An Improved Mampel Model of the Non-Isothermal Crystallization Kinetics of Fiber-Reinforced Thermoplastic Composites
Abstract
1. Introduction
2. The Improved Non-Isothermal Crystallization Kinetic Model for Thermoplastic Composites
2.1. Introduction to Non-Isothermal Crystallization Kinetics Models
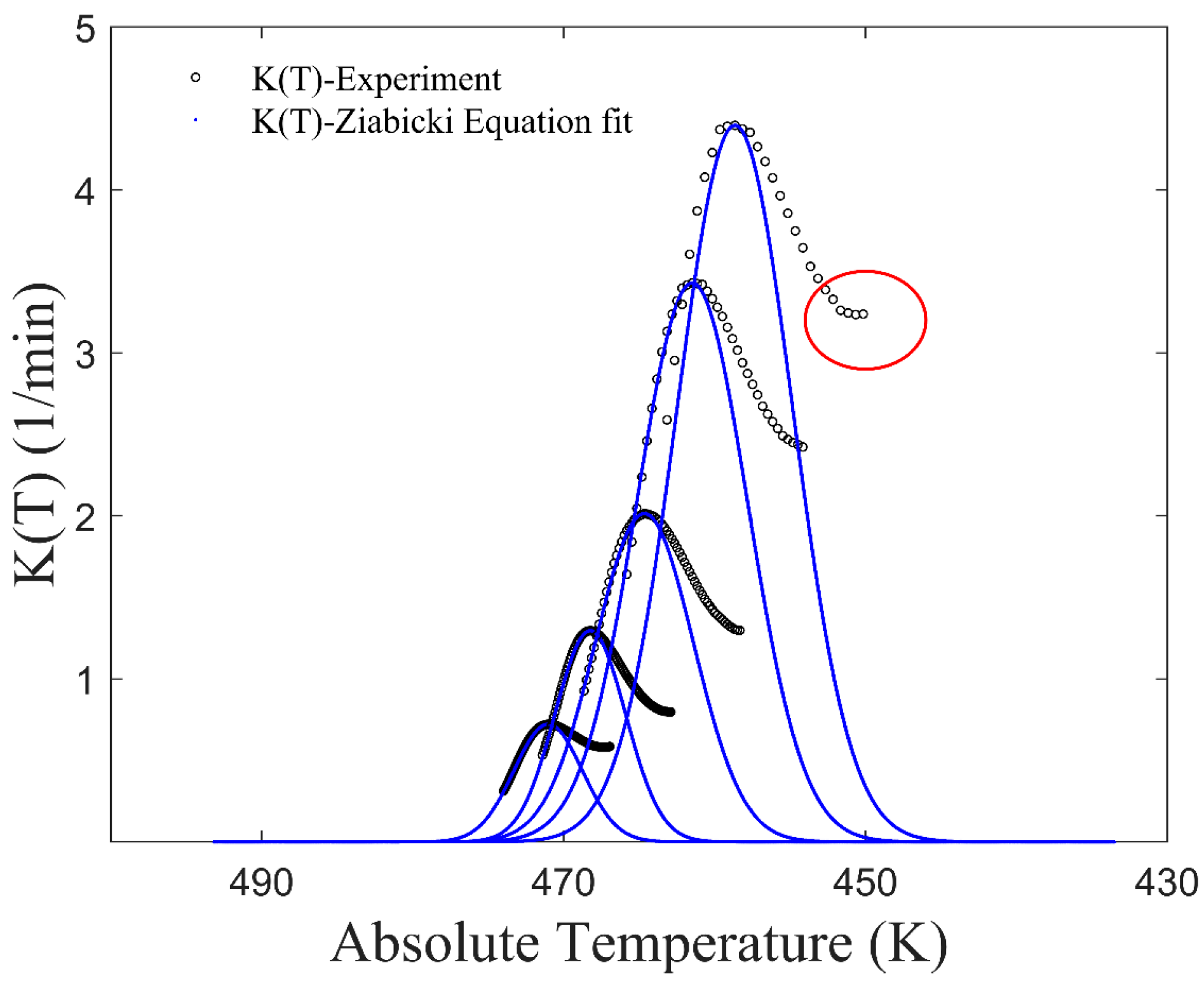
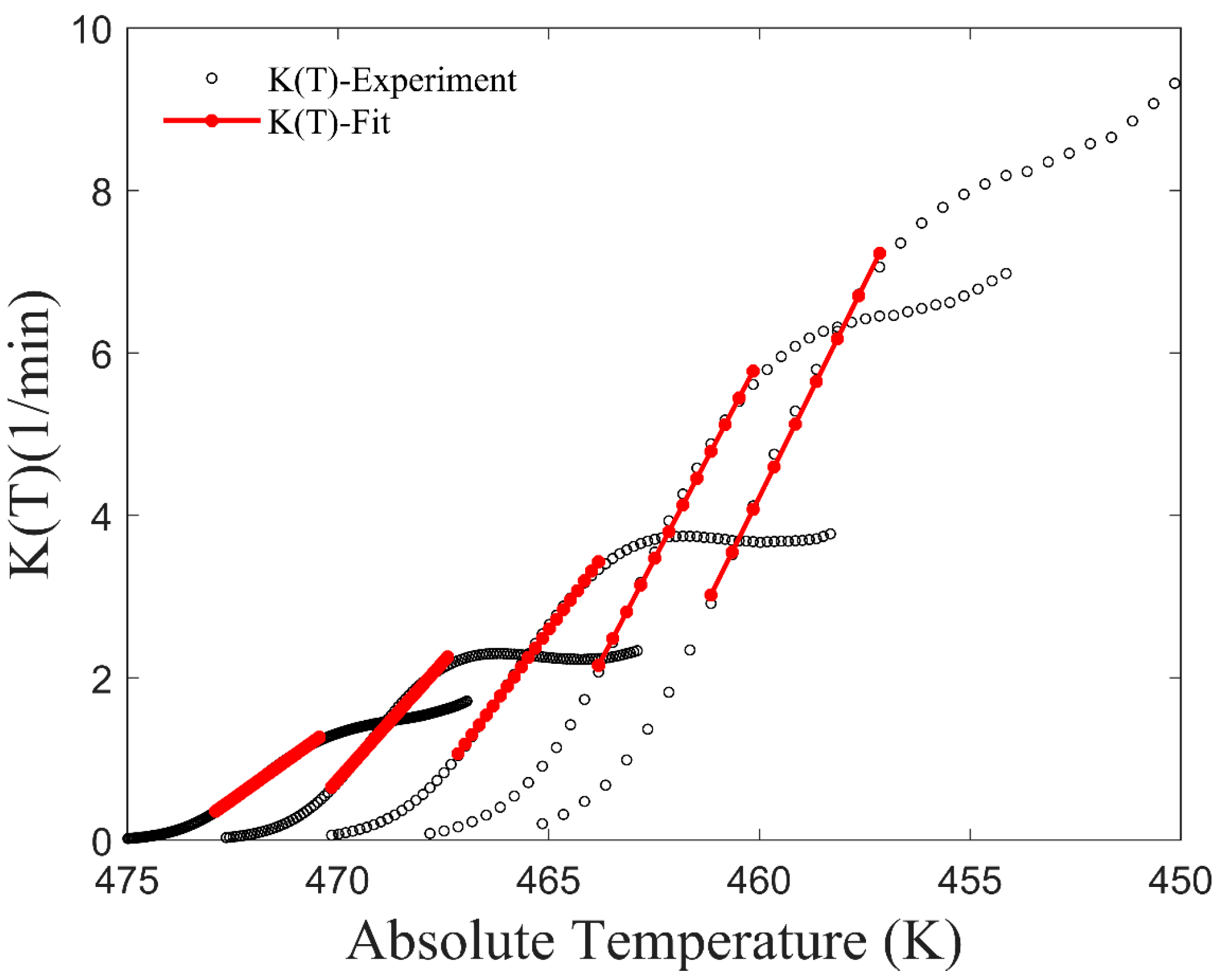
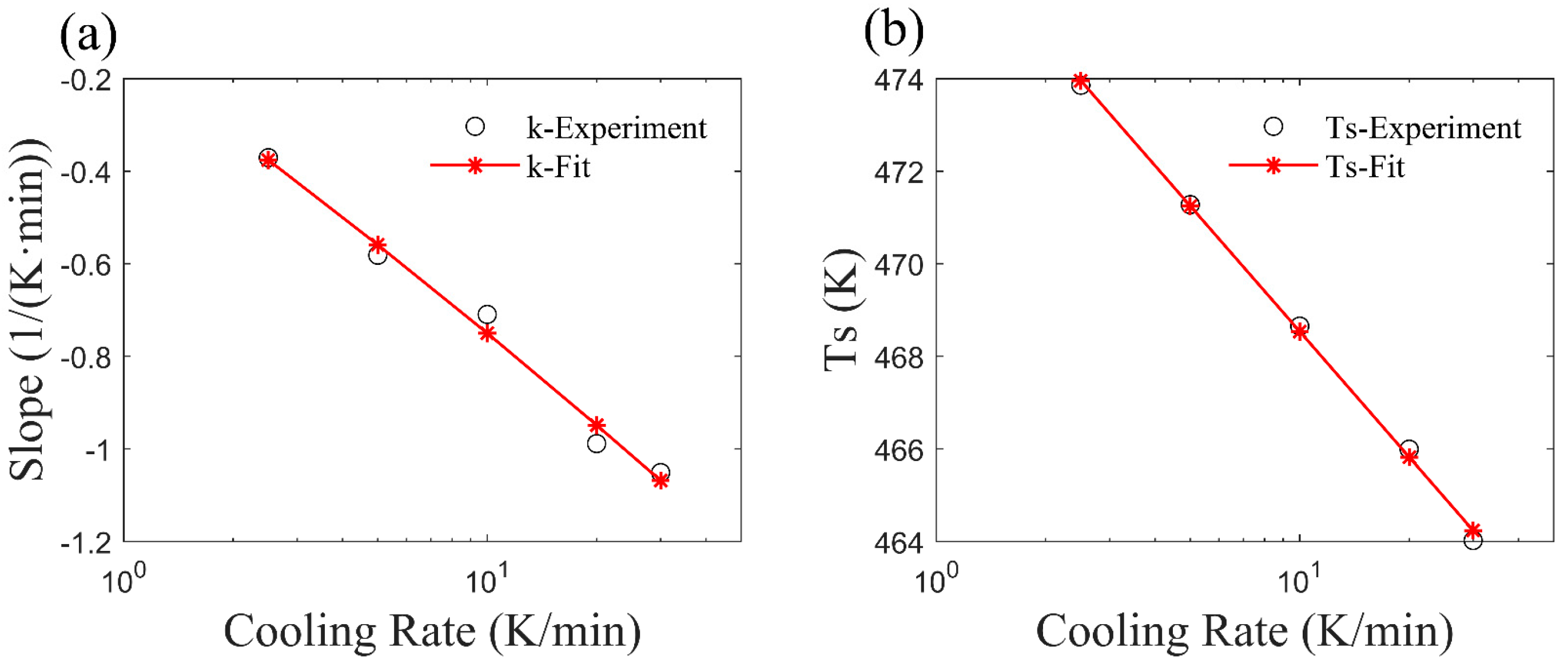
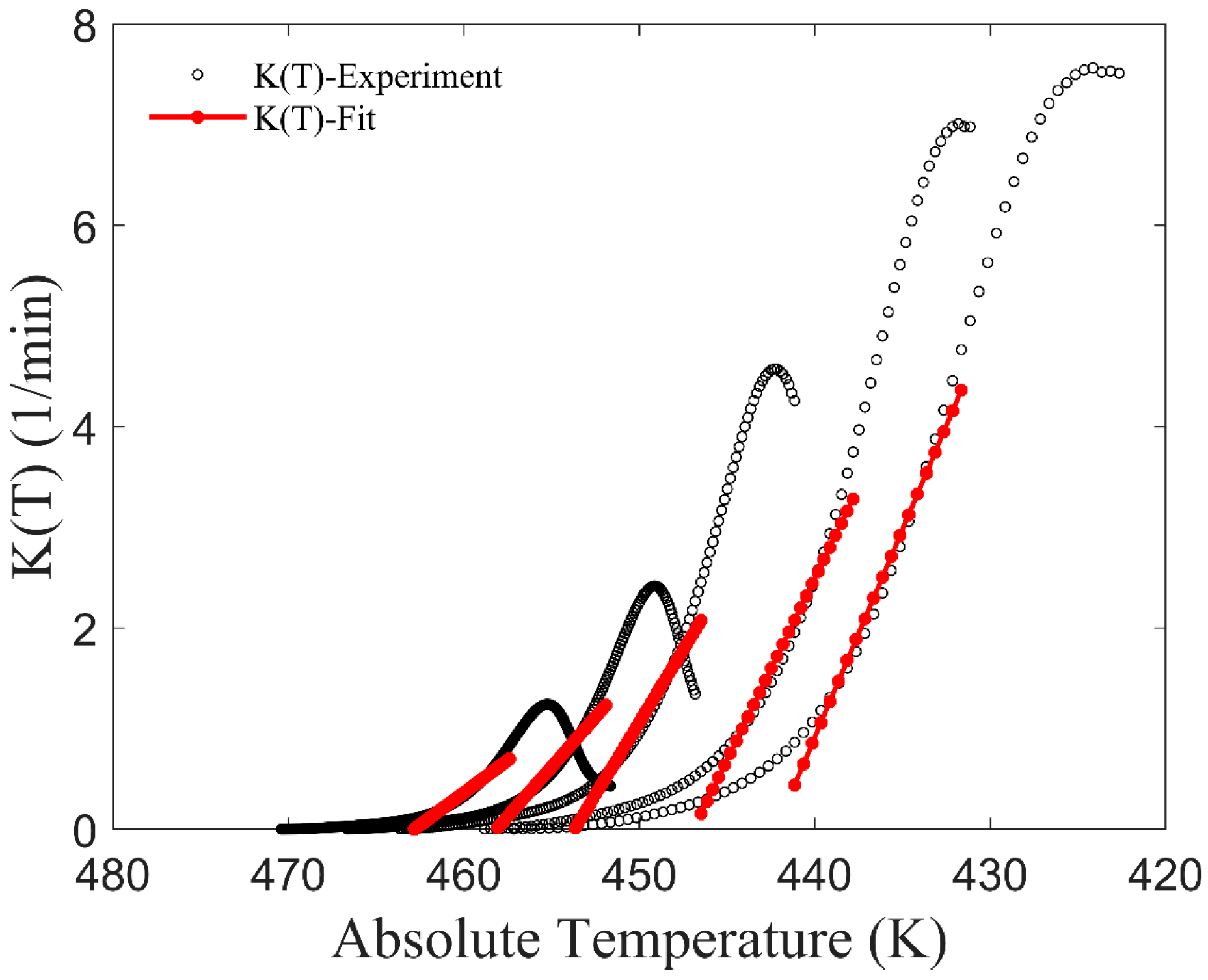
2.2. The Improved Mampel Model Based on Piecewise-Linear K(T)
3. Verification of the Improved Model
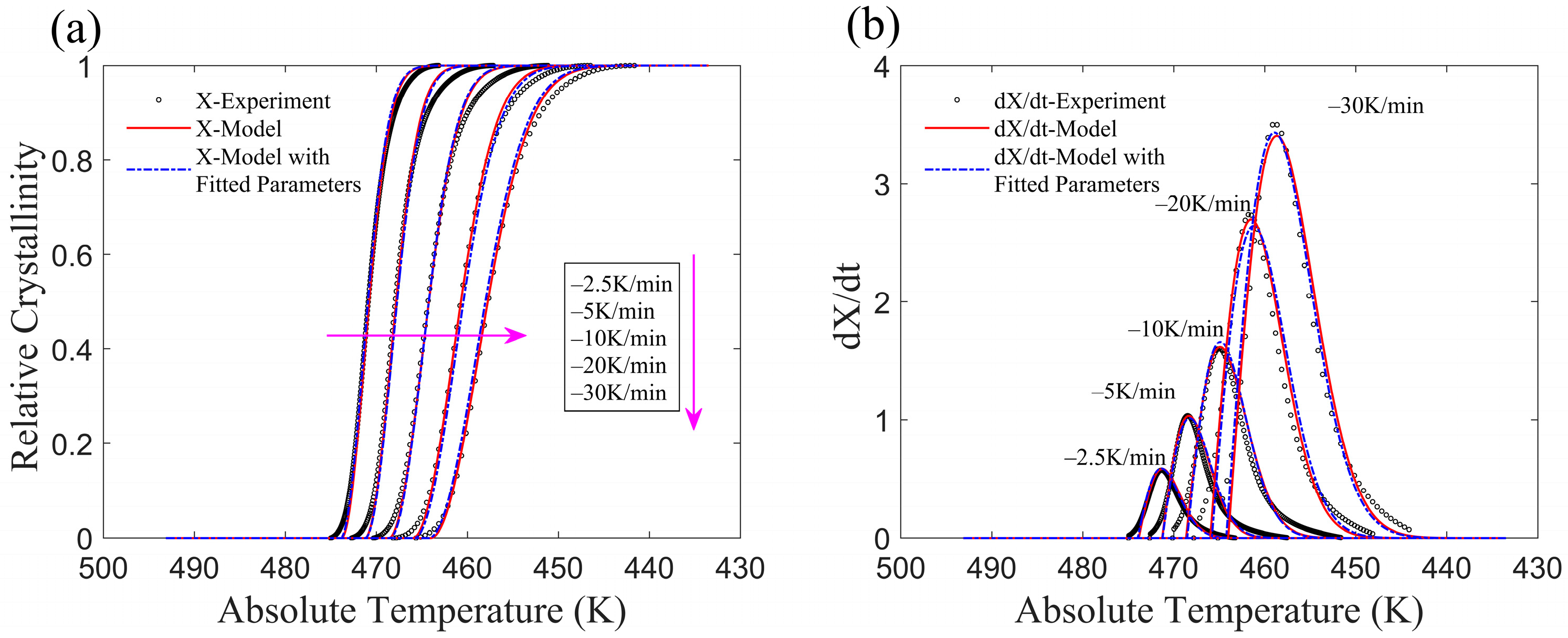
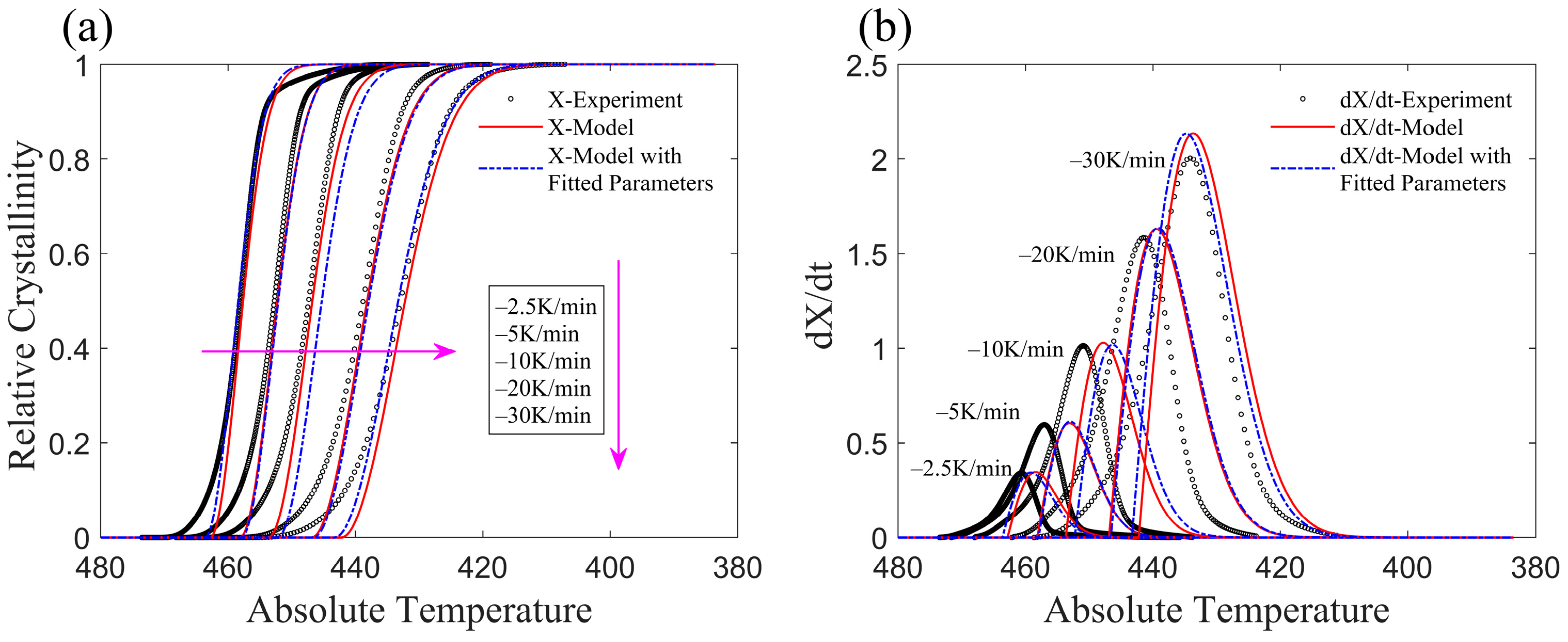
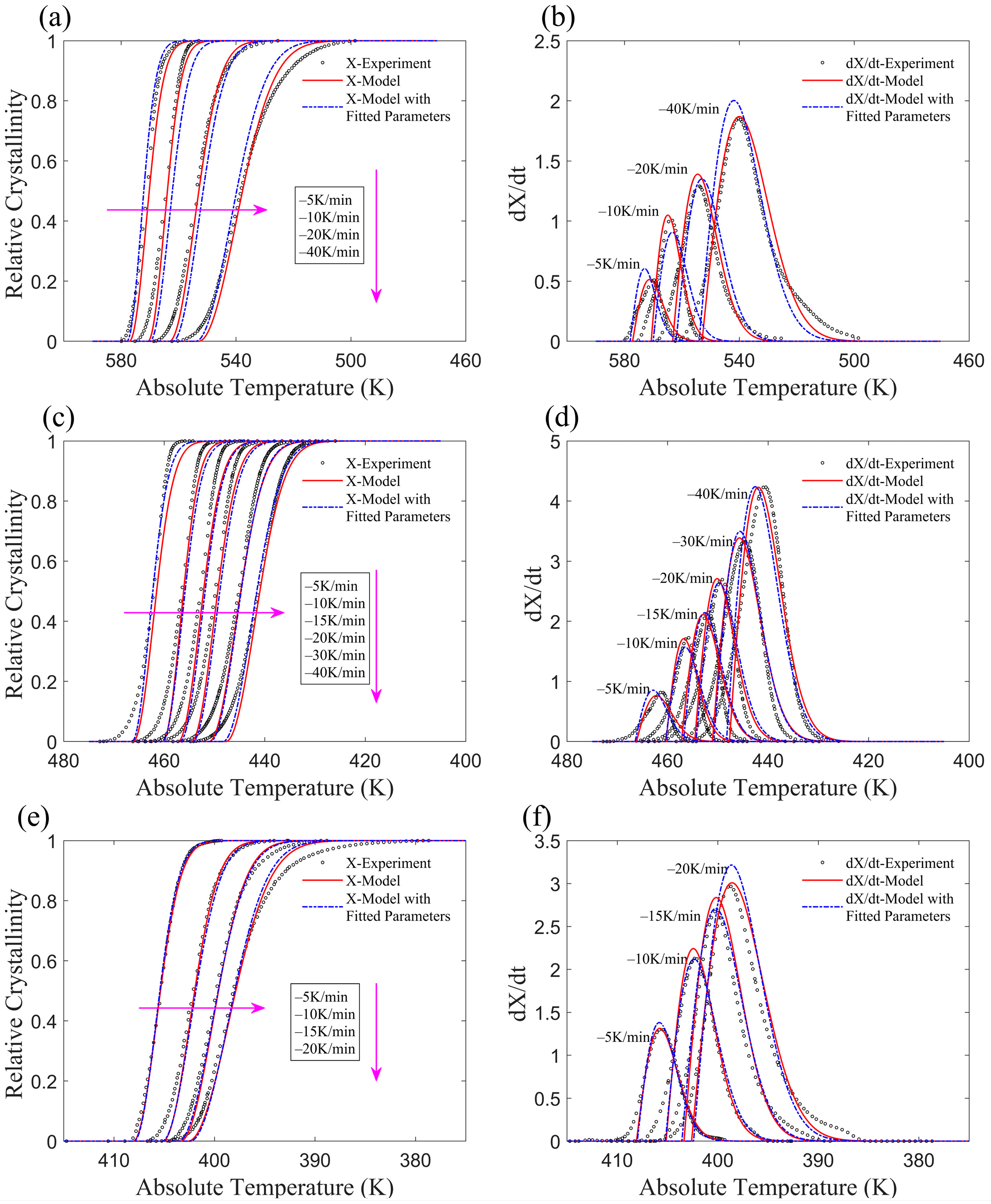
3.1. Application of the Improved Model to Non-Isothermal Crystallization of PA6
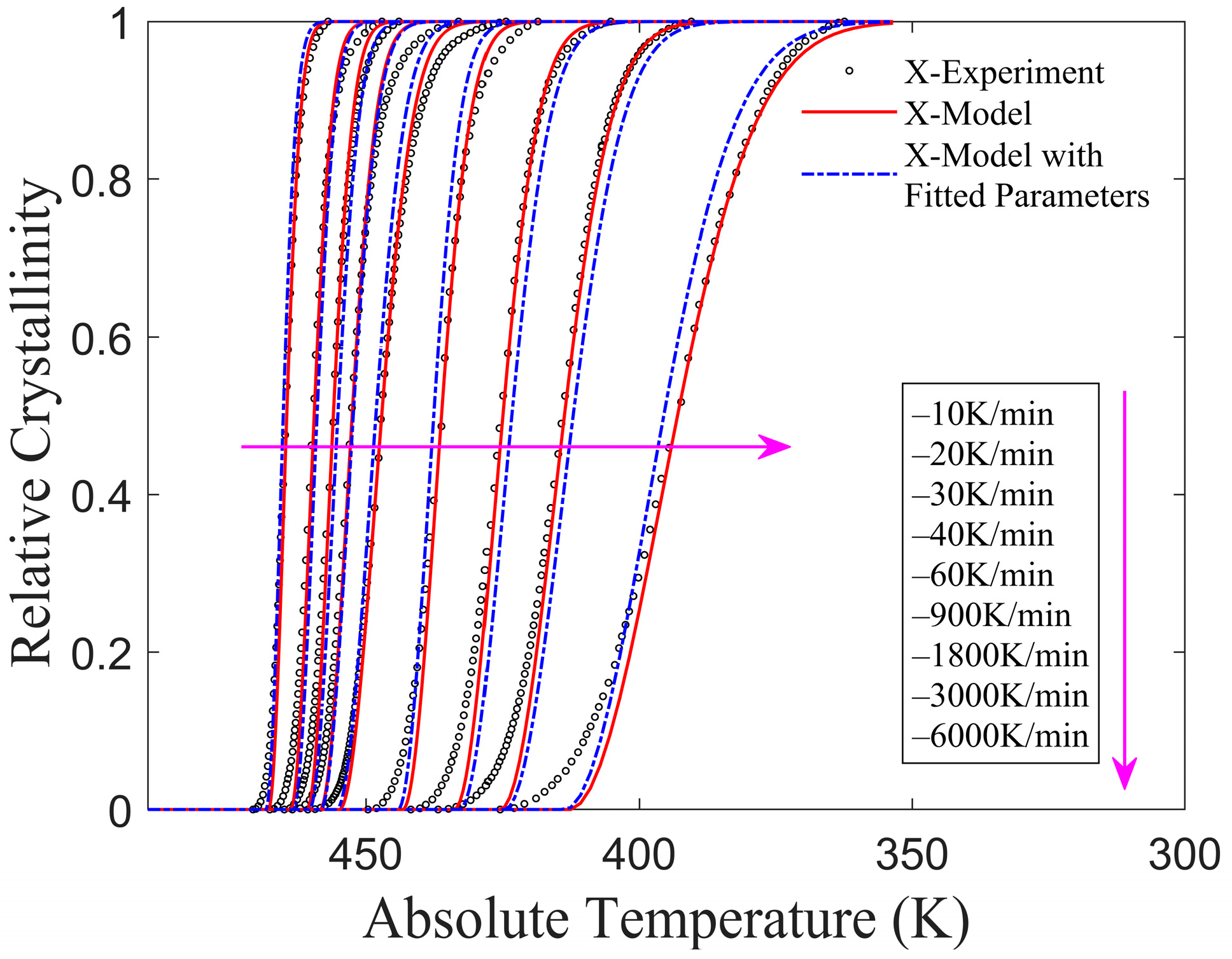
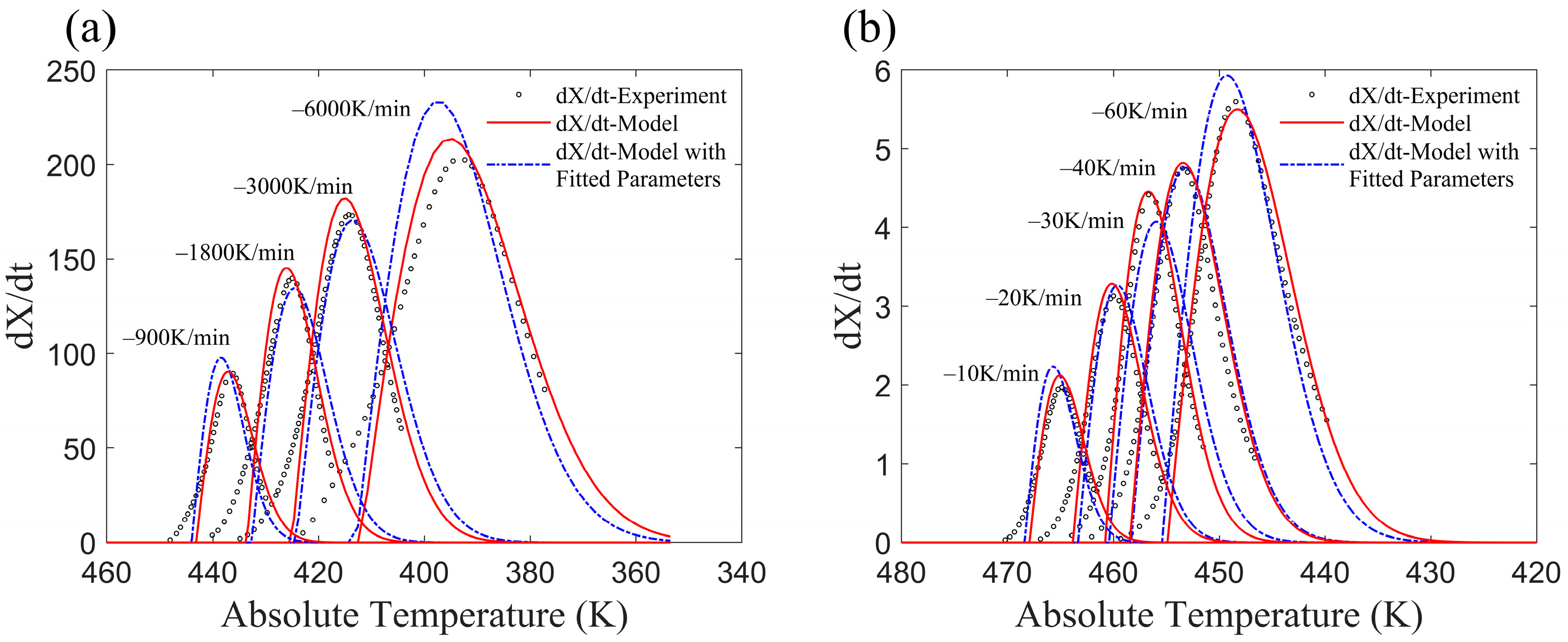
3.2. Application of the Improved Model to Published Experiments
- (1)
- CF/PA6 (Fiber volume fraction 50%)
- (2)
- Other materials
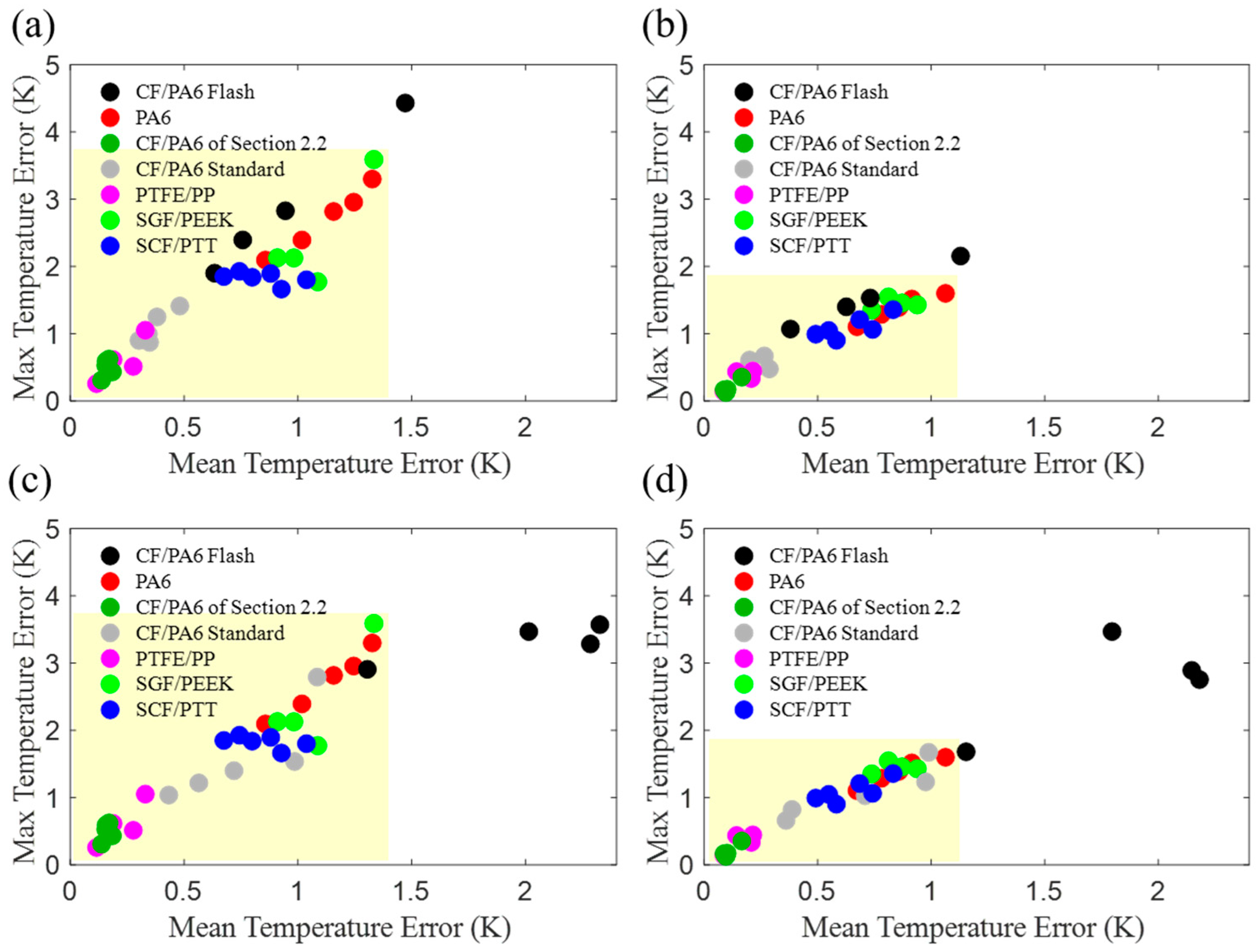
3.3. Assessment of Model Applicability
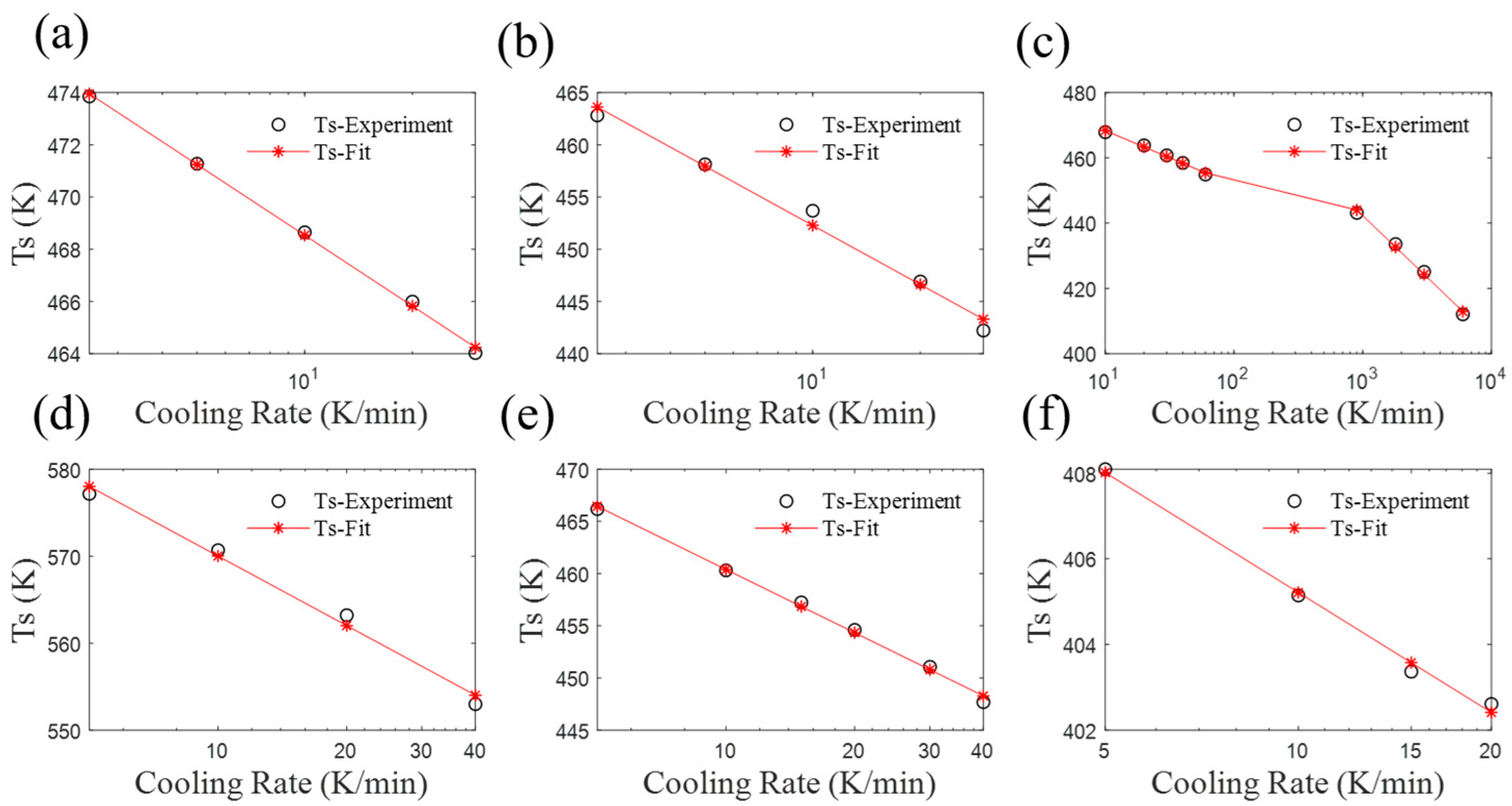
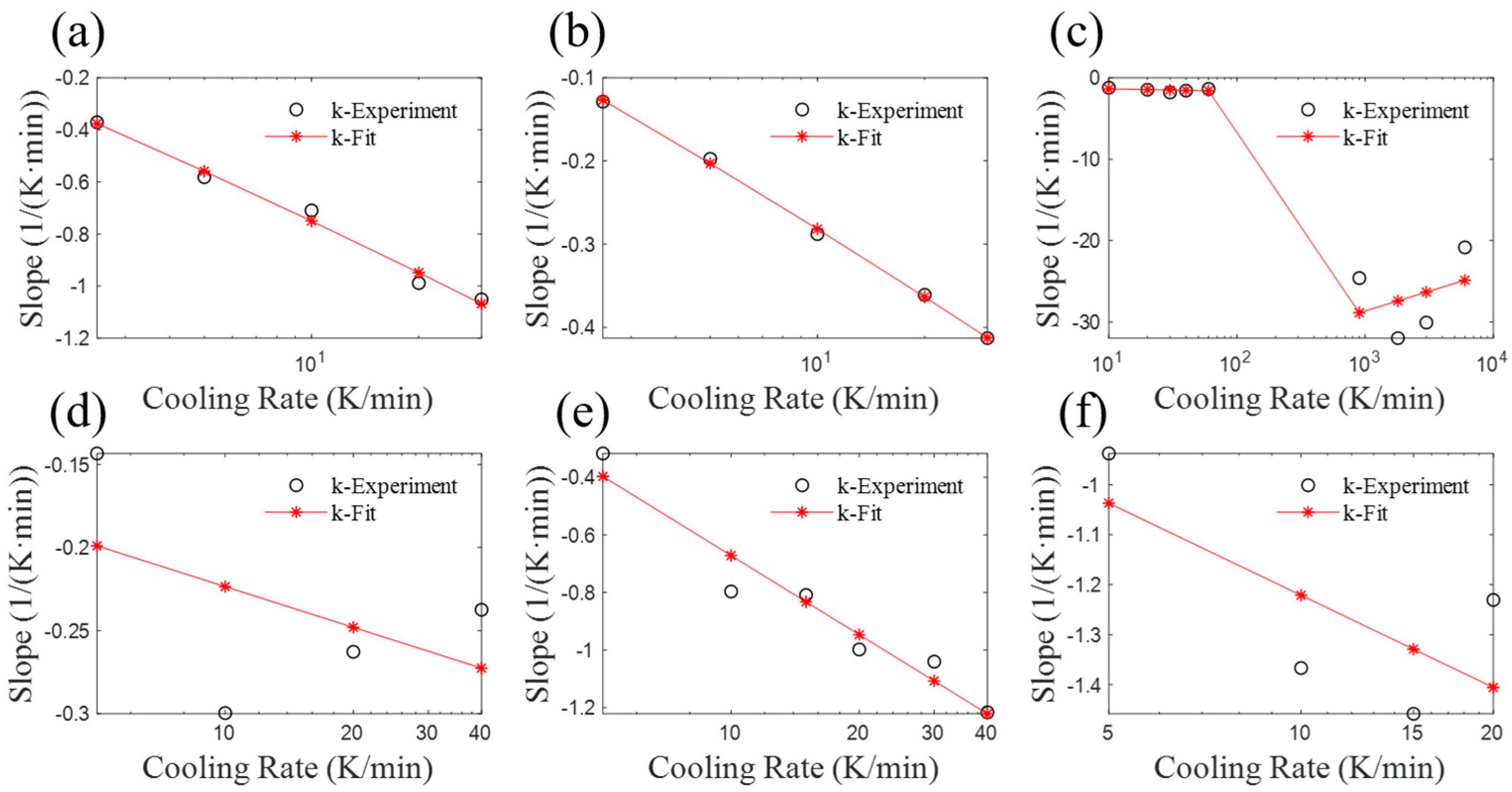
4. Parameter Analysis of the Revised Mampel Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Y.; Song, Z.; Ning, H.; Hu, N.; Peng, X.; Wu, X.; Zou, R.; Liu, F.; Weng, S.; Liu, Q. A comprehensive review of characterization and simulation methods for thermo-stamping of 2D woven fabric reinforced thermoplastics. Compos. Part B Eng. 2020, 203, 108462. [Google Scholar] [CrossRef]
- Choupin, T.; Debertrand, L.; Fayolle, B.; Régnier, G.; Paris, C.; Cinquin, J.; Brulé, B. Influence of thermal history on the mechanical properties of poly(ether ketone ketone) copolymers. Polym. Cryst. 2019, 2, e10086. [Google Scholar] [CrossRef]
- Gao, S.L.; Kim, J.K. Cooling rate influences in carbon fibre/PEEK composites. Part 1. Crystallinity and interface adhesion. Compos. Part A-Appl. Sci. Manuf. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Brauner, C.; Peters, C.; Brandwein, F.; Herrmann, A.S. Analysis of process-induced deformations in thermoplastic composite materials. J. Compos. Mater. 2014, 48, 2779–2791. [Google Scholar] [CrossRef]
- Lessard, H.; Lebrun, G.; Benkaddour, A.; Pham, X.T. Influence of process parameters on the thermostamping of a [0/90]12 carbon/polyether ether ketone laminate. Compos. Part A-Appl. Sci. Manuf. 2015, 70, 59–68. [Google Scholar] [CrossRef]
- Whitfield, T.; Kuboki, T.; Wood, J.; Ugresic, V.; Sathyanarayana, S.; Dagnon, K. Effects of process parameters on thermal properties of glass fiber reinforced polyamide 6 composites throughout the direct long-fiber-reinforced thermoplastics process. Polym. Eng. Sci. 2018, 58, E114–E123. [Google Scholar] [CrossRef]
- Han, W.; Govignon, Q.; Cantarel, A.; Schmidt, F. Efficient polymerization and crystallization kinetics coupling of polyamide 6 synthesis for liquid composite molding process modeling. Polym. Eng. Sci. 2022, 62, 999–1012. [Google Scholar] [CrossRef]
- Ruan, C.L.; Ouyang, J.; Liu, S.G. Multi-scale modeling and simulation of crystallization during cooling in short fiber reinforced composites. Int. J. Heat Mass Transf. 2012, 55, 1911–1921. [Google Scholar] [CrossRef]
- Fang, H.D.; Wang, X.Y.; Gu, J.F.; Li, Z.; Ruan, S.L.; Shen, C.Y. A novel crystallization kinetics model of transcrystalline used for crystallization behavior simulation of short carbon fiber-reinforced polymer composites. Polym. Eng. Sci. 2019, 59, 854–862. [Google Scholar] [CrossRef]
- Fang, H.D.; Gu, J.F.; Li, Z.; Ruan, S.L.; Shen, C.Y. An analytical model for temperature and crystalline evolution analysis of carbon fiber reinforced polymer composites during cooling. Polym. Compos. 2020, 41, 4074–4083. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly (ethylene terephthalate) determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Lagarinhos, J.; da Silva, S.M.; Oliveira, J.M. Non-Isothermal Crystallization Kinetics of Polyamide 6/Graphene Nanoplatelets Nanocomposites Obtained via In Situ Polymerization: Effect of Nanofiller Size. Polymers 2023, 15, 4109. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.F.; Yang, X.J.; Wei, K.; Wu, Y.Z.; Fang, W.J. Non-isothermal crystallization kinetics of continuous glass fiber-reinforced poly(ether ether ketone) composites. J. Therm. Anal. Calorim. 2019, 138, 369–378. [Google Scholar] [CrossRef]
- Zou, P.; Tang, S.W.; Fu, Z.Z.; Xiong, H.G. Isothermal and non-isothermal crystallization kinetics of modified rape straw flour/high-density polyethylene composites. Int. J. Therm. Sci. 2009, 48, 837–846. [Google Scholar] [CrossRef]
- Run, M.T.; Song, H.Z.; Yao, C.G.; Wang, Y.J. Crystal morphology and nonisothermal crystallization kinetics of short carbon Fiber/Poly(trimethylene terephthalate) composites. J. Appl. Polym. Sci. 2007, 106, 868–877. [Google Scholar] [CrossRef]
- Li, J.; Fang, Z.P.; Tong, L.F.; Gu, A.J.; Liu, F. Effect of multi-walled carbon nanotubes on non-isothermal crystallization kinetics of polyamide 6. Eur. Polym. J. 2006, 42, 3230–3235. [Google Scholar] [CrossRef]
- Wu, B.Z.; Gong, Y.; Yang, G.S. Non-isothermal crystallization of polyamide 6 matrix in all-polyamide composites: Crystallization kinetic, melting behavior, and crystal morphology. J. Mater. Sci. 2011, 46, 5184–5191. [Google Scholar] [CrossRef]
- Layachi, A.; Frihi, D.; Satha, H.; Seguela, R.; Gherib, S. Non-isothermal crystallization kinetics of polyamide 66/glass fibers/carbon black composites. J. Therm. Anal. Calorim. 2016, 124, 1319–1329. [Google Scholar] [CrossRef]
- Regis, M.; Zanetti, M.; Pressacco, M.; Bracco, P. Opposite role of different carbon fiber reinforcements on the non-isothermal crystallization behavior of poly(etheretherketone). Mater. Chem. Phys. 2016, 179, 223–231. [Google Scholar] [CrossRef]
- Weng, W.G.; Chen, G.H.; Wu, D.J. Crystallization kinetics and melting behaviors of nylon 6/foliated graphite nanocomposites. Polymer 2003, 44, 8119–8132. [Google Scholar] [CrossRef]
- Shi, N.; Dou, Q. Non-isothermal cold crystallization kinetics of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/treated calcium carbonate composites. J. Therm. Anal. Calorim. 2015, 119, 635–642. [Google Scholar] [CrossRef]
- Qiao, Y.H.; Jalali, A.; Yang, J.N.; Chen, Y.G.; Wang, S.W.; Jiang, Y.C.; Hou, J.H.; Jiang, J.; Li, Q.; Park, C.B. Non-isothermal crystallization kinetics of polypropylene/polytetrafluoroethylene fibrillated composites. J. Mater. Sci. 2021, 56, 3562–3575. [Google Scholar] [CrossRef]
- Perez-Martin, H.; Mackenzie, P.; Baidak, A.; Bradaigh, C.M.O.; Ray, D. Crystallisation behaviour and morphological studies of PEKK and carbon fibre/PEKK composites. Compos. Part A-Appl. Sci. Manuf. 2022, 159, 106992. [Google Scholar] [CrossRef]
- Kugele, D.; Dörr, D.; Wittemann, F.; Hangs, B.; Rausch, J.; Kärger, L.; Henning, F. Modeling of the non-isothermal crystallization kinetics of polyamide 6 composites during thermoforming. AIP Conf. Proc. 2017, 1896, 030005. [Google Scholar]
- Dörr, D.; Joppich, T.; Kugele, D.; Henning, F.; Kärger, L. A coupled thermomechanical approach for finite element forming simulation of continuously fiber-reinforced semi-crystalline thermoplastics. Compos. Part A-Appl. Sci. Manuf. 2019, 125, 105508. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Simple approximate analytical solution for nonisothermal single-step transformations: Kinetic analysis. Aiche J. 2008, 54, 2145–2154. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Modification of the Kolmogorov–Johnson–Mehl–Avrami rate equation for non-isothermal experiments and its analytical solution. Acta Mater. 2006, 54, 5573–5579. [Google Scholar] [CrossRef]
- Mampel, K. Time–conversion formulae for heterogenous reactions in phase limits of solid bodies. 2. The time–conversion formulae for a powder from globular particles. Phys. Chem. A 1940, 187, 235–249. [Google Scholar]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Hoffman, J.D. Regime III crystallization in melt-crystallized polymers: The variable cluster model of chain folding. Polymer 1983, 24, 3–26. [Google Scholar] [CrossRef]
- Ziabicki, A. Studies on orientation phenomena by fiber formation from polymer melts. Part II. Theoretical considerations. J. Appl. Polym. Sci. 1960, 2, 24–31. [Google Scholar] [CrossRef]
- Kulkarni, S.; Reuvers, M.C.; Brepols, T.; Reese, S.; Johlitz, M.; Lion, A. Characterization of crystallization kinetics in Polyamide 6 with a focus on modeling the thermoforming process: Experiments, modeling, simulations. Contin. Mech. Thermodyn. 2023, 36, 415–431. [Google Scholar] [CrossRef]
- Huang, J.W.; Chang, C.C.; Kang, C.C.; Yeh, M.Y. Crystallization kinetics and nucleation parameters of Nylon 6 and poly(ethylene-co-glycidyl methacrylate) blend. Thermochim. Acta 2008, 468, 66–74. [Google Scholar] [CrossRef]
- Guo, B.C.; Zou, Q.L.; Lei, Y.D.; Du, M.L.; Liu, M.X.; Jia, D.M. Crystallization behavior of polyamide 6/halloysite nanotubes nanocomposites. Thermochim. Acta 2009, 484, 48–56. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Nakamura, K.; Katayama, K.; Amano, T. Some aspects of nonisothermal crystallization of polymers. II. Consideration of the isokinetic condition. J. Appl. Polym. Sci. 1973, 17, 1031–1041. [Google Scholar] [CrossRef]
- Yang, X.J.; Wu, Y.Z.; Wei, K.; Fang, W.J.; Sun, H.F. Non-Isothermal Crystallization Kinetics of Short Glass Fiber Reinforced Poly (Ether Ether Ketone) Composites. Materials 2018, 11, 2094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Ning, H.; Liu, F.; Hu, N.; Gong, Y. An Improved Mampel Model of the Non-Isothermal Crystallization Kinetics of Fiber-Reinforced Thermoplastic Composites. Materials 2024, 17, 4747. https://doi.org/10.3390/ma17194747
Song Z, Ning H, Liu F, Hu N, Gong Y. An Improved Mampel Model of the Non-Isothermal Crystallization Kinetics of Fiber-Reinforced Thermoplastic Composites. Materials. 2024; 17(19):4747. https://doi.org/10.3390/ma17194747
Chicago/Turabian StyleSong, Zengrui, Huiming Ning, Feng Liu, Ning Hu, and Youkun Gong. 2024. "An Improved Mampel Model of the Non-Isothermal Crystallization Kinetics of Fiber-Reinforced Thermoplastic Composites" Materials 17, no. 19: 4747. https://doi.org/10.3390/ma17194747
APA StyleSong, Z., Ning, H., Liu, F., Hu, N., & Gong, Y. (2024). An Improved Mampel Model of the Non-Isothermal Crystallization Kinetics of Fiber-Reinforced Thermoplastic Composites. Materials, 17(19), 4747. https://doi.org/10.3390/ma17194747







