Effect of Butadiene Rubber Crystallization on Low-Temperature Properties of Butadiene/Silicone Rubber Blends with Potential for Mars Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Elastomer Blends Preparation
2.3. Vulcanization Kinetics
2.4. Vulcanization
2.5. Static Mechanical Properties at Various Temperatures
2.6. Dynamic Mechanical Analysis
2.7. Thermal Shrinkage Measurement
2.8. Differential Scanning Calorimetry
3. Results and Discussion
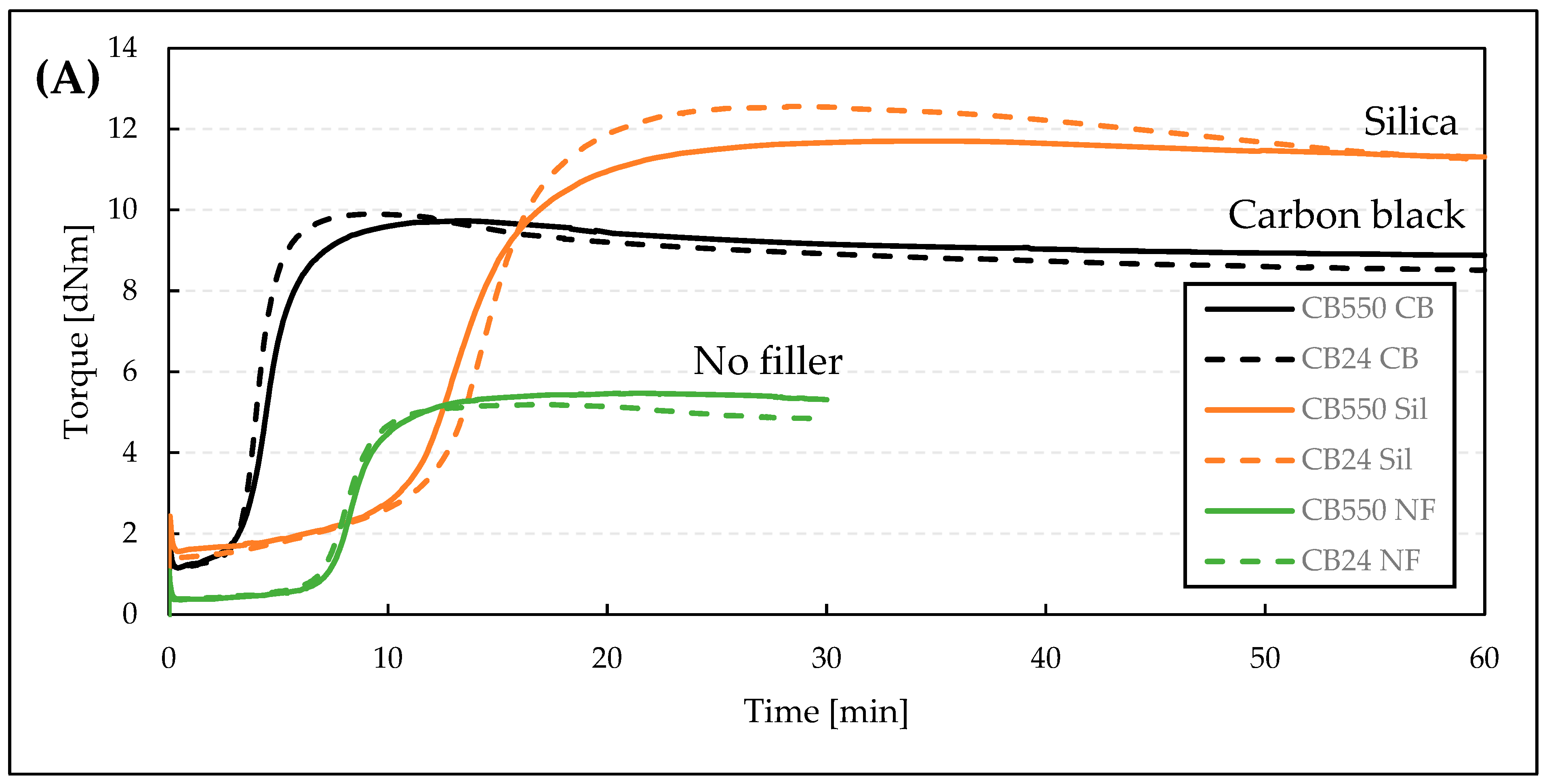
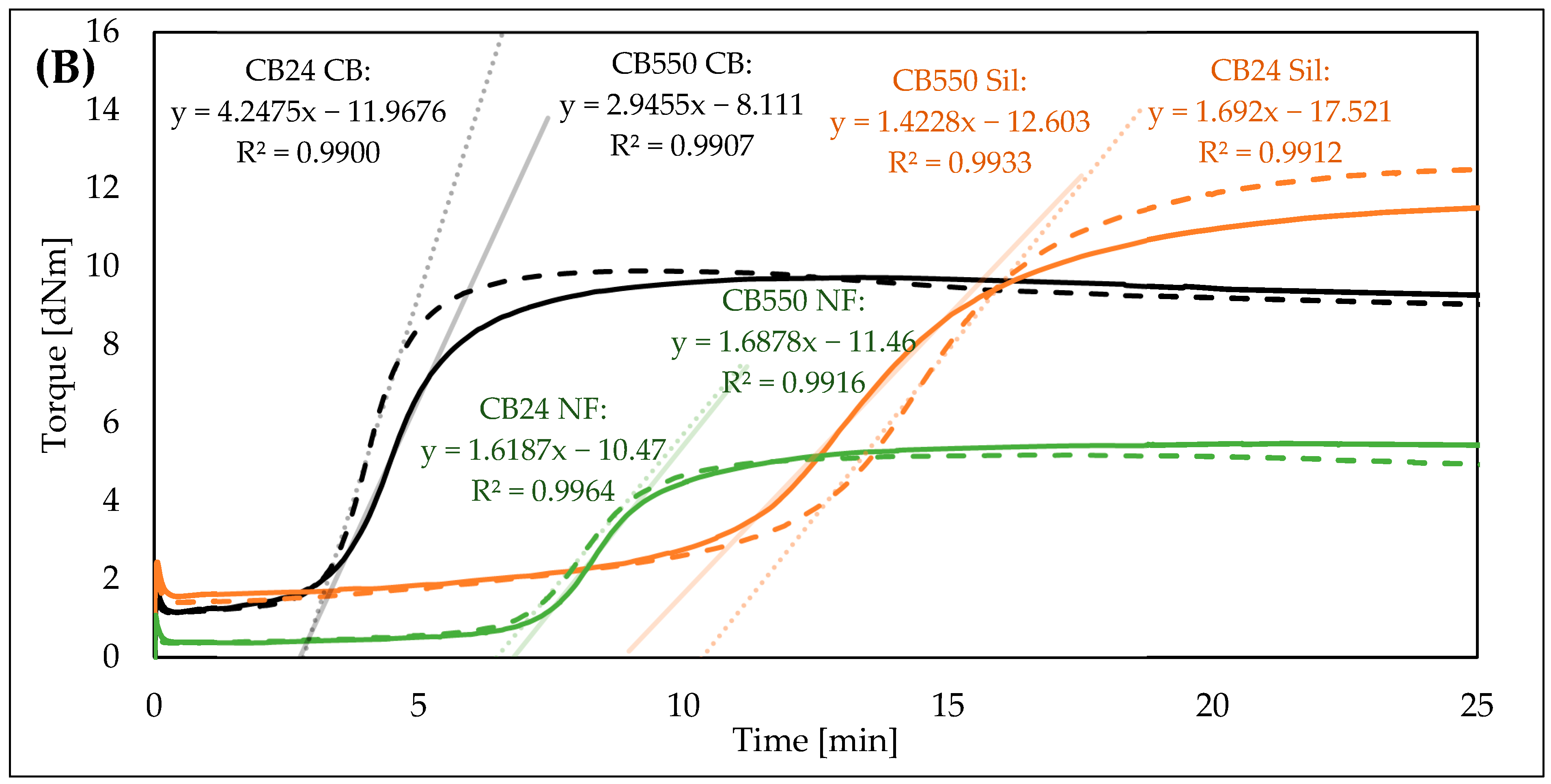
3.1. Vulcanization Kinetics
| Sample Denotation | t05 [min] | t90 [min] | Mmax [dNm] | Mmin [dNm] | ΔM [dNm] | CRI [%/min] |
|---|---|---|---|---|---|---|
| CB550 CB | 2.6 | 6.9 | 9.7 | 1.2 | 8.6 | 23.42 |
| CB24 CB | 2.8 | 5.5 | 9.9 | 1.2 | 8.8 | 37.04 |
| CB550 Sil | 6.8 | 18.8 | 11.7 | 1.6 | 10.1 | 8.35 |
| CB24 Sil | 6.4 | 18.7 | 12.6 | 1.4 | 11.2 | 8.15 |
| CB550 NF | 6.2 | 11.5 | 5.5 | 0.4 | 5.1 | 18.83 |
| CB24 NF | 5.5 | 10.1 | 5.2 | 0.4 | 4.8 | 21.65 |
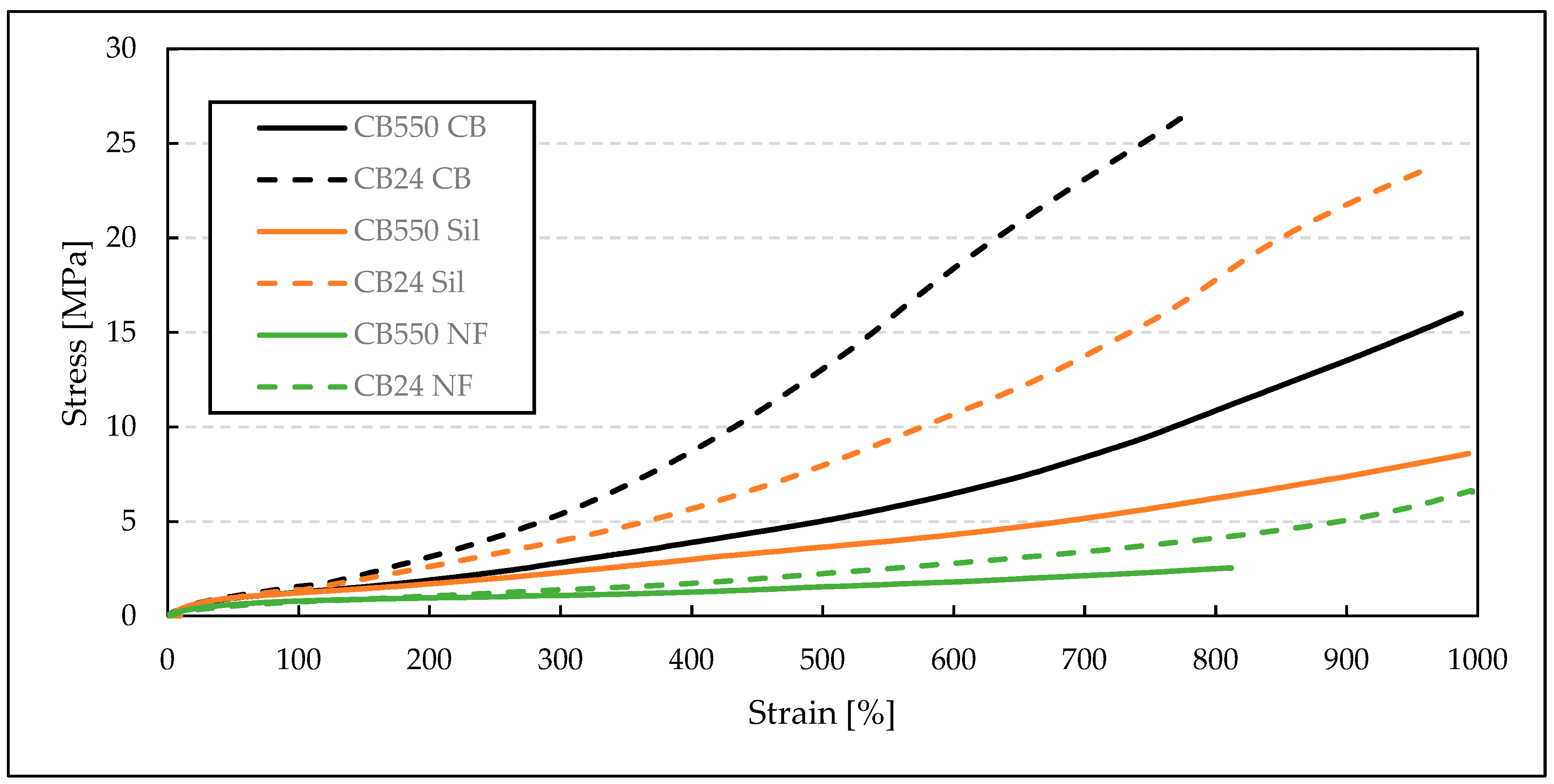
3.2. Static Mechanical Properties at Various Temperatures
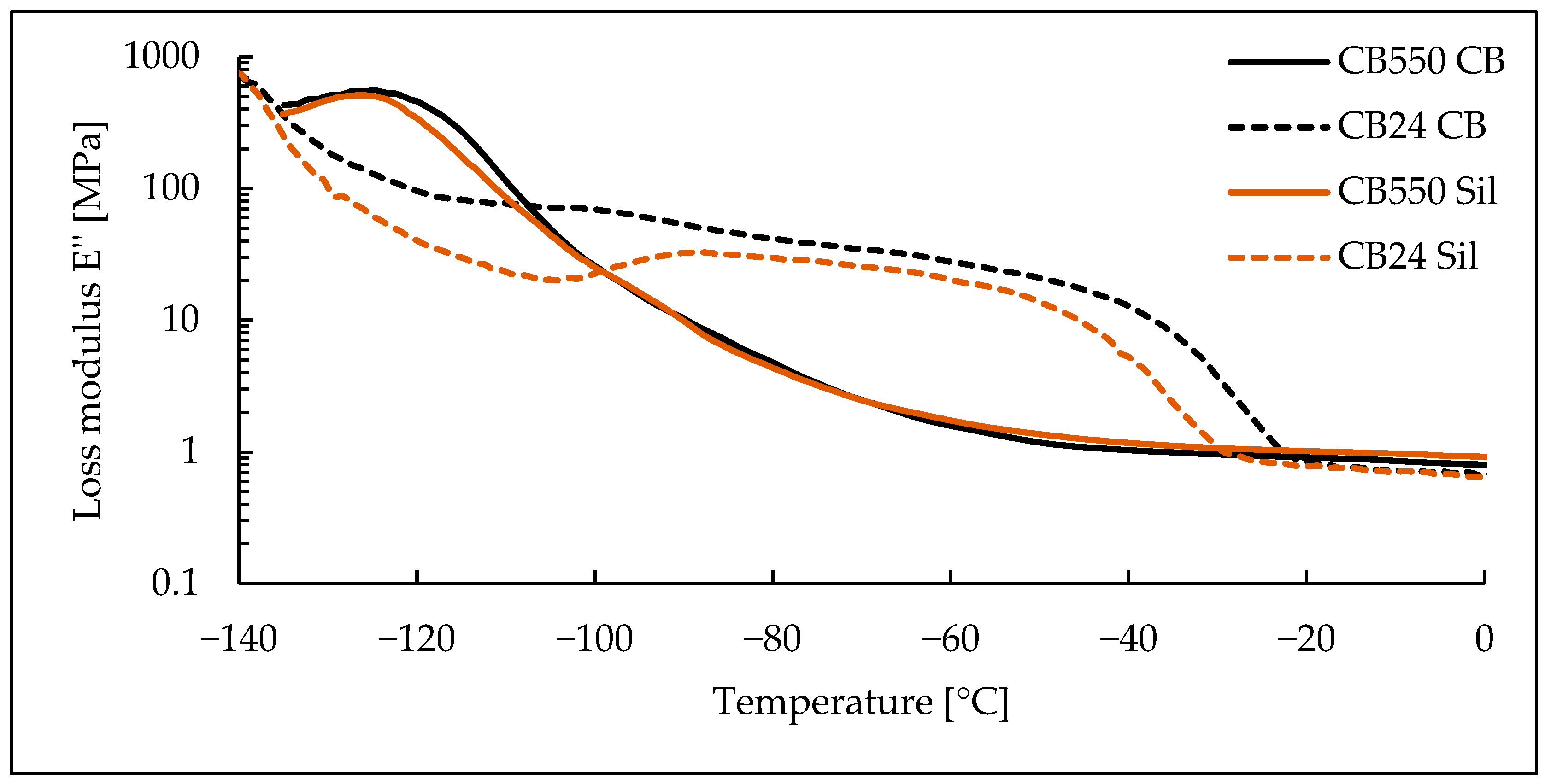
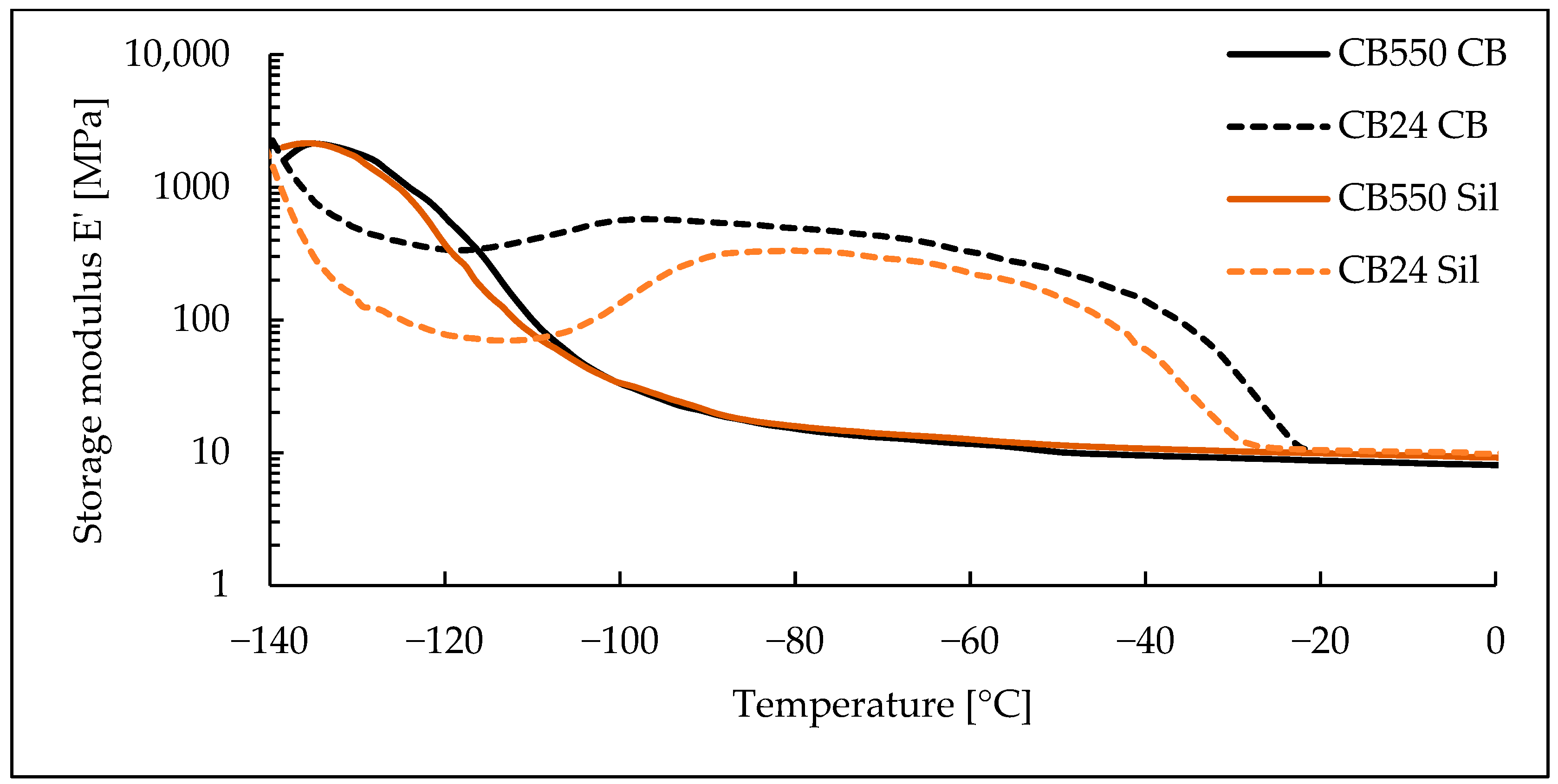
3.3. Dynamic Mechanical Analysis
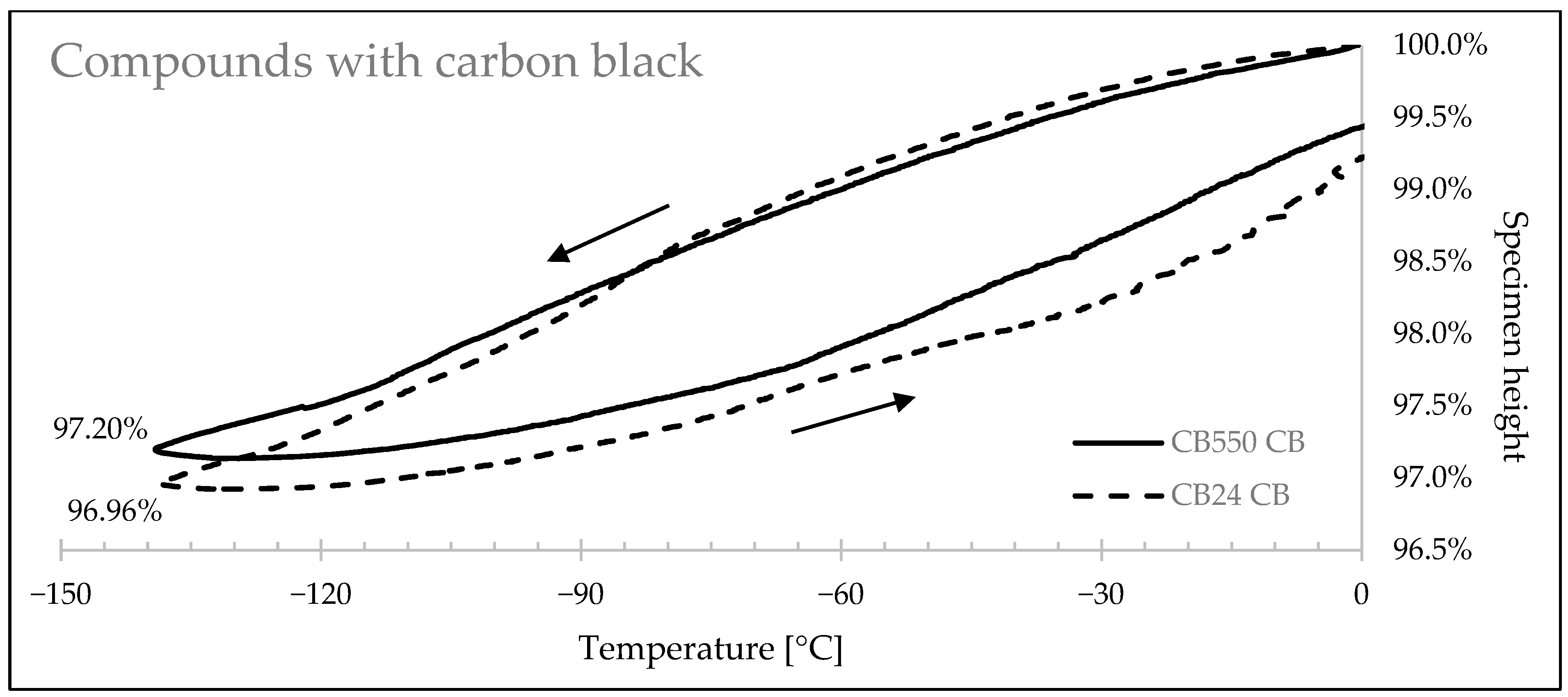
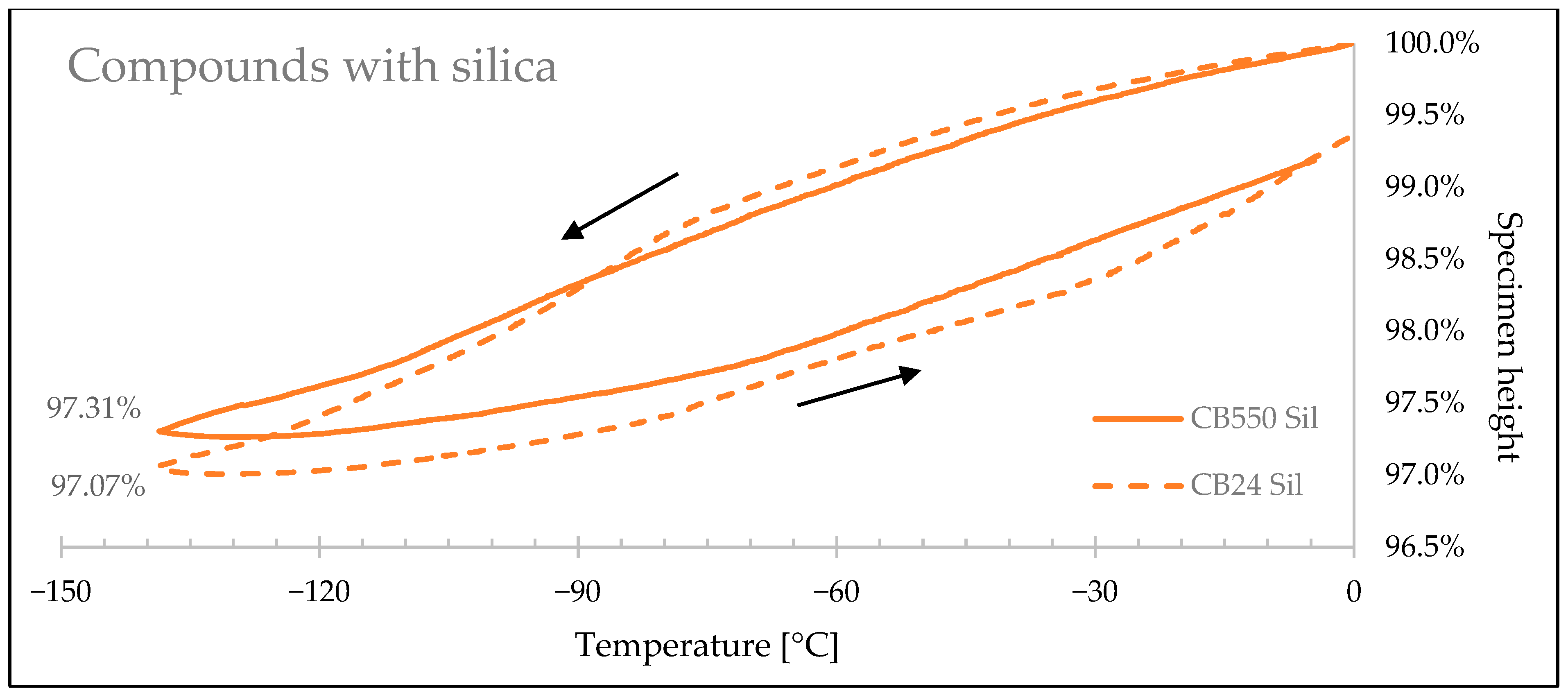
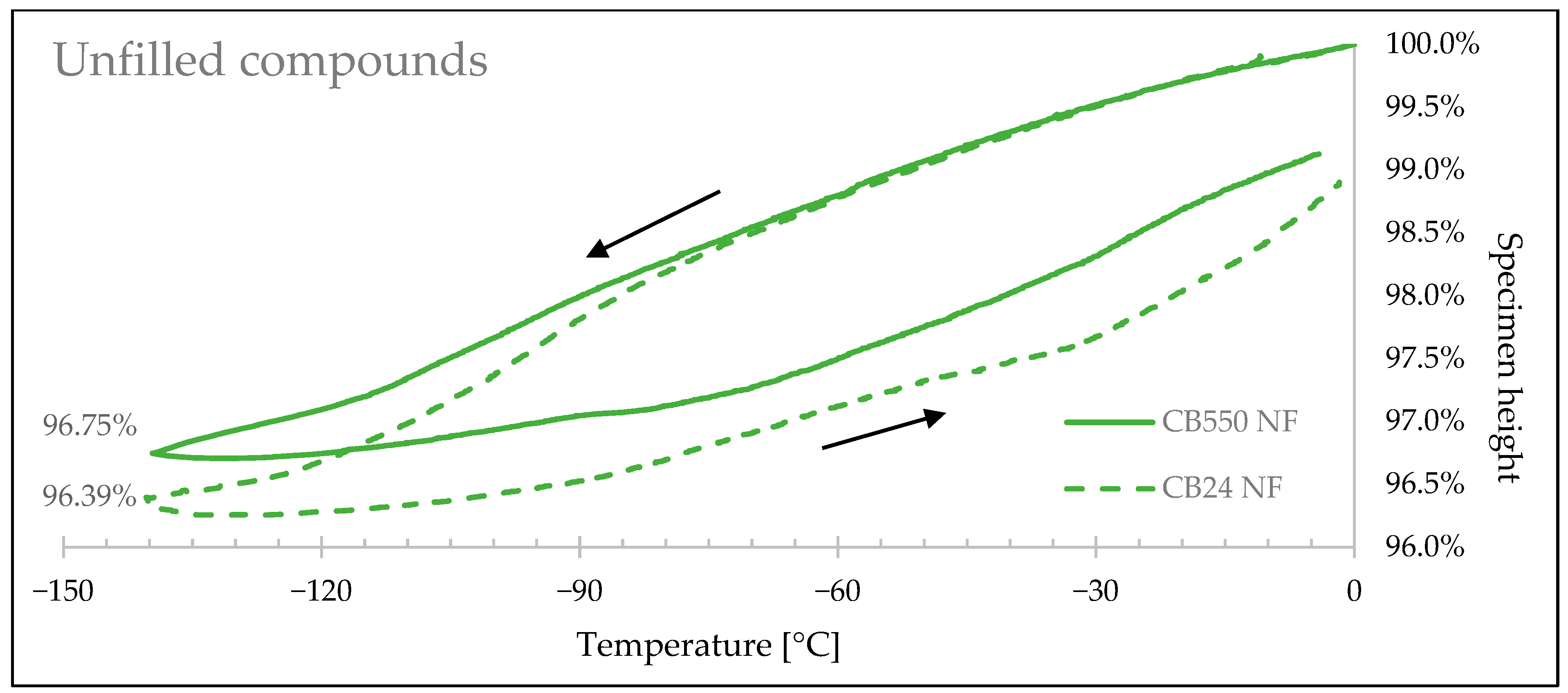
3.4. Thermal Shrinkage Measurement
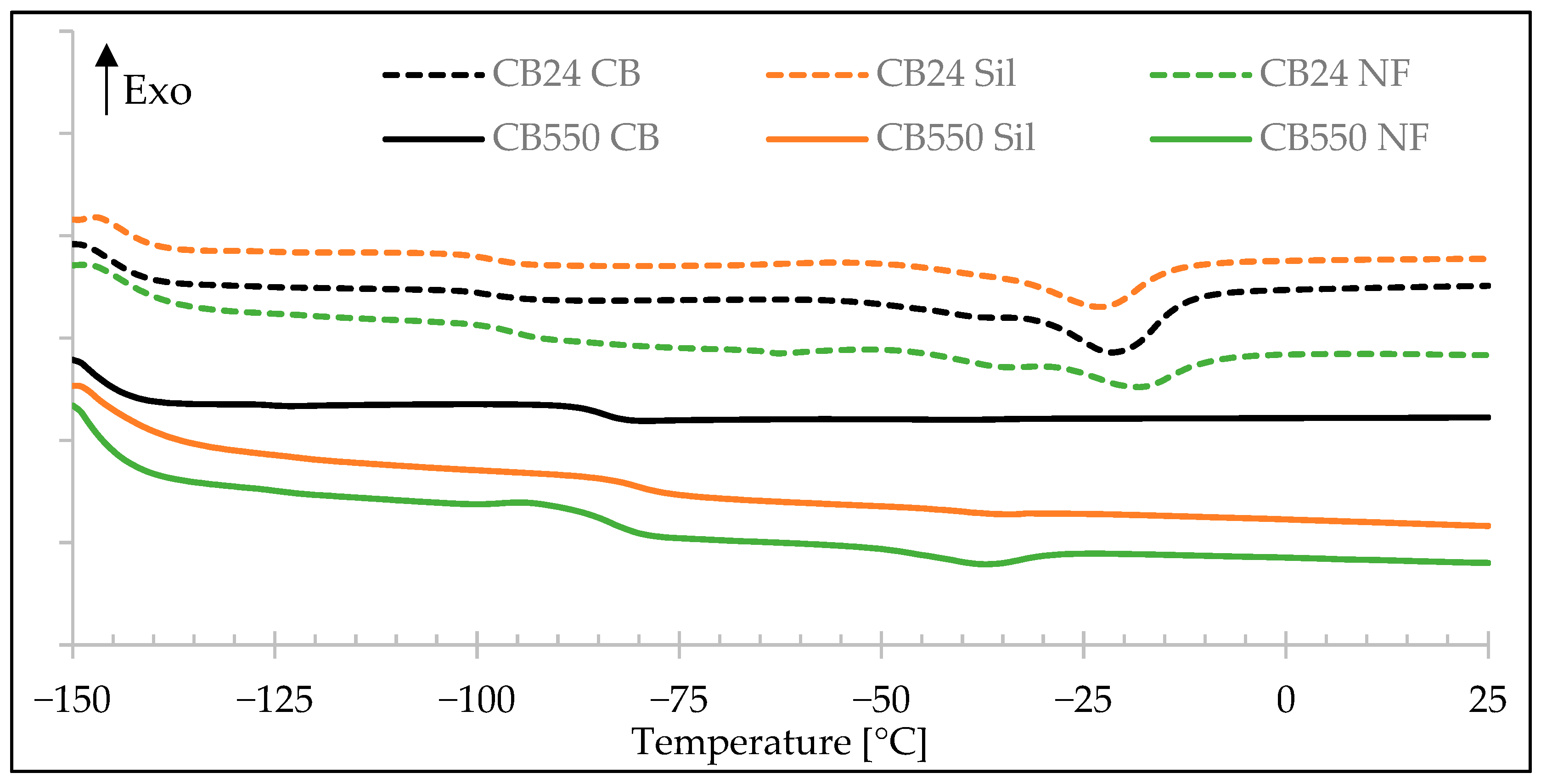
3.5. Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NASA. The International Space Station Transition Plan. Available online: https://www.nasa.gov/faqs-the-international-space-station-transition-plan/ (accessed on 5 July 2024).
- ESA. Preparing for European Commercial Presence in Low Earth Orbit. Available online: https://www.esa.int/Science_Exploration/Human_and_Robotic_Exploration/Preparing_for_European_commercial_presence_in_low_Earth_orbit (accessed on 5 July 2024).
- NASA. Premature Wear of the MSL Wheels. NASA Lessons Learned System. Available online: https://llis.nasa.gov/lesson/22401 (accessed on 22 June 2024).
- Owen, T.; Biemann, K.; Rushneck, D.R.; Biller, J.E.; Howarth, D.W.; Lafleur, A.L. The composition of the atmosphere at the surface of Mars. J. Geophys. Res. 1977, 82, 4635–4639. [Google Scholar] [CrossRef]
- Nizel, N.; Szajerski, P.; Maciejewska, M.; Bieliński, D.M.; Anyszka, R. Low glass transition temperature BR/VMQ blends for Martian environment applications. Adv. Space Res. 2023, 72, 4449–4460. [Google Scholar] [CrossRef]
- Mars Exploration Rover Mission: Spotlight. Available online: https://web.archive.org/web/20131102112312/http:/marsrover.nasa.gov/spotlight/20070612.html (accessed on 4 June 2024).
- NASA. Mars: Facts—NASA Science. Available online: https://science.nasa.gov/mars/facts/ (accessed on 21 September 2024).
- Zhang, X.; Zhu, W.; Li, X.; Xie, X.; Ji, H.; Wei, Y.; Bi, J. Study on the Crystallization Behavior of Neodymium Rare-Earth Butadiene Rubber Blends and Its Effect on Dynamic Mechanical Properties. Materials 2024, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Bekkedahl, N. Forms of rubber as indicated by temperature-volume relationship. Rubber Chem. Technol. 1935, 8, 5–22. [Google Scholar] [CrossRef]
- Magill, J.H. Crystallization and Morphology of Rubber. Rubber Chem. Technol. 1995, 68, 507–539. [Google Scholar] [CrossRef]
- Akulichev, A.G.; Echtermeyer, A.T.; Persson, B.N.J. Interfacial leakage of elastomer seals at low temperatures. Int. J. Pres. Ves. Pip. 2018, 160, 14–23. [Google Scholar] [CrossRef]
- Nizel, N. Development of Butadiene-Silicone Rubber Blends (BR/VMQ) for Mars Applications. Master’s Thesis, Lodz University of Technology, Lodz, Poland, September 2023. [Google Scholar] [CrossRef]
- Yehia, A.A.; Stoll, B. Kinetics of vulcanization of nitrile-butadiene rubber. Kauts Gummi Kunsts 1987, 40, 950–952. [Google Scholar]
- ISO 37:2024; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. Available online: https://www.iso.org/standard/86892.html (accessed on 29 September 2024).
- Marzocca, A.J.; Garraza, A.R.; Sorichetti, P.; Mosca, H.O. Cure kinetics and swelling behaviour in polybutadiene rubber. Polym. Test. 2010, 29, 477–482. [Google Scholar] [CrossRef]
- Dogadkin, B.A.; Dobromyslova, A.V.; Tolstukhina, F.S.; Samsonova, N.G. The influence of structure on the chemical activity and vulcanizability of butadiene polymers. Rubber Chem. Technol. 1958, 31, 569–580. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Razzaghi-Kashani, M. Catalytic and networking effects of carbon black on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber. Soft Matter 2018, 14, 9194–9208. [Google Scholar] [CrossRef] [PubMed]
- Kosmalska, A.; Zaborski, M.; Ślusarski, L. Adsorption of curatives and activity of silica toward elastomers. Macromol. Symp. 2003, 194, 269–276. [Google Scholar] [CrossRef]
- Sowińska-Baranowska, A.; Maciejewska, M. Influence of the silica specific surface area and ionic liquids on the curing characteristics and performance of styrene–butadiene rubber composites. Materials 2021, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; van Swaaij, A.P.; Noordermeer, J.W.; Blume, A.; Dierkes, W.K. On the various roles of 1, 3-DIPHENYL guanidine in silica/silane reinforced SBR/BR blends. Polym. Test. 2021, 93, 106858. [Google Scholar] [CrossRef]
- Gleichentheil, S.; Neumann, W. The Gough-Joule Effect in Vulcanizates. Rubber Chem. Technol. 1938, 11, 508–509. [Google Scholar] [CrossRef]
- Anyszka, R.; Jia, L.; Blume, A. Tires for Mars Rovers: Reinforcing BR and BR/Vinyl-Methyl Silicone Rubber Compounds with Carbon Black, Nano-CaCO3, or Silica for Good Low-Temperature Dynamic-Mechanical Performance. Tire Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Rivera, M.R.; Nájera, R.H.; Tapia, J.J.B.; Guerrero, L.R. Structure and properties of model polybutadienes-Effect of microstructure on the dynamic mechanical properties of rubber. J. Elastom. Plast. 2005, 37, 267–278. [Google Scholar] [CrossRef]
- Bordeaux, D.; Cohen-Addad, J.P. Silica-filled polydimethylsiloxane: Crystallization process of the adsorbed layer. Polymer 1990, 31, 743–748. [Google Scholar] [CrossRef]
- Boonstra, B.B.; Cochrane, H.; Dannenberg, E.M. Reinforcement of silicone rubber by particulate silica. Rubber Chem. Technol. 1975, 48, 558–576. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, X.; Su, W.; Zhao, J.; Zhou, C. Molecular insights into sequence distributions and conformation-dependent properties of high-phenyl polysiloxanes. Polymers 2019, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Q: Silicone Rubber—NETZSCH Analyzing & Testing. Available online: https://analyzing-testing.netzsch.com/es/polymers-netzsch-com/elastomers/hnbr-hydrogenated-acrylonitrile-butadiene-rubber-1 (accessed on 5 July 2024).
- BR: Butadiene Rubber—NETZSCH Polymers. Available online: https://polymers.netzsch.com/Materials/Details/52 (accessed on 5 July 2024).
- Poor, S.M. Study of the Mechanical Behavior of High Cis-Polybutadiene Rubber: Structure to Property Correlation. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, December 2015. [Google Scholar]




| Samples Denotation Component [phr] | CB24 CB | CB550 CB | CB24 Sil | CB550 Sil | CB24 NF | CB550 NF |
|---|---|---|---|---|---|---|
| BR CB24 (semi-crystalline) | 80 | - | 80 | - | 80 | - |
| BR CB550 (amorphous) | - | 80 | - | 80 | - | 80 |
| VMQ polymer MV 1,0 | 20 | 20 | 20 | 20 | 20 | 20 |
| Carbon Black N330 | 30 | 30 | - | - | - | - |
| Silica Ultrasil 7000 | - | - | 30 | 30 | - | - |
| Si 266 (TESPD) | - | - | 3 | 3 | - | - |
| Time [min] | Action | Rotor Speed [rpm] | Temperature [°C] |
|---|---|---|---|
| Step 1 | |||
| 0:00 | Add BR and VMQ | 20 | Room temp. |
| 1:00 | Increase rotor speed | 60 | Room temp. |
| 6:00 | Add filler, silane (if applicable), ZnO, stearic acid, 6PPD | 60 | 100 |
| 7:00 | Increase rotor speed, reach 130 °C | 80–90 | 100 |
| 12:30 | Decrease rotor speed | 60 | 130 |
| 15:30 | Stop mixing | 0 | 130 |
| Step 2 | |||
| 0:00 | Add pre-mix | 30 | 50 |
| 1:00 | Increase rotor speed | 60 | 50 |
| 3:00 | Add CBS, sulfur | 60 | 70 |
| 5:00 | Stop mixing | 0 | 70 |
| Samples Denotation | Temperature [°C] | M100 [MPa] | M200 [MPa] | M300 [MPa] | TS or TFmax [MPa] | EB [%] |
|---|---|---|---|---|---|---|
| CB24 CB | Room Temp. | 1.6 ± 0.1 | 2.8 ± 0.1 | 4.1 ± 0.2 | 7.5 ± 0.7 | 520 ± 49 |
| 0 | 1.6 ± 0.03 | 2.8 ± 0.1 | 4.3 ± 0.1 | 11.1 ± 1.6 | 649 ± 58 | |
| −20 | 1.6 ± 0.1 | 2.9 ± 0.1 | 4.6 ± 0.1 | 11.6 ± 0.7 | 552 ± 18 | |
| −40 | 1.6 ± 0.1 | 3.3 ± 0.2 | 5.7 ± 0.3 | 25.6 ± 1.2 | 742 ± 33 | |
| CB550 CB | Room Temp. | 1.3 ± 0.04 | 2.0 ± 0.1 | 2.9 ± 0.1 | 6.8 ± 0.8 | 654 ± 55 |
| 0 | 1.2 ± 0.01 | 1.95 ± 0.01 | 2.85 ± 0.01 | 10.7 ± 0.6 | 821 ± 25 | |
| −20 | 1.2 ± 0.03 | 1.9 ± 0.1 | 2.9 ± 0.1 | 13.7 ± 1.8 | 897 ± 64 | |
| −40 | 1.3 ± 0.04 | 1.94 ± 0.04 | 2.87 ± 0.03 | 14.3 ± 2.8 | >1000 | |
| CB24 Sil | Room Temp. | 1.5 ± 0.05 | 2.3 ± 0.1 | 3.1 ± 0.1 | 4.8 ± 0.9 | 489 ± 97 |
| 0 | 1.6 ± 0.03 | 2.5 ± 0.1 | 3.6 ± 0.1 | 7.8 ± 1.2 | 619 ± 76 | |
| −20 | 1.4 ± 0.1 | 2.5 ± 0.1 | 3.6 ± 0.1 | 18.2 ± 0.9 | 960 ± 28 | |
| −40 | 1.4 ± 0.02 | 2.68 ± 0.04 | 4.06 ± 0.04 | 23.0 ± 1.1 | 943 ± 26 | |
| CB550 Sil | Room Temp. | 1.3 ± 0.03 | 1.84 ± 0.04 | 2.46 ± 0.04 | 5.1 ± 0.8 | 686 ± 114 |
| 0 | 1.2 ± 0.03 | 1.80 ± 0.03 | 2.41 ± 0.04 | 7.7 ± 0.2 | 952 ± 32 | |
| −20 | 1.2 ± 0.04 | 1.73 ± 0.07 | 2.34 ± 0.09 | 8.2 ± 0.4 | >1000 | |
| −40 | 1.2 ± 0.03 | 1.70 ± 0.02 | 2.33 ± 0.02 | 8.6 ± 0.2 | >1000 | |
| CB24 NF | Room Temp. | 0.8 ± 0.04 | 1.16 ± 0.03 | 1.46 ± 0.04 | 1.4 ± 0.2 | 291 ± 49 |
| 0 | 0.8 ± 0.01 | 1.15 ± 0.02 | 1.43 ± 0.03 | 2.7 ± 0.4 | 677 ± 108 | |
| −20 | 0.8 ± 0.03 | 1.08 ± 0.02 | 1.36 ± 0.02 | 4.3 ± 0.3 | 954 ± 22 | |
| −40 | 0.8 ± 0.01 | 1.07 ± 0.01 | 1.31 ± 0.01 | 6.6 ± 0.1 | >1000 | |
| CB550 NF | Room Temp. | 0.8 ± 0.02 | - | - | 1.0 ± 0.1 | 181 ± 35 |
| 0 | 0.8 ± 0.01 | 0.99 ± 0.01 | - | 1.2 ± 0.1 | 282 ± 30 | |
| −20 | 0.7 ± 0.01 | 0.95 ± 0.03 | 1.14 ± 0.05 | 1.23 ± 0.01 | 348 ± 13 | |
| −40 | 0.8 ± 0.03 | 0.95 ± 0.03 | 1.10 ± 0.03 | 2.5 ± 0.3 | 803 ± 64 |
| Samples Denotation | CB24 CB | CB550 CB | CB24 Sil | CB550 Sil | CB24 NF | CB550 NF |
|---|---|---|---|---|---|---|
| TS before TC [MPa] | 7.5 ± 0.7 | 6.8 ± 0.8 | 4.8 ± 0.9 | 5.1 ± 0.8 | 1.4 ± 0.2 | 1.0 ± 0.1 |
| TS after TC [MPa] | 5.6 ± 0.2 | 7.0 ± 1.0 | 5.2 ± 0.2 | 4.2 ± 0.4 | 1.5 ± 0.2 | 1.0 ± < 0.1 |
| EB before TC [%] | 520 ± 49 | 654 ± 55 | 489 ± 97 | 686 ± 114 | 291 ± 49 | 181 ± 35 |
| EB after TC [%] | 407 ± 15 | 613 ± 53 | 469 ± 37 | 484 ± 72 | 271 ± 50 | 166 ± 10 |
| Sample Denotation | Height Change at −140 °C | Linear Shrink Difference | Volume Change at −140 °C | Volumetric Shrink Difference |
|---|---|---|---|---|
| CB24 CB | 3.08% | 6.91% | 8.95% | 6.71% |
| CB550 CB | 2.86% | 8.35% | ||
| CB24 Sil | 2.99% | 8.59% | 8.71% | 8.36% |
| CB550 Sil | 2.73% | 7.98% | ||
| CB24 NF | 3.75% | 12.03% | 10.82% | 11.63% |
| CB550 NF | 3.30% | 9.56% |
| Samples Denotation | Tg(VMQ) [°C] | Tm(VMQ) [°C] | ΔHm(VMQ) [J/gVMQ] | Tg(BR) [°C] | Tm(BR) [°C] | ΔHm(BR) [J/gBR] |
|---|---|---|---|---|---|---|
| CB550 CB | −125.2 | −40.4 | 0.78 | −84.8 | n/a | |
| CB24 CB | −126.6 | −36.1 | 12.53 | −98.0 | −21.5 | 25.05 |
| CB550 Sil | −125.4 | −36.9 | 1.91 | −81.0 | n/a | |
| CB24 Sil | −125.5 | n/a | −98.1 | −23.2 | 20.19 | |
| CB550 NF | −124.5 | −37.9 | 14.51 | −85.5 | n/a | |
| CB24 NF | −124.5 | −34.8 | 17.48 | −96.2 | −18.6 | 9.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizel, N.; Bieliński, D.M.; Pawlak, A.; Maciejewska, M.; Wręczycki, J.; Masłowski, M.; Anyszka, R. Effect of Butadiene Rubber Crystallization on Low-Temperature Properties of Butadiene/Silicone Rubber Blends with Potential for Mars Applications. Materials 2024, 17, 4857. https://doi.org/10.3390/ma17194857
Nizel N, Bieliński DM, Pawlak A, Maciejewska M, Wręczycki J, Masłowski M, Anyszka R. Effect of Butadiene Rubber Crystallization on Low-Temperature Properties of Butadiene/Silicone Rubber Blends with Potential for Mars Applications. Materials. 2024; 17(19):4857. https://doi.org/10.3390/ma17194857
Chicago/Turabian StyleNizel, Norbert, Dariusz M. Bieliński, Andrzej Pawlak, Magdalena Maciejewska, Jakub Wręczycki, Marcin Masłowski, and Rafał Anyszka. 2024. "Effect of Butadiene Rubber Crystallization on Low-Temperature Properties of Butadiene/Silicone Rubber Blends with Potential for Mars Applications" Materials 17, no. 19: 4857. https://doi.org/10.3390/ma17194857
APA StyleNizel, N., Bieliński, D. M., Pawlak, A., Maciejewska, M., Wręczycki, J., Masłowski, M., & Anyszka, R. (2024). Effect of Butadiene Rubber Crystallization on Low-Temperature Properties of Butadiene/Silicone Rubber Blends with Potential for Mars Applications. Materials, 17(19), 4857. https://doi.org/10.3390/ma17194857










