Magnetic Phase-Change Microcapsules with High Encapsulation Efficiency, Enhancement of Infrared Stealth, and Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleic Acid-Modified Fe3O4 (Fe3O4(m))
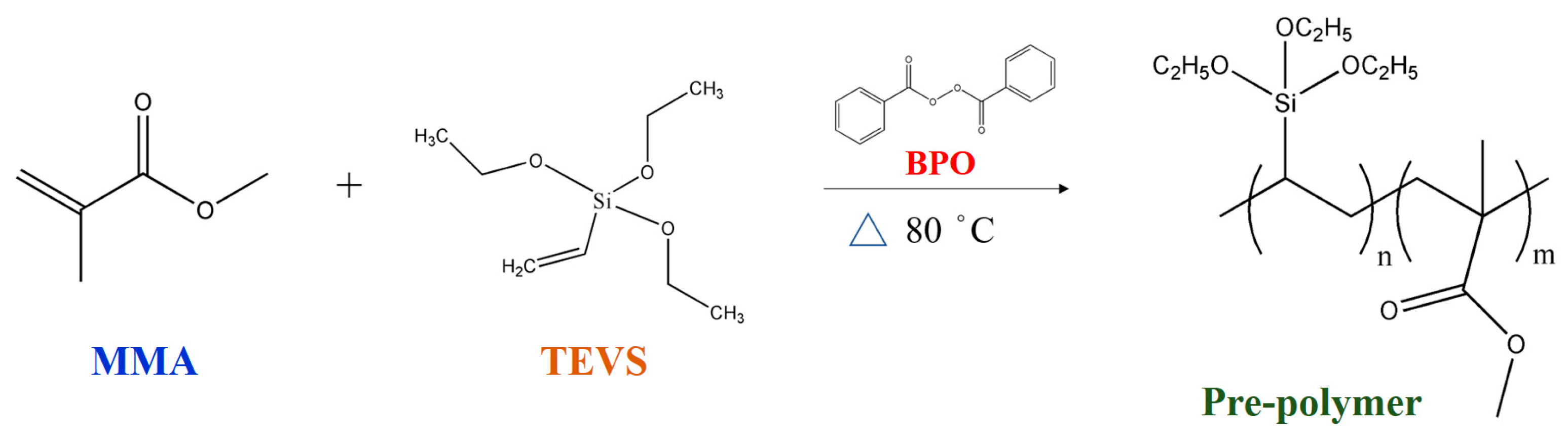
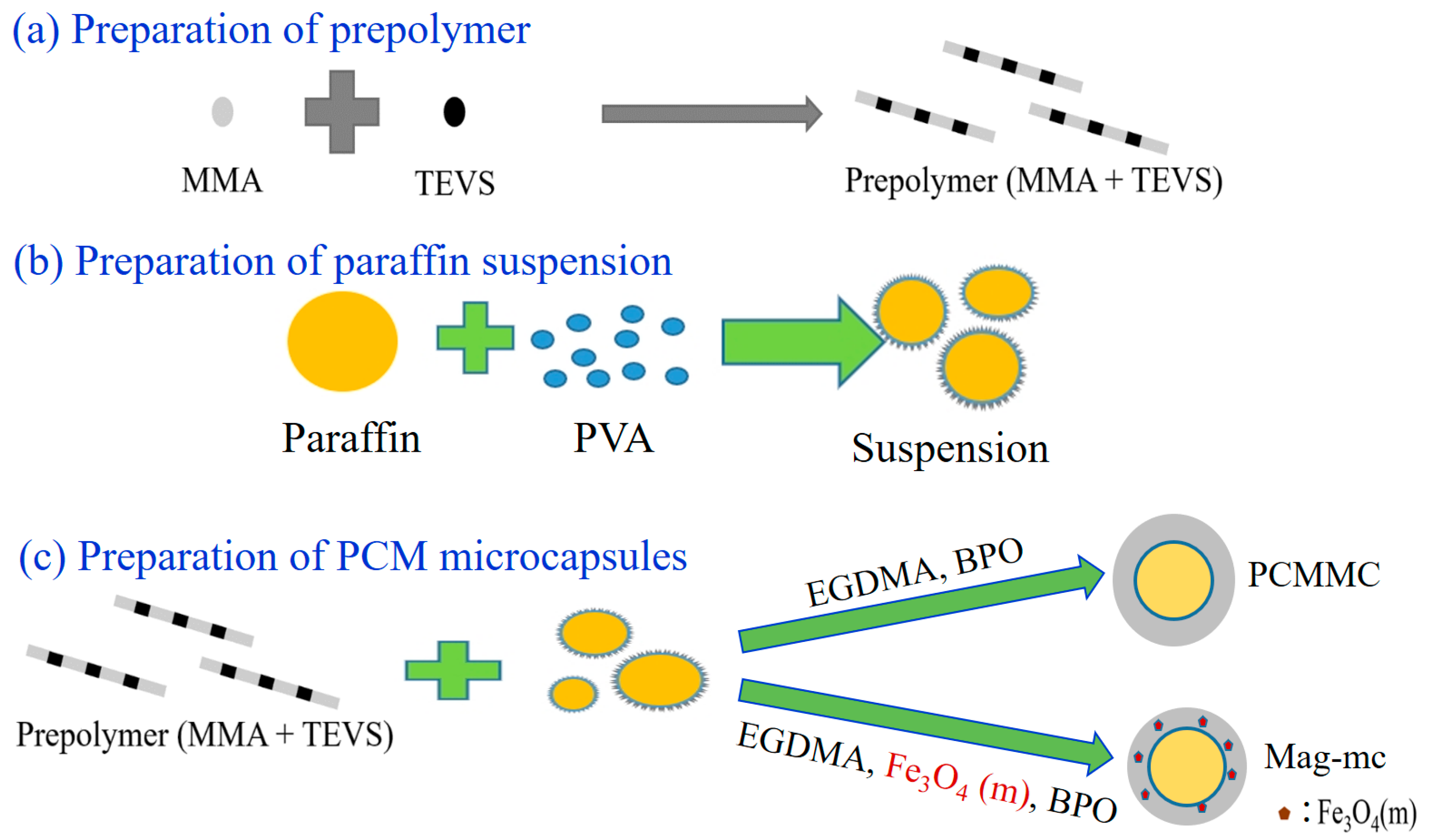
2.3. Preparation of Phase Change Material (PCM) Microcapsules
2.4. Preparation of PCM Microcapsule-Containing Films
2.5. Characterization and Measurement
3. Results and Discussion
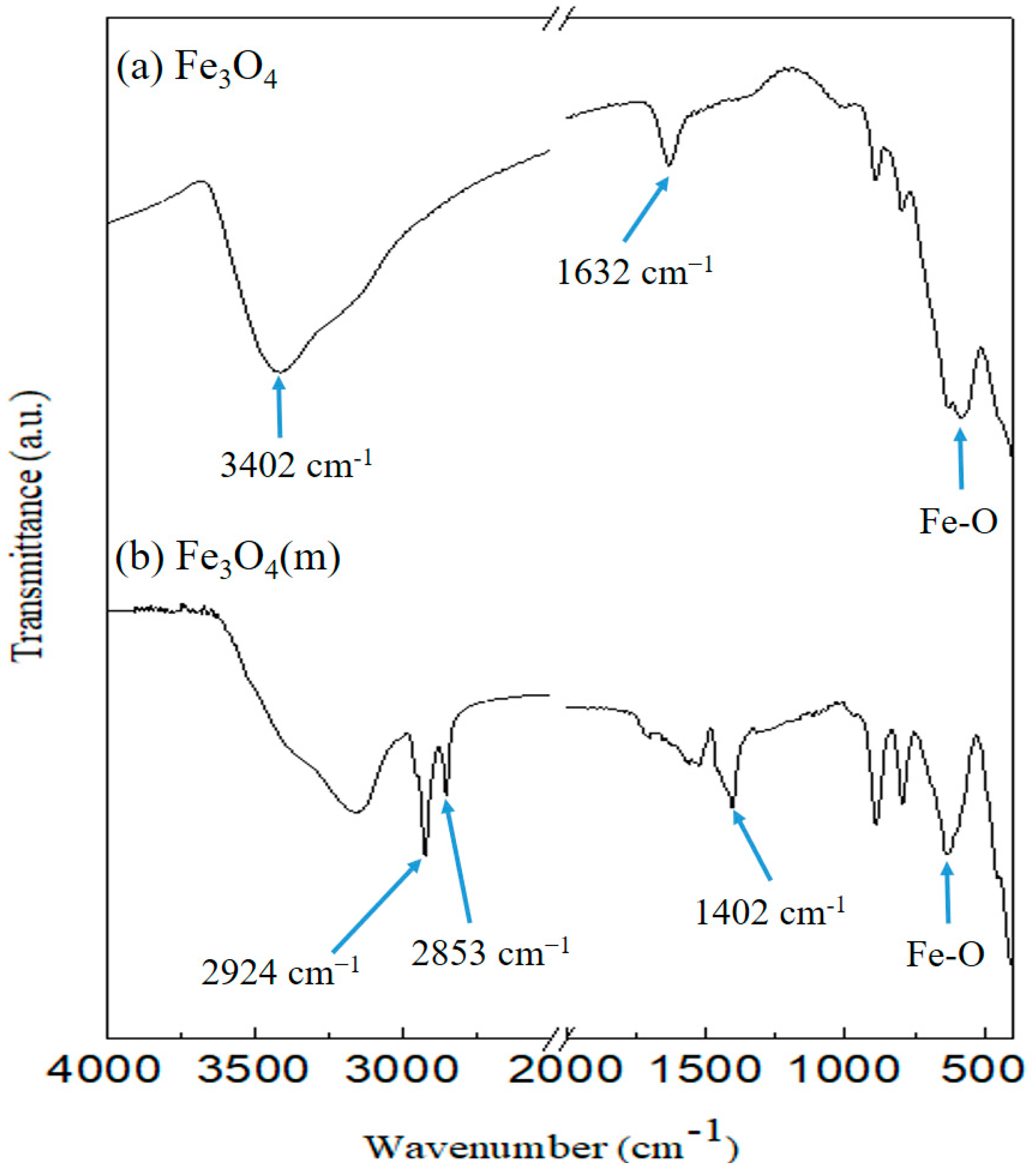
3.1. FT-IR Analysis
3.2. X-ray Diffraction Analysis
3.3. Magnetic Properties
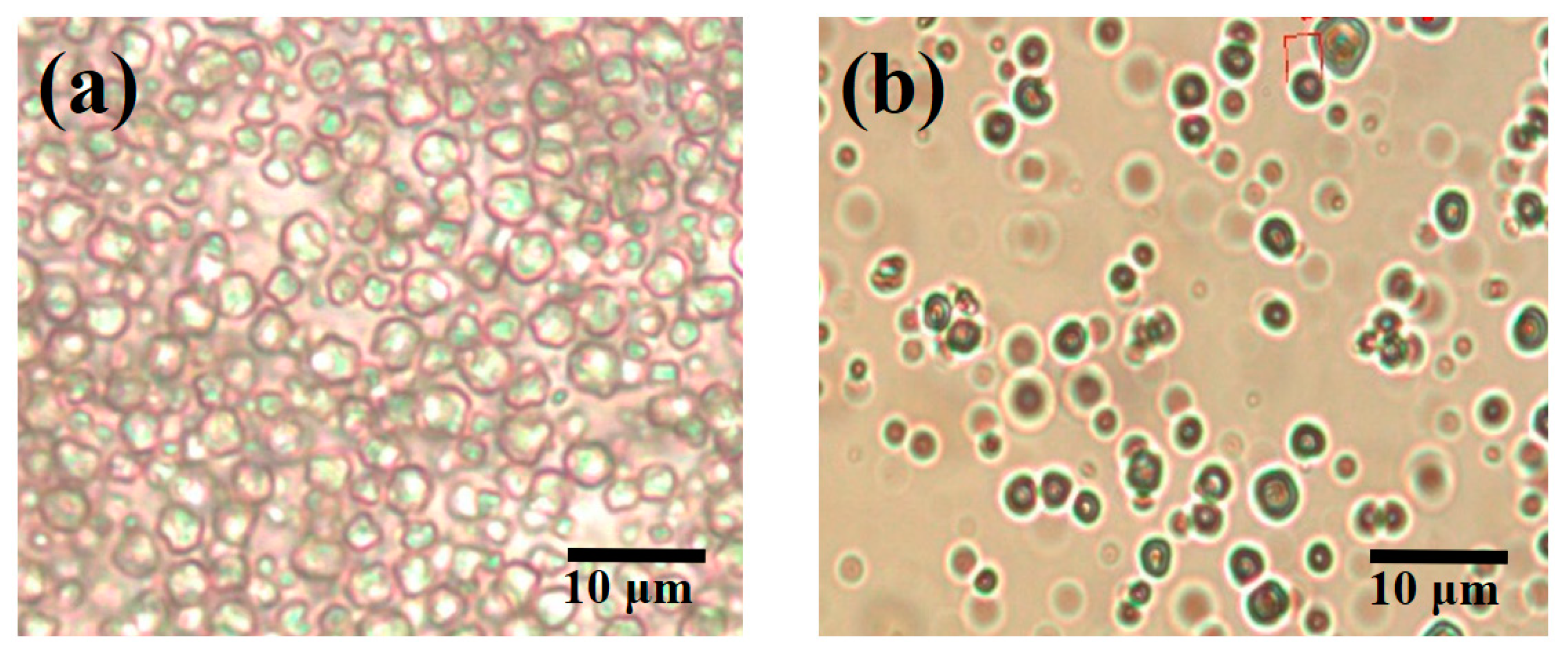
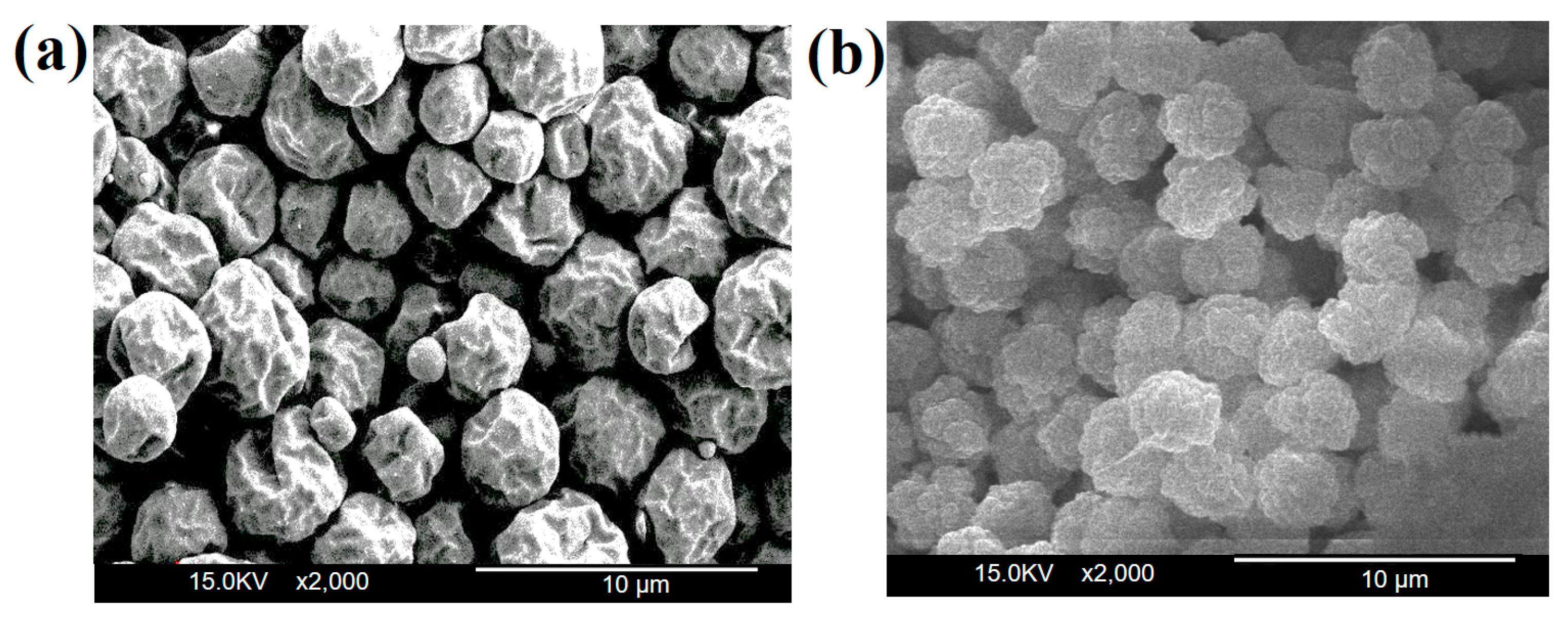
3.4. Morphology Analysis of Microcapsules
3.5. Particle Size Analysis of Microcapsules
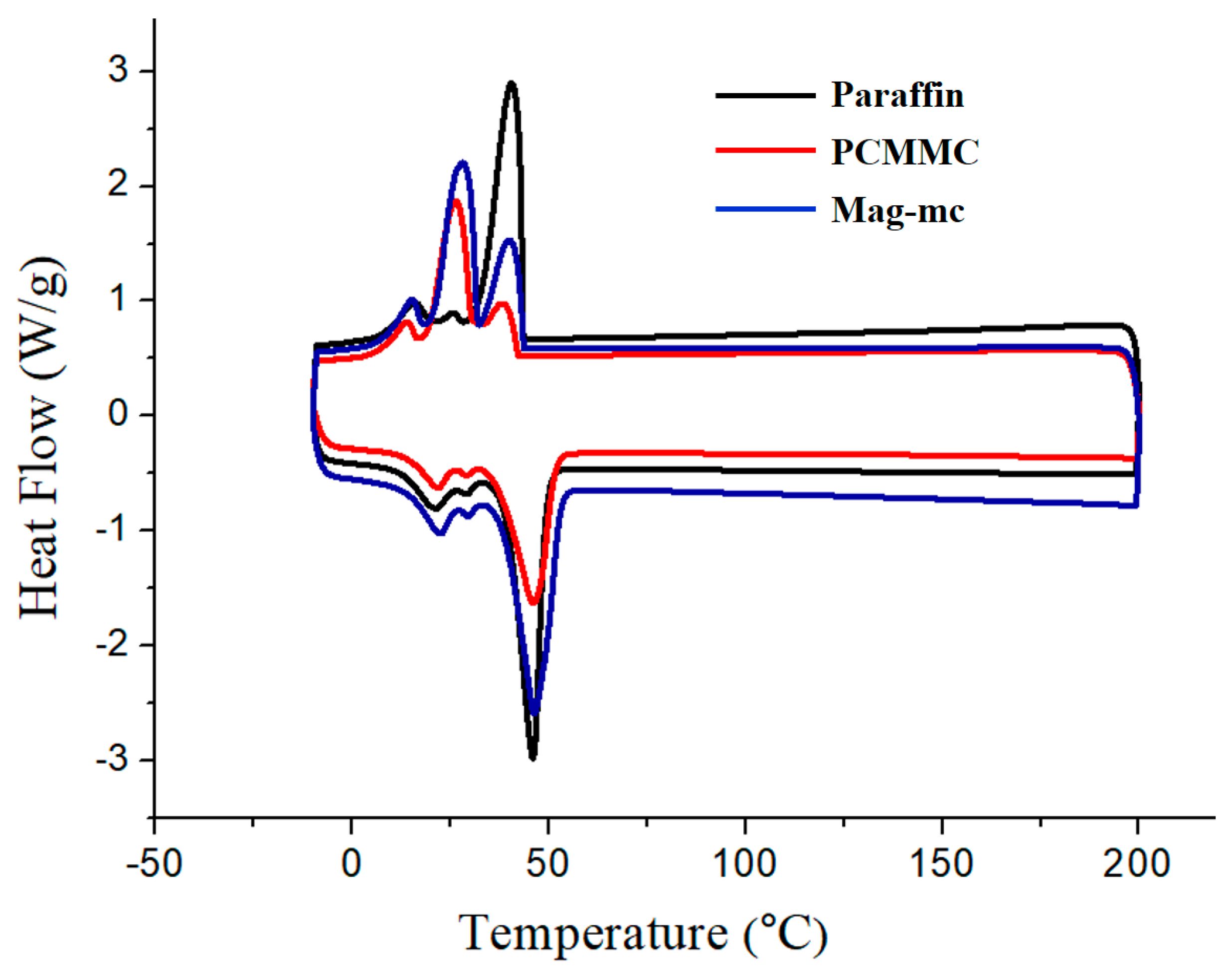
3.6. DSC Analysis
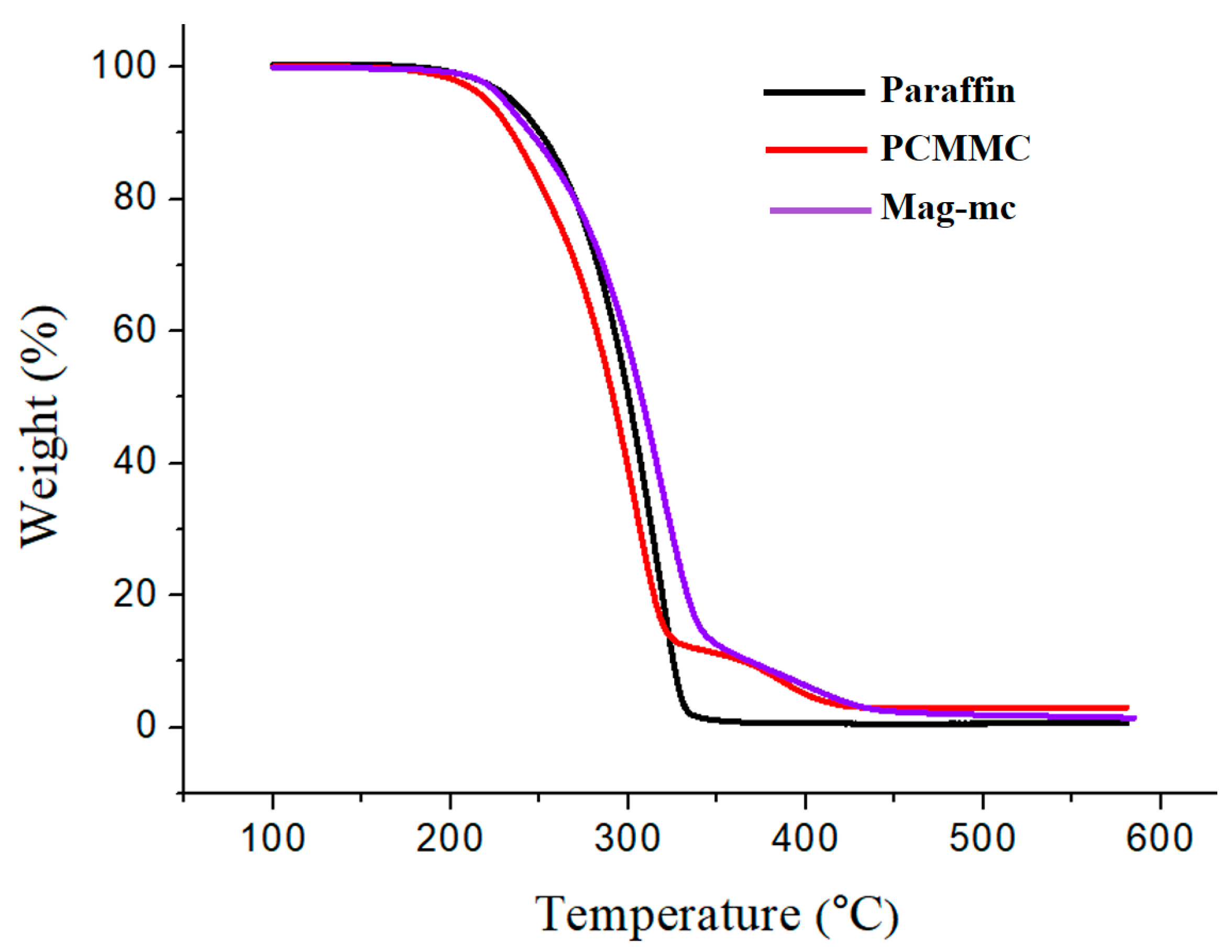
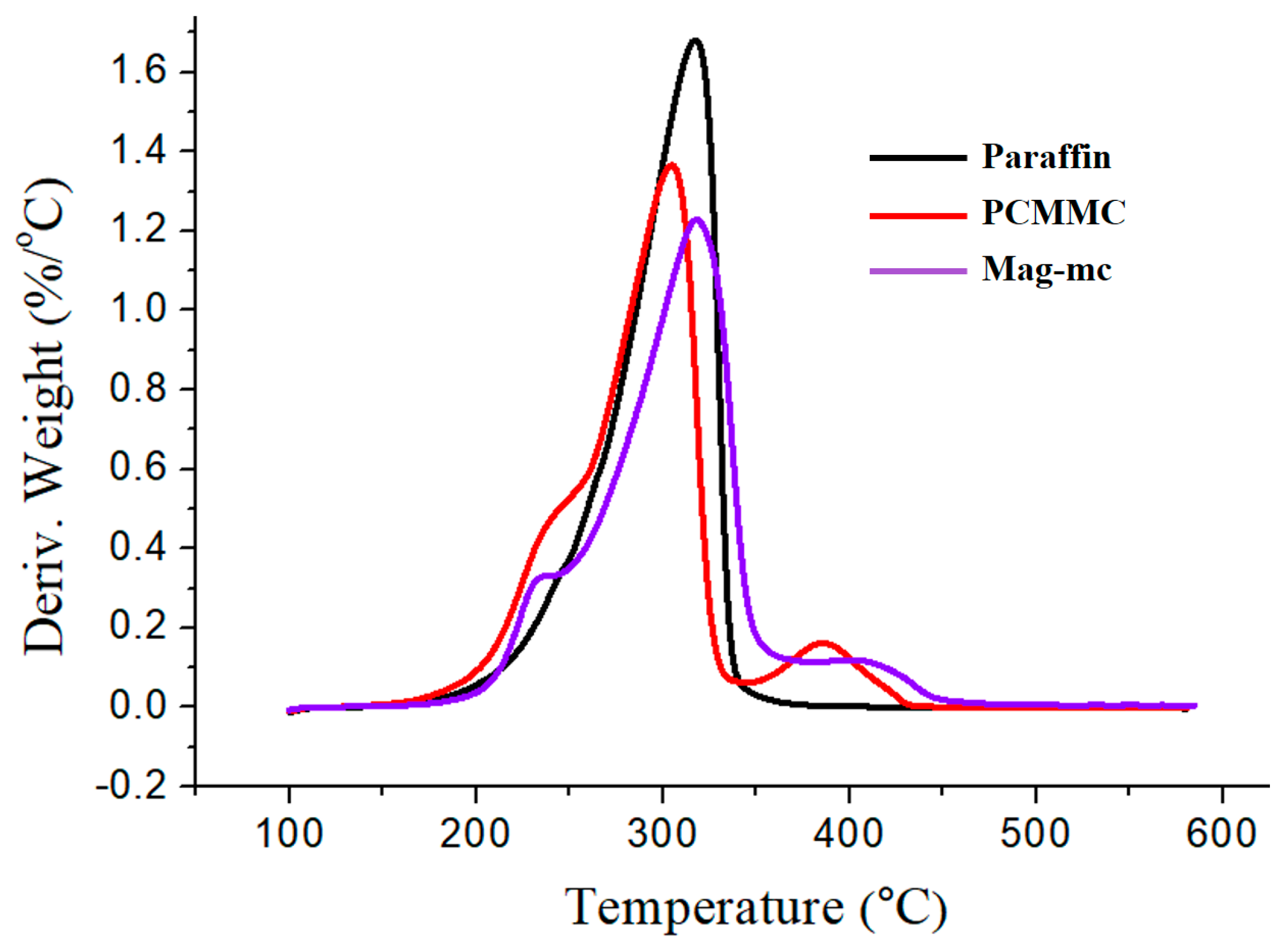
3.7. TGA Analysis
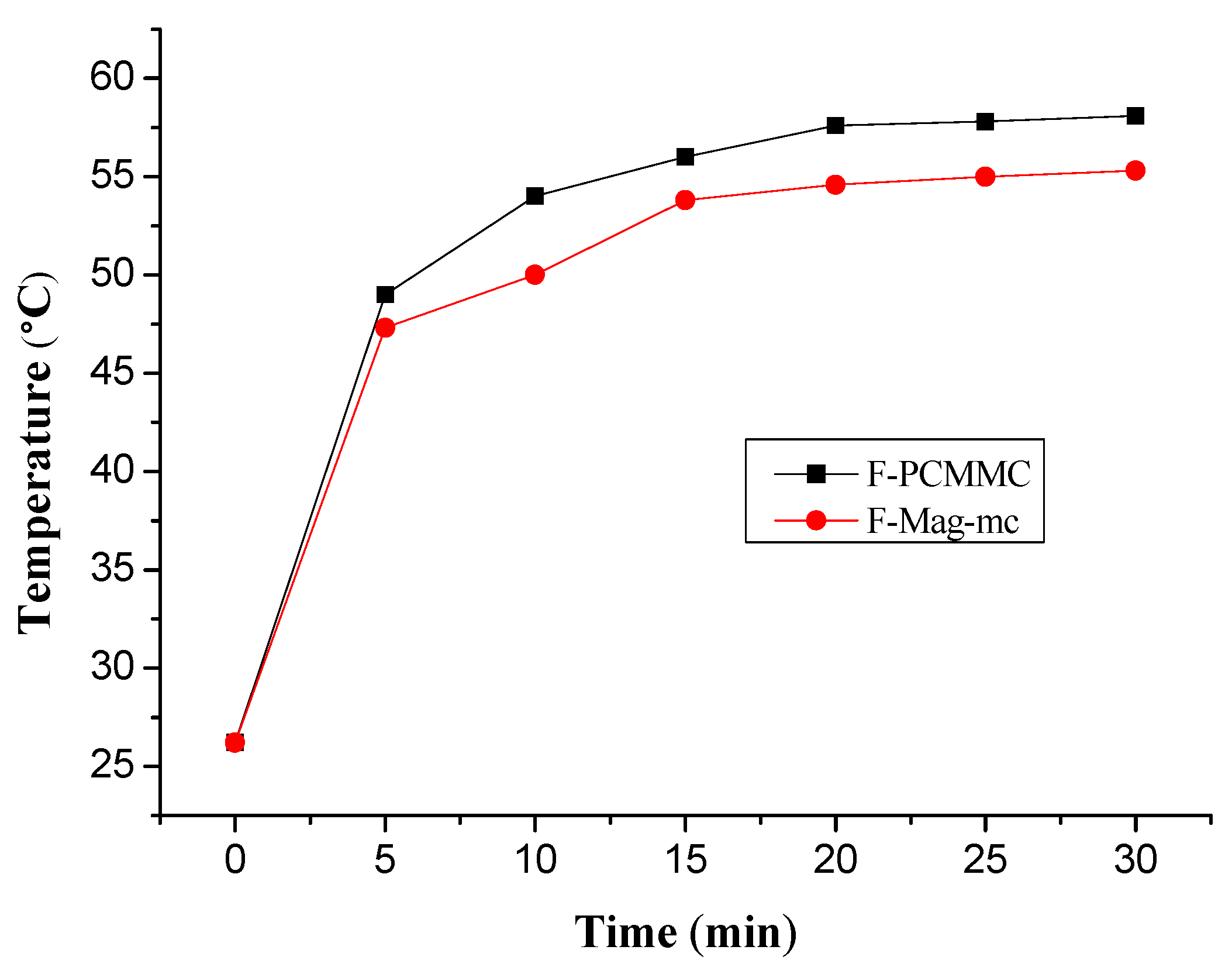
3.8. Thermal Buffering Capacity Analysis
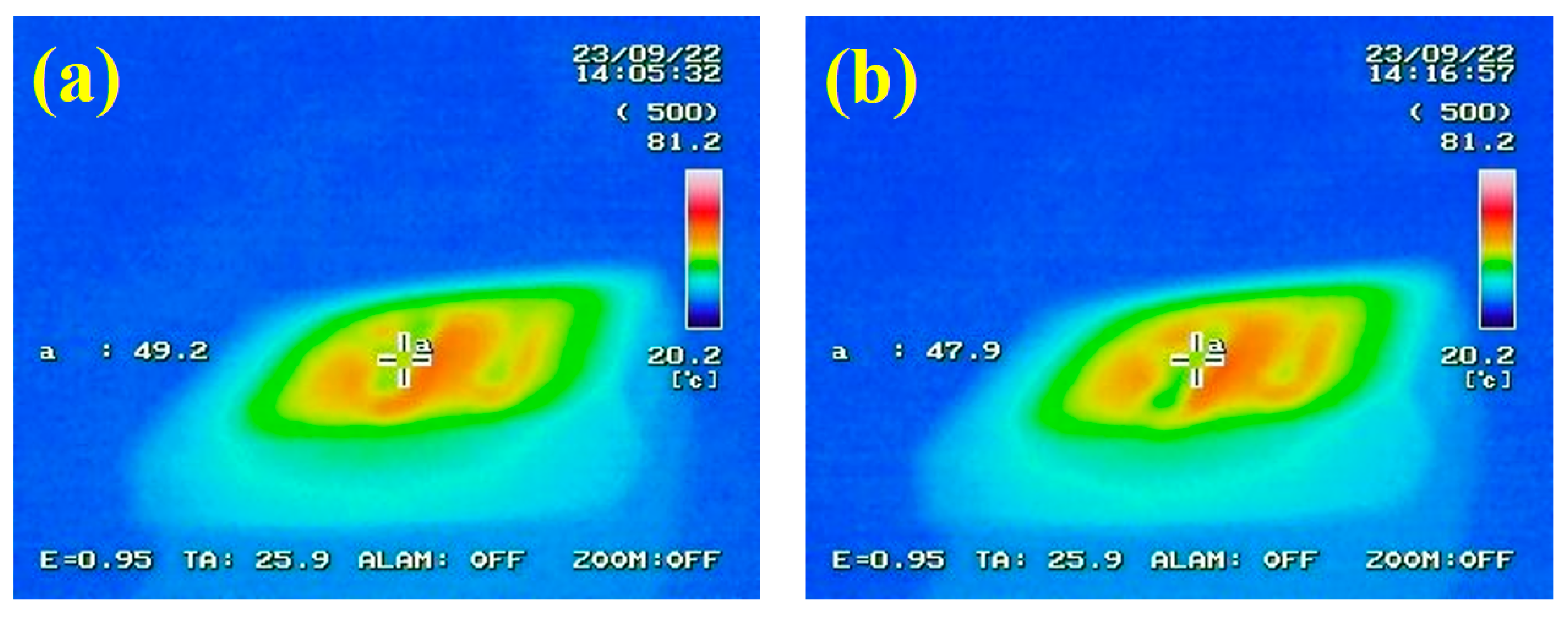
3.9. Infrared Stealth Effect Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.; Jeong, H.J.; Ji, H.Y.; Park, S.Y.; Chung, D.-Y.; Kang, C.; Kim, K.M.; Park, D. Peak Load Shifting Control on Hot Water Supplied from District Heating Using Latent Heat Storage System in Apartment Complex. Case Stud. Therm. Eng. 2022, 34, 101993. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.Y.; Markides, C.N.; Wang, H.; Li, W. Medium- and High-Temperature Latent and Thermochemical Heat Storage Using Metals and Metallic Compounds as Heat Storage Media: A Technical Review. Appl. Energy 2020, 280, 115950. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on Thermal Energy Storage with Phasechange: Materials, Heat Transfer Analysis and Applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A Review on Current Status and Challenges of Inorganic Phase Change Materials for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, L.; Huang, Y.; Ding, Y.; Chen, H. Latent Heat of Molten Salt Transport across Graphite Induced Anisotropic Interface. Sol. Energy Mater. Sol. Cells 2022, 236, 111496. [Google Scholar] [CrossRef]
- Tuncbilek, K.; Sari, A.; Tarhan, S.; Ergunes, G.; Kaygusuz, K. Lauric and Palmitic Acids Eutectic Mixture as Latent Heat Storage Material for Low Temperature Heating Applications. Energy 2005, 30, 677–692. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.Ö. Polystyrene-Based Caprylic Acid Microencapsulation for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2017, 159, 235–242. [Google Scholar] [CrossRef]
- Mohammadi, B.; Najafi, F.S.; Ranjbar, H.; Mohammadi, J.; Zakaryazadeh, M. Nanoencapsulation of Butyl Palmitate in Polystyrene-Co-Methyl Methacrylate Shell for Thermal Energy Storage Application. Energy Build. 2016, 118, 99–105. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Huang, R.; Wang, N.; Wang, J.; Zhang, X. Microstructure Regulation of Microencapsulated Bio-Based N-Dodecanol as Phase Change Materials Via in Situ Polymerization. New J. Chem. 2017, 41, 14696–14707. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, X.; Luo, J.; Song, G.; Liu, Y.; Tang, G. Polymethyl Methacrylate Based Phase Change Microencapsulation for Solar Energy Storage with Silicon Nitride. Solar Energy 2015, 115, 289–296. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Wang, X.; Wu, D. Innovative Design of Superhydrophobic Thermal Energy-Storage Materials by Microencapsulation of N-Docosane with Nanostructured ZnO/SiO2 Shell. Appl. Energy 2019, 237, 549–565. [Google Scholar] [CrossRef]
- Ma, S.; Song, G.; Li, W.; Fan, P.; Tang, G. UV Irradiation-Initiated MMA Polymerization to Prepare Microcapsules Containing Phase Change Paraffin. Sol. Energy Mater. Sol. Cells 2010, 94, 1643–1647. [Google Scholar] [CrossRef]
- Sami, S.; Sadrameli, S.M.; Etesami, N. Thermal Properties Optimization of Microencapsulated a Renewable and Non-Toxic Phase Change Material with a Polystyrene Shell for Thermal Energy Storage Systems. Appl. Therm. Eng. 2018, 130, 1416–1424. [Google Scholar] [CrossRef]
- Zhang, D.X.; Li, B.X.; Zhang, X.P.; Zhang, Z.Q.; Wang, W.C.; Liu, F. Phoxim Microcapsules Prepared with Polyurea and Urea-Formaldehyde Resins Differ in Photostability and Insecticidal Activity. J. Agric. Food Chem. 2016, 64, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kim, B. Synthesis of Graphene Oxide–Epoxy Resin Encapsulated Urea-Formaldehyde Microcapsule by in Situ Polymerization Process. Polym. Compos. 2016, 39, 636–644. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.M. A Novel Graphite-Pcm Composite Sphere with Enhanced Thermo-Physical Properties. Appl. Therm. Eng. 2018, 142, 401–409. [Google Scholar] [CrossRef]
- Wan, X.; Guo, B.; Xu, J. A Facile Hydrothermal Preparation for Phase Change Materials Microcapsules with a Pliable Self-Recovering Shell and Study on Its Thermal Energy Storage Properties. Powder Technol. 2017, 312, 144–151. [Google Scholar] [CrossRef]
- Özonur, Y.; Mazman, M.; Paksoy, H.Ö.; Evliya, H. Microencapsulation of Coco Fatty Acid Mixture for Thermal Energy Storage with Phase Change Material. Int. J. Energy Res. 2006, 30, 741–749. [Google Scholar] [CrossRef]
- Lashgari, S.; Arabi, H.; Mahdavian, A.R.; Ambrogi, V. Thermal and Morphological Studies on Novel Pcm Microcapsules Containing N-Hexadecane as the Core in a Flexible Shell. Appl. Energy 2017, 190, 612–622. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, Z. Synthesis and Thermal Properties of N-Tetradecane Phase Change Microcapsules for Cold Storage. J. Energy Storage 2022, 52, 104959. [Google Scholar] [CrossRef]
- Ng, D.-Q.; Tseng, Y.-L.; Shih, Y.-F.; Lian, H.-Y.; Yu, Y.-H. Synthesis of Novel Phase Change Material Microcapsule and Its Application. Polymer 2017, 133, 250–262. [Google Scholar] [CrossRef]
- Fu, E.B.; Yao, Y.P. Research on the Application of Microencapsulated Phase Change Materials to Thermal Infrared Camouflage. Appl. Mech. Mater. 2013, 328, 855–859. [Google Scholar] [CrossRef]
- Qiao, Z.; Mao, J. Multifunctional Poly (Melamine-Urea-Formaldehyde)/Graphene Microcapsules with Low Infrared Emissivity and High Thermal Conductivity. Mater. Sci. Eng. B 2017, 226, 86–93. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.; Di, X.; Yang, Z.; Yang, T.; Yu, Q.; Liu, F.; Li, J.; Li, G.; Wang, C. Multifunctional Wood Based Composite Phase Change Materials for Magnetic-Thermal and Solar-Thermal Energy Conversion and Storage. Energy Convers. Manag. 2019, 200, 112029. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite Phase Change Materials with Good Reversible Thermochromic Ability in Delignified Wood Substrate for Thermal Energy Storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Liu, H.; Tian, D.; Zheng, Z.; Wang, X.; Qian, Z. Mxene-Decorated Magnetic Phase-Change Microcapsules for Solar-Driven Continuous Seawater Desalination with Easy Salt Accumulation Elimination. Chem. Eng. J. 2023, 458, 141395. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Y.; Cai, C.; Zhang, J.; Zhu, Y. Design the Magnetic Microencapsulated Phase Change Materials with Poly(MMA-MAA) @ N-Octadecane Modified by Fe3O4. Sci. Rep. 2018, 8, 16379. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, S.; Mahdavian, A.R.; Arabi, H.; Ambrogi, V.; Marturano, V. Preparation of Acrylic Pcm Microcapsules with Dual Responsivity to Temperature and Magnetic Field Changes. Eur. Polym. J. 2018, 101, 18–28. [Google Scholar] [CrossRef]
- Ke, W.-D.; Wu, X.-W.; Zhang, J.-L. In Situ Polymerization of Organic and Inorganic Phase Change Microcapsule and Enhancement of Infrared Stealth Via Nano Iron. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127124. [Google Scholar] [CrossRef]
- Ashjari, M.; Mahdavian, A.R.; Ebrahimi, N.G.; Mosleh, Y. Efficient Dispersion of Magnetite Nanoparticles in the Polyurethane Matrix through Solution Mixing and Investigation of the Nanocomposite Properties. J. Inorg. Organomet. Polym. Mater. 2010, 20, 213–219. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and Their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Dallas, P.; Georgakilas, V.; Niarchos, D.; Komninou, P.; Kehagias, T.; Petridis, D. Synthesis, Characterization and Thermal Properties of Polymer/Magnetite Nanocomposites. Nanotechnology 2006, 17, 2046–2053. [Google Scholar] [CrossRef]
- Mürbe, J.; Rechtenbach, A.; Töpfer, J. Synthesis and Physical Characterization of Magnetite Nanoparticles for Biomedical Applications. Mater. Chem. Phys. 2008, 110, 426–433. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum muticum) Aqueous Extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef]
- Urus, S. Synthesis of Fe3O4@SiO2@OSi(CH2)3NHRN(CH2PPh2)2PdCl2 Type Nanocomposite Complexes: Highly Efficient and Magnetically-Recoverable Catalysts in Vitamin K3 Synthesis. Food Chem. 2016, 213, 336–343. [Google Scholar] [CrossRef]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing Magnetic and Inductive Thermal Properties of Various Surfactants Functionalised Fe3O4 Nanoparticles for Hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef]
- Mahdieh, A.; Mahdavian, A.R.; Salehi-Mobarakeh, H. Chemical Modification of Magnetite Nanoparticles and Preparation of Acrylic-Base Magnetic Nanocomposite Particles Via Miniemulsion Polymerization. J. Magn. Magn. Mater. 2017, 426, 230–238. [Google Scholar] [CrossRef]
- Sadchaiyaphum, J.; Phapugrangkul, P.; Chaiyasat, P.; Chaiyasat, A. High Encapsulation Efficiency of Magnetite Nanoparticles in Hydrophobic Polymer Microcapsules Using Microsuspension Conventional Radical Polymerization. Orient. J. Chem. 2019, 35, 516–522. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Qudeiri, J.A. Photocatalytic Removal of Methylene Blue Using Titania- and Silica-Coated Magnetic Nanoparticles. Mater. Res. Express 2018, 5, 065518. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on Thermal Degradation Properties of Oleic Acid and Its Methyl and Ethyl Esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of Thermal Decomposition of PMMA at Different Heating Rates and in a Wide Temperature Range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M.; Radhika, N. Thermal Characteristics Enhancement of Paraffin Wax Phase Change Material (PCM) for Thermal Storage Applications. Renew. Energy 2024, 222, 119986. [Google Scholar] [CrossRef]
- Gu, J.; Wang, W.; Yu, D. Temperature Control and Low Infrared Emissivity Double-Shell Phase Change Microcapsules and Their Application in Infrared Stealth Fabric. Prog. Org. Coat. 2021, 159, 106439. [Google Scholar] [CrossRef]







| PCMMC (μm) | Mag-mc (μm) | |
|---|---|---|
| Q1 | 1.00 | 0.86 |
| Q2 | 2.20 | 1.92 |
| Q3 | 4.20 | 3.63 |
| Average size | 3.48 | 2.09 |
| Sample | Tm (°C) | ΔHm (J/g) | Encapsulation Efficiency (%) |
|---|---|---|---|
| Paraffin | 45.86 | 119.6 | -- |
| PCMMC | 46.04 | 95.4 | 79.77 |
| Mag-mc | 45.26 | 115.0 | 96.63 |
| Sample | Td1 (°C) | Td2 (°C) | Td3 (°C) | Char Yield (%) |
|---|---|---|---|---|
| Fe3O4 | 64.4 | 217.143 | -- | 91.91 |
| Fe3O4(m) | 64.8 | 247.942 | 368.7 | 79.39 |
| Paraffin | 317.4 | -- | -- | 0.69 |
| PCMMC | 304.6 | 386.1 | -- | 3.064 |
| Mag-mc | 317.9 | 413.2 | -- | 1.486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-W.; Chen, Z.-T.; Shih, Y.-F. Magnetic Phase-Change Microcapsules with High Encapsulation Efficiency, Enhancement of Infrared Stealth, and Thermal Stability. Materials 2024, 17, 4778. https://doi.org/10.3390/ma17194778
Chang C-W, Chen Z-T, Shih Y-F. Magnetic Phase-Change Microcapsules with High Encapsulation Efficiency, Enhancement of Infrared Stealth, and Thermal Stability. Materials. 2024; 17(19):4778. https://doi.org/10.3390/ma17194778
Chicago/Turabian StyleChang, Chun-Wei, Zheng-Ting Chen, and Yeng-Fong Shih. 2024. "Magnetic Phase-Change Microcapsules with High Encapsulation Efficiency, Enhancement of Infrared Stealth, and Thermal Stability" Materials 17, no. 19: 4778. https://doi.org/10.3390/ma17194778
APA StyleChang, C.-W., Chen, Z.-T., & Shih, Y.-F. (2024). Magnetic Phase-Change Microcapsules with High Encapsulation Efficiency, Enhancement of Infrared Stealth, and Thermal Stability. Materials, 17(19), 4778. https://doi.org/10.3390/ma17194778







