Utilization of Municipal Solid Waste Incineration (MSWIFA) in Geopolymer Concrete: A Study on Compressive Strength and Leaching Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. MSWIFA Preparation
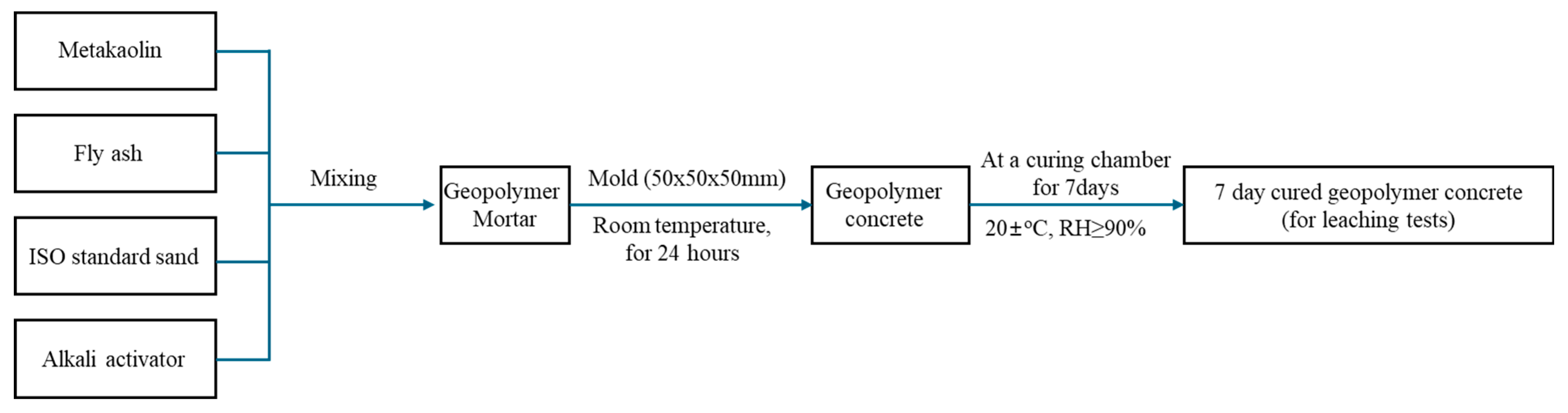
2.2. Preparation of Geopolymer Concrete
2.3. Orthogonal Experiment for Geopolymer Concrete
2.4. Compressive Strength Test
2.5. Long-Term Leaching Test
2.6. Analytical Method
3. Results and Discussion
3.1. Orthogonal Experiment Range Analysis
3.2. The Effects of Washing on MSWIFA Characteristics
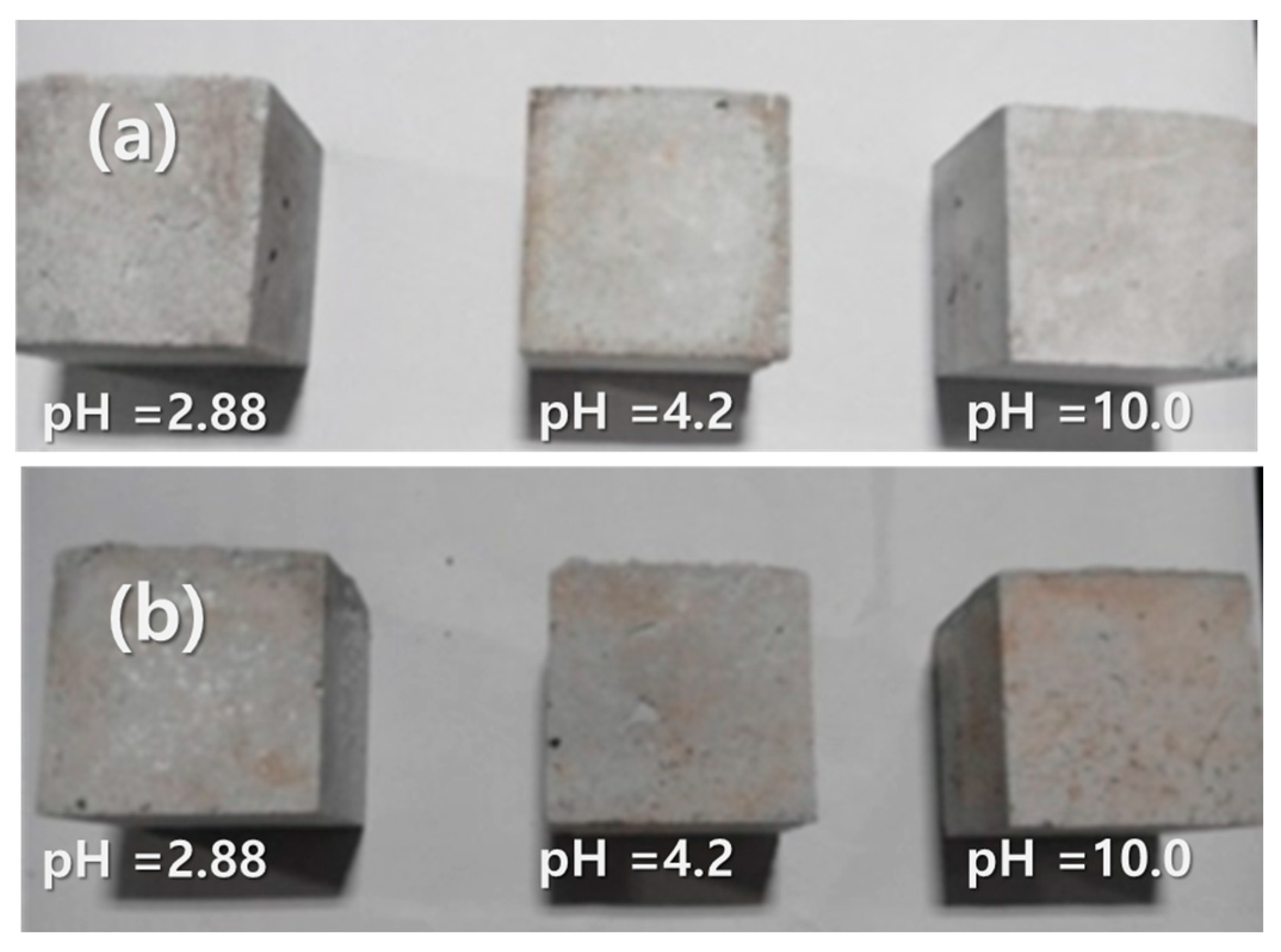
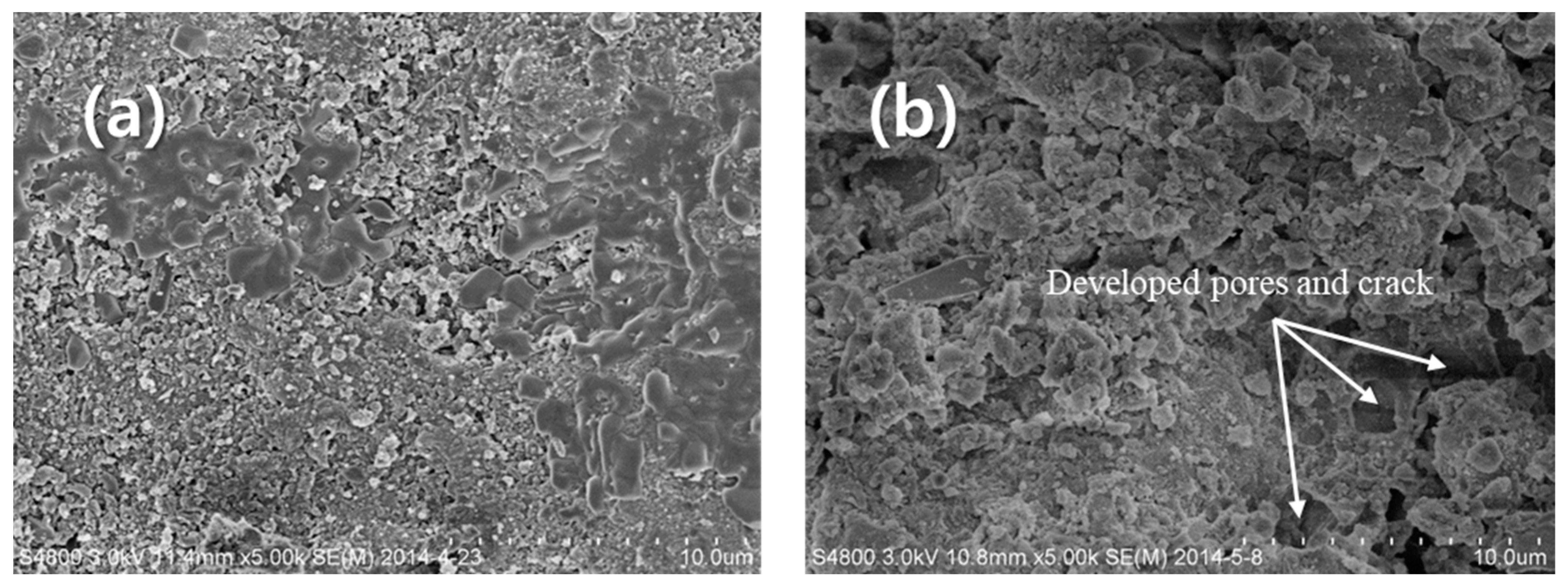
3.3. Surface Characteristics of Geopolymer in Different Leaching Conditions
3.4. Compressive Strength Development
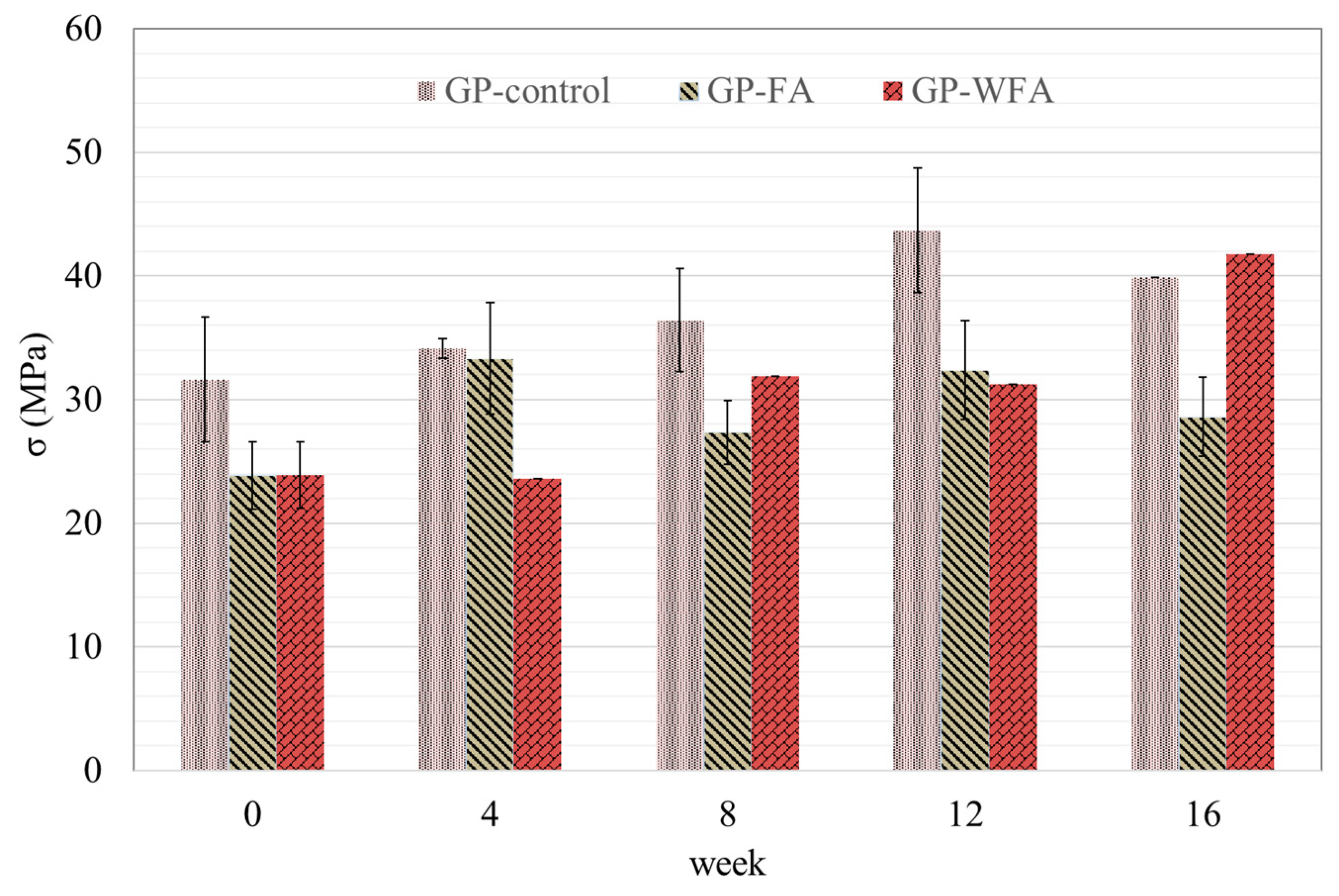
3.4.1. Compressive Strength Development of GP-Control, GP-FA and GP-WFA
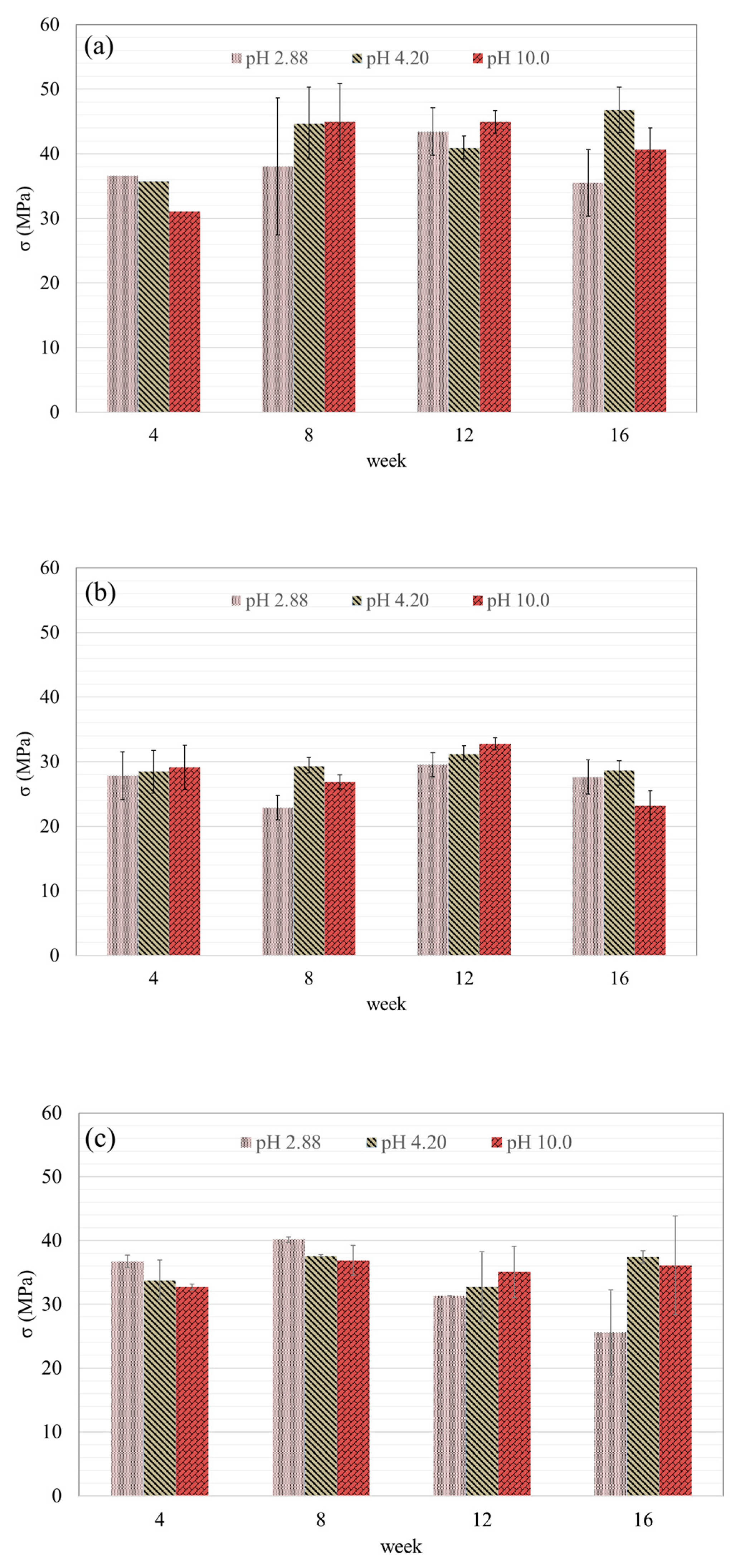
3.4.2. Compressive Strength Development in Different pH Conditions
3.5. Leaching Characteristics under Different pH Conditions
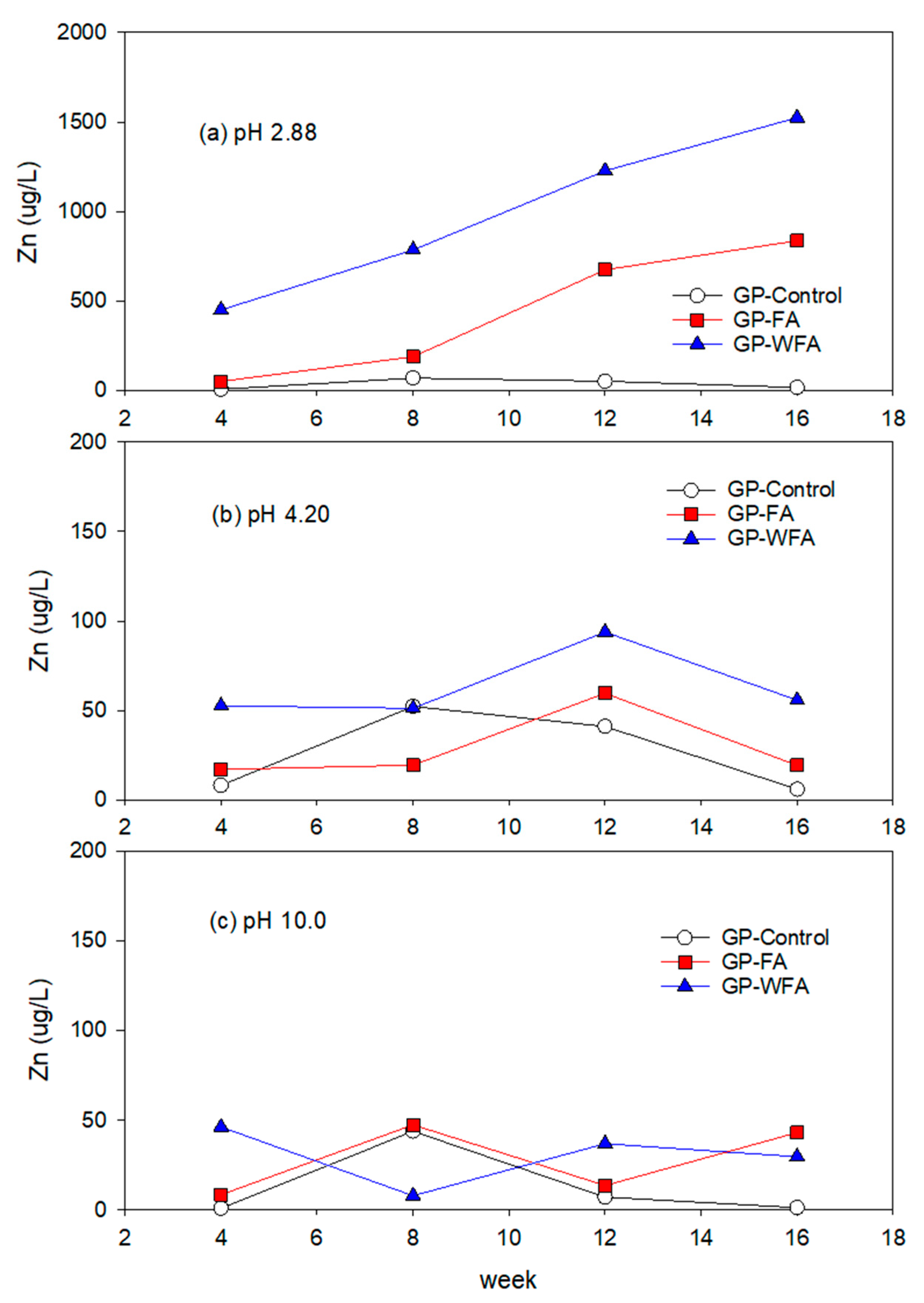
3.5.1. Zn Leachability under Different pH Conditions
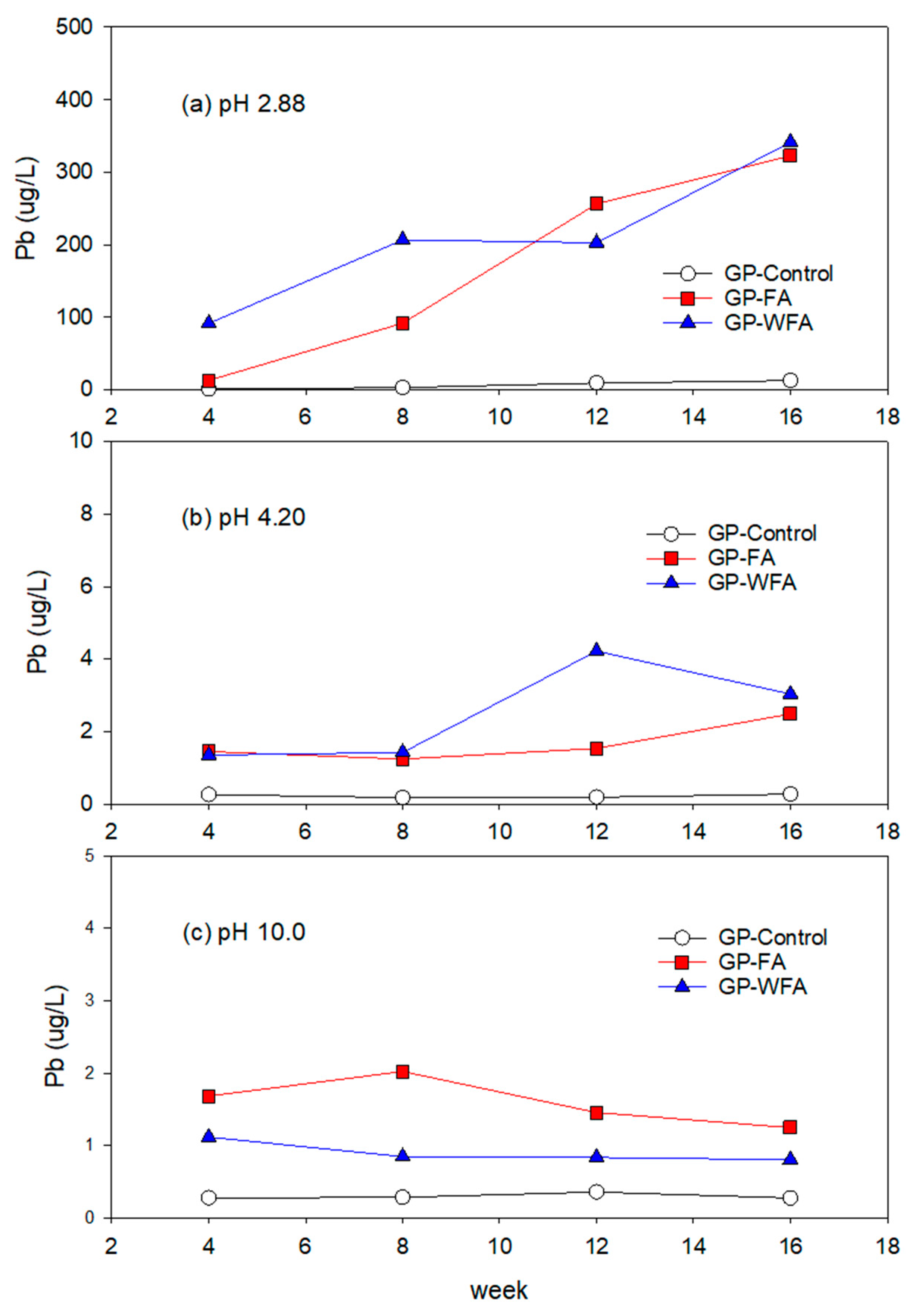
3.5.2. Pb Leachability under Different pH Conditions
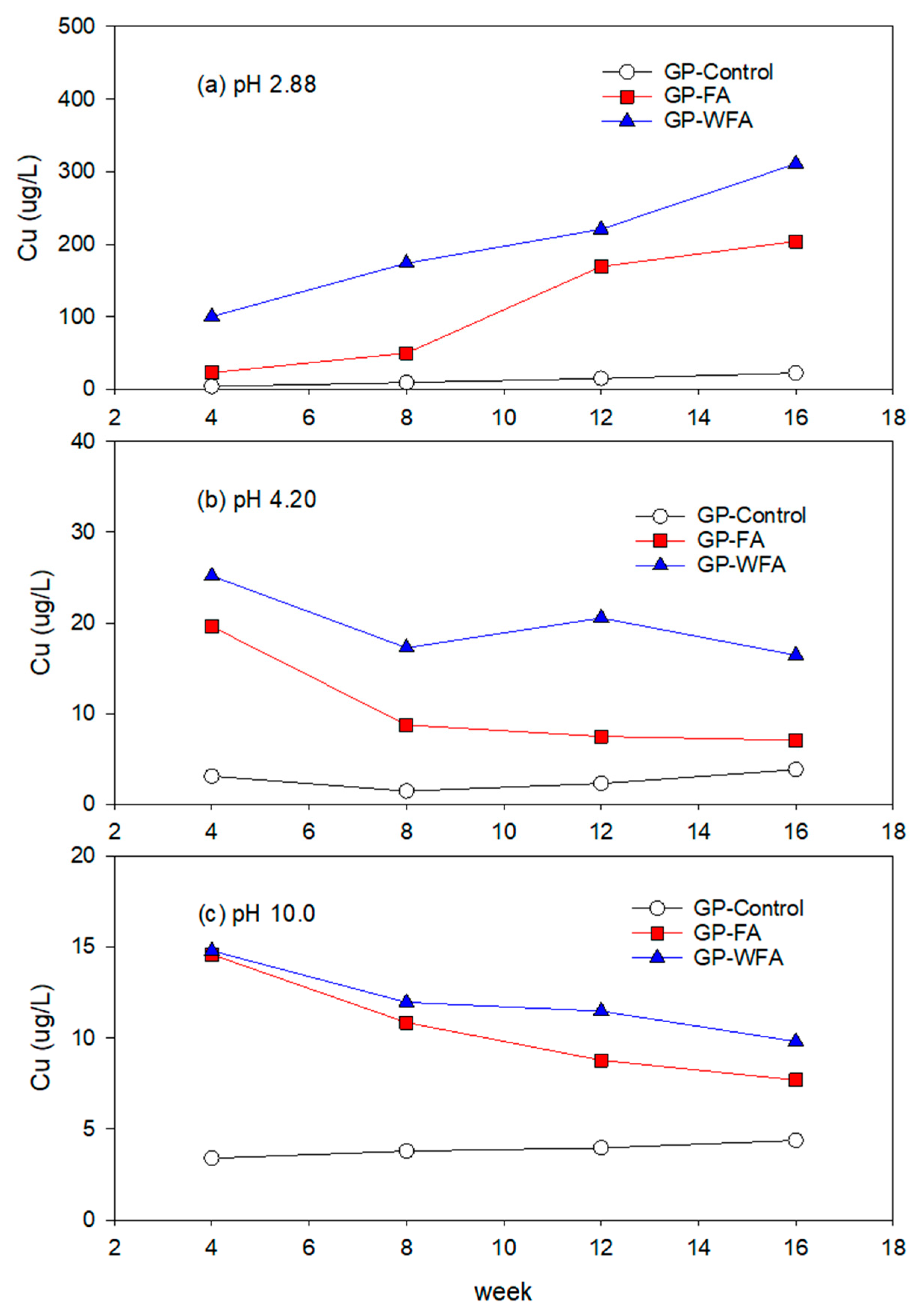
3.5.3. Cu Leachability under Different pH Conditions
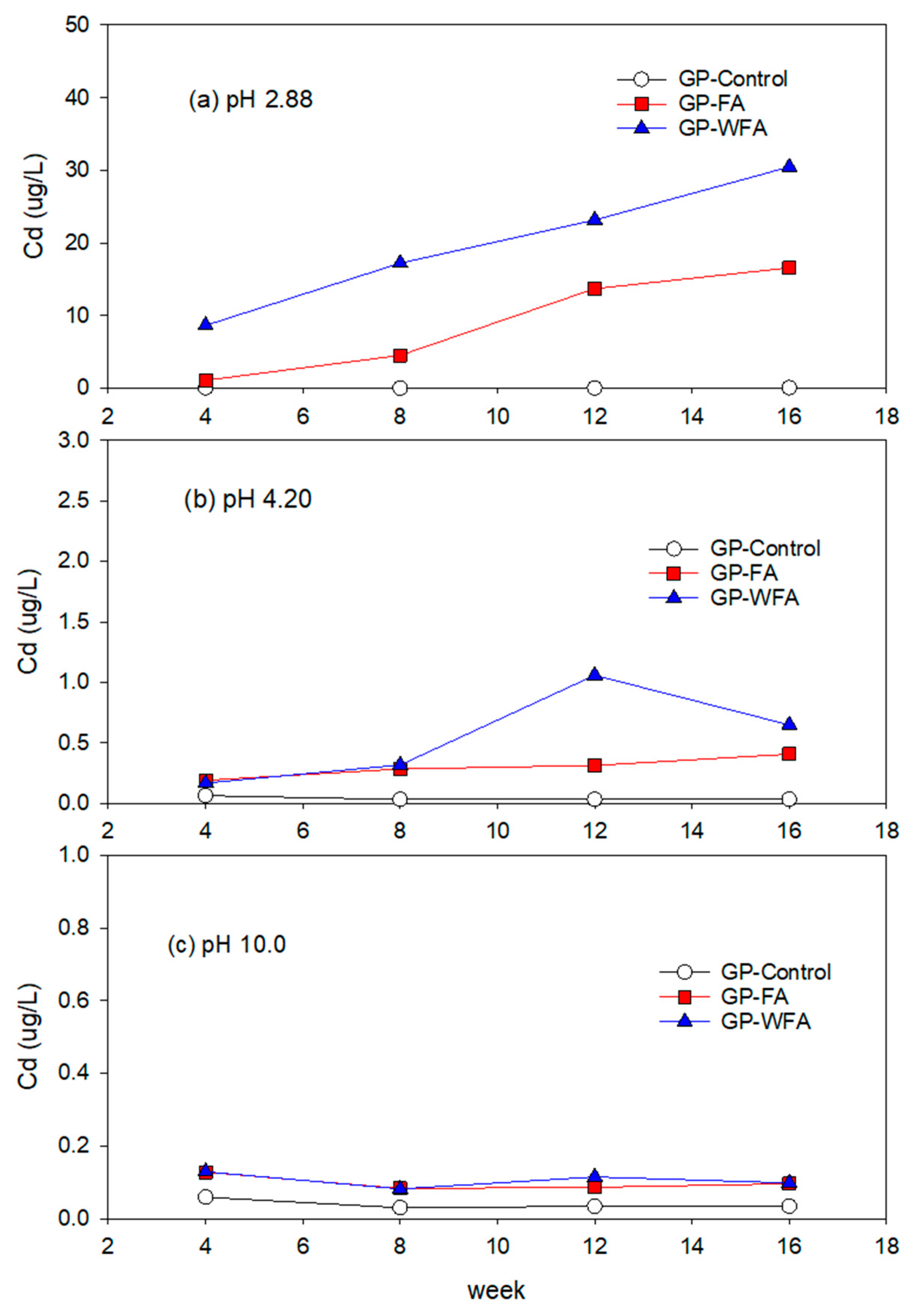
3.5.4. Cd Leachability under Different pH Conditions
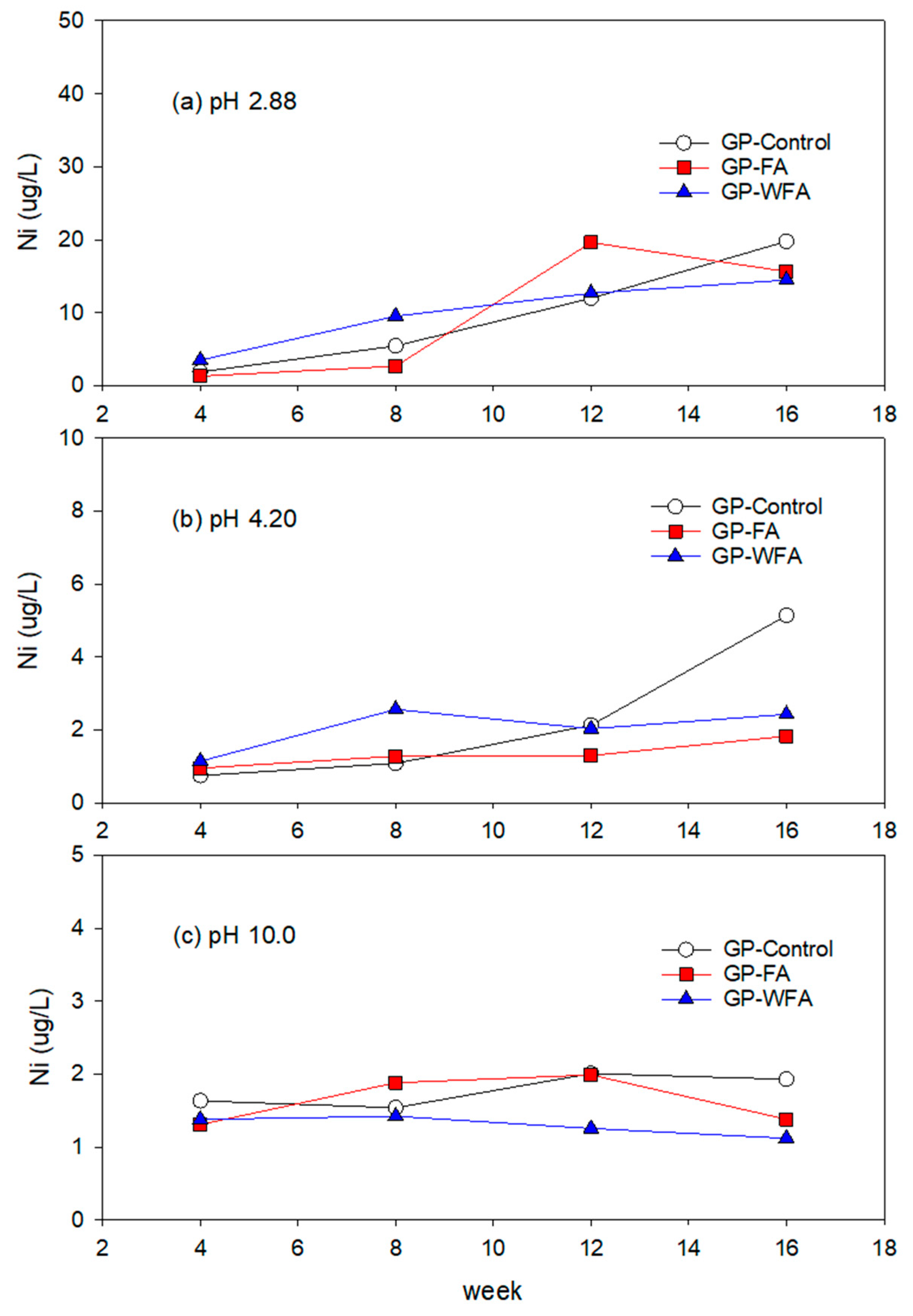
3.5.5. Ni Leachability under Different pH Conditions
3.5.6. Cr Leachability under Different pH Conditions
4. Conclusions
- A Si/Al ratio of 1.5, water/Na ratio of 10, and sand-binder ratio of 0.6 were identified as optimal parameters for achieving the highest compressive strength in geopolymer concrete. The influence of the Si/Al ratio was particularly significant, with a higher Si/Al ratio leading to a stronger geopolymer matrix.
- The washing pretreatment of MSWIFA significantly enhanced the compressive strength of geopolymer concrete, especially under alkaline conditions. This improvement can be attributed to the removal of soluble salts, which reduces the detrimental impact of contaminants.
- Geopolymer concrete containing washed MSWIFA (GP-WFA) showed an improved long-term compressive strength compared to unwashed MSWIFA (GP-FA), with GP-WFA reaching a compressive strength comparable to control geopolymer concrete (GP-control) after 16 weeks. Compressive strength was more stable under alkaline conditions (pH 10), while acidic conditions (pH 2.88) led to surface degradation and reduced strength.
- The leachability of heavy metals such as Zn, Pb, Cu, Cd, and Cr varied significantly with pH levels. Strong acidic conditions (pH 2.88) resulted in a higher leachability, whereas alkaline conditions (pH 10) greatly reduced the leaching of heavy metals. The washing pretreatment increased the leachability of some metals, particularly Zn and Cu, under acidic conditions due to the concentration of these metals in the washed residue.
- The reduction in heavy metal leachability under alkaline conditions highlights the potential for the safe usage of MSWIFA in construction applications, particularly in environments with higher pH levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, S.; Liu, B. Degradation technologies and mechanisms of dioxins in municipal solid waste incineration fly ash: A review. J. Clean. Prod. 2020, 250, 119507. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zhou, Y.; Tyrer, M.; Yu, Y. Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manag. 2014, 34, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liao, W.-P.; Wu, P.-H. Basic characteristics of leachate produced by various washing processes for MSWI ashes in Taiwan. J. Environ. Manag. 2012, 104, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Sun, M.; Ma, L.; Dai, Q.; Yang, J.; Xie, L.; Hu, Y.; Duan, L.; Yan, X.; Zhou, G.; Zeng, L.; et al. Preparation of functional geopolymers from municipal solid waste incineration fly ash: An approach combining experimental and computational simulation studies. J. Environ. Manag. 2024, 355, 120226. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Meng, Y.; Ju, T.; Chen, Z.; Du, Y.; Deng, Y.; Song, M.; Han, S.; Jiang, J. Synthesis and application of geopolymers from municipal waste incineration fly ash (MSWI FA) as raw ingredient—A review. Resour. Conserv. Recycl. 2022, 182, 106308. [Google Scholar] [CrossRef]
- Liang, D.; Tao, L.; Wang, F.; Lv, G. Synthesis of geopolymers using municipal solid waste incineration fly ash and construction and demolition waste: Mechanical and thermal activation. J. Environ. Chem. Eng. 2023, 11, 111249. [Google Scholar] [CrossRef]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Ganguly, A.; Hug, G. Microstructural Evidence of Reconstituted Limestone Blocks in the Great Pyramids of Egypt. J. Am. Ceram. Soc. 2006, 89, 3788–3796. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 2nd ed.; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Elemam, W.E.; Tahwia, A.M.; Abdellatief, M.; Youssf, O.; Kandil, M.A. Durability, Microstructure, and Optimization of High-Strength Geopolymer Concrete Incorporating Construction and Demolition Waste. Sustainability 2023, 15, 15832. [Google Scholar] [CrossRef]
- Xue, Y.; Arulrajah, A.; Horpibulsuk, S.; Chu, J. Strength and stiffness performance of geopolymer stabilized washed recycled sands derived from demolition wastes in pavement subgrades. Constr. Build. Mater. 2023, 369, 130618. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Tao, Z. A comprehensive review on performance of cementitious and geopolymeric concretes with recycled waste glass as powder, sand or cullet. Resour. Conserv. Recycl. 2021, 172, 105664. [Google Scholar] [CrossRef]
- Yashwanth Reddy, M.; Harihanandh, M. Experimental studies on strength and durability of alkali activated slag and coal bottom ash based geopolymer concrete. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, H.; Zhou, X.; Gao, J.; Liu, J.; Lu, G.; Guo, Y.; Duan, S. Feasibility analysis of the preparation of geopolymers from different types of coal based ash: Reaction, synthesis, and properties. Case Stud. Constr. Mater. 2024, 20, e03234. [Google Scholar] [CrossRef]
- Guo, X.; Yu, H.; Yao, H.; Lin, F.; Salama, E.; Ossman, M.; Yan, B.; Chen, G. Green transformation of oily sludge through geopolymer: Material properties and hydration mechanisms. Chemosphere 2024, 364, 143132. [Google Scholar] [CrossRef]
- Hoe-Woon, T.; Cheng-Yong, H.; Yun-Ming, L.; Qi-Hwa, N.; Wei-Ken, P.; Chin-Yii, Y.; Darshinder; Jia-Ni, L.; Yong-Jie, H. Assessing viability and leachability in fly ash geopolymers incorporated with rubber sludge. J. Ind. Eng. Chem. 2024. [Google Scholar] [CrossRef]
- Duan, S.; Guo, H.; Sun, H.; Zhou, X.; Lu, G.; Guo, Y.; Ma, Z. Effect of synthesis conditions on mechanical properties and microstructure of coal gasification slag-based geopolymer. Constr. Build. Mater. 2024, 443, 137736. [Google Scholar] [CrossRef]
- Hamed, E.; Demiröz, A. Optimization of geotechnical characteristics of clayey soils using fly ash and granulated blast furnace slag-based geopolymer. Constr. Build. Mater. 2024, 441, 137488. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, T.; Yang, Q.; Vandeginste, V.; Li, J. Influence of ground granulated blast furnace slag on recycled concrete powder-based geopolymer cured at ambient temperature: Rheology, mechanical properties, reaction kinetics and air-void characteristics. Constr. Build. Mater. 2024, 438, 137190. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Qin, Z.; Yuan, Y.; Chen, Z.; Li, Y.; Xia, Y. Combined preparation and application of geopolymer pavement materials from coal slurry-slag powder-fly ash mining solid waste: A case study. Constr. Build. Mater. 2024, 441, 137510. [Google Scholar] [CrossRef]
- Singh, S.; Kant Sharma, S.; Abdul Akbar, M. Developing zero carbon emission pavements with geopolymer concrete: A comprehensive review. Transp. Res. Part D Transp. Environ. 2022, 110, 103436. [Google Scholar] [CrossRef]
- Das, S.; Saha, P.; Prajna Jena, S.; Panda, P. Geopolymer concrete: Sustainable green concrete for reduced greenhouse gas emission—A review. Mater. Today Proc. 2022, 60, 62–71. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Utilization of blended fluidized bed combustion (FBC) ash and pulverized coal combustion (PCC) fly ash in geopolymer. Waste Manag. 2010, 30, 667–672. [Google Scholar] [CrossRef]
- Nazari, A.; Bagheri, A.; Riahi, S. Properties of geopolymer with seeded fly ash and rice husk bark ash. Mater. Sci. Eng. A 2011, 528, 7395–7401. [Google Scholar] [CrossRef]
- Tanu, H.M.; Unnikrishnan, S. Utilization of industrial and agricultural waste materials for the development of geopolymer concrete—A review. Mater. Today Proc. 2022, 65, 1290–1297. [Google Scholar]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Luan, C.; Zhou, A.; Li, Y.; Zou, D.; Gao, P.; Liu, T. CO2 avoidance cost of fly ash geopolymer concrete. Constr. Build. Mater. 2024, 416, 135193. [Google Scholar] [CrossRef]
- Bie, Y.; Yin, X.; Chen, S. The durability of slag and tannery sludge based geopolymer corroded by acid and base solutions. Constr. Build. Mater. 2024, 433, 136746. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I. Geopolymers: A New Generation of Inorganic Polymeric Novel Materials. In Proceedings of the 1st International Conference on Advances in Mineral Resources Management and Environmental Geotechnology, Hania, Greece, 7–9 June 2004; National Technical University of Athens: Athens, Greece, 2004; AMIREG; pp. 407–412. [Google Scholar]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Provis, J.L.; Harrex, R.M.; Bernal, S.A.; Duxson, P.; van Deventer, J.S.J. Dilatometry of geopolymers as a means of selecting desirable fly ash sources. J. Non-Cryst. Solids 2012, 358, 1930–1937. [Google Scholar] [CrossRef]
- Allahverdi, A.; Kani, E.N. Construction Wastes as Raw Materials for Geopolymer Binders. Int. J. Civ. Eng. 2009, 7, 154–160. [Google Scholar]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jangra, P.; Pham, T.M.; Lim, Y.Y. Enhancing the Durability Properties of Sustainable Geopolymer Concrete Using Recycled Coarse Aggregate and Ultrafine Slag at Ambient Curing. Sustainability 2022, 14, 10948. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Miner. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Yao, R.; Chen, S.; Gao, J.; Shimaoka, T. Removal of harmful components from MSWI fly ash as a pretreatment approach to enhance waste recycling. Waste Manag. 2022, 150, 110–121. [Google Scholar] [CrossRef]
- Wei, Y.; Du, X.; Liu, S.; Wen, Y.; Liao, Q.; Jiao, G.; Shimaoka, T.; Tang, S. Promotion of chloride removal from MSWI fly ash by an accelerated wet-carbonation process to enhance ash recycling in cement manufacture. J. Environ. Chem. Eng. 2024, 12, 112591. [Google Scholar] [CrossRef]
- Tian, Y.; Themelis, N.J.; Bourtsalas, A.C. Effects of water, acid, or alkali washing on Waste-to-Energy (WTE) bottom ash, fly ash, and combined ash. J. Environ. Chem. Eng. 2024, 12, 111936. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.-S.; Poon, C.-S.; Jiang, W.-H.; Ma, Z.-H.; Chen, X.; Lu, J.-X.; Dong, H.-X. Physicochemical and pozzolanic properties of municipal solid waste incineration fly ash with different pretreatments. Waste Manag. 2023, 160, 146–155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Nie, Q.; Yue, Y.; He, J.; Qian, G. Potential and durability of supplementary cementitious material prepared by incineration fly ash: Co-sintering and water-washing treatment. Chem. Eng. J. 2024, 496, 153992. [Google Scholar] [CrossRef]
- Ma, X.; He, T.; Da, Y.; Xu, Y.; Luo, R.; Yang, R. Improve toxicity leaching, physicochemical properties of incineration fly ash and performance as admixture by water washing. Constr. Build. Mater. 2023, 386, 131568. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mangialardi, T.; Paolini, A.E.; Polettini, A.; Sirini, P. Optimization of the solidification/stabilization process of MSW fly ash in cementitious matrices. J. Hazard. Mater. 1999, 70, 53–70. [Google Scholar] [CrossRef]
- Mangialardi, T. Disposal of MSWI fly ash through a combined washing-immobilisation process. J. Hazard. Mater. 2003, 98, 225–240. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Dinh, H.L.; Ong, D.E.L.; Zi, G.; You, I. Effect of Si/Al molar ratio on the strength behavior of geopolymer derived from various industrial waste: A current state of the art review. Constr. Build. Mater. 2022, 329, 127134. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Zhang, L.; Yue, Y.; Zhou, J.; Xu, Y.; Qian, G. Can washing-pretreatment eliminate the health risk of municipal solid waste incineration fly ash reuse? Ecotoxicol. Environ. Saf. 2015, 111, 177–184. [Google Scholar] [CrossRef]
- Chuewangkam, N.; Kidkhunthod, P.; Pinitsoontorn, S. Direct evidence for the mechanism of early-stage geopolymerization process. Case Stud. Constr. Mater. 2024, 21, e03539. [Google Scholar] [CrossRef]
- Tan, J.; Dan, H.; Ma, Z. Metakaolin based geopolymer mortar as concrete repairs: Bond strength and degradation when subjected to aggressive environments. Ceram. Int. 2022, 48, 23559–23570. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Kumar, S.; Maradani, L.S.R.; Mohapatra, A.K.; Pradhan, B. Effect of mix parameters on chloride content, sulfate ion concentration, and microstructure of geopolymer concrete. Constr. Build. Mater. 2024, 435, 136864. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; He, X.; Hu, X. Resistance and durability of fly ash based geopolymer for heavy metal immobilization: Properties and mechanism. RSC Adv. 2024, 14, 12580–12592. [Google Scholar] [CrossRef]
- Tochetto, G.A.; Simão, L.; Pavan Korf, E.; de Oliveira, D.; Hotza, D.; Serafini Immich, A.P. How acid attack and high temperature affect the microstructure of adsorbent geopolymers. Open Ceram. 2024, 17, 100532. [Google Scholar] [CrossRef]
- Gu, L.; Visintin, P.; Bennett, T. Evaluation of accelerated degradation test methods for cementitious composites subject to sulfuric acid attack; application to conventional and alkali-activated concretes. Cem. Concr. Compos. 2018, 87, 187–204. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of Geopolymer in Stabilization/Solidification of Hazardous Pollutants: A Review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Dou, X.; Ren, F.; Nguyen, M.Q.; Ahamed, A.; Yin, K.; Chan, W.P.; Chang, V.W.-C. Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew. Sustain. Energy Rev. 2017, 79, 24–38. [Google Scholar] [CrossRef]
- Malolepszy, J.; Deja, J. Effect of heavy metals immobilization on the properties of alkali activated slag mortars. Spec. Publ. 1995, 153, 1087–1102. [Google Scholar]
- Pierrard, J.-C.; Rimbault, J.; Aplincourt, M. Experimental study and modelling of lead solubility as a function of pH in mixtures of ground waters and cement waters. Water Res. 2002, 36, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Grba, N.; Grengg, C.; Petronijević, M.; Dietzel, M.; Baldermann, A. Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange. Polymers 2023, 15, 1971. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Immobilization efficiency and mechanism of metal cations (Cd2+, Pb2+ and Zn2+) and anions (AsO43− and Cr2O72−) in wastes-based geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashqbeh, A.; Abuali, S.; El-Eswed, B.; Khalili, F.I. Immobilization of toxic inorganic anions (Cr2O72−, MnO4− and Fe(CN)63−) in metakaolin based geopolymers: A preliminary study. Ceram. Int. 2018, 44, 5613–5620. [Google Scholar] [CrossRef]

| No. | Si/Al Ratio | Water/Na Ratio | Sand/Binder Ratio | Ca(OH)2 Addition |
|---|---|---|---|---|
| 1 | 1.5 | 7.5 | 0.6 | 0 |
| 2 | 1.5 | 8.5 | 1.0 | 0.1 |
| 3 | 1.5 | 9.5 | 1.4 | 0.2 |
| 4 | 1.9 | 7.5 | 1.4 | 0.1 |
| 5 | 1.9 | 8.5 | 0.6 | 0.2 |
| 6 | 1.9 | 9.5 | 1.0 | 0 |
| 7 | 2.3 | 7.5 | 1.0 | 0.2 |
| 8 | 2.3 | 8.5 | 1.4 | 0 |
| 9 | 2.3 | 9.5 | 0.6 | 0.1 |
| Level * | Si/Al Ratio | Water/Na Ratio | Sand/Binder Ratio | Ca(OH)2 Addition |
|---|---|---|---|---|
| Level 1 | 27.4 (±8.9) ** | 22.5 (±12.8) | 19.1 (±16.1) | 14.6 (±19.5) |
| Level 2 | 10.6 (±5.7) | 14.3 (±11.5) | 15.7 (±11.9) | 14.9 (±10.0) |
| Level 3 | 8.6 (±7.7) | 9.8 (±8.4) | 11.7 (±8.5) | 17.1 (±2.5) |
| Range * | 18.79 | 12.66 | 7.4 | 2.49 |
| Component (wt. %) | Al2O3 | CaO | Cl | Fe2O3 | K2O | MgO | Na2O | SiO2 | SO3 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSWIFA | unwashed | 1.0 | 38.9 | 9.9 | 0.5 | 6.5 | 0.3 | 6.9 | 6.8 | 7.0 | 19.6 |
| washed | 1.8 | 48.2 | 1.2 | 0.9 | 0.7 | 1.3 | 1.1 | 5.6 | 7.9 | 26.3 | |
| Metakaolin | 45.4 | 0.3 | - | - | 0.04 | - | 0.1 | 51.4 | - | 1.5 | |
| Heavy Metal | Unwashed MSWIFA | Washed MSWIFA | Metakaolin |
|---|---|---|---|
| (mg·kg−1) | |||
| Zn | 6096 | 9416 | 94 |
| Pb | 1555 | 1472 | 20 |
| Cu | 1144 | 2205 | 60 |
| Cd | 174 | 341 | 3 |
| Cr | 53 | 83 | 21 |
| Ni | 436 | 190 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Shang, N.; Ko, J.H. Utilization of Municipal Solid Waste Incineration (MSWIFA) in Geopolymer Concrete: A Study on Compressive Strength and Leaching Characteristics. Materials 2024, 17, 4609. https://doi.org/10.3390/ma17184609
Xu Q, Shang N, Ko JH. Utilization of Municipal Solid Waste Incineration (MSWIFA) in Geopolymer Concrete: A Study on Compressive Strength and Leaching Characteristics. Materials. 2024; 17(18):4609. https://doi.org/10.3390/ma17184609
Chicago/Turabian StyleXu, Qiyong, Ning Shang, and Jae Hac Ko. 2024. "Utilization of Municipal Solid Waste Incineration (MSWIFA) in Geopolymer Concrete: A Study on Compressive Strength and Leaching Characteristics" Materials 17, no. 18: 4609. https://doi.org/10.3390/ma17184609






