Developing a Cobalt Phosphide Catalyst with Combined Cobalt Defects and Phosphorus Vacancies to Boost Oxygen Evolution Reaction
Abstract
1. Introduction
2. Experimental Section
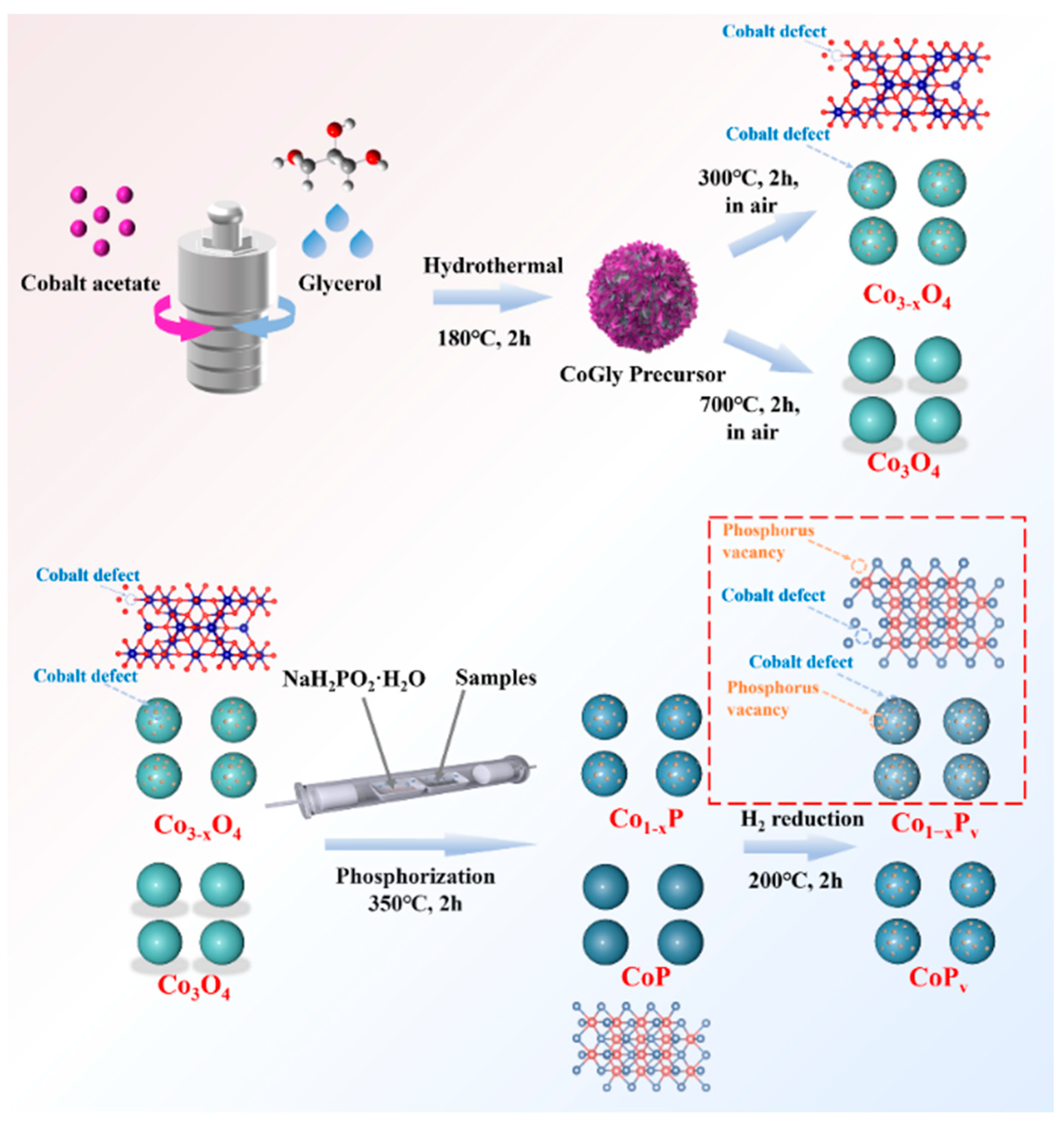
2.1. Catalyst Preparation
2.2. Structure Characterization
2.3. Electrode Preparation
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Morphology Analysis
3.2. Electrochemical Oxygen Evolution Reaction Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalew, S.G.; van Vliet, M.T.H.; Gernaat, D.E.H.J.; Ludwig, F.; Miara, A.; Park, C.; Byers, E.; De Cian, E.; Piontek, F.; Iyer, G.; et al. Impacts of climate change on energy systems in global and regional scenarios. Nat. Energy 2020, 5, 794–802. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Comparative assessment of renewable energy-based hydrogen production methods. Renew. Sustain. Energy Rev. 2021, 135, 110192. [Google Scholar] [CrossRef]
- Le, P.-A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The current status of hydrogen energy: An overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S.X.; Yao, R.; Wu, Y.H.; Qiu, J.S. Progress and prospects of hydrogen production: Opportunities and challenges. J. Electron. Sci. Technol. 2021, 19, 100080. [Google Scholar] [CrossRef]
- Li, L.G.; Wang, P.T.; Shao, Q.; Huang, X.Q. Recent Progress in Advanced Electrocatalyst Design for Acidic Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef] [PubMed]
- Hess, F. Corrosion mechanism and stabilization strategies for RuO2 and IrO2 catalysts in the electrochemical oxygen evolution reaction. Curr. Opin. Electrochem. 2023, 41, 101349. [Google Scholar] [CrossRef]
- Gao, J.J.; Tao, H.B.; Liu, B. Progress of Nonprecious-Metal-Based Electrocatalysts for Oxygen Evolution in Acidic Media. Adv. Mater. 2021, 33, 2003786. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zou, R.Q. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Jin, S. Are Metal Chalcogenides, Nitrides, and Phosphides Oxygen Evolution Catalysts or Bifunctional Catalysts? ACS Energy Lett. 2017, 2, 1937–1938. [Google Scholar] [CrossRef]
- Wu, J.; Ye, T.; Wang, Y.C.; Yang, P.Y.; Wang, Q.C.; Kuang, W.Y.; Chen, X.L.; Duan, G.H.; Yu, L.M.; Jin, Z.Q.; et al. Understanding the Catalytic Kinetics of Polysulfide Redox Reactions on Transition Metal Compounds in Li-S Batteries. ACS Nano 2022, 16, 15734–15759. [Google Scholar] [CrossRef]
- Ai, L.; Luo, Y.; Huang, W.; Tian, Y.; Jiang, J. Cobalt/cerium-based metal-organic framework composites for enhanced oxygen evolution electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 12893–12902. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Song, D.; Wang, L.; Zhang, Y.; Wang, Y. Cerium-Based Electrocatalysts for Oxygen Evolution/Reduction Reactions: Progress and Perspectives. Nanomaterials 2023, 13, 1921. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Han, C.D.; Gao, J.; Pan, L.; Wu, J.T.; Zhu, X.D.; Zou, J.J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Liu, X.-M.; Cui, X.; Dastafkan, K.; Wang, H.-F.; Tang, C.; Zhao, C.; Chen, A.; He, C.; Han, M.; Zhang, Q. Recent advances in spinel-type electrocatalysts for bifunctional oxygen reduction and oxygen evolution reactions. J. Energy Chem. 2021, 53, 290–302. [Google Scholar] [CrossRef]
- Saad, A.; Cheng, Z.X.; Shen, H.J.; Thomas, T.; Yang, M.H. Recent Advances in Nanocasting Cobalt-Based Mesoporous Materials for Energy Storage and Conversion. Electrocatalysis 2021, 11, 465–484. [Google Scholar] [CrossRef]
- Sun, S.N.; Sun, Y.M.; Zhou, Y.; Xi, S.B.; Ren, X.; Huang, B.C.; Liao, H.B.; Wang, L.Y.P.; Du, Y.H.; Xu, Z.C. Shifting Oxygen Charge Towards Octahedral Metal: A Way to Promote Water Oxidation on Cobalt Spinel Oxides. Angew. Chem. Int. Ed. 2019, 58, 6042–6047. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, N.; Ge, R.; Liu, J.; Li, W.; Chen, Y.; Feng, L.; Che, R. Porous Mn-doped cobalt phosphide nanosheets as highly active electrocatalysts for oxygen evolution reaction. Chem. Eng. J. 2021, 425, 131642. [Google Scholar] [CrossRef]
- Guo, Z.T.; Liu, L.; Wang, J.Q.; Cao, Y.; Tu, J.C.; Zhang, X.L.; Ding, L. Recent progress in CoP-based materials for electrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 34194–34215. [Google Scholar] [CrossRef]
- Ji, L.L.; Wei, Y.J.; Wu, P.R.; Xu, M.Z.; Wang, T.; Wang, S.; Liang, Q.F.; Meyer, T.J.; Chen, Z.F. Heterointerface Engineering of Ni2P-Co2P Nanoframes for Efficient Water Splitting. Chem. Mater. 2021, 33, 9165–9173. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Yang, S.X.; Li, B.; Lan, M.Q.; Liu, L.S.; Sun, Y.M.; Xiao, F.; Xiao, J.W. Heterostructuring cobalt sulfide with highly oxophilic 1T-tungsten sulfide for durable and efficient oxygen electrocatalysis. J. Mater. Chem. A 2022, 10, 19811–19820. [Google Scholar] [CrossRef]
- Cai, W.W.; Zhang, X.L.; Shi, J.W.; Li, J.; Liu, Z.; Zhou, S.F.; Jia, X.M.; Xiong, J.; Qu, K.G.; Huang, Y.J. Contribution of carbon support in cost-effective metal oxide/carbon composite catalysts for the alkaline oxygen evolution reaction. Catal. Commun. 2019, 127, 5–9. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.J.; Hang, X.D.; Zhang, X.D.; Xie, J.F.; Pan, B.C.; Xie, Y. Half-Metallicity in Single-Layered Manganese Dioxide Nanosheets by Defect Engineering. Angew. Chem. Int. Ed. 2015, 54, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, S.B.; Xie, J.W.; Wang, L.; Zhang, X.W.; Zou, J.J. Constructing TiO2 p-n homojunction for photoelectrochemical and photocatalytic hydrogen generation. Nano Energy 2016, 28, 296–303. [Google Scholar] [CrossRef]
- Yu, X.L.; Wu, X.M.; Chen, Z.Y.; Huang, Z.W.; Jing, G.H. Oxygen vacancy defect engineering in Mn-doped CeO2 nanostructures for, nitrogen oxides emission abatement. Mol. Catal. 2019, 476, 110512. [Google Scholar] [CrossRef]
- Wang, X.X.; Yang, Y.; Diao, L.C.; Tang, Y.; He, F.; Liu, E.Z.; He, C.N.; Shi, C.S.; Li, J.J.; Sha, J.W.; et al. CeOx-Decorated NiFe-Layered Double Hydroxide for Efficient Alkaline Hydrogen Evolution by Oxygen Vacancy Engineering. ACS Appl. Mater. Interfaces 2018, 10, 35145–35153. [Google Scholar] [CrossRef]
- Zhang, R.R.; Zhang, Y.C.; Pan, L.; Shen, G.Q.; Mahmood, N.; Ma, Y.H.; Shi, Y.; Jia, W.Y.; Wang, L.; Zhang, X.W.; et al. Engineering Cobalt Defects in Cobalt Oxide for Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Yan, W.S.; Ma, H.; Zhao, X.T.; Zhang, Y.; Vishniakov, P.; Wang, X.; Zhong, X.H.; Hong, Z.; Maximov, M.Y.; Song, L.; et al. P and Se Binary Vacancies and Heterostructures Modulated MoP/MoSe2 Electrocatalysts for Improving Hydrogen Evolution and Coupling Electricity Generation. Small 2023, 19, 2208270. [Google Scholar] [CrossRef]
- Eckberg, R.P.; Hatfield, W.E.; Losee, D.B. Unusual magnetic properties of polymeric cobalt(II) monoglycerolate, a compound containing alkoxo-bridged cobalt(II) ions. Inorg. Chem. 1974, 13, 740–742. [Google Scholar] [CrossRef]
- Pan, L.; Wang, S.B.; Mi, W.B.; Song, J.J.; Zou, J.J.; Wang, L.; Zhang, X.W. Undoped ZnO abundant with metal vacancies. Nano Energy 2014, 9, 71–79. [Google Scholar] [CrossRef]
- Wang, S.B.; Pan, L.; Song, J.J.; Mi, W.B.; Zou, J.J.; Wang, L.; Zhang, X.W. Titanium-Defected Undoped Anatase TiO2 with p-Type Conductivity, Room-Temperature Ferromagnetism, and Remarkable Photocatalytic Performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yu, H.F.; Cai, L.L.; Chen, L.; Li, C.Z.; Jiang, H. Unique insights into the design of low- strain single- crystalline Ni- rich cathodes with superior cycling stability. Proc. Natl. Acad. Sci. USA 2024, 121, e2317282121. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Zhao, L.L.; Xu, H.R.; Huang, Q.S.; Wang, Y.Q.; Hou, C.X.; Hou, Y.Y.; Wang, J.; Dang, F.; Zhang, J.T. Tunable Cationic Vacancies of Cobalt Oxides for Efficient Electrocatalysis in Li-O2 Batteries. Adv. Energy Mater. 2020, 10, 2001415. [Google Scholar] [CrossRef]
- Wang, J.K.; Gao, R.; Zhou, D.; Chen, Z.J.; Wu, Z.H.; Schumacher, G.; Hu, Z.B.; Liu, X.F. Boosting the Electrocatalytic Activity of Co3O4 Nanosheets for a Li-O2Battery through Modulating Inner Oxygen Vacancy and Exterior Co3+/Co2+Ratio. ACS Catal. 2017, 7, 6533–6541. [Google Scholar] [CrossRef]
- Zhai, G.J.; Wang, J.G.; Chen, Z.M.; An, W.; Men, Y. Boosting soot combustion efficiency of Co3O4 nanocrystals via tailoring crystal facets. Chem. Eng. J. 2018, 337, 488–498. [Google Scholar] [CrossRef]
- Qu, J.; Liu, W.; Liu, R.; He, J.; Liu, D.; Feng, Z.; Feng, Z.; Li, R.; Li, C. Evolution of oxygen vacancies in cerium dioxide at atomic scale under CO2 reduction. Chem. Catal. 2023, 3, 100759. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering Cobalt Oxide with Coexisting Cobalt Defects and Oxygen Vacancies for Enhanced Catalytic Oxidation of Toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Wang, Y.; Huang, Y.C.; Wei, Z.X.; Dong, C.L.; Ma, J.M.; Shen, S.H.; Li, Y.F.; Wang, S.Y. Filling the oxygen vacancies in Co3O4 with phosphorus: An ultra-efficient electrocatalyst for overall water splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Gu, Z.H.; Cheng, C.; Yan, T.R.; Liu, G.L.; Jiang, J.S.; Mao, J.; Dai, K.H.; Li, J.; Wu, J.P.; Zhang, L. Synergistic effect of Co3Fe7 alloy and N-doped hollow carbon spheres with high activity and stability for high-performance lithium-sulfur batteries. Nano Energy 2021, 86, 106111. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Li, G.R.; Fu, J.; Chen, Z.W.; Peng, X.S. Strings of Porous Carbon Polyhedrons as Self-Standing Cathode Host for High-Energy-Density Lithium-Sulfur Batteries. Angew. Chem.-Int. Ed. 2017, 56, 6176–6180. [Google Scholar] [CrossRef]
- Li, G.X.; Han, Y. Two-in-One MOF Structure with Tunable Porosity for Enhanced Separation. ACS Cent. Sci. 2022, 8, 150–152. [Google Scholar] [CrossRef]
- Sun, S.N.; Li, H.Y.; Xu, Z.C.J. Impact of Surface Area in Evaluation of Catalyst Activity. Joule 2018, 2, 1024–1027. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Zhu, J.L.; Zhao, S.Z.; Li, Y.Y.; Zhang, B.; Jiang, J.J.; Ji, X.; Zhang, H.Y.; Shen, P.K. Chestnut-like copper cobalt phosphide catalyst for all-pH hydrogen evolution reaction and alkaline water electrolysis. J. Mater. Chem. A 2019, 7, 14271–14279. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chang, Y.C.; Lee, J.F.; Pao, C.W.; Huang, H.C.; Wang, C.H. Operando Identification of Hydrangea-like and Amorphous Cobalt Oxyhydroxide Supported by Thin-Layer Copper for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2021, 9, 12300–12310. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.Q.; Xiao, Z.H.; Li, X.Y.; Huo, J.; Wang, S.Y.; Dai, L.M. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Qian, J.M.; Ran, J.Q.; Xi, P.X.; Yang, L.J.; Gao, D.Q. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Li, Y.; Nidamanuri, N.P.; Jiang, A.; Wang, Z.; Li, Q.; Dong, M. In situ construction of tandem nitrogen-doped MoP nanocrystals for high-efficient electrocatalytic hydrogen evolution. Electrochim. Acta 2020, 342, 136059. [Google Scholar] [CrossRef]
- Gao, Y.H.; Wang, K.P.; Xu, C.; Fang, H.; Yu, H.L.; Zhang, H.; Li, S.K.; Li, C.H.; Huang, F.Z. Enhanced electrocatalytic nitrate reduction through phosphorus-vacancy-mediated kinetics in heterogeneous bimetallic phosphide hollow nanotube array. Appl. Catal. B-Environ. 2023, 330, 122627. [Google Scholar] [CrossRef]
- Huang, H.P.; Liu, K.; Yang, F.L.; Cai, J.L.; Wang, S.P.; Chen, W.Z.; Wang, Q.X.; Fu, L.H.; Xie, Z.X.; Xie, S.F. Breaking Surface Atomic Monogeneity of Rh2P Nanocatalysts by Defect-Derived Phosphorus Vacancies for Efficient Alkaline Hydrogen Oxidation. Angew. Chem. Int. Ed. 2023, 62, 2315752. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, L.Z.; He, T.W.; Jia, Y.; Zhang, L.Z.; Yen, X.C.; Gao, M.R.; Du, A.J.; Zhu, Z.H.; Yao, X.D.; et al. Grafting Cobalt Diselenide on Defective Graphene for Enhanced Oxygen Evolution Reaction. Iscience 2018, 7, 145–153. [Google Scholar] [CrossRef]
- Jin, X.X.; Wang, R.Y.; Zhang, L.X.; Si, R.; Shen, M.; Wang, M.; Tian, J.J.; Shi, J.L. Electron Configuration Modulation of Nickel Single Atoms for Elevated Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, W.X.; Peng, Y.F.; Wei, Y.M. Application of Electrochemical Impedance Spectroscopy to Degradation and Aging Research of Lithium-Ion Batteries. J. Phys. Chem. C 2023, 127, 4465–4495. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A-Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Huang, J.Z.; Borca, C.N.; Huthwelker, T.; Yuezbasi, N.S.; Baster, D.; El Kazzi, M.; Schneider, C.W.; Schmidt, T.J.; Fabbri, E. Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment. Nat. Commun. 2024, 15, 3067. [Google Scholar] [CrossRef]
- Ou, L.; Yang, F.; Liu, Y.; Chen, S. First-Principle Study of the Adsorption and Dissociation of O2 on Pt(111) in Acidic Media. J. Phys. Chem. C 2009, 113, 20657–20665. [Google Scholar] [CrossRef]
- Wu, Q.K.; Zhao, D.K.; Yu, X.L.; Xu, J.C.; Wang, N.; Zhou, W.; Li, L.G. Vapor-assisted engineering heterostructure of 1D Mo3N2 nanorod decorated with nitrogen-doped carbon for rapid pH-Universal hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 5064–5073. [Google Scholar] [CrossRef]
- Gong, S.Y.; Zhang, T.Y.; Meng, J.; Sun, W.M.; Tian, Y. Advances in the mechanism investigation for the oxygen evolution reaction: Fundamental theory and monitoring techniques. Mater. Chem. Front. 2024, 8, 603–626. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, Q.; Wang, Y. Dynamic surface reconstruction of perovskite oxides in oxygen evolution reaction and its impacts on catalysis: A critical review. Mater. Today Chem. 2023, 34, 101800. [Google Scholar] [CrossRef]
- Hao, Y.; Cao, X.; Lei, C.; Chen, Z.; Yang, X.; Gong, M. Chemical oxygen species on electrocatalytic materials during oxygen evolution reaction. Mater. Today Catal. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper Cobalt Sulfide Nanosheets Realizing a Promising Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Guo, Y.N.; Yuliarto, B.; Jiang, X.C.; Ide, Y.; Nugraha, N.; Dipojono, H.K.; Yu, A.B.; Sugahara, Y.; et al. Holey Assembly of Two-Dimensional Iron-Doped Nickel-Cobalt Layered Double Hydroxide Nanosheets for Energy Conversion Application. Chemsuschem 2020, 13, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Tang, B.; Gu, X.C.; Feng, L.G. Surface chemical state evaluation of CoSe2 catalysts for the oxygen evolution reaction. Chem. Commun. 2019, 55, 10928–10931. [Google Scholar] [CrossRef] [PubMed]
- Zai, S.F.; Zhou, Y.T.; Yang, C.C.; Jiang, Q. Al, Fe-codoped CoP nanoparticles anchored on reduced graphene oxide as bifunctional catalysts to enhance overall water splitting. Chem. Eng. J. 2021, 421, 127856. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, J.P.; Chen, X.J.; Yu, S.; Yu, L.; Si, R.; Wang, Y.; Wang, S.H.; Meng, X.G.; Wang, Y.; et al. Reaction Mechanisms of Well-Defined Metal-N4 Sites in Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 16339–16342. [Google Scholar] [CrossRef]
- Thiyagarajan, D.; Gao, M.Y.; Sun, L.; Dong, X.C.; Zheng, D.H.; Wahab, M.A.; Will, G.; Lin, J.J. Nanoarchitectured porous Cu-CoP nanoplates as electrocatalysts forefficient oxygen evolution reaction. Chem. Eng. J. 2022, 432, 134303. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, W.; Li, L.; Zhou, W.; Chen, M.; Zhu, C.; Zhu, X.; Yuan, K. Developing a Cobalt Phosphide Catalyst with Combined Cobalt Defects and Phosphorus Vacancies to Boost Oxygen Evolution Reaction. Materials 2024, 17, 4647. https://doi.org/10.3390/ma17184647
Ou W, Li L, Zhou W, Chen M, Zhu C, Zhu X, Yuan K. Developing a Cobalt Phosphide Catalyst with Combined Cobalt Defects and Phosphorus Vacancies to Boost Oxygen Evolution Reaction. Materials. 2024; 17(18):4647. https://doi.org/10.3390/ma17184647
Chicago/Turabian StyleOu, Weihua, Ligui Li, Wei Zhou, Minzhe Chen, Chuheng Zhu, Xiaoyan Zhu, and Ke Yuan. 2024. "Developing a Cobalt Phosphide Catalyst with Combined Cobalt Defects and Phosphorus Vacancies to Boost Oxygen Evolution Reaction" Materials 17, no. 18: 4647. https://doi.org/10.3390/ma17184647
APA StyleOu, W., Li, L., Zhou, W., Chen, M., Zhu, C., Zhu, X., & Yuan, K. (2024). Developing a Cobalt Phosphide Catalyst with Combined Cobalt Defects and Phosphorus Vacancies to Boost Oxygen Evolution Reaction. Materials, 17(18), 4647. https://doi.org/10.3390/ma17184647






