Cu-Al-Ni Nanocrystalline Compacts Obtained by Spark Plasma Sintering of Mechanically Alloyed Powders
Abstract
:1. Introduction
2. Experimental
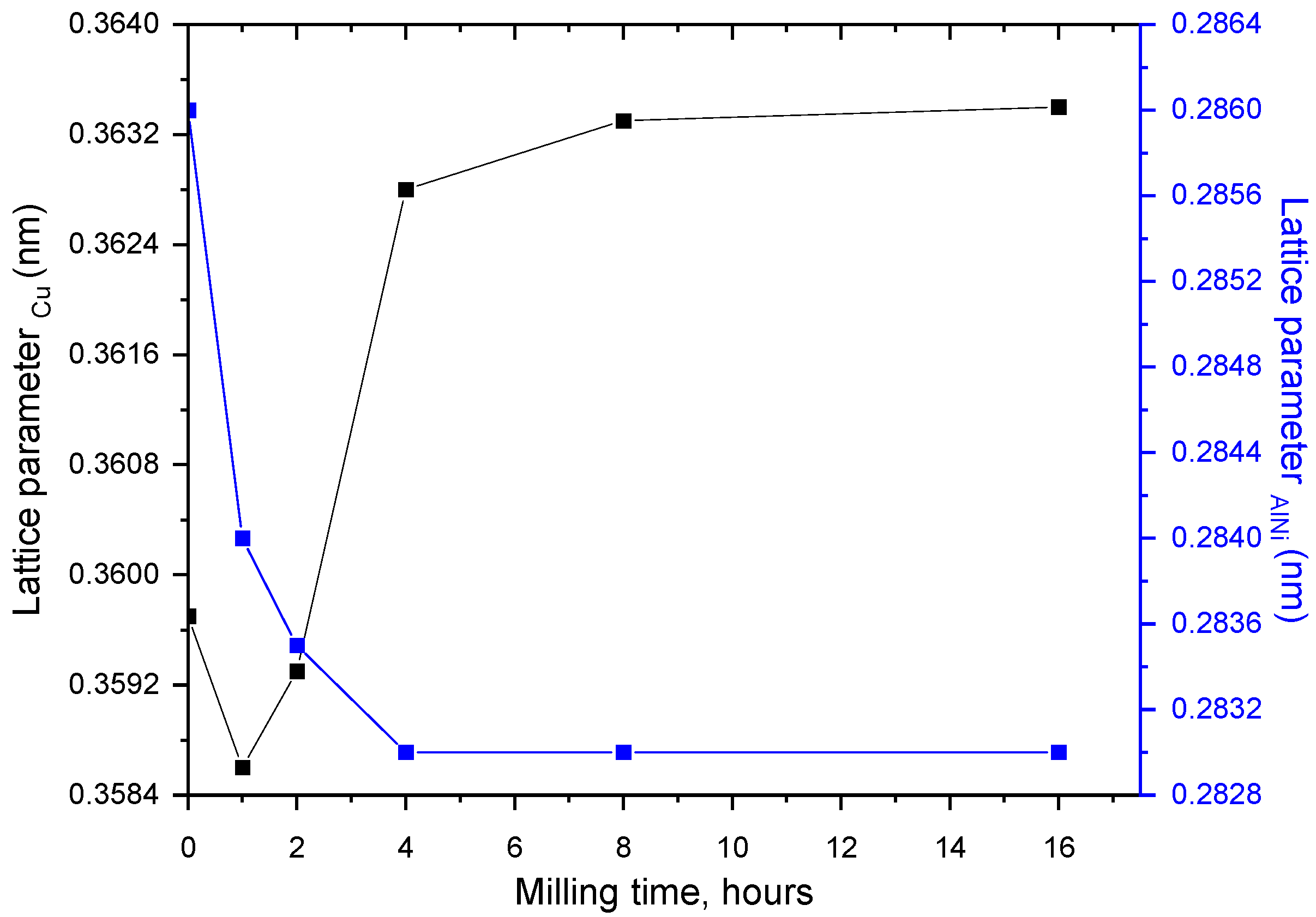
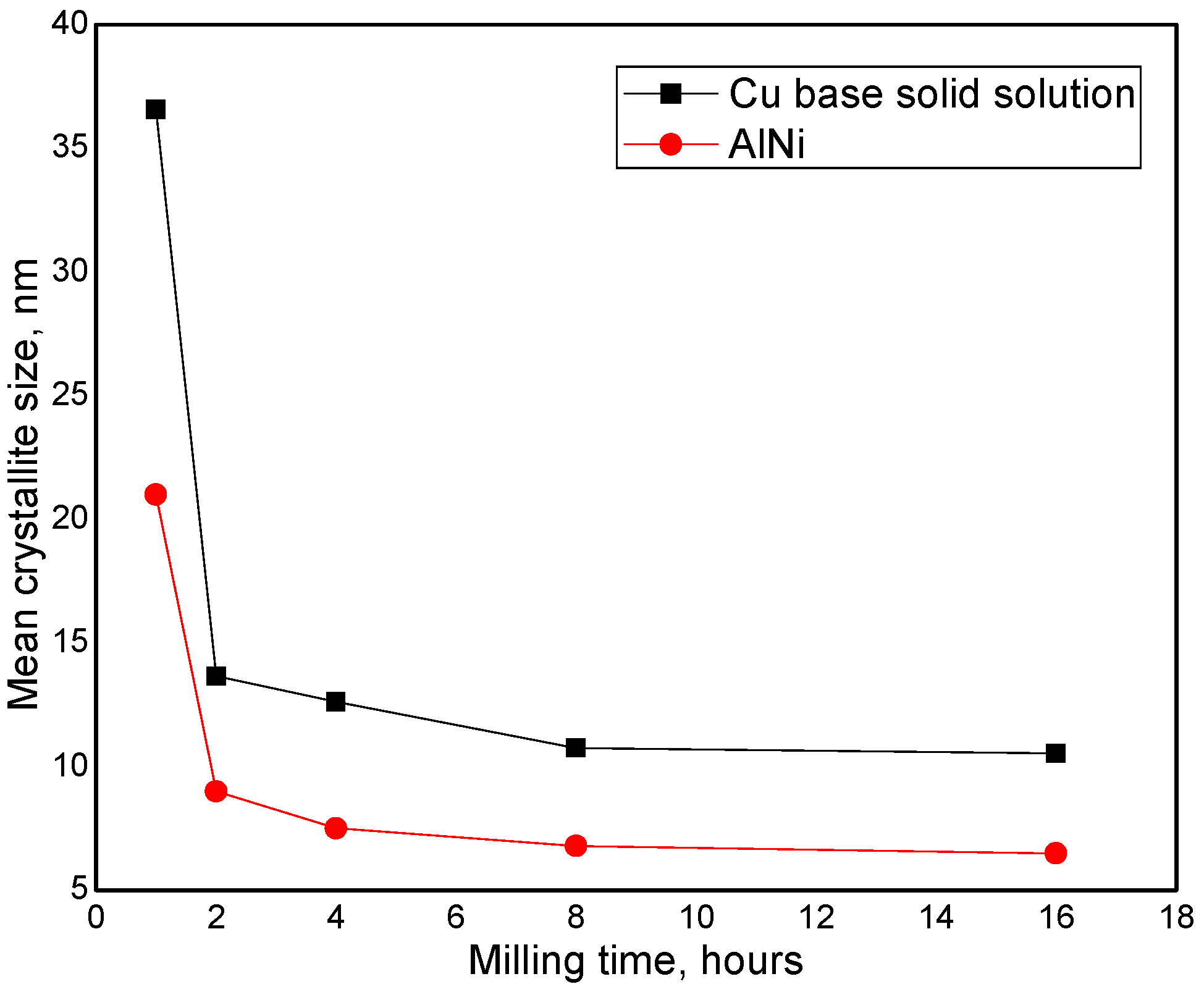
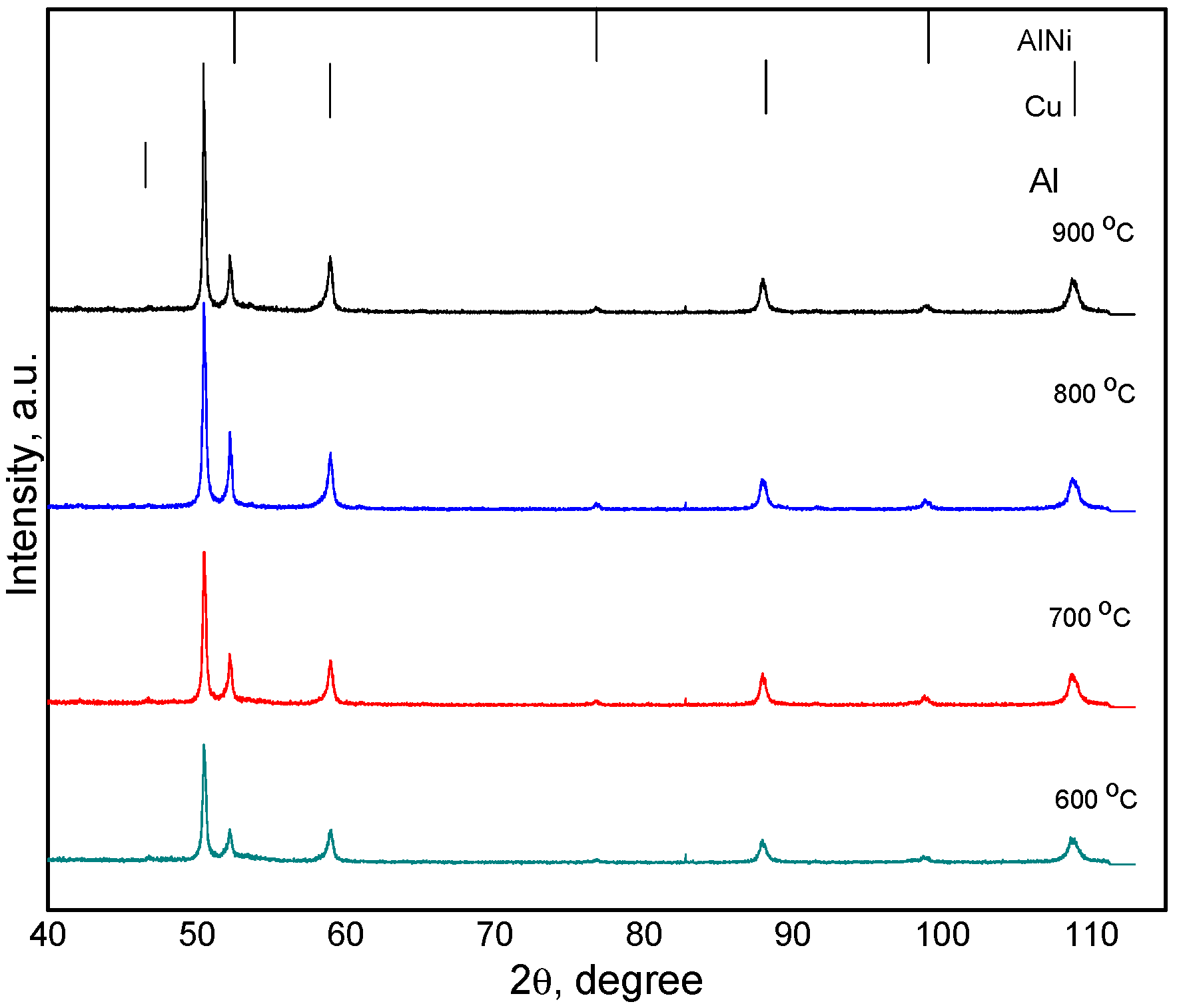
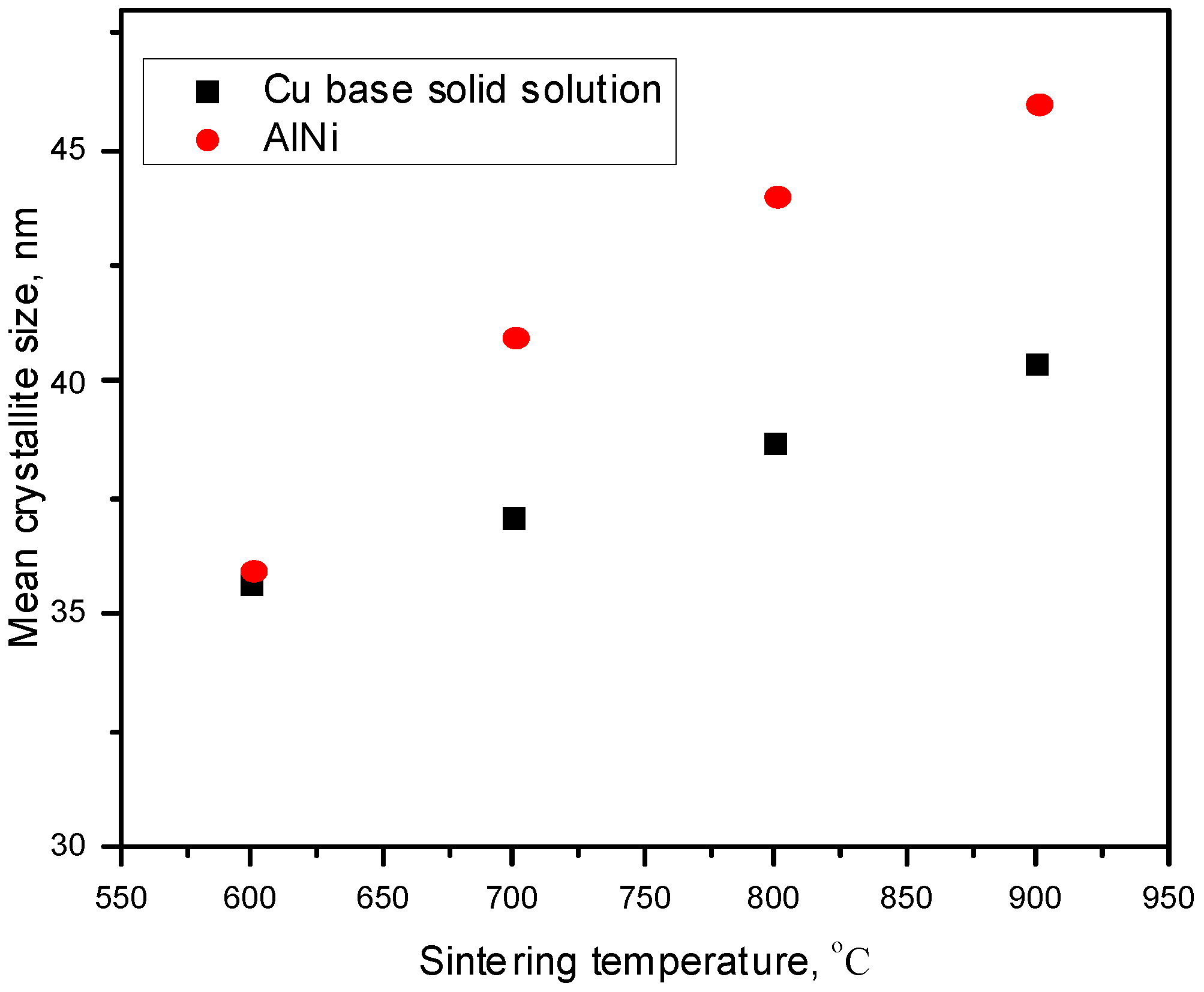
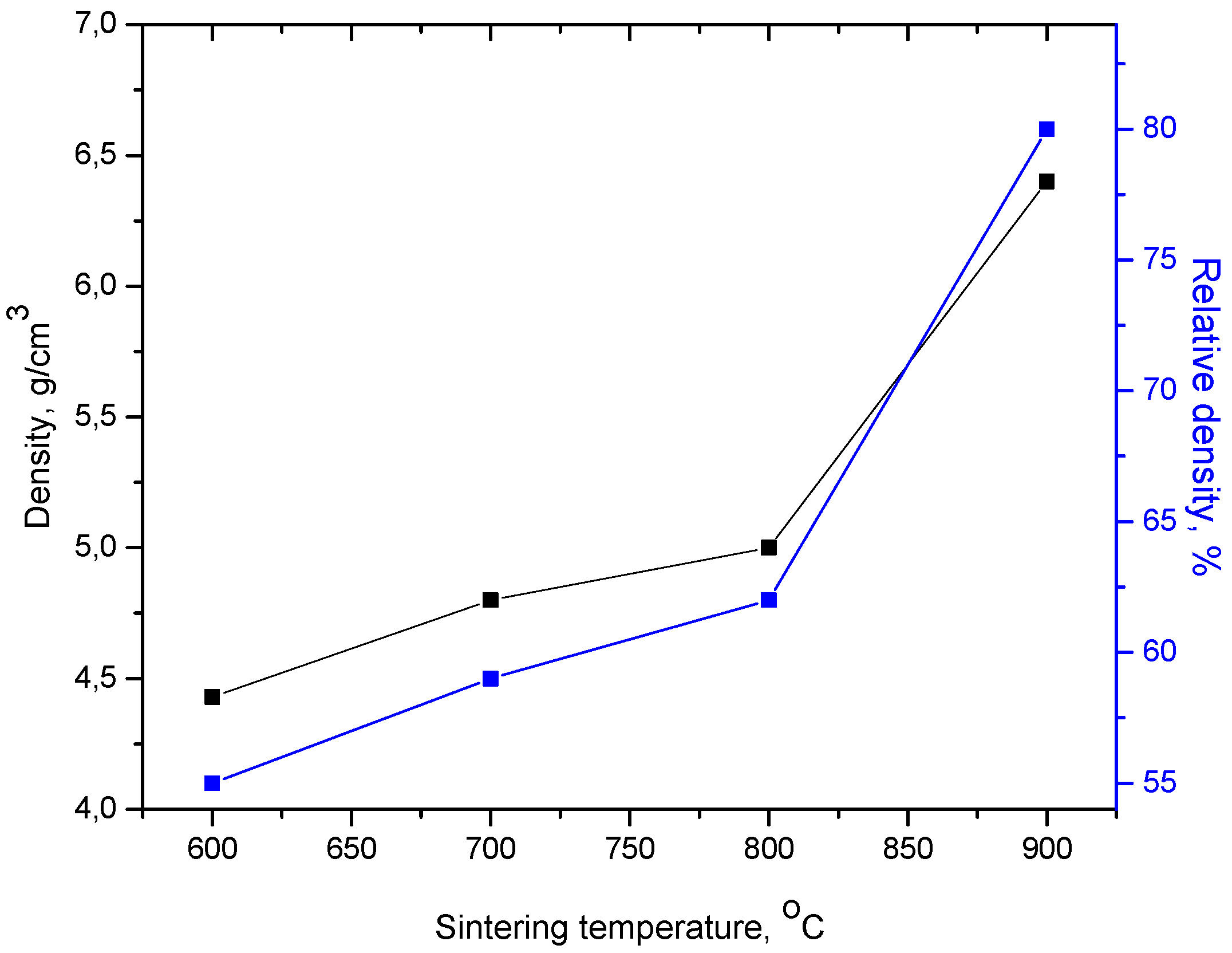
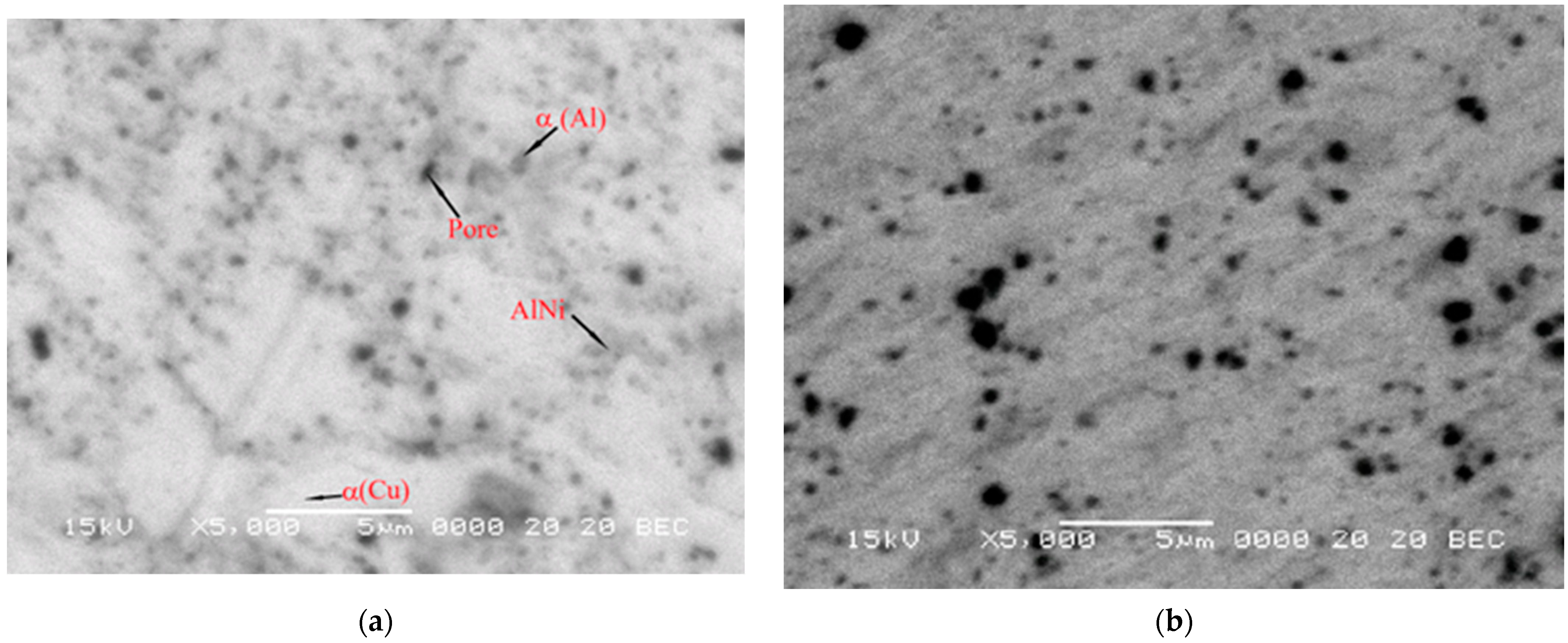
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wharton, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel–aluminum bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, Y.; Gu, T.; Guo, Y.; Xu, F.; Hou, H. Cooperative effect of strength and ductility processed by thermomechanical treatment for Cu–Al–Ni alloy. Mater. Sci. Eng. A 2022, 849, 143485. [Google Scholar] [CrossRef]
- Sharma, M.; Vajpai, S.K.; Dube, R.K. Processing and Characterization of Cu-Al-Ni Shape Memory Alloy Strips Prepared from Elemental Powders via a Novel Powder Metallurgy Route. Met. Mater. Trans. A 2010, 41, 2905–2913. [Google Scholar] [CrossRef]
- Dasgupta, R. A look into Cu-based shape memory alloys: Present scenario and future prospects. J. Mater. Res. 2014, 29, 1681–1698. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Z.Y.; Tang, N.; Jiang, Y.B.; Liu, N.; Fang, M.; Zheng, F. Cu–Al–Ni–Mn shape memory alloy processed by mechanical alloying and powder metallurgy. Mater. Sci. Eng. A 2006, 417, 225–229. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.-S. Influence of Alloying Element Addition on Cu–Al–Ni High-Temperature Shape Memory Alloy without Second Phase Formation. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 884–888. [Google Scholar] [CrossRef]
- Ramaiah, K.V.; Saikrishna, C.N.; Gouthama; Bhaumik, S.K. Microstructure and transformation behavior of Ni24.7Ti50.3Pd25 high temperature shape-memory alloy with Sc micro-addition. Mater. Charact. 2015, 106, 36–43. [Google Scholar] [CrossRef]
- Buenconsejo, P.J.S.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Shape memory behavior of Ti–Ta and its potential as a high-temperature shape memory alloy. Acta Mater. 2009, 57, 1068–1077. [Google Scholar] [CrossRef]
- Gama, J.L.L.; Dantas, C.C.; Quadros, N.F.; Ferreira, R.A.S.; Yadava, Y.P. Microstructure-mechanical property relationship to copper alloys with shape memory during thermomechanical treatments. Met. Mater. Trans. A 2006, 37, 77–87. [Google Scholar] [CrossRef]
- Abbass, M.K.; Radhi, M.M.; Adnan, R.S.A. The effect of Germanium addition on mechanical properties& microstructure of Cu-Al-Ni shape memory alloy. Mater. Today Proc. 2017, 4, 224–233. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.R.; Farahany, S.; Abdolahi, A.; Taheri, M.M. Influence of Silver nanoparticles addition on the phase transformation, mechanical properties and corrosion behaviour of Cu–Al–Ni shape memory alloys. J. Alloys Compd. 2014, 612, 471. [Google Scholar] [CrossRef]
- Amini, A.; Beladi, H.; Hameed, N.; Will, F. Effects of dynamic loading on nano-scale depth-recovery and damping property of single crystal CuAlNi shape memory alloy. J. Alloys Compd. 2012, 545, 222–224. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Ibrahim, M.K.; Bahador, A. Effect of a fourth alloying element on the microstructure and mechanical properties of Cu–Al–Ni shape memory alloys. J. Mater. Res. 2015, 30, 2258–2269. [Google Scholar] [CrossRef]
- Lee, J.S.; Wayman, C.M. Grain refinement of a Cu-Al-Ni shape memory alloy by Ti and Zr additions. Trans. Jpn. Inst. Met. 1986, 27, 584–591. [Google Scholar] [CrossRef]
- Gohar, G.A.; Manzoor, T.; Ahmad, A.; Raza, H.; Farooq, A.; Karim, I.; Iftikhar, W.; Umar, M.; Asad, F. Synthesis and investigate the properties of Cu–Al–Ni alloys with Ag addition using powder metallurgy technique. J. Alloys Compd. 2020, 817, 153281. [Google Scholar] [CrossRef]
- Rajeshkumar, R.; Vigraman, T. Formation of Cu-Al-Ni shape memory alloy with the addition of alloying elements Mn and Si. Int. J. Sci. Eng. Res. 2014, 5, 179–182. [Google Scholar]
- Agrawal, A.; Dube, R.K. Methods of fabricating Cu-Al-Ni shape memory alloys. J. Alloys Compd. 2018, 750, 235–247. [Google Scholar] [CrossRef]
- Prica, C.-V.; Marinca, T.F.; Popa, F.; Sechel, N.A.; Isnard, O.; Chicinas, I. Synthesis of nanocrystalline Ni3Fe powder by mechanical alloying using an extreme friction mode. Adv. Powder Technol. 2016, 27, 395–402. [Google Scholar] [CrossRef]
- Tang, S.M.; Chung, C.Y.; Liu, W.G. Preparation of CuAlNi-based shape memory alloys by mechanical alloying and powder metallurgy method. J. Mater. Process. Technol. 1997, 63, 307–312. [Google Scholar] [CrossRef]
- Elsayed, A.; Ibrahim, R.; Bahlol, M.; Dawood, O. Synthesis of Cu-Ni-Al Shape Memory Alloy by Powder Metallurgy. Mater. Sci. Forum 2018, 941, 1618–1622. [Google Scholar] [CrossRef]
- da Silva, M.D.C.A.; de Lima, S.J.G. Evaluation of Mechanical Alloying to obtain Cu-Al-Nb Shape Memory Alloy. Mater. Res. 2005, 8, 169–172. [Google Scholar] [CrossRef]
- Shou, D.; Deng, Z.; Zhang, C.; Yan, J.; Chen, X. Microstructure and mechanical properties of Cu-10Al-4Ni-4.8Fe with MoS2 content prepared by powder metallurgy. AIP Adv. 2024, 14, 035136. [Google Scholar] [CrossRef]
- Prică, C.V.; Neamţu, B.V.; Popa, F.; Marinca, T.F.; Sechel, N.; Chicinaş, I. Invar-type nanocrystalline compacts obtained by spark plasma sintering from mechanically alloyed powders. J. Mater. Sci. 2018, 53, 3735–3743. [Google Scholar] [CrossRef]
- Diouf, S.; Molinari, A. Densification mechanisms in spark plasma sintering: Effect of particle size and pressure. Powder Technol. 2012, 221, 220–227. [Google Scholar] [CrossRef]
- Galy, J.; Dolle, M.; Hungria, T.; Rozier, P.; Monchoux, J.-P. A new way to make solid state chemistry: Spark plasma synthesis of copper or silver vanadium oxide bronzes. Solid State Sci. 2008, 10, 976–981. [Google Scholar] [CrossRef]
- Zhu, K.N.; Godfrey, A.; Hansen, N.; Zhang, X.D. Microstructure and mechanical strength of near- and sub-micrometre grain size copper prepared by spark plasma sintering. Mater. Des. 2017, 117, 95–103. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Kuskov, K.V.; Shkodich, N.F.; Moskovskikh, D.O.; Orlov, A.O.; Usenko, A.A.; Karpov, A.V.; Kovalev, I.D.; Mukasyan, A.S. Influence of high-energy ball milling on electrical resistance of Cu and Cu/Cr nanocomposite materials produced by Spark Plasma Sintering. J. Alloys Compd. 2016, 688, 468–474. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Zhang, P.; Zhou, M.; Liu, X.; Zhou, L. Microstructure, mechanical and tribological properties of nickel-aluminium bronze alloys developed via gas-atomization and spark plasma sintering. Mater. Sci. Eng. A 2017, 707, 325–336. [Google Scholar] [CrossRef]
- Scherrer, P. Göttinger Nachrichten Math. Phys 1918, 2, 98–100. [Google Scholar]
- Lee, G.-J.; Lee, J.-H.; Lee, D.; Park, K.-I.; Jeong, C.K.; Park, J.-J.; Lee, M.-K. Synthesis and characterization of carbon-coated Cu-Ni alloy nanoparticles and their application in conductive films. Appl. Surf. Sci. 2021, 566, 150672. [Google Scholar] [CrossRef]




| Powder | Composition, % wt. |
|---|---|
| Cu | 82.5 |
| Al | 13.5 |
| Ni | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prica, C.-V.; Marinca, T.F.; Popa, F.; Sechel, A.N.; Neamțu, B.V.; Chicinaș, H.F.; Chicinaș, I. Cu-Al-Ni Nanocrystalline Compacts Obtained by Spark Plasma Sintering of Mechanically Alloyed Powders. Materials 2024, 17, 4847. https://doi.org/10.3390/ma17194847
Prica C-V, Marinca TF, Popa F, Sechel AN, Neamțu BV, Chicinaș HF, Chicinaș I. Cu-Al-Ni Nanocrystalline Compacts Obtained by Spark Plasma Sintering of Mechanically Alloyed Powders. Materials. 2024; 17(19):4847. https://doi.org/10.3390/ma17194847
Chicago/Turabian StylePrica, Calin-Virgiliu, Traian Florin Marinca, Florin Popa, Argentina Niculina Sechel, Bogdan Viorel Neamțu, Horea Florin Chicinaș, and Ionel Chicinaș. 2024. "Cu-Al-Ni Nanocrystalline Compacts Obtained by Spark Plasma Sintering of Mechanically Alloyed Powders" Materials 17, no. 19: 4847. https://doi.org/10.3390/ma17194847






