The Adsorption of Pb(II) from Aqueous Solution Using KOH-Modified Banana Peel Hydrothermal Carbon: Adsorption Properties and Mechanistic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Methods
2.2.1. Preparation of Banana Peel Hydrothermal Carbon
2.2.2. Preparation of KOH-Modified Hydrothermal Carbon
2.2.3. Adsorption Experiment
2.2.4. Experimental Equipment
3. Results and Analysis
3.1. Surface Structure and Performance Analysis of Hydrothermal Carbon before and after Modification
3.1.1. SEM Analysis
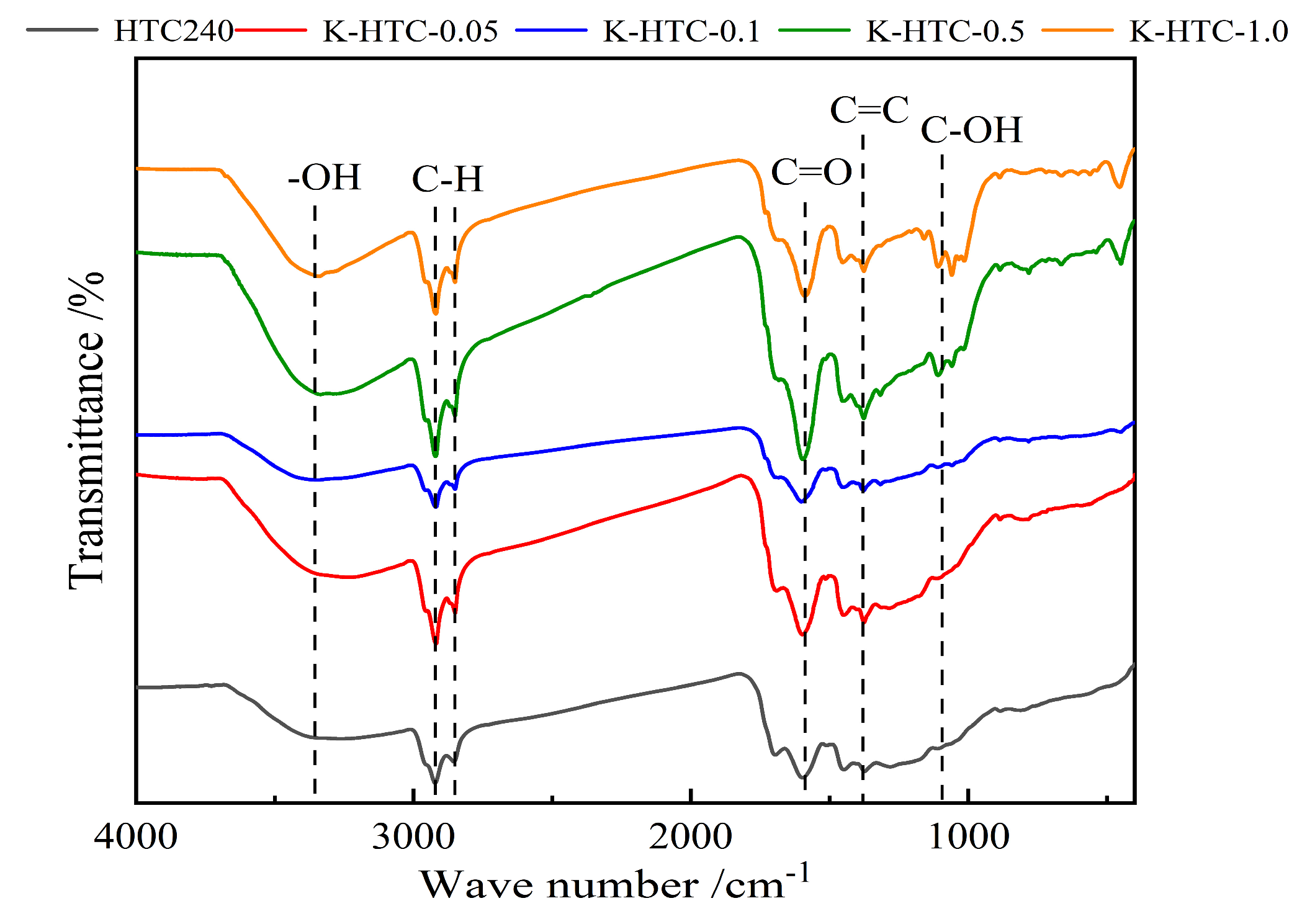
3.1.2. FT-IR Analysis
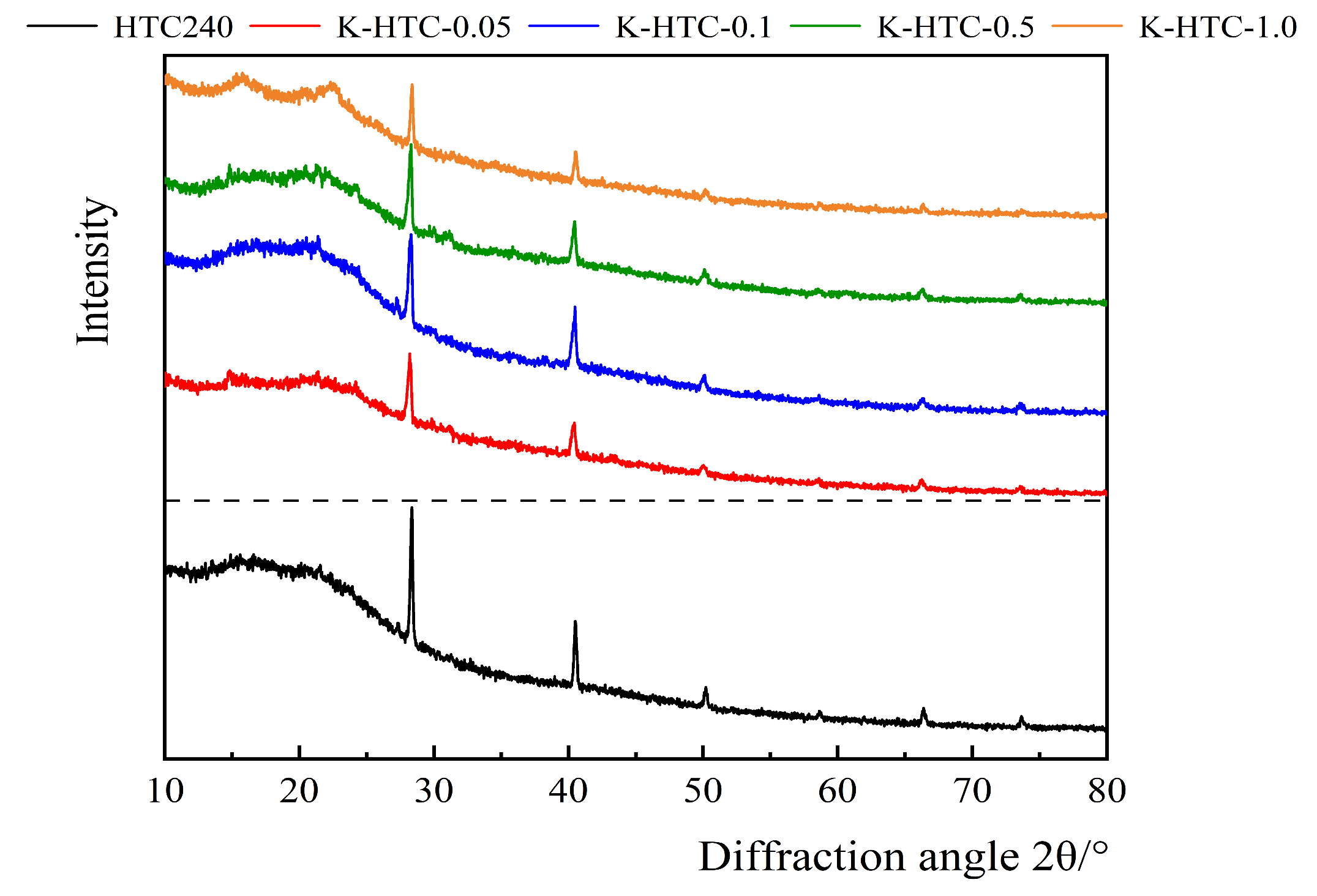
3.1.3. XRD Analysis
3.1.4. BET Analysis
3.1.5. Element Analysis
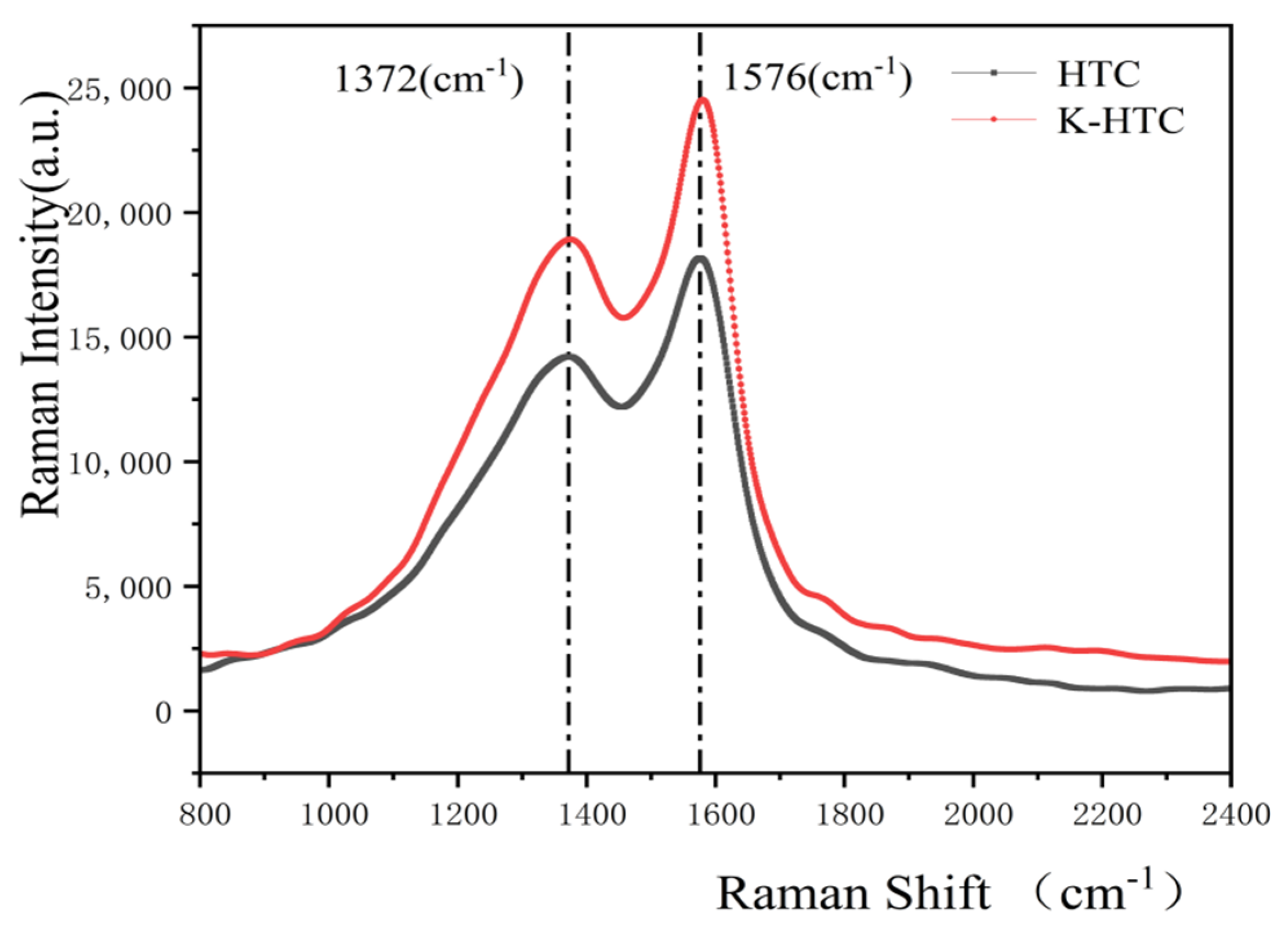
3.1.6. Raman Analysis
3.2. Analysis of the Lead Ion Adsorption Performance of Hydrothermal Carbon
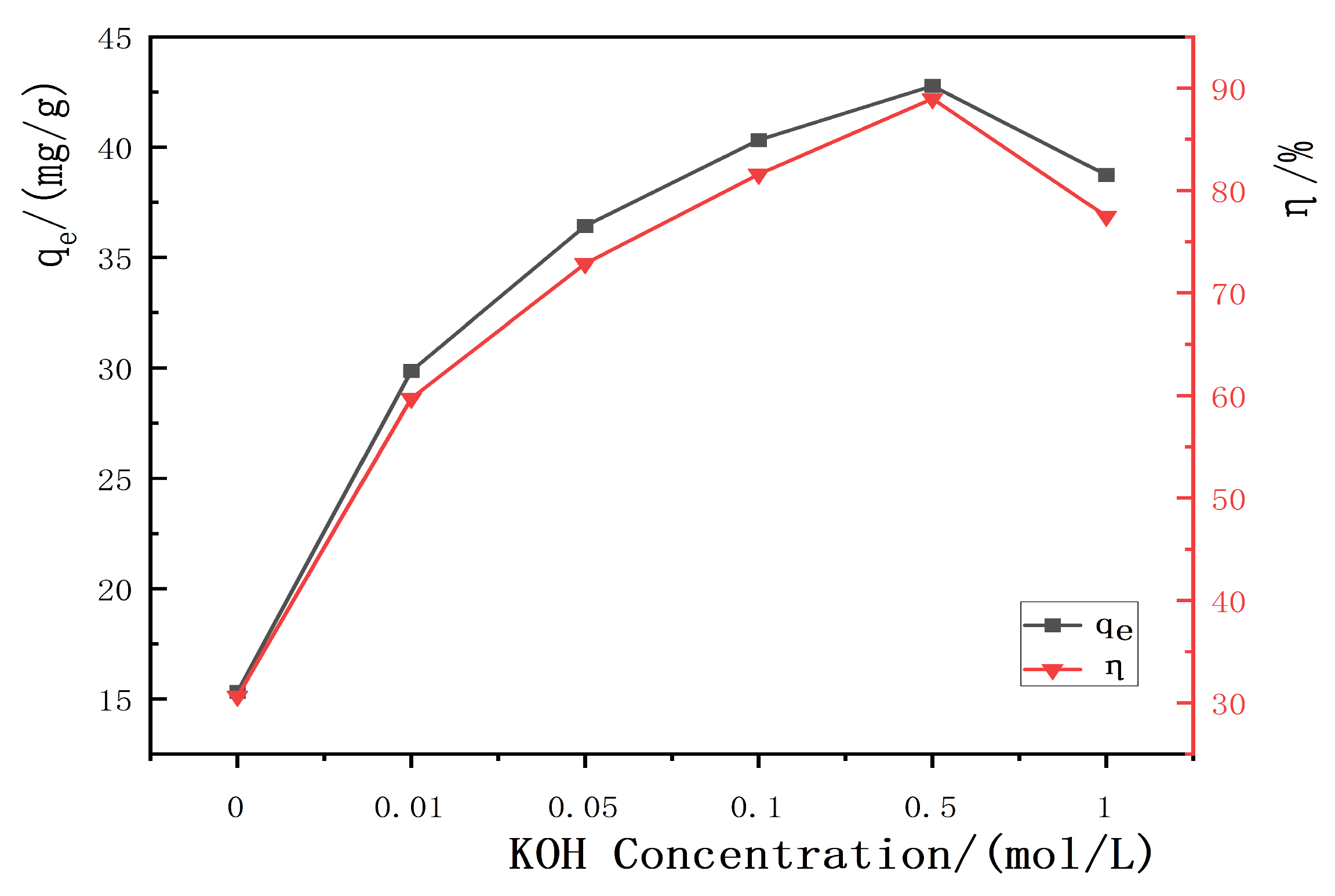
3.2.1. Influence of KOH Concentration on the Adsorption Performance of Hydrothermal Carbon
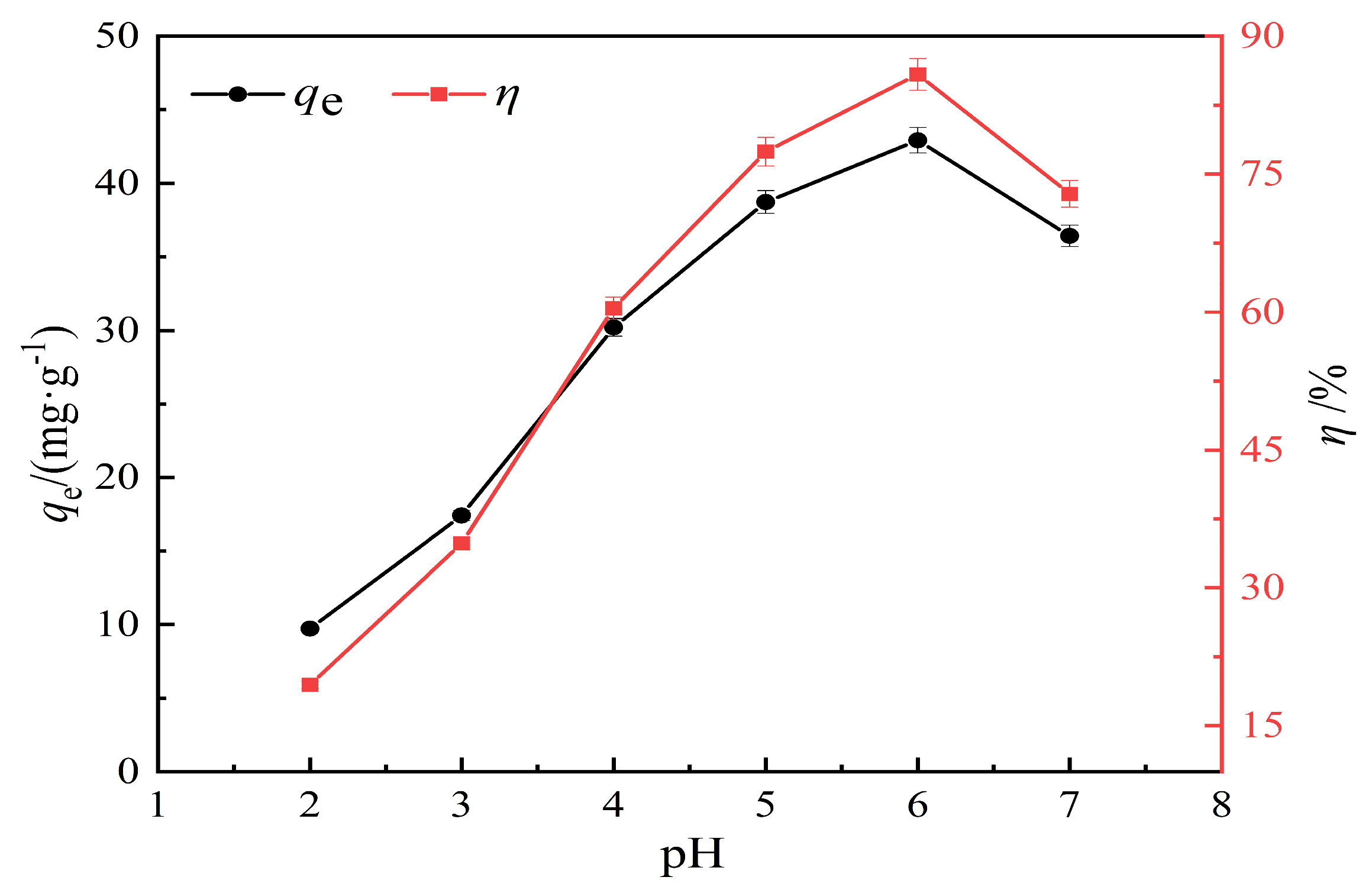
3.2.2. Effect of pH on Lead Ion Adsorption Performance
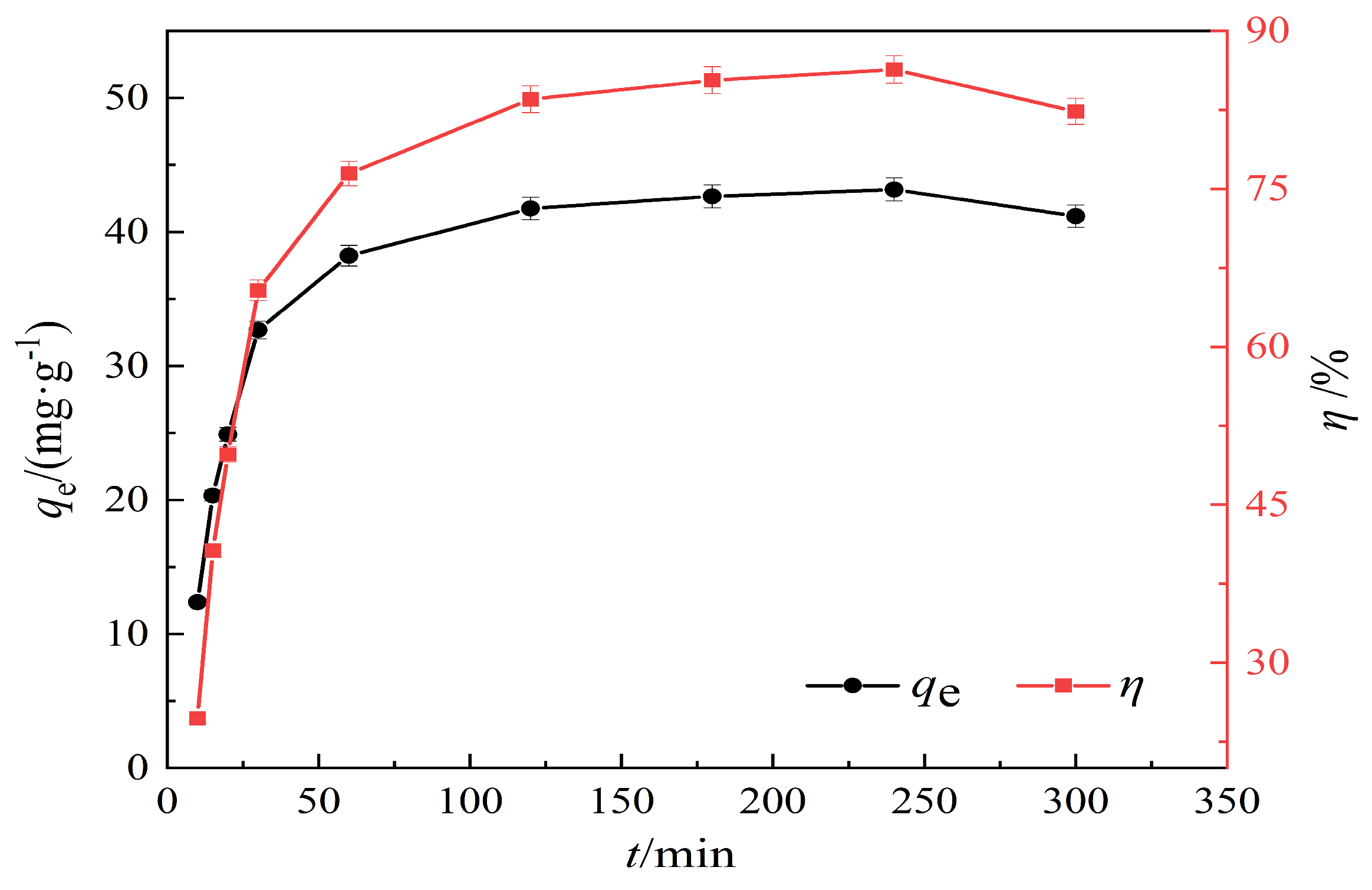
3.2.3. Effect of Adsorption Time on the Adsorption Performance of Hydrothermal Carbon
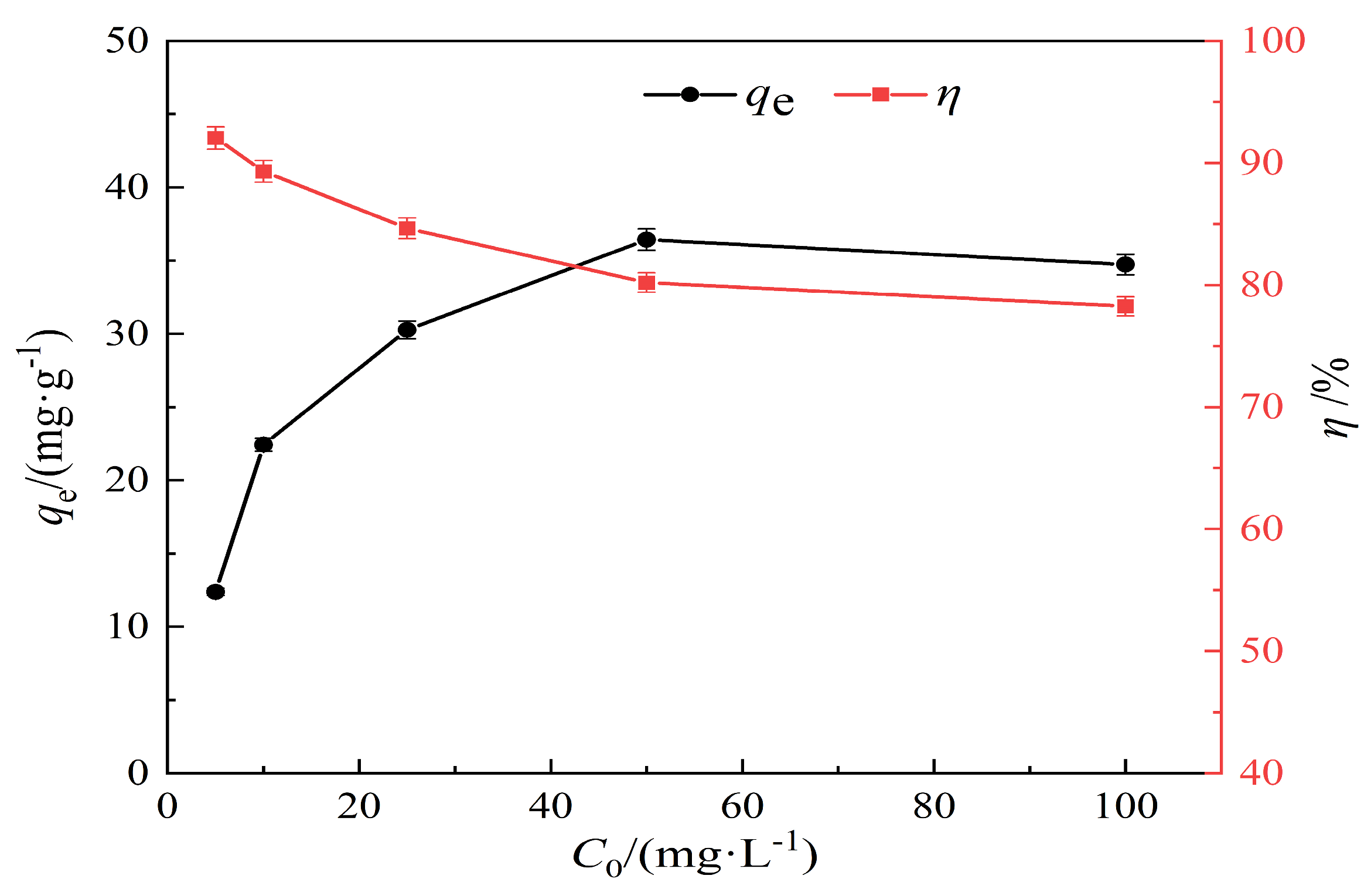
3.2.4. Effect of Lead Solution at Different Concentrations on the Adsorption Properties of Hydrothermal Carbon
3.2.5. Adsorption Kinetics of Modified Hydrothermal Carbon
3.2.6. Modified Hydrothermal Carbon Isothermal Adsorption Analysis
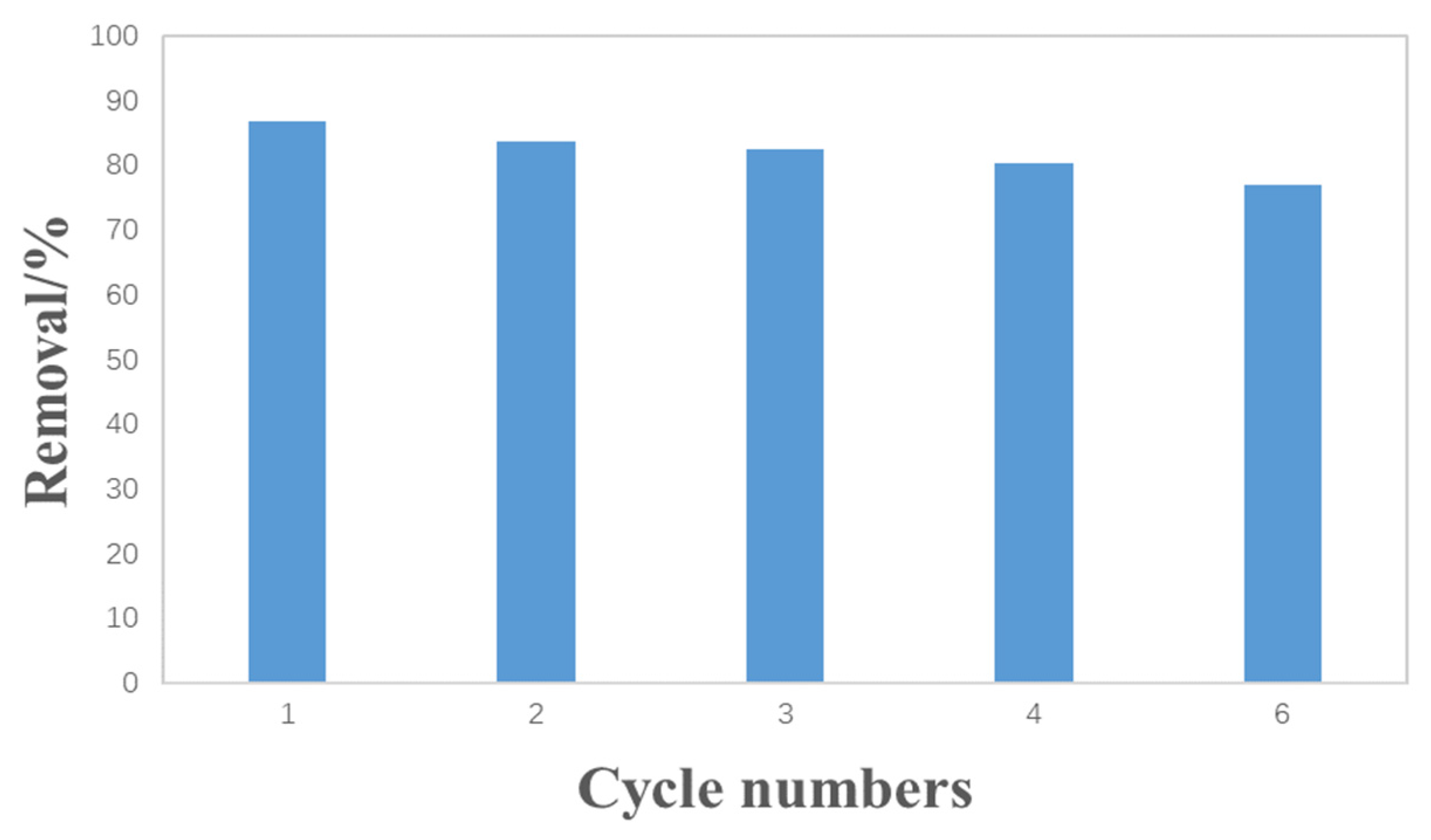
3.3. The Reusability of Hydrothermal Carbon
4. Conclusions
- (1)
- K-HTC is a carbon material obtained by KOH modification of banana peel hydrothermal carbon. This mainly included amorphous carbon with a large number of oxygen-containing functional groups on its surface, which could provide active sites for adsorption;
- (2)
- An adsorption capacity of 42.92 mg/g and a removal rate of 86.84% were achieved when the banana peel hydrothermal carbon was modified with a KOH solution with a pH of 6 and a solid–liquid ratio of 1 g/L;
- (3)
- The Redlich–Peterson model in the adsorption thermodynamics model was more suitable for the adsorption process of modified hydrothermal carbon on Pb(II), with correlation coefficients (R2) of 0.98, 0.99, and 0.99 at 293 K, 303 K, and 313 K, respectively, indicating a multilayer adsorption process. The second-order kinetic model in the adsorption kinetics model better represented the adsorption process of modified hydrothermal carbon on Pb(II), indicating chemical–physical adsorption;
- (4)
- Hydrothermal carbon is a new type of adsorbent with the advantages of high adsorption capacity, good adsorption effects, and simple regeneration. The hydrothermal carbon method could effectively aid in removing heavy metal pollutants in wastewater and has a wide range of application prospects.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, L.; Wang, P.; Liu, H.; Xiong, M.; Wang, H. Research progress on the adsorption and removal of lead ions in wastewater using functionalized metal-organic framework materials. Mater. Guide 2022, 36, 40–50. [Google Scholar]
- Li, X.; Pan, F.; Sun, C.; Zhang, M.; Wang, Z.; Du, J.; Wang, J.; Xiao, M.; Xue, L.; Zhang, Z.-G.; et al. Simplified synthetic routes for low cost and high photovoltaic performance n-type organic semiconductor acceptors. Nat. Commun. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.B.; Zhao, J.B.; Zhou, S.P.; Wu, C.H.; Zhao, D. Performance and adsorption mechanism of peanut shell biochar for removing lead and cadmium ions from water. J. Southwest For. Univ. (Nat. Sci.) 2022, 42, 78–86. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Luo, C.; Routh, J.; Luo, D.; Wei, L.; Liu, Y. Arsenic in the Pearl River Delta and its related waterbody, South China: Occurrence and sources, a review. Geosci. Lett. 2021, 8, 12. [Google Scholar] [CrossRef]
- Van der Kuijp, T.J.; Huang, L.; Cherry, C.R. Health hazards of China’s lead-acid battery industry: A review of its market drivers, production processes, and health impacts. Environ. Health 2013, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U., Jr.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Elisadiki, J.; Jande, Y.A.C.; Kibona, T.E.; Machunda, R.L. Highly porous biomass based capacitive deionization electrodes for water defluoridation. Ionics 2020, 26, 2477–2492. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Research on the Reaction Mechanism and Product Formation Mechanism of Cow Dung Hot Water Treatment; Central China Agricultural University: Wuhan, China, 2019; Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CDFD&dbname=CDFDLAST2020&filename=1019065795.nh&v= (accessed on 1 January 2023).
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Baloch, H.A.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A. Upgradation of chemical, fuel, thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environ. Sci. Pollut. Res. 2018, 25, 17529–17539. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of oily sludge for solid fuel recovery—Investigation of chemical characteristics and combustion behaviour. J. Anal. Appl. Pyrolysis 2021, 157, 105235. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Cao, D.; Hu, S.; Wang, Y.; Zhang, Y.; Huang, X.; Zhao, H.; Wu, C.; Li, J.; Ding, Y.; et al. High-pressure carbon dioxide-hydrothermal enhance yield and methylene blue adsorption performance of banana pseudo-stem activated carbon. Bioresour. Technol. 2022, 354, 127137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Putra, H.E.; Damanhuri, E.; Dewi, K.; Pasek, A.D. Hydrothermal carbonization of biomass waste under low temperature condition. MATEC Web of Conferences. EDP Sci. 2018, 154, 01025. [Google Scholar]
- Johnson, V.E.; Liao, Q.; Jallawide, B.W., Jr.; Anaman, R.; Amanze, C.; Huang, P.; Cao, W.; Ding, C.; Shi, Y. Simultaneous removal of As (V) and Pb (II) using highly-efficient modified dehydrated biochar made from banana peel via hydrothermal synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131115. [Google Scholar] [CrossRef]
- Mohd, N.S.; Mohamed, R.M. The initial ion effect of heavy metals adsorption by using hydrothermal carbonization banana peels. Environ. Contam. Rev. 2021, 3, 8–10. [Google Scholar]
- Anwar, J.; Shafique, U.; Zaman, W.U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Castro, R.S.D.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana peel applied to the solid phase extraction of copper and lead from river water: Preconcentration of metal ions with a fruit waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Yasim, N.S.E.M.; Ismail, Z.S.; Zaki, S.M.; Azis, M.F.A. Adsorption of Cu, As, Pb and Zn by banana trunk. Malays. J. Anal. Sci. 2016, 20, 103–112. [Google Scholar]
- Zhang, X.; Zhang, L.; Li, A. Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J. Environ. Manag. 2018, 206, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.T.; Ding, Y.; Shao, Y.; Cai, L.; Jin, Z.Y.; Liu, Z.; Zhao, J.; Li, F.; Pan, Z.; Li, X.; et al. Banana peel biochar with nanoflake-assembled structure for cross contamination treatment in water: Interaction behaviors between lead and tetracycline. Chem. Eng. J. 2021, 420, 129807. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Krishnan, B.; Sagadevan, S.; Rafique, R.F.; Hamizi, N.A.B.; Wahab, Y.A.; Khan, A.A.; Bin Johan, R.; Al-Douri, Y.; Kazi, S.N.; et al. Effect of temperature on the physical, electro-chemical and adsorption properties of carbon micro-spheres using hydrothermal carbonization process. Nanomaterials 2018, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Fortenberry, R.C. The unsolved issue with out-of-plane bending frequencies for CC multiply bonded systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zu, J.; Feng, Q.; Chen, H.; Li, J.; Zhong, M.-E.; Zhou, Z.; Zhuang, S. Effect of pyrolysis condition on the adsorption mechanism of heavy metals on tobacco stem biochar in competitive mode. Environ. Sci. Pollut. Res. 2019, 26, 26947–26962. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Shah, M.U.H.; Reddy, A.V.B.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, J.P.; Zhao, X.Y.; Hao, Z.Q.; Zhuang, Q.Q.; Zhu, J.S.; Wang, X.Y.; Wei, X.Y. Preparation of porous carbons by hydrothermal carbonization and KOH activation of lignite and their performance for electric double layer capacitor. Electrochim. Acta 2017, 252, 397–407. [Google Scholar] [CrossRef]
- Deliyanni, E.; Arabatzidou, A.; Tzoupanos, N.; Matis, K.A. Adsorption of Pb2+ using mesoporous activated carbon and its effects on surface modifications. Adsorpt. Sci. Technol. 2012, 30, 627–645. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Teng, F.; Zhou, C. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem. Eng. J. 2021, 405, 126989. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zhang, H. Comparative research on selective adsorption of Pb (II) by biosorbents prepared by two kinds of modifying waste biomass: Highly-efficient performance, application and mechanism. J. Environ. Manag. 2021, 288, 112388. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Song, F.; Li, F.; Xiao, S.; Liu, F.; Jiang, L. Solvent-free molten co-assembly of ordered mesoporous carbon for efficiently supported adsorption and separation of SO2. J. Mater. Chem. A 2022, 10, 8817–8825. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb (II) and Cd (II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef]
- Kowanga, K.D.; Gatebe, E.; Mauti, G.O.; Mauti, E.M. Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu (II) and Pb (II) using defatted Moringa oleifera seed powder. J. Phytopharm. 2016, 5, 71–78. [Google Scholar] [CrossRef]
- Prasad, R.K.; Srivastava, S.N. Sorption of distillery spent wash onto fly ash: Kinetics and mass transfer studies. Chem. Eng. J. 2009, 146, 90–97. [Google Scholar]



| BET Surface Area /(m2∙g−1) | Total Pore Volume /(cm3∙g−1) | Average Pore Size /nm | |
|---|---|---|---|
| HTC240 | 7.3709 | 0.026279 | 14.2607 |
| K-HTC-0.1 | 5.6700 | 0.034832 | 24.5729 |
| C/% | H/% | N/% | O/% | |
|---|---|---|---|---|
| HTC240 | 69.688 | 6.229 | 3.107 | 15.730 |
| K-HTC-0.1 | 66.832 | 5.928 | 2.843 | 18.029 |
| Type of Dynamic Model | Parameters and Correlation Coefficients | |||
|---|---|---|---|---|
| First-order kinetics | k1 | qe | h0 | R2 |
| 1/min | mg/g | mg/(min∙g) | ||
| 4.45 | 42.03 | 187.03 | 0.91 | |
| Second-order kinetics | k2 | qe | h0 | R2 |
| g/(mg∙min) | mg/g | mg/(min∙g) | ||
| 0.14 | 45.48 | 289.58 | 0.98 | |
| Elovich | α | β | - | R2 |
| 1802.09 | 0.15 | - | 0.89 | |
| Isotherm Model | Experimental Conditions | Parameters and Related Parameters | |||
|---|---|---|---|---|---|
| Langmuir | T/K | KL/L∙mg−1 | qm/mg∙g−1 | - | R2 |
| 293 | 0.036 | 101.27 | - | 0.96 | |
| 303 | 0.044 | 120.19 | - | 0.96 | |
| 313 | 0.055 | 131.53 | - | 0.95 | |
| Freundlich | T/K | KF (mg/g(1/mg)1/n) | 1/n | - | R2 |
| 293 | 19.61 | 0.28 | - | 0.97 | |
| 303 | 23.33 | 0.28 | - | 0.98 | |
| 313 | 28.27 | 0.27 | - | 0.98 | |
| Redlich–Peterson | T/K | KR (mg/g(1/mg)1/n) | g | aR | R2 |
| 293 | 14.64 | 0.79 | 0.51 | 0.98 | |
| 303 | 20.23 | 0.78 | 0.60 | 0.99 | |
| 313 | 65.57 | 0.77 | 1.88 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, T.; Zhao, J.; Tian, L.; Zhang, L.; Jin, Z. The Adsorption of Pb(II) from Aqueous Solution Using KOH-Modified Banana Peel Hydrothermal Carbon: Adsorption Properties and Mechanistic Studies. Materials 2024, 17, 311. https://doi.org/10.3390/ma17020311
Bai T, Zhao J, Tian L, Zhang L, Jin Z. The Adsorption of Pb(II) from Aqueous Solution Using KOH-Modified Banana Peel Hydrothermal Carbon: Adsorption Properties and Mechanistic Studies. Materials. 2024; 17(2):311. https://doi.org/10.3390/ma17020311
Chicago/Turabian StyleBai, Tao, Jiaxin Zhao, Laixin Tian, Luming Zhang, and Zhiping Jin. 2024. "The Adsorption of Pb(II) from Aqueous Solution Using KOH-Modified Banana Peel Hydrothermal Carbon: Adsorption Properties and Mechanistic Studies" Materials 17, no. 2: 311. https://doi.org/10.3390/ma17020311






