Boosting Zn2+ Storage Kinetics by K-Doping of Sodium Vanadate for Zinc-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
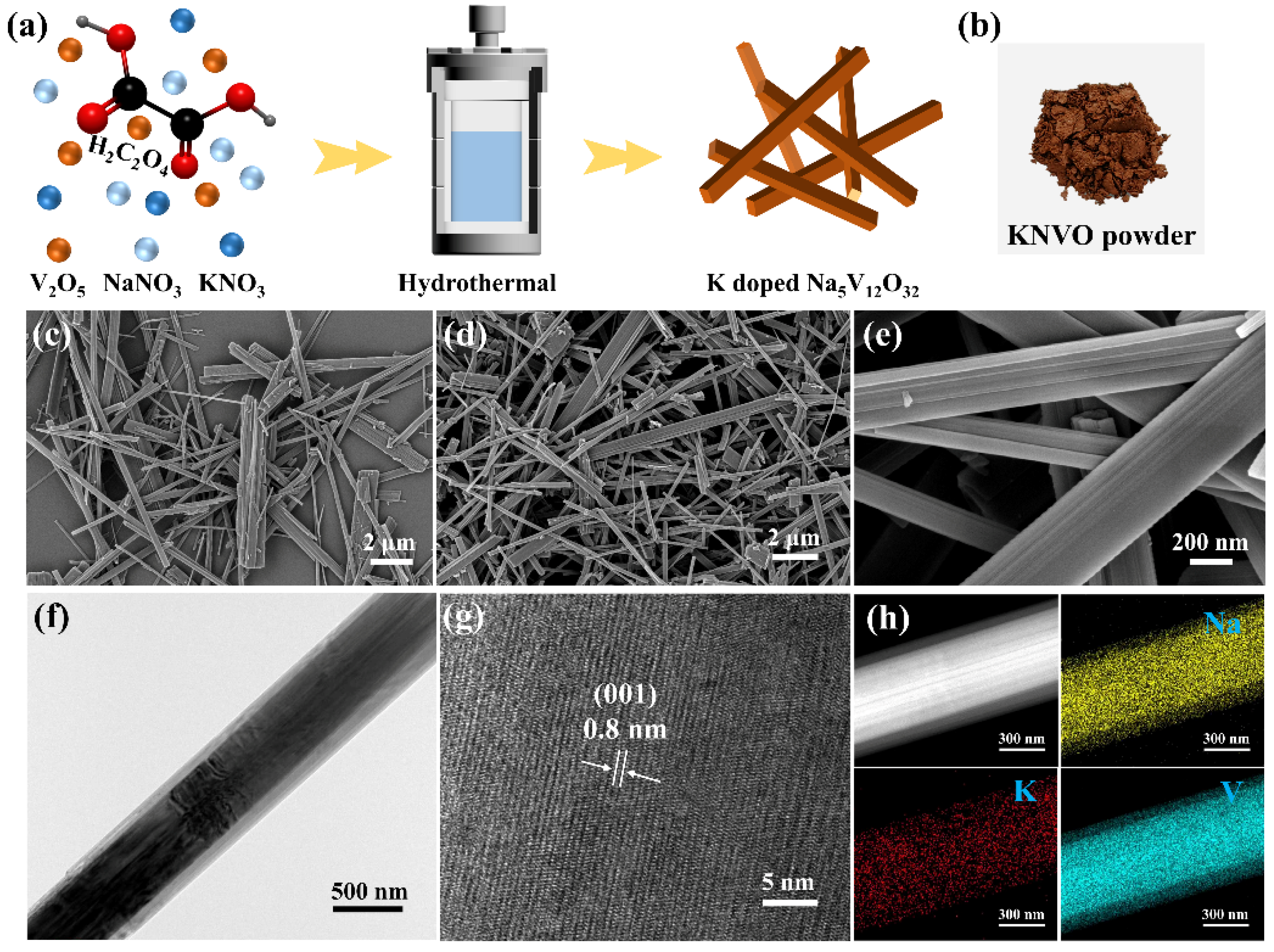
2.1. Material Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
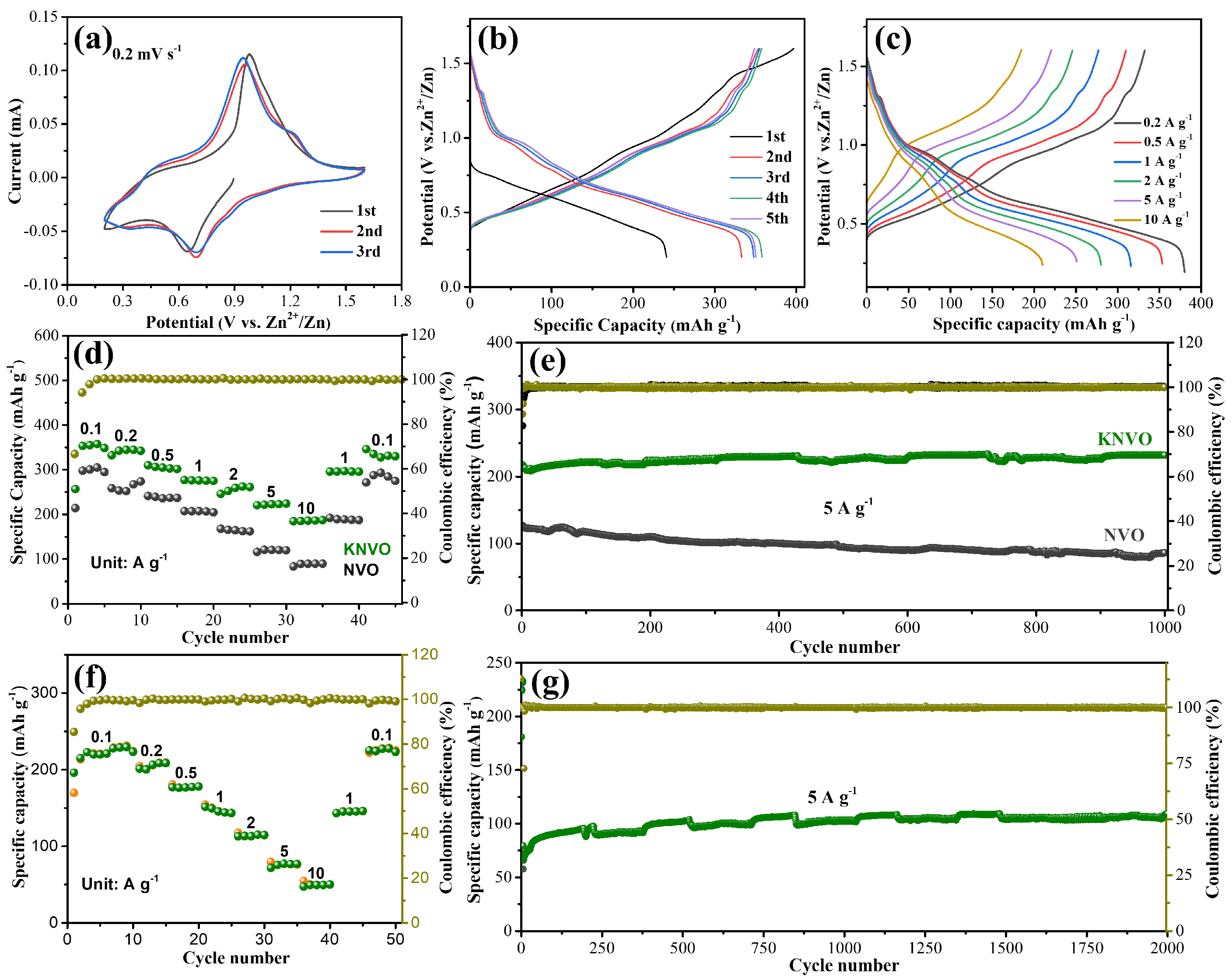
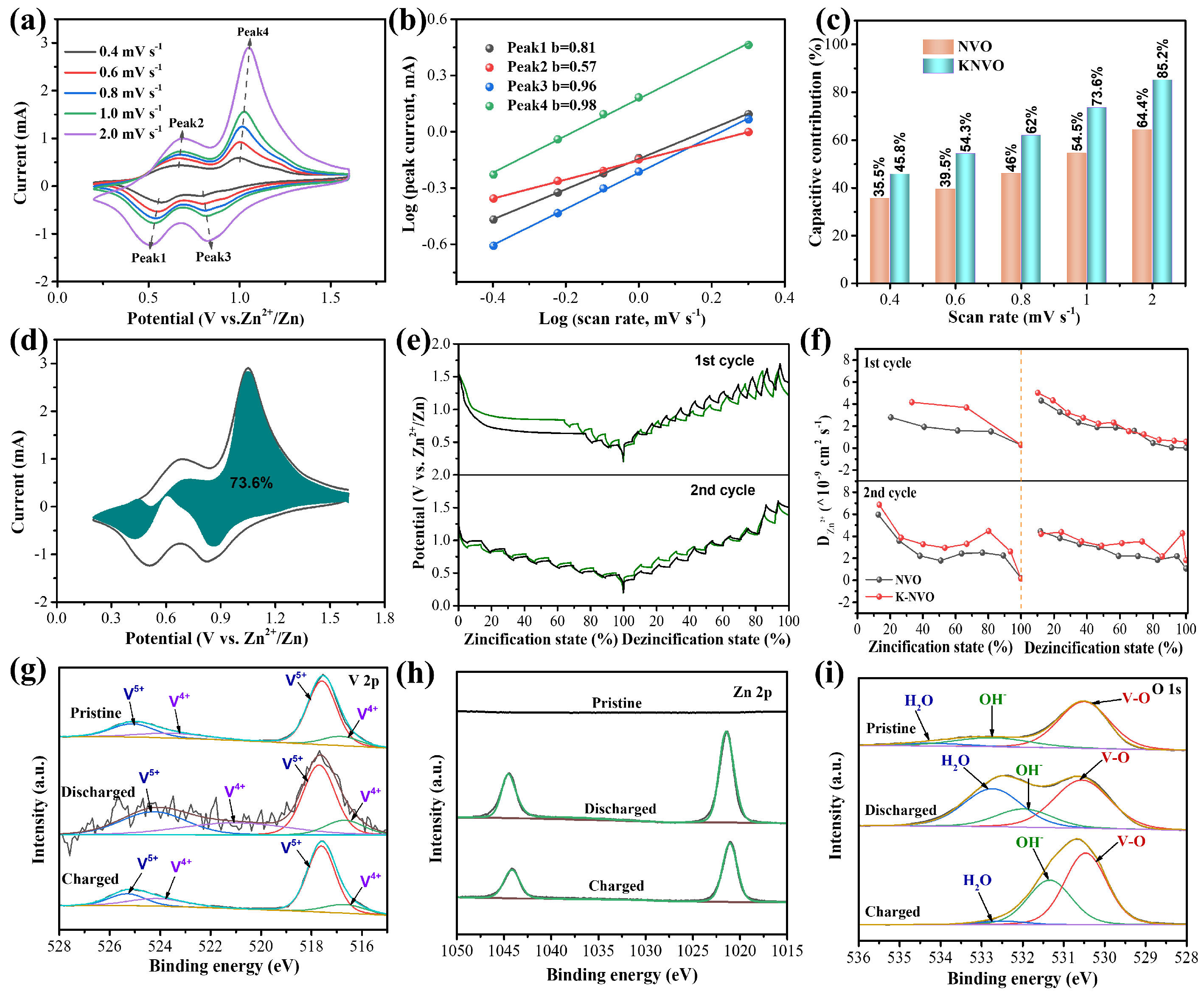
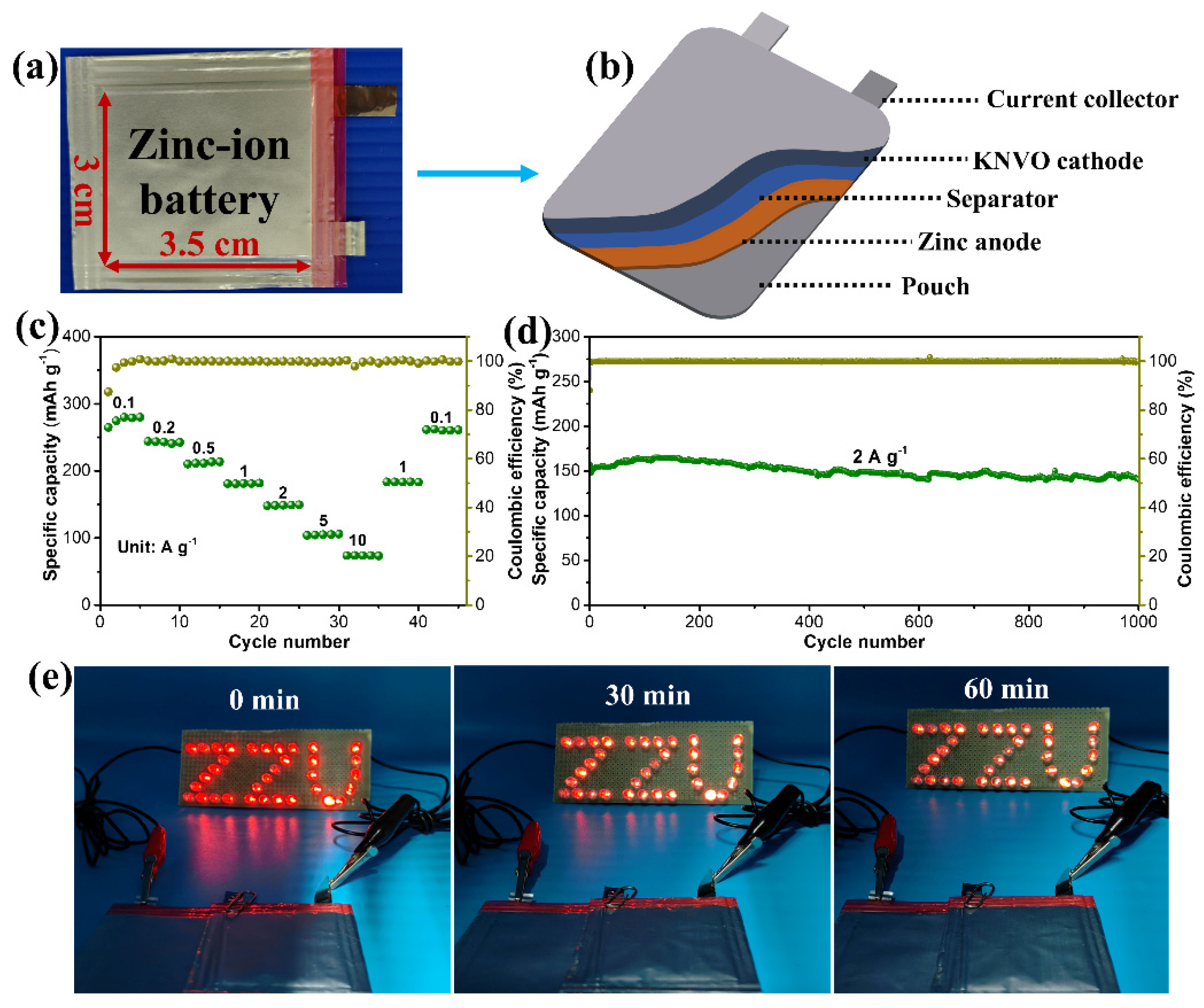
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Jin, S.; Archer, L.A.; Nazar, L.F. Toward practical aqueous zinc-ion batteries for electrochemical energy storage. Joule 2022, 6, 1727–1742. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, C.; Li, J.; Gao, X.; Hu, P.; Ye, C.; He, H.; Zhu, J.; Zhang, W.; Chen, R.; et al. Inhibition of vanadium cathodes dissolution in aqueous Zn-ion batteries. Adv. Mater. 2024, 36, 2310645. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Niu, Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- Bai, Y.; Qin, Y.; Hao, J.; Zhang, H.; Li, C.M. Advances and perspectives of ion-intercalated vanadium oxide cathodes for high-performance aqueous zinc ion battery. Adv. Funct. Mater. 2023, 34, 2310393. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Chen, J.; Gao, L.; Guo, P.; Wei, C.; Fu, J.; Xu, Q. Sulfur-bridged bonds enabled structure modulation and space confinement of MnS for superior sodium-ion capacitors. J. Colloid Interface Sci. 2024, 664, 360–370. [Google Scholar] [CrossRef]
- Li, S.; Tian, Q.; Chen, J.; Chen, Y.; Guo, P.; Wei, C.; Cui, P.; Jiang, J.; Li, X.; Xu, Q. An intrinsically non-flammable organic electrolyte for wide temperature range supercapacitors. Chem. Eng. J. 2023, 457, 141265. [Google Scholar] [CrossRef]
- Gao, L.; Ma, Y.; Zhang, C.; Cao, M. Nitrogen-doped carbon trapped MnMoO4 microrods toward high performance aqueous zinc-ion battery. J. Alloys Compd. 2023, 968, 172008. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, S.; Jiao, R.; Hao, G.; Liu, Y.; He, W.; Chen, J.; Jia, G.; Cui, J.; Li, S. Lignite-based hierarchical porous C/SiOx composites as high-performance anode for potassium-ion batteries. Energy Environ. Mater. 2023, 7, e12674. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Jiang, Z.; Chen, J.; Wei, C.; Wu, W.; Li, S.; Xu, Q. Cation-anion redox active organic complex for high performance aqueous zinc ion battery. Energy Environ. Mater. 2023, 7, e12507. [Google Scholar] [CrossRef]
- Zhao, F.; Li, C.; Li, S.; Wang, B.; Huang, B.; Hu, K.; Liu, L.; Yu, W.W.; Li, H. Continuous solar energy conversion windows integrating zinc anode-based electrochromic device and IoT system. Adv. Mater. 2024, 36, 2405035. [Google Scholar] [CrossRef]
- Ji, F.; Yu, J.; Hou, S.; Hu, J.; Li, S. Doping engineering in manganese oxides for aqueous zinc-ion batteries. Materials 2024, 17, 33327. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, C.; Gao, L.; Lin, M.-F.; Eh, A.L.-S.; Chen, J.; Li, S. Recent advances in electrospun nanostructured electrodes in zinc-ion batteries. Batteries 2024, 10, 22. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Zhao, X. K-ion intercalated V6O13 with advanced high-rate long-cycle performance as cathode for Zn-ion batteries. J. Mater. Chem. C 2022, 10, 590–597. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Huang, Q.; Guan, C.; Zong, W.; Dai, Y.; Du, Z.; Zhang, Z.; Li, J.; Guo, F.; et al. Trace amounts of triple-functional additives enable reversible aqueous zinc-ion batteries from a comprehensive perspective. Nano-Micro Lett. 2023, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guo, P.; Chen, J.; Li, S.; Li, H. Recent advances in multifunctional electrochromic devices. Responsive Mater. 2024, 2, e20240013. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Zhao, B.; Zhang, S.; Zheng, X.; Wang, Y.; Jin, X.; Dai, C.; Wang, J.; Xie, J.; et al. Ultrafast synthesis of metal-layered hydroxides in a dozen seconds for high-performance aqueous Zn (micro-) battery. Nano-Micro Lett. 2023, 15, 32. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Naveed, A.; Li, G.; Zhang, H.; Zhou, Y.; Dou, A.; Su, M.; Liu, Y.; Guo, R.; et al. Magnesium ion doping and micro-structural engineering assist NH4V4O10 as a high-performance aqueous zinc ion battery cathode. Adv. Funct. Mater. 2023, 33, 2306205. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Zhou, J.; Wang, L.; Lei, Y.; Wang, C.; Lin, T.; Tang, Y.; Liang, S. Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 2018, 51, 579–587. [Google Scholar] [CrossRef]
- Xia, H.; Xu, G.; Cao, X.; Miao, C.; Zhang, H.; Chen, P.; Zhou, Y.; Zhang, W.; Sun, Z. Single-ion-conducting hydrogel electrolytes based on slide-ring pseudo-polyrotaxane for ultralong-cycling flexible zinc-ion batteries. Adv. Mater. 2023, 35, 2301996. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-energy batteries: Beyond lithium-ion and their long road to commercialisation. Nano-Micro Letters 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Y.; Ruan, P.; Niu, X.; Zheng, D.; Xu, X.; Gao, X.; Cai, Y.; Liu, W.; Shi, W.; et al. Fe-doping enabled a stable vanadium oxide cathode with rapid Zn diffusion channel for aqueous zinc-ion batteries. Mater. Today Energy 2021, 21, 100842. [Google Scholar] [CrossRef]
- Lv, T.; Peng, Y.; Zhang, G.; Jiang, S.; Yang, Z.; Yang, S.; Pang, H. How about vanadium-based compounds as cathode materials for aqueous zinc ion batteries? Adv. Sci. 2023, 10, 2206907. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, C.; Wang, Z.; Huang, D.; Cao, G. Weakly polarized organic cation-modified hydrated vanadium oxides for high-energy efficiency aqueous zinc-ion batteries. Nano-Micro Lett. 2024, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Lulu, W.; Kuo-Wei, H.; Jitao, C.; Junrong, Z. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 2019, 5, eaax4279. [Google Scholar]
- Rao, L.; Zhou, Z.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. In-situ electrochemical conversion of Na5V12O32@graphene for enhanced cycle stability in aqueous zinc ion batteries. J. Colloid Interface Sci. 2023, 629, 473–481. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Wang, X.; Wei, X.; Yan, M.; Li, J.; Luo, W.; Yang, W.; Zhang, W.; Zhou, L.; et al. Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery. Nano Lett. 2018, 18, 1758–1763. [Google Scholar] [CrossRef]
- Zhou, T.; Xie, L.; Han, Q.; Yang, X.; Zhu, L.; Cao, X. Investigation of Na6V10O28 as a promising rechargeable aqueous zinc-ion batteries cathode. Chem. Eng. J. 2022, 445, 136789. [Google Scholar] [CrossRef]
- Kong, X.; Luo, S.; Rong, L.; Wan, Z.; Li, S. Rational Design of Hierarchical Mn-doped Na5V12O32 nanorods with low crystallinity as advanced cathodes for aqueous zinc ion batteries. Energy Fuels 2021, 35, 13483–13490. [Google Scholar] [CrossRef]
- Wei, M.; Luo, W.; Yu, D.; Liang, X.; Wei, W.; Gao, M.; Sun, S.; Zhu, Q.; Liu, G. Layered Ni0.22V2O5·nH2O as high-performance cathode material for aqueous zinc-ion batteries. Ionics 2021, 27, 4801–4809. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Geng, H.; Yang, Y.; Rui, X.; Li, C.C. Zinc ions pillared vanadate cathodes by chemical pre-intercalation towards long cycling life and low-temperature zinc ion batteries. J. Power Sources 2019, 441, 227192. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, H.; Ren, L.; Ji, M.; Shen, X.; Li, S. Mn0.26V2O5·nH2O nanoribbons with fast ion diffusion channels and high electrical conductivity for intercalation pseudocapacitive Zn2+ storage. Energy Fuels 2021, 35, 17948–17955. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, G.; Yu, X.; Bai, X.; Chen, A.; Zhao, C.; Lyu, P.; Zhang, N. Reconstructing fast ion transport channels of Zn3V2O7 (OH)2·2H2O to realize enhanced Zn2+ storage performance. Nano Energy 2023, 110, 108336. [Google Scholar] [CrossRef]
- Lv, T.; Zhu, G.; Dong, S.; Kong, Q.; Peng, Y.; Jiang, S.; Zhang, G.; Yang, Z.; Yang, S.; Dong, X.; et al. Co-intercalation of dual charge carriers in metal-ion-confining layered vanadium oxide nanobelts for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2022, 62, 2216089. [Google Scholar]
- Huang, J.; Zhou, J.; Liang, S. Guest Pre-intercalation strategy to boost the electrochemical performance of aqueous zinc-ion battery cathodes. Acta Phys. Chim. Sin. 2021, 37, 2005020. [Google Scholar] [CrossRef]
- Deng, S.; Li, H.; Chen, B.; Xu, Z.; Jiang, Y.; Li, C.; Xiao, W.; Yan, X. High performance of Mn-doped VO2 cathode for aqueous zinc-ion batteries: An insight into Zn2+ storage mechanism. Chem. Eng. J. 2023, 452, 139115. [Google Scholar] [CrossRef]
- Geng, H.; Cheng, M.; Wang, B.; Yang, Y.; Zhang, Y.; Li, C.C. Electronic structure regulation of layered vanadium oxide via interlayer doping strategy toward superior high-rate and low-temperature zinc-ion batteries. Adv. Funct. Mater. 2019, 30, 1907684. [Google Scholar] [CrossRef]
- Qian, l.; Wei, T.; Ma, K.; Yang, G.; Wang, C. Boosting the cyclic stability of aqueous zinc-ion battery based on Al-doped V10O24·12H2O cathode materials. ACS Appl. Mater. Interfaces 2019, 11, 20888–20894. [Google Scholar] [CrossRef]
- He, D.; Sun, T.; Wang, Q.; Ma, T.; Zheng, S.; Tao, Z.; Liang, J. Multi-functional potassium ion assists ammonium vanadium oxide cathode for high-performance aqueous zinc-ion batteries. Batteries 2022, 8, 84. [Google Scholar] [CrossRef]
- Pronin, I.A.; Averin, I.A.; Karmanov, A.A.; Yakushova, N.D.; Komolov, A.S.; Lazneva, E.F.; Sychev, M.M.; Moshnikov, V.A.; Korotcenkov, G. Control over the surface properties of zinc oxide powders via combining mechanical, electron beam, and thermal processing. Nanomaterials 2022, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Komolov, A.; Lazneva, E.; Gerasimova, N.; Panina, Y.A.; Sobolev, V.; Koroleva, A.; Pshenichnyuk, S.; Asfandiarov, N.; Modelli, A.; Handke, B. Conduction band electronic states of ultrathin layers of thiophene/phenylene co-oligomers on an oxidized silicon surface. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 40–45. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A deep-cycle aqueous zinc-ion battery containing an oxygen-deficient vanadium oxide cathode. Angew. Chem. 2019, 132, 2293–2298. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.-R. High performance aqueous zinc battery enabled by potassium ion stabilization. J. Colloid Interface Sci. 2022, 628, 33–40. [Google Scholar] [CrossRef]
- Song, X.; Li, J.; Li, Z.; Li, X.; Ding, Y.; Xiao, Q.; Lei, G. Effect of K-doping on the sodium-storage performance of sodium vanadate nanoplates. Acta Chim. Sin. 2019, 77, 625–633. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, D.; He, J.; Li, J.; Chen, H.; Chen, Y.; Chao, D. A scalable top-down strategy toward practical metrics of Ni–Zn aqueous batteries with total energy densities of 165 W h kg−1 and 506 W h L−1. Energy Environ. Sci. 2020, 13, 4157–4167. [Google Scholar] [CrossRef]
- Argyrou, M.; Christodoulides, P.; Kalogirou, S.A. Energy storage for electricity generation and related processes: Technologies appraisal and grid scale applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Jin, C.; Yu, J.; Li, S. Boosting Zn2+ Storage Kinetics by K-Doping of Sodium Vanadate for Zinc-Ion Batteries. Materials 2024, 17, 4703. https://doi.org/10.3390/ma17194703
Jia M, Jin C, Yu J, Li S. Boosting Zn2+ Storage Kinetics by K-Doping of Sodium Vanadate for Zinc-Ion Batteries. Materials. 2024; 17(19):4703. https://doi.org/10.3390/ma17194703
Chicago/Turabian StyleJia, Mengting, Chen Jin, Jiamin Yu, and Shaohui Li. 2024. "Boosting Zn2+ Storage Kinetics by K-Doping of Sodium Vanadate for Zinc-Ion Batteries" Materials 17, no. 19: 4703. https://doi.org/10.3390/ma17194703






