Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Particles and Morphology Extraction
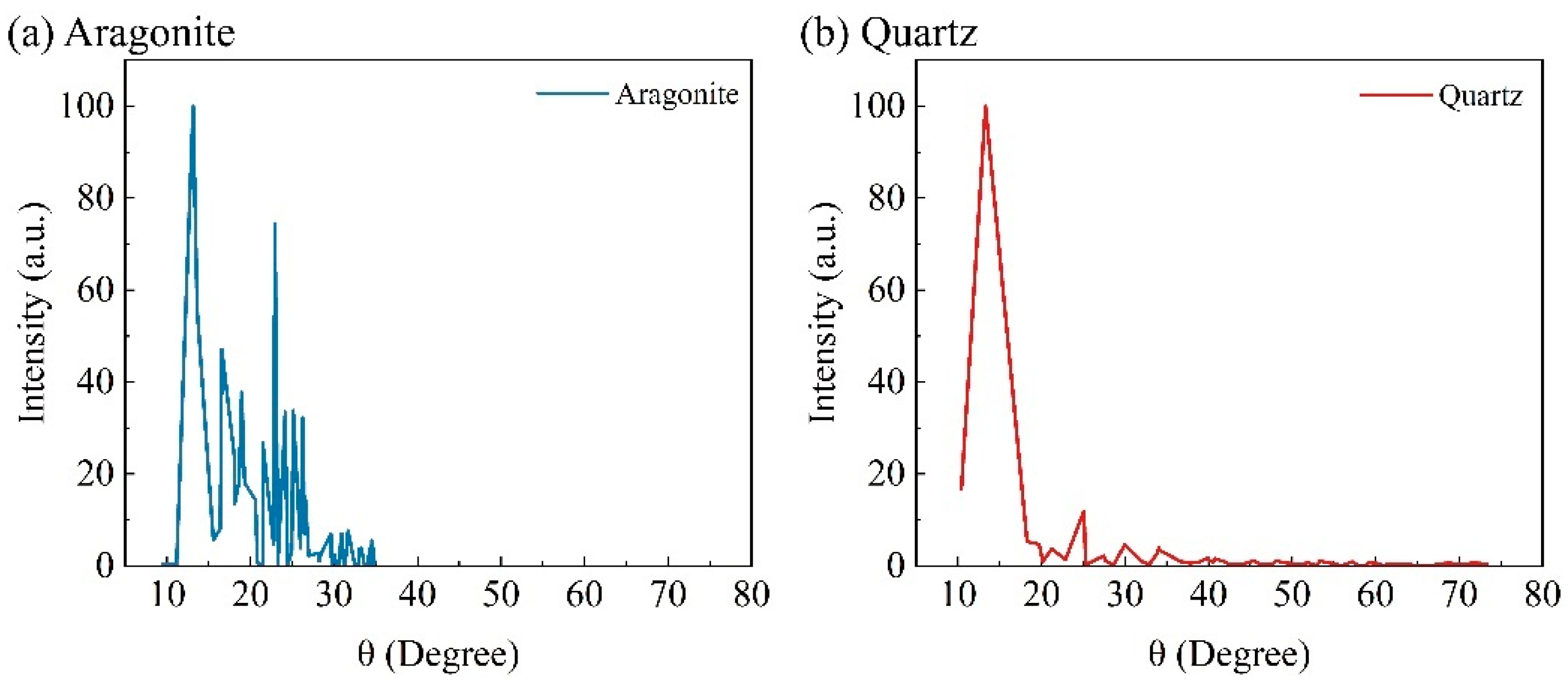
2.1.1. General Chemical and Physical Features
2.1.2. Morphology Extraction Tests
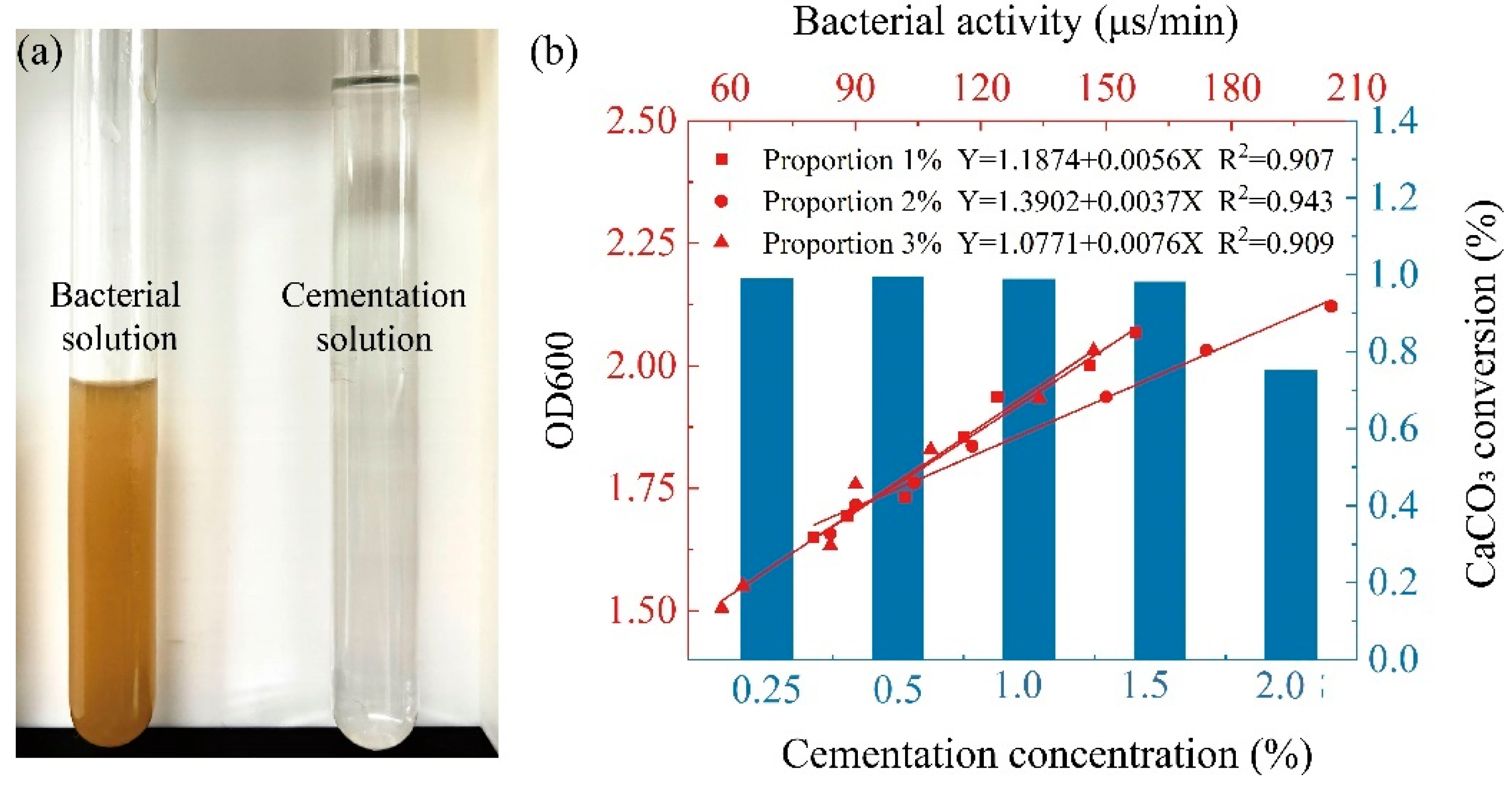
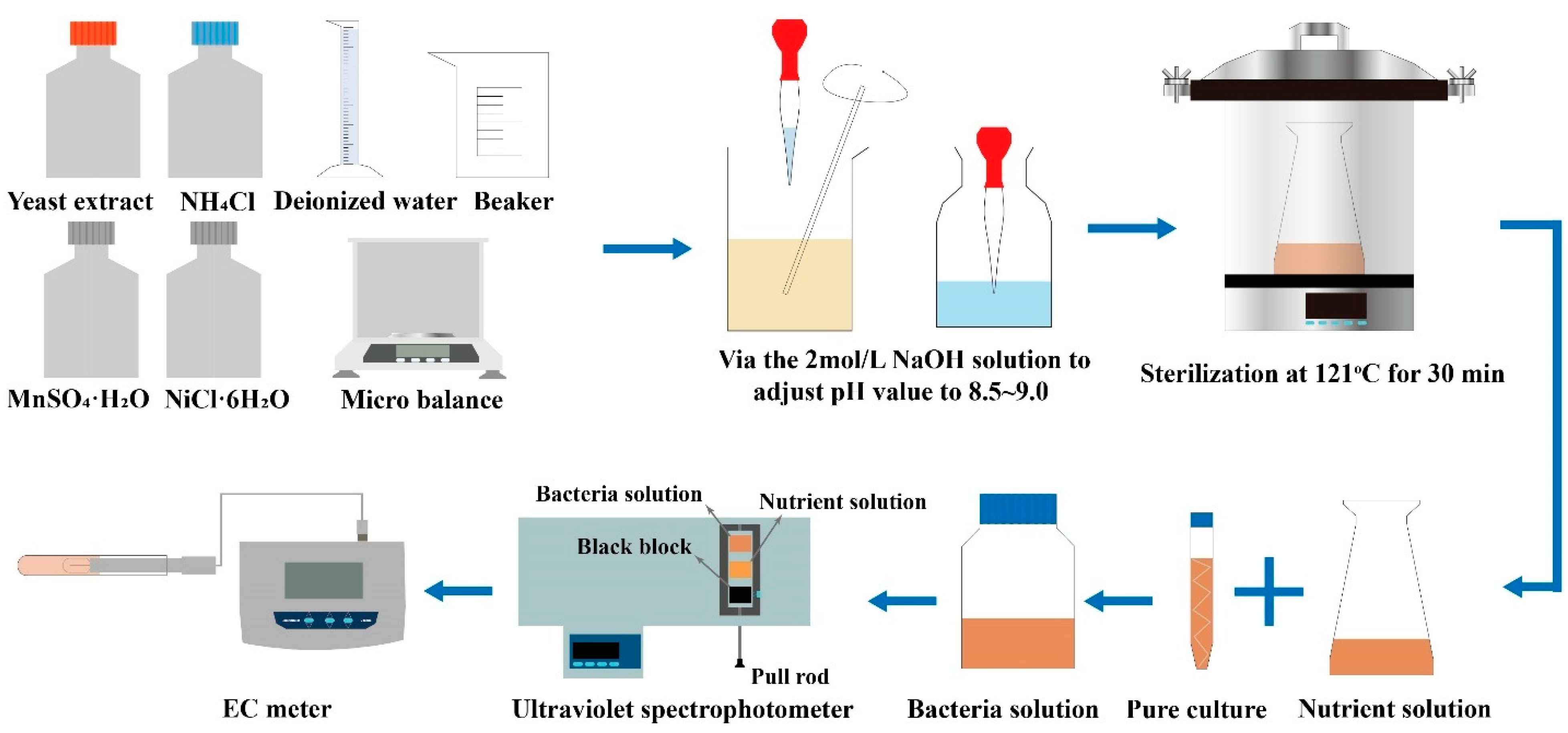
2.2. Bacteria Solution and Cementation Solution
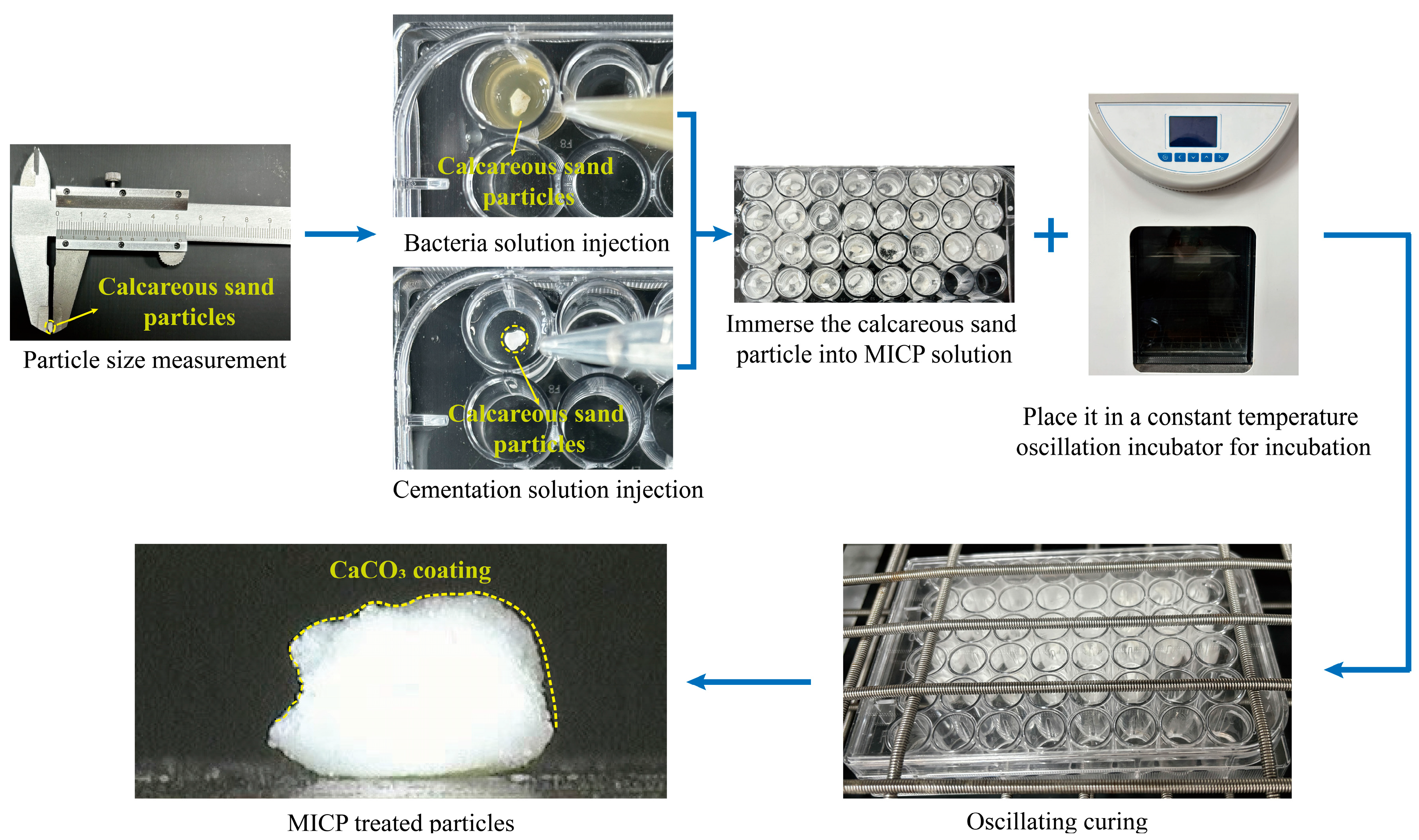
2.3. MICP Treatment for Individual Particles
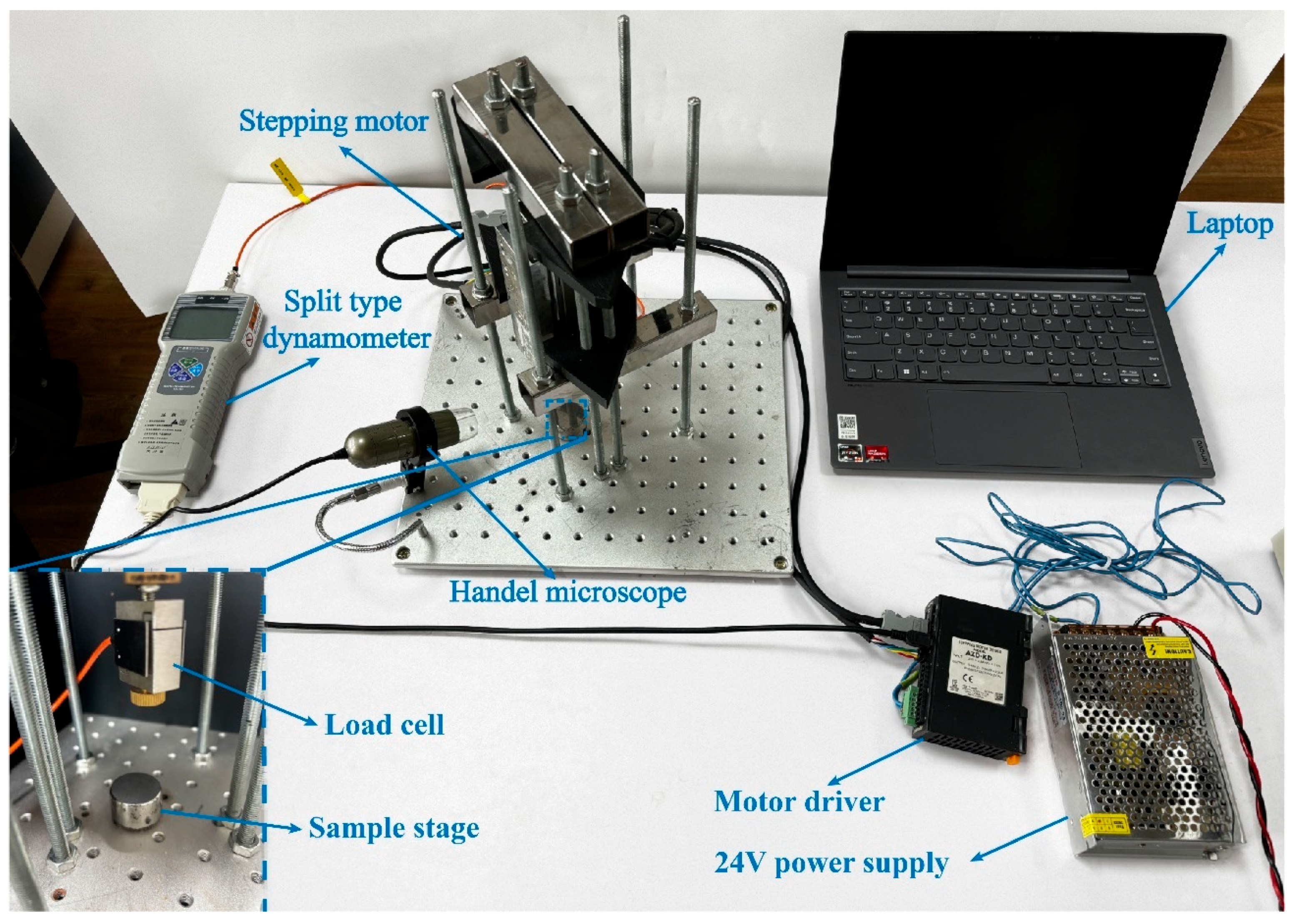
2.4. Single-Particle Crushing Tests
2.5. Scanning Electron Microscopy (SEM) Tests
3. Results and Discussion
3.1. Morphologies of Coated Calcareous Sand
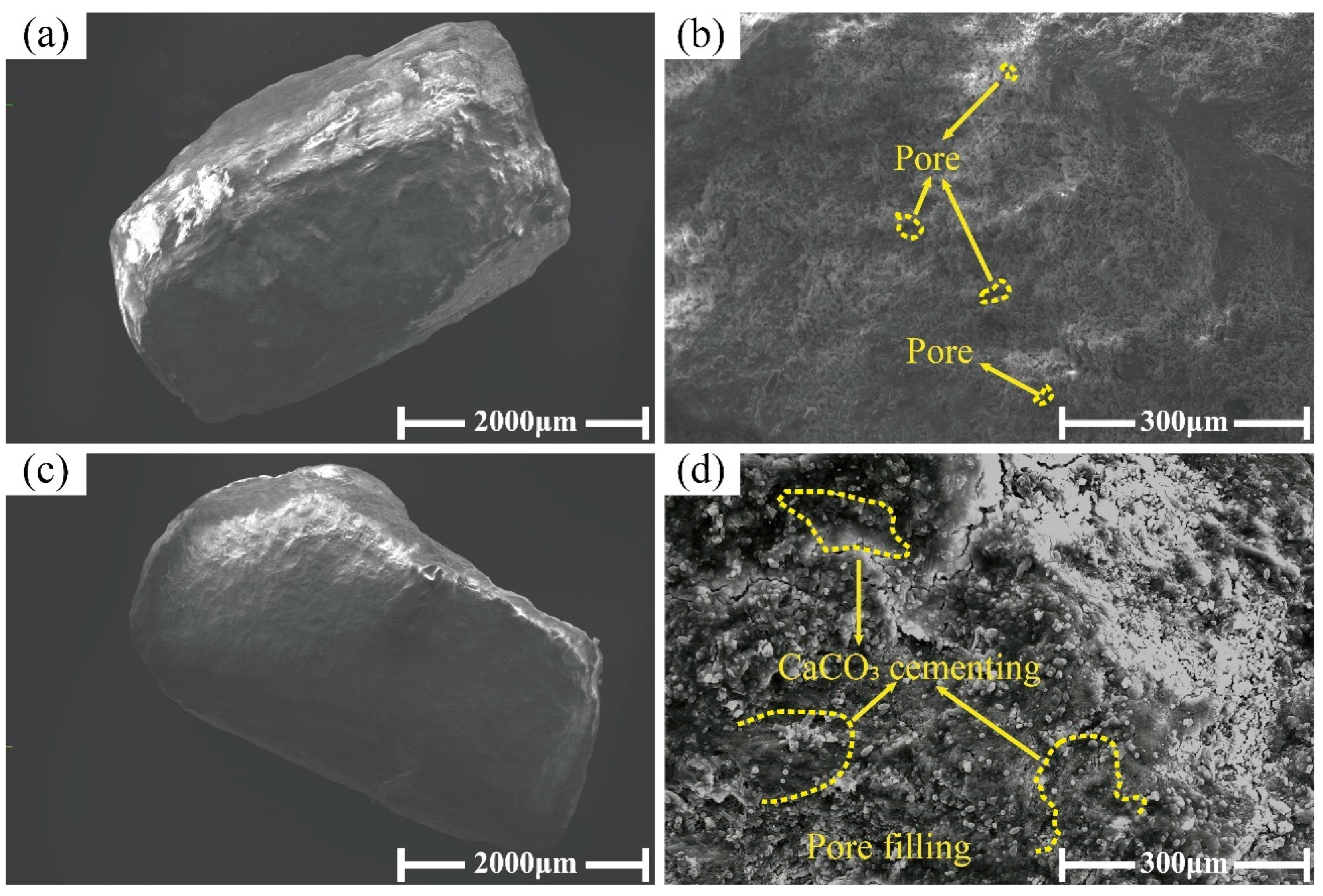
3.2. Microscopic Structure of Coated Calcareous Sand
3.3. The Impact of Coating Thickness on Crushing Behavior
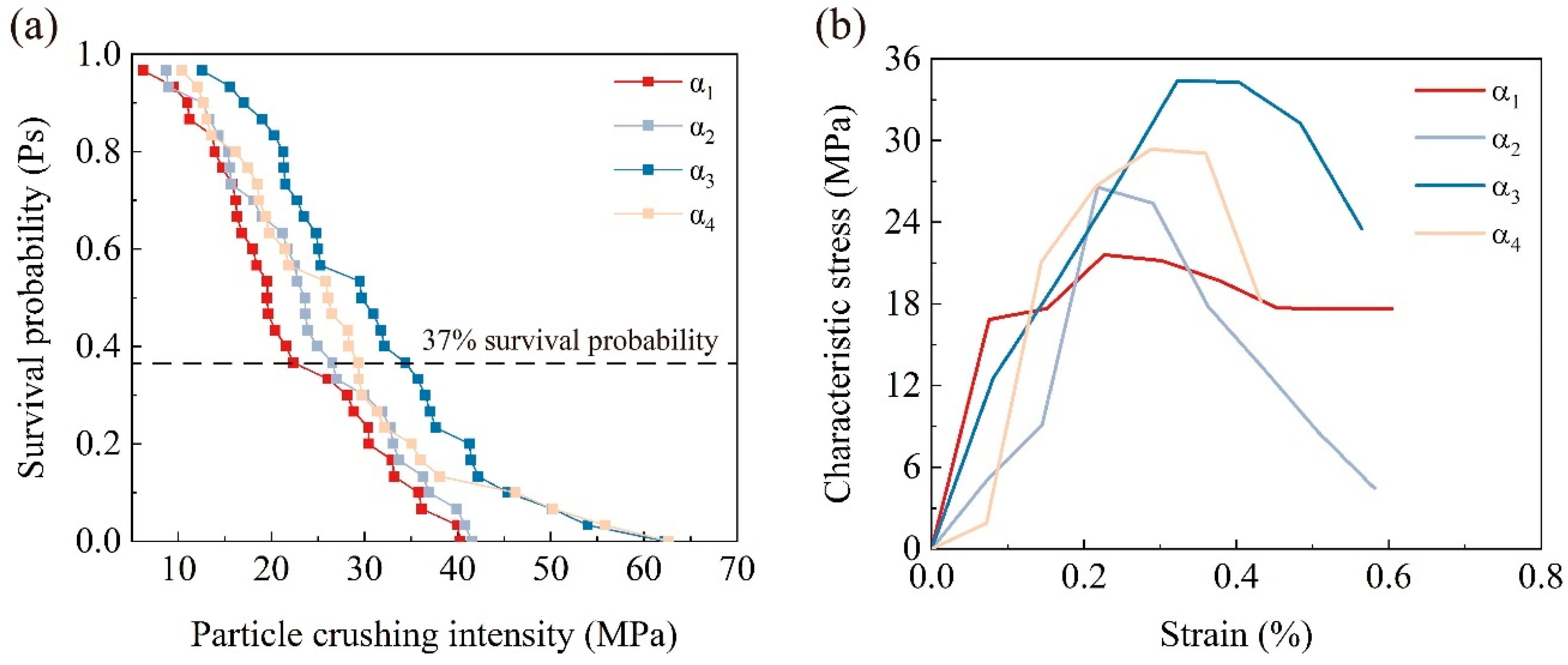
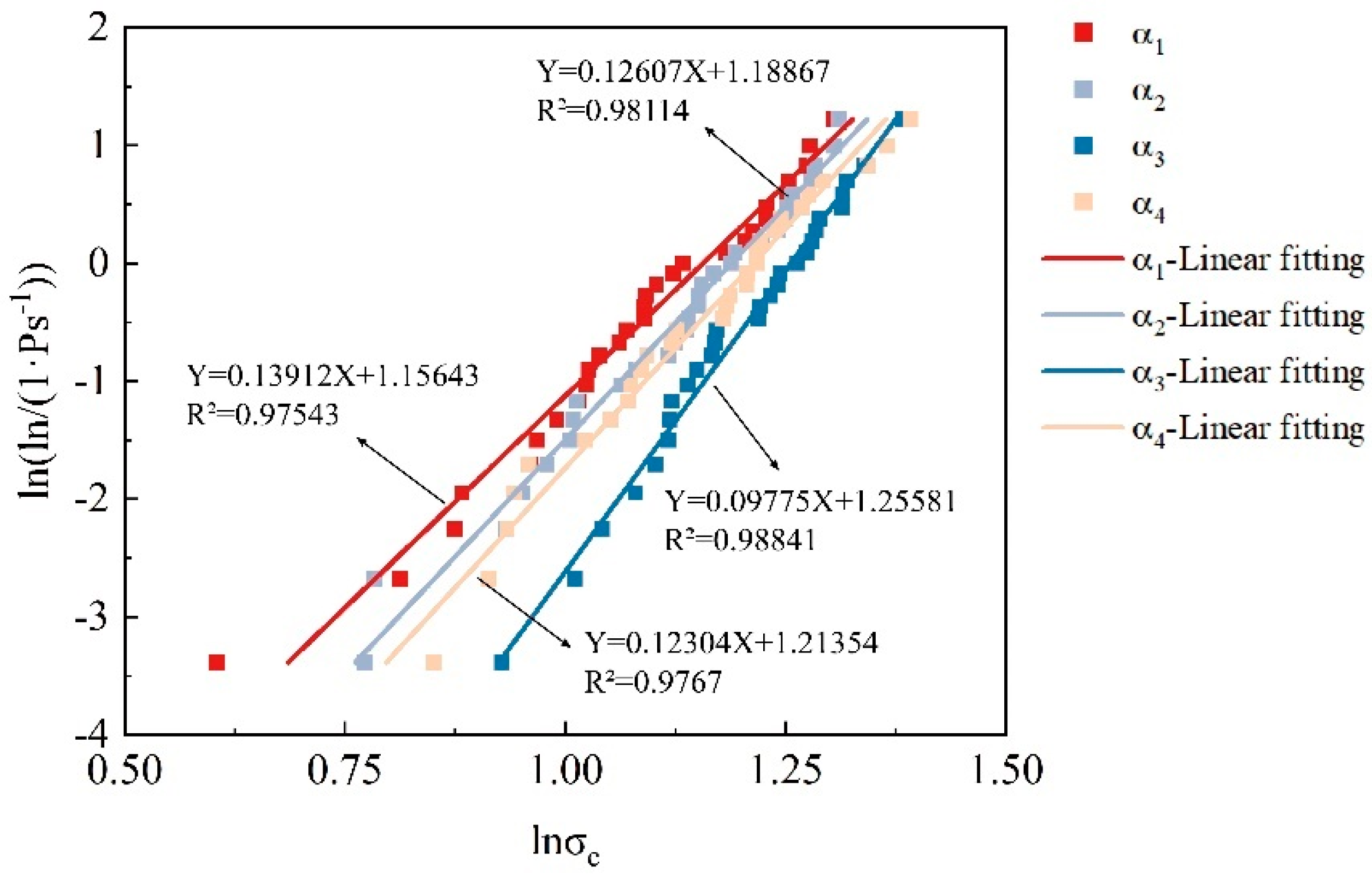
3.3.1. Residual Probability and Characteristic Strength
3.3.2. The Dispersion of Particle Strength Distribution
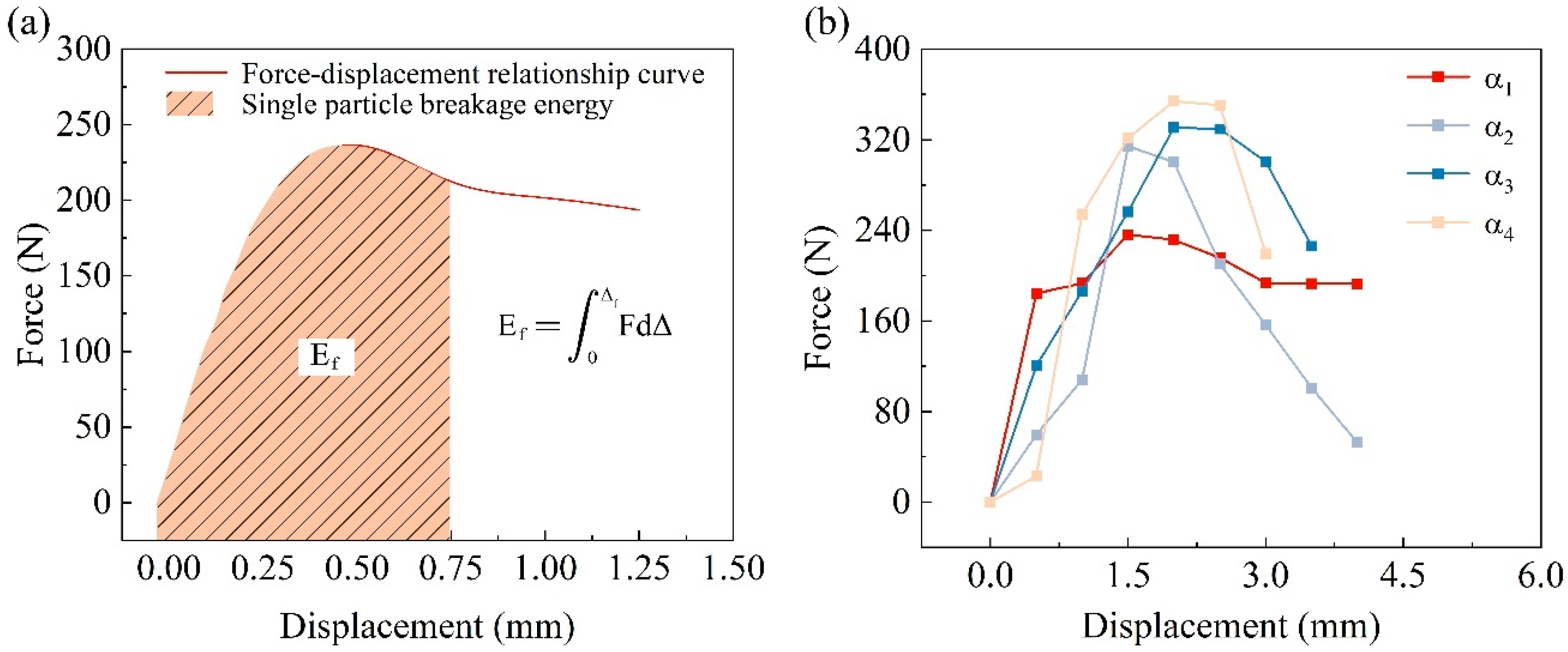
3.3.3. Single-Particle Crushing Energy
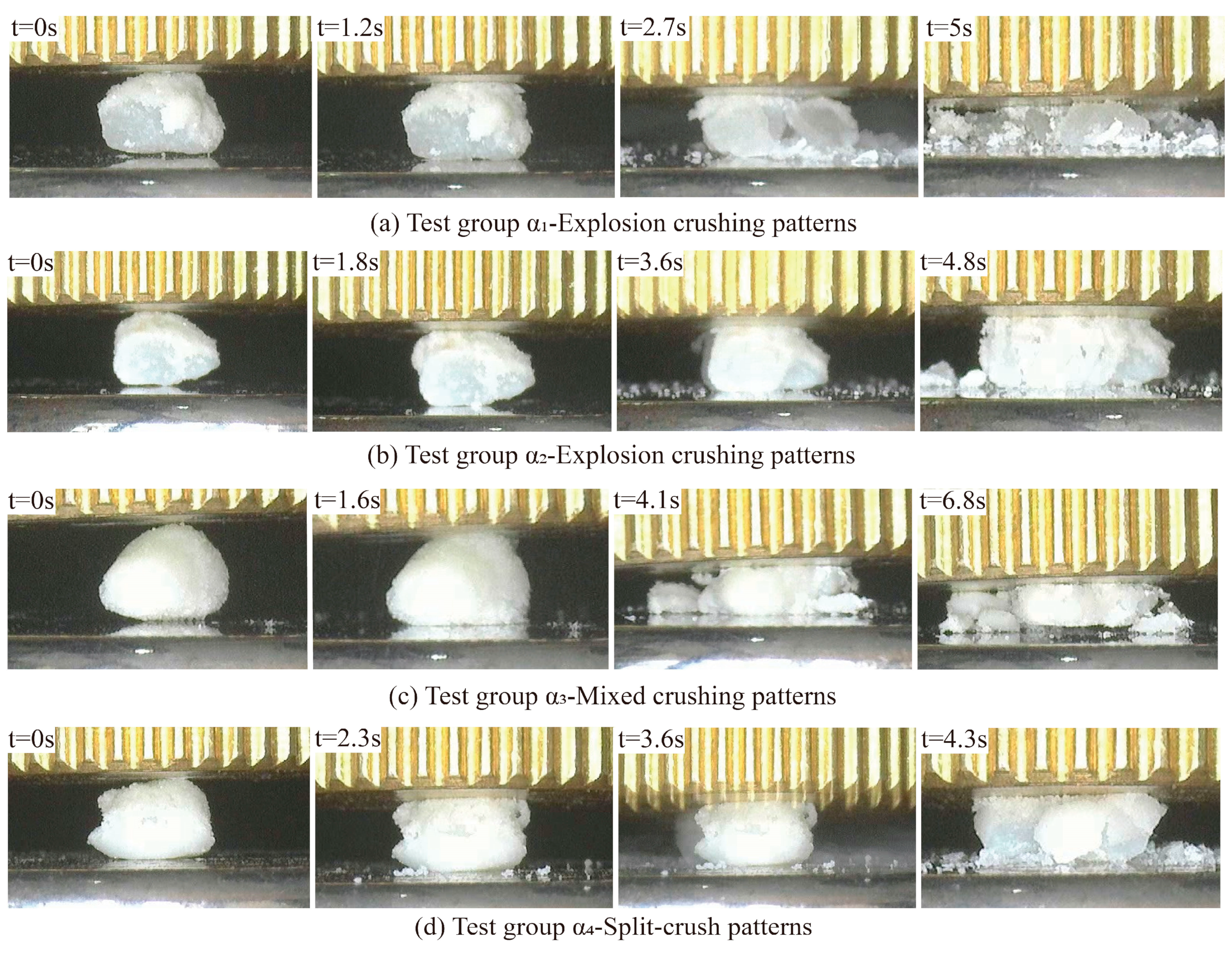
3.4. Crushing Process and Pattern
3.5. Fragment Characteristics of Coated Calcareous Sand after Crushing
4. Conclusions
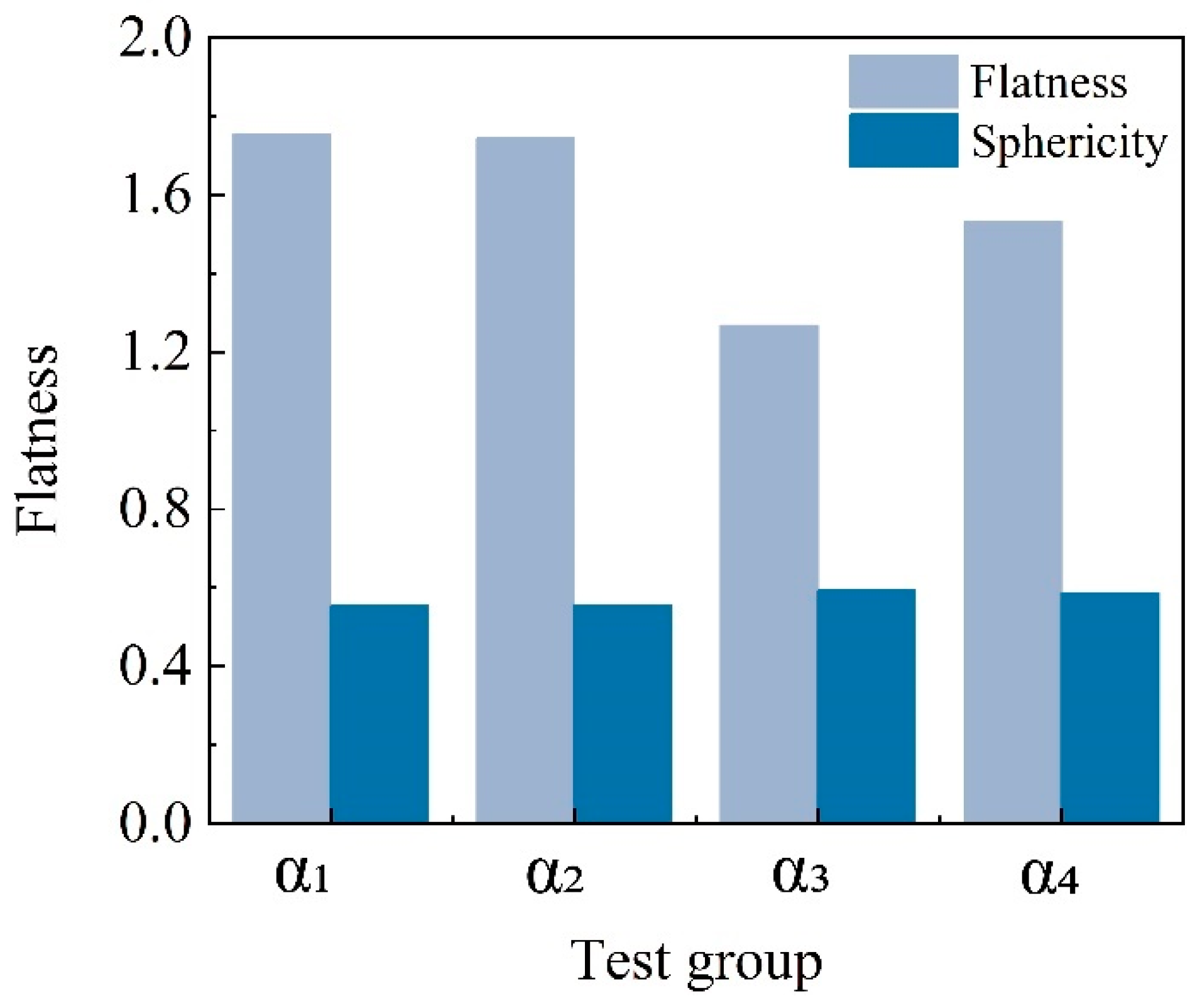
- The basic size parameters of MICP-treated coated calcareous sand particles were precisely quantified using 3D scanning technology. The results showed that the MICP treatment significantly altered the surface structure of calcareous sand particles, leading to increases in particle size, surface area, and volume. Notably, the calcium carbonate coating formed by microbial mineralization on the particle surface significantly reduced the overall flatness of the particles and somewhat increased their sphericity. This provides an intuitive understanding of the changes in particle size and morphology due to MICP treatment.
- The calcium carbonate coating formed by microbial mineralization on the particle surface significantly increased the coating thickness. SEM results indicated that the surfaces of MICP-treated, coated calcareous sand particles became smoother, with the original unevenness reduced and surface pores filled with calcium carbonate, all contributing to enhanced mechanical properties.
- The concentration of the cementation solution can regulate the thickness of the calcium carbonate coating, directly influencing the crushing behavior. At a cementation solution concentration of 0.5 mol/L, the calcium carbonate coating was thinner, resulting in a lower particle crushing strength. When increased to 1 mol/L, the coating thickness and crushing strength were maximized, with some particles exhibiting crushing strengths exceeding 40 MPa, demonstrating optimal microbial mineralization effects. Further increasing the concentration to 1.5 mol/L inhibited microbial activity due to the excessively high concentration, reducing coating thickness and crushing strength, but most particles still maintained relatively high crushing strength. These findings highlight the crucial role of controlling the cementation solution concentration in optimizing the mechanical properties of calcareous sand. Specifically, at 1 mol/L, the thickening of the coating significantly enhanced the particles’ crushing strength and energy, whereas at 1.5 mol/L, despite a decrease in crushing energy, the initial larger particle size maintained high crushing energy levels. Additionally, with increasing coating thickness, the Weibull modulus decreased, reflecting increased dispersion in the crushing strength, further proving the direct and significant impact of calcium carbonate coating thickness on the crushing characteristics of the particles.
- In compression experiments, coated calcareous sand particles exhibited different crushing modes, including explosive crushing, mixed crushing, and splitting crushing, closely related to the presence of the calcium carbonate coating. As compression increased, particles first showed elastic deformation, quickly reaching peak stress and fracturing. The filling effect of the calcium carbonate enhanced the overall crushing strength of the particles, causing them to tend to break into larger fragments, especially when the coating was thicker. This phenomenon underscores that the MICP treatment, by altering the coating thickness, not only improved the mechanical properties of calcareous sand but also effectively controlled the crushing behavior of the particles.
- During single-particle crushing, the fractal characteristics of coated calcareous sand particles were expressed by the relationship between the number of fragments and particle size, following a fractal distribution. Changes in the fractal dimension indicated that with increasing coating thickness, the fractal degree of local crushing decreased, related to the increased thickness of the calcium carbonate coating formed after the MICP treatment. This variation in coating thickness had a direct impact on the local crushing characteristics of the particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brandes, H.G. Simple shear behavior of calcareous and quartz sands. Geotech. Geol. Eng. 2011, 29, 113–126. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Y.; Zuo, D. Effects of particle size on dynamic constitutive relation and energy absorption of calcareous sand. Powder Technol. 2019, 356, 21–30. [Google Scholar] [CrossRef]
- Wang, S.; Lei, X.-W.; Meng, Q.-S.; Xu, J.-L.; Xie, L.-F.; Li, Y.-J. Influence of Particle Shape on the Density and Compressive Performance of Calcareous Sand. KSCE J. Civ. Eng. 2020, 24, 49–62. [Google Scholar] [CrossRef]
- Wei, H.; Liu, H.; Zhao, T.; Zhang, S.; Ma, L.; Yin, M.; Meng, Q. Particle breakage and morphology changes of calcareous sands under one-dimensional compression loading. Mar. Geophys. Res. 2022, 43, 45. [Google Scholar] [CrossRef]
- Al-Homoud, A.S.; Wehr, W. Experience of Vibrocompaction in Calcareous Sand of UAE. Geotech. Geol. Eng. 2006, 24, 757–774. [Google Scholar] [CrossRef]
- Van Impe, P.; Van Impe, W.; Manzotti, A.; Mengé, P.; Broeck, M.V.D.; Vinck, K. Compaction control and related stress–strain behaviour of off-shore land reclamations with calcareous sands. Soils Found. 2015, 55, 1474–1486. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, D.-P.; Wang, F.-J.; Lyu, H.-B. Investigation of Engineering Properties of Cement-Stabilized Calcareous Sand Foundation. Adv. Mater. Sci. Eng. 2021, 2021, 9188560. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, H.; Zhou, F.; Dai, G. Axial Bearing Mechanism of Post-Grouted Piles in Calcareous Sand. Appl. Sci. 2022, 12, 2731. [Google Scholar] [CrossRef]
- Opukumo, A.W. Sodium Silicate Stabilization of Collapsible Clayey Calcareous Soils. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2020. [Google Scholar]
- Tao, G.; Yuan, J.; Chen, Q.; Peng, W.; Yu, R.; Basack, S. Chemical stabilization of calcareous sand by polyurethane foam adhesive. Constr. Build. Mater. 2021, 295, 123609. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, A.; Li, N.; Wang, W.; Hu, J. Mechanical properties and microscopic characterization of cement stabilized calcareous sand modified by nano SiO2. Case Stud. Constr. Mater. 2022, 17, e01636. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Wang, H. Theoretical quantification for cracks repair based on microbially induced carbonate precipitation (MICP) method. Cem. Concr. Compos. 2021, 118, 103950. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wang, S.; Evans, T.M.; Stuedlein, A.W.; Chu, J.; Zhao, C.; Wu, H.; Liu, H. Homogeneity and mechanical behaviors of sands improved by a temperature-controlled one-phase MICP method. Acta Geotech. 2021, 16, 1417–1427. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, C.; Sun, Y.; Wang, S.; Wu, H.; Chen, H.; Liu, H. Compression behavior of MICP-treated sand with various gradations. Acta Geotech. 2020, 16, 1391–1400. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, J.; Tong, H.; Yuan, J.; Lei, D.; Li, Y. Effects of activated carbon on liquefaction resistance of calcareous sand treated with microbially induced calcium carbonate precipitation. Soil Dyn. Earthq. Eng. 2022, 161, 107419. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Shan, Y.; Tong, H.; Zhao, J. Effect of Magnesium Ions on the Mechanical Properties of Soil Reinforced by Microbially Induced Carbonate Precipitation. J. Mater. Civ. Eng. 2023, 35, 04023413. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Li, S.-K.; Li, Z.-Y.; Garg, A. Exploring the application of the MICP technique for the suppression of erosion in granite residual soil in Shantou using a rainfall erosion simulator. Acta Geotech. 2023, 18, 3273–3285. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, C.; Gao, Y. Improving the Erosion Resistance Performance of Pisha Sandstone Weathered Soil Using MICP Technology. Crystals 2021, 11, 1112. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Dapaah, M.F.; Niu, Q.; Cheng, L. Bio-Remediation of Heavy Metal-Contaminated Soil by Microbial-Induced Carbonate Precipitation (MICP)—A Critical Review. Sustainability 2023, 15, 7622. [Google Scholar] [CrossRef]
- Bhutange, S.P.; Latkar, M.V. Microbially Induced Calcium Carbonate Precipitation in Construction Materials. J. Mater. Civ. Eng. 2020, 32, 03120001. [Google Scholar] [CrossRef]
- Lapierre, F.M.; Schmid, J.; Ederer, B.; Ihling, N.; Büchs, J.; Huber, R. Revealing nutritional requirements of MICP-relevant Sporosarcina pasteurii DSM33 for growth improvement in chemically defined and complex media. Sci. Rep. 2020, 10, 22448. [Google Scholar] [CrossRef]
- Ferris, F.; Stehmeier, L.; Kantzas, A.; Mourits, F. Bacteriogenic Mineral Plugging. J. Can. Pet. Technol. 1997, 36. [Google Scholar] [CrossRef] [PubMed]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Liu, H.; Zhang, Z.; Ding, X. Performance evaluation of a MICP-treated calcareous sandy foundation using shake table tests. Soil Dyn. Earthq. Eng. 2019, 129, 105959. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Zhang, X.; Sarajpoor, S.; Zhang, S.; Yao, X. Experimental study on permeability and strength characteristics of MICP-treated calcareous sand. Biogeotechnics 2023, 1, 100034. [Google Scholar] [CrossRef]
- Wang, B.; Guo, L.; Luo, X.; Jiang, Y.; Li, Q.; Xie, J. Identification and extraction of cementation patterns in sand modified by MICP: New insights at the pore scale. PLoS ONE 2024, 19, e0296437. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, W.M.; Liu, Z.R.; Wang, Q.; Chen, Y.G. Advances in soil cementation by biologically induced calcium carbonate precipitation. Rock Soil Mech. 2022, 43, 3. [Google Scholar]
- Zhang, X.; Zhou, B.; You, L.; Wu, Z.; Wang, H. Cementation anisotropy associated with microbially induced calcium-carbonate precipitation and its treatment effect on calcareous and quartz sands. Constr. Build. Mater. 2023, 395, 132237. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Jiao, Y.-Y.; Wang, R.; Hu, M.-J.; Meng, Q.-S.; Tan, F.-Y. Engineering characteristics of the calcareous sand in Nansha Islands, South China Sea. Eng. Geol. 2011, 120, 40–47. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Stuedlein, A.W.; Evans, T.M.; Xiao, Y. Strength, stiffness, and microstructure characteristics of biocemented cal-careous sand. Can. Geotech. J. 2019, 56, 1502–1513. [Google Scholar] [CrossRef]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- Qabany, A.A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. In Bio-and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013; ICE Publishing: London, UK, 2014; pp. 107–115. [Google Scholar] [CrossRef]
- Ma, L.; Pang, A.-P.; Luo, Y.; Lu, X.; Lin, F. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Influence of native ureolytic microbial community on biocementation potential of Sporosarcina pasteurii. Sci. Rep. 2021, 11, 20856. [Google Scholar] [CrossRef] [PubMed]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e0271761. [Google Scholar] [CrossRef]
- McDowell, G.; Amon, A. The Application of Weibull Statistics to the Fracture of Soil Particles. Soils Found. 2000, 40, 133–141. [Google Scholar] [CrossRef]
- Lim, W.L.; McDowell, G.R.; Collop, A.C. The application of Weibull statistics to the strength of railway ballast. Granul. Matter 2004, 6, 229–237. [Google Scholar] [CrossRef]
- Xiao, Y.; Meng, M.; Daouadji, A.; Chen, Q.; Wu, Z.; Jiang, X. Effects of particle size on crushing and deformation behaviors of rockfill materials. Geosci. Front. 2020, 11, 375–388. [Google Scholar] [CrossRef]
- McDowell, G.R. Statistics of soil particle strength. Géotechnique 2001, 51, 897–900. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Z.; Wang, C.; He, X.; Xiao, Y. Size effect on mudstone strength during the freezing–thawing cycle. Environ. Geotech. 2019, 10, 353–368. [Google Scholar] [CrossRef]
- Jayatilaka, A.D.S.; Trustrum, K. Statistical approach to brittle fracture. J. Mater. Sci. 1977, 12, 1426–1430. [Google Scholar] [CrossRef]
- Weibull, W. A Statistical Distribution Function of Wide Applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar] [CrossRef]
- Shan, Z.; Zhang, P.; Kou, H. Mechanical and Engineering Behavior of MICP-Treated Coarse Siliceous Sands. KSCE J. Civ. Eng. 2022, 26, 79–87. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Soriano, M.; Ramos-Cormenzana, A.; Delgado, R. Precipitation of carbonates by Nesterenkonia halobia in liquid media. Chemosphere 2000, 41, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, C.; Xu, Y. Fractal crushing of solid particles. KSCE J. Civ. Eng. 2017, 21, 987–993. [Google Scholar] [CrossRef]
- Meng, M.Q.; Wang, L.; Jiang, X.; Wang, C.G.; Liu, H.L.; Xiao, Y. Single-particle crushing test and numerical simulation of coarse grained soil based on size effect. Rock Soil Mech. 2021, 41, 5. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; WH Freeman: New York, NY, USA, 1982. [Google Scholar]
- Yu, F. Particle breakage in triaxial shear of a coral sand. Soils Found. 2018, 58, 866–880. [Google Scholar] [CrossRef]
- Chen-xi, T.; Sheng, Z.; Xi, L.; Dai-chao, S. Evolution and ultimate state of breakage for uniformly graded granular materials. Rock Soil Mech. 2015, 36, 260–264. [Google Scholar]
- Turcotte, D.L. Fractals and fragmentation. J. Geophys. Res. 1986, 91, 1921–1926. [Google Scholar] [CrossRef]
- Xu, Y. Explanation of scaling phenomenon based on fractal fragmentation. Mech. Res. Commun. 2005, 32, 209–220. [Google Scholar] [CrossRef]





| Components | Dosage |
|---|---|
| Yeast extract | 20 g/L |
| NH4Cl | 20 g/L |
| MnSO4·H2O | 12 mg/L |
| NiCl2·6H2O | 24 mg/L |
| Agar | 15 g/L (Solid medium) |
| NaOH or HCl (pH control) | pH: 8.5~9.0 |
| Test Group | Coating Thickness (mm) | Characteristic Stress (MPa) | Weibull Modulus (m) |
|---|---|---|---|
| α1 | 0 | 21.607 | 0.13912 |
| α2 | 0.219 | 26.568 | 0.12607 |
| α3 | 0.369 | 34.402 | 0.09775 |
| α4 | 0.361 | 29.357 | 0.12304 |
| Test Group | Coating Thickness (mm) | Test Particles Crushing Force (N) | Test Particles Crushing Energy (mJ) |
|---|---|---|---|
| α1 | 0 | 236.3 | 473.2 |
| α2 | 0.219 | 314.4 | 628.8 |
| α3 | 0.369 | 330.6 | 826.5 |
| α4 | 0.361 | 354.1 | 1416.4 |
| Test Number | Coating Thickness (mm) | Weibull Modulus (m) | Fractal Dimension |
|---|---|---|---|
| α1 | 0 | 0.13912 | 0.366 |
| α2 | 0.219 | 0.12607 | 0.336 |
| α3 | 0.369 | 0.09775 | 0.267 |
| α4 | 0.361 | 0.12304 | 0.329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Gong, L.; Hu, Z.; Xu, Y.; He, Y.; Long, Y. Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP. Materials 2024, 17, 4690. https://doi.org/10.3390/ma17194690
Zhu S, Gong L, Hu Z, Xu Y, He Y, Long Y. Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP. Materials. 2024; 17(19):4690. https://doi.org/10.3390/ma17194690
Chicago/Turabian StyleZhu, Shuyue, Linxian Gong, Zhazha Hu, Yan Xu, Yuanyuan He, and Yunyi Long. 2024. "Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP" Materials 17, no. 19: 4690. https://doi.org/10.3390/ma17194690
APA StyleZhu, S., Gong, L., Hu, Z., Xu, Y., He, Y., & Long, Y. (2024). Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP. Materials, 17(19), 4690. https://doi.org/10.3390/ma17194690







