The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste
Abstract
1. Introduction
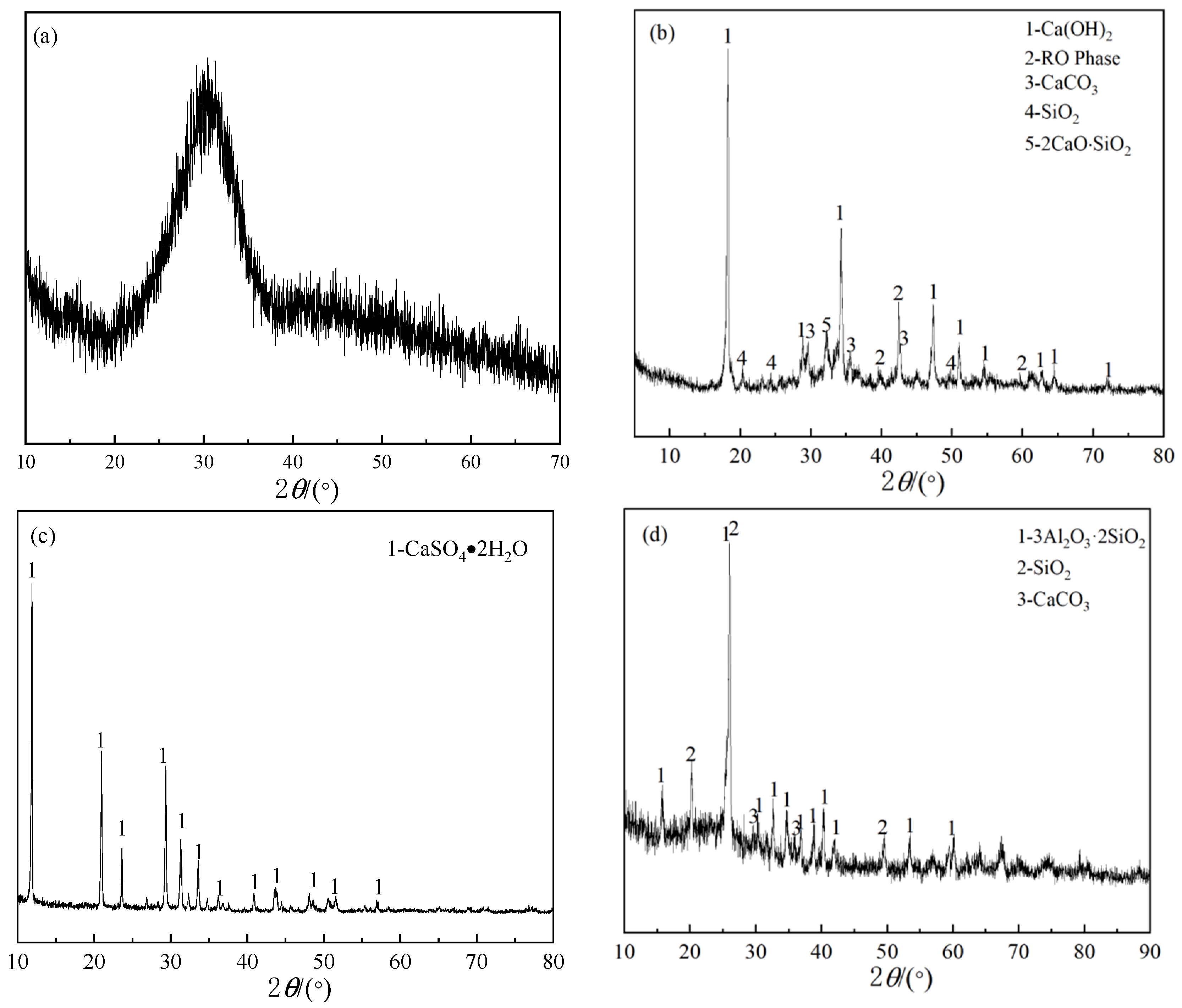
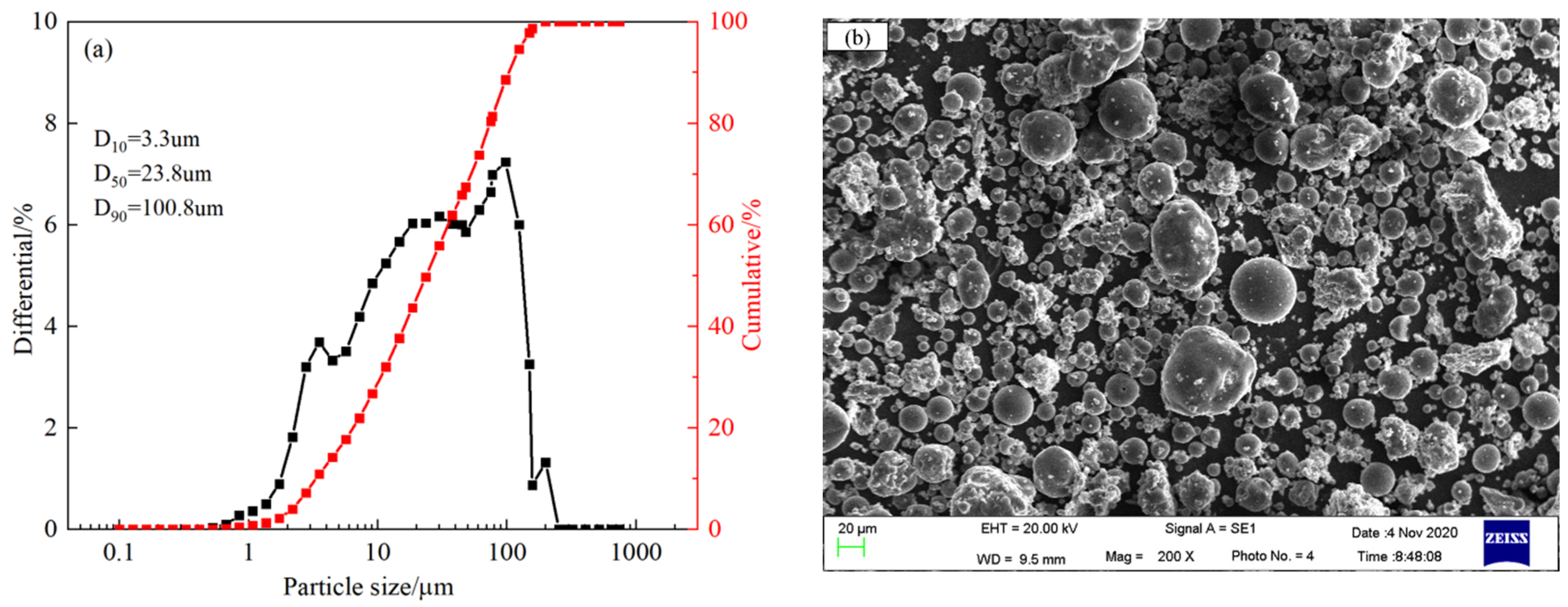
2. Materials
3. Methods
3.1. Methods in This Study
3.2. Characterization Methods
4. Results and Discussion
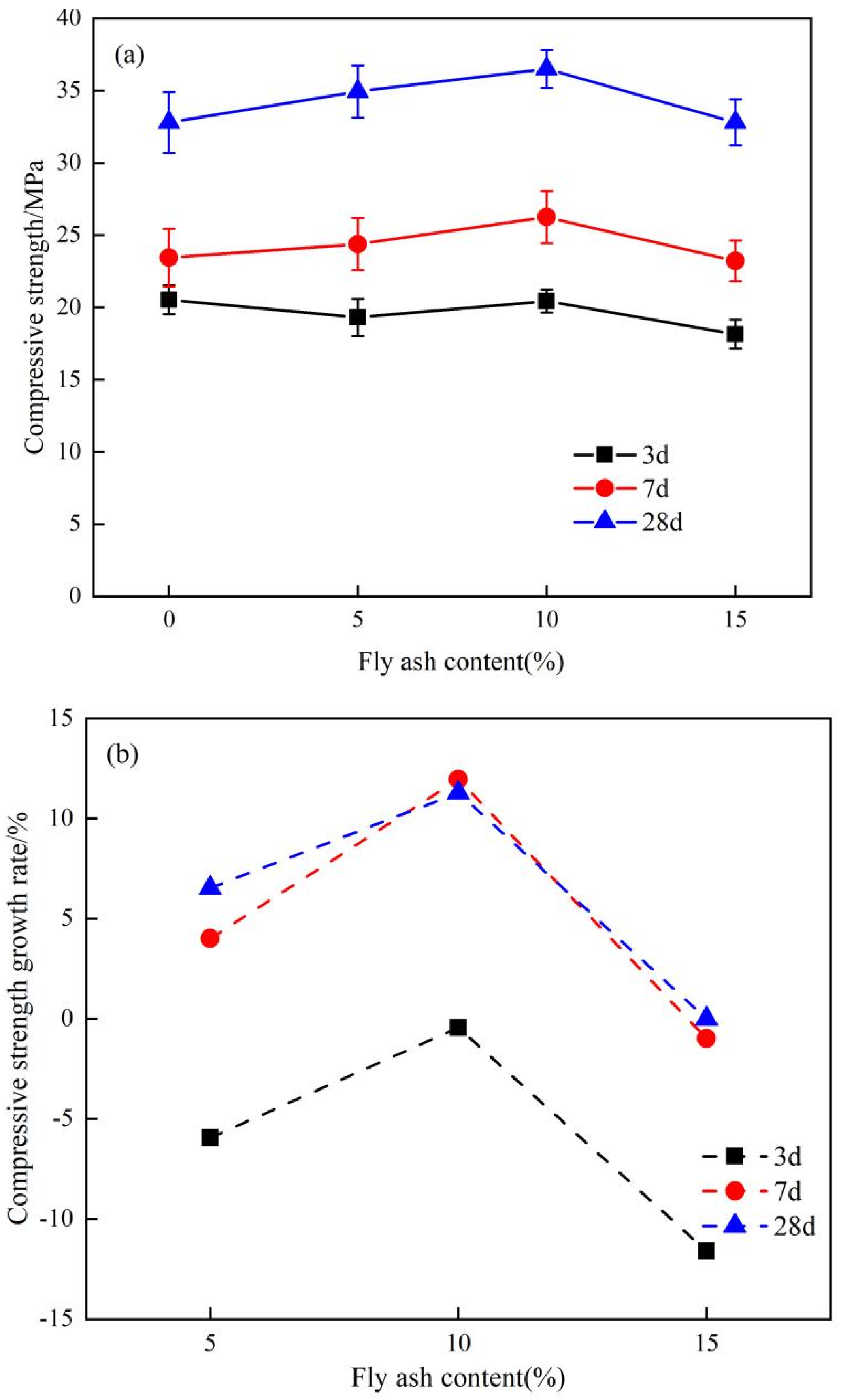
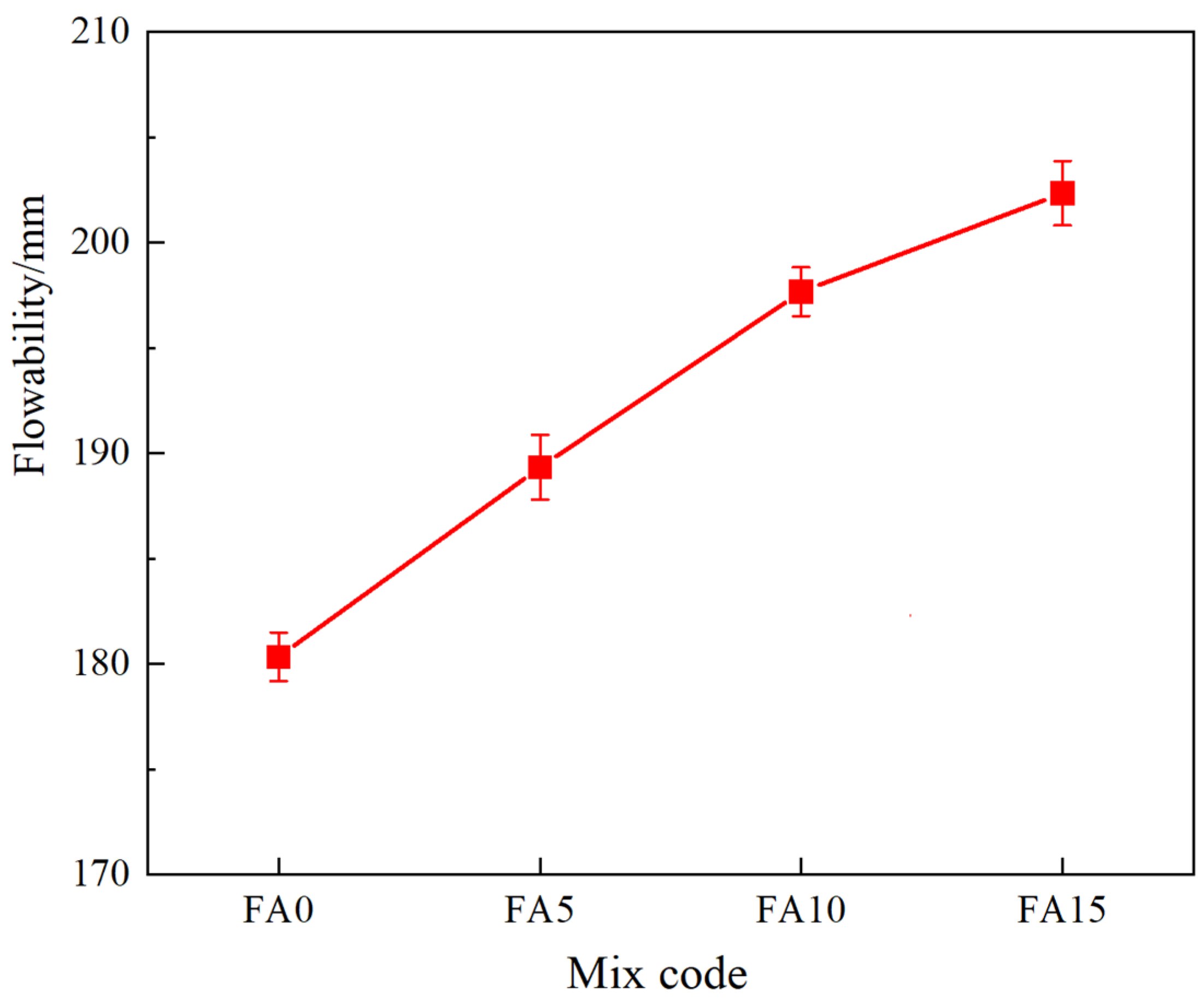
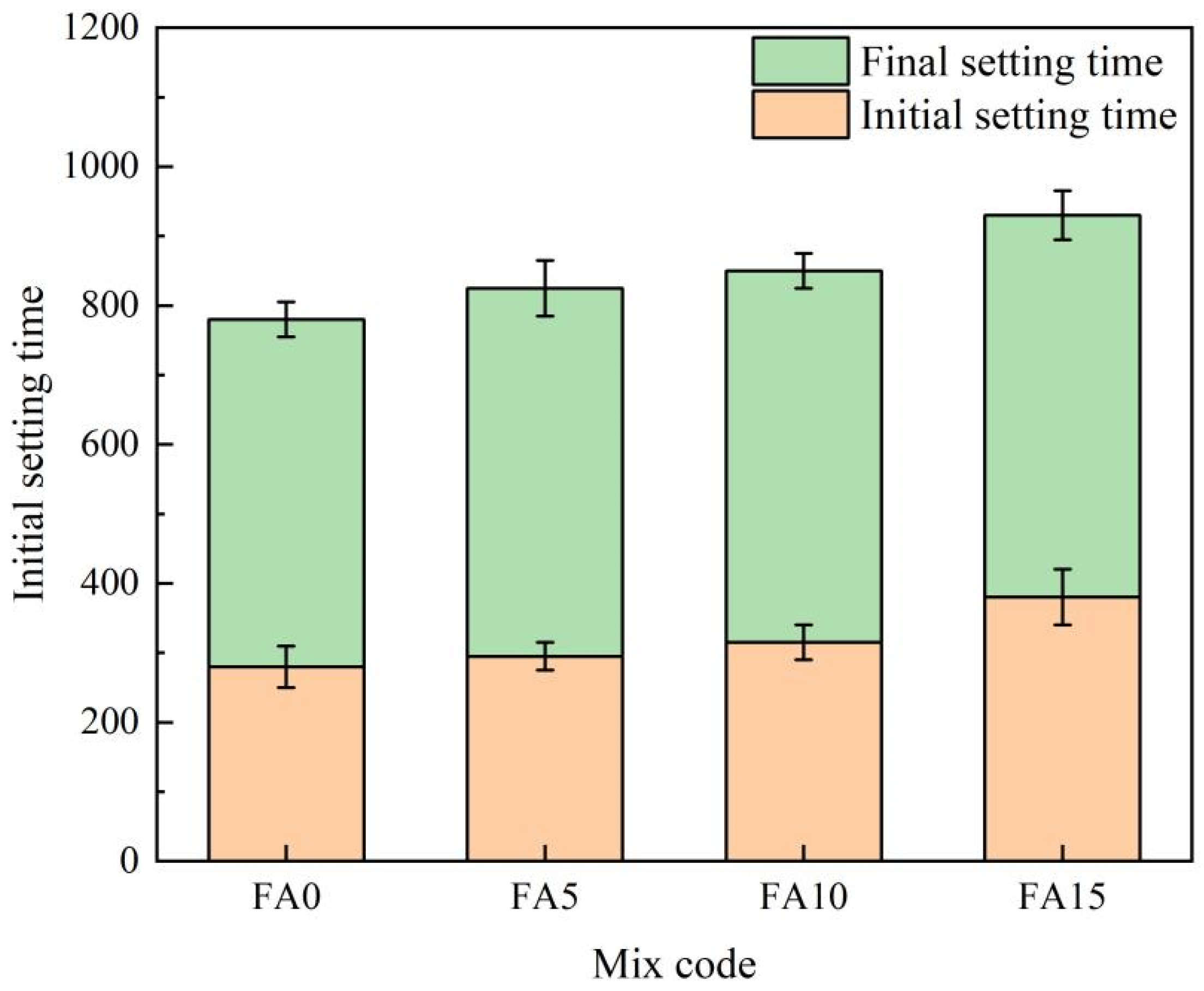
4.1. The Impact of Fly Ash Content on the Macroscopic Properties of Cementitious Materials
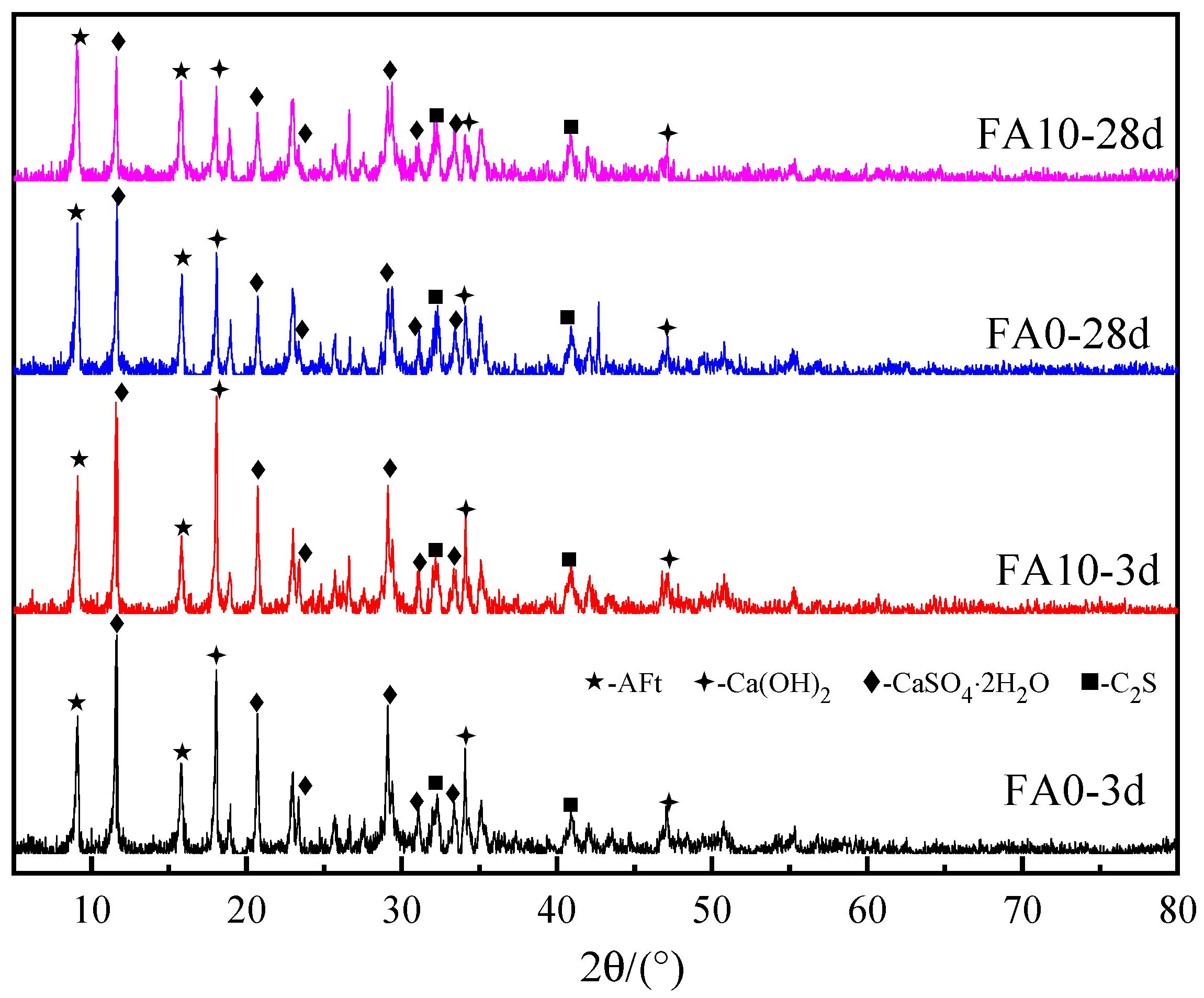
4.2. The Effect of Fly Ash Content on the Phase Composition of Cementitious Materials
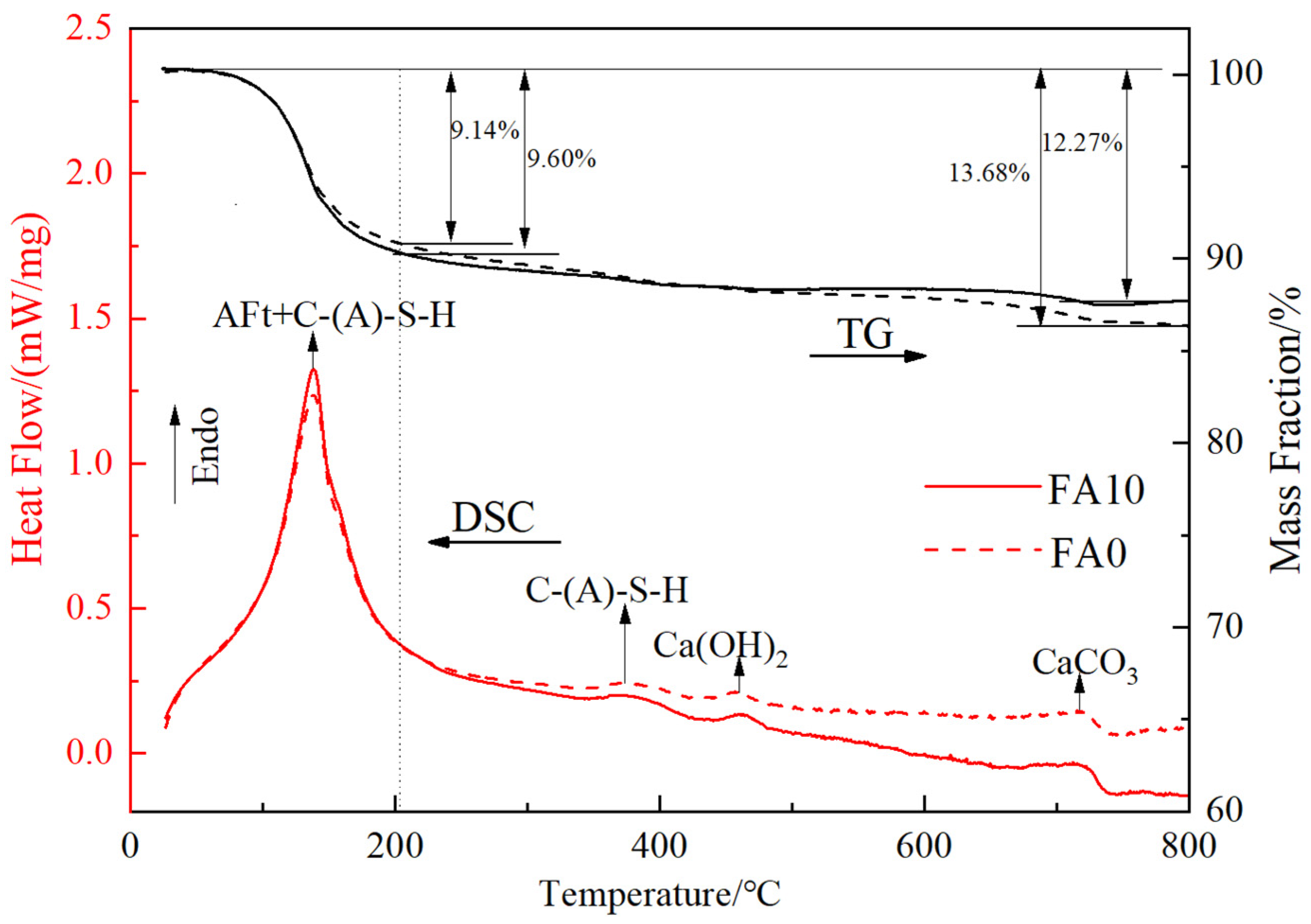
4.3. The Influence of Fly Ash Content on the Hydration Process of Cementitious Materials
4.4. The Influence of Fly Ash Content on the Leaching Ion Concentration of Cementitious Materials
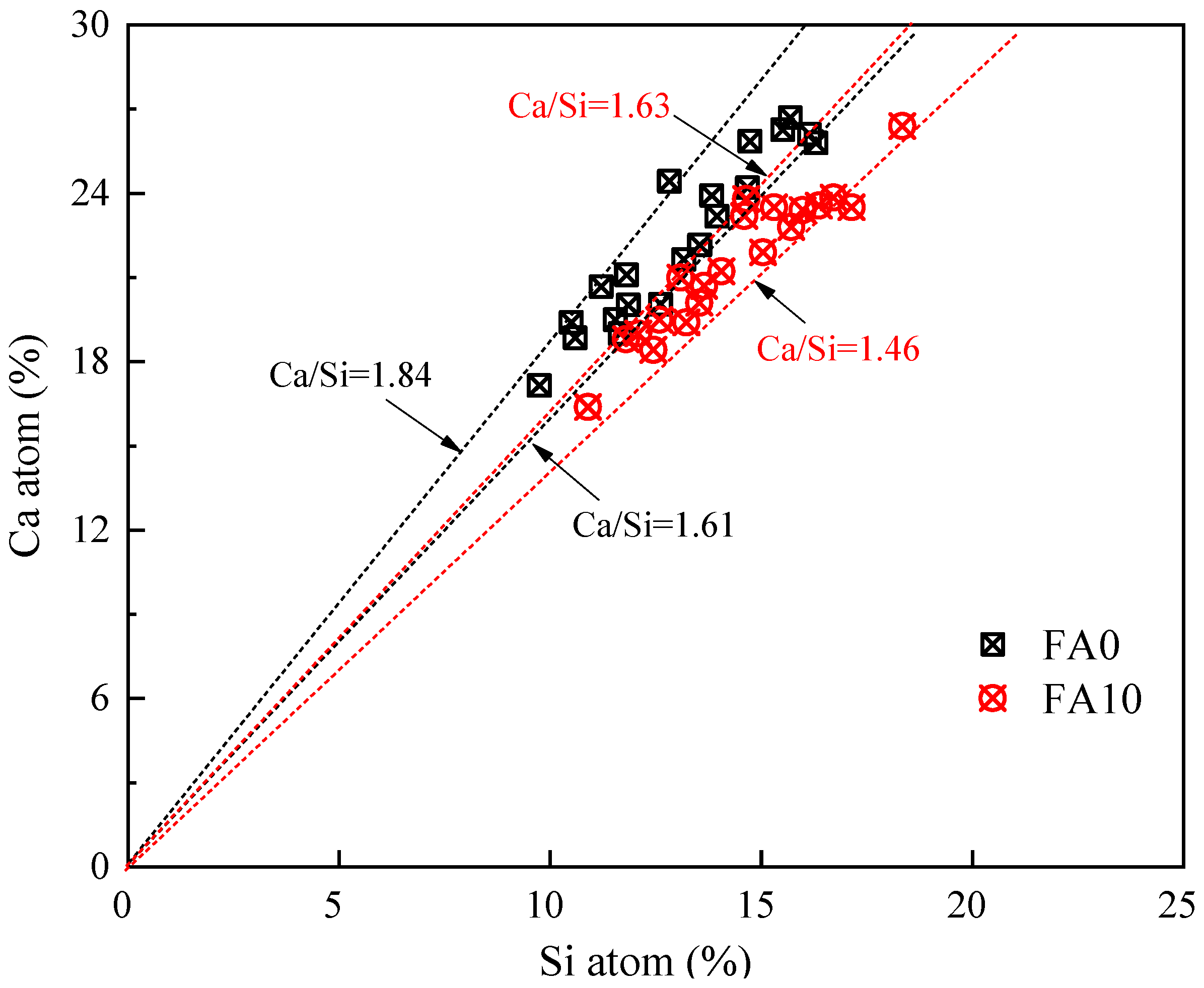
4.5. The Influence of Fly Ash Content on the Microstructure of Cementitious Materials
5. Discussion
- The impact on macroscopic performance
- 2.
- The impact on microscopic products and structures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Churkina, G.; Organschi, A.; Reyer, C.P.O.; Ruff, A.; Vinke, K.; Liu, Z.; Reck, B.K.; Graedel, T.E.; Schellnhuber, H.J. Buildings as a global carbon sink. Nat. Sustain. 2020, 3, 269–276. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Busch, P.; Kendall, A.; Murphy, C.W.; Miller, S.A. Literature review on policies to mitigate GHG emissions for cement and concrete. Resour. Conserv. Recycl. 2022, 182, 106278. [Google Scholar] [CrossRef]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Orsini, F.; Marrone, P. Approaches for a low-carbon production of building materials: Areview. J. Clean. Prod. 2019, 241, 118380. [Google Scholar] [CrossRef]
- Soni, A.; Das, P.K.; Hashmi, A.W.; Yusuf, M.; Kamyab, H.; Chelliapan, S. Challenges and opportunities of utilizing municipal solid waste as alternative building materials for sustainable development goals: A review. Sustain. Chem. Pharm. 2022, 27, 100706. [Google Scholar] [CrossRef]
- Tang, B.; Wu, H.; Wu, Y. Evaluation of arbon footprint of compression cast waste rubber concrete based on LCA approach. J. Build. Eng. 2024, 86, 108818. [Google Scholar] [CrossRef]
- Ghamari, A.; Powęzka, A.; Kytinou, V.K.; Amini, A. An Innovative fire-resistant lightweight concrete infill wall reinforced with waste glass. Buildings 2024, 14, 626. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z. Investigation the synergistic effects in quaternary binder containing red mud, blast furnace slag, steel slag and flue gas desulfurization gypsum based on artificial neural networks. J. Clean. Prod. 2020, 273, 122972. [Google Scholar] [CrossRef]
- Du, H.; Xu, D.; Li, X.; Li, J.; Ni, W.; Li, Y.; Fu, P. Application of molten iron desulfurization slag to replace steel slag as an alkaline component in solid waste-based cementitious materials. J. Clean. Prod. 2022, 377, 134353. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlangen, E.; Opurolu, O. Effect of slags of different origins and the role of sulfur in slag on the hydration characteristics of cement-slag systems. Constr. Build. Mater. 2022, 316, 125266. [Google Scholar] [CrossRef]
- Bssaa, B. Fly ash properties, characterization, and applications: A review. J. King Saud Univ.-Sci. 2021, 33, 101536. [Google Scholar]
- Liu, Y.; Sun, S.; Yang, Y. Effect of particle size distribution of fly ash on hydration property of silicate cement. Chin. Powder Technol. 2017, 23, 83–86. [Google Scholar]
- Wang, X. Effect of fly ash on concrete properties and engineering application. Bulk Cem. 2022, 1, 24–26. [Google Scholar]
- Yong, J.; Jia, L.J.; Wen, M.Y.; Niu, Y.H. Preparation of alkali-activated fly ash/steel slag cementitious materials. Bull. Chin. Ceram. Soc. 2019, 38, 2152–2156. [Google Scholar]
- Sun, X.; Zhao, Y.; Xing, J.; Qiu, J.; Li, H. Preparation of binding materials for backfilling with alkali activated blast furnace slag and fly ash. Met. Mine 2016, 11, 189–192. [Google Scholar]
- Chen, X.; Yang, C. Fly ash backfilling cementitious materials and application. Compr. Util. Fly Ash 2014, 3, 44–46. [Google Scholar]
- Wang, Y.; Wen, Z.; Yang, X.; Gao, Q. Study on development and ratio optimization of cementing material of fly ssh-slag based consolidated powder. Min. Res. Dev. 2019, 39, 88–94. [Google Scholar]
- Feng, C.; Li, D. Reaction degree of fly ssh in the cement paste. Bull. Chin. Ceram. Soc. 2015, 34, 3202–3208. [Google Scholar]
- Geng, F.; Yin, W.; Xi, Y.; Shao, C.; Xie, J.; Liu, C.; Zhou, L. Experimental study on pore structure of foam Concrete. Bull. Chin. Ceram. Soc. 2017, 36, 526–532. [Google Scholar]
- Longarini, N.; Crespi, P.G.; Zucca, M.; Giordano, N.; Silvestro, G. The advantages of fly ash use in concrete structures. Inżynieria Miner. 2014, 15, 141–145. [Google Scholar]
- Li, Q.; Zhang, C.; Chen, Q.; Sun, K. Relationship between reaction degree and compressive strength of cement and fly ash regenerated micro-powder system at different temperatures. Concr. Cem. Prod. 2017, 3, 9–14. [Google Scholar]
- Yi, L.; Wang, H.; Wang, X.; Peng, J. Research progress of utilizing fly ssh as resource of building material. Bull. Chin. Ceram. Soc. 2012, 31, 88–91. [Google Scholar]
- Cho, B.; Choi, H. Physical and chemical properties of concrete using GGBFS-KR slag-gypsum binder. Constr. Build. Mater. 2016, 123, 436–443. [Google Scholar] [CrossRef]
- Yang, Z.; Saiyin, B.; Shi, Z.; Yin, Y.; Guo, J.; Wang, J.; Fu, J. Study of the properties of C30 concrete prepared by multi-solid waste coupled cement. Environ. Eng. 2023, 41, 143–147. [Google Scholar]
- ISO 679:2009; Cement—Test Methods—Determination of Strength. ISO: Geneva, Switzerland, 2009.
- GB/T 203-2008; Granulated Blast Furnace Slag Used for Cement Production. National Standardization Technical Committee Cement: Beijing, China, 2009.
- Du, H.; Li, J.; Ni, W.; Xu, D.; Li, N.; Mu, X.; Hou, Y.; Li, Y.; Fu, P. Optimization of the Whole-Waste Binder Containing Molten Iron Desulfurization Slag from Kambara Reactor for Concrete Production. J. Build. Eng. 2022, 54, 104594. [Google Scholar] [CrossRef]
- Shi, C.; Qian, J. High performance cementing materials from industrial slags—A review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). The National Standards of the People’s Republic of China: Beijing, China, 2021.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. National Standardization Technical Committee Cement: Beijing, China, 2005.
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Li, K. Ammonia-soda residue and metallurgical slags from iron and steel industries as cementitious materials for clinker-free concretes. J. Clean. Prod. 2021, 307, 127262. [Google Scholar] [CrossRef]
- HJ 557-2009; Solid Waste Extraction Procedure for Leaching Toxicity—Horizontal Vibration Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2010.
- Han, X.; Feng, J.; Sun, C.; Wang, S. Research on properties of ultrafine fly ash and cement cementitious materials under curing at 50 °C. J. Build. Mater. 2021, 24, 473–482. [Google Scholar]
- Song, S.; Liu, Z.; Fan, J. Effect of the Fineness and Proportion of Fly Ash on the Water Demand Behavior of Cementitious Material. Conccrete 2014, 10, 70–71, 76. [Google Scholar]
- Bosque, I.F.S.D.; Medina, J.M.; Frías, M.; de Rojas, M.S.; Medina, C. Use of biomass-fired power plant bottom ash as an addition in new blended cements: Effect on the structure of the C-S-H gel formed during hydration. Constr. Build. Mater. 2019, 228, 117081. [Google Scholar] [CrossRef]
- Qiao, C.; Suranei, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Cao, X.; Liu, Q.; Zang, H. Influence of the combination of calcium oxide and sodium carbonate on the hydration reactivity of alkali-activated slag binders. J. Clean. Prod. 2018, 171, 622–629. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Feng, J. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, H.; Liu, X. Hydration mechanism and leaching behavior of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Yang, H.; Chen, X. Research of fly ash on microstructure of cementitious materials paste. Concrete 2013, 1, 69–72. [Google Scholar]
- Scrivener, K.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Abdelrazig, B.; Bonner, D.; Nowell, D.; Dransfield, J.; Egan, P. The solution chemistry and early hydration of ordinary portland cement pastes with and without admixtures. Thermochim. Acta 1999, 340, 417–430. [Google Scholar] [CrossRef]


| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | P2O5 | MnO | TiO2 | SO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Slag | 39.37 | 29.83 | 14.22 | 0.36 | 8.97 | 0.02 | 0.17 | 3.91 | 2.45 |
| Steel slag | 53.46 | 9.85 | 3.43 | 17.97 | 6.49 | 0.88 | 1.17 | - | 3.96 |
| Gypsum | 42.64 | 1.87 | 0.52 | 0.51 | 0.79 | - | 0.02 | - | 35.57 |
| Fly ash | 5.74 | 38.6 | 28.0 | 4.06 | 0.73 | - | 0.07 | 1.14 | 0.59 |
| Materials | Solid Waste-Based Cementitious Materials | Water/g | ISO Sand/g | Admixture/g | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Slag | Steel Slag | Gypsum | Fly Ash | ||||
| FA0 | 52% | 33% | 15% | 0 | 135 | 1350 | 1.35 | |
| FA5 | 52% | 33% | 15% | 5% | 135 | 1350 | 1.35 | |
| FA10 | 52% | 33% | 15% | 10% | 135 | 1350 | 1.35 | |
| FA15 | 52% | 33% | 15% | 15% | 135 | 1350 | 1.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Du, H.; Zheng, Z.; Xu, D.; Wang, Y.; Li, N.; Ni, W.; Ren, C. The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste. Materials 2024, 17, 4696. https://doi.org/10.3390/ma17194696
Wang F, Du H, Zheng Z, Xu D, Wang Y, Li N, Ni W, Ren C. The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste. Materials. 2024; 17(19):4696. https://doi.org/10.3390/ma17194696
Chicago/Turabian StyleWang, Fei, Huihui Du, Zhong Zheng, Dong Xu, Ying Wang, Ning Li, Wen Ni, and Chao Ren. 2024. "The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste" Materials 17, no. 19: 4696. https://doi.org/10.3390/ma17194696
APA StyleWang, F., Du, H., Zheng, Z., Xu, D., Wang, Y., Li, N., Ni, W., & Ren, C. (2024). The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste. Materials, 17(19), 4696. https://doi.org/10.3390/ma17194696







