Mechanism and Performance Control Methods of Sulfate Attack on Concrete: A Review
Abstract
:1. Introduction
2. Mechanism of Concrete Deterioration under Sulfate Attack Conditions
2.1. Physical Attack
2.1.1. Solid Volume Expansion
2.1.2. Pressure of Crystalline Water
2.1.3. Pressure of Crystal Expansion
2.2. Chemical Attack
3. Influence of External Environmental Factors on the Sulfate Attack Resistance of Concrete
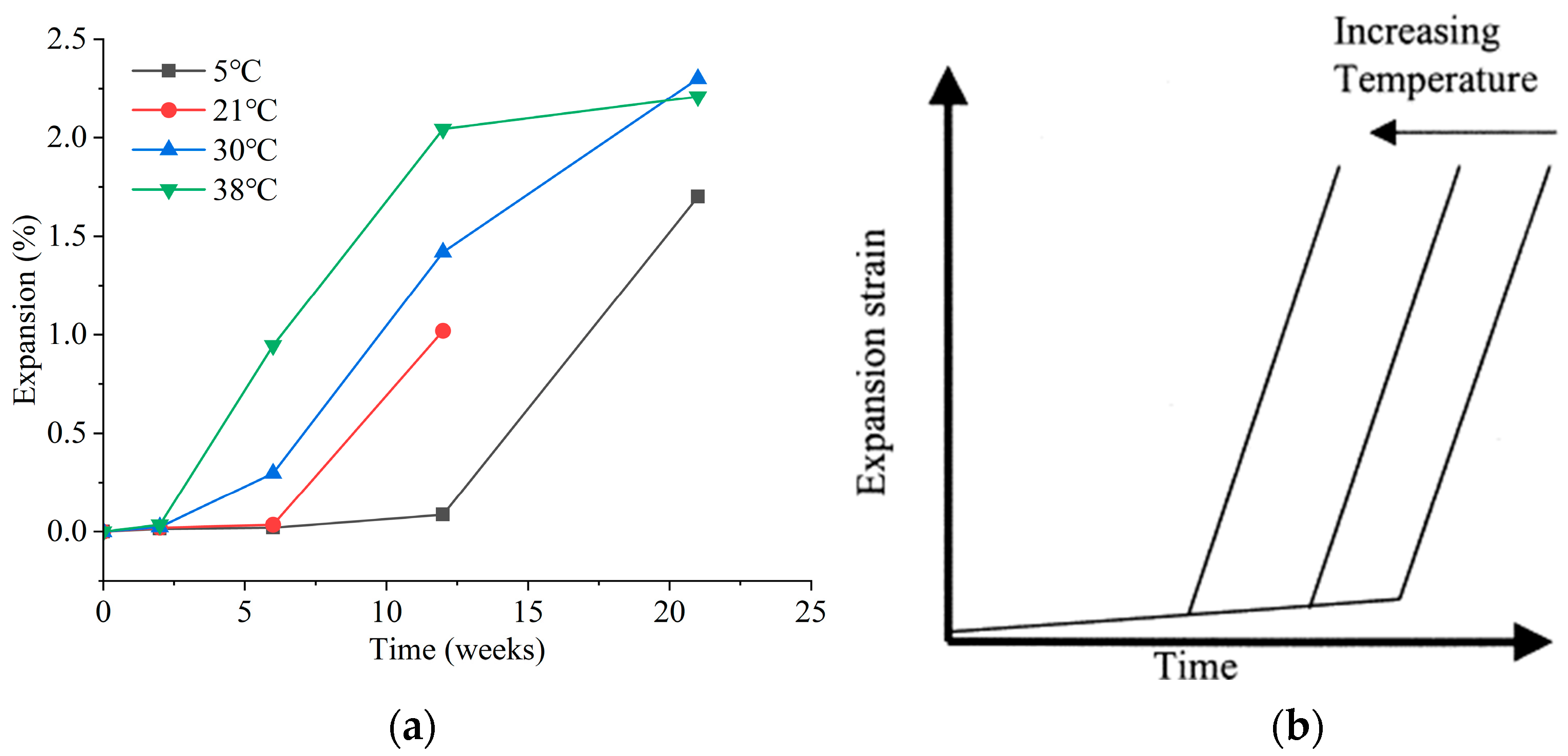
3.1. Environmental Temperature
3.2. Environmental Humidity
3.3. Concentration of Sulfate Solution
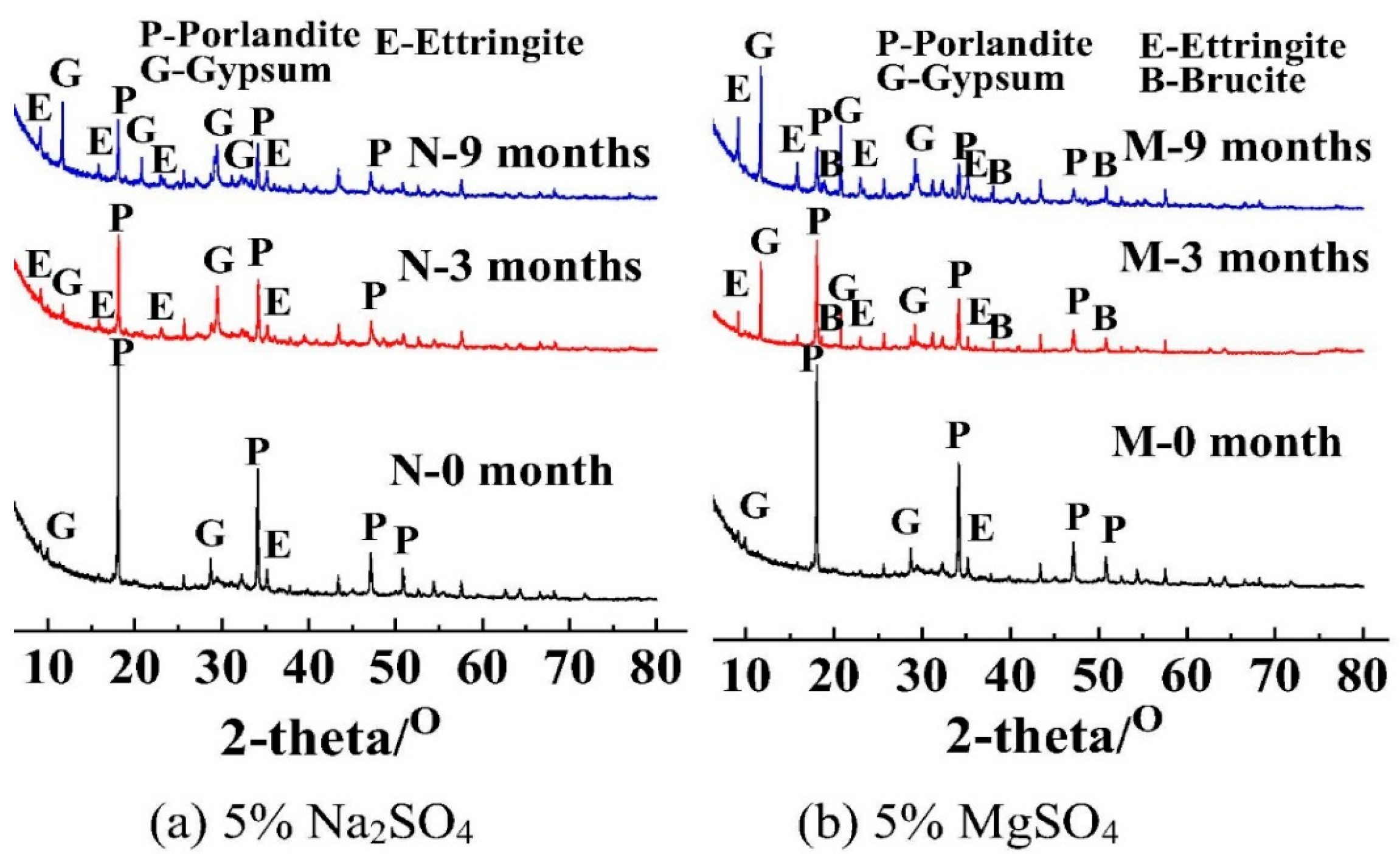
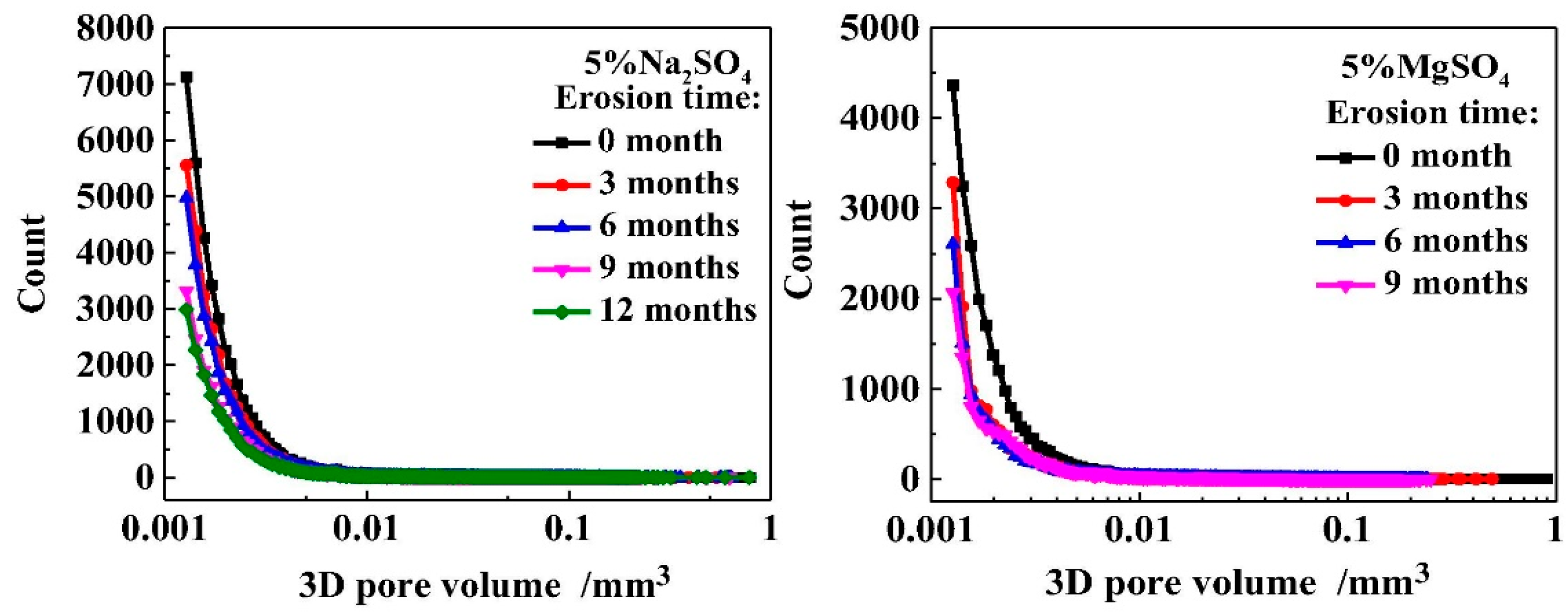
3.4. Types of Sulfates
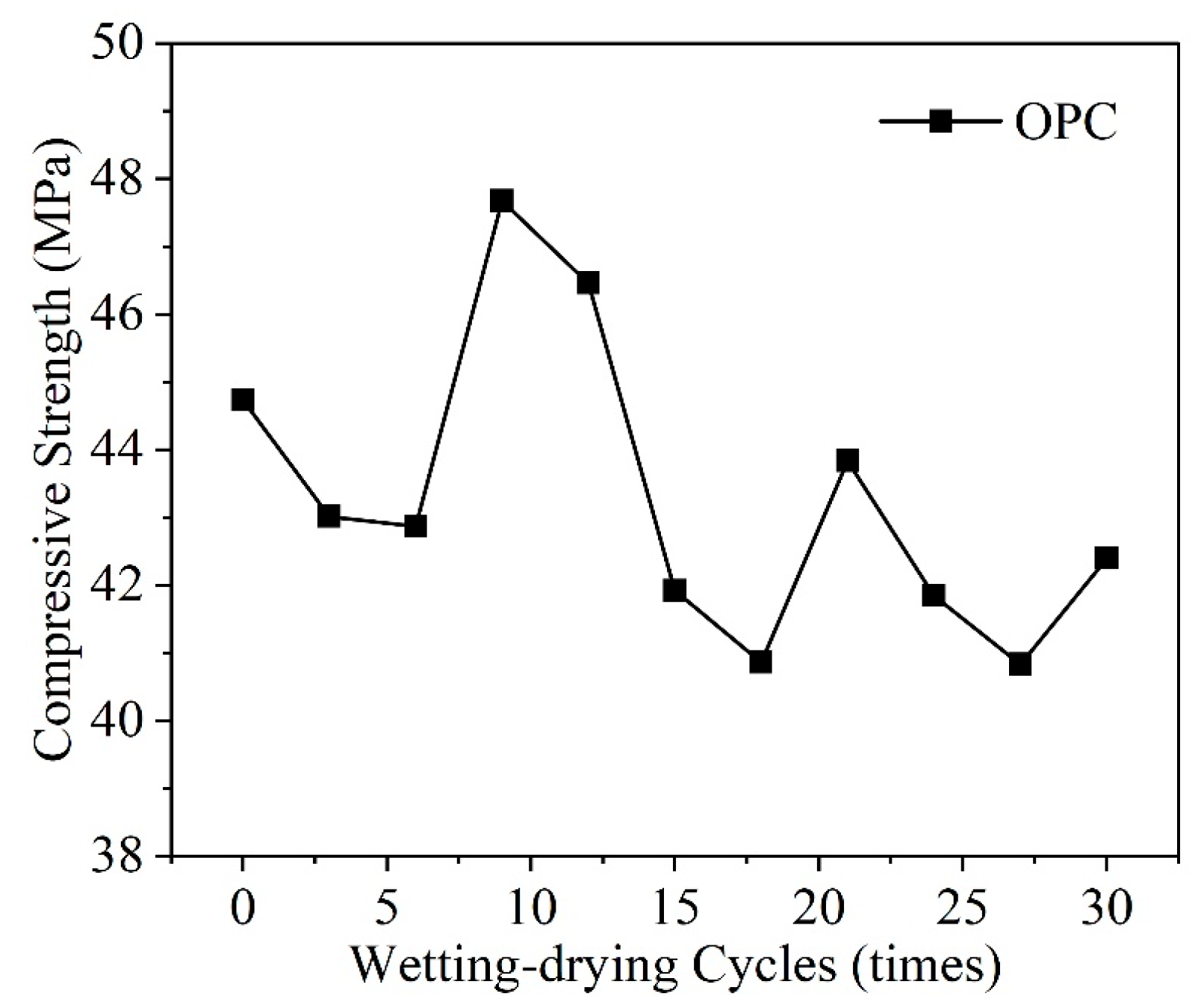
3.5. Wet-Dry Cycles
3.6. Freeze-Thaw Cycle
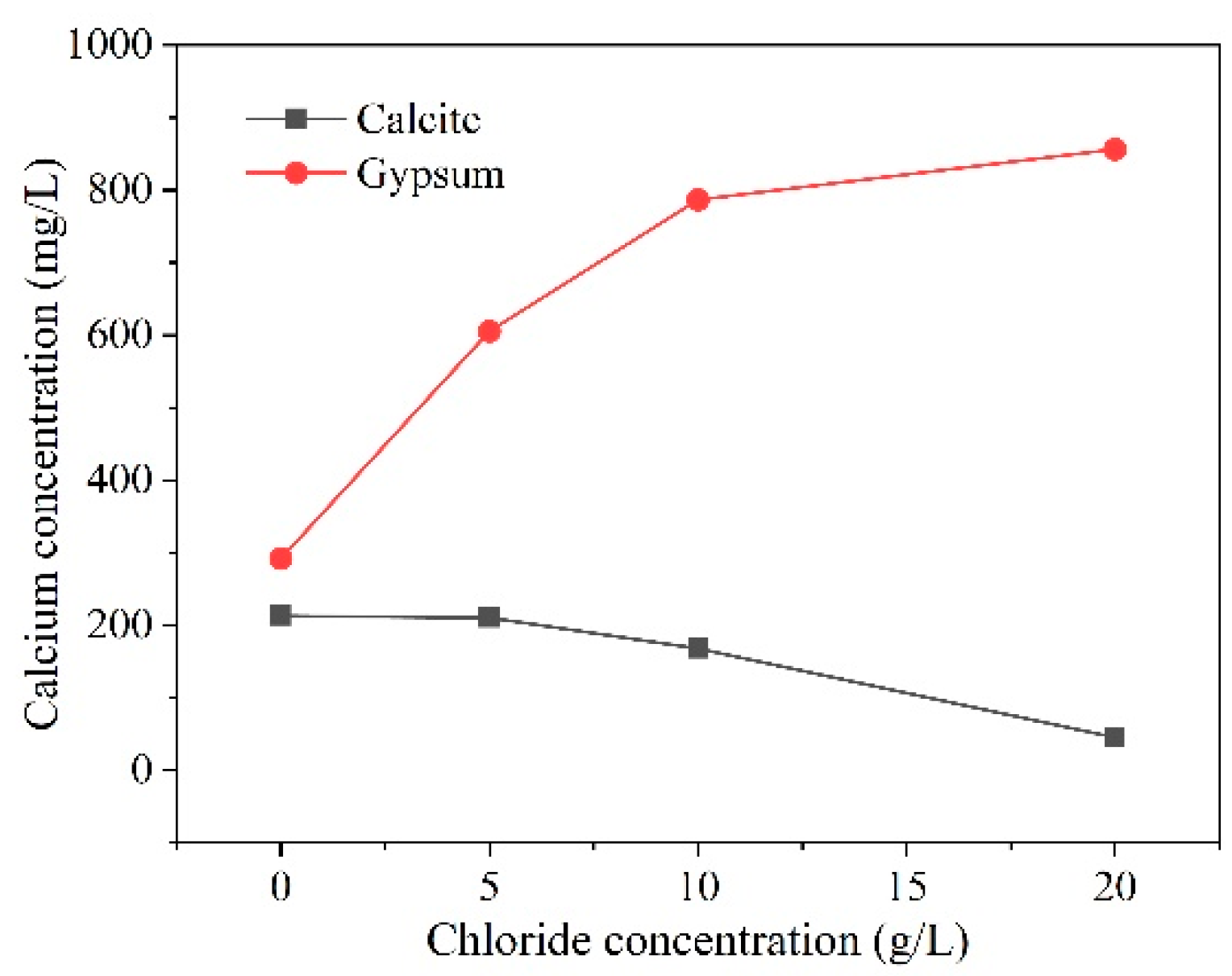
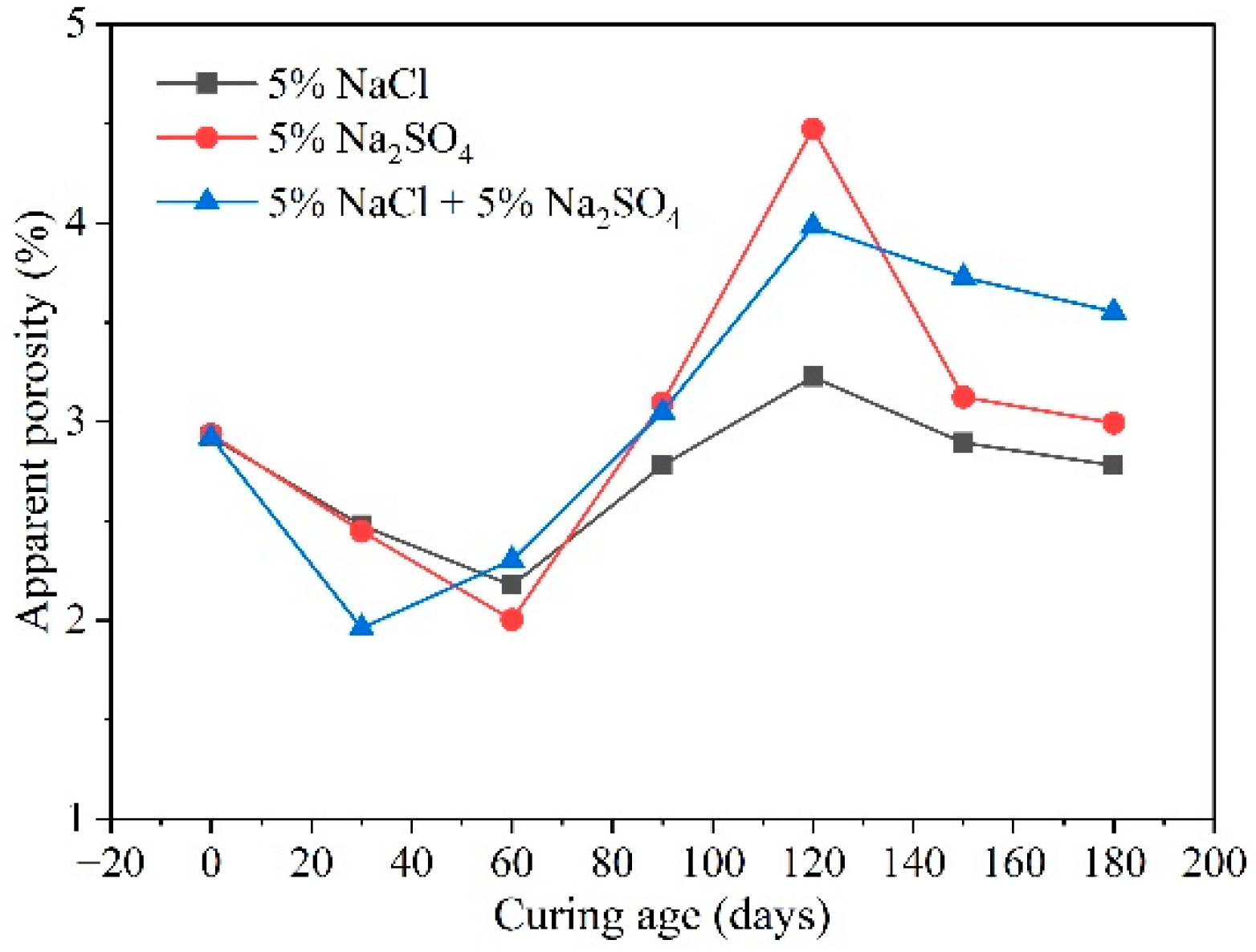
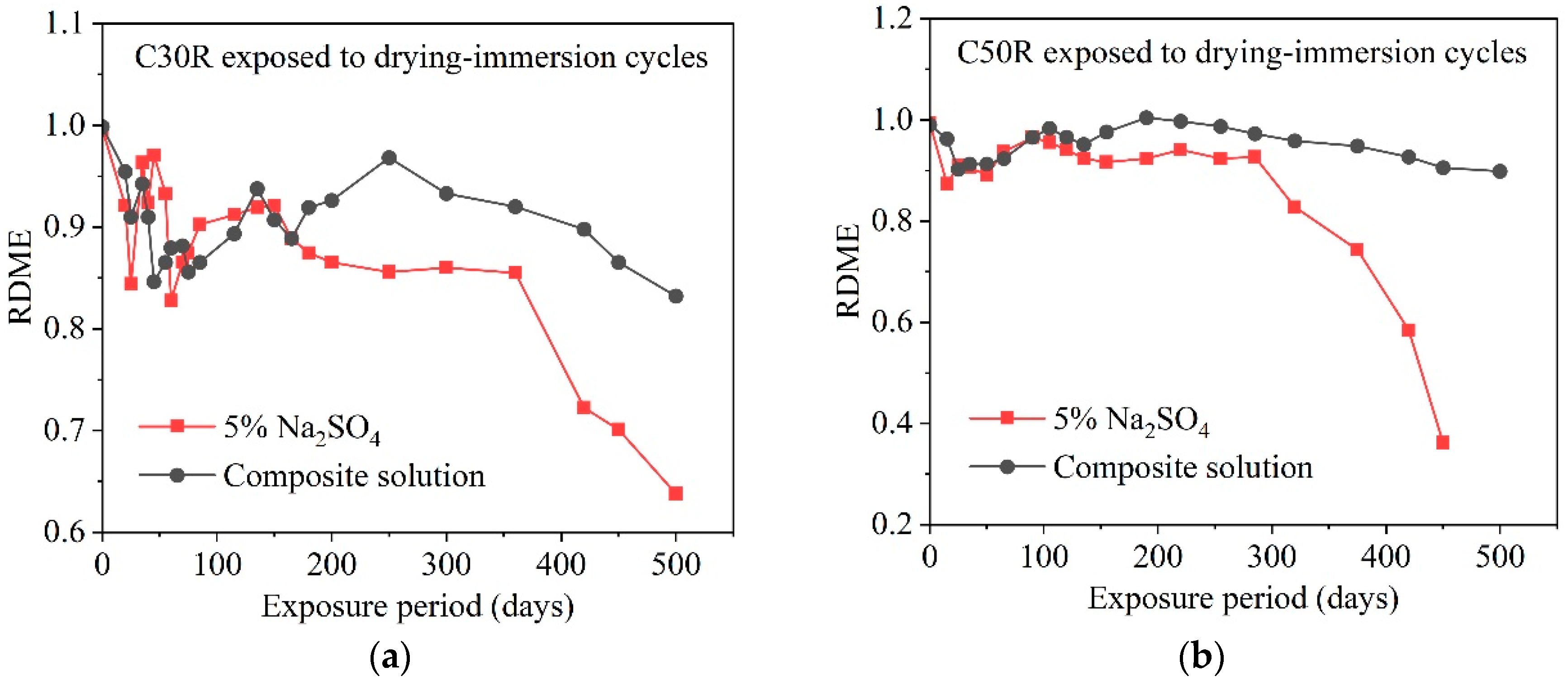
3.7. Chloride Coupling
3.8. Stray Current
4. Influence of Internal Environmental Factors on the Sulfate Attack Resistance of Concrete
4.1. Internal Sources of Corrosion
4.2. Cement Chemical Composition
4.3. Water-Cement Ratio
5. Methods to Improve Corrosion Resistance
5.1. Mineral Admixture
5.2. Surface Coating
5.3. Nanomaterials

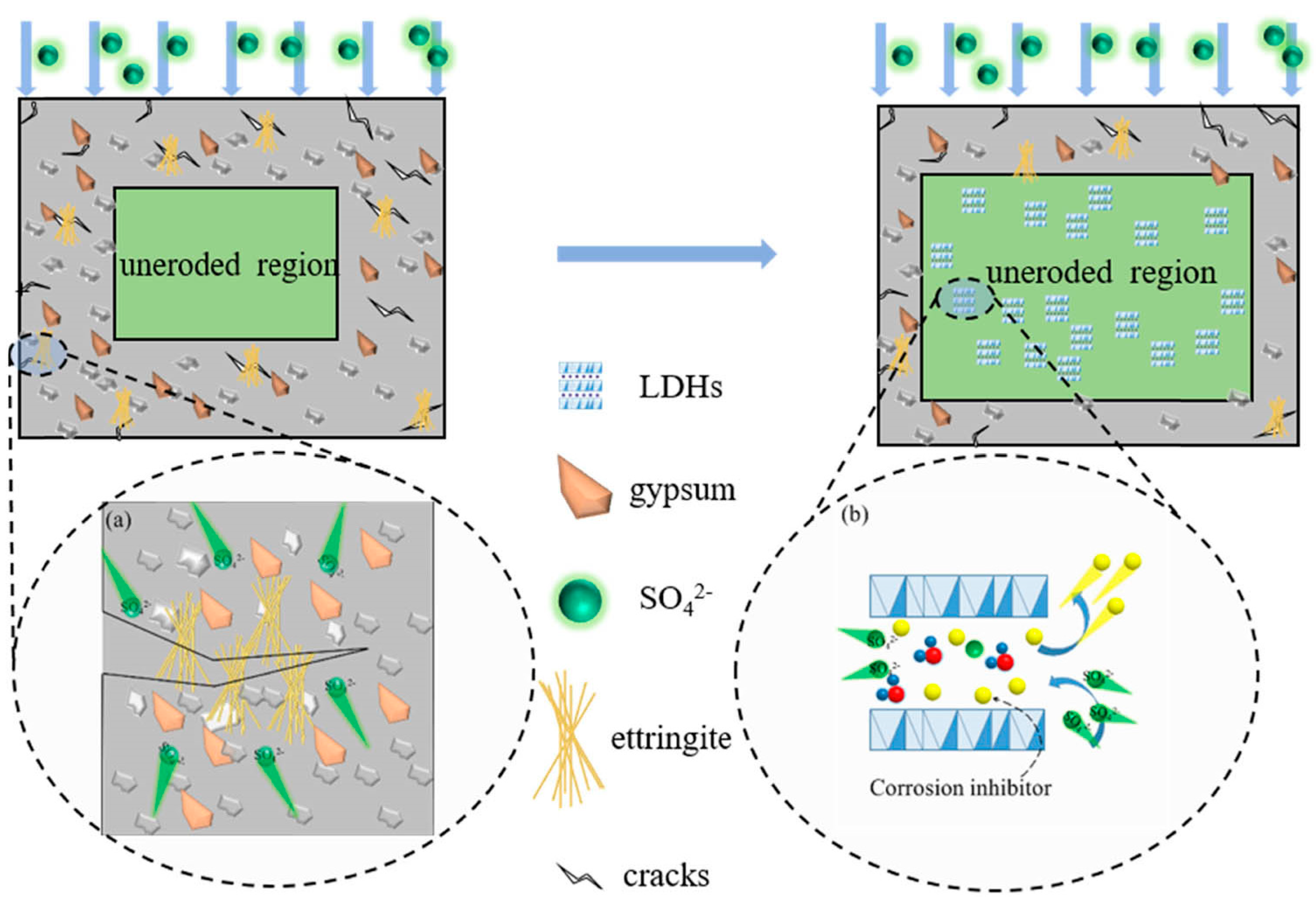
5.4. Layered Double Hydroxides (LDHs)
5.5. Geopolymers
6. Conclusions and Future Outlook
- (1)
- Currently, the mechanisms causing performance degradation in concrete due to sulfate attack are primarily divided into physical and chemical damage. Theories supporting physical damage suggest that the precipitation of sulfate crystals or volumetric expansion create pressure within the concrete, leading to deterioration of concrete properties. In contrast, chemical damage theories posit that SO42− ions penetrate the concrete’s pores, participating in hydration reactions and forming expansive products like ettringite and gypsum, which cause cracking. In practical engineering, the durability of cement-based materials is usually controlled by multiple factors; therefore, a deeper understanding of the coupling mechanisms between various factors under composite corrosion is essential.
- (2)
- Numerous environmental factors significantly influence the resistance of concrete structures to sulfate attack, and can even alter the mechanisms of sulfate attack in concrete. The effect of ambient temperature change on the production of expansion in sulfate damaged concrete shows a two-phase pattern. Firstly, variations in environmental humidity, sulfate solution concentration, and type can change the mechanisms and reaction products of sulfate attack in concrete, leading to different types of damage. Then, cycles of wetting-drying and freezing-thawing both accelerate the rate of sulfate attack in concrete, significantly speeding up performance deterioration. Moreover, the presence of chlorides can impact concrete deterioration to varying degrees. The presence of stray current will significantly accelerate the transmission of SO42−, which will have a negative impact on the performance of concrete.
- (3)
- The characteristics of concrete materials to some extent determine their resistance to sulfate attack. Internal sources of corrosion can lead to the early production of more degradation products, and the accumulation and embedding of these products can inhibit strength development and produce more cracks. Adjusting the content of various components in cement clinker or adding supplementary cementitious materials can enhance the sulfate resistance of concrete. At present, the effect of water-cement ratio on sulfate-corroded concrete is controversial. As geopolymer has a denser pore structure and more stable hydration products, these advantages result in a significantly higher salt corrosion resistance of geopolymer concrete than silicate cement concrete. There is a lack of research on the sulfate resistance of low-carbon cementitious materials such as magnesium-based cements (e.g., potassium magnesium phosphate cement), improved silicate cements (e.g., C3S2 cementitious materials), and limestone-calcined clay cements (LC3 cements), and the research and development of high-corrosion-resistant low-carbon cementitious materials is of great significance to the promotion of the sustainable development of the cement industry.
- (4)
- At present, relatively effective methods for improving the sulfate resistance of concrete that have attracted more attention from scholars mainly include the use of supplementary cementitious materials (SCM), nanomaterials, layered double hydroxides (LDHs), and coatings on concrete. There are three different mechanisms for improving the sulfate resistance of concrete by adding SCM. In addition, the type and proportion of auxiliary cementing materials will also affect the modification effect. The two modification methods, nanomaterials addition and concrete surface coating, mainly fill the internal pores of concrete and prevent the transport of aggressive ions to achieve the purpose of improving the performance of concrete. The modification mechanism of layered double hydroxides (LDHs) is to adsorb the aggressive ions inside the concrete. LDHs have a better modification effect, while SCM has the advantage that the material is easier to obtain and the price is lower.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Amoudi, O.S.B. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Yao, M.B.; Li, J.P. Distribution behavior of sulfate erosion in concrete piles under water pressure. J. Tongji Univ. (Nat. Sci.) 2019, 47, 1131–1136. [Google Scholar]
- Gao, J.M.; Yu, Z.X.; Song, L.G.; Wang, T.X.; Wei, S. Durability of concrete exposed to sulfate attack under flexural loading and drying-wetting cycles. Constr. Build. Mater. 2013, 39, 33–38. [Google Scholar] [CrossRef]
- Schneider, U.; Chen, S.W. The chemomechanical effect and the mechanochemical effect on high-performance concrete subjected to stress corrosion. Cem. Concr. Res. 1998, 28, 509–522. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Nehdi, M.L. Durability of self-consolidating concrete to sulfate attack under combined cyclic environments and flexural loading. Cem. Concr. Res. 2009, 39, 206–226. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhao, Y.T.; Yang, H.Y.; Xu, L.L. Research progress on resistance of calcium aluminate cement to seawater erosion. New Build. Mater. 2018, 45, 7–11. [Google Scholar]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.M.; Roussel, N.; Divet, L.; de Lacaillerie, J.B.D. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Sun, X.H.; Tang, Y.J. Macro-microscopically numerical analysis on expansion response of hardened cement paste under external sulfate attack. Constr. Build. Mater. 2019, 207, 600–615. [Google Scholar] [CrossRef]
- Wang, P.G.; Mo, R.; Sui, X.Y.; Tian, S.; Xu, J.; Jin, Z.Q. Chemo-Damage-Transport Model of Combined Chloride-sulfate attack in concrete. J. Chin. Ceram. Soc. 2022, 50, 512–521. [Google Scholar]
- Guo, J.J.; Liu, P.Q.; Wu, C.L.; Wang, K. Effect of Dry-Wet Cycle Periods on Properties of Concrete under Sulfate Attack. Appl. Sci. 2021, 11, 888. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Y.Z.; Liu, H.W.; Hu, T.F. Deterioration mechanism of recycled composite powder concrete under dry-wet cycles of sulfate. J. Build. Mater. 2022, 25, 1128–1135. [Google Scholar]
- Tian, W.; Gao, F.F. Damage and degradation of concrete under coupling action of freeze-thaw cycle and sulfate attack. Adv. Mater. Sci. Eng. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, S.L.; Xu, J.; Bai, J.J.; Quan, X.Y.; Xu, W.F. Durability and piezoresistivity of self-sensing cement mortar under sulfate freeze-thaw cycles. Bull. Chin. Ceram. Soc. 2022, 41, 777–786. [Google Scholar]
- Ikumi, T.; Segura, I.; Cavalaro, S.H.P. Effects of biaxial confinement in mortars exposed to external sulfate attack. Cem. Concr. Compos. 2019, 95, 111–127. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Han, F.; Shi, M.; Fan, H. Sulfate-induced degradation of cast-in-situ concrete influenced by magnesium. Constr. Build. Mater. 2019, 199, 194–206. [Google Scholar] [CrossRef]
- Thaulow, N.; Sahu, S. Mechanism of concrete deterioration due to salt crystallization. Mater. Charact. 2004, 53, 123–127. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Shearer, C.R.; Naser Rashid Reza, A.; Saha, A.K.; Khan, M.N.N.; Hossain, M.M.; Sarker, P.K. Improving the sulfate attack resistance of concrete by using supplementary cementitious materials (SCMs): A review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Chatterji, S.; Thaulow, N. Unambiguous demonstration of destructive crystal growth pressure. Cem. Concr. Res. 1997, 27, 811–816. [Google Scholar] [CrossRef]
- Flatt, R.J. Salt damage in porous materials: How high supersaturations are generated. J. Cryst. Growth 2002, 242, 435–454. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W. Hydration and crystallization pressure of sodium sulfate: A critical review. MRS Online Proc. Libr. (OPL) 2002, 712, 22. [Google Scholar] [CrossRef]
- Mehta, P.K. Evaluation of sulfate-resisting cements by a new test method. J. ACI 1975, 72, 573–575. [Google Scholar]
- Theoulakis, P.; Moropoulou, A. Salt crystal growth as weathering mechanism of porous stone on historic masonry. J. Porous Mater. 1999, 6, 345–358. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Liu, X.H.; Chen, Z.X.; Yu, Z.C.; Chen, P.; Zhang, Y.Z. A review on ultra-high performance seawater sea sand concrete: Hydration, microstructure and properties. Constr. Build. Mater. 2024, 438, 136945. [Google Scholar] [CrossRef]
- Yang, D.Y.; Guo, R. Experimental study on modulus and hardness of ettringite. Exp. Tech. 2014, 38, 6–12. [Google Scholar] [CrossRef]
- Collepardi, M. A state-of-the-art review on delayed ettringite attack on concrete. Cem. Concr. Compos. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Wu, P.Y. Test of Anti-sulfate erosion of cement mortar at different temperatures. J. Gansu Sci. 2020, 32, 83–88. [Google Scholar]
- Chen, D.; Yu, X.T.; Liao, Y.D.; Wang, Q.F.; Wang, X. Progress of study on sulfate attack on concrete materials. J. Chongqing Jiaotong Univ. (Nat. Sci.) 2016, 35, 24–30. [Google Scholar]
- Nie, S.F.; Ma, Y.X.; Guo, J.Q.; Chai, M.X.; Lu, S.B. Diffusion pattern of sulfate ions in concrete under different temperature conditions. Water Resour. Hydropower Eng. 2022, 55, 186–196. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Modeling the effects of solution temperature and concentration during sulfate attack on cement mortars. Cem. Concr. Res. 2002, 32, 585–592. [Google Scholar] [CrossRef]
- Fang, X.W.; Shen, C.N.; Yang, D.B.; Chen, Z.H.; Zhang, Z.F. Investigations of influence factor on the rate of concrete sulfate attack. J. Build. Mater. 2007, 10, 89–96. [Google Scholar]
- Ma, K.L. Mechanism and Evaluation Method of Salt Crystallization Attack on Concrete. Ph.D. Thesis, Central South University, Changsha, China, 2009. [Google Scholar]
- Nehdi, M.; Hayek, M. Behavior of blended cement mortars exposed to sulfate solutions cycling in relative humidity. Cem. Concr. Res. 2005, 35, 731–742. [Google Scholar] [CrossRef]
- Zhang, G.H. Sulfate corrosion of concrete structure review. Concrete 2012, 1, 49–54+61. [Google Scholar]
- Tixier, R.; Mobasher, B. Modeling of damage in cement-based materials subjected to external sulfate attack. II: Comparison with experiments. J. Mater. Civ. Eng. 2003, 15, 314–322. [Google Scholar] [CrossRef]
- Ou, X.; Jiao, C.J.; He, S.S.; Huang, W.S. Experimental study on resistance sulfate attack of plant concrete under the action of dry-wet circulation. Concrete 2022, 7, 174–177. [Google Scholar]
- Fan, H.J.; Xu, W.; Shen, L.; Wang, X.; Hu, X.Y.; Gao, C.; Zhang, H.W. Pore characteristics and simulation of mechanical behavior of concrete under uniaxial compression with sulfate attack. Bull. Chin. Ceram. Soc. 2022, 41, 818–824. [Google Scholar]
- Chen, Q. Study on the Experiments of Micro and Dynamic Properties of Concrete under the Seawater Erosion; Ningbo University: Ningbo, China, 2012. [Google Scholar]
- Yu, Z.X.; Gao, J.M.; Song, L.G.; Wang, X.T.; Xue, B.F.; Sun, W. Damage process of concrete exposed to sulfate attack under drying-wetting cycles and loading. J. Southeast Univ. (Nat. Sci. Ed.) 2012, 42, 487–491. [Google Scholar]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, G.Z.; Sun, D.S.; Liu, K.W. Progress of the mechanism of sulfate attack on cement-based materials. Mater. Rep. Rev. 2018, 32, 1174–1180. [Google Scholar]
- Yang, Y.G.; Zhang, Y.S.; She, W.; Liu, N.D.; Liu, Z.Y. In situ observing the erosion process of cement pastes exposed to different sulfate solutions with X-ray computed tomography. Constr. Build. Mater. 2018, 176, 556–565. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.T. Damage of concrete under sulfate attack exposed to different types of sulfate solution. Bull. Chin. Ceram. Soc. 2017, 36, 51–56. [Google Scholar]
- Xiong, C.S.; Jiang, L.H.; Song, Z.J.; Liu, R.G.; You, L.S.; Chu, H.Q. Influence of cation type on deterioration process of cement paste in sulfate environment. Constr. Build. Mater. 2014, 71, 158–166. [Google Scholar] [CrossRef]
- Bonakdar, A.; Mobasher, B.; Chawla, N. Diffusivity and micro-hardness of blended cement materials exposed to external sulfate attack. Cem. Concr. Compos. 2012, 34, 76–85. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.L.; Li, T.; Liu, B.; Li, B.B.; Zhou, Y. The mechanical properties of tailing recycled aggregate concrete and its resistance to the coupled deterioration of sulfate attack and wetting-drying cycles. Structures 2020, 27, 2208–2216. [Google Scholar] [CrossRef]
- Ren, J.G.; Lai, Y.M.; Bai, R.Q.; Qin, Y.H. The damage mechanism and failure prediction of concrete under wetting-drying cycles with sodium sulfate solution. Constr. Build. Mater. 2020, 264, 120525. [Google Scholar] [CrossRef]
- Li, J.P.; Xie, F.; Zhao, G.W.; Li, L. Experimental and numerical investigation of cast-in-situ concrete under external sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118789. [Google Scholar] [CrossRef]
- Xia, X.G. Static and Dynamic Mechanical Properties of Cement Soil under Dry-Wet Cycles and Sulfate Erosion; Anhui University of Science and Technology: Huainan, China, 2021. [Google Scholar]
- Wu, X.J.; Zhang, Y.B. Study on the sulfate erosion and dry-wet cycling effects on the mechanical performance and microstructure of concrete. Concrete 2021, 10, 10–13. [Google Scholar]
- Han, Y.D.; Liu, C.; Wang, Z.B.; Zuo, J.P.; Hong, Z.J. Uniaxial compressive behavior of ECC under sulfate erosion in drying-wetting cycles. J. Build. Mater. 2020, 23, 846–851. [Google Scholar]
- Wang, K.; Guo, J.J.; Yang, L.; Zhang, P.; Xu, H.Y. Multiphysical damage characteristics of concrete exposed to external sulfate attack: Elucidating effect of drying-wetting cycles. Constr. Build. Mater. 2022, 329, 127143. [Google Scholar] [CrossRef]
- Yang, Y.G. Deterioration Mechanism and Life Prediction of Damaged Concrete in Sulfate Environment. Ph.D. Thesis, Southeast University, Nanjing, China, 2019. [Google Scholar]
- Liu, F.; You, Z.P.; Xiong, R.; Yang, X. Effects of sodium sulfate attack on concrete incorporated with drying-wetting cycles. Adv. Civ. Eng. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.H.; Luo, T.; Zhou, M.Z.; Zhang, K.K.; Ma, W.W. Study on the deterioration of concrete under dry-wet cycle and sulfate attack. Materials 2020, 13, 4095. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Y.; Tan, Z.C.; Zhang, B.K. Investigating the failure process of concrete under the coupled actions between sulfate attack and drying-wetting cycles by using X-ray CT. Constr. Build. Mater. 2016, 108, 129–138. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, C.X.; Chen, H.C.; Liang, C.Y.; Kang, W.C. Calculation of expansion stresses and strains in concrete under sulfate crystallization attack in dry-wet cycles environments. J. Mater. Civ. Eng. 2021, 33, 04020479. [Google Scholar] [CrossRef]
- He, P.; Yu, J.Y.; Wan, Y.; Wang, R.Y.; Han, X.B.; Gu, S.J.; Liu, Q.T. Effect of ion chelator on microstructure and properties of cement-based materials under sulfate dry-wet cycle attack. Constr. Build. Mater. 2020, 257, 119527. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.T. Study on constitutive relation of concrete under sulfate attack and freeze-thaw environment. J. Sichuan Univ. (Eng. Sci. Ed.) 2016, 48, 71–78. [Google Scholar]
- Zhang, W.L.; Wang, L.Q.; Song, Y.H.; Tan, Y.W. Experimental study on the mechanical properties of grouted rock bolts subjected to sulfate attack and freeze-thaw cycling. Constr. Build. Mater. 2021, 291, 123391. [Google Scholar] [CrossRef]
- Li, Y. Study on the anti erosion of fly ash concrete under freezing and thawing environment. Highw. Eng. 2017, 42, 237–239+281. [Google Scholar]
- Xiao, Q.H.; Cao, Z.Y.; Guan, X.; Qiu, J.S. Degradation law of recycled concrete under the coupling of freeze—Thaw and sulfate erosion. Bull. Chin. Ceram. Soc. 2020, 39, 352–358. [Google Scholar]
- Abdalkader, A.H.M.; Lynsdale, C.J.; Cripps, J.C. The effect of chloride on cement mortar subjected to sulfate exposure at low temperature. Constr. Build. Mater. 2015, 78, 102–111. [Google Scholar] [CrossRef]
- Li, G.X.; Zhang, A.; Song, Z.P.; Liu, S.J.; Zhang, J.B. Ground granulated blast furnace slag effect on the durability of ternary cementitious system exposed to combined attack of chloride and sulfate. Constr. Build. Mater. 2018, 158, 640–648. [Google Scholar] [CrossRef]
- Ukpata, J.O.; Ogirigbo, O.R.; Black, L. Resistance of Concretes to External Chlorides in the Presence and Absence of Sulphates: A Review. Appl. Sci. 2023, 13, 182. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B.; Mohammed, M. The effect of chloride and sulfate ions on reinforcement corrosion. Cem. Concr. Res. 1993, 23, 139–146. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Abdul-Al, Y.A.B. Role of chloride ions on expansion and strength reduction in plain and blended cements in sulfate environments. Constr. Build. Mater. 1995, 9, 25–33. [Google Scholar] [CrossRef]
- Sun, H.F.; Zou, H.; Li, X.W.; Memon, S.A.; Yuan, B.Y.; Xing, F.; Zhang, X.G.; Ren, J. Combined Effects of Sulfate and Chloride Attack on Steel Reinforced Mortar under Drying-Immersion Cycles. Buildings 2022, 12, 1252. [Google Scholar] [CrossRef]
- Jin, Z.P.; Sun, W.; Zhang, Y.S.; Jiang, J.Y.; Lai, J.Z. Interaction between sulfate and chloride solution attack of concretes with and without fly ash. Cem. Concr. Res. 2007, 37, 1223–1232. [Google Scholar]
- Ran, B.; Omikrine-Metalssi, O.; Fen-Chong, T.; Dangla, P.; Li, K. Pore crystallization and expansion of cement pastes in sulfate solutions with and without chlorides. Cem. Concr. Res. 2023, 166, 107099. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Luo, C.Q.; Yang, C.H.; Luo, Y.L.; Xie, H. Effect of mineral admixtures on sulfate resistance of mortars under electrical field. Adv. Cem. Res. 2017, 29, 45–53. [Google Scholar] [CrossRef]
- Luo, Y.L.; Wang, C.; Luo, C.Q.; Huang, Q.; Wang, S.P.; Peng, X.Q. Effect of electrical field on TSA failure of cement-based materials. Cem. Concr. Res. 2016, 90, 19–26. [Google Scholar] [CrossRef]
- Lorente, S.; Yssorche-Cubaynes, M.P.; Auger, J. Sulfate transfer through concrete: Migration and diffusion results. Cem. Concr. Compos. 2011, 33, 735–741. [Google Scholar] [CrossRef]
- Campos, A.; López, C.M.; Aguado, A. Diffusion–reaction model for the internal sulfate attack in concrete. Constr. Build. Mater. 2016, 102, 531–540. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Shi, M.; Fan, H.; Cui, J.; Xie, F. Degradation mechanisms of cast-in-situ concrete subjected to internal-external combined sulfate attack. Constr. Build. Mater. 2020, 248, 118683. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, D.; Hafiz, R.B.; Han, Q.; Ma, H. Effects of internally introduced sulfate on early age concrete properties: Active acoustic monitoring and molecular dynamics simulation. Constr. Build. Mater. 2018, 188, 1014–1024. [Google Scholar] [CrossRef]
- Gomez, V.G.; Garcia, A.C.; Castillo, J.G.; Roig, I.B.; Rosado, M.V.B.; Bernabeu, J.J.P. Optimized ultrasonic attenuation measures for internal sulphate attack monitoring in portland cement mortars. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- González, M.A.; Irassar, E.F. Ettringite formation in low C3A portland cement exposed to sodium sulfate solution. Cem. Concr. Res. 1997, 27, 1061–1071. [Google Scholar] [CrossRef]
- Tosun-Felekoğlu, K. The effect of C3A content on sulfate durability of Portland limestone cement mortars. Constr. Build. Mater. 2012, 36, 437–447. [Google Scholar] [CrossRef]
- Huang, X.; Hu, S.G.; Wang, F.Z.; Yang, L.; Rao, M.J.; Tao, Y. Enhanced Sulfate Resistance: The Importance of Iron in Aluminate Hydrates. ACS Sustain. Chem. Eng. 2019, 7, 6792–6801. [Google Scholar] [CrossRef]
- Wan, D.W.; Zhang, W.Q.; Tao, Y.; Wan, Z.H.; Wang, F.Z.; Hu, S.G.; He, Y.J. The impact of Fe dosage on the ettringite formation during high ferrite cement hydration. J. Am. Ceram. Soc. 2021, 104, 3652–3664. [Google Scholar] [CrossRef]
- Huang, X.; Hu, S.G.; Wang, F.Z.; Yang, L.; Rao, M.J.; Mu, Y.D.; Wang, C.X. The effect of supplementary cementitious materials on the permeability of chloride in steam cured high-ferrite Portland cement concrete. Constr. Build. Mater. 2019, 197, 99–106. [Google Scholar] [CrossRef]
- Han, S.W.; Zhong, J.; Yu, Q.S.; Yan, L.; Ou, J.P. Sulfate resistance of eco-friendly and sulfate-resistant concrete using seawater sea-sand and high-ferrite Portland cement. Constr. Build. Mater. 2021, 305, 124753. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M.S.H.; Mahmood, A.H. Durability of calcium aluminate and sulphate resistant Portland cement based mortars in aggressive sewer environment and sulphuric acid. Cem. Concr. Res. 2019, 124, 105852. [Google Scholar] [CrossRef]
- Guo, Z.H.; Hou, P.K.; Xu, Z.H.; Gao, J.M.; Zhao, Y.S. Sulfate attack resistance of tricalcium silicate modified with nano-silica and supplementary cementitious materials. Constr. Build. Mater. 2022, 321, 126332. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.C.; Liu, H.Y. An overview on sulfate corrosion of cement concrete. Mater. Rep. 2013, 27, 67–71. [Google Scholar]
- Long, G.C.; Xie, Y.J.; Tang, X.Q.A. Evaluating deterioration of concrete by sulfate attack. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2007, 22, 572–576. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, X.; Luo, W. Long-term behaviors of concrete under low-concentration sulfate attack subjected to natural variation of environmental climate conditions. Cem. Concr. Res. 2019, 116, 217–230. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Pei, M.; Li, Y.L.; Yuan, Q. Sulfate attacks on uncarbonated fly ash plus cement pastes partially immersed in Na2SO4 solution. Materials 2020, 13, 4920. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.M. Performance of plain and blended cements exposed to varying concentrations of sodium sulphate. Adv. Cem. Res. 2007, 19, 177–184. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Zhao, M.L.; Wang, S.Q.; Li, A.X. The study on sulfate resistance of concrete containing admixture mineral. Appl. Mech. Mater. 2012, 174–177, 1265–1268. [Google Scholar] [CrossRef]
- Shi, Z.G.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate resistance of calcined clay—Limestone—Portland cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Sezer, G.I.; Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar] [CrossRef]
- Almoudi, O.S.B.; Maslehuddin, M.; Saadi, M.M. Effects of magnesium-sulfate and sodium-sulfate on the durability performance of plain and blended cements. ACI Mater. J. 1995, 92, 15–24. [Google Scholar]
- Ye, Q.; Zhang, Z.N.; Kong, D.Y.; Chen, R.S. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar]
- Mukharjee, B.B.; Barai, S.V. Assessment of the influence of Nano-Silica on the behavior of mortar using factorial design of experiments. Constr. Build. Mater. 2014, 68, 416–425. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.G.; Yuan, J.; Ou, J.P. Microstructure of cement mortar with nano-particles. Compos. Part B Eng. 2004, 35, 185–189. [Google Scholar] [CrossRef]
- Cao, H.T.; Bucea, L.; Ray, A.; Yozghatlian, S. The effect of cement composition and pH of environment on sulfate resistance of portland cements and blended cements. Cem. Concr. Compos. 1997, 19, 161–171. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface treatments for concrete: Assessment methods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Huang, B.; Deng, D.H.; Liao, N.F.; Du, H.W.; Zhong, L. Improvement of concrete resistance to sulfate attack by rmo-cement paste coating. Ind. Constr. 2011, 41, 23–26. [Google Scholar]
- Nia, S.M.; Othman, F. Evaluation of organic coating materials efficiency in sewage concrete pipe. In Proceeding of the International Conference for Technical Postgraduates (TECHPOS 2009), Kuala Lumpur, Malaysia, 14–15 December 2009. [Google Scholar]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Price, A.R. A field trial of waterproofing systems for concrete bridge decks. In Protection of Concrete: Proceedings of the International Conference, Scotland, UK, 11–13 September 1990; University of Dundee: Scotland, UK, 1990. [Google Scholar]
- Sharkawi, A.M.; Abd-Elaty, M.A.; Khalifa, O.H. Synergistic influence of micro-nano silica mixture on durability performance of cementious materials. Constr. Build. Mater. 2018, 164, 579–588. [Google Scholar] [CrossRef]
- Zhang, A.; Ge, Y.; Du, S.; Wang, G.; Chen, X.; Liu, X.; Li, H. Durability effect of nano-SiO2/Al2O3 on cement mortar subjected to sulfate attack under different environments. J. Build. Eng. 2023, 64, 105642. [Google Scholar] [CrossRef]
- Xu, C.; Liao, H.H.; Chen, Y.L.; Du, X.; Peng, B.; Fernandez-Steeger, T.M. Corrosion performance of nano-TiO2-modified concrete under a dry–wet sulfate environment. Materials 2021, 14, 5900. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Hakuzweyezu, T.; Yang, B.; Li, K. Experimental study on sulfate erosion resistance of nano-CaCO3 modified concrete. Can. J. Civ. Eng. 2022, 49, 590–596. [Google Scholar] [CrossRef]
- Lu, J.G.; Pei, W.S.; Zhang, M.Y.; Wan, X.S.; Zhang, J.C.; Wang, Y.D. Coupled effect of the freeze-thaw cycles and salt erosion on the performance of concretes modified with nanoparticles. Cold Reg. Sci. Technol. 2024, 217, 104046. [Google Scholar] [CrossRef]
- Li, D.M.; Wang, H.Z.; Wang, L.Q.; Zhao, Z.P. Removal of sulfate from aqueous solution by adsorption of it on layered double hydroxides. ACTA Mineral. Sin. 2007, 27, 109–114. [Google Scholar]
- Zhai, M.Y.; Zhao, J.H.; Wang, D.M.; Gao, X.; Wang, Q.B.; Li, Z.H.; Zhang, M. Layered double hydroxides (LDHs) modified cement-based materials: A systematic review. Nanotechnol. Rev. 2022, 11, 2857–2874. [Google Scholar] [CrossRef]
- Parker, L.M.; Milestone, N.B.; Newman, R.H. The use of hydrotalcite as an anion absorbent. Ind. Eng. Chem. Res. 1995, 34, 1196–1202. [Google Scholar] [CrossRef]
- Tatematsu, H.; Nakamura, T.; Koshimizu, H.; Morishita, T.; Kotaki, H. Cement-Additive for Inhibiting Concrete-Deterioration. U.S. Patent 5,435,864, 25 July 1995. [Google Scholar]
- Tsujimura, A.; Uchida, M.; Okuwaki, A. Synthesis and sulfate ion-exchange properties of a hydrotalcite-like compound intercalated by chloride ions. J. Hazard. Mater. 2007, 143, 582–586. [Google Scholar] [CrossRef]
- Guimarães, D.; da Rocha, N.C.; de Morais, R.A.; Resende, A.D.-L.B.; Lima, R.M.; da Costa, G.M.; Leão, V.A. Precipitation of a layered double hydroxide comprising Mg2+ and Al3+ to remove sulphate ions from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 102815. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.Y.; Duan, P.; Zhang, Z.H. Improving sulfate attack resistance of concrete by using calcined Mg-Al-CO3 LDHs: Adsorption behavior and mechanism. Constr. Build. Mater. 2020, 232, 117256. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H. Compressive stress–strain behavior of slag-based alkali-activated seawater coral aggregate concrete after exposure to seawater environments. Constr. Build. Mater. 2023, 367, 130294. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, B. Research progress on the durability of geopolymer concrete. J. Chang. Univ. Sci. Technol. Nat. Sci. 2023, 20, 1–24. [Google Scholar]
- Saavedra, W.G.V.; Angulo, D.E.; de Gutiérrez, R.M. Fly ash slag geopolymer concrete: Resistance to sodium and magnesium sulfate attack. J. Mater. Civ. Eng. 2016, 28, 04016148. [Google Scholar] [CrossRef]
- Özcan, A.; Karakoç, M.B. Evaluation of sulfate and salt resistance of ferrochrome slag and blast furnace slag-based geopolymer concretes. Struct. Concr. 2019, 20, 1607–1621. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Sulfate resistance of alkali-activated slag and Portland cement based reactive powder concrete. J. Build. Eng. 2021, 43, 103205. [Google Scholar] [CrossRef]
- Özdal, M.; Karakoç, M.B.; Özcan, A. Investigation of the properties of two different slag-based geopolymer concretes exposed to freeze-thaw cycles. Struct. Concr. 2019, 22, 332–340. [Google Scholar] [CrossRef]
- Pan, J.Y.; Jiang, Y.; Wang, L.M.; Ding, F.Y.; Guo, L.; Duan, P. Sulfate attack resistance of sisal-pva hybrid fiber reinforced geopolymer. Bull. Chin. Ceram. Soc. 2020, 39, 1430–1437+1443. [Google Scholar]

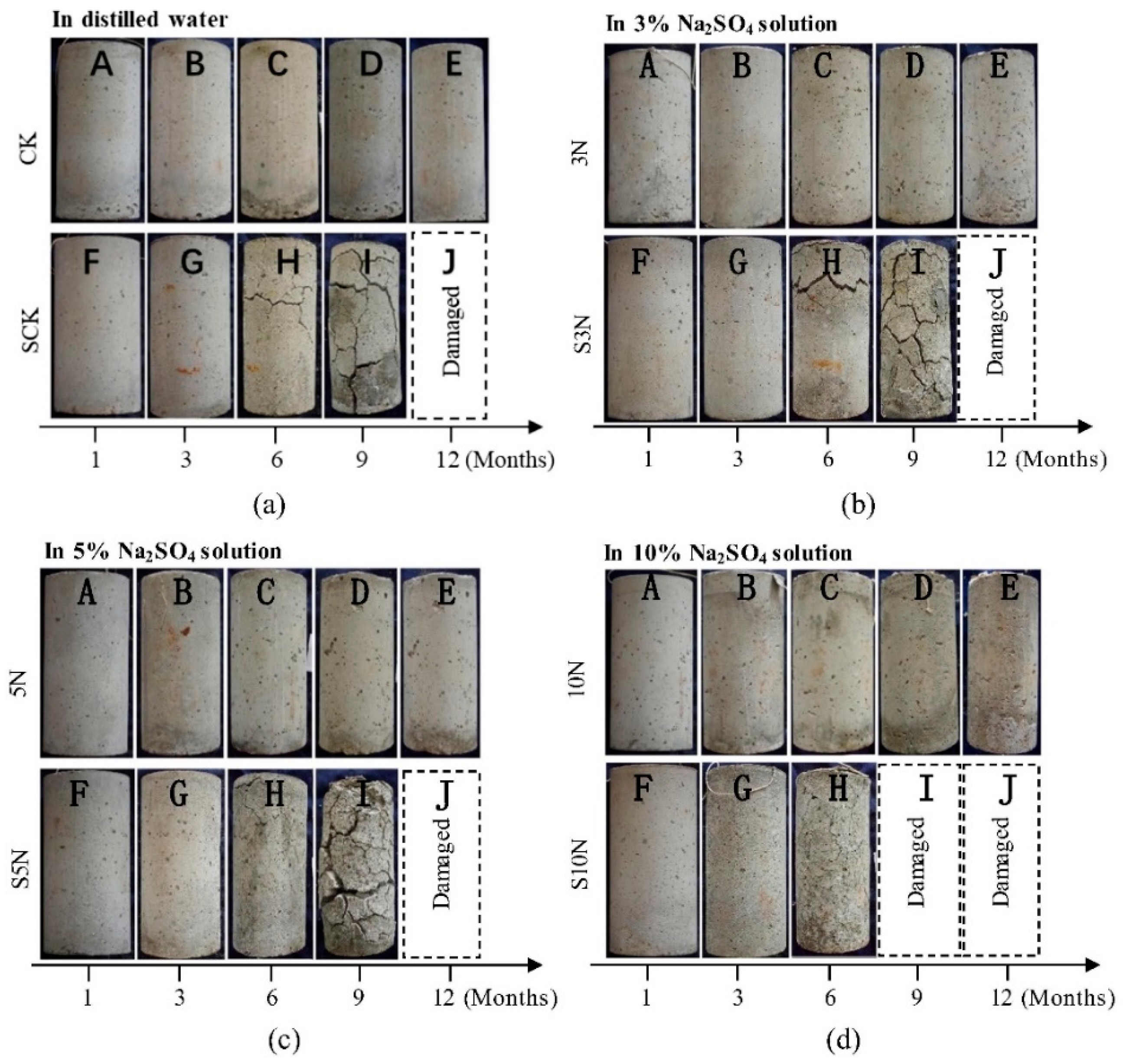
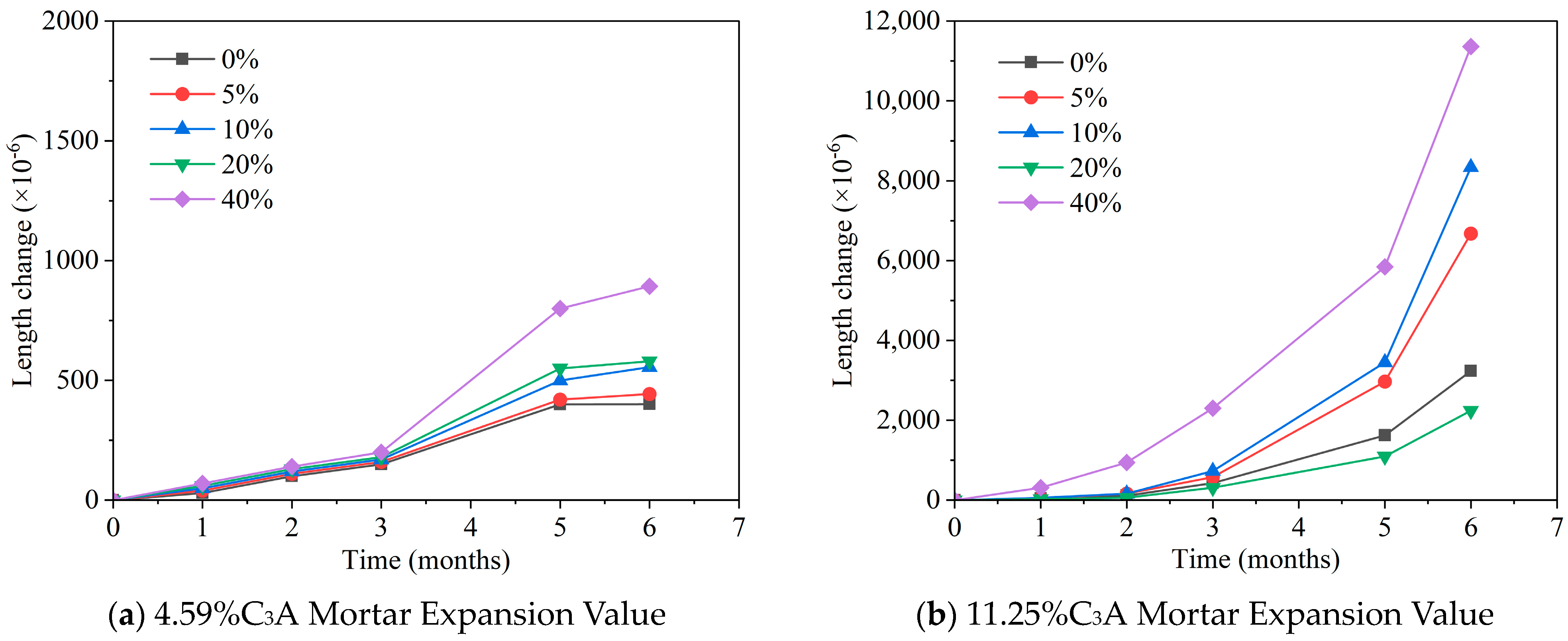
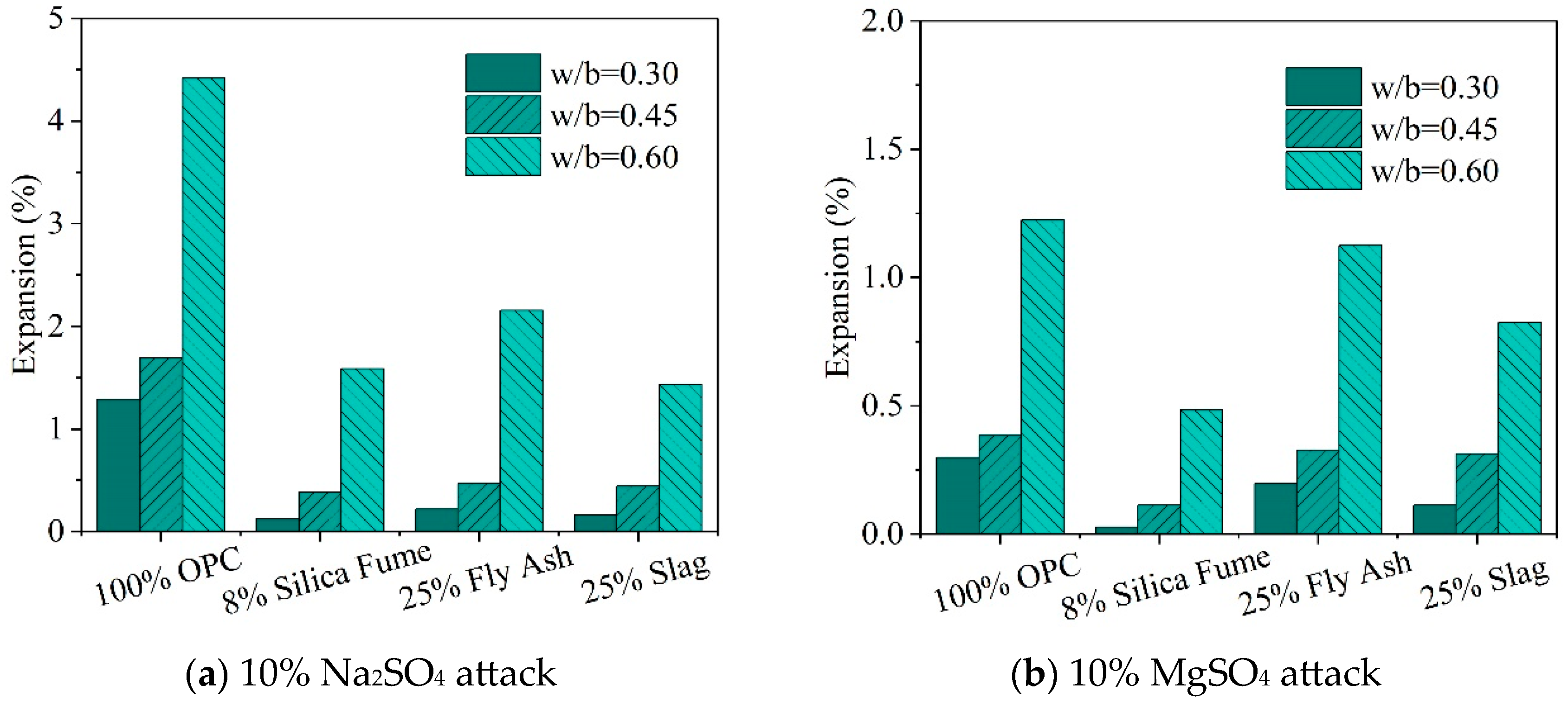
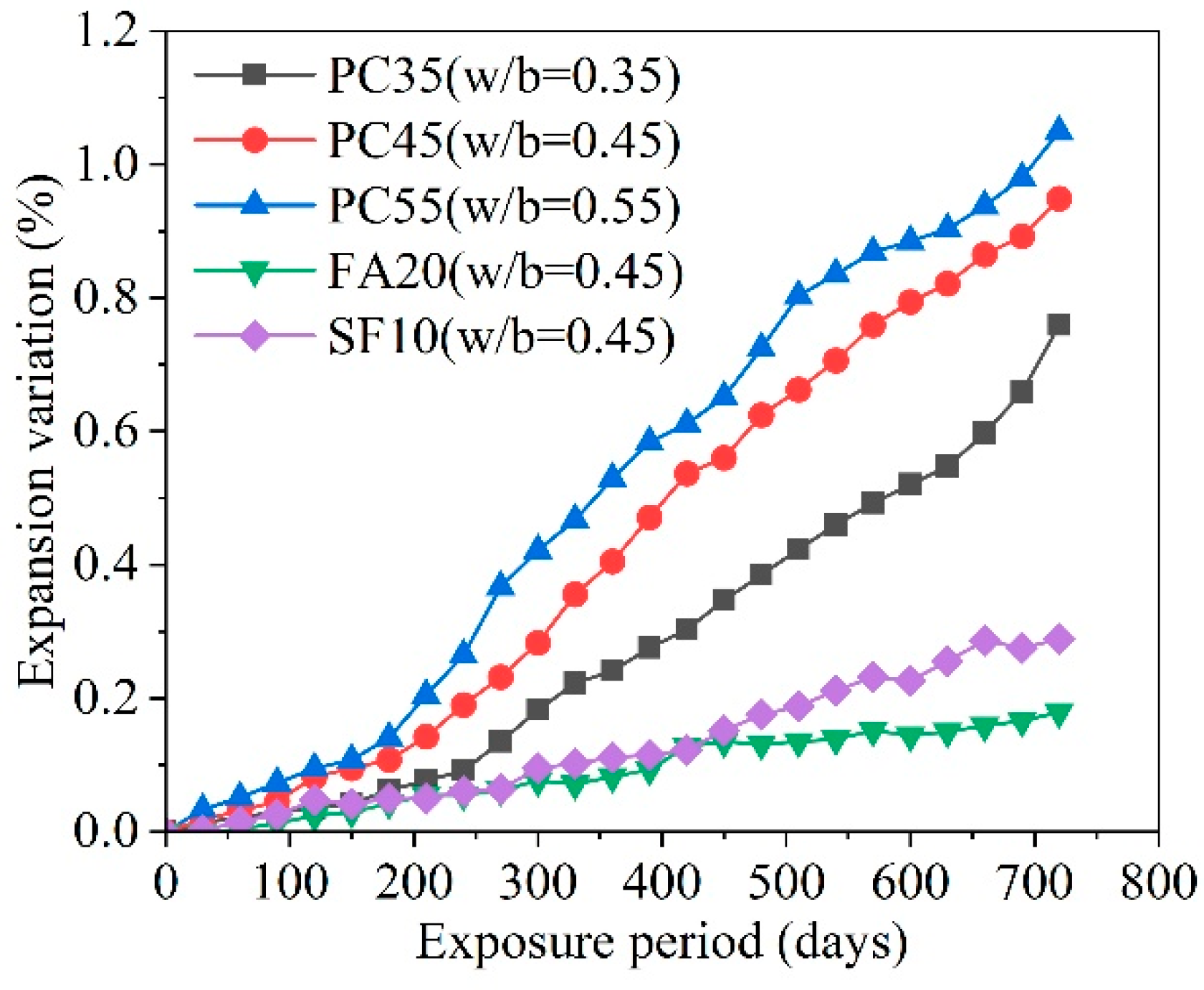
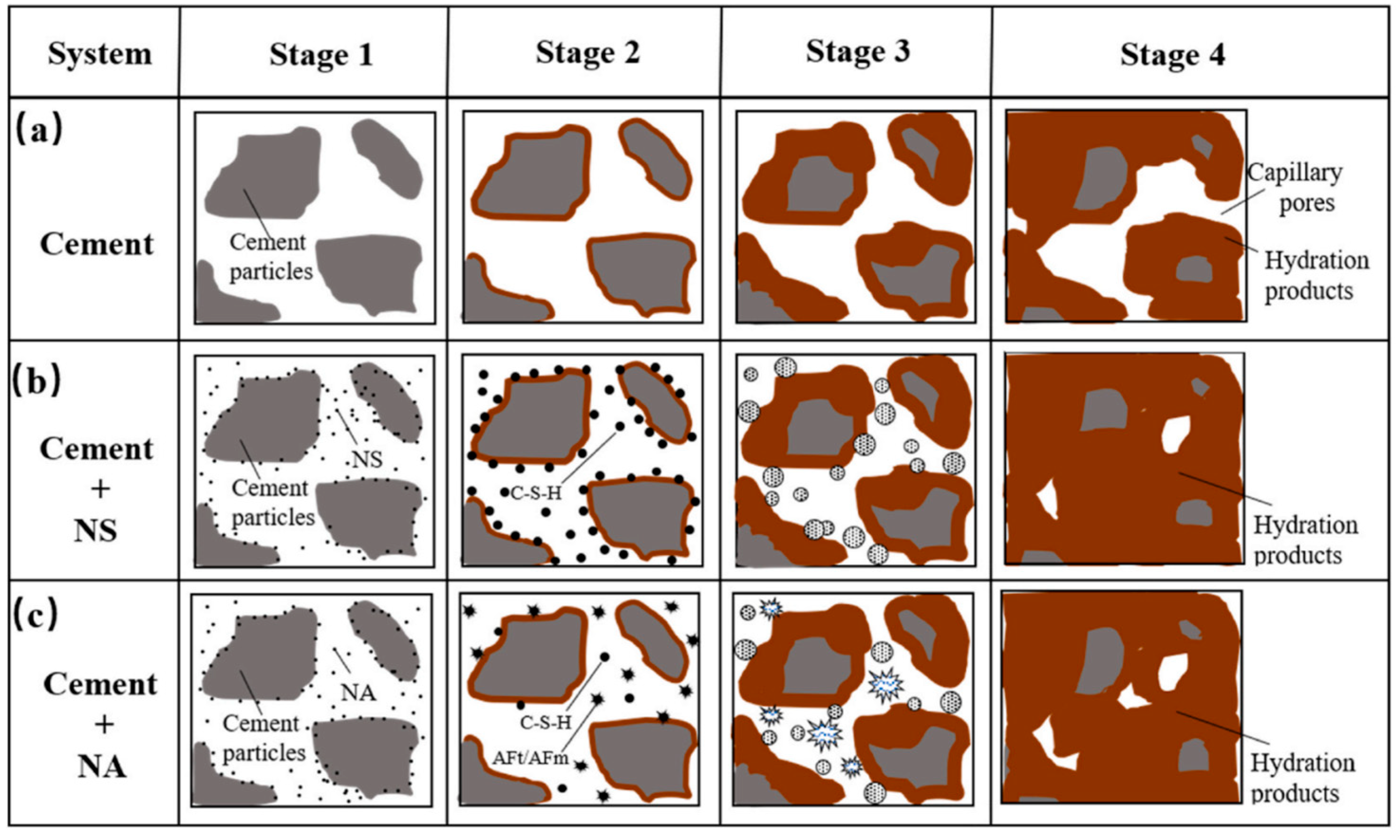
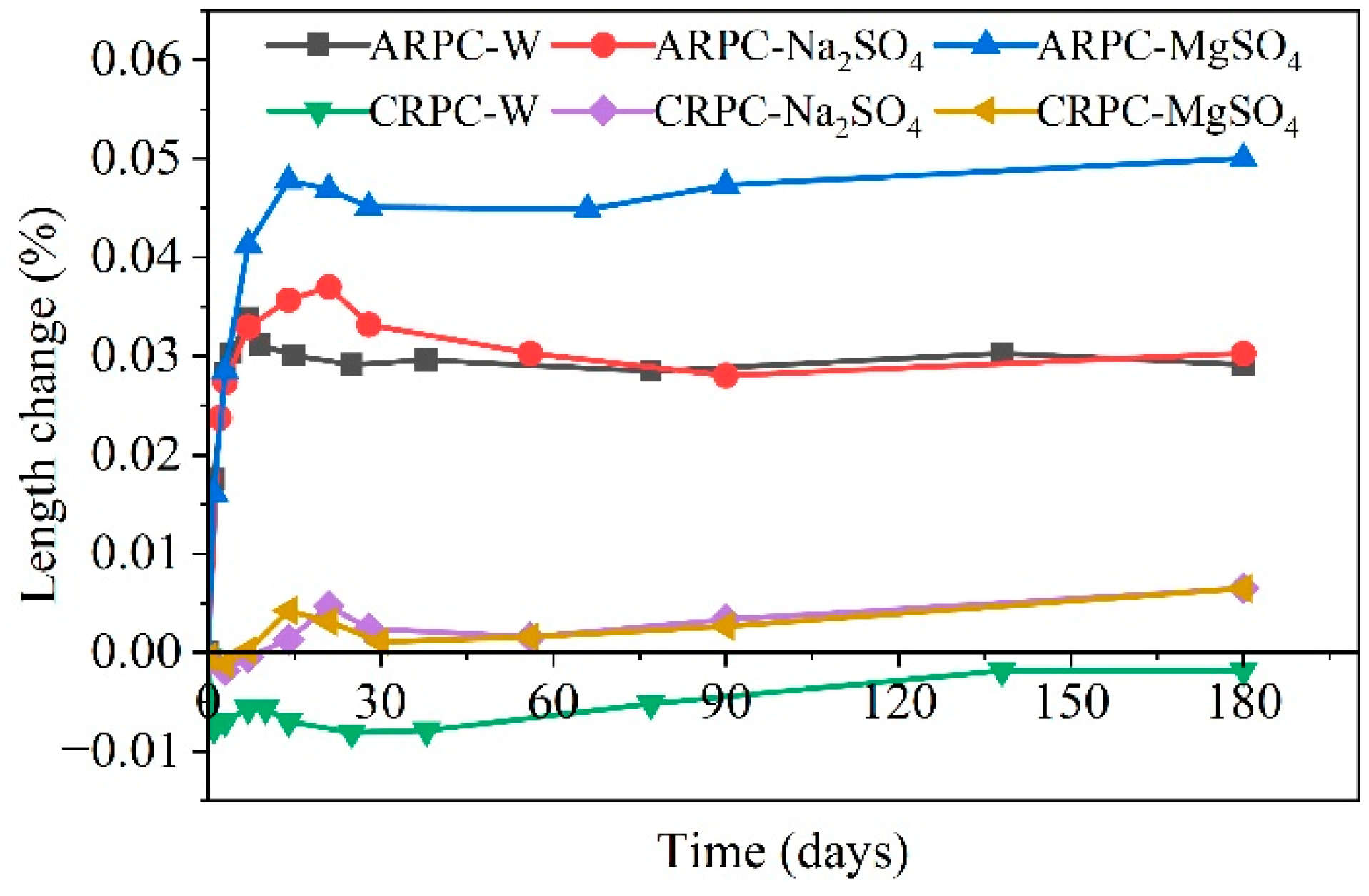
| Ref. | Concentration of Sulfate Solution | Corrosion Age | Conclusion |
|---|---|---|---|
| Ou et al. [38] | 3%, 5%, and 7% Na2SO4 solution | 14 d, 28 d, 55 d, 83 d, 110 d | Concrete resistance to sulfate attack increases progressively in damage with increasing sodium sulfate concentration. |
| Fang et al. [33] | 15% and saturated Na2SO4 solution; 13% and saturated MgSO4 solution | 0 d, 28 d, 56 d, 84 d, 154 d | When the concentration of sodium sulfate solution is less than 15%, the higher the concentration, the faster the degradation rate of the flexural and compressive resistance coefficients of the specimens. Specimens in a 15% sodium sulfate solution exhibit the fastest rate of deterioration in their resistance coefficients. However, the rate of decline in the flexural and compressive resistance coefficients of specimens immersed in a saturated sodium sulfate solution is slower compared to those in a 15% sodium sulfate solution. When the concentration of magnesium sulfate solution is below 13%, an increase in concentration leads to a faster degradation rate in both the flexural and compressive resistance coefficients of the specimens. Specimens in a 13% magnesium sulfate solution experience the most rapid decline in their resistance coefficients. However, the rate of decline in the flexural and compressive resistance coefficients of specimens in a saturated magnesium sulfate solution is slower than that in a 13% magnesium sulfate solution. |
| Fan et al. [39] | 0%, 10%, 15%, and 20% Na2SO4 solution | 210 d | As sulfate concentrations increase, the peak stress in concrete decreases, and the surface exhibits an increased density of pores. Concurrently, the size and number of internal pores within the concrete expand, providing a more direct route for sulfate ion infiltration. This process facilitates the accumulation and expansion of corrosive products, which compromises the internal structure of the concrete. Consequently, there is a macroscopic reduction in both the peak stress and the elastic modulus, effects that become increasingly pronounced with higher sulfate concentrations. The concrete specimens demonstrate shear failure, marked by a diagonal crack that extends through the entire sample. As the concentration of corrosion increases, the severity of the material’s crushing also intensifies. |
| Chen [40] | 0%, 3%, 4%, 5%, and 6% Na2SO4 solution | 24 d, 45 d, 60 d | Cement with a high water–cement ratio produces more ettringite when immersed in a solution with sulfate ion concentrations of 3–4%. Conversely, cement with a low water–cement ratio generates a greater amount of ettringite at a sulfate ion concentration of 6%. High-concentration sulfate solutions continuously influence the formation of ettringite in cement with a high water–cement ratio and accelerate the formation of ettringite in cement with a low water–cement ratio. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, J.; Yu, M.; Lu, Y.; Liu, S. Mechanism and Performance Control Methods of Sulfate Attack on Concrete: A Review. Materials 2024, 17, 4836. https://doi.org/10.3390/ma17194836
Zhang C, Li J, Yu M, Lu Y, Liu S. Mechanism and Performance Control Methods of Sulfate Attack on Concrete: A Review. Materials. 2024; 17(19):4836. https://doi.org/10.3390/ma17194836
Chicago/Turabian StyleZhang, Chuanchuan, Julun Li, Miao Yu, Yue Lu, and Shizhong Liu. 2024. "Mechanism and Performance Control Methods of Sulfate Attack on Concrete: A Review" Materials 17, no. 19: 4836. https://doi.org/10.3390/ma17194836




