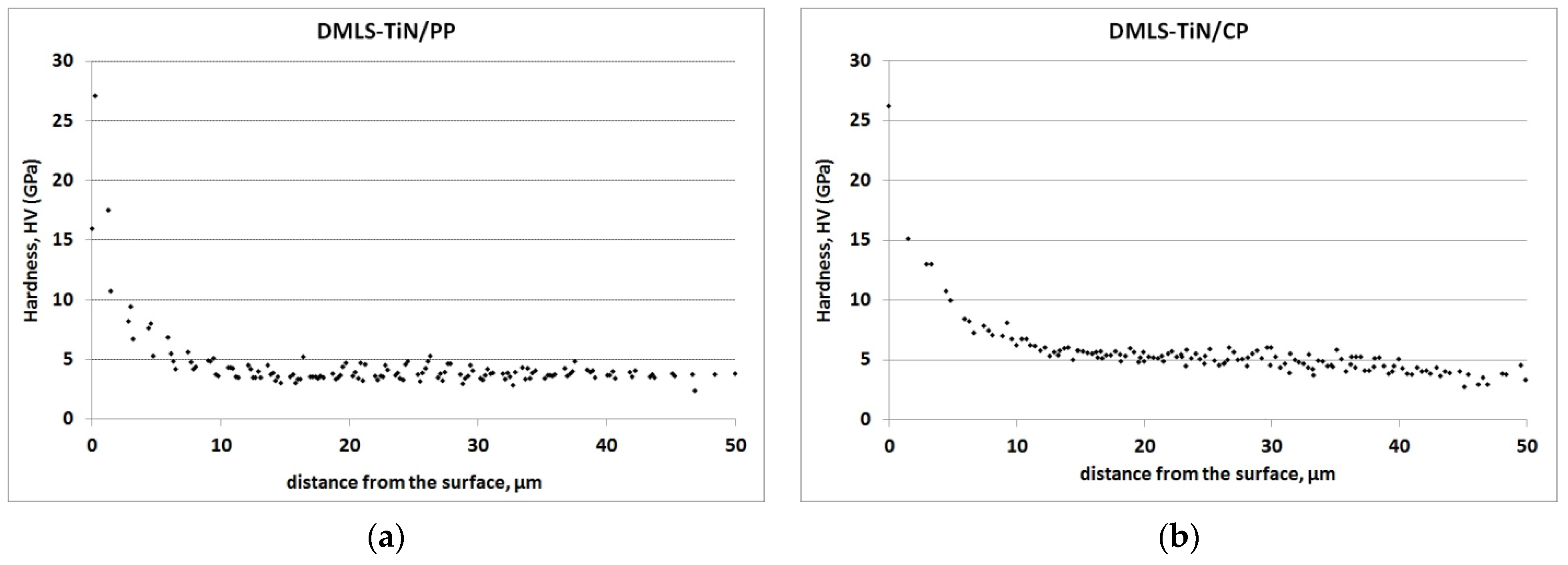
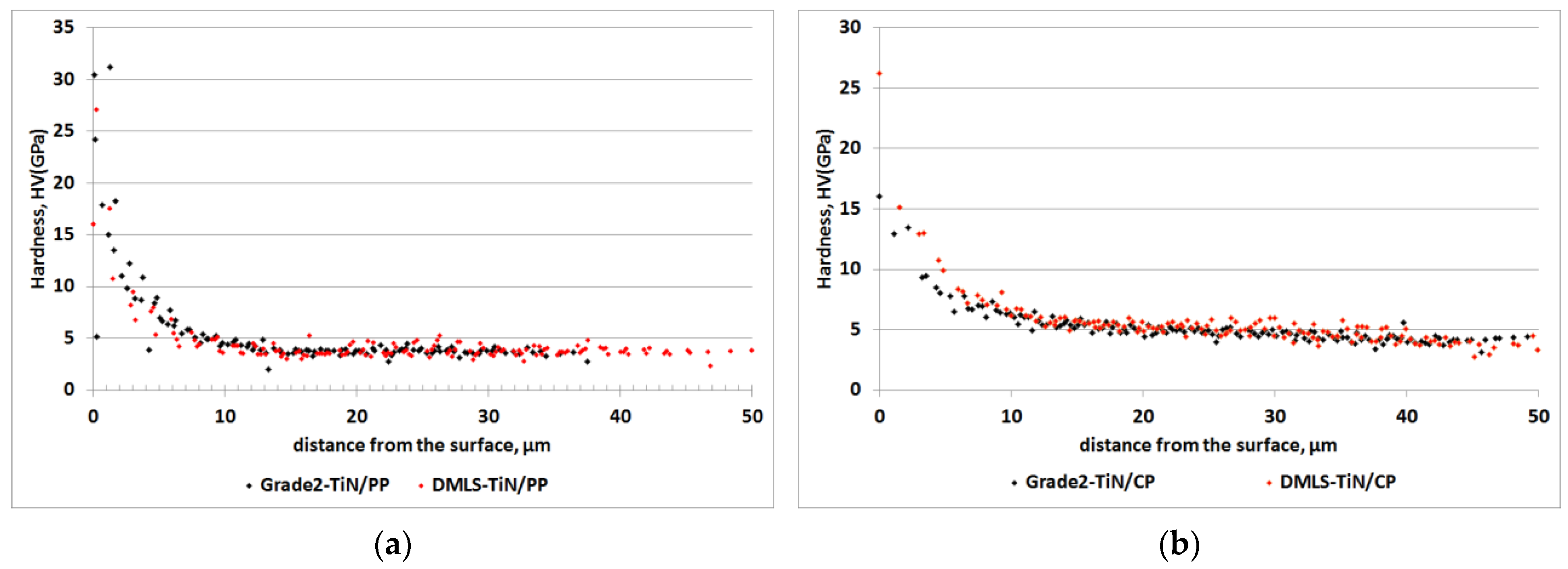
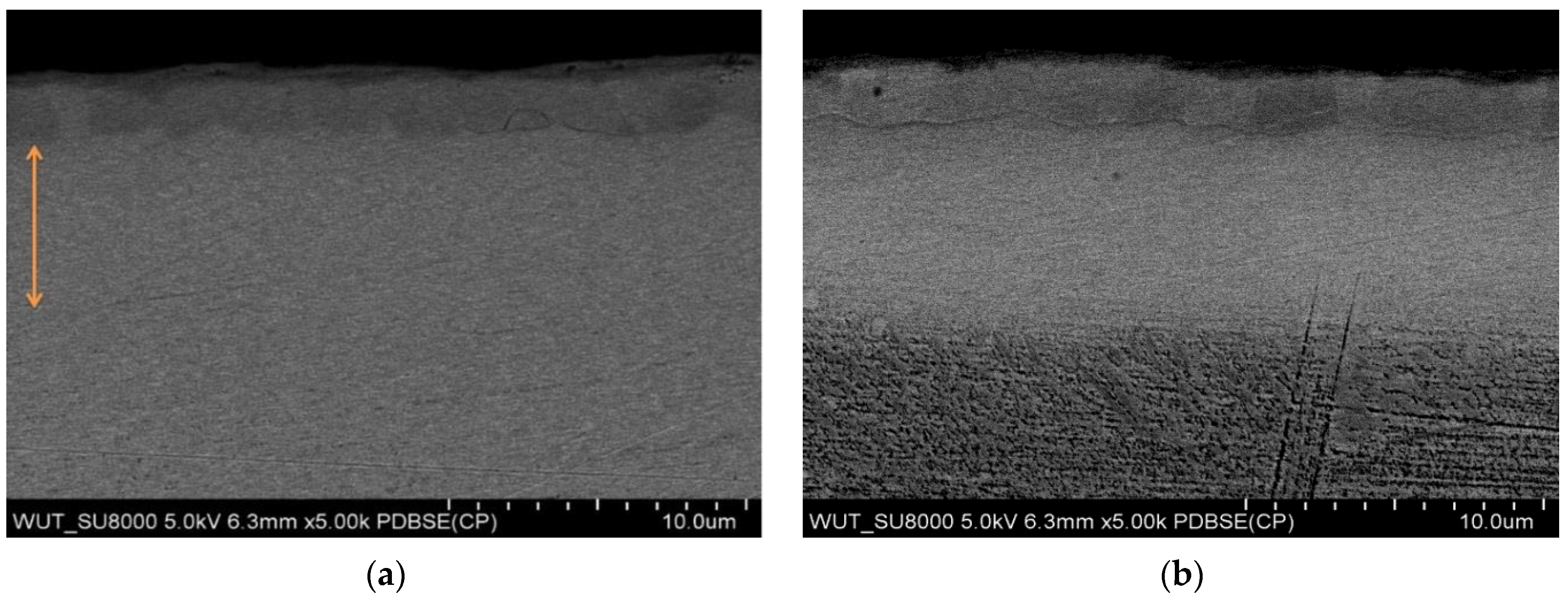
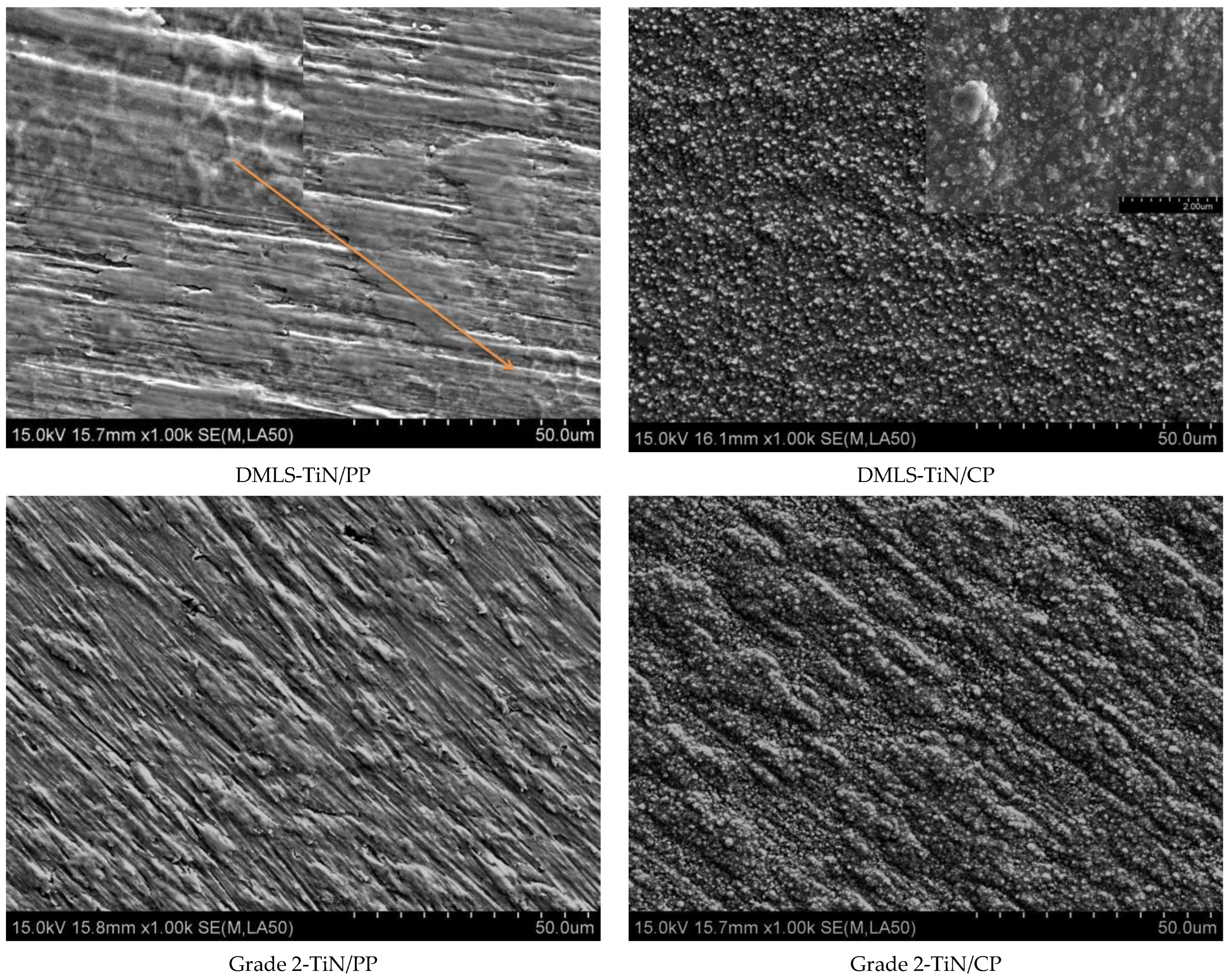

| Sa (µm) | SD | Sp (µm) | SD | Sv (µm) | SD | Sz (µm) | SD | |
|---|---|---|---|---|---|---|---|---|
| DMLS-IS | 0.221 | 0.012 | 1.053 | 0.021 | 1.300 | 0.068 | 2.354 | 0.081 |
| DMLS-TiN/PP | 0.224 | 0.005 | 1.110 | 0.026 | 1.279 | 0.043 | 2.390 | 0.039 |
| DMLS-TiN/CP | 0.275 | 0.018 | 1.371 | 0.079 | 1.554 | 0.085 | 2.926 | 0.162 |
| Grade 2-IS | 0.197 | 0.01 | 1.015 | 0.035 | 1.020 | 0.079 | 2.035 | 0.055 |
| Grade 2-TiN/PP | 0.198 | 0.006 | 1.008 | 0.056 | 1.059 | 0.039 | 2.068 | 0.068 |
| Grade 2-TiN/CP | 0.234 | 0.006 | 1.124 | 0.022 | 1.311 | 0.069 | 2.435 | 0.078 |
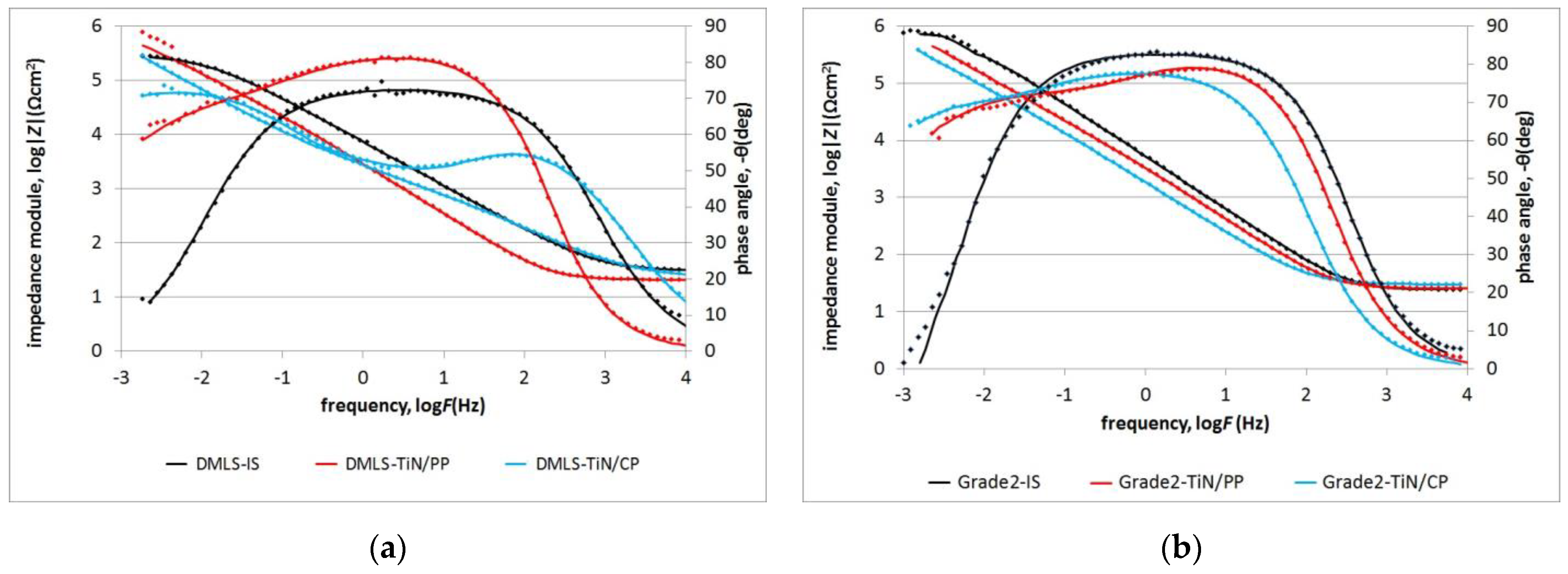
| Dielectric Layer | Error (%) | Double Layer | Error (%) | |||
|---|---|---|---|---|---|---|
| DMLS-IS | R (Ωcm2) | 2.98 × 105 | 0.8 | |||
| QCPE | Y0CPE(Fcm−2sn−1) | 3.00 × 10−5 | 0.3 | |||
| n | 0.82 | 0.1 | ||||
| DMLS TiN/PP | R (Ωcm2) | 5.99 × 104 | 11.7 | 2.92 × 106 | 35.9 | |
| QCPE | Y0CPE(Fcm−2sn−1) | 6.29 × 10−5 | 0.5 | 1.77 × 10−5 | 6.0 | |
| n | 0.92 | 0.1 | 0.60 | 4.2 | ||
| DMLS TiN/CP | R (Ωcm2) | 2.57 × 103 | 7.2 | 7.36 × 106 | 45.5 | |
| QCPE | Y0CPE(Fcm−2sn−1) | 1.66 × 10−4 | 4.7 | 8.42 × 10−5 | 17.8 | |
| n | 0.63 | 2.8 | 0.81 | 4.2 | ||
| Grade 2 IS | R (Ωcm2) | 9.44 × 105 | 1.8 | |||
| QCPE | Y0CPE(Fcm−2sn−1) | 2.75 × 10−5 | 0.4 | |||
| n | 0.92 | 0.1 | ||||
| Grade 2 TiN/PP | R (Ωcm2) | 2.74 × 104 | 15.1 | 2.46 × 106 | 18.4 | |
| QCPE | Y0CPE(Fcm−2sn−1) | 5.40 × 10−5 | 1.2 | 1.92 × 10−5 | 3.9 | |
| n | 0.91 | 0.2 | 0.67 | 2.5 | ||
| Grade 2 TiN/CP | R (Ωcm2) | 1.56 × 105 | 8.2 | 2.43 × 106 | 11.9 | |
| QCPE | Y0CPE(Fcm−2sn−1) | 1.07 × 10−4 | 0.3 | 2.36 × 10−5 | 8.9 | |
| n | 0.87 | 0.1 | 0.69 | 3.4 | ||
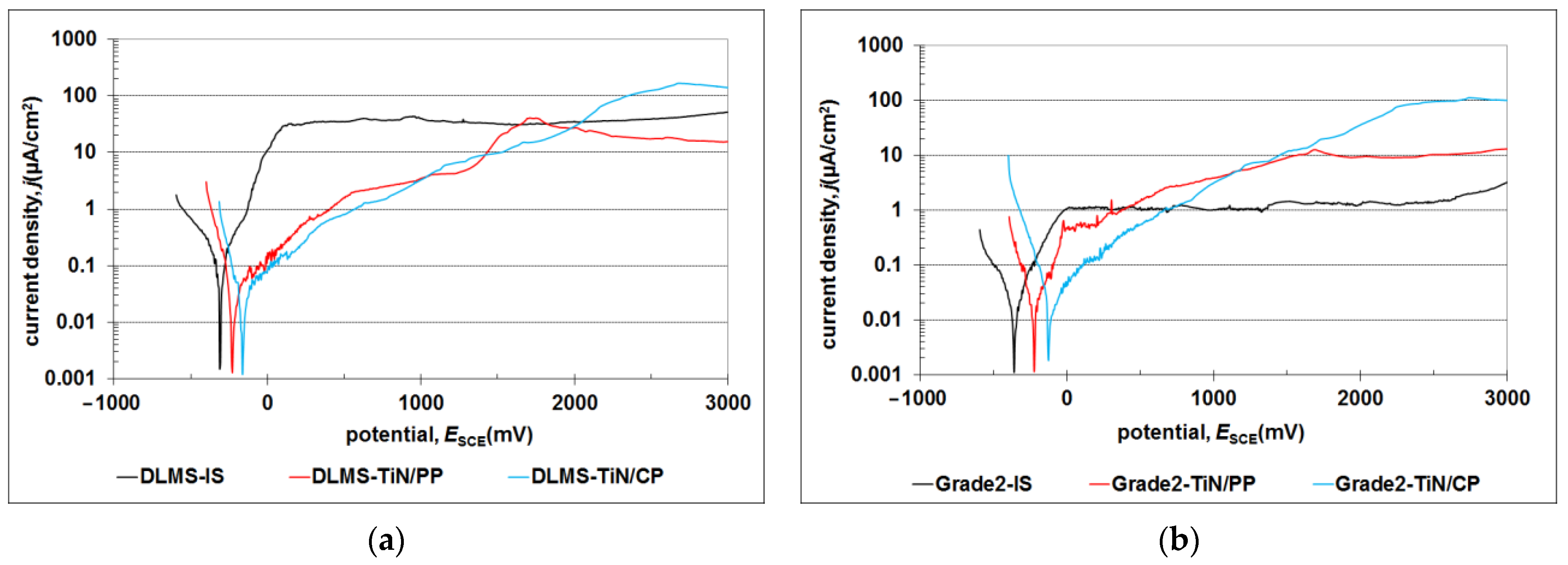
| DMLS | Conventional Grade 2 | |||||
|---|---|---|---|---|---|---|
| Rp (kΩcm2) | Ecorr (SCE) (mV) | Icorr (µA cm−2) | Rp (kΩcm2) | Ecorr (SCE) (mV) | Icorr (µA cm−2) | |
| TiN/CP | 1122 | −165 | 2.65 × 10−2 | 792 | −125 | 2.53 × 10−2 |
| TiN/PP | 1194 | −230 | 1.90 × 10−2 | 1603 | −225 | 1.42 × 10−2 |
| IS | 275 | −310 | 7.13 × 10−2 | 1369 | −360 | 1.91 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiński, J.; Sitek, R.; Adamczyk-Cieślak, B.; Kulikowski, K. Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy. Materials 2024, 17, 4592. https://doi.org/10.3390/ma17184592
Kamiński J, Sitek R, Adamczyk-Cieślak B, Kulikowski K. Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy. Materials. 2024; 17(18):4592. https://doi.org/10.3390/ma17184592
Chicago/Turabian StyleKamiński, Janusz, Ryszard Sitek, Bogusława Adamczyk-Cieślak, and Krzysztof Kulikowski. 2024. "Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy" Materials 17, no. 18: 4592. https://doi.org/10.3390/ma17184592
APA StyleKamiński, J., Sitek, R., Adamczyk-Cieślak, B., & Kulikowski, K. (2024). Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy. Materials, 17(18), 4592. https://doi.org/10.3390/ma17184592






