Ternary ZnS/ZnO/Graphitic Carbon Nitride Heterojunction for Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of gCN
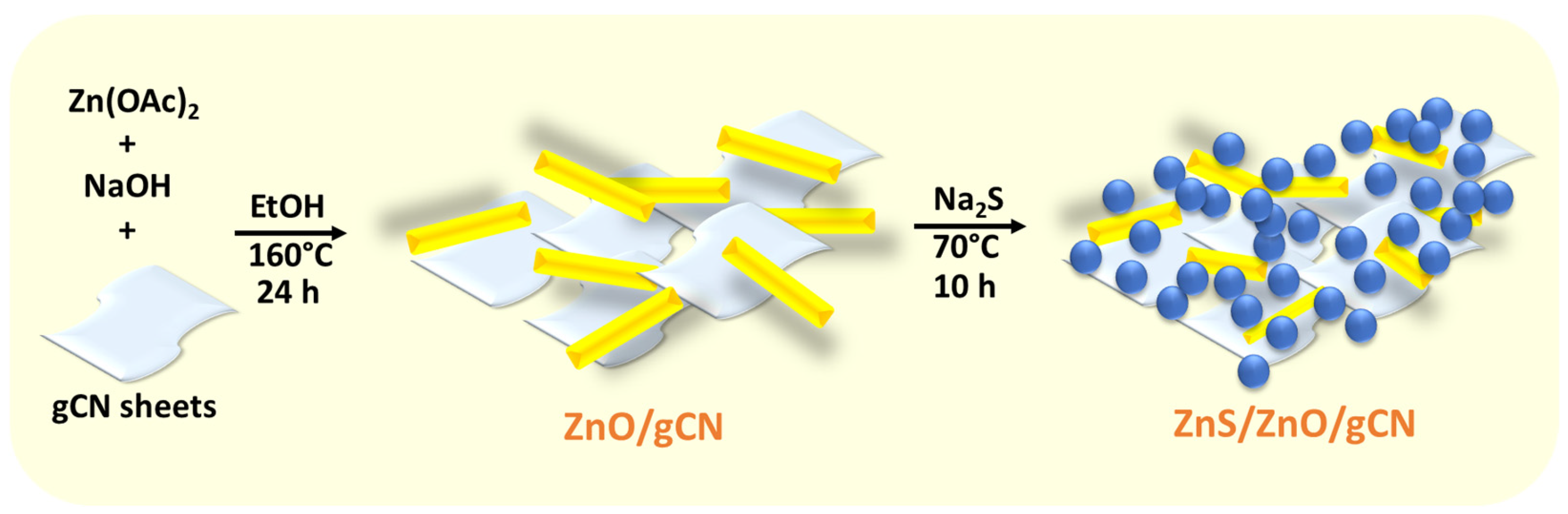
2.3. Synthesis of ZnO/gCN Photocatalysts
2.4. Sulfuration of ZnO/gCN. Preparation of ZnS/ZnO/gCN Catalysts
2.5. Photocatalytic Hydrogen Production
2.6. Characterizations
3. Results
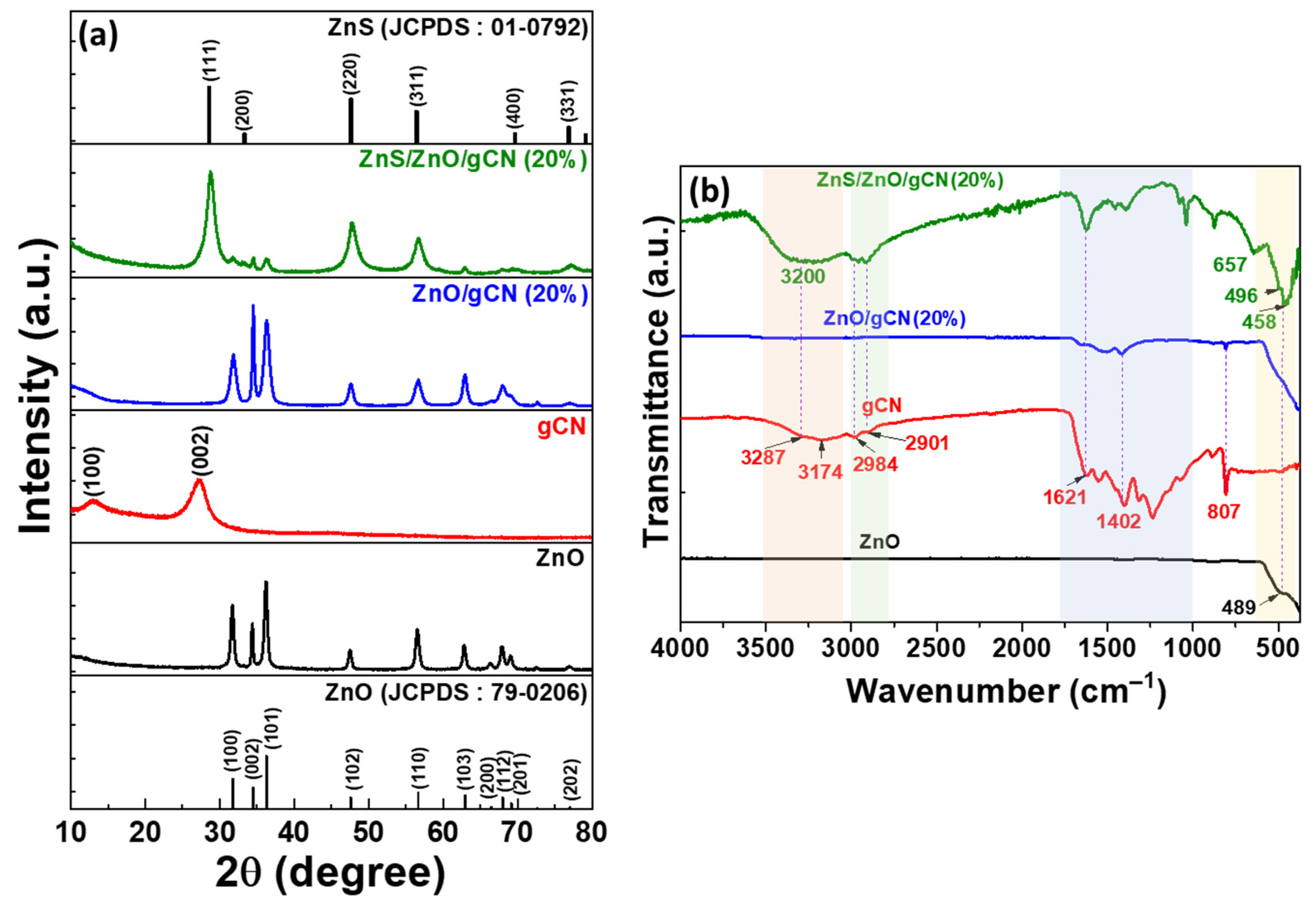
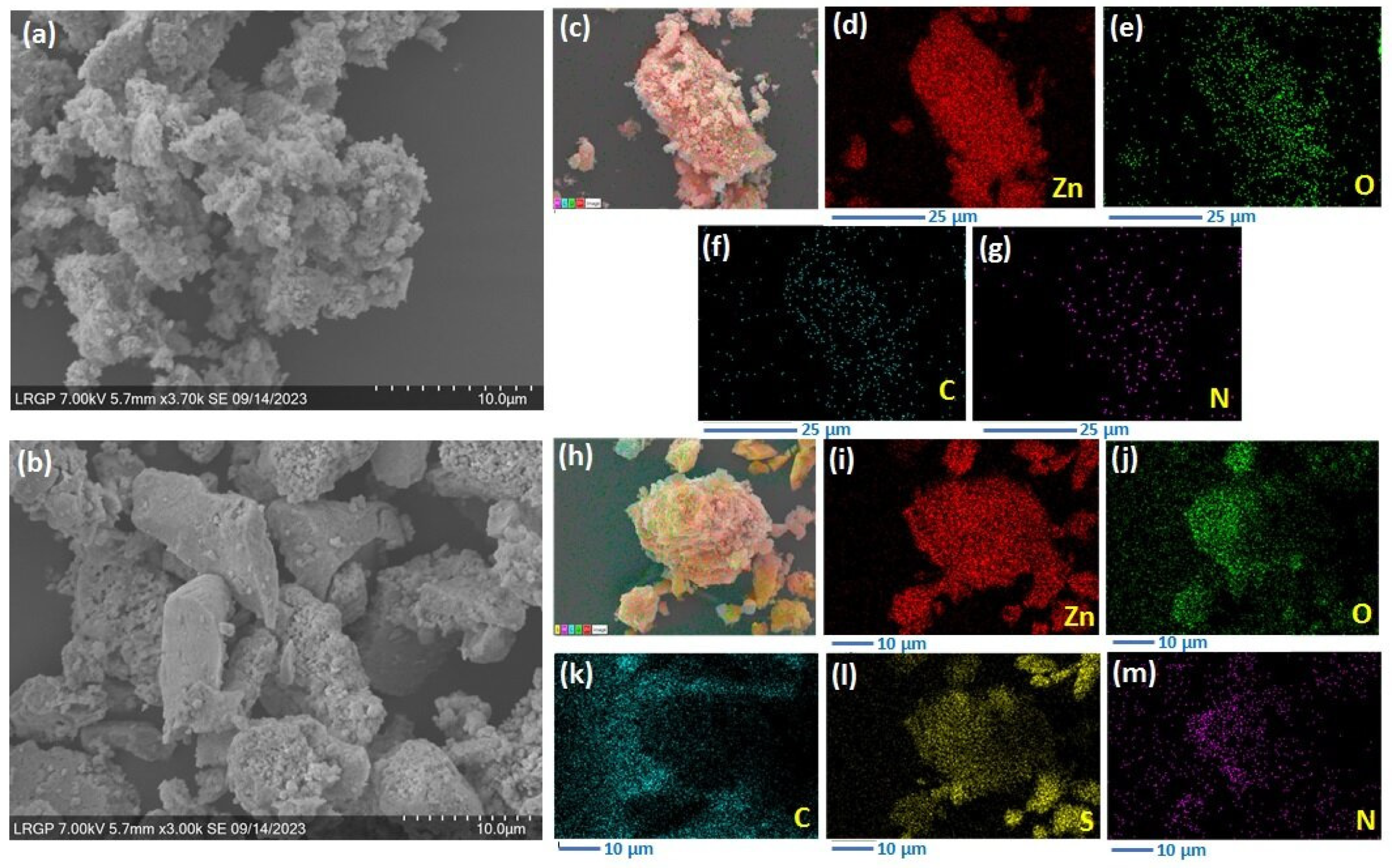
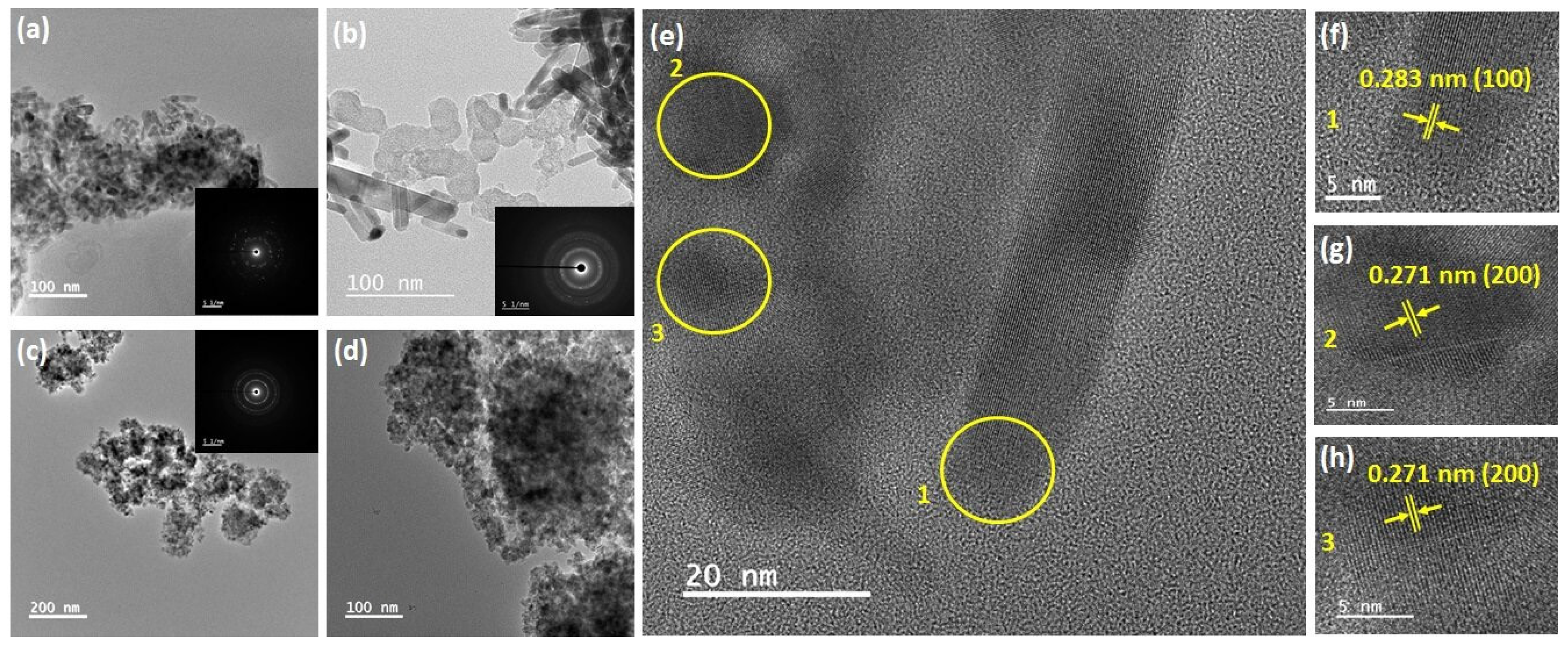
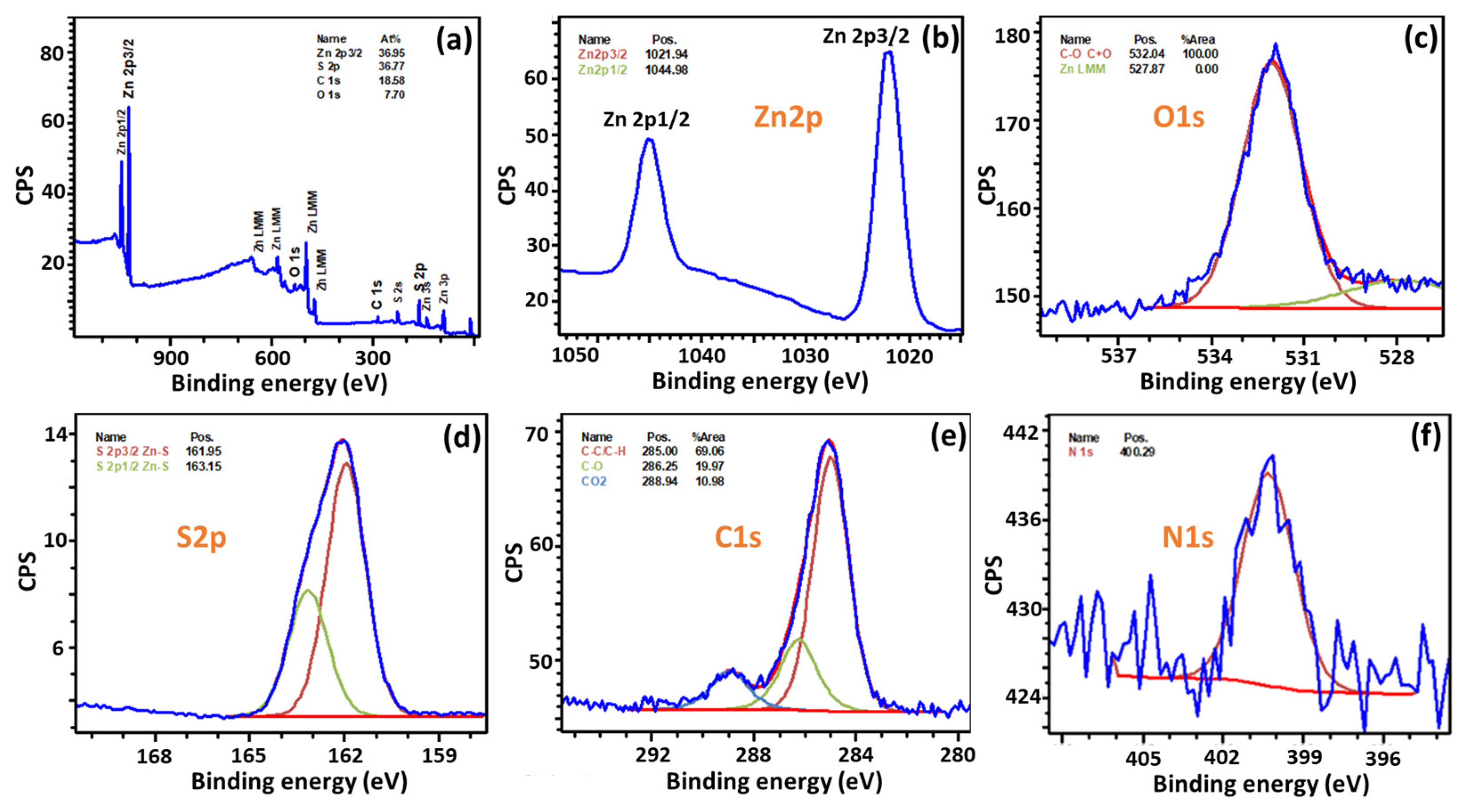
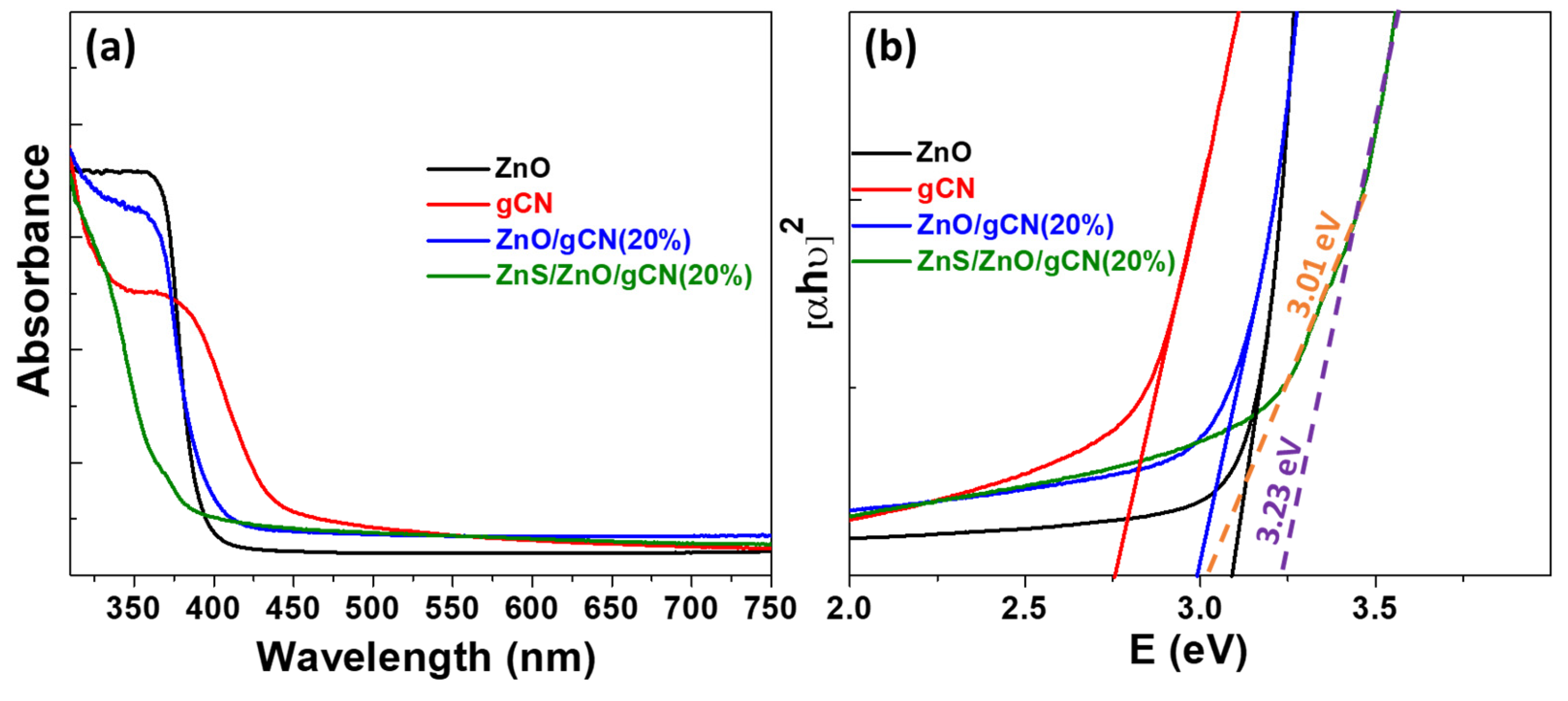
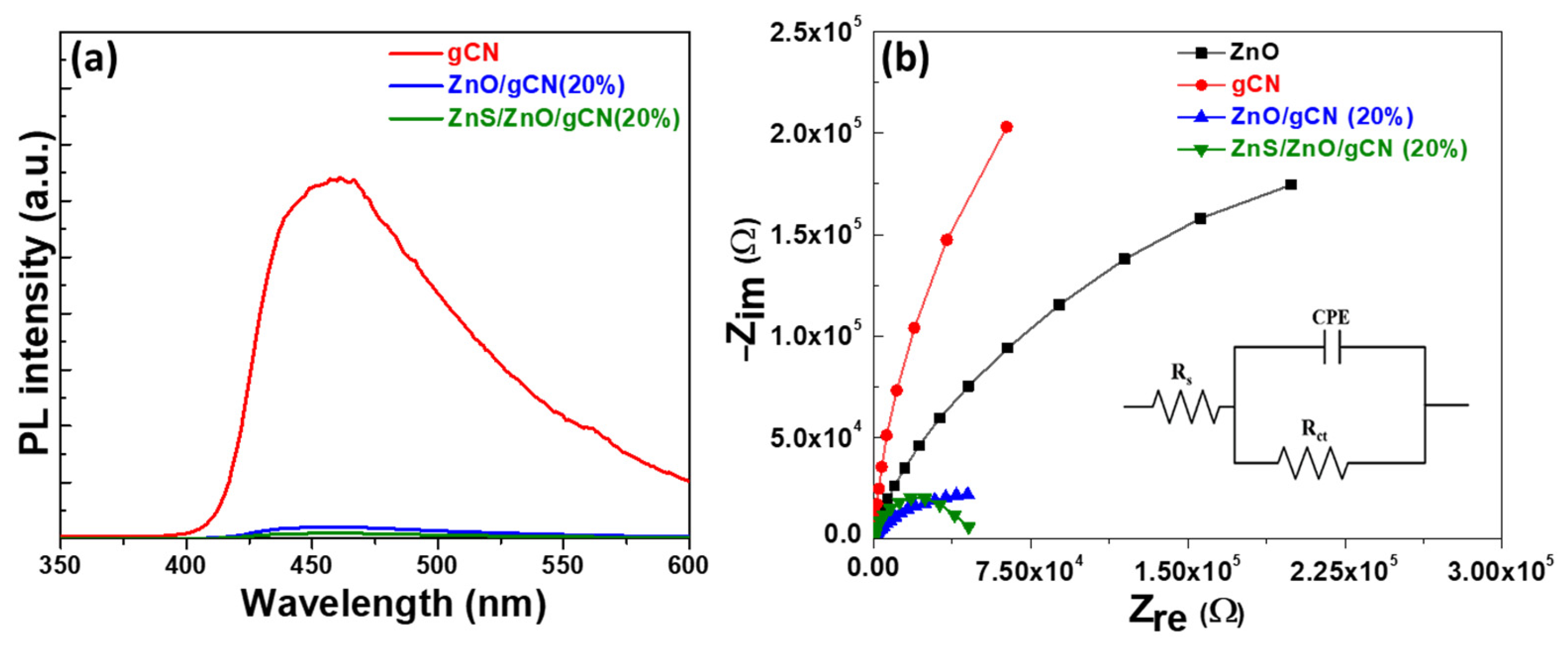
3.1. Photocatalyst Synthesis and Characterization
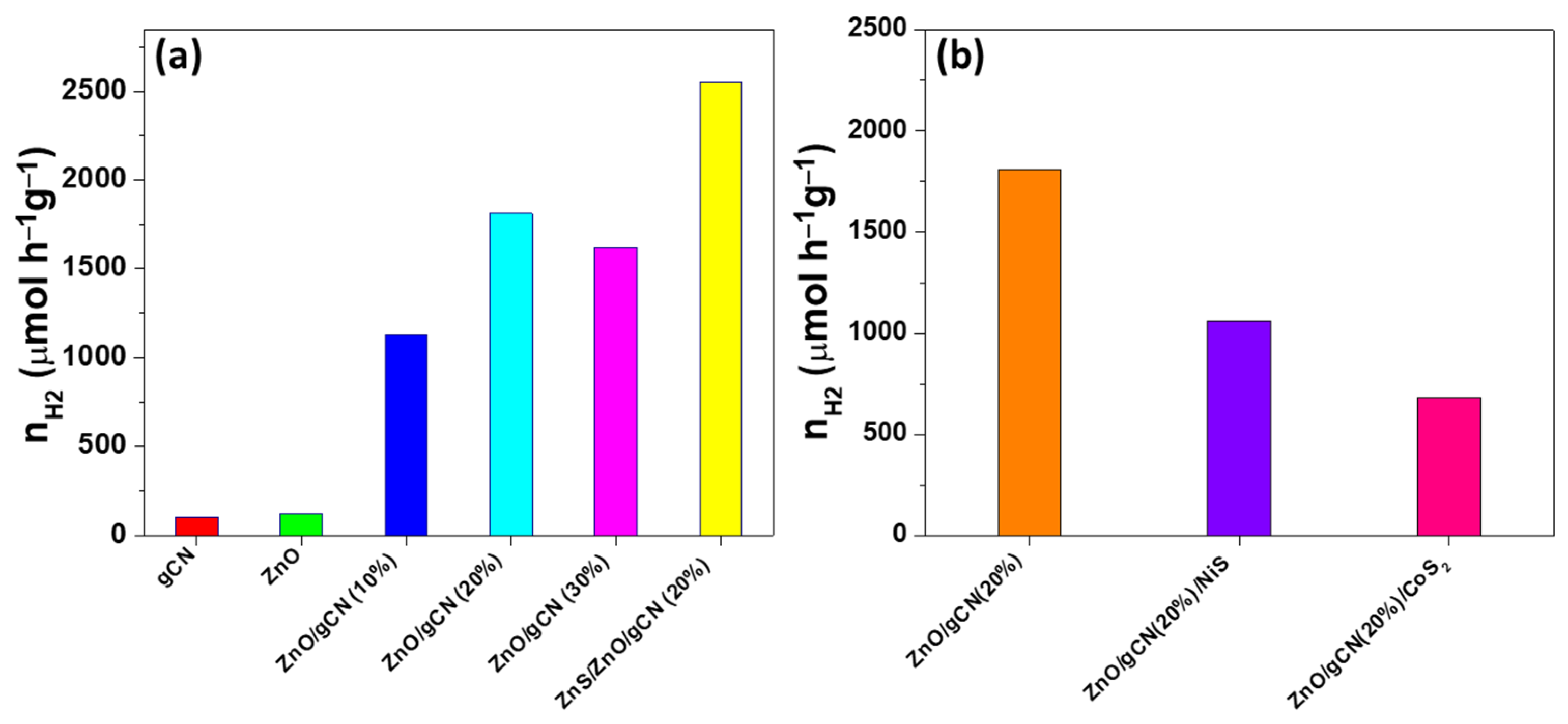
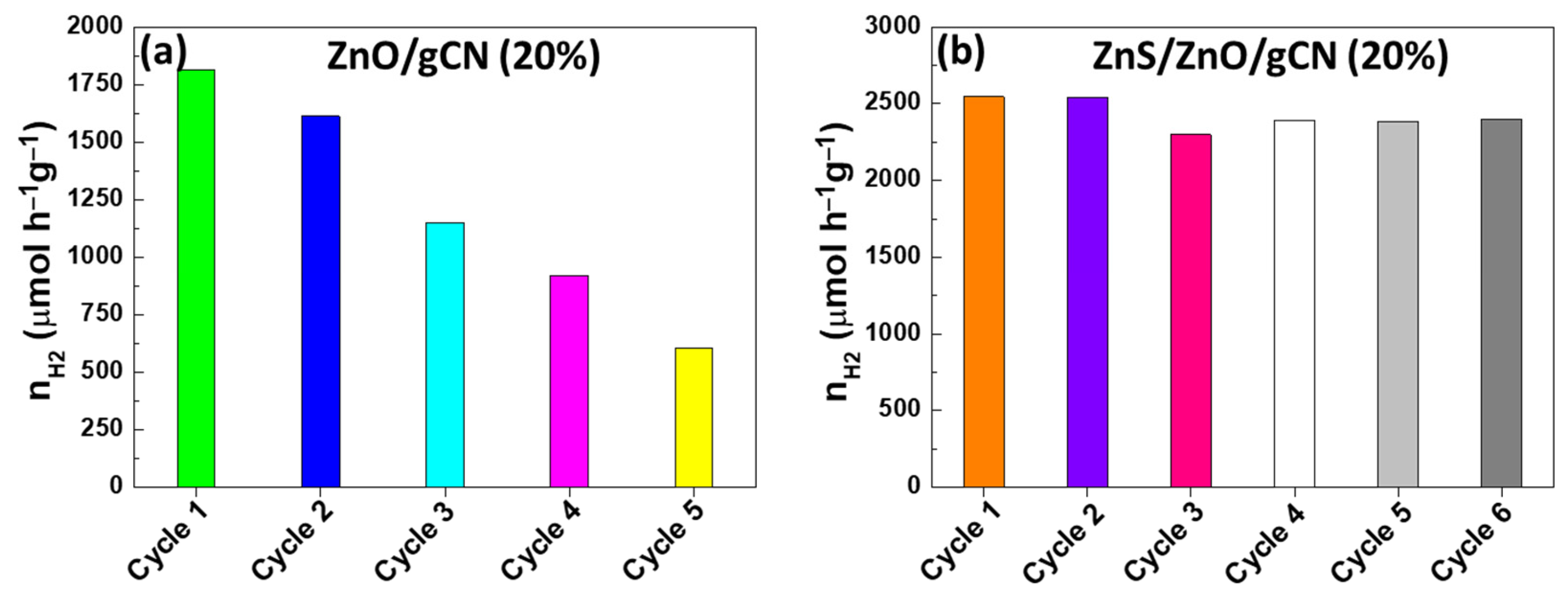
3.2. Photocatalytic Performance and Stability
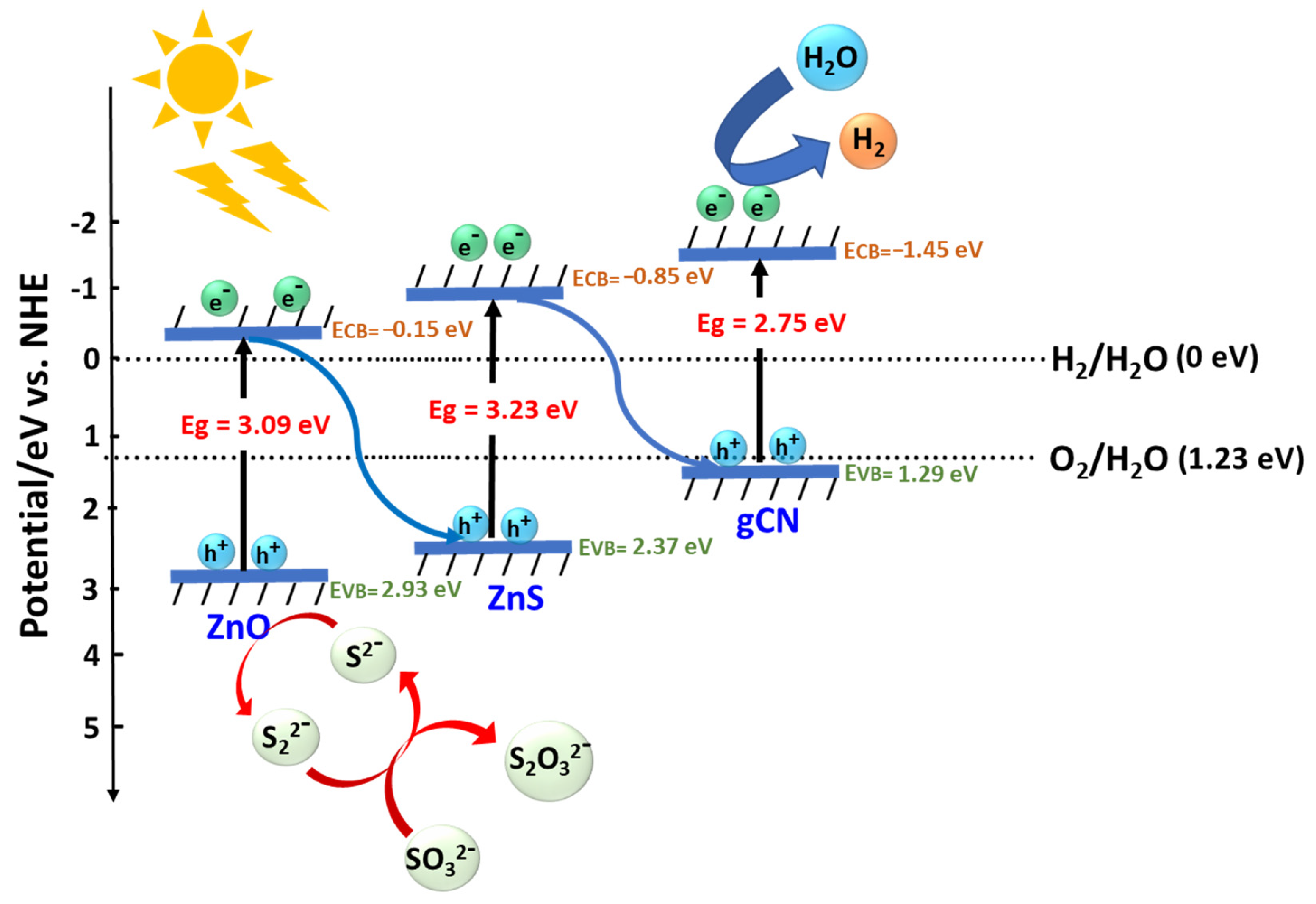
3.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Zuhair Sameen, A.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipinski, W.; Khalilpou, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Kumar Lakhera, S.; Rajan, A.; Rugma, T.P.; Bernaurdshaw, N. A review on particulate photocatalytic hydrogen production system: Progress made in achieving high energy conversion efficiency and key challenges ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Sharma, R.; Almáši, M.; Pal Nehra, S.; Singh Rao, V.; Panchal, P.; Rattan Paul, D.; Prabh Jain, I.; Sharma, A. Photocatalytic hydrogen production using graphitic carbon nitride (GCN): A precise review. Renew. Sustain. Energy Rev. 2022, 168, 112776. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2020, 59, 1295–1301. [Google Scholar] [CrossRef]
- Cline, E.D.; Adamson, S.E.; Bernhard, S. Homogeneous Catalytic System for Photoinduced Hydrogen Production Utilizing Iridium and Rhodium Complexes. Inorg. Chem. 2008, 47, 10378–10388. [Google Scholar] [CrossRef]
- Li, T.; Tsubaki, N.; Jin, Z. S-scheme heterojunction in photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 169, 82–104. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production. Adv. Mater. 2018, 30, 1804282. [Google Scholar] [CrossRef]
- Lu, L.; Wu, B.; Shi, W.; Cheng, P. Metal–organic framework-derived heterojunctions as nanocatalysts for photocatalytic hydrogen production. Inorg. Chem. Front. 2019, 6, 3456–3467. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Dinh, C.-T.; Béland, F.; Do, T.-O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; Chouchene, B.; Gries, T.; Balan, L.; Mozet, K.; Medjahdi, G.; Schneider, R. Growth of ZnO Nanorods on Graphitic Carbon Nitride gCN Sheets for the Preparation of Photocatalysts with High Visible-Light Activity. ChemCatChem 2018, 10, 4973–4983. [Google Scholar] [CrossRef]
- Behera, P.; Ray, A.; Tripathy, S.R.; Acharya, L.; Subudhi, S.; Parida, K. ZIF-8 derived porous C, N co-doped ZnO modified B-g-C3N4: A Z-Scheme charge dynamics approach operative towards photocatalytic hydrogenevolution and ciprofloxacin degradation. J. Photochem. Photobiol. A Chem. 2023, 436, 114415. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Byrappa, N.M.; Alnaggar, G.; Hezam, A.; Nagaraju, G.; Pramoda, K.; Byrappa, K. Rapid and facile synthesis of Z -scheme ZnO/g-C3N4 heterostructure as efficient visible light-driven photocatalysts for dye degradation and hydrogen evolution reaction. J. Hazard. Mater. Adv. 2023, 9, 100230. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Ismael, M. The photocatalytic performance of the ZnO/g-C3N4 composite photocatalyst toward degradation of organic pollutants and its inactivity toward hydrogen evolution: The influence of light irradiation and charge transfer. Chem. Phys. Lett. 2020, 739, 136992. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Yao, Z.; Yang, Z.; Xu, H. Enhanced visible-light-driven photocatalytic hydrogen evolution and NO photo-oxidation capacity of ZnO/g-C3N4 with N dopant. Colloids Surf. A 2020, 599, 124869. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; LWang, L.; Li, Y.; Yu, J. Hierarchically Porous ZnO/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient H2O2 Production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, T.; Zhang, L. A Z-scheme mechanism of N-ZnO/g-C3N4 for enhanced H2 evolution and photocatalytic degradation. Appl. Surf. Sci. 2019, 466, 133–140. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.-T.; Qin, X.-S.; Wu, S.-J.; Zhao, H.; Yu, W.-B.; Chen, L.-H.; Li, Y.; Su, B.-L. Light-assisted preparation of heterostructured g-C3N4/ZnO nanorods arrays for enhanced photocatalytic hydrogen performance. Catal. Today 2020, 355, 932–936. [Google Scholar] [CrossRef]
- Rakikuddin, M.; Kim, H. Samarium(III)-doped ZnO/graphitic-C3N4 composites for enhanced hydrogen generation from water under visible light photocatalysis. J. Alloys Compd. 2020, 832, 154887. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Hussain, Z.; Ali Shah, M.I.; Ateeq, M.; Ullah, M.; Ali, N.; Shaheen, S.; Yasmeen, H.; Ali Shah, S.N.; et al. Extended visible light driven photocatalytic hydrogen generation by electron induction from g-C3N4 nanosheets to ZnO through the proper heterojunction. Z. Phys. Chem. 2022, 236, 53–66. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119538. [Google Scholar] [CrossRef]
- Liang, S.; Sui, G.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Li, J.; Wang, C. g-C3N4-wrapped nickel doped zinc oxide/carbon core-double shell microspheres for high-performance photocatalytic hydrogen production. J. Colloid Interface Sci. 2023, 635, 83–93. [Google Scholar] [CrossRef]
- Khamdang, C.; Singsen, S.; Ngoipala, A.; Fongkaew, I.; Junkaew, A.; Suthirakun, S. Computational Design of a Strain-Induced 2D/2D g-C3N4/ZnO S-scheme Heterostructured Photocatalyst for Water Splitting. ACS Appl. Energy Mater. 2022, 5, 13997–14007. [Google Scholar] [CrossRef]
- Girish Kumar, S.; Kavitha, R.; Manjunatha, C. Review and Perspective on Rational Design and Interface Engineering of g-C3N4/ZnO: From Type-II to Step-Scheme Heterojunctions for Photocatalytic Applications. Energy Fuels 2023, 37, 14421–14472. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Machado Filho, M.A.; Hsiao, C.-L.; Batista dos Santos, R.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Self-Induced Core-Shell InAlN Nanorods: Formation and Stability Unraveled by Ab Initio Simulations. ACS Nanosci. Au 2023, 3, 84–93. [Google Scholar] [CrossRef]
- Alaya, Y.; Chouchene, B.; Medjahdi, G.; Balan, L.; Bouguila, N.; Schneider, R. Heterostructured S-TiO2/g-C3N4 Photocatalysts with High Visible Light Photocatalytic Activity. Catalysts 2024, 14, 226. [Google Scholar] [CrossRef]
- Dworschak, D.; Brunnhofer, C.; Valtiner, M. Photocorrosion of ZnO Single Crystals during Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 51530–51536. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wu, Y.; Thirugnanam, N. Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 2018, 430, 293–3000. [Google Scholar] [CrossRef]
- Prabhu Yendrapati, T.; Prasad Ega, S.; Moses Abraham, B.; Pal, U. Hydrothermally decorated robust bimetallic sulfides with heterojunction interfaces for efficient hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 40254–40263. [Google Scholar] [CrossRef]
- Wu, T.; He, Q.; Liu, Z.; Shao, B.; Liang, Q.; Pan, Y.; Huang, J.; Peng, Z.; Liu, Y.; Zhao, C.; et al. Tube wall delamination engineering induces photogenerated carrier separation to achieve photocatalytic performance improvement of tubular g-C3N4. J. Hazard. Mater. 2022, 424, 127177. [Google Scholar] [CrossRef]
- Jothibas, M.; Johnson Jayakumar, S.; Manoharan, C.; Kartharinal Punithavathy, I.; Praveen, P.; Prince Richard, J. Structural and optical properties of zinc sulphide nanoparticles synthesized via solid state reaction method. J. Mater. Sci. Mater. Electron. 2017, 28, 1889–1894. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J.; Li, Y. Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles. Appl. Catal. A Gen. 2011, 409–410, 215–222. [Google Scholar] [CrossRef]
- Vu, M.-H.; Sakar, M.; Nguyen, C.-C.; Do, T.-O. Chemically Bonded Ni Cocatalyst onto the S Doped g-C3N4 Nanosheets and Their Synergistic Enhancement in H2 Production under Sunlight Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 4194–4203. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-scheme 2D/3D g-C3N4@ZnO with enhanced photocatalytic activity for cephalexin oxidation under solar light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Liu, X.; Willander, M. Hydrothermal Synthesis of Nanoclusters of ZnS Comprised on Nanowires. Nanomaterials 2013, 3, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Yang, Y.; Guo, Y. Controllable Approach to Carbon-Deficient and Oxygen-Doped Graphitic Carbon Nitride: Robust Photocatalyst Against Recalcitrant Organic Pollutants and the Mechanism Insight. Adv. Funct. Mater. 2021, 31, 2010763. [Google Scholar] [CrossRef]
- Sang, H.X.; Wang, X.T.; Fan, C.C.; Wang, F. Enhanced photocatalytic H2 production from glycerol solution aver ZnO/ZnS core/shell nanorods prepared by a low temperature route. Int. J. Hydrogen Energy 2012, 37, 1348–1355. [Google Scholar] [CrossRef]
- Lin, H.; Wei, L.; Wu, C.; Chen, Y.; Yan, S.; Mei, L.; Jiao, J. High-Performance Self-powered Photodetectors Based on ZnO/ZnS Core-Shell Nanorod Arrays. Nanoscale Res. Lett. 2016, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, H.; Cui, G.; Dong, Y.; Wang, G.; Jiang, P.; Wu, X.; Zhao, N. A photochemical synthesis route to typical transition metal sulfides as highly efficient cocatalyst for hydrogen evolution: From the case of NiS/g-C3N4. Appl. Catal. B Environ. 2018, 225, 284–290. [Google Scholar] [CrossRef]
- Yang, S.; Guo, X.; Liu, K.; Li, Y.; Li, T.; Gu, X.; Arenal, R.; Zheng, X.; Li, W.; Sun, C.; et al. Size effect of CoS2 cocatalyst on photocatalytic hydrogen evolution performance of g-C3N4. J. Colloid Interface Sci. 2023, 635, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Wang, X. Post-annealing reinforced hollow carbon nitride nanospheres for hydrogen photosynthesis. Nanoscale 2015, 7, 465–470. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
| Catalyst | Light Source | Sacrificial Substrate | Co-Catalyst | H2 Production Rate (μmol h−1 g−1) or H2 Amount (μmol) | Reference |
|---|---|---|---|---|---|
| gCN/O-defective ZnO | 300 W Xe lamp with UV filter | TEOA a | - | 322 μmol h−1 g−1 | [17] |
| gCN/N-doped ZnO | 300 W Xe lamp | MeOH | - | 152.7 μmol h−1 g−1 | [21] |
| gCN/ZnO NRs arrays | Simulated solar light | Na2S/Na2SO3 | - | 33 μmol after 3 h | [22] |
| gCN/Sm-doped ZnO | 300 W Xe lamp with UV filter | Lactic acid | - | 10,250 μmol h−1 g−1 | [23] |
| gCN/N-doped ZnO | 300 W Xe lamp with UV filter | TEOA | Pt (1.5 wt%) | 780 μmol h−1 g−1 | [19] |
| gCN/ZnO | 300 W Xe lamp with UV filter | MeOH | - | 70 μmol after 1 h | [24] |
| B-doped gCN/ZnO | 300 W Xe lamp | TEOA | Pt (2 wt%) | 357 μmol h−1 g−1 | [25] |
| B-doped gCN/C,N-doped ZnO | 125 W Xe lamp | MeOH | - | 7020 μmol h−1 g−1 | [15] |
| gCN/ZnO + eosin Y as sensitizer | 400 W Xe lamp | TEOA | - | 1358 μmol h−1 g−1 | [16] |
| gCN/Ni-doped ZnO/C microspheres | 300 W Xe lamp | TEOA | Pt (2 wt%) | 336 μmol h−1 g−1 | [26] |
| gCN/ZnO/ZnS | 300 W Xe lamp | Na2S/Na2SO3 | - | 301 μmol h−1 g−1 | [32] |
| gCN/ZnO/ZnS | 300 W Xe lamp | Na2S/Na2SO3 | - | 2548 μmol h−1 g−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolatov, A.; Manjovelo, A.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Uralbekov, B.; Schneider, R. Ternary ZnS/ZnO/Graphitic Carbon Nitride Heterojunction for Photocatalytic Hydrogen Production. Materials 2024, 17, 4877. https://doi.org/10.3390/ma17194877
Bolatov A, Manjovelo A, Chouchene B, Balan L, Gries T, Medjahdi G, Uralbekov B, Schneider R. Ternary ZnS/ZnO/Graphitic Carbon Nitride Heterojunction for Photocatalytic Hydrogen Production. Materials. 2024; 17(19):4877. https://doi.org/10.3390/ma17194877
Chicago/Turabian StyleBolatov, Asset, Alida Manjovelo, Bilel Chouchene, Lavinia Balan, Thomas Gries, Ghouti Medjahdi, Bolat Uralbekov, and Raphaël Schneider. 2024. "Ternary ZnS/ZnO/Graphitic Carbon Nitride Heterojunction for Photocatalytic Hydrogen Production" Materials 17, no. 19: 4877. https://doi.org/10.3390/ma17194877









