Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution
Abstract
1. Introduction
2. Experimental
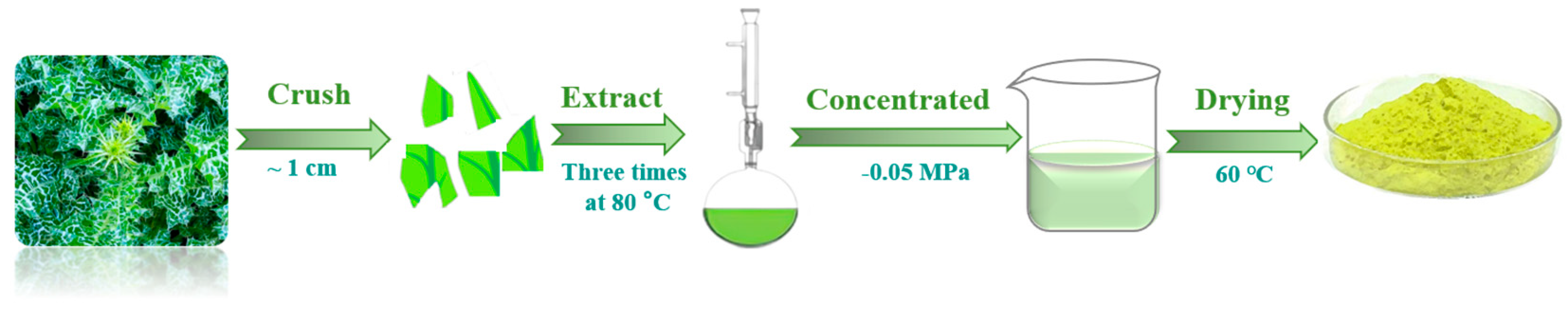
2.1. Preparation of Materials
2.2. Mass Loss Measurement
2.3. Electrochemical Measurements
2.4. Surface Characterizations
2.5. Theoretical Calculations
3. Results and Discussion
3.1. Corrosion Inhibition Performance
3.2. Morphology and Composition of Corrosion Products
3.3. Corrosion Inhibition Mechanism
4. Conclusions
- Waste leaves of Silybum marianum (L.) Gaertn can be utilized as raw materials to prepare novel green corrosion inhibitors for carbon steel in 0.5 mol/L H2SO4.
- SMGE showed superior corrosion inhibition ability for carbon steel in H2SO4 solution. The corrosion inhibition efficiency increased with the inhibitor dosage, and the maximum value reached 93.06% with the addition of 800 ppm SMGE and 60 ppm KI.
- SMGE can act as a mixed-type corrosion inhibitor and physico-chemically adsorb onto a metal surface, forming a hydrophobic protective film, thus protecting carbon steel from corrosion. The adsorption sites of inhibitor molecules for chemical bonding were mainly distributed on heteroatoms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials Science: Share Corrosion Data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, R.; Duan, J.; Greenfield, D.N. Corrosion Is a Global Menace to Crucial Infrastructure—Act to Stop the Rot Now. Nature 2024, 629, 41. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The Cost of Corrosion in China. Npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Zeng, C.; Zhou, Z.-Y.; Mai, Z.W.; Chen, Q.; He, J.; Liao, B. Exploration on the corrosion inhibition performance of Salvia miltiorrhiza extract as a green corrosion inhibitor for Q235 steel in HCl environment. J. Mater. Res. Technol. 2024, 32, 3857–3870. [Google Scholar] [CrossRef]
- Gao, L.; Cao, J.; Qiu, J.; Chen, L.; Yang, J.; Li, H.; Liao, B.; Guo, X. Effect of Sodium Dodecyl Sulfate Adsorption and Desorption Behavior on the Self-corrosion and Discharge Performances of AZ31B as Anode for Seawater Battery. J. Power Sources 2024, 617, 235115. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Liao, B.; Guo, X. Enhanced anti-corrosive capability of waterborne epoxy coating by ATT exfoliated boron nitride nanosheets composite fillers. Prog. Org. Coat. 2024, 186, 108089. [Google Scholar] [CrossRef]
- Min, X.; Ma, S.; Zhou, Z.; Wu, D.; Liao, B. Ti3 Alc2 Mxene Nanosheets as a Novel Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid Solution. RSC Adv. 2024, 14, 4335–4338. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.-F.; Liao, B.-K.; Guo, X.-P. Modified Nano-Lignin as a Novel Biomass-Derived Corrosion Inhibitor for Enhanced Corrosion Resistance of Carbon Steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Liao, B.-K.; Quan, R.-X.; Feng, P.-X.; Wang, H.; Wang, W.; Niu, L. Carbon Steel Anticorrosion Performance and Mechanism of Sodium Lignosulfonate. Rare Met. 2024, 43, 356–365. [Google Scholar] [CrossRef]
- Wan, S.; Wei, H.; Quan, R.; Luo, Z.; Wang, H.; Liao, B.; Guo, X. Soybean Extract Firstly Used as a Green Corrosion Inhibitor with High Efficacy and Yield for Carbon Steel in Acidic Medium. Ind. Crops Prod. 2022, 187, 115354. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, T.; Chen, H.; Liao, B.; Guo, X. Kapok Leaves Extract and Synergistic Iodide as Novel Effective Corrosion Inhibitors for Q235 Carbon Steel in H2SO4 Medium. Ind. Crops Prod. 2022, 178, 114649. [Google Scholar] [CrossRef]
- Liao, B.; Luo, Z.; Wan, S.; Chen, L. Insight into the Anti-Corrosion Performance of Acanthopanax Senticosus Leaf Extract as Eco-Friendly Corrosion Inhibitor for Carbon Steel in Acidic Medium. J. Ind. Eng. Chem. 2023, 117, 238–246. [Google Scholar] [CrossRef]
- Liao, B.; Ma, S.; Zhang, S.; Li, X.; Quan, R.; Wan, S.; Guo, X. Fructus Cannabis Protein Extract Powder as a Green and High Effective Corrosion Inhibitor for Q235 Carbon Steel in 1 M HCl Solution. Int. J. Biol. Macromol. 2023, 239, 124358. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Chen, H.; Zhang, T.; Liao, B.; Guo, X. Anti-Corrosion Mechanism of Parsley Extract and Synergistic Iodide as Novel Corrosion Inhibitors for Carbon Steel-Q235 in Acidic Medium by Electrochemical, Xps and Dft Methods. Front. Bioeng. Biotechnol. 2021, 9, 815953. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Zheng, H.; Liu, L.; Wu, X.; Zhao, C.; Zhou, X.; Sun, Y.; Yan, Z.; Li, X. Insight into Anti-Corrosion Behavior of Protein Extract as Eco–Friendly Corrosion Inhibitor. Sustain. Chem. Pharm. 2023, 34, 101177. [Google Scholar] [CrossRef]
- Tan, B.; Liu, Y.; Gong, Z.; Zhang, X.; Chen, J.; Guo, L.; Xiong, J.; Liu, J.; Marzouki, R.; Li, W. Pyracantha Fortuneana Alcohol Extracts as Biodegradable Corrosion Inhibitors for Copper in H2SO4 Media. J. Mol. Liq. 2024, 397, 124117. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum Marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Corchete, P. Silybum Marianum (L.) Gaertn: The Source of Silymarin. In Bioactive Molecules and Medicinal Plants; Springer: Berlin, Germany, 2008; pp. 123–148. [Google Scholar]
- Lucini, L.; Kane, D.; Pellizzoni, M.; Ferrari, A.; Trevisi, E.; Ruzickova, G.; Arslan, D. Phenolic Profile and in Vitro Antioxidant Power of Different Milk Thistle [Silybum Marianum (L.) Gaertn.] Cultivars. Ind. Crops Prod. 2016, 83, 11–16. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, M.A.; Fernández-Tárrago, J.; Corchete, P. Yeast Extract and Methyl Jasmonate-Induced Silymarin Production in Cell Cultures of Silybum Marianum (L.) Gaertn. J. Biotechnol. 2005, 119, 60–69. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of Milk Thistle (Silybum Marianum L. Gaertn.), a Medicinal Weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Sayyah, M.; Boostani, H.; Pakseresht, S.; Malayeri, A. Comparison of Silybum Marianum (L.) Gaertn. With Fluoxetine in the Treatment of Obsessive-Compulsive Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 362–365. [Google Scholar] [CrossRef] [PubMed]
- ASTM G103; Standard Practice for Evaluating Stress-Corrosion Cracking Resistance of Low Copper 7XXX Series Al-Zn-Mg-Cu Alloys in Boiling 6% Sodium Chloride Solution. ASTM: West Conshohocken, PA, USA, 2016.
- Elateeq, A.A.; Sun, Y.; Nxumalo, W.; Gabr, A.M. Biotechnological Production of Silymarin in Silybum Marianum L.: A Review. Biocatal. Agric. Biotechnol. 2020, 29, 101775. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.; Ebenso, E. Challenges and Advantages of Using Plant Extract as Inhibitors in Modern Corrosion Inhibition Systems: Recent Advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Seman, A.A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Sobri, S.A.; Ali, A.; et al. Plant Extracts as Green Corrosion Inhibitor for Ferrous Metal Alloys: A Review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Moschona, A.; Plesu, N.; Mezei, G.; Thomas, A.G.; Demadis, K.D. Corrosion Protection of Carbon Steel by Tetraphosphonates of Systematically Different Molecular Size. Corros. Sci. 2018, 145, 135–150. [Google Scholar] [CrossRef]
- Diaz-Ramos, M.; Roche, V.; Song, R.; Fan, H.; Bureau, C.; Lepretre, J.C. Electrochemical Impedance Spectroscopy (Eis) of Parylene Coated Magnesium Stents in Organic Solvent to Study Early Corrosion Control. Corros. Sci. 2023, 213, 110932. [Google Scholar] [CrossRef]
- Ferrah, D.; Renault, O.; Petit-Etienne, C.; Okuno, H.; Berne, C.; Bouchiat, V.; Cunge, G. Xps Investigations of Graphene Surface Cleaning Using H2-and Cl2-Based Inductively Coupled Plasma. In Proceedings of the 16th European Conference on Applications of Surface and Interface Analysis, Granada, Spain, 28 September–1 October 2015; Volume 48, pp. 451–455. [Google Scholar]
- Hu, C.; Ruan, R.; Wang, W.; Gao, A.; Xu, L. Electrochemical Grafting of Poly (Glycidyl Methacrylate) on a Carbon-Fibre Surface. RSC Adv. 2020, 10, 10599–10605. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Langell, M. Xps Analysis of Oleylamine/Oleic Acid Capped Fe3O4 Nanoparticles as a Function of Temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, P.; Wang, Q.; Huo, X.; Huang, X.; Ye, Q.; Xu, H.; Zhou, G.; Liu, Y.; Zhang, J. Fullerol Mediated Enhancement of Chloramphenicol Degradation In Fe(III)/H2O2 System by Accelerating Fe(III)/Fe(II) Cycle Via a Non-Photochemical Pathway. Chem. Eng. J. 2020, 402, 126176. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmahdy, A.M.; Abdel-Khalek, N.A.; El-Aref, M.; Elmanawi, A.E. Xps and Ftir Spectroscopic Study on Microwave Treated High Phosphorus Iron Ore. Appl. Surf. Sci. 2015, 345, 127–140. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, C.; Lu, H.; Gao, F.; Ma, H. Influence of Phytic Acid on the Corrosion Behavior of Iron under Acidic and Neutral Conditions. Electrochim. Acta 2014, 150, 188–196. [Google Scholar] [CrossRef]
- Nagaraju, P.; Srilakshmi, C.; Pasha, N.; Lingaiah, N.; Suryanarayana, I.; Prasad, P. Effect of P/Fe Ratio on the Structure and Ammoxidation Functionality of Fe-P-O Catalysts. Appl. Catal. A Gen. 2008, 334, 10–19. [Google Scholar] [CrossRef]
- Marshall-Roth, T.; Libretto, N.J.; Wrobel, A.T.; Anderton, K.J.; Pegis, M.L.; Ricke, N.D.; Voorhis, T.V.; Miller, J.T.; Surendranath, Y. A Pyridinic Fe-N4 Macrocycle Models the Active Sites in Fe/N-Doped Carbon Electrocatalysts. Nat. Commun. 2020, 11, 5283. [Google Scholar] [CrossRef]
- Lü, F.; Zhao, S.; Guo, R.; He, J.; Peng, X.; Bao, H.; Fu, J.; Han, L.; Qi, G.; Luo, J.; et al. Nitrogen-Coordinated Single Fe Sites for Efficient Electrocatalytic N2 Fixation in Neutral Media. Nano Energy 2019, 61, 420–427. [Google Scholar] [CrossRef]
- Li, X.; Qi, T.; Wang, J.; She, W.; Mao, G.; Yan, P.; Li, W.; Li, G. Enhanced Catalytic Performance of Nitrogen-Doped Carbon Supported Feox-Based Catalyst Derived from Electrospun Nanofiber Crosslinked N, Fe-Containing Mofs for Efficient Hydrogenation of Nitroarenes. Mol. Catal. 2019, 477, 110544. [Google Scholar] [CrossRef]
- Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-Containing Graphene Analyzed by X-Ray Photoelectron Spectroscopy. Carbon 2014, 70, 59–74. [Google Scholar] [CrossRef]
- Kan, Y.; Ning, G.; Ma, X. Sulfur-Decorated Nanomesh Graphene for High-Performance Supercapacitors. Chin. Chem. Lett. 2017, 28, 2277–2280. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Zi, F.; Hu, X.; Chen, S.; He, P.; Zhao, L.; Li, X.; Li, J.; Lin, Y.; et al. Making Untreated Carbon Effective in Cleaner Thiosulfate System: A New and High-Efficiency Method Including Gold Adsorption and Desorption. J. Clean. Prod. 2022, 334, 130185. [Google Scholar] [CrossRef]





| Concentration of Corrosion Inhibitor | W1 (g) | W2 (g) | ∆W (g) | ηw (%) |
|---|---|---|---|---|
| 0 | 11.3388 | 11.1251 | 0.2137 | -- |
| 60 ppm KI | 11.2362 | 11.0531 | 0.1831 | 14.05 |
| 50 ppm SMGE + 60 ppm KI | 11.2772 | 11.2063 | 0.0709 | 65.64 |
| 100 ppm SMGE + 60 ppm KI | 11.3704 | 11.3218 | 0.0486 | 76.14 |
| 200 ppm SMGE + 60 ppm KI | 11.3749 | 11.3312 | 0.0437 | 78.92 |
| 800 ppm SMGE + 60 ppm KI | 11.3283 | 11.2952 | 0.0330 | 84.52 |
| Concentration of Corrosion Inhibitor | Rs (Ω·cm2) | CPEdl − T (Sn·Ω−1·cm−2) | n | Rp (Ω·cm2) | σ2 | ηEIS (%) |
|---|---|---|---|---|---|---|
| 0 | 2.51 | 0.0020231 | 0.85 | 3.50 | 6.63 × 10−4 | - |
| 60 ppm KI | 2.38 | 0.0012002 | 0.93 | 4.04 | 4.63 × 10−4 | 13.36 |
| 50 ppm SMGE + 60 ppm KI | 3.19 | 0.0016129 | 0.86 | 4.11 | 1.08 × 10−4 | 14.91 |
| 100 ppm SMGE + 60 ppm KI | 3.12 | 0.00061973 | 0.84 | 14.55 | 4.84 × 10−4 | 75.92 |
| 200 ppm SMGE + 60 ppm KI | 2.94 | 0.00048374 | 0.86 | 14.94 | 1.93 × 10−4 | 76.55 |
| 800 ppm SMGE + 60 ppm KI | 2.11 | 0.00018701 | 0.93 | 22.96 | 2.47 × 10−4 | 84.74 |
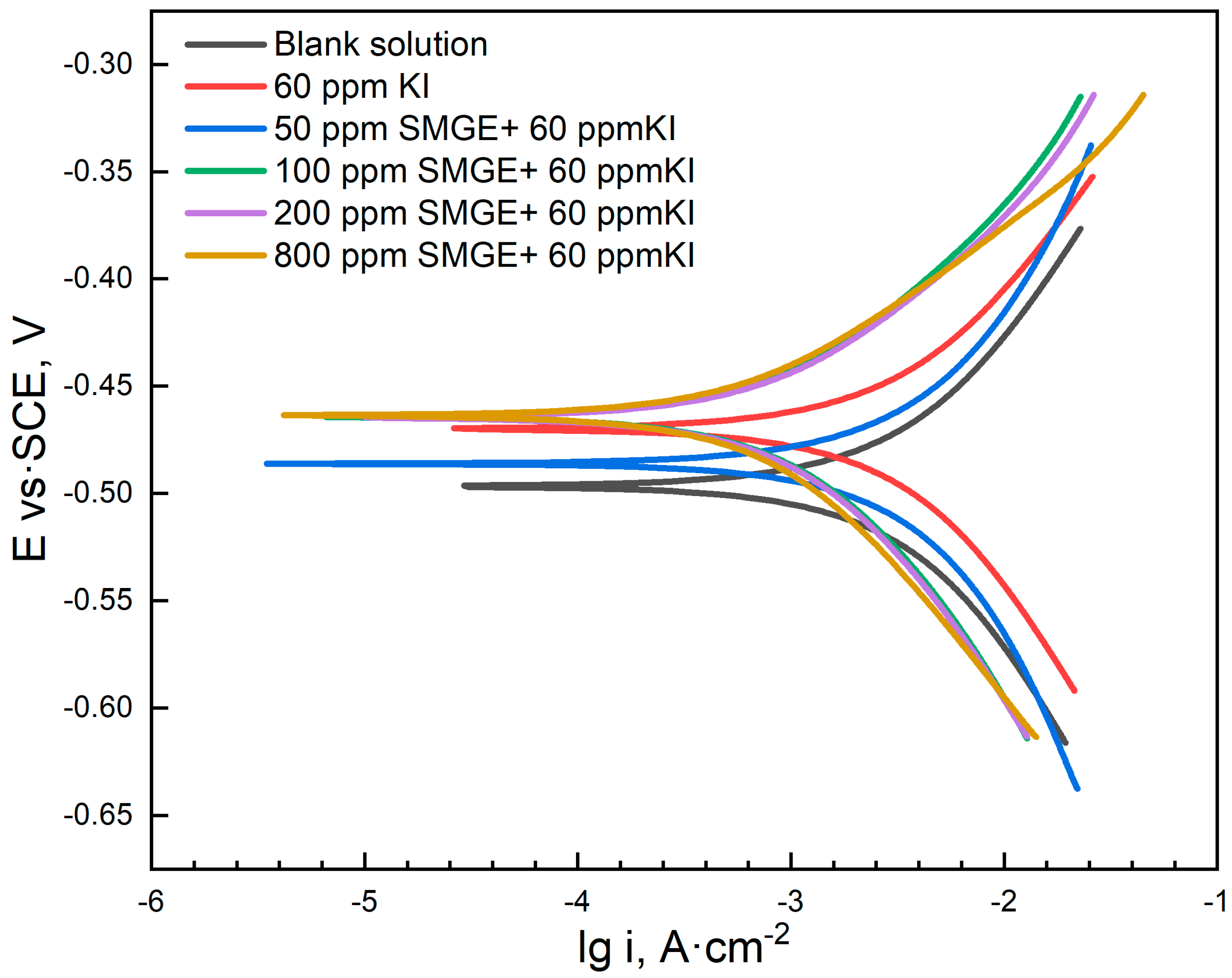
| Concentration of Corrosion Inhibitor | βa (mV/dec) | βc (mV/dec) | icorr (A/cm2) | Ecorr (V vs. SCE) | ηPDP (%) |
|---|---|---|---|---|---|
| 0 | 244 | −282 | 8.4718 × 10−3 | −0.4967 | -- |
| 60 ppm KI | 147 | −171 | 4.2634 × 10−3 | −0.4699 | 49.68 |
| 50 ppm SMGE + 60 ppm KI | 173 | −189 | 3.8892 × 10−3 | −0.4878 | 54.09 |
| 100 ppm SMGE + 60 ppm KI | 105 | −140 | 1.1764 × 10−3 | −0.4622 | 86.11 |
| 200 ppm SMGE + 60 ppm KI | 102 | −135 | 1.1443 × 10−3 | −0.4665 | 86.49 |
| 800 ppm SMGE + 60 ppm KI | 86 | −121 | 5.8882 × 10−4 | −0.4911 | 93.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, L.; He, J.; Sun, B. Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution. Materials 2024, 17, 4794. https://doi.org/10.3390/ma17194794
Wang Y, Li L, He J, Sun B. Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution. Materials. 2024; 17(19):4794. https://doi.org/10.3390/ma17194794
Chicago/Turabian StyleWang, Yubin, Lingjie Li, Jinbei He, and Baojiang Sun. 2024. "Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution" Materials 17, no. 19: 4794. https://doi.org/10.3390/ma17194794
APA StyleWang, Y., Li, L., He, J., & Sun, B. (2024). Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution. Materials, 17(19), 4794. https://doi.org/10.3390/ma17194794





